Abstract
Quail have emerged as a potential intermediate host in the spread of avian influenza A viruses in poultry in Hong Kong. To better understand this possible role, we tested the replication and transmission in quail of influenza A viruses of all 15 HA subtypes. Quail supported the replication of at least 14 subtypes. Influenza A viruses replicated predominantly in the respiratory tract. Transmission experiments suggested that perpetuation of avian influenza viruses in quail requires adaptation. Swine influenza viruses were isolated from the respiratory tract of quail at low levels. There was no evidence of human influenza A or B virus replication. Interestingly, a human–avian recombinant containing the surface glycoprotein genes of a quail virus and the internal genes of a human virus replicated and transmitted readily in quail; therefore, quail could function as amplifiers of influenza virus reassortants that have the potential to infect humans and/or other mammalian species.
Keywords: Virus, Influenza, Avian, North American, Quail, Reassortant
Introduction
Aquatic birds are the natural reservoir of influenza A viruses (Hinshaw et al., 1980a), which replicate in the gastrointestinal tract of waterfowl and are transmitted by the fecal– oral route (Hinshaw et al., 1980b). Influenza A viruses in other hosts, including humans, have ancestral links to waterfowl influenza viruses (reviewed in Webster et al., 1992; Webby and Webster, 2001). However, influenza viruses from waterfowl replicate poorly in humans (Beare and Webster, 1991) and other primates (Murphy et al., 1982), and human viruses replicate poorly in ducks (Hinshaw et al., 1983). Therefore, waterfowl viruses must undergo change before they can cross the species barrier. Because of the segmented nature of their genome, influenza viruses can reassort. Human influenza viruses are thought to be able to acquire genes from waterfowl influenza viruses through reassortment or adaptation in a mammalian intermediate host. Pigs, which are susceptible to infection with both avian and human influenza viruses (Kida et al., 1994), are postulated to be an important intermediate host that acts as a “mixing vessel” in which such reassortment takes place (Scholtissek, 1990). In nature, a limited number of avian and human influenza viruses have established stable lineages in pigs. Occasional transmission of influenza viruses from pigs to humans, with resulting respiratory disease, has also been documented (reviewed by Brown, 2000).
A new picture emerged in 1997, when H5N1 viruses circulating in poultry in Hong Kong were transmitted directly to humans. Six of 18 people known to be infected died (reviewed by Shortridge, 1999). In 1999, viruses of the H9N2 subtype, which are endemic in poultry species in Asia, were transmitted to humans and pigs; they caused mild respiratory disease in some humans but were not lethal (Peiris et al., 1999; Lin et al., 2000; Guo et al., 1999). These incidents raised the possibility that land-based poultry species are a potential source of influenza viruses that can cross to humans (Shortridge et al., 1998; Webby and Webster, 2001).
Recent observations suggest that the potential role of quail (Coturnix coturnix) as intermediate hosts in the interspecies transmission of influenza viruses has been underestimated. The first reported cases of influenza A respiratory disease in quail occurred in Italy during 1966–1968 (Nardelli et al., 1970). Mortality was observed in young birds in 13 different flocks. Influenza viruses of several subtypes (H5N2, H7N2, H7N3, H9N2, and H10N8) have since been isolated from quail in North America, Europe, and Asia in the course of sporadic surveillance (Guan et al., 1999; Guo et al., 2000; Saito et al., 1993; Suarez et al., 1999). Interestingly, quail infected with the highly pathogenic virus Turkey/Ontario/7732/66 (H5N9) show no signs of disease but can transmit the virus to chickens, which die of the infection (Tashiro et al., 1987). Recently, Guan et al. (1999) showed that the H5N1 influenza viruses isolated from humans and poultry in Hong Kong in 1997 possessed internal genes phylogenetically related to those of the quail influenza virus quail/Hong Kong/G1/97 (H9N2). The quail/Hong Kong/G1/97-like influenza viruses continue to circulate in quail and in other minor land-based poultry in Hong Kong (Guan et al., 2000). Quail in Hong Kong also have a high incidence of infection with H6N1 influenza viruses whose NA and internal genes are phylogenetically indistinguishable from those of the H5N1/1997 viruses (Chin et al., 2002). Interestingly, quail infected with either H6N1 or H9N2 viruses show no signs of disease, although they shed virus from the respiratory tract at high concentrations (Perez et al., 2002b and unpublished results). Quail are also more susceptible than chickens to experimental infection with goose H5N1 influenza viruses from southeastern China. The goose H5N1 viruses replicate in the respiratory tract of quail and are transmitted by aerosol (Webster et al., 2002). Quail infected with the goose H5N1 viruses take longer than chickens to show signs of disease and to die, thus increasing the probability of transmission. These observations highlight the need for a better understanding of the role of quail as an intermediate host of influenza A viruses.
We recently showed that quail are susceptible to influenza viruses of the H2, H3, and H4 subtypes isolated from domestic ducks in a live-bird market in Nanchang, China (Liu et al., 2003). Such viruses were also isolated from other land-based bird species in the same market, suggesting that they may already be adapted to land-based birds. In this study, we sought to better understand the susceptibility of quail to influenza A viruses circulating in the wild aquatic bird reservoir. We used influenza A viruses isolated from wild ducks and shorebirds in North America and Asia, although a limited number of virus subtypes from wild birds of the Eurasian lineage is available. We examined these viruses’ ability to replicate and transmit in quail and tested the replication and transmission in quail of influenza viruses from humans and swine. Our results suggest that quail can act as an intermediate host in which influenza viruses adapt and in which avian–mammalian reassortant viruses can be amplified before transmission to other species.
Results
Susceptibility of quail to avian influenza viruses isolated from aquatic birds
We determined the susceptibility of quail to avian influenza viruses isolated from aquatic birds and representing all 15 HA subtypes (Table 1). The viruses tested were primarily derived from the North American lineage due to the limited number of strains available isolated from wild birds of the Eurasian lineage. Nevertheless, four isolates belonging to the Eurasian lineage were included in these studies represented by viruses of the H3, H4, H14, and H15 subtypes. Groups of three quail were inoculated by the oral, nasal, and ocular routes with 2.5 × 106 50% egg infectious dose (EID50) of virus per bird. Tracheal and cloacal swabs were obtained daily for virus titration.
Table 1.
Viruses used
| Subtype | Virus | Virus stock titer (log10EID50/ml) |
| Avian influenza viruses | ||
| H1 | A/Mallard/Alberta/119/98 (H1N1) | 8.0 |
| H2 | A/Mallard/Alberta/33/01 (H2N4) | 8.3 |
| H3 | A/Mallard/Alberta/31/01 (H3N9) | 8.3 |
| H3 | A/Duck/Siberia/01 (H3N8) | 8.0 |
| H4 | A/Mallard/Alberta/119/00 (H4N6) | 9.5 |
| H4 | A/Duck/Mongolia/52/01 (H4N6) | 8.5 |
| H5 | A/Mallard/Alberta/271/88 (H5N3) | 8.3 |
| H6 | A/Mallard/Alberta/206/96 (H6N8) | 7.5 |
| H7 | A/Mallard/Alberta/24/01 (H7N3) | 8.3 |
| H8 | A/Mallard/Alberta/194/92 (H8N4) | 7.8 |
| H9 | A/Shorebird/DE/9/96 (H9N8) | 9.0 |
| H10 | A/Pintail/Alberta/202/00 (H10N7) | 8.5 |
| H10 | A/Shorebird/DE/260/00 (H10N4) | 8.3 |
| H11 | A/Mallard/Alberta/122/99 (H11N9) | 7.8 |
| H12 | A/Mallard/Alberta/238/96 (H12N5) | 8.0 |
| H13 | A/Mallard/Alberta/146/01 (H13N6) | 7.8 |
| H14 | A/Mallard/Gurjev/263/82 (H14N5) | 8.3 |
| H15 | A/Duck/Australia/341/83 (H15N8) | 8.3 |
| Subtype | Virus | (log10PFU/ml) |
| Mammalian influenza viruses—Human influenza A viruses | ||
| A/USSR/90/77 | 7.9 | |
| H1N1 | A/Nanchan/1/01 | 5.3 |
| A/HK/1/68 | 6.7 | |
| H3N2 | A/Memphis/14/98 | 5.7 |
| Mammalian influenza viruses—Swine influenza A viruses | ||
| H1N1 | A/Sw/NE/22806/92 | 5.9 |
| H3N2 | A/Sw/TX/4199-2/98 | 9.2 |
| H1N2 | A/Sw/MN/40318/99 | 7.0 |
| Mammalian influenza viruses—Influenza B viruses | ||
| B/Lee/40 | 5.0 | |
| B/Memphis/2/01 | 5.4 | |
As shown in Table 2,14 of the 15 influenza subtypes tested replicated in quail; the exception was the H15 subtype, A/duck/Australia/341/83 (H15N8). The viruses replicated predominantly in the respiratory tract, although virus was occasionally isolated from the cloaca. Maximum virus titers were observed 3 to 4 days after inoculation. The virus yield in tracheal samples allowed the separation of the viruses into three groups. The first group (with the highest virus yield) comprised the H2, H3, H4, H5, H7, H10, and H13 subtypes. Viruses of the H4, H7, H10, and H13 subtypes and the H3 virus from the Eurasian lineage were detected during days 3, 4, and 5 after inoculation. On day 3, titers of shed virus were above 105 EID50/ml in some birds. Viruses of the H2 and H5 subtypes and the H3 virus from the North American lineage were shed at slightly lower titers (104 EID50/ml) but were detected for at least 3 days, starting on day 3 after inoculation. The second group of viruses included the H1, H6, and H9 subtypes, which were shed for at least 2 days, starting on day 3 after inoculation, at a maximum titer of approximately 102.5 EID50/ml. The third group comprised the H8, H11, H12, and H14 subtypes, which replicated poorly (detected only on day 3 after inoculation and only in some birds). As expected, this group shed little virus (~101.75 EID50/ml). Although a single isolate of each subtype does not allow conclusions about the relative efficiency of replication of influenza viruses in quail, these results show that a broad range of influenza viruses from aquatic birds can replicate in the respiratory tract of quail.
Table 2.
Growth of waterfowl influenza A viruses in quail
| HA subtype |
Number positives/ Total N—trachea/ cloaca/N inoculated |
Virus shed 3 dpi (range log10 EID50/ml) |
|||
|---|---|---|---|---|---|
| Day 3 | Day 4 | Day 5 | Trachea | Cloaca | |
| H1 | 2/0/3 | 2/0/3 | 1/0/3 | 1.8–2.8 | 0 |
| H2a | 7/0/9 | 5/0/9 | 1/0/9 | 1.8–4.3 | 0 |
| H3 | 3/1/3 | 3/1/3 | 2/0/3 | 3.5–4.5 | 2.5 |
| H3b | 2/0/3 | 2/0/3 | 2/0/3 | 5.3–5.8 | ND |
| H4 | 3/0/3 | 3/0/3 | 3/0/3 | 5.5–5.8 | 0 |
| H4b | 2/0/3 | 2/0/3 | 2/0/3 | 5.3–5.0 | ND |
| H5c | 6/0/6 | 5/0/6 | 5/0/6 | 2.5–4.8 | 0 |
| H6 | 2/1/3 | 2/1/3 | 0/0/3 | 1.8–2.5 | 1.3 |
| H7c | 6/1/6 | 6/1/6 | 5/1/6 | 2.3–6.5 | 2.5 |
| H8 | 1/0/3 | 1/0/3 | ND | 1.8 | 0 |
| H9shorebird | 2/0/3 | 1/0/3 | 1/0/3 | 1.8–3.5 | 0 |
| H10c,d | 4/0/6 | 2/0/4 | 2/0/4 | 4.8–5.8 | 0 |
| H10shorebird | 3/2/3 | 3/2/3 | 3/1/3 | 5.5–6.8 | 3.8 |
| H11 | 1/0/3 | 0/0/3 | 0/0/3 | 1.8 | 0 |
| H12 | 1/0/3 | 0/0/3 | 0/0/3 | 1.8 | 0 |
| H13 | 3/0/3 | 3/0/3 | 3/0/3 | 2.3–5.8 | 0 |
| H14b | 2/0/3 | 1/0/3 | 0/0/3 | 1.8 | 0 |
| H15b | 0/0/3 | 0/0/3 | 0/0/3 | 0 | 0 |
Note. Birds were inoculated with 2.5 × 106 EID50/bird (volume 500 µl). DPI, days postinoculation; ND, Not done.
Results of three independent experiments.
Viruses of the Eurasian lineage.
Results of two independent experiments.
Two birds showed signs of disease and died 4 days after inoculation.
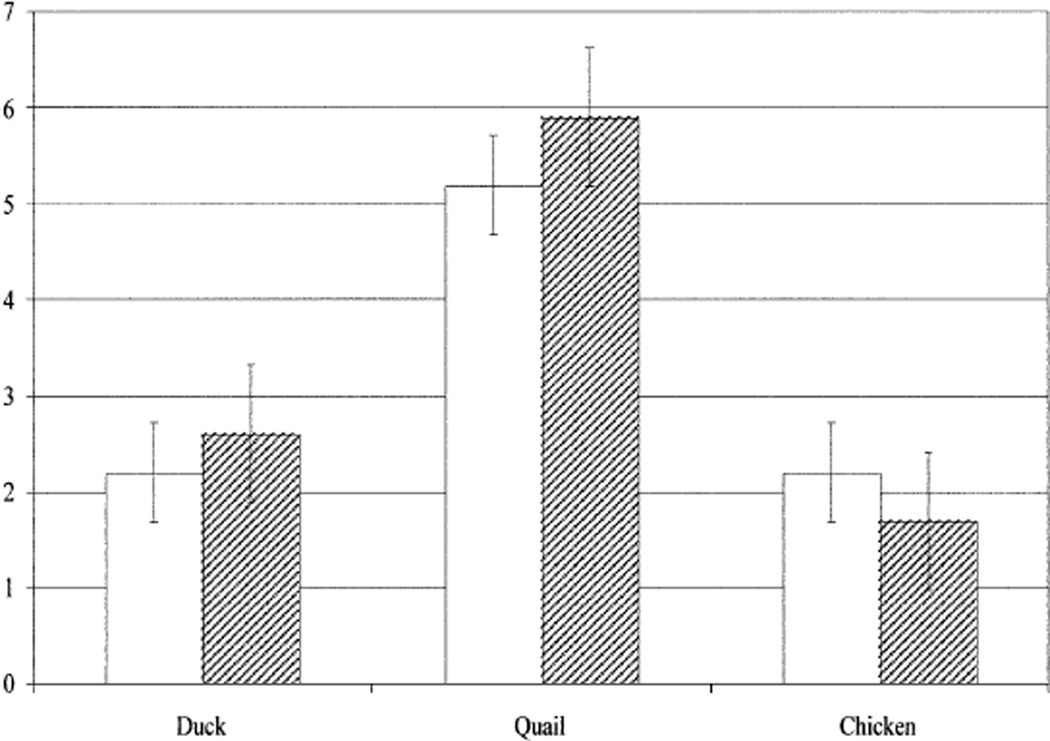
Only one of the viruses tested caused signs of disease in quail. Otherwise, quail remained healthy and gained weight during the 10 days of observation. The exception was the H10 virus, which caused disease signs and death in two of six birds tested. The high yield of the H10 virus in the respiratory tract of quail and its ability to cause disease in some of them prompted us to investigate whether this virus could replicate in the respiratory tract of other avian hosts and whether quail are also susceptible to H10 virus isolated from a different wild bird species. We therefore tested the replication of the H10 viruses A/pintail/Alberta/202/00 (H10N7) and A/shorebird/Delaware/260/00 (H10N4) in quail, mallard ducks, and white Leghorn chickens. Although chickens and ducks shed both viruses from the trachea, quail were particularly susceptible to respiratory infection with both H10 viruses (Table 2 and Fig. 1). In quail, the mean titer of virus shed from the trachea was ~1000 times the tracheal titer observed in ducks and in chickens 3 days after inoculation.
Fig. 1.
Growth of H10 influenza virus subtypes isolated from a duck and a shorebird in the tracheas of quail, mallard ducks, and white Leghorn chickens. Four-week-old birds were inoculated by the oral, nasal, and ocular routes, as indicated under Materials and methods. The bars show the mean (±SD) titer of virus obtained from three inoculated birds 3 days after inoculation. White bars: A/Pintail/Alberta/202/00 (H10N7); hatched bars: A/Shorebird/DE/260/00 (H10N4).
Transmission of avian influenza viruses among quail
Virus transmission in wild aquatic birds is thought to occur mainly through the fecal– oral route, because viruses replicate in the intestine of ducks and are excreted at high concentrations in the feces. However, the respiratory infection we had observed in quail suggested that these aquatic avian influenza viruses could be transmitted among quail via aerosol. For transmission experiments, we used the virus subtypes that had shown the highest yield in the replication experiments (H3, H4, H5, H7, and H10) and one subtype that had shown an intermediate yield (H2). Groups of three quail were inoculated with these viruses and placed in direct physical contact with three uninfected quail 1 day after inoculation. Three additional uninfected quail were placed in aerosol contact in an adjacent cage. Daily tracheal and cloacal samples were obtained. As shown in Table 3, there was no evidence of transmission of the H2, H3, H4, and H5 viruses by direct contact, although the inoculated quail shed approximately 104.25 EID50/ml of virus on day 3 after inoculation (not shown). One quail in each direct contact group was infected with the H10 and H7 viruses. These birds shed virus from the trachea at titers of 101 to 102 EID50/ml; the identity of the H10 and H7 viruses was confirmed by hemagglutinin inhibition assays and by RT-PCR with specific primers (not shown). The birds placed in aerosol contact showed no evidence of infection, suggesting that the direct contacts may have been infected through the drinking water. Therefore, although quail can be experimentally infected with at least 14 subtypes of influenza A virus, additional adaptation is required for efficient transmission of these viruses among quail.
Table 3.
Transmission of aquatic avian influenza viruses by direct contact in quail
| HA subtype | Number of quail infected via direct contact—trachea/ cloaca/No. contacts (log10 EID50/ml) |
|||
|---|---|---|---|---|
| Day 2 | Day 3 | Day 4 | Day 5 | |
| H10 | 1/0/3 (<1.0) | 1/0/3 (1.5) | 0/0/3 | 0/0/3 |
| H7 | 0/0/3 | 0/0/3 | 1/0/3 (1.5) | 1/0/3 (1.5) |
| H5 | 0/0/3 | 0/0/3 | 0/0/3 | 0/0/3 |
| H4 | 0/0/3 | 0/0/3 | 0/0/3 | 0/0/3 |
| H3 | 0/0/3 | 0/0/3 | 0/0/3 | 0/0/3 |
| H2 | 0/0/3 | 0/0/3 | 0/0/3 | 0/0/3 |
Replication of human and swine viruses in quail
Liu et al. (2003) reported the isolation of a human influenza A virus from the trachea of one quail. This finding and our observation that avian influenza viruses that are not adapted to quail can establish respiratory infections in these birds led us to test whether other human and swine influenza viruses could replicate in quail. In addition, we tested the replication of influenza B viruses in quail. We used both old and recently isolated H1N1 and H3N2 human influenza virus (Table 1) to ensure that antigenic drift during adaptation to humans had not affected the ability of these viruses to replicate in quail. Quail inoculated with old strains of human influenza virus showed traces of virus in the trachea (Table 4); A/USSR/90/77 (H1N1) virus was detected on day 1 after inoculation and A/HK/1/68 (H3N2) virus was detected on days 1 and 2 after inoculation. It was not possible to ascertain whether the trace quantities of these viruses were the product of replication or merely remnants of the inoculum. The recent human influenza A and B viruses showed no evidence of replication in quail. It should be noted that the 1977 H1N1 and 1968 H3N2 human viruses may be considered more avian-like than currently circulating human influenza viruses. However, they had undergone numerous passages in eggs, a process that may have generated variants more or less adapted for replication in avian hosts. These results show that human influenza viruses undergo very limited replication in quail and that replication is unlikely to occur in nature, in the absence of prior molecular alteration.
Table 4.
Replication of mammalian influenza A and B viruses in quail
| Virusesa | Number of quail infected—trachea/cloaca/N inoculated (log10PFU/ml) | |||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | |
| A/USSR/90/77 (H1N1) | 1/0/3 (1.25) | 0/0/3 | 0/0/3 | 0/0/3 |
| A/Nanchang/1/01 (H1N1) | 0/0/3 | 0/0/3 | 0/0/3 | 0/0/3 |
| A/HK/1/68 (H3N2) | 3/0/3 (2.5) | 1/0/3 (1.25) | 0/0/3 | 0/0/3 |
| A/Memphis/14/98 (H3N2) | 0/0/3 | 0/0/3 | 0/0/3 | 0/0/3 |
| B/Lee/40 | 0/0/3 | 0/0/3 | 0/0/3 | 0/0/3 |
| B/Memphis/5/01 | 0/0/3 | 0/0/3 | 0/0/3 | 0/0/3 |
| A/Swine/NE/22806/92 (H1N1) | 1/0/3 (1.0) | 2/0/3 (2.5) | 1/0/3 (1.25) | 0/0/3 |
| A/Sw/TX/4199-2/98 (H3N2) | 3/0/3 (2.5) | 3/0/3 (1.0) | 2/0/3 (1.75) | 1/0/3 (1.5) |
| A/Sw/MN/40318/99 (H1N2) | 3/0/3 (<1.0) | 0/0/3 | 0/0/3 | 0/0/3 |
Birds were inoculated with 2 × 105 PFU/ml (influenza A viruses) or 1 × 105 PFU/ml (influenza B viruses).
To determine whether swine influenza viruses replicate in quail, we tested three recent American isolates (Table 1) representing the classical swine H1N1 viruses and the H3N2 and H1N2 triple reassortants that have emerged recently in the U.S. swine population (Webby et al., 2001). Unlike the human influenza viruses, all three swine viruses replicated, albeit inefficiently, in the respiratory tract of quail. Virus shedding was observed for 3 days after inoculation with A/Sw/NE/22806/92 (H1N1) and for 4 days after inoculation with A/Sw/TX/4199-2/98 (H3N2). Traces of influenza A/Sw/MN/40318/99 (H1N2) virus were detected in the trachea of quail on day 1 after inoculation. All cloacal samples were negative, and no transmission by direct contact was observed (data not shown). Thus, although swine viruses appear to replicate in quail more readily than human viruses, additional molecular alterations would be required to allow efficient replication and transmission in quail.
The internal gene constellation of human influenza viruses is compatible with replication and transmission in quail
The molecular factors that determine the host range of influenza A viruses are polygenic. The specific factors that determine host range in quail are not known. To better understand the factors that contribute to influenza host range restriction in quail, we cloned and rescued by reverse genetics the recent human H3N2 influenza virus isolate A/Memphis/14/98 (H3N2) and replaced its surface glycoprotein genes with those of A/quail/Hong Kong/A28945/88 (H9N2) virus. Three quail were inoculated with this recombinant by oral, nasal, and ocular routes and were placed in direct contact with three uninfected quail. Cloacal and tracheal swabs were obtained daily from all birds for 12 days. As shown in Table 5, the recombinant was able to replicate and transmit in quail. Five days after inoculation, all birds in direct contact showed signs of infection, indicating efficient transmission of the recombinant virus. Similar results were obtained with a recombinant containing the surface genes of the same quail virus and the internal genes of A/Sw/TX/4199-2/98 (H3N2) virus (Perez et al., 2002a). Therefore, the replication and transmission of human and swine influenza viruses in quail are not restricted by their internal genes. In contrast, chickens inoculated with these recombinants did not transmit the viruses to other chickens, although traces of viral replication were observed (not shown). Similar experiments have shown that the internal genes of human viruses restrict their replication in the intestine of ducks (Hatta et al., 2002).
Table 5.
Replication of an H9N2 avian-human recombinant influenza A virus in quail
| Number with positive tracheal swab/total N |
Maximum infectivity titer of each quaila |
|||||
|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 10 | ||
| Inoculated quail | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 | 1.8, 2.8, 2.5 |
| Quail in direct contact | 0/3 | 2/3 | 3/3 | 3/3 | 1/3 | 1.5, 3.5, 1.8 |
log10 EID50/ml 5 days postinoculation.
Discussion
Several reports suggest that quail played a role in the spread of influenza viruses in the live poultry markets of Hong Kong and mainland China (Chin et al., 2002; Guan et al., 2002). The determinants of the host range of influenza viruses in quail are poorly understood. We showed that avian influenza viruses from wild aquatic birds representing 14 of 15 HA subtypes replicate in quail after experimental inoculation by natural routes (oral, nasal, and ocular inoculation). In this study, we tested mainly influenza A viruses isolated from wild aquatic birds in North America, because of the greater variety of isolates and subtypes available. The viruses replicated mainly in the respiratory tract; virus was isolated from the cloaca in only a few cases. Thus, the preferred site of replication of influenza virus in quail differs from that observed in natural hosts (intestinal epithelial cells).
The ability of these viruses to replicate in quail differed. We identified three groups that differed on the basis of the quantity of virus shed. These results do not permit general conclusions about the influenza subtypes’ relative efficiency of replication in quail. Interestingly, the H9 virus tested did not replicate efficiently in quail, although viruses of this subtype have established permanent lineages in quail in Asia (Guan et al., 2000). This finding suggests that adaptation is needed to allow influenza viruses to replicate and transmit in quail. Our observations are consistent with the possibility that quail can be an intermediate host in which influenza viruses can adapt and generate variants that have the potential to cross to other animal species. In Hong Kong, public health officials no longer permit the sale of live quail in the poultry markets, where they are suspected of transmitting influenza viruses to other birds. However, live poultry markets in mainland China and other parts of the world (including the U.S.) continue to mix many different species of aquatic and land-based birds (including quail). In one such market in Nanchang, China, this environment has been shown to be ideal for the perpetuation, reassortment, and diversification of influenza viruses (Liu et al., 2003). Interestingly, the same study found quail to be susceptible to all of the influenza virus subtypes circulating in the market. Influenza virus was isolated from quail less frequently than from ducks or chickens. This apparent discrepancy can be explained by the fact that samples were taken only from the fecal material under the cages, whereas the viruses replicate mostly in the respiratory tract of quail and chickens. Alternatively, quail may be infected early in life and be free of virus by the time they reach the market. Future surveillance studies in the live bird markets should consider sampling the trachea and cloaca of birds and performing serologic tests.
In their natural hosts—ducks and shorebirds—influenza viruses replicate predominantly in the intestinal tract (Webster et al., 1978). In quail, however, all of the avian influenza viruses we tested replicated preferentially in the respiratory tract, which is the site of replication in mammalian hosts. Quail also supported the replication of at least two of the three swine influenza viruses tested in this study, although virus titers were low. It is important to note that the two swine viruses that replicated in quail contain PB2 and PA genes derived from the avian reservoir, which may provide a growth advantage in avian hosts. Thus, avian and swine viruses may be able to reassort in quail.
The human viruses tested in our study did not replicate in quail, although our previous findings (Liu et al., 2003) suggest that they could eventually do so. To investigate which genes are responsible for this host range restriction, we used reverse genetics to create a recombinant human H3N2 virus with the HA (H9) and NA (N2) of a quail virus. This recombinant transmitted in quail, although the virus did not replicate to high titers in either the inoculated quail or the contact quail. These results are consistent with the idea that transmission of a virus is determined more by its “transmissibility” features than by its efficiency of replication. Thus, emerging influenza viruses may acquire the surface characteristics that allow efficient transmission to a given host even before they replicate to high titers in that host. Efficient replication (and the ability to cause disease) would then result from several rounds of adaptation in the host. In conclusion, our results support the hypothesis that quail could play an important role as an intermediate host that permits the adaptation of influenza viruses from wild birds and the generation of variants that can cross to other species, such as chickens, pigs, or humans. In addition, quail could provide an environment in which avian–mammalian reassortant viruses could be amplified, thereby increasing the likelihood of interspecies transmission.
Materials and methods
Influenza viruses
The viruses used for this study (Table 1) were obtained from the repository at St. Jude Children’s Research Hospital. Avian influenza viruses were isolated from wild ducks, with the exception of the H9N8 and H10N4 viruses, which were isolated from shorebirds. Viruses were propagated in 10-day-old embryonated chicken eggs and stored at −70°C. Avian influenza A viruses were titrated to determine the 50% egg infectious dose by the method of Reed and Muench (1938). Human and swine influenza isolates were propagated in Madin–Darby canine kidney (MDCK) cells and stored at −70°C. The concentration of the human and swine influenza viruses was determined by plaque assay.
Animals and experimental infections
Four-week-old Japanese quail (C. coturnix) (B & D Game Farm, Harrah, OK), 4-week-old Mallard ducks (IDE-AL Poultry and Breeding Farms, Inc., Cameron, TX), and 4-week-old specific-pathogen-free (SPF) white Leghorn chickens (Spafas, CT) were used. Groups of three birds were inoculated through the nares, mouth, and eyes with a concentration of 5 × 106 EID50/ml of avian influenza viruses or with 2 × 105 PFU/ml of human or swine influenza A viruses or with 1 × 105 PFU/ml of human influenza B viruses. The volume of virus inoculum was 0.5 ml for each quail and 1.0 ml for each chicken or duck. Tracheal and cloacal swabs were obtained daily for 10 days after inoculation of quail with avian viruses and daily for 7 days after inoculation of quail with human and swine influenza viruses. Swab samples were diluted in 1 ml of freezing medium (50% glycerol, 1 × PBS, antibiotics, and antimycotics) as described previously (Guan et al., 2000). Viruses in swab samples were titrated for infectivity in embryonated chicken eggs (avian viruses) or in MDCK cells (human and swine viruses) (Palmer et al., 1975). Undiluted positive samples with no HA activities at the 10−1 dilution in EID50 or plaque assays were scored as positive with the notation of “<1.0 EID50/ml (or PFU/ml).” The birds were weighed daily and observed for overt signs of disease. Samples from ducks and chickens were obtained and analyzed essentially as described above. Experiments with recombinant viruses derived by reverse genetics were done in BL3+ facilities by staff wearing appropriate protective equipment.
Transmission of influenza viruses in quail
In each experiment, three quail were inoculated as described above. On day 1 after inoculation, three quail were placed in the same cage with inoculated birds to allow direct contact. Transmission of viruses by aerosol was tested by placing three quail in a cage 30 cm from the side of the cage housing the inoculated birds. Tracheal and cloacal samples were collected daily and tested as described above.
RT-PCR, cloning, and sequencing
Viral RNA was extracted from allantoic or tissue culture supernatant by using the RNeasy Mini Kit (Qiagen, Valencia, CA) as directed by the manufacturer. RT-PCR amplification and cloning of the eight genes of A/Memphis/14/98 into pHW2000 was performed as described previously (Hoffmann et al., 2001, Hoffmann et al., 2002). The full-length HA and NA genes of A/Quail/HK/A28945/88 influenza virus were cloned into pHW2000 as previously described (Perez et al., 2002b). Plasmids containing the HA and NA genes of A/Quail/HK/A28945/88 were designated pRGHAQaHK/88 and pRGNAQaHK/88. The eight plasmids containing full-length cDNA of the gene segments of A/Memphis/14/98 virus were also cloned into pHW2000 and designated pRGPB2Mem14/98, pRGPB1Mem14/98, pRGPAMem14/98, pRGHAMem14/98, pRGNPMem14/98, pRGNAMem14/98, pRGMMem14.98, and pRGNS-Mem14/98. Each cloned influenza virus segment was sequenced with segment-specific synthetic oligonucleotides at the St. Jude Children’s Research Hospital Center for Biotechnology by using Rhodamine or dRhodamine dye-terminator cycle sequencing ready reaction kit with AmpliTaq DNA polymerase FS (Perkin–Elmer Applied Biosystem Inc., Foster City, CA).
Generation of viruses by reverse genetics
Recombinant viruses were generated by using the 8-plasmid system described previously (Hoffmann et al., 2000). The recombinant virus RG-A/Quail/HK/A28945/88 (H9N2) × A/Memphis/14/98 (H3N2), whose HA and NA genes came from A/Quail/HK/A28945/88 (H9N2) and whose remaining gene segments came from A/Memphis/14/98 (H3N2), was further amplified in MDCK cells, titrated to determine the EID50, and maintained at −70°C. The recombinant virus stock concentration was 108 EID50/ml. Recombinant viruses were generated and handled under the BL3+ containment conditions recommended by the NIH.
Acknowledgments
These studies were supported in part by Public Health Service Grants AI-29680, AI-95357, and CA-21765 and by the American Lebanese Syrian Associated Charities (AL-SAC). We thank Patrick Seiler, Scott Krauss, and Ashley Baker for excellent technical assistance, Richard Webby for helpful discussions, and Sharon Naron for editorial assistance.
References
- Beare AS, Webster RG. Replication of avian influenza viruses in humans. Arch. Virol. 1991;119:37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- Brown IH. The epidemiology and evolution of influenza viruses in pigs. Vet. Microbiol. 2000;74:29–46. doi: 10.1016/s0378-1135(00)00164-4. [DOI] [PubMed] [Google Scholar]
- Chin PS, Hoffmann E, Webby R, Webster RG, Guan Y, Peiris M, Shortridge KF. Molecular evolution of H6 influenza viruses from poultry in Southeastern China: prevalence of H6N1 influenza viruses possessing seven A/Hong Kong/156/97 (H5N1)-like genes in poultry. J. Virol. 2002;76:507–516. doi: 10.1128/JVI.76.2.507-516.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Peiris JS, Lipatov AS, Ellis TM, Dyrting KC, Krauss S, Zhang LJ, Webster RG, Shortridge KF. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc. Natl. Acad. Sci. USA. 2002;99:8950–8955. doi: 10.1073/pnas.132268999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Shortridge KF, Krauss S, Chin PS, Dyrting KC, Ellis TM, Webster RG, Peiris M. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 2000;74:9372–9380. doi: 10.1128/jvi.74.20.9372-9380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Shortridge KF, Krauss S, Webster RG. Molecular characterization of H9N2 influenza viruses: were they the donors of the"internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YJ, Krauss S, Senne DA, Mo IP, Lo KS, Xiong XP, Norwood M, Shortridge KF, Webster RG, Guan Y. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology. 2000;267:279–288. doi: 10.1006/viro.1999.0115. [DOI] [PubMed] [Google Scholar]
- Guo Y, Li J, Cheng X, Wang M, Zhou Y, Li XH, Guo F, et al. Discovery of men infected by avian influenza A (H9N2) virus. Chin. J. Exp. Clin. Virol. 1999;13:105–108. [PubMed] [Google Scholar]
- Hatta M, Halfmann P, Wells K, Kawaoka Y. Human influenza A viral genes responsible for the restriction of its replication in duck intestine. Virology. 2002;295:250–255. doi: 10.1006/viro.2002.1358. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS, Webster RG, Bean WJ, Sriram G, et al. The ecology of influenza viruses in ducks and analysis of influenza viruses with monoclonal antibodies. Comp. Immunol. Microbiol. Infect. Dis. 1980a;3:155–164. doi: 10.1016/0147-9571(80)90051-x. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS, Webster RG, Turner B, et al. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can. J. Microbiol. 1980b;26:622–629. doi: 10.1139/m80-108. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS, Webster RG, Naeve CW, Murphy BR. Altered tissue tropism of human-avian reassortant influenza viruses. Virology. 1983;128:260–263. doi: 10.1016/0042-6822(83)90337-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20:3165–3170. doi: 10.1016/s0264-410x(02)00268-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. USA. 2000;97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Couceiro JN, Kelm S, Baum LG, Krauss S, Castrucci MR, Donatelli I, Kida H, Paulson JC, Webster RG, Kawaoka Y. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 1998;72:7367–7373. doi: 10.1128/jvi.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kawaoka Y. Host-range barrier of influenza A viruses. Vet. Microbiol. 2000;74:71–75. doi: 10.1016/s0378-1135(00)00167-x. [DOI] [PubMed] [Google Scholar]
- Kida H, Ito T, Yasuda J, Shimizu Y, Itakura C, Shortridge KF, Kawaoka Y, Webster RG. Potential for transmission of avian influenza viruses to pigs. J. Gen. Virol. 1994;75:2183–2188. doi: 10.1099/0022-1317-75-9-2183. [DOI] [PubMed] [Google Scholar]
- Lin YP, Shaw M, Gregory V, Cameron K, Lim W, Klimov A, Subbarao K, Guan Y, Krauss S, Shortridge K, Webster R, Cox N, Hay A. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc. Natl. Acad. Sci. USA. 2000;97:9654–9658. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, He S, Walker D, Zhou N, Perez DR, Mo B, Li F, Huang X, Webster RG, Webby RJ. The influenza virus gene pool in a poultry market in South central china. Virology. 2003;305:267–275. doi: 10.1006/viro.2002.1762. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Hinshaw VS, Sly DL, London WT, Hosier NT, Wood FT, Webster RG, Chanock RM. Virulence of avian influenza A viruses for squirrel monkeys. Infect. Immunol. 1982;37:1119–1126. doi: 10.1128/iai.37.3.1119-1126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BR, Sly DL, Tierney EL, Hosier NT, Massicot JG, London WT, Chanock RM, Webster RG, Hinshaw VS. Reassortant virus derived from avian and human influenza A viruses is attenuated and immunogenic in monkeys. Science. 1982;218:1330–1332. doi: 10.1126/science.6183749. [DOI] [PubMed] [Google Scholar]
- Nardelli L, Rinaldi A, Pereira HG, Mandelli G. Influenza virus infections in Japanese quails. Arch. Exp. Veterinarmed. 1970;24:231–249. [PubMed] [Google Scholar]
- Palmer DF, Coleman M, Dowdle WR, Schild GG. Advanced laboratory techniques for influenza diagnosis. U.S. Department of Health, Education, and Welfare, Washington, DC. Immunol. Ser. 1975;6:51–52. [Google Scholar]
- Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, Orr WK, Shortridge KF. Human infection with influenza H9N2. Lancet. 1999;354:916–917. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- Pérez DR, Lim W, Seiler JP, Guan Y, Peiris M, Shortridge KF, Webster RG. Role of quail in the interspecies transmission of influenza H9 viruses: molecular changes that correspond to adaptation from ducks to chickens. J. Virol. 2002b;00:000–000. doi: 10.1128/JVI.77.5.3148-3156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DR, Lim W, Seiler JP, Yi G, Peiris M, Shortridge KF, Webster RG. Role of quail in the interspecies transmission of H9 influenza A viruses: molecular changes on HA that correspond to adaptation from ducks to chickens. J. Virol. 2003;77:3148–3156. doi: 10.1128/JVI.77.5.3148-3156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez DR, Webby R, Hoffmann E, Webster RG. Land-based birds as disseminators of avian/mammalian reassortant influenza A viruses? Proceedings of the 5th International Symposium of Avian Influenza. Avian Dis. 2002a;00:000–000. doi: 10.1637/0005-2086-47.s3.1114. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method for estimating 50 per cent endpoints. Am. J. Hig. 1938;37:493. [Google Scholar]
- Rohm C, Zhou N, Suss J, Mackenzie J, Webster RG. Characterization of a novel influenza hemagglutinin, H15: criteria for determination of influenza A subtypes. Virology. 1996;217:508–516. doi: 10.1006/viro.1996.0145. [DOI] [PubMed] [Google Scholar]
- Saito T, Kawaoka Y, Webster RG. Phylogenetic analysis of the N8 neuraminidase gene of influenza A viruses. Virology. 1993;193:868–876. doi: 10.1006/viro.1993.1196. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Pigs as the “mixing vessel” for the creation of new pandemic influenza A viruses. Med. Princip. Pract. 1990;2:65–71. [Google Scholar]
- Shortridge KF. Pandemic influenza: a zoonosis? Semin. Respir. Infect. 1992;7:11–25. [PubMed] [Google Scholar]
- Shortridge KF. Poultry and the influenza H5N1 outbreak in Hong Kong, 1997: abridged chronology and virus isolation. Vaccine. 1999;17(Suppl. 1):S26–S29. doi: 10.1016/s0264-410x(99)00102-4. [DOI] [PubMed] [Google Scholar]
- Shortridge KF, Zhou NN, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti KG, Norwood M, Senne D, Sims L, Takada A, Webster RG. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- Suarez DL, Garcia M, Latimer J, Senne D, Perdue M. Phylogenetic analysis of H7 avian influenza viruses isolated from the live bird markets of the Northeast United States. J. Virol. 1999;73:3567–3573. doi: 10.1128/jvi.73.5.3567-3573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne DE, Slemons RD. Comparative pathology of a chicken-origin and two duck-origin influenza virus isolates in chickens: the effect of route of inoculation. Vet. Pathol. 1994;31:237–245. doi: 10.1177/030098589403100211. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Reinacher M, Rott R. Aggravation of pathogenicity of an avian influenza virus by adaptation to quails. Arch. Virol. 1987;93:81–95. doi: 10.1007/BF01313895. [DOI] [PubMed] [Google Scholar]
- Webby R, Swenson SL, Krauss S, Goyal SM, Rossow KD, Webster R. Evolving H3N2 and emerging H1N2 swine influenza viruses in the United States. In: Osterhaus ADME, Cox N, Hampson AW, editors. Proceedings of the World Congress on Options for the Control of Influenza IV; 23–28 September 2000; Crete, Greece. 2001. [Google Scholar]
- Webby RJ, Webster RG. Emergence of influenza A viruses. Philos. Trans.R. Soc. Lond. B Biol. Sci. 2001;356:1817–1828. doi: 10.1098/rstb.2001.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby RJ, Woolcock PR, Krauss SL, Webster RG. Reassortment and interspecies transmission of North American H6N2 influenza viruses. Virology. 2002;295:44–53. doi: 10.1006/viro.2001.1341. [DOI] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992;5:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Guan Y, Peiris M, Walker D, Krauss S, Zhou NN, Govorkova EA, Ellis TM, Dyrting KC, Sit T, Perez DR, Shortridge KF. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J. Virol. 2002;76:118–126. doi: 10.1128/JVI.76.1.118-126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Yakhno M, Hinshaw VS, Bean WJ, Murti KG. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology. 1978;84(2):268–278. doi: 10.1016/0042-6822(78)90247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]