Abstract
The Asian cockroach (Blattella asahinai Mizukubo) was introduced to Florida in 1986 and has since spread throughout the Southeastern United States. Blattella asahinai is a peridomestic pest and high population densities in residential areas can become a nuisance, especially when adults fly into homes. Few studies to date have been conducted on Asian cockroach control, and we evaluated the efficacy of Zyrox Fly Granular Bait and Maxforce Complete Granular Insect Bait against this species in the laboratory compared with the closely related German cockroach (Blattella germanica (L.)). In no-choice and two-choice assays with both species, Zyrox bait and Maxforce bait achieved nearly 100% mortality within two and five days, respectively. We also tested Zyrox bait against B. asahinai in an invasive field population in North Carolina at the label rate (2 g/m2) and at approximately three times the label rate (6.9 g/m2), and found that broadcast applications at both rates reduced populations by an average of 64 and 92%, respectively, for 35 d after the initial application. Zyrox Fly Bait appears to be effective against the Asian and German cockroaches, and could be another tool in an integrated pest management program, if its label could be extended or the active ingredient (cyantraniliprole) formulated into a cockroach bait.
Keywords: Blattella asahinai, Blattella germanica, Zyrox Fly Granular Bait, cyantraniliprole, Maxforce Complete Granular Insect Bait
The Asian cockroach (Blattella asahinai Mizukubo), first described in 1981 in Okinawa, Japan (Mizukubo 1981), was introduced into Florida in 1986 (Roth 1986) and has been spreading throughout Florida and the Southeastern United States along major highway routes to Alabama, Georgia, South Carolina, and Texas (Koehler 1999, Sitthicharoenchai 2002, Pfannenstiel et al. 2008, Snoddy and Appel 2008). Blattella asahinai frequently inhabits leaf litter and dense grass in shaded areas (Brenner et al. 1988), and mulch with small to medium interstitial spaces (Snoddy and Appel 2013). Populations of the Asian cockroach can reach high densities in residential settings where adults may often fly into homes when attracted to lights, becoming a nuisance (Brenner et al. 1988). Additionally, B. asahinai can damage strawberry fruit by creating excavations, and densities of up to 54,000 per acre have been reported in Florida (Price and Nagle 2008). The Asian cockroach has also been found in citrus groves, where it has been observed to prevent the emergence of parasitoids by feeding on parasitized brown citrus aphids (Persad and Hoy 2004). Nevertheless, because B. asahinai also reaches exceptionally high densities in soybean fields in Texas and it is an efficient predator of lepidopteran eggs, it may be considered a beneficial insect in this and related crops (Pfannenstiel et al. 2008).
Few studies have been conducted on control of Asian cockroach populations, although they appear to be susceptible to most insecticides (Valles et al. 1999). Recently however, several insecticides readily available to consumers were evaluated against B. asahinai, revealing that fipronil granules and β-cyfluthrin EC are effective in achieving significant reduction in field population levels (Snoddy and Appel 2014), and a suggested integrated pest management program has been developed (Snoddy 2012). A common control method of other peridomestic pest cockroach species, such as the smokybrown cockroach (Periplaneta fuliginosa Serville), is to treat a narrow band around the perimeter of a residence with a broad-spectrum insecticide (Appel and Smith 2002). However, these perimeter sprays can cause large populations to build up directly outside the treated band and the insecticide can degrade rapidly, especially during hot summer months when cockroach populations are highest (Appel and Smith 2002).
In the past few decades, baits have become an increasingly popular and effective control method for cockroaches and other urban insect pests. Baits are preferred because they use smaller amounts of active ingredient and are longer lasting than residual sprays, have low mammalian toxicity, are odorless, and can be used in a diverse range of conditions (Reierson 1995). Moreover, effective active ingredients that cannot be labeled in spray formulations or are photolabile have been formulated in baits (Harpaz 1987). One of the newest active ingredients, cyantraniliprole, is a second-generation anthranilic diamide insecticide (Selby et al. 2013). Cyantraniliprole has low mammalian toxicity. It binds to ryanodine receptors causing calcium ions to be depleted, thus interfering with muscle contraction and leading to paralysis and death (Selby et al. 2013). Cyantraniliprole is being used in agricultural crops against lepidopteran, hemipteran, and coleopteran pests (Selby et al. 2013), and has now been incorporated into a new granular bait for use against the house fly (Musca domestica L.), which causes significant reduction in lab and field populations (Murillo et al. 2014). A cyantraniliprole-containing bait, Zyrox Fly Granular Bait (Syngenta, EPA Reg number 100-1541) is registered for use in commercial and residential areas (Syngenta 2015), the same types of areas that B. asahinai frequently inhabit.
The aims of this study were to evaluate the efficacy of Zyrox Fly Granular Bait against B. asahinai 1) in the laboratory compared with the closely related German cockroach (Blattella germanica L.) and 2) in the field. If Zyrox bait is effective in reducing B. asahinai populations in the field, it could be used as a novel control method for this nuisance pest in residential and commercial settings and could reduce the use of broad-spectrum insecticides.
Materials and Methods
Insects
Blattella germanica were from a laboratory colony (Orlando Normal = American Cyanamid strain, collected >60 yr ago in Florida, maintained in the Schal lab since 1989) provided ad libitum with rat chow (LabDiet 5001, PMI Nutrition International, Brentwood, MO) and water. Cockroaches were maintained in an incubator at 27°C, 40–70% relative humidity (RH), and a photoperiod of 12:12 (L:D) h.
Blattella asahinai were maintained in laboratory colonies (originally field collected from Auburn, AL and Florida) and provided continuously with rat chow and water. Cockroaches were raised in an incubator at 27°C, 45–55% RH, and a photoperiod of 14:10 (L:D) h.
Laboratory Assays of Mortality
Insecticides
In laboratory assays, we tested Zyrox Fly Granular Bait (0.5% cyantraniliprole; Syngenta Crop Protection, Greensboro, NC, EPA Reg number 100-1541) and Maxforce Complete Brand Granular Insect Bait (1% hydramethylnon; Bayer Environmental Science, Research Triangle Park, NC, EPA Reg number 432-1255), an industry standard for cockroach control.
Experimental Design
Blattella germanica males ranged in age from 10–30 d. The age was unknown for B. asahinai males, but only males with no apparent damage and full-length antennae were used. Males were separated into treatment groups, provided with water, and starved for 24 h prior to the start of assays. For B. germanica, equal numbers of same-aged insects were put into each treatment group. Equal proportions of B. asahinai from the Florida and Alabama colonies were put into each treatment group.
No-Choice Assay
Assays were set up in 15-cm by 26-mm Petri dishes (Thermo Fisher Scientific, Waltham, MA) with the bottom third of an egg section of an egg carton for harborage, a glass test tube (10- by 75-mm disposable culture tube, Thermo Fisher Scientific) filled with water and plugged with cotton, and a cap for a 7-ml glass scintillation vial (Thermo Fisher Scientific) which held ∼0.5 g of bait or rat chow. The egg carton and vial cap were held in place with labeling tape without leaving any part of the sticky side exposed, and the water was held in place with modeling clay (Crayola, Easton, PA). Treatment groups received Zyrox bait, Maxforce bait, or rat chow crushed to the approximate size of bait granules as a negative control.
Males were anesthetized briefly for 10 min in a refrigerator, and released into assay arenas. Thirty B. asahinai were assigned to the control and the Zyrox bait treatments, and 23 were assigned to the Maxforce treatment. Fifty B. germanica were assigned to each treatment group. Insects were kept in a walk-in incubator at 27°C, 40% RH, and a photoperiod of 12:12 (L:D) h. Mortality was monitored every 2 h for the first 8 h, and 20–24 h. After 24 h, mortality was monitored three times daily. Individuals that could not right themselves or grasp the egg carton harborage were considered dead. Dead individuals were left in the arenas. Assays were terminated when all individuals in the bait treatment groups were dead.
Two-Choice Assay
Two-choice assays received the same setup as the no-choice assays, except with two vial caps on opposite ends of the arena equidistant from the egg carton harborage and water. The first treatment group received Zyrox bait and crushed rat chow. The second treatment group received Maxforce bait and crushed rat chow. The final treatment (negative control) received two vials of crushed rat chow. The same number of insects was used as in the no-choice assays, with one exception: one B. asahinai rat chow treatment served as the control for both the no-choice and two-choice assays, as they were conducted concurrently.
Ingestion Versus Contact Assay
Assays were conducted to differentiate ingestion of bait from contact using the same setup as the no-choice assay. These assays were conducted only with B. germanica, and males were starved for 18 h before their mouthparts were glued to prevent ingestion. Males were anesthetized briefly using carbon dioxide and placed immediately on ice. Loctite Superglue (Henkel Corporation, Westlake, OH) was applied using the head of an insect pin laterally across the mouthparts and vertically on the ventral side of the mouthparts. Males were placed back on ice, and the glue was allowed to dry. A second layer of glue was applied in the same fashion. Each cockroach was examined, and the mouthparts were tapped to ensure they could not move. Treatment groups included Zyrox bait and a crushed rat chow control. An additional sham control was performed with glue placed between the eyes using the procedure described above to ensure that mortality was not caused by the glue itself. Males were isolated in their treatment groups for six more hours before the start of the assay to allow for recovery. Sample size was 30 males per treatment. The assay was conducted at 25°C, 40% RH, and a photoperiod of 12:12 (L:D) h, and terminated when all individuals in both the control and treatment group were dead.
Field Trial
Experiments were conducted on the North Carolina State University campus with a recently established B. asahinai population with well-defined boundaries in a wooded area beside railroad tracks (Matos and Schal 2015). Density of the population was determined via timed counts along a transect in a wooded area beside railroad tracks on North Carolina State University’s main campus where a B. asahinai population had established. Transects were performed on a nonrainy day on 5 August 2014 between (−78.67169667, 35.78528333) and (−78.66710167, 35.78352667). Samples were taken every 20–30 m along the transect in favorable habitats (i.e., leaf litter and trees present). Unfavorable habitats (i.e., rocks, concrete) were not used. At each sampling site, a visual scan was performed in an ∼8 m2 area for 3–5 min. The location with the highest density of B. asahinai was noted, and a 1.5 m by 4.5 m area (9 m2) was marked off within this area. A timed count of total individuals was performed for 5 min within this area.
The sites with the highest timed counts were chosen and designated as high, medium, and low density. On a nonrainy day, 13 August 2014, one site of each density group was treated with Zyrox bait, and one site was an untreated control. In the treated groups, a 36 m2 area was staked off and 250 g Zyrox Fly Granular Bait was applied evenly at a rate of 6.94 g/m2 using a Scatter Box applicator (PlantMates LLC, Pasadena, TX). Seven days after the original application, the treatment and control sites were monitored for population density, and bait was reapplied as above. The population density was monitored every 7 d for four weeks after the reapplication.
The same procedure described above was performed again in 2015. On a nonrainy day, 31 July 2015, four sites of each density group were treated with Zyrox bait and the other four served as untreated controls. In the treated groups, 72 g Zyrox bait was applied evenly at the label rate of 2 g/m2 to a 36 m2 area. Seven days after the original application, the treatment and control sites were monitored for population density, and bait was reapplied as above. The population density was monitored every 7 d for four weeks after the reapplication.
Data Analysis
For all laboratory assays, Kaplan–Meier survivorship curves (Parmar and Machin 1995) were used to analyze data instead of regression due to the presence of censored responses, and generated using SPSS Version 22 (IBM Corporation 2013). The log rank option was selected in order to identify differences among treatment groups, and pairwise comparisons were conducted across treatments.
Field data were log10-transformed and analyzed using a mixed analysis of variance (ANOVA; General linear model with repeated measures by treatment) in SPSS Version 22. Independent t-tests between treatments were conducted at all time points.
Results
Laboratory Assays of Mortality
In the laboratory, Zyrox bait acted quickly against both B. asahinai and B. germanica with little difference between the two species or between the no-choice and two-choice assays. Overall, all the Zyrox treatments caused high mortality sooner than Maxforce treatments. Little mortality occurred in the control group that received rat chow. In the no-choice assays, there was a difference in survivorship times among treatments and species (χ2 = 218.98, df = 4, P < 0.0001; Fig. 1A) . As expected, significant differences were found in pairwise comparisons of the B. asahinai control and all other treatment and species combinations (P < 0.0001 for all). Pairwise comparisons of the Maxforce treatments and the Zyrox treatments for both species revealed that differences in survivorship time were highly significant (P < 0.0001 for all) and that cockroaches exposed to Maxforce survived longer. Survivorship times for the B. asahinai and B. germanica Zyrox treatments were not significantly different (P = 0.644), but B. asahinai in the Maxforce treatment survived longer than B. germanica in the Maxforce treatment (P = 0.024).
Fig. 1.
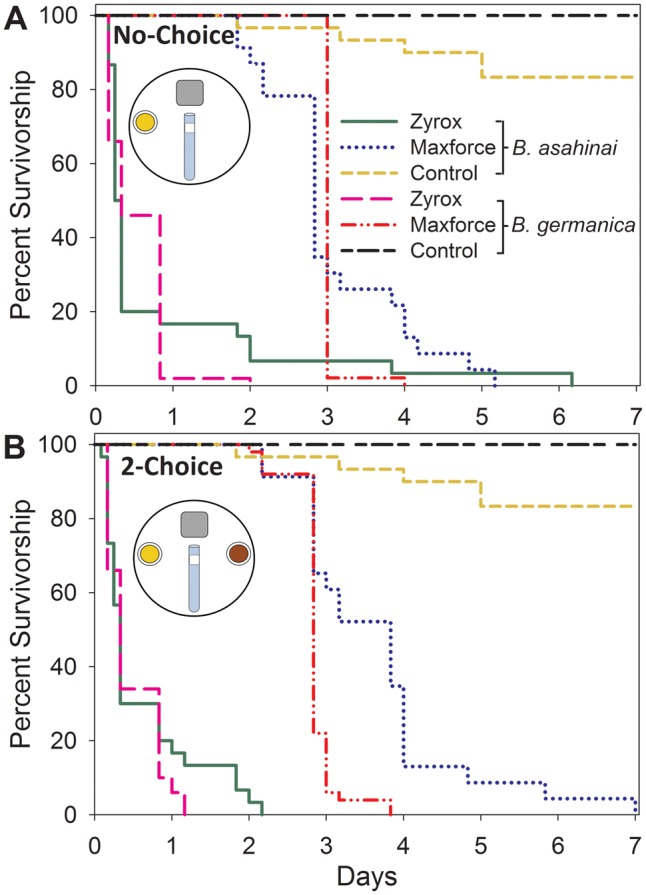
Kaplan–Meier survivorship curves of B. asahinai and B. germanica offered Zyrox Fly Granular Bait, Maxforce Complete Granular Insect Bait, and crushed rat chow in no-choice (A) and two-choice (B) assays. Each curve represents an independent species and treatment combination. Sample size was 50 B. germanica in all assays, 30 B. asahinai in control and Zyrox treatments in all assays, and 23 B. asahinai for Maxforce treatments. (Color online only.)
In the two-choice assays, overall comparisons among treatments and species revealed significant differences in survivorship times (χ2 = 269.35, df = 4, P < 0.0001; Fig. 1B). As in the no-choice assay, pairwise comparisons of the B. asahinai control and all other treatment and species combinations were highly significant (P < 0.0001 for all). Similarly, comparisons of Maxforce and Zyrox treatments for both species revealed that insects in the Maxforce treatments survived longer than those in the Zyrox treatments (P < 0.0001 for all). Survivorship times were not significantly different in B. asahinai and B. germanica Zyrox treatments (P = 0.415), but male B. asahinai survived the Maxforce treatment longer than B. germanica males (P < 0.0001).
Ingestion Versus Contact Assays
Zyrox bait appeared to have minimal contact activity against B. germanica with glued mouthparts within four days. An overall comparison revealed a significant difference in survivorship time when cockroaches ingested bait or were prevented from ingesting bait (χ2 = 123.67, df = 2, P < 0.0001; Fig. 2). No difference was evident between cockroaches with glued mouthparts (contact only) that were exposed to Zyrox and those exposed to rat chow (P = 0.567), and a significant difference was apparent among all other pairs (P < 0.0001).
Fig. 2.
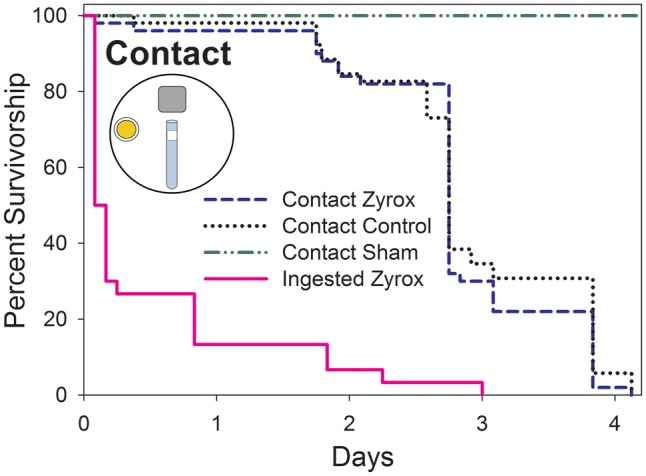
Kaplan–Meier survivorship curves of B. germanica offered Zyrox Fly Granular Bait via ingestion or via contact. Each curve represents an independent treatment. In contact-only assays, insect mouthparts were glued to prevent ingestion. Glue was applied to the head between the eyes as a sham control. Sample size was 30 insects per treatment. (Color online only.)
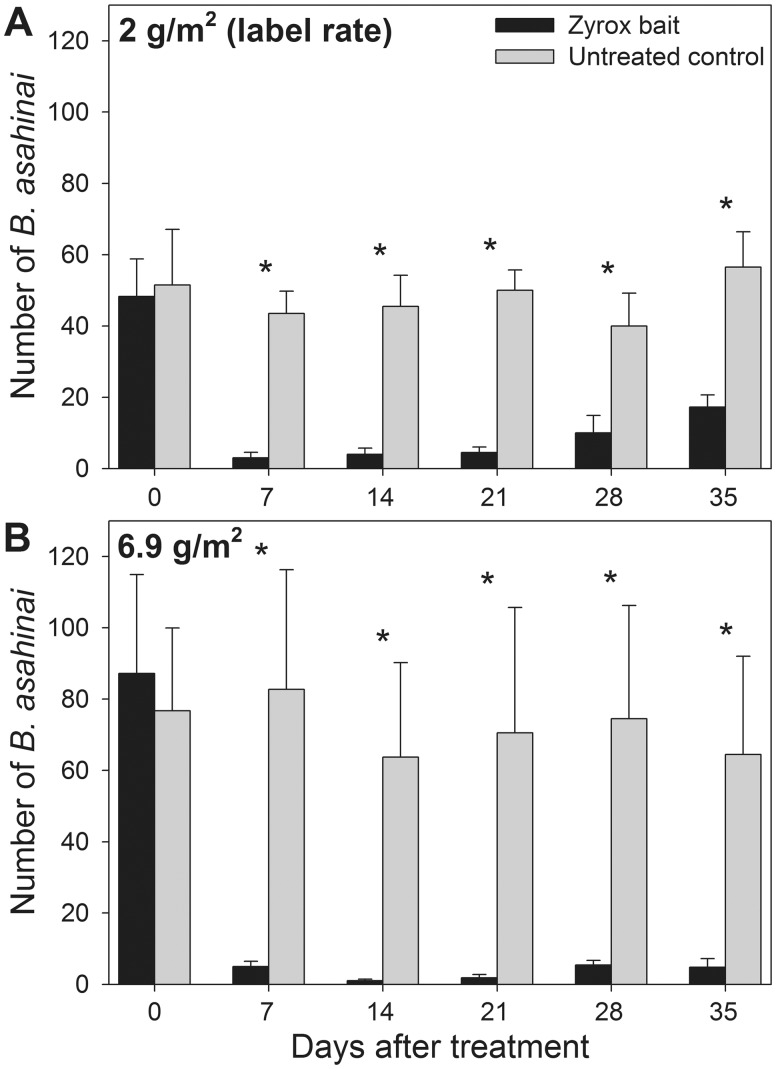
Field Trial
Application of Zyrox bait reduced the B. asahinai population in treated plots by over 90%. When the label rate (2 g/m2) of Zyrox was applied, a mixed ANOVA revealed significant differences between treated and control plots (F = 28.38; df = 1; P = 0.002; Fig. 3A). An independent t-test conducted between treatments on day 0 indicated the pretreatment B. asahinai populations did not differ between Zyrox and control plots (t(6) = −0.030; P = 0.977; Fig. 3A). Independent t-tests conducted weekly on days 7 (t(6) = 5.542; P = 0.001), 14 (t(6) = 5.854; P = 0.001), 21 (t(6) = 6.688; P = 0.001), 28 (t(6) = 2.617; P = 0.040), and 35 (t(6) = 4.479; P = 0.004) indicated significant reductions in the B. asahinai populations between treatments, which remained for at least four weeks after re-application of Zyrox bait (Fig. 3A).
Fig. 3.
Mean (± SE) B. asahinai found in timed counts in field sites before and after treatment with Zyrox Fly Granular Bait or an untreated control. (A) Plots treated with 2 g/m2 Zyrox bait; (B) Plots treated with 6.9 g/m2 Zyrox bait. All plots were treated on day 0 following the initial timed count, and bait was re-applied on day 7. In (A) and (B), asterisks denote significant differences between treatments in mean B. asahinai numbers. Sample size was four plots each for Zyrox and control (A), five plots for Zyrox and four plots for control (B). Data were analyzed with a mixed ANOVA.
In plots treated with 6.9 g/m2 Zyrox bait, a similar pattern emerged. A mixed ANOVA indicated differences between treated and control plots (F = 34.08; df = 1; P = 0.001; Fig. 3B). Independent t-tests conducted on day 0 revealed no significant differences pretreatment (t(7) = 0.008; P = 0.994) and a significant reduction in B. asahinai populations on days 7 (t(7) = 5.924; P = 0.001), 14 (t(7) = 5.921; P = 0.001), 21 (t(7) = 4.483; P = 0.003), 28 (t(7) = 4.663; P = 0.002), and 35 (t(7) = 3.324; P = 0.013) (Fig. 3B).
Discussion
Overall, Zyrox bait and Maxforce bait showed significant toxicity against B. asahinai and B. germanica in the laboratory. Zyrox bait achieved near 100% mortality within two days, and Maxforce bait within five days in both species. In no-choice and two-choice assays, Zyrox bait killed males of both species more quickly than Maxforce bait. This difference was expected due to the modes of action of the active ingredients—as cyantraniliprole (Zyrox) targets the nerve–muscle interface, it is expected to act more quickly than hydramethylnon (Maxforce), which disrupts the mitochondrial electron transport chain. There was little difference in the survivorship curves between the no-choice and two-choice assays between species and among treatments, suggesting that both Zyrox and Maxforce are attractive and palatable baits, even when cockroaches were offered their usual food source of rat chow as an alternative food. As B. asahinai survived longer than B. germanica in Maxforce treatments, and a few individual B. asahinai survived up to four days longer than B. germanica in the Zyrox treatments, it is plausible that low levels of resistance exist in the B. asahinai population. Blattella asahinai might have had more exposure to insecticides in the Alabama and Florida sites where they were collected than our nonresistant B. germanica strain, which has had no insecticide exposure since the advent of modern synthetic insecticides. Indeed, Valles et al. (1999) showed that a B. asahinai population collected in 1986 (just after its detection in Florida) was less tolerant of carbamate and organophosphate insecticides than the same Orlando Normal colony of B. germanica reared in our lab, suggesting that B. asahinai populations might have become more resistant to insecticides. As likely, however, is the possibility that B. asahinai has some intrinsic protection against xenobiotics (e.g., lower penetration, greater metabolism, or excretion) related to its phytophagous habits, or that ingestion of bait was lower than in B. germanica.
Our assays to uncouple ingestion-based from contact-based toxicity of Zyrox bait were conducted with B. germanica and were limited to four days because males with glued mouthparts started dying within 2 d. When deprived of food and water, male B. germanica live about 8 d at 40% RH (Willis and Lewis 1957). In our assays, cockroaches survived only 4 d, which could be due to stress induced by having glued mouthparts. However, as cockroaches with glue placed on the head survived throughout the length of the assay, it is unlikely that mortality was caused by the glue. The mouth is also used for hygienic behavior and grooming (Böröczky et al. 2013), and it is possible that disruption of these behaviors contributed to lower survivorship. We attempted similar experiments with Zyrox bait or crushed rat chow spread across the bottom of a Petri dish in order to maximize contact with the bait. However, despite several attempts, high mortality occurred in both the treatment and control groups, even after smaller particles were sifted and eliminated. Upon examination under a microscope, dead cockroaches displayed a significant accumulation of particles on the cuticle (YKM, personal observation). These observations support the idea that the inability of the cockroach to groom because of its glued mouthparts contributed to unexpected low survival.
Within the four day experiment Zyrox bait did not appear to have contact activity against B. germanica males. Ingestion, on the other hand, was highly effective at delivering the active ingredient to the target site, as almost all males died within two days.
Zyrox bait was very effective against the B. asahinai field population at the NC State University campus, and effects lasted for weeks. We did not include Maxforce bait in these field trials because of the limited availability of field sites. Application of the label rate (2 g/m2) of Zyrox bait maintained an average of 64% population reduction 35 days after the first treatment. Application of approximately three times the label rate (6.9 g/m2) was even more effective, maintaining an average of 92% reduction 35 d after the first treatment. These are likely underestimates of the potential efficacy of Zyrox bait in an area-wide treatment. The overall infested area occupied a small and relatively contiguous wooded area along a railroad track, with no natural or artificial barriers. Our experimental design assigned treatment plots within an untreated landscape. Therefore, there were no barriers to prevent cockroaches from untreated areas from entering the plots after treatment. This likely occurred, given the small size of each treated plot (9 m2) relative to its perimeter (12 m), resulting in rather conservative estimates of efficacy. Likewise, if Zyrox were repellent, cockroaches from the treated plots could readily move to adjacent untreated areas. We contend that this is unlikely because cockroaches readily consumed Zyrox bait in the presence of rat chow in the two-choice assays.
Cyantraniliprole appears to be an effective active ingredient against B. asahinai, B. germanica, and likely other cockroaches. Zyrox bait could be used against B. asahinai if the label is extended to include this species, and Maxforce Complete may be an effective control option pending field trials. Human tolerance of the Asian cockroach is low when it enters residences (Brenner et al. 1988) and there is a need to reduce populations in the peridomestic environment before they enter homes. This need is reinforced because B. asahinai shares human allergens with B. germanica which can trigger allergies and exacerbate asthma (Helm et al. 1990). Granular baits, such as Zyrox and Maxforce, are a preferable control option for the Asian cockroach because they can be effective for longer durations, have low mammalian toxicity and thus pose less risk to humans and companion animals, and use less active ingredient than broadcast perimeter sprays, which are the predominant control strategy for peridomestic cockroach pests (Appel and Smith 2002). In future studies, it would be advantageous to conduct Zyrox and Maxfoce bait field trials against other peridomestic cockroach pest species, such as the smokybrown cockroach. This species often inhabits similar habitats to the Asian cockroach (Appel and Smith 2002), and we have shown Zyrox bait to be an attractive and palatable bait for two other pest cockroach species.
Cyantraniliprole could be formulated against the German cockroach, or the Zyrox bait label could be extended, as we have shown it to be effective against this species in the laboratory. It is permissible to use Zyrox bait indoors, as long as it is within bait stations (Syngenta 2015). Zyrox bait, or a re-formulated cyantraniliprole bait, could be used as an alternate control to rotate with other baits used in B. germanica control, which could aid in insecticide resistance management. A word of caution, however, about the use of Zyrox bait against German cockroach populations: house fly baits are often formulated with large amounts of sugar, and specific sugar aversions have arisen independently in multiple B. germanica populations (Silverman and Bieman 1993, Wada-Katsumata et al. 2013). Additionally, nontarget species such as ants, which are often attracted to sugars produced in extrafloral nectaries (Koptur 1992) may be affected by Zyrox bait.
Acknowledgments
We would like to thank David Stephan for discovering the Blattella asahinai population on the North Carolina State University campus. This research was funded in part by the Blanton J. Whitmire Endowment, the David R. Nimocks, Jr. Fellowship, and a graduate assistantship from the Structural Pest Management Training and Research Facility (all at North Carolina State University), the U.S. Department of Housing and Urban Development Healthy Homes program (NCHHU0017-13), the Alfred P. Sloan Foundation (2013-5-35 MBE), and the National Institute of Environmental Health Sciences under award (P30ES025128) to the Center for Human Health and the Environment.
References Cited
- Appel A. G., Smith L. M. 2002. Biology and management of the smokybrown cockroach. Annu. Rev. Entomol. 47: 33–35. [DOI] [PubMed] [Google Scholar]
- Böröczky K., Wada-Katsumata A., Batchelor D., Zhukovskaya M., Schal C. 2013. Insects groom their antennae to enhance olfactory acuity. Proc. Natl. Acad. Sci. USA. 110: 3615–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R. J., Patterson R. S., Koehler P. G. 1988. Ecology, behavior, and distribution of Blattella asahinai (Orthoptera: Blattellidae) in Central Florida. Ann. Entomol. Soc. Am. 81: 432–436. [Google Scholar]
- Harpaz I. 1987. Improving the effectiveness of insect pathogens for pest control. pp. 451–455. In Maramorosch K. (ed.), Biotechnology in invertebrate pathology and cell culture. Academic Press, Inc., San Diego, CA. [Google Scholar]
- Helm R. M., Squillace D. L., Jones R. T., Brenner R. J. 1990. Shared allergenic activity in Asian (Blattella asahinai), German (Blattella germanica), American (Periplaneta americana), and oriental (Blatta orientalis) cockroach species. Int. Arch. Allergy Immunol. 92: 154–161. [DOI] [PubMed] [Google Scholar]
- IBM. 2013. IBM SPSS Advanced Statistics 22 user’s manual. IBM Corporation, Armonk, NY. [Google Scholar]
- Koehler P. G. 1999. Asian cockroach. Entomology and Nematology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Document ENY-202. Gainsville, FL. [Google Scholar]
- Koptur S. 1992. Extrafloral nectary-mediated interactions between insects and plants. pp. 81–129. In Bernays E. (ed.), Insect-Plant Interactions. CRC Press, Boca Raton, FL. [Google Scholar]
- Matos Y. K., Schal C. 2015. Electroantennogram responses and field trapping of Asian cockroach (Dictyoptera: Blattellidae) with blattellaquinone, sex pheromone of the German cockroach (Dictyoptera: Blattellidae). Environ. Entomol. 44: 1155–1160. [DOI] [PubMed] [Google Scholar]
- Mizukubo T. 1981. A revision of the genus Blattella (Blattaria: Blattellidae) of Japan. I. Terminology of the male genitalia and description of a new species from Okinawa Island. Esakia 17: 149–159. [Google Scholar]
- Murillo A. C., Gerry A. C., Gallagher N. T., Peterson N. G., Mullens B. A. 2014. Laboratory and field assessment of cyantraniliprole relative to existing fly baits. Pest Manag. Sci. 71: 752–758. (DOI: 10.1002/ps.3847) [DOI] [PubMed] [Google Scholar]
- Parmar M.K.B., Machin D. 1995. Survival analysis: A practical approach, John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- Persad A. B., Hoy M. A. 2004. Predation by Solenopsis invicta and Blattella asahinai on Toxoptera citricida parasitized by Lysiphlebus testaceipes and Lipolexis oregmae on citrus in Florida. Biol. Control 30: 531–537. [Google Scholar]
- Pfannenstiel R. S., Booth W., Vargo E. L., Schal C. 2008. Blattella asahinai (Dictyoptera: Blattellidae): A new predator of lepidopteran eggs in South Texas soybean. Ann. Entomol. Soc. Am. 101: 763–768. [Google Scholar]
- Price J. F., Nagle C. A. 2008. Asian Cockroaches are eating strawberry fruit. Berry/Vegetable Times, Institute of Food and Agricultural Sciences Extension, University of Florida, Gainsville, FL. 4 December. [Google Scholar]
- Reierson D. A. 1995. Baits for German cockroach control, pp. 231–265. In Rust M. K., Owens J. M., Reierson D. A. (eds.), Understanding and Controlling the German Cockroach. Oxford University Press, New York, NY. [Google Scholar]
- Roth L. M. 1986. Blattella asahinai introduced into Florida (Blattaria: Blattellidae). Psyche 93: 371–374. [Google Scholar]
- Selby T. P., Lahm G. P., Stevenson T. M., Hughes K. A., Cordova D., Annan I. B., Barry J. D., Benner E. A., Currie M. J., Pahutski T. F. 2013. Discovery of cyantraniliprole, a potent and selective anthranilic diamide ryanodine receptor activator with cross-spectrum insecticidal activity. Bioorg. Med. Chem. Lett. 23: 6341–6345. [DOI] [PubMed] [Google Scholar]
- Silverman J., Bieman D. N. 1993. Glucose aversion in the German cockroach, Blattella germanica. J. Insect Physiol. 39: 925–933. [Google Scholar]
- Sitthicharoenchai D. 2002. Ecology and behavior of the Asian cockroach, Blattella asahinai Mizukubo (Blattodea: Blattellidae), in Charleston County, South Carolina. Ph.D. dissertation, Clemson University, Clemson, SC. [Google Scholar]
- Snoddy E. T. 2012. Evaluations of integrated pest management control techniques of the Asian cockroach (Blattella asahinai Mizukubo) in the urban environment. Ph.D. dissertation, Auburn University, Auburn, AL. [Google Scholar]
- Snoddy E. T., Appel A. G. 2008. Distribution of Blattella asahinai (Dietyoptera: Blattellidae) in Southern Alabama and Georgia. Ann. Entomol. Soc. Am. 101: 397–401. [Google Scholar]
- Snoddy E. T., Appel A. G. 2013. Mulch preferences of the Asian cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 106: 322–328. [DOI] [PubMed] [Google Scholar]
- Snoddy E. T., Appel A. G. 2014. Field and laboratory efficacy of three insecticides for population management of the Asian cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 107: 326–332. [DOI] [PubMed] [Google Scholar]
- Syngenta Crop Protection, LLC. 2015. Zyrox Fly Granular Bait. Greensboro, NC. SCP 1541A-L1A 0215. [Google Scholar]
- Valles S. M., Koehler P. G., brenner R. J. 1999. Comparative insecticide susceptibility and detoxification enzyme activities among pestiferous Blattodea. Comp. Biochem. Physiol. C. 124: 227–232. [DOI] [PubMed] [Google Scholar]
- Wada-Katsumata A., Silverman J., Schal C. 2013. Changes in taste neurons support the emergence of an adaptive behavior in cockroaches. Science 340: 972–975. [DOI] [PubMed] [Google Scholar]
- Willis E. R., Lewis N. 1957. The longevity of starved cockroaches. J. Econ. Entomol. 50: 438–440. [Google Scholar]