Abstract
Thyroid hormone replacement has been used for more than a century to treat hypothyroidism. Natural thyroid preparations (thyroid extract, desiccated thyroid, or thyroglobulin), which contain both thyroxine (T4) and triiodothyronine (T3), were the first pharmacologic treatments available and dominated the market for the better part of the 20th century. Dosages were adjusted to resolve symptoms and to normalize the basal metabolic rate and/or serum protein-bound iodine level, but thyrotoxic adverse effects were not uncommon. Two major developments in the 1970s led to a transition in clinical practice: 1) The development of the serum thyroid-stimulating hormone (TSH) radioimmunoassay led to the discovery that many patients were overtreated, resulting in a dramatic reduction in thyroid hormone replacement dosage, and 2) the identification of peripheral deiodinase-mediated T4-to-T3 conversion provided a physiologic means to justify l-thyroxine monotherapy, obviating concerns about inconsistencies with desiccated thyroid. Thereafter, l-thyroxine mono-therapy at doses to normalize the serum TSH became the standard of care. Since then, a subgroup of thyroid hormone–treated patients with residual symptoms of hypothyroidism despite normalization of the serum TSH has been identified. This has brought into question the inability of l-thyroxine monotherapy to universally normalize serum T3 levels. New research suggests mechanisms for the inadequacies of l-thyroxine monotherapy and highlights the possible role for personalized medicine based on deiodinase polymorphisms. Understanding the historical events that affected clinical practice trends provides invaluable insight into formulation of an approach to help all patients achieve clinical and biochemical euthyroidism.
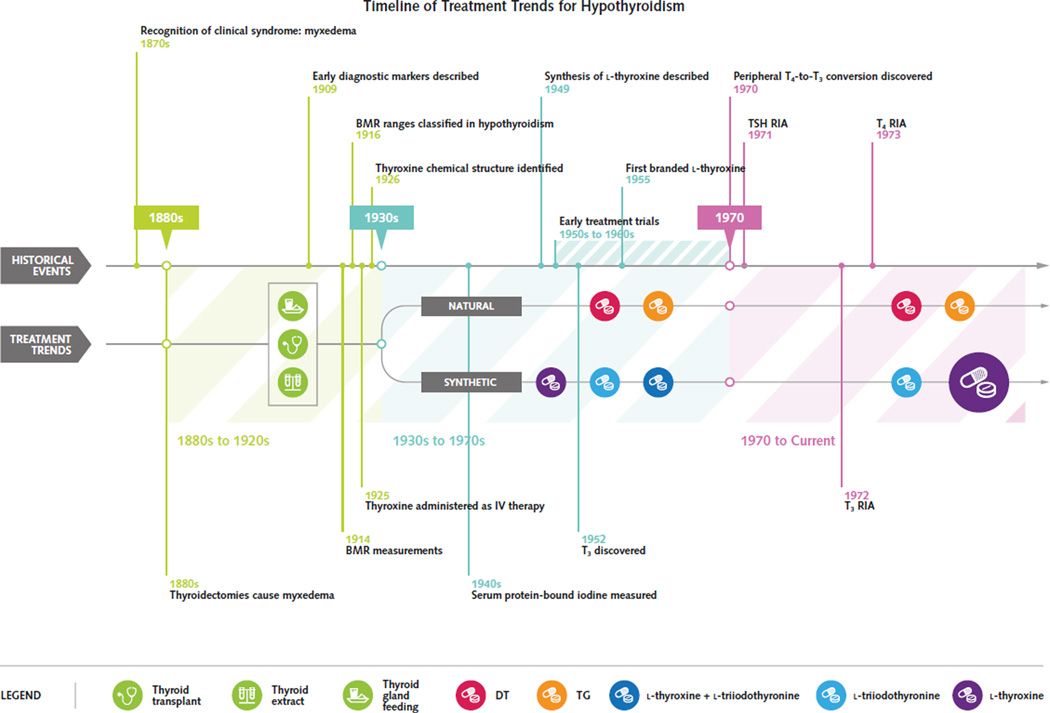
Major diagnostic and therapeutic advancements in the early 20th century dramatically changed the prognosis of hypothyroidism from a highly morbid condition to one that could be successfully managed with safe, effective therapies. These advancements dictated treatment trends that have led to the adoption of l-thyroxine monotherapy, administered at doses to normalize serum thyroid-stimulating hormone (TSH), as the contemporary standard of care (Figure). Most patients do well with this approach, which both normalizes serum TSH levels and leads to symptomatic remission (1).
Figure. Events influencing the evolution of treatment trends in hypothyroidism.
Initial strategies for thyroid hormone replacement included thyroid transplantation, but efficacious pharmacologic strategies soon won favor. Natural thyroid preparations containing T4 and T3, such as desiccated thyroid, thyroid extracts, or thyroglobulin, were the initial pharmacologic agents. Synthetic agents were synthesized later. Early clinical trials demonstrated the efficacy of synthetic and natural agents, but concerns arose regarding consistency of natural thyroid preparations and adverse effects associated with T3-containing preparations (natural or synthetic). With the demonstration of peripheral T4-to-T3 conversion and the availability of the serum TSH radioimmunoassay in the early 1970s, there was a major trend in prescribing preference toward l-thyroxine monotherapy. BMR = basal metabolic rate; DT = desiccated thyroid; IV = intravenous; RIA = radioim-munoassay; T3 = triiodothyronine; T4 = thyroxine; TG = thyroglobulin; TSH = thyroid-stimulating hormone.
Despite these successes, authors have questioned the efficacy of l-thyroxine monotherapy because about 10% to 15% of patients are dissatisfied as a result of residual symptoms of hypothyroidism (1, 2), including neurocognitive impairment (3), and about 15% of patients do not achieve normal serum triiodothyronine (T3) levels (4). Studies of several animal models indicate that maintaining normal serum T3 levels is a biological priority (5). Although the clinical significance of relatively low serum T3 in humans is not well-defined (1), evidence shows that elevating serum T3 through the administration of both l-thyroxine and l-triiodothyronine has benefited some patients (6, 7). However, this has not been consistently demonstrated across trials (1). Novel findings highlight the molecular mechanisms underlying the inability of l-thyroxine monotherapy to universally normalize measures of thyroid hormone signaling (8, 9), and new evidence may lay the foundation for a role of personalized medicine (10). Understanding the historical rationale for the trend toward l-thyroxine monotherapy allows us to identify scientific and clinical targets for future trials.
Establishing the Need for Thyroid Replacement
Cases of myxedema were reported in the mid–19th century but were not initially connected with a deficiency from the thyroid gland until surgeons identified incident myxedema after thyroidectomy (11). Initial treatment strategies were largely insufficient and primarily symptom directed, including hot baths and institutionalization (12). The significant morbidity and mortality in the absence of efficacious treatment were clear, and thus the need to “replace” the thyroid through surgical transplantation or oral or intravenous routes was established. Thyroid transplant had some early successes, but for many patients symptoms recurred and the procedure even had to be repeated (13). Because of the rapidity and transiency of improvement (12), it was hypothesized that symptoms improved by absorption of the “juice” of the donor gland (14).
Trials of the first pharmacologic strategies included intravenous or subcutaneous (12) or oral (15) administration of thyroid extract, in addition to “thyroid feeding,” the consumption of raw or cooked thyroid gland (16), with sustainable successes. Oral replacement strategies quickly won favor, although “alarming symptoms” associated with treatment were noted; however, the details were not fully described (17). Thyroid transplant may one day reemerge as a viable treatment option given that functional thyroid tissue can be generated from stem cells (18).
Role of Basal Metabolic Rate and Serum Protein-Bound Iodine in Diagnosis and Treatment
The association between hypothyroidism and energy expenditure was suspected clinically, and the discovery of lower O2 consumption in myxedema provided an early diagnostic tool (19). The development of a device to assess energy expenditure through measurement of the basal metabolic rate (BMR) in humans proved to be useful for not only diagnosis but also titration of therapy (20). The scale was calibrated so that a normal BMR reference range would be around 0%, whereas athyreotic individuals could have a BMR of about −40% (21). Because of lack of specificity (for example, low BMR in malnutrition), BMR was used in conjunction with the overall clinical impression; a low BMR in the setting of high clinical suspicion would secure a diagnosis and justify treatment (21, 22).
l-Thyroxine was the first synthetic molecule used to treat hypothyroidism (23) and was shown to be efficacious as monotherapy for myxedema (24). Around that time, serum protein-bound iodine (PBI) emerged as a diagnostic test and therapeutic marker; serum PBI quantitation was the only valid way to biochemically assess thyroid hormone status (25). This tool was limited in terms of treatment monitoring because the effect on serum PBI varied by agent (26). For example, l-triiodothyronine corrected BMR without much increase in serum PBI, l-thyroxine increased serum PBI sometimes to above normal, and combination l-thyroxine and l-triiodothyronine and desiccated thyroid had the advantage of normalizing serum PBI (27). In addition to BMR and serum PBI, other surrogates for treatment response included cholesterol levels, symptoms, and deep tendon reflexes, but their lack of sensitivity was always recognized (28).
Evidence of Overtreatment in Early Trials
With the availability of multiple forms of thyroid hormone replacement, early clinical trials were designed to assess efficacy and dose equivalency among natural thyroid (typically desiccated), synthetic l-thyroxine, and/or l-triiodothyronine. These were not designed as superiority trials, their therapeutic goals were the normalization of serum PBI or BMR, and doses were dramatically higher than used today. For example, desiccated thyroid and intravenous l-thyroxine monotherapy normalized BMR, pulse, and body weight in myxedema (29), l-triiodothyronine monotherapy was likewise effective (30), and the potency of l-triiodothyronine exceeded that of l-thyroxine (31).
These clinical trials also began to define the adverse-effect profiles associated with these agents; thyrotoxicosis was frequently encountered. Patients treated with l-triiodothyronine3 (100 to 175 mcg/d) normalized BMR faster than did those receiving desiccated thyroid (120 to 210 mg/d) or l-thyroxine (200 to 350 mcg/d) but were more likely to experience angina (32). Desiccated thyroid was also associated with adverse symptoms in other studies; muscle stiffness, psychosis, and angina all occurred (33). In a crossover study of l-triiodothyronine monotherapy (75 to 100 mcg/d), l-thyroxine monotherapy (200 to 300 mcg/d), and desiccated thyroid (1.5 to 3 grains/d), all of these therapies restored BMR and serum PBI; with l-triiodothyronine, however, angina and heart failure occurred. Dose reduction corrected these adverse effects, but authors concluded that l-thyroxine monotherapy or thyroid extract was preferred (34). In a trial of l-thyroxine monotherapy at doses of 200 to 300 mcg/d versus l-thyroxine (80 mcg) plus l-triiodothyronine (20 mcg) daily, patients receiving the combination had such symptoms as palpitations, nervousness, tremor, and perspiration (35). Some early proponents of l-thyroxine monotherapy emerged because of less frequent thyrotoxic effects (24), but it is difficult to determine whether such adverse effects were related to the agent used or its high dosage. Thyrotoxic adverse effects were typically remediable by simple dose reduction (36), so desiccated thyroid remained the preparation of choice (37).
Rise and Fall of Natural Thyroid Products
From the early 1890s through the mid-1970s, desiccated thyroid was the preferred form of therapy for hypothyroidism (Appendix Table, available at www.annals.org). This preference was reinforced by the unique ability of desiccated thyroid to reproduce a normal serum PBI (33). The predominance of natural thyroid products was illustrated by prescribing patterns in the United States: In 1965, approximately 4 of every 5 prescriptions for thyroid hormone were for natural thyroid preparations (38). Concerns about inconsistencies in the potency of these tablets arose (26) after the discovery that some contained anywhere from double to no detectable metabolic activity (39). The shelf-life of desiccated tablets was limited, especially if the tablets were kept in humid conditions (36). There were reports of patients not responding to desiccated thyroid altogether because their tablets contained no active thyroid hormone. It was not until 1985 that the revision of the U.S. Pharmacopeia standard from iodine content to T3/thyroxine (T4) content resulted in stable potency (38), but by then the reputation of natural thyroid products was tarnished (40).
Physicians hesitated to use l-thyroxine monotherapy over concern that it could result in a relative T3 deficiency, despite growing discontent with potency of natural thyroid products (39) and reduced cost of l-thyroxine, such that the 2 treatments were approximately equivalent (36, 41). The seminal discovery of peripheral T4-to-T3 conversion in athyreotic individuals largely obviated this concern (42). This laid the foundation for the corollary that treatment with l-thyroxine could replace thyroid hormone in such a way that the prohormone pool would be restored and the deiodinases would regulate the pool of active T3. Within a decade there was a major transition toward l-thyroxine monotherapy as first-line therapy (Appendix Table and Figure) (38).
Effect of Radioimmunoassay-Based Thyroid Function Tests
The development of TSH radioimmunoassay (43) provided the first sensitive and specific marker of systemic thyroid hormone status (Figure). Clinicians could now titrate therapy to achieve a serum TSH within the normal range as a specific marker of replacement adequacy (44). For patients who were once treated with doses that normalized their symptoms, BMR, or serum PBI, the use of serum TSH revealed such doses to be typically supratherapeutic (45, 46). Maintenance doses of l-thyroxine ranged from 200 to 500 mcg/d before the institution of the TSH assay and then became typically closer to 100 to 150 mcg/d (Appendix Table). Implementation of the TSH radioimmunoassay also provided a means to diagnose much milder, or even subclinical, cases of hypothyroidism that may have been undiagnosed with earlier, less sensitive, diagnostic methods (47).
Radioimmunoassays for measurement of serum T3 (48) and T4 (49) were soon developed, and it was observed that l-thyroxine monotherapy could normalize both T4 and T3 levels at the expense of a high T4:T3 ratio. In contrast, l-triiodothyronine, desiccated thyroid, thyroglobulin, and l-thyroxine/l-triiodothyronine combination all typically resulted in low or low-normal serum T4 values with usually elevated serum T3 levels, and thus a low T4:T3 ratio (28). Desiccated thyroid resulted in a T3 peak about 2 to 5 hours after administration that corresponded to thyrotoxic symptoms in some patients (50). That a single daily dose of l-thyroxine resulted in stable blood levels of T4 and T3 throughout the day (48) was understood to result from a steady rate of conversion of T4 to T3 (51).
l-Thyroxine monotherapy, the novel and physiologically savvy method for treatment of hypothyroidism, contrasted with the traditional approach of natural thyroid preparations that was marred by potency concerns. In less than a decade, there was a major shift in treatment of hypothyroidism such that normalization of TSH with l-thyroxine monotherapy became the new standard of care (Appendix Table) (52). Many clinicians advocated for this to be first-line therapy and for patients previously treated with desiccated thyroid to be transitioned to l-thyroxine monotherapy (50).
l-Thyroxine Monotherapy Fails to Restore All Markers of Hypothyroidism
Clinicians noted several differences in the ability of l-thyroxine monotherapy to normalize markers of hypothyroidism at doses that normalized serum TSH (45). For instance, in many l-thyroxine-treated patients with a normal serum TSH, the BMR remained at about 10% less than that of normal controls even after 3 months of therapy (53). At the same time, doses of l-thyroxine that normalize the BMR can suppress serum TSH and cause iatrogenic thyrotoxicosis (28, 45, 46). The clinical significance of this was not fully understood because many patients appeared clinically euthyroid with a BMR between −20% and −10% (36, 37).
Hypothyroidism is a secondary cause of dyslipidemia, typically manifesting in elevation of low-density lipoprotein and total cholesterol levels. It is clear that treatment resulting in the normalization of the serum TSH is associated with reduction in total cholesterol levels (54), but whether total cholesterol is fully normalized by l-thyroxine monotherapy is less well-defined. An analysis of 18 studies on the effect of thyroid hormone replacement on total cholesterol levels in overt hypothyroidism showed a reduction in the total cholesterol level in all 18 studies; however, in 14 of the 18 studies, the mean post treatment total cholesterol level remained above the normal range (>200 mg/dL [>5.18 mmol/L]) (55). These findings suggest that lipid measures are not fully restored despite normalization of the serum TSH (56). Whether the degree of dyslipidemia remaining in l-thyroxine-treated patients with a normal TSH is clinically significant is unknown, given that the benefit of thyroid hormone replacement in subclinical hypothyroidism is itself controversial (57, 58).
Although relatively low serum T3 levels could contribute to these residual manifestations, the higher serum T4:T3 ratio should also be considered. This has been well-established for 4 decades (28, 50, 59), but only recently has it been recognized as a relevant measure given that higher serum T4 levels will impair systemic T3 production via downregulation of a deiodinase pathway (9). Thus, some emphasis has recently been directed toward establishing the clinical significance of this ratio (1, 5).
The normal values for the serum T4:T3 ratio are seldom discussed in the literature because measurement of serum T3 levels is not a recommended outcome in hypothyroidism (1). In a large study of approximately 3800 healthy individuals (4), the serum free T4:free T3 ratio was around 3, as opposed to a ratio of 4 in more than 1800 patients who had undergone thyroidectomy and were receiving l-thyroxine monotherapy. The corresponding serum free T4:free T3 ratio in patients continuing to receive desiccated thyroid is not well-defined, but the serum total T4:T3 ratio is known to be low (28, 50). In one study, the serum total T4:total T3 was about 40 in patients receiving desiccated thyroid and about 100 in those taking l-thyroxine monotherapy (60). Of course, this is affected by the timing of blood collection in relation to the timing of l-triiodothyronine administration, which is not commonly reported. Other key factors are the well-known poor reproducibility of the serum total T3 assay (61) and the interferences with direct measurement of free T3 (5).
Thus, neither desiccated thyroid nor l-thyroxine monotherapy recreates a biochemical state of euthyroidism as defined by the serum T4:T3 ratio. l-Thyroxine and l-triiodothyronine combination therapy theoretically could be titrated to restore this measure, but such a method would be challenging because of the frequent dosing schedule needed to achieve stable serum T3 levels (5). New technology is needed to allow for steady delivery of l-thyroxine; only then would high-quality clinical trials best investigate the utility of the serum T4:T3 ratio as an outcome measure in hypothyroidism.
The “Euthyroid“ Yet Symptomatic Patient
There is little mention of patients who did not respond symptomatically to treatment despite having normalization of their other measured variables, such as BMR or serum PBI, in the early clinical trials in the 1940s through 1960s. After the 1970s (38, 52), a new category of hypothyroid patient was recognized: the patient who received thyroid hormone replacement therapy, had normal serum TSH, and exhibited residual symptoms of hypothyroidism. Initially, such symptoms were largely dismissed as unrelated to the thyroid condition (62). Indeed, hypothyroidism is prevalent, and symptoms overlap with those of other common conditions, including menopause, depression, and chronic fatigue syndrome. Likewise, thyroid hormone had been administered for nonthyroid disorders, including obesity and psychiatric disease, for decades. Thus, it was difficult to assess whether patients with residual symptoms had been misdiagnosed. Residual symptoms were even attributed to nonadherence (63).
Although the implementation of sensitive TSH assays resulted in dose reduction, it also fueled the discovery of subclinical states of hypothyroidism (i.e., serum TSH <10 mIU/L and normal serum free T4); this state is 20 times more prevalent than overt hypothyroidism (64). Hence, many patients with vague symptoms, such as depressed mood and fatigue, are commonly screened and found to have subclinical hypothyroidism. In many cases, this finding prompts the conclusion that the subclinical hypothyroidism is the cause of the nonspecific symptoms, and thyroid hormone therapy is initiated. The patients in whom the cause–effect relationship was incorrect contribute to the increasing number of euthyroid but symptomatic patients (57). The marked increase in prescribing of thyroid hormone with decreasing TSH thresholds amplifies this problem (47).
To document that this was a result of trends toward lower doses, an unblinded study tracked well-being according to various doses and found that the highest well-being was achieved at supraoptimal doses, resulting in a suppressed TSH (65). However, a blinded trial did not reproduce this finding (66). In a call to the public, a 1997 British Thyroid Foundation newsletter asked readers to recount personal history of residual hypothyroid symptoms. More than 200 patients responded, 54 of whom specifically mentioned that they did not feel well despite normal serum markers of thyroid function (67, 68). Because of this surge in symptomatic patients, some clinicians advocated titrating dose by symptoms rather than serum TSH, reminiscent of the period before the 1970s (69).
A clinical trial investigating symptoms found that patients receiving l-thyroxine monotherapy, even with a normal TSH, displayed substantial impairment in psychological well-being compared with controls of similar age and sex (3). Because some hypothesized that this phenomenon came about only after adoption of l-thyroxine monotherapy, a study assessed combination therapy with l-thyroxine and l-triiodothyronine. Remarkably, the latter study showed that psychological measures improve in patients receiving combination therapy until serum TSH level is normal (6). In another study comparing l-thyroxine monotherapy versus desiccated thyroid, in which both groups had a normal TSH, many patients preferred desiccated thyroid and lost weight (60). Unfortunately, the solution to this complex problem is not as simple as reverting to combination therapy; the more than a dozen clinical trials on the subject have not shown benefit of superiority and preference for combination therapy, as previously reviewed (1, 3, 70).
Changing Themes in Treatment Guidelines
In the 1995 American Thyroid Association (ATA) guidelines, biological and synthetic thyroid hormone preparations containing T4 plus T3 were not recommended out of concern for fluctuating and often elevated serum T3 concentrations (71). In conjunction with the American Association of Clinical Endocrinologists in 2012, the ATA continued to recommend l-thyroxine monotherapy and noted that evidence does not support using synthetic combination therapies; in addition, they stated that “desiccated thyroid hormone should not be used for the treatment of hypothyroidism” (72). In 2014, the ATA recommendations evolved with the recognition that 1) serum T3 levels might not be normalized in all l-thyroxine–treated hypothyroid patients and 2) some patients remain symptomatic while receiving l-thyroxine monotherapy. Titration of l-thyroxine dose to achieve normal TSH concentrations remains a first-line approach, but trials with combination therapy can be considered. In addition, the guidelines recognize that although superiority data are lacking, some patients do experience a clinical response with desiccated thyroid preparations or combination therapy with l-thyroxine plus l-triiodothyronine (1). The European Thyroid Association has similar recommendations (2).
The Future
l-Thyroxine monotherapy for athyreotic rats results in a high T4:T3 ratio at doses sufficient to normalize serum TSH levels (8). Yet, the brain, liver, and skeletal muscle tissues of these l-thyroxine–treated animals continue to exhibit markers of hypothyroidism (9), probably because of the inability of l-thyroxine monotherapy to restore tissue levels of T3 (8). This is probably a direct consequence of lower serum T3 levels and the relatively high T4 concentration in these tissues, which inactivates the type 2 iodothyronine deiodinase (D2). In the hypothalamus, loss of D2 is minimal in the presence of T4, which increases sensitivity to T4 levels and explains TSH normalization, despite relatively lower levels of serum T3. Only combination therapy with l-thyroxine plus l-triiodothyronine normalized all thyroid hormone–dependent measures (9), including serum and tissue T3 levels (8). Whether tissue-specific markers of hypothyroidism are restored with l-thyroxine monotherapy in humans remains to be determined, as does the ability of l-thyroxine plus l-triiodothyronine combination therapy to normalize the serum T4:T3 ratio without adverse events. The development of a novel drug delivery system for l-triiodothyronine would facilitate these studies (5).
In humans, a factor associated with response to combination therapy in a large clinical trial is the Thr92Ala polymorphism in the type 2 deiodinase gene (DIO2), wherein the subpopulation of patients with this genetic alteration had improved well-being and preference for combination therapy (7). This has led investigators to consider whether this polymorphism could confer a defect in the D2 pathway, but normal Thr92AlaD2 enzyme kinetics have been demonstrated (73). Only recently has the Thr92AlaD2 protein been found to have a longer half-life, ectopically localize in the Golgi apparatus, and significantly alter the genetic fingerprint in cultured cells and in the temporal pole of the human brain without evidence of reduced thyroid hormone signaling (74). The significance of these studies transcends the thyroid field—this polymorphism has now been associated with a constellation of diseases, including mental retardation, bipolar disorder, and low IQ (75). If hypothyroid carriers of Thr92AlaD2 benefit from alternate therapeutic strategies in replicate studies, then personalized medicine—based on genotype— may have a role.
Conclusions
The development of TSH assays led to a dramatic reduction in thyroid hormone replacement dosage and the ability to diagnose with certainty milder forms of hypothyroidism. Discovery of peripheral T4-to-T3 conversion gave a physiologic means to justify l-thyroxine monotherapy. In combination with the concerns over consistency and safety of natural thyroid preparations, synthetic l-thyroxine was perceived as a more reliable therapy. These findings laid the foundation for the clinical practice trend away from natural thyroid preparations and toward l-thyroxine monotherapy at doses to normalize the serum TSH. Later, a subpopulation of patients with residual symptoms of hypothyroidism was recognized. It remains to be determined whether this is due to a trend of attributing nonspecific symptoms to minimal thyroid dysfunction, relatively low serum T3 levels and/or high T4:T3 ratio, or the role of Thr92AlaD2 polymorphism, and whether combination therapy with l-thyroxine plus l-triiodothyronine will be beneficial.
Acknowledgments
The authors thank Dr. Martin Surks, Dr. Jorge Mestman, and Dr. Colum Gorman for providing additional historical insight for this project; they did not read or endorse the final text.
Appendix Table
Trends in the Treatment Recommendations for Hypothyroidism*
| Year (Reference) | Agent Recommended | Adverse Effects and Highlighted Notes | Suggested Daily Maintenance Dose |
|---|---|---|---|
| 1929(22) | “The treatment consists of giving thyroid extract until the symptoms and physical findings disappear and the basal metabolic rate is normal.” |
- | Start with 3 grains and then increase to normalize BMR |
| 1948(76) | Dried thyroid “is the simplest and atthe same time the best method of treatment” and “is actually superior to pure crystalline thyroxine because it contains a hormone in a more soluble and assimilable form.” |
“Cardiac symptoms of pain or palpitation are not uncommon.” “The isolation and synthesis of thyroxine are of vast importance to physiology. They have contributed nothing to therapeutics.” |
60–200 mg DT |
| “Curative treatment of adult myxedema is as perfect a form of therapy as any known to medicine.” | |||
| 1955(33) | “Most of the patients are maintained in good condition with 1½ or 2 grains (U.S.P.) of desiccated thyroid daily.” |
“Pharmaceutical companies have recently made available thyroxin and triiodothyronine for oral use instead of desiccated thyroid. Because the former two are pure crystalline compounds, there probably is a more reliable dosage-response, but since such precise dosage is not necessary, nor easily measured, and since pure compounds are more expensive, thyroidologists have continued to use desiccated thyroid in most patients.” |
1.5–2 grains DT |
| “Should the pure hormone become much less expensive, they probably should be used instead of desiccated thyroid.” | |||
| 1958(77) | Available agents are DT, T4, and T3; all “are most satisfactory” | - | 2–3 grains DT 200–500 mcg l-T4 70–105 mcg l-T3 |
| 1962(78) | No “standard of care” | - | 90–240 mcg DT 140–400 mcg l-T4 50–150 mcg l-T3 |
| 1963(79) | “Desiccated thyroid or thyroid extract is the most satisfactory for longterm treatment. It is relatively cheap, easily administered, welTabsorbed, stable on storage for considerable periods (up to three months or more at room temperature, dry), and different batches are usually of comparable potency.” |
“There is a growing tendency to use thyroxine initially instead of thyroid or to switch patients who are not doing well on thyroid to thyroxine instead.” |
130–260 mg (2–4 grains) DT 200–400 mcg l-T4 75–125 mcg l-T3 |
| 1968(27) | “Treatment is carried out with one or two general types of preparation, either synthetic hormone or thyroprotein derived from animal thyroid glands.” |
“Despite their theoretical disadvantages and the occasional instances of ineffectiveness or excessive potency, the preparations of natural origin are generally reliable agents that sustain a normal metabolic state in association with a normal PBI.” |
120–180 mgDT |
| “Levothyroxine is capable of reversing all the known abnormalities of the adult hypothyroid state. This, together with its uniform potency, makes it an entirely satisfactory preparation.” | |||
| “Liothyronine should not be used in the treatment of hypothyroidism.” | |||
| “Recent studies indicate that preparations of natural origin vary considerably in regard to the proportion of total organic iodine present as T4 and T3, as well as the ratio between these hormones themselves. Consequently, variations in biological potency may occur among different preparations or different batches of the same preparation, despite their conforming to prescribed standards. It would appear entirely feasible at present for standardization ofthyroid extracts to be based upon their absolute content of T4 and T3; trends in this direction are evident. If this were done, the major reason for employing synthetic hormone in preference to material of natural origin would no longer exist.” | |||
| 1971 (36) | “Desiccated thyroid remains the preparation of choice in the treatment of myxedema, largely because it is the least expensive.” |
Animal product criticisms: “iodothyronine content varies among species, season, location of the animal, brand and potency”; iodothyronines “break down within the tablets in the presence of moisture.” |
90–180 mg DT 200–400 mcg l-T4 50–100 mcg l-T3 |
| “The use of desiccated thyroid or a T4-T3 mixture is probably preferable” as combination therapies restore both the plasma T3 and T4 to the normal range. | |||
| 1974(59) | “There has been a distinct trend away from the use of the natural preparations and towards the newer synthetic preparations, in view of their uniform potency and, as a result, their more predictable effects… levothyroxine is the agent of choice.” |
Regarding natural or synthetic preparations containing T3 “peaks make assessment of the proper dosage through measurement of hormone concentrations extremely difficult and, moreover, may have adverse effects, especially in the older patient or in the patient with cardiac disease. |
Dose to bring TSH into normal range, as low as 200 mcg l-T4 |
| “The recent demonstration that most of the T3 in the serum is derived from the metabolism of T4 and, as a corollary, that serum T3 concentrations are nearly normal in patients receiving replacement doses of T4 has to a large extent eliminated the rationale both for the use of Liotrix and for the maintenance of elevated serum T4 concentrations when levothyroxine is employed.” | |||
| 1978(63) | “The use of l-thyroxine sodium by mouth is tending to supersede desiccated thyroid, liothyronine sodium (sodium triiodo-l-thyronine), and sodium thyroxine-triiodothyronine mixtures.” |
- | 100–150 mcg l-T4 |
| 1979(52) | “There is now nearly universal agreement that synthetic levothyroxine is the agent of choice.” | - | Minimum dose to normalize TSH |
| 1986(80) | “Most physicians… use synthetic T4 in preference to thyroid” | - | 100–150 mcg l-T4 1–1.5 grains DT |
| 1989(81) | “Levothyroxine is the drug of choice” | - | 100–200 mcg l-T4 90–180 mgDT |
| 1996(40) |
l-Thyroxine is “preferred due to its long half-life, its ready quantitation in the blood, ease of absorption, and the availability of multiple tablet strengths” |
“There are patients who have taken DTE for many decades and are reluctant to consider a change.” |
100–200 mcg l-T4 |
| “The difficulties that led to the development of LT4 as a replacement preparation, namely inconstant and subpotent desiccated thyroid tables, have largely been obviated by new USP standards for quantitation of T3 and T4 in DTE tabs.” | |||
BMR = basal metabolic rate; DT = desiccated thyroid; DTE = desiccated thyroid extract; l-fT3 = l-triiodothyronine; l-fT4 or (LT4) = l-thyroxine; PBI = protein-bound iodine; T3 = triiodothyronine; T4 = thyroxine; TSH = thyroid-stimulating hormone; USP (or U.S.P.) = United States Pharmacopeia.
In the years prior to the discovery of peripheral T4-to-T3 conversion, most groups recommended treatment with natural thyroid preparations, such as desiccated thyroid, thyroid extract, or thyroglobulin, which contain both T4 and T3. However with the discovery of T4-to-T3 conversion and the development of the radioimmunoassay for TSH in the early 1 970s, not only was there a trend toward l-thyroxine monotherapy, but the recommended daily maintenance doses decreased significantly. These trends led to the adoption of the contemporary standard of care: l-thyroxine monotherapy administered at doses to maintain a normal serum TSH level.
Footnotes
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M15-1799.
Author Contributions: Conception and design: E.A. McAn-inch, A.C. Bianco.
Analysis and interpretation of the data: E.A. McAninch, A.C. Bianco.
Drafting of the article: E.A. McAninch, A.C. Bianco. Critical revision of the article for important intellectual content: E.A. McAninch, A.C. Bianco.
Final approval of the article: E.A. McAninch, A.C. Bianco. Obtaining of funding: A.C. Bianco. Collection and assembly of data: E.A. McAninch, A.C. Bianco.
References
- 1.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [PMID: 25266247] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. 2012;1:55–71. doi: 10.1159/000339444. [PMID: 24782999] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf) 2002;57:577–585. doi: 10.1046/j.1365-2265.2002.01654.x. [PMID: 12390330] [DOI] [PubMed] [Google Scholar]
- 4.Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One. 2011;6:e22552. doi: 10.1371/journal.pone.0022552. [PMID: 21829633] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdalla SM, Bianco AC. Defending plasma T3 is a biological priority. Clin Endocrinol (Oxf) 2014;81:633–641. doi: 10.1111/cen.12538. [PMID: 25040645] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ., Jr Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med. 1999;340:424–429. doi: 10.1056/NEJM199902113400603. [PMID: 9971866] [DOI] [PubMed] [Google Scholar]
- 7.Panicker V, Saravanan P, Vaidya B, Evans J, Hattersley AT, Frayling TM, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94:1623–1629. doi: 10.1210/jc.2008-1301. [PMID: 19190113] [DOI] [PubMed] [Google Scholar]
- 8.Escobar-Morreale HF, del Rey FE, Obregón MJ, de Escobar GM. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. 1996;137:2490–2502. doi: 10.1210/endo.137.6.8641203. [PMID: 8641203] [DOI] [PubMed] [Google Scholar]
- 9.Werneck de Castro JP, Ueta CB, McAninch EA, Abdalla S, Wittmann G, et al. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest. 2015;125:769–781. doi: 10.1172/JCI77588. [PMID: 25555216] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAninch EA, Jo S, Preite NZ, Farkas E, Mohácsik P, Fekete C, et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab. 2015;100:920–933. doi: 10.1210/jc.2014-4092. [PMID: 25569702] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindholm J, Laurberg P. Hypothyroidism and thyroid substitution: historical aspects. J Thyroid Res. 2011;2011:809341. doi: 10.4061/2011/809341. [PMID: 21760981] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundie RA. The treatment of myxoedema. Edinburgh Medical Journal. 1893;38:996–1005. [Google Scholar]
- 13.Kocher A. The treatment of hypothyroidism by thyroid transplantation. Br Med J. 1923;2:560–561. doi: 10.1136/bmj.2.3274.560. [PMID: 20771297] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettencourt R, Serrano J. Un cas de myxoedè me traité par la greffe hypodermique du corps thyroïde d’un mouton. La Semaine Médicale. 1890;10:294. [Google Scholar]
- 15.Ord WM, White E. Clinical remarks on certain changes observed in the urine in myxoedema after the administration of glycerine extract of thyroid gland. Br Med J. 1893;2:217. doi: 10.1136/bmj.2.1700.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackenzie HW. A case of myxoedema treated with great benefit by feeding with fresh thyroid glands. Br Med J. 1892;2:940–941. doi: 10.1136/bmj.2.1661.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson R. Correspondence. Montreal Medical Journal. 1893;22:437–439. [Google Scholar]
- 18.Ma R, Latif R, Davies TF. Human embryonic stem cells form functional thyroid follicles. Thyroid. 2015;25:455–461. doi: 10.1089/thy.2014.0537. [PMID: 25585054] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnus-Levy A. Energy metabolism in health and disease. J Hist Med Allied Sci. 1947;2:307–320. doi: 10.1093/jhmas/ii.3.307. [PMID: 20266805] [DOI] [PubMed] [Google Scholar]
- 20.Stanbury JB. A Constant Ferment. Ipswich, MA: Ipswich Pr; 1991. [Google Scholar]
- 21.Palmer WW. Metabolism in hyperthyroidism and hypothyroidism. Bull N Y Acad Med. 1934;10:52–64. [PMID: 19311910] [PMC free article] [PubMed] [Google Scholar]
- 22.Chesky VE. Diseases of the Thyroid Gland. 2nd. St. Louis: CV Mosby; 1929. The Hospital Management of Goiter Patients; pp. 209–229. [Google Scholar]
- 23.Chalmers JR, Dickson GT, Elks J, Hems BA. The synthesis of thyroxine and related substances. Part V. A synthesis of L-thyroxine from L-tyrosine. Journal of the Chemical Society. 1949:3424–3438. [Google Scholar]
- 24.Hart FD, Maclagan NF. Synthetic thyroxine in the treatment of myxoedema. J Endocrinol. 1950;6:xxxiv. [PMID: 14774506] [PubMed] [Google Scholar]
- 25.Williams RH. Relation of obesity to the function of the thyroid gland, especially as indicated by the protein-bound iodine concentration in the plasma. J Clin Endocrinol Metab. 1948;8:257–261. doi: 10.1210/jcem-8-3-257. [PMID: 18912537] [DOI] [PubMed] [Google Scholar]
- 26.Braverman LE, Ingbar SH. Anomalous effects of certain preparations of desiccated thyroid on serum protein-bound iodine. N Engl J Med. 1964;270:439–442. doi: 10.1056/NEJM196402272700903. [PMID: 14163222] [DOI] [PubMed] [Google Scholar]
- 27.Ingbar SH, Woeber KA. Thyroid hormone deficiency. In: Williams RH, editor. Textbook of Endocrinology. 4th. Philadelphia: WB Saunders; 1968. pp. 232–258. [Google Scholar]
- 28.Cobb WE, Jackson IM. Drug therapy reviews: management of hypothyroidism. Am J Hosp Pharm. 1978;35:51–58. [PMID: 341699] [PubMed] [Google Scholar]
- 29.Thompson WO, McLellan LL, Thompson PK, Dickie LF. The rates of utilization of thyroxine and of desiccated thyroid in man: the relation between the iodine in desiccated thyroid and in thyroxine. J Clin Invest. 1933;12:235–246. doi: 10.1172/JCI100492. [PMID: 16694117] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross J, Pitt-Rivers R, Trotter WR. Effect of 3:5:3’-L-triiodothyronine in myxoedema. Lancet. 1952;1:1044–1045. doi: 10.1016/s0140-6736(52)90695-8. [PMID: 14928556] [DOI] [PubMed] [Google Scholar]
- 31.Asper SP, Jr, Selenkow HA, Plamondon CA. A comparison of the metabolic activities of 3,5,3-l-triiodothyronine and Lthyroxine in myxedema. Bull Johns Hopkins Hosp. 1953;93:164–198. [PMID: 13094265] [PubMed] [Google Scholar]
- 32.McGavack TH, Reckendorf HK. Therapeutic activity of desiccated thyroid substance, sodium l-thyroxine and D,L-triiodothyronine; a comparative study. Am J Med. 1956;20:774–777. doi: 10.1016/0002-9343(56)90159-0. [PMID: 13313575] [DOI] [PubMed] [Google Scholar]
- 33.Williams RH. The thyroid. In: Williams RH, editor. Textbook of Endocrinology. 2nd. Philadelphia: WB Saunders; 1955. pp. 99–220. [Google Scholar]
- 34.Frawley TF, McClintock JC, Beebe RT, Marthy GL. Metabolic and therapeutic effects of triiodothyronine. J Am Med Assoc. 1956;160:646–652. doi: 10.1001/jama.1956.02960430036007. [PMID: 13286110] [DOI] [PubMed] [Google Scholar]
- 35.Smith RN, Taylor SA, Massey JC. Controlled clinical trial of combined triiodothyronine and thyroxine in the treatment of hypothyroidism. Br Med J. 1970;4:145–148. doi: 10.1136/bmj.4.5728.145. [PMID: 4097650] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werner SC. Treatment; myxedema coma; nonspecific uses of thyroid medication. In: Werner SC, Ingbar SH, editors. The Thyroid: A Fundamental and Clinical Text. 3rd. New York: Harper & Row; 1971. pp. 832–838. [Google Scholar]
- 37.Werner SC. Pharmacology; treatment. In: Werner SC, editor. The Thyroid: A Fundamental and Clinical Text. 2nd. New York: Harper & Row; 1962. pp. 817–825. [Google Scholar]
- 38.Kaufman SC, Gross TP, Kennedy DL. Thyroid hormone use: trends in the United States from 1960 through 1988. Thyroid. 1991;1:285–291. doi: 10.1089/thy.1991.1.285. [PMID: 1841728] [DOI] [PubMed] [Google Scholar]
- 39.Mangieri CN, Lund MH. Potency of United States Pharmacopeia dessicated thyroid tablets as determined by the antigoitrogenic assay in rats. J Clin Endocrinol Metab. 1970;30:102–104. doi: 10.1210/jcem-30-1-102. [PMID: 5409525] [DOI] [PubMed] [Google Scholar]
- 40.DeGroot LJ, Larsen PR, Hennemann G. The Thyroid and Its Diseases. 6th. London: Churchill Livingstone; 1996. Treatment of the patient with primary hypothyroidism; pp. 351–356. [Google Scholar]
- 41.Lavietes PH, Epstein FH. Thyroid therapy of myxedema: a comparison of various agents with a note on the composition of thyroid secretion in man. Ann Intern Med. 1964;60:79–87. doi: 10.7326/0003-4819-60-1-79. [PMID: 14104859] [DOI] [PubMed] [Google Scholar]
- 42.Braverman LE, Ingbar SH, Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970;49:855–864. doi: 10.1172/JCI106304. [PMID: 4986007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Utiger RD. Thyrotrophin radioimmunoassay: another test of thyroid function. Ann Intern Med. 1971;74:627–629. doi: 10.7326/0003-4819-74-4-627. [PMID: 5551168] [DOI] [PubMed] [Google Scholar]
- 44.Hershman JM, Pittman JA., Jr Utility of the radioimmunoassay of serum thyrotrophin in man. Ann Intern Med. 1971;74:481–490. doi: 10.7326/0003-4819-74-4-481. [PMID: 4994544] [DOI] [PubMed] [Google Scholar]
- 45.Cotton GE, Gorman CA, Mayberry WE. Suppression of thyrotropin (h-TSH) in serums of patients with myxedema of varying etiology treated with thyroid hormones. N Engl J Med. 1971;285:529–533. doi: 10.1056/NEJM197109022851001. [PMID: 5109216] [DOI] [PubMed] [Google Scholar]
- 46.Evered D, Young ET, Ormston BJ, Menzies R, Smith PA, Hall R. Treatment of hypothyroidism: a reappraisal of thyroxine therapy. Br Med J. 1973;3:131–134. doi: 10.1136/bmj.3.5872.131. [PMID: 4720761] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor PN, Iqbal A, Minassian C, Sayers A, Draman MS, Greenwood R, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med. 2014;174:32–39. doi: 10.1001/jamainternmed.2013.11312. [PMID: 24100714] [DOI] [PubMed] [Google Scholar]
- 48.Surks MI, Schadlow AR, Oppenheimer JH. A new radioimmunoassay for plasma l-triiodothyronine: measurements in thyroid disease and in patients maintained on hormonal replacement. J Clin Invest. 1972;51:3104–3113. doi: 10.1172/JCI107137. [PMID: 4539287] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsen PR, Dockalova J, Sipula D, Wu FM. Immunoassay of thyroxine in unextracted human serum. J Clin Endocrinol Metab. 1973;37:177–182. doi: 10.1210/jcem-37-2-177. [PMID: 4198255] [DOI] [PubMed] [Google Scholar]
- 50.Jackson IM, Cobb WE. Why does anyone still use desiccated thyroid USP? Am J Med. 1978;64:284–288. doi: 10.1016/0002-9343(78)90057-8. [PMID: 629277] [DOI] [PubMed] [Google Scholar]
- 51.Surks MI, Schadlow AR, Stock JM, Oppenheimer JH. Determination of iodothyronine absorption and conversion of l-thyroxine (T4) to l-triiodothyronine (T3) using turnover rate techniques. J Clin Invest. 1973;52:805–811. doi: 10.1172/JCI107244. [PMID: 4693647] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woeber KA, Braverman LE. The thyroid. In: Ingbar SH, editor. Contemporary Endocrinology. New York: Plenum; 1979. pp. 77–117. [Google Scholar]
- 53.Gorman CA, Jiang NS, Ellefson RD, Elveback LR. Comparative effectiveness of dextrothyroxine and levothyroxine in correcting hypothyroidism and lowering blood lipid levels in hypothyroid patients. J Clin Endocrinol Metab. 1979;49:1–7. doi: 10.1210/jcem-49-1-1. [PMID: 447807] [DOI] [PubMed] [Google Scholar]
- 54.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–293. doi: 10.1089/10507250252949405. [PMID: 12034052] [DOI] [PubMed] [Google Scholar]
- 55.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PMID: 12485966] [PubMed] [Google Scholar]
- 56.Tanis BC, Westendorp GJ, Smelt HM. Effect of thyroid substitution on hypercholesterolaemia in patients with subclinical hypothyroidism: a reanalysis of intervention studies. Clin Endocrinol (Oxf) 1996;44:643–649. doi: 10.1046/j.1365-2265.1996.739560.x. [PMID: 8759176] [DOI] [PubMed] [Google Scholar]
- 57.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142–1154. doi: 10.1016/S0140-6736(11)60276-6. [PMID: 22273398] [DOI] [PubMed] [Google Scholar]
- 58.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [PMID: 14722150] [DOI] [PubMed] [Google Scholar]
- 59.Ingbar SH, Woeber KA. Thyroid hormone deficiency. In: Williams RH, editor. Textbook of Endocrinology. 5th. Philadelphia: WB Saunders; 1974. pp. 191–212. [Google Scholar]
- 60.Hoang TD, Olsen CH, Mai VQ, Clyde PW, Shakir MK. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982–1990. doi: 10.1210/jc.2012-4107. [PMID: 23539727] [DOI] [PubMed] [Google Scholar]
- 61.Kazerouni F, Amirrasouli H. Performance characteristics of three automated immunoassays for thyroid hormones. Caspian J Intern Med. 2012;3:400–104. [PMID: 24358433] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffenberg R. Primary hypothyroidism. In: Ingbar SH, Braverman LE, editors. Werner’s The Thyroid: A Fundamental and Clinical Text. 5th. Philadelphia: JB Lippincott; 1978. pp. 1255–1266. [Google Scholar]
- 63.Werner SC. Treatment. In: Werner SC, Ingbar SH, editors. The Thyroid: A Fundamental and Clinical Text. 4th. Baltimore: Harper & Row; 1978. pp. 965–970. [Google Scholar]
- 64.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [PMID: 10695693] [DOI] [PubMed] [Google Scholar]
- 65.Carr D, McLeod DT, Parry G, Thornes HM. Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol (Oxf) 1988;28:325–333. doi: 10.1111/j.1365-2265.1988.tb01219.x. [PMID: 3139338] [DOI] [PubMed] [Google Scholar]
- 66.Walsh JP, Ward LC, Burke V, Bhagat CI, Shiels L, Henley D, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab. 2006;91:2624–2630. doi: 10.1210/jc.2006-0099. [PMID: 16670161] [DOI] [PubMed] [Google Scholar]
- 67.Roberts N. Psychological problems in thyroid disease. British Thyroid Foundation Newsletter. 1996:3. [Google Scholar]
- 68.Saravanan P, Visser TJ, Dayan CM. Psychological well-being correlates with free thyroxine but not free 3,5,3’-triiodothyronine levels in patients on thyroid hormone replacement. J Clin Endocrinol Metab. 2006;91:3389–3393. doi: 10.1210/jc.2006-0414. [PMID: 16804044] [DOI] [PubMed] [Google Scholar]
- 69.Skinner GR, Thomas R, Taylor M, Sellarajah M, Bolt S, Krett S, et al. Thyroxine should be tried in clinically hypothyroid but biochemically euthyroid patients [Letter] BMJ. 1997;314:1764. doi: 10.1136/bmj.314.7096.1764. [PMID: 9202524] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiersinga WM. Paradigm shifts in thyroid hormone replacement therapies for hypothyroidism. Nat Rev Endocrinol. 2014;10:164–174. doi: 10.1038/nrendo.2013.258. [PMID: 24419358] [DOI] [PubMed] [Google Scholar]
- 71.Singer PA, Cooper DS, Levy EG, Ladenson PW, Braverman LE, Daniels G, Cooper DS, et al. Treatment guidelines for patients with hyperthyroidism and hypothyroidism. Standards of Care Committee, American Thyroid Association. JAMA. 1995;273:808–812. [PMID: 7532241] [PubMed] [Google Scholar]
- 72.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: co-sponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200–1235. doi: 10.1089/thy.2012.0205. [PMID: 22954017] [DOI] [PubMed] [Google Scholar]
- 73.Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, et al. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [PMID: 12788902] [DOI] [PubMed] [Google Scholar]
- 74.McAninch EA, Jo S, Preite NZ, Farkas E, Mohácsik P, Fekete C, et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab. 2015;100:920–933. doi: 10.1210/jc.2014-4092. [PMID: 25569702] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bianco AC, Casula S. Thyroid hormone replacement therapy: three ‘simple’ questions, complex answers. Eur Thyroid J. 2012;1:88–98. doi: 10.1159/000339447. [PMID: 24783002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Web-Only References
- 76.Means JH. The Thyroid and Its Diseases. 2nd. Philadelphia: JB Lippincott; 1948. Myxedema; pp. 241–273. [Google Scholar]
- 77.Soffer LJ. Diseases of the Endocrine Glands. 2nd. Philadelphia: Lea & Febiger; 1956. Hypothyroidism: cretinism, juvenile and adult myx-edema (Gulls’ disease) pp. 829–868. [Google Scholar]
- 78.Williams RH, Bakke JL. General consideration of the treatment of thyroid disease. In: Williams RH, editor. Textbook of Endocrinology. 3rd. Philadelphia: WB Saunders; 1962. pp. 133–135. [Google Scholar]
- 79.Pittman JA. Diagnosis and Treatment of Thyroid Diseases. Philadelphia: FA Davis; 1963. Hypothyroidism; pp. 56–78. [Google Scholar]
- 80.Hoffenberg R. Primary hypothyroidism. In: Ingbar SH, Braverman LE, editors. Werner’s The Thyroid: A Fundamental and Clinical Text. 5th. Philadelphia: JB Lippincott; 1986. pp. 1255–1265. [Google Scholar]
- 81.Volpe R. Hypothyroidism. In: Burrow GN, Oppenheimer JH, Volpe R, editors. Thyroid Function & Disease. Philadelphia: Saun-ders; 1989. pp. 274–291. [Google Scholar]