Abstract
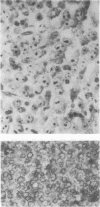
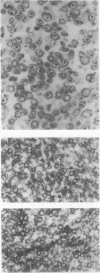
Fifteen cases of large-cell lymphoma, diagnosed as centroblastic (5), B-immunoblastic (5) or true histiocytic (5). lymphoma and one case of malignant histiocytosis were studied with monoclonal antibodies. Each diagnosis was based on morphological as well as marker studies. A panel of monoclonal and heterologous antibodies against T lymphocyte differentiation antigens (Leul, Leu2a, Leu3a, OKT4, OKT8, TA1), B lymphocyte subsets (BA1, BA2, HLA-DR, alpha C3b receptor antiserum, surface immunoglobulins), the common acute lymphoblastic leukaemia antigen (CALLA), monocytes/macrophages (OKM1, anti-human monocyte 1, TA1, Mac1, HLA-DR, anti-C3b receptor), myeloid cells (VIM-D5, elastase, OKM1) and the cells of the Langerhans cell/interdigitating reticulum cell series (OKT6, NA1/34). The results show a specific staining pattern for true histiocytic lymphoma (histiocytic sarcoma). Centroblastic and B-immunoblastic lymphomas showed gradual differences with mostly strong staining for HLA-DR and weak with anti C3b receptor for B-immunoblastic lymphomas in contrast to centroblastic lymphomas. Staining with BA1 and BA2 indicated immunological heterogeneity in these lymphomas. The number of admixed cells was usually low with few B cells and a shift in the ratio helper/inducer to suppressor/cytotoxic T cells in favour of the suppressor/cytotoxic subset.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosman F. T., Lindeman J., Kuiper G., van der Wal A., Kreunig J. The influence of fixation on immunoperoxidase staining of plasmacells in paraffin sections of intestinal biopsy specimens. Histochemistry. 1977 Jul 18;53(1):57–62. doi: 10.1007/BF00511210. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Brown G., Capellaro D., Greaves M. Leukemia-associated antigens in man. J Natl Cancer Inst. 1975 Dec;55(6):1281–1289. doi: 10.1093/jnci/55.6.1281. [DOI] [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBien T. W., Kersey J. H. A monoclonal antibody (TA-1) reactive with human T lymphocytes and monocytes. J Immunol. 1980 Nov;125(5):2208–2214. [PubMed] [Google Scholar]
- Lukes R. J., Collins R. D. New approaches to the classification of the lymphomata. Br J Cancer Suppl. 1975 Mar;2:1–28. [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Meijer C. J., Lindeman J. A modified method for tissue localization of cells bearing a complement receptor. J Immunol Methods. 1975 Nov;9(1):59–65. doi: 10.1016/0022-1759(75)90035-6. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Velde J. T., Burkhardt R., Kleiverda K., Leenheers-Binnendijk L., Sommerfeld W. Methyl-methacrylate as an embedding medium in histopathology. Histopathology. 1977 Sep;1(5):319–330. doi: 10.1111/j.1365-2559.1977.tb01671.x. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Good R. A., Ammirati P., Dymbort G., Evans R. L. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J Exp Med. 1980 Jun 1;151(6):1539–1544. doi: 10.1084/jem.151.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke R., Levy R. Detection of T and B cell antigens hybridoma monoclonal antibodies: a biotin-avidin-horseradish peroxidase method. J Histochem Cytochem. 1980 Aug;28(8):771–776. doi: 10.1177/28.8.7003003. [DOI] [PubMed] [Google Scholar]
- Warnke R., Miller R., Grogan T., Pederson M., Dilley J., Levy R. Immunologic phenotype in 30 patients with diffuse large-cell lymphoma. N Engl J Med. 1980 Aug 7;303(6):293–300. doi: 10.1056/NEJM198008073030601. [DOI] [PubMed] [Google Scholar]
- van der Valk P., Hermans J., Brand R., Cornelisse C. J., Spaander P. J., Meijer C. J. Morphometric characterization of diffuse large-cell (histiocytic) lymphomas. Am J Pathol. 1982 Jun;107(3):327–335. [PMC free article] [PubMed] [Google Scholar]
- van der Valk P., te Velde J., Jansen J., Ruiter D. J., Spaander P. J., Cornelisse C. J., Meijer C. J. Malignant lymphoma of true histiocytic origin: histiocytic sarcoma. A morphological, ultrastructural, immunological, cytochemical and clinical study of 10 cases. Virchows Arch A Pathol Anat Histol. 1981;391(3):249–265. doi: 10.1007/BF00709158. [DOI] [PubMed] [Google Scholar]