Abstract
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are available online to subscribers. Monographs can be customized to meet the needs of a facility. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, contact Wolters Kluwer customer service at 866-397-3433. The July 2016 monograph topics are pimavanserin, venetoclax, defibrotide, lifitegrast ophthalmic solution 5%, and atezolizumab. The Safety MUE is on pimavanserin.
INDICATIONS
Sugammadex is approved by the US Food and Drug Administration (FDA) for the reversal of neuromuscular blockade induced by rocuronium bromide or vecuronium in adults undergoing surgery.1 It has not been studied for reversal following rocuronium or vecuronium administration in the intensive care unit.1 Sugammadex should not be used to reverse blockade induced by nonsteroidal neuromuscular blocking agents such as succinylcholine or benzylisoquinolinium compounds or to reverse neuromuscular blockade induced by steroidal neuromuscular blocking agents other than rocuronium or vecuronium.1
Other agents currently used for reversal of neuromuscular blockade in surgical patients include edrophonium, pyridostigmine, and neostigmine, although only edrophonium and pyridostigmine are FDA approved for this use.2
CLINICAL PHARMACOLOGY
Sugammadex reverses the effects of nondepolarizing neuromuscular-blocking drugs by sequestering the free molecules of the muscle relaxant. The molecular weight of sugammadex is 2,178 daltons. Although it was specifically designed to sequester free molecules of rocuronium, it is also able to sequester those of vecuronium.3 The resulting reduction in concentration of free muscle relaxant leads to a rapid offset of neuromuscular block.3
Sugammadex, a highly water-soluble synthetic gamma-cyclodextrin derivative, structurally consists of a ring with 8 negative charges.3–5 Rocuronium and vecuronium fit into the cavity of the ring, forming a 1:1 complex.3 Vecuronium has a 3-fold lower affinity for sugammadex than does rocuronium.4 Higher doses of sugammadex are likely to be necessary to reverse pancuronium.6 Muscle relaxants from the benzylisoquinoline group (eg, cisatracurium, mivacurium) fit poorly or not at all.3
Sugammadex has no effect on acetylcholinesterase, nicotinic receptors, or muscarinic receptors.5
Following a rocuronium 0.6 mg/kg dose, sugammadex 8 mg/kg reduced the train-of-four (TOF) ratio to 0.9 in healthy volunteers within 2 minutes of administration.7
PHARMACOKINETICS
Dose-linear pharmacokinetics have been observed over a dose range of 0.1 to 16 mg/kg.1,7 Sugammadex volume of distribution is approximately 11 to 14 L in adult patients with normal renal function.1 Sugammadex and the sugammadex-rocuronium complex do not bind to plasma proteins.1 The mean plasma clearance of sugammadex is 0.084 to 0.12 L/min.1,7,8 The elimination half-life is approximately 2 hours; however, in nonclinical drug distribution studies, sugammadex was retained in sites of active mineralization such as bone and teeth, with a mean half-life of 172 and 8 days, respectively.1,7 More than 90% of the dose is excreted within 24 hours, with 96% excreted in the urine.1,9 Renal excretion (as unchanged drug) is the only observed route of elimination. No metabolism has been observed.1,9
Following sugammadex administration, rocuronium clearance was reduced and the area under the curve (AUC) was increased; however, the volume of distribution was also reduced, and the percentage excreted in the urine was increased.7 The mean plasma clearance of rocuronium has ranged from 0.135 to 0.234 L/min following sugammadex administration, compared with 0.327 L/min when administered alone.7,8 The excretion of unchanged rocuronium in the urine is increased following sugammadex administration. Median urine excretion over 24 hours increased from 14% to 26% in placebo recipients and from 44% to 74% in patients receiving sugammadex 4 to 8 mg/kg.7,8,10
Rocuronium and vecuronium plasma concentrations decline more rapidly than do those of sugammadex.4 The half-life of sugammadex in patients with mild, moderate, and severe renal impairment is 4, 6, and 19 hours, respectively.1 In patients with severe renal function impairment, exposure to sugammadex was prolonged and was 17-fold higher. Low levels were detectable for at least 48 hours, and up to 7 days, postdose in patients with severe renal impairment.1,11 Hemodialysis produced reductions of 69% and 75% in the plasma concentrations of sugammadex and rocuronium, respectively, after the first dialysis session in patients with severe renal impairment and reductions of about 50% during sequential dialysis sessions.1,12
COMPARATIVE EFFICACY
Indications: Reversal of Neuromuscular Blockade Induced by Rocuronium or Vecuronium
Studies
Drug: Sugammadex vs Neostigmine
Reference: Abad-Gurumeta A, et al, 201513
Study Design: Systematic review and meta-analysis of randomized controlled trials
Study Funding: Not provided
Patients: 1,553 patients in 17 randomized controlled trials comparing sugammadex with neostigmine for the reversal of neuromuscular blockade generated by rocuronium or vecuronium in adults.
Intervention: Patients received sugammadex 0.0625 to 4 mg/kg (most studies assessed doses of 2 mg/kg or 4 mg/kg) or neostigmine 40 to 70 mg/kg (most studies assessed doses of 50 mg/kg) plus atropine or glycopyrrolate. Reversal was initiated either at the reappearance of the second twitch (T2) after the last dose of rocuronium or vecuronium (moderate blockade) or at 1 to 2 post-tetanic counts (PTCs) after the last dose of rocuronium or vecuronium (deep blockade).
Results
Primary Endpoint(s)
All signs of residual postoperative paralysis were reduced to a greater extent with sugammadex (relative risk [RR], 0.46; 95% confidence interval (CI), 0.29 to 0.71; p < .001).
Secondary Endpoint(s)
The risk of minor signs of postoperative residual paralysis was reduced to a greater extent with sugammadex (RR, 0.51; 95% CI, 0.32 to 0.8; p = .003).
There was no difference in rate of critical respiratory events requiring tracheal reintubation (RR, 0.13; 95% CI, 0.02 to 1.06; p = .06).
Drug-related adverse effects occurred less frequently with sugammadex (RR, 0.72; 95% CI, 0.54 to 0.95; p = .02).
There were no differences in rates of postoperative nausea (p = .53) or postoperative vomiting (p = .36).
Comments: Overall, fewer patients who received sugammadex had clinical signs of residual paralysis compared with those who received neostigmine, although there was no difference in the risk of severe residual paralysis. The authors reported that for sugammadex, the number needed to treat to avoid 1 patient showing signs of residual paralysis was between 21 and 22.
Reference: Bridion Prescribing Information, 20161
Study Design: Randomized, open-label (safety-assessor blinded), multicenter study
Study Funding: Not provided
Patients: 189 patients with neuromuscular blockade (87 women and 102 men) who were randomly assigned to rocuronium or vecuronium groups and underwent elective surgical procedures under general anesthesia that required endotracheal intubation and maintenance of neuromuscular blockade. Patients were mostly White (99%), with a median age of 50 years.
Intervention: Sugammadex 2 mg/kg or neostigmine 50 mcg/kg was administered as a single bolus injection at the reappearance of T2 after the last dose of rocuronium or vecuronium (moderate blockade).
Results
Primary Endpoint(s)
Time from start of sugammadex or neostigmine administration to recovery of the TOF (T4:T1) ratio to 0.9, which correlates with recovery from neuromuscular blockade, was faster with sugammadex than with neostigmine following either rocuronium- or vecuronium-induced blockade. The median recovery time was 1.4 minutes with sugammadex and 21.5 minutes with neostigmine in patients who had received rocuronium. The median recovery time was 2.1 minutes with sugammadex and 29 minutes with neostigmine in patients who had received vecuronium.
Comments: Similar results were observed in another study comparing sugammadex 2 mg/kg with neostigmine 50 mcg/kg plus glycopyrrolate 10 mcg/kg for reversal of rocuronium and vecuronium neuromuscular blockade when initiated at the reappearance of T2. The median recovery time in the rocuronium study arms was 1.5 minutes (95% CI, 1.3 to 1.6) with sugammadex and 18.6 minutes (95% CI, 14.2 to 24.4) with neostigmine (p < .001).14 The mean recovery time in the vecuronium study arms was 2.7 minutes (95% CI, 2.2 to 3.3) with sugammadex and 17.9 minutes (95% CI, 13.1 to 24.3) with neostigmine plus glycopyrrolate (p < .001).15 Sugammadex was also assessed in a randomized, placebo-controlled, dose-finding study enrolling 80 patients undergoing elective surgery and requiring muscle relaxation for intubation. Patients were randomly assigned to receive rocuronium 0.6 mg/kg or vecuronium 0.1 mg/kg. Patients received sugammadex 0.5, 1, 2, 3, 4, or 8 mg/kg or placebo at reappearance of T2 on TOF stimulation. In the rocuronium group, the mean time to recovery of TOF ratio to 0.9 was 31.8 minutes with placebo, compared with 3.7 minutes in the sugammadex 0.5 mg/kg group, 2.3 minutes in the 1 mg/kg group, 1.7 minutes in the 2 mg/kg group, 1.9 minutes in the 3 mg/kg group, and 1.1 minutes in the 4 mg/kg group. In the vecuronium group, the mean time to recovery of TOF ratio to 0.9 was 48.8 minutes with placebo, compared with 7.7 minutes in the sugammadex 0.5 mg/kg group, 2.5 minutes in the 1 mg/kg group, 2.3 minutes in the 2 mg/kg group, 1.5 minutes in the 4 mg/kg group, and 1.4 minutes in the 8 mg/kg group.16 Sugammadex was also evaluated in a similar randomized, placebo-controlled study enrolling 27 male surgical patients. All patients received rocuronium 0.6 mg/kg. Sugammadex 0.5, 1, 2, 3, or 4 mg/kg or placebo was administered at the reappearance of T2 in response to TOF stimulation. The median recovery time to TOF ratio of 0.9 was 21 minutes in the placebo group, compared with 4.3 minutes in the sugammadex 0.5 mg/kg group, 3.3 minutes in the 1 mg/kg group, 1.3 minutes in the 2 mg/kg group, 1.2 minutes in the 3 mg/kg group, and 1.1 minutes in the 4 mg/kg group.10 Sugammadex was also evaluated in a randomized, assessor-blinded, dose-finding study enrolling 30 surgical patients receiving rocuronium to maintain a deep block for at least 2 hours. Patients received a sugammadex dose of 0.5, 1, 2, 4, or 6 mg/kg. Mean time to TOF ratio of 0.9 was 6.49 minutes with the 0.5 mg/kg dose, 2.43 minutes with the 1 mg/kg dose, 1.46 minutes with the 2 mg/kg dose, 1.22 minutes with the 4 mg/kg dose, and 2.37 minutes with the 6 mg/kg dose.17 In another study, sugammadex demonstrated equivalent activity for reversal of rocuronium-induced neuromuscular block in 42 patients under maintenance anesthesia with propofol or sevoflurane. All patients received propofol for induction, followed by randomization to propofol or sevoflurane for maintenance. Rocuronium 0.6 mg/kg was administered to facilitate tracheal intubation. Sugammadex 2 mg/kg was administered at reappearance of T2 on TOF stimulation. Mean time to recovery of TOF ratio to 0.9 with sugammadex was 1.8 minutes in both the sevoflurane and propofol groups.18 In a study enrolling 70 morbidly obese patients undergoing surgery and receiving rocuronium, faster reversal was observed with sugammadex 2 mg/kg than with neostigmine 50 mcg/kg; the mean time to recovery was 2.7 minutes with sugammadex and 9.6 minutes with neostigmine (p < .05).19 Another study of 84 patients compared sugammadex 2 mg/kg for reversal of blockade induced by rocuronium 0.6 mg/kg with neostigmine 50 mcg/kg for reversal of blockade induced by cisatracurium 0.15 mg/kg. Reversal was initiated at reappearance of T2. The time to recovery of the T4:T1 ratio to 0.9 was 1.9 minutes with sugammadex, compared to 9 minutes with neostigmine (p < .001).20 In a dose-finding study assessing sugammadex after pipecuronium-induced neuromuscular blockade, mean time to recovery from moderate blockade to TOF ratio of 0.9 was 1.7 minutes with sugammadex 2 mg/kg.21
Limitations: The results of this study were only reported in the Bridion prescribing information and the FDA briefing document from the 2008 submission for FDA approval.
Reference: Bridion Prescribing Information, 20161
Study Design: Randomized, open-label, safety-assessor blinded, multicenter study
Study Funding: Not provided
Patients: 157 patients (86 women and 71 men) randomly assigned to rocuronium or vecuronium groups and who underwent elective surgical procedures under general anesthesia that required endotracheal intubation and maintenance of neuromuscular blockade. Median age was 55 years, and 79% of patients were White.
Intervention: Sugammadex 4 mg/kg or neostigmine 70 mcg/kg was administered as a single bolus injection at 1 to 2 PTCs after the last dose of rocuronium or vecuronium (deep blockade).
Results
Primary Endpoint(s)
Time from start of administration of sugammadex or neostigmine to recovery of the TOF (T4:T1) ratio to 0.9, which correlates with recovery from neuromuscular blockade, was 2.7 minutes with sugammadex after rocuronium and 3.3 minutes with sugammadex after vecuronium. Neostigmine was not expected to reverse neuromuscular blockade at a depth of 1 to 2 PTCs; results for neostigmine were not reported.
Comments: Similar results were observed in another study comparing sugammadex 4 mg/kg with neostigmine 70 mcg/kg plus glycopyrrolate administered at reappearance of 1 to 2 PTCs to reverse rocuroniumor vecuronium-induced neuromuscular blockade. In the rocuronium-treated patients, the mean time to recovery of the TOF ratio to 0.9 was 2.9 minutes with sugammadex compared with 50.4 minutes with neostigmine (p < .001).22 In the vecuronium arm, the time to recovery of the T4:T1 ratio to 0.9 was 4.5 minutes in the sugammadex group compared with 66.2 minutes in the neostigmine group (p < .001).23 Sugammadex was also evaluated in a randomized, assessor-blinded, dose-finding study enrolling 50 patients undergoing elective surgery requiring tracheal intubation. Patients received 1 of 2 doses of rocuronium (0.6 or 1.2 mg/kg) and 1 of 5 doses of sugammadex (0.5, 1, 2, 4, or 8 mg/kg) at PTC of 1 to 2. Reversal of neuromuscular blockade was consistently observed in the groups receiving sugammadex 4 or 8 mg/kg; at the 2 mg/kg dose, all patients experienced reversal, but there was substantial patient variability in the time to recovery (1.8 to 15.2 minutes). Patient variability decreased and speed of recovery increased in a dose-dependent manner. At the 8 mg/kg dose, mean time to recovery of TOF ratio to 0.9 was 1.2 minutes (range, 0.8 to 2.1 minutes).24
Limitations: The results of this study were only reported in the Bridion prescribing information and the FDA briefing document from the 2008 submission for FDA approval.
Drug: Sugammadex vs Neostigmine/Glycopyrrolate
Reference: Brueckmann B, et al, 201525
Study Design: Randomized, open-label, single-center study
Study Funding: Merck and Co., Inc.
Patients: 154 adult patients undergoing abdominal surgery with rocuronium-induced neuromuscular blockade. Mean age was 57 years, and 60% of patients were male.
Intervention: Patients received sugammadex 2 or 4 mg/kg (n = 74) or neostigmine/glycopyrrolate (n = 77) at a dose consistent with the center's usual care practice. Timing of administration was at the discretion of the provider, either at moderate blockade (defined as TOF count 1 to 3 in response to TOF stimulation) or deep blockade (defined as no response to TOF stimulation but a response to PTC of at least 1). Median levels of blockade before reversal did not differ between the 2 groups; median TOF count was 2.5 with sugammadex and 3 with neostigmine (p = .312). Sugammadex was given at a dose of 2 mg/kg if spontaneous recovery had reached moderate blockade, or 4 mg/kg if recovery had reached deep neuromuscular blockade. Neostigmine dose did not exceed 5 mg, and the median dose was 51.6 mcg/kg (range, 17.1 to 84.8 mcg/kg).
Results
Primary Endpoint(s)
Presence of residual neuromuscular blockade (defined as TOF ratio less than 0.9) at postanesthesia care unit arrival was evident in 33 patients (43%) in the neostigmine/glycopyrrolate group compared with no patients (0%) in the sugammadex group (p < .001). The average level of neuromuscular blockade recovery was greater in the sugammadex group (mean TOF ratio of 1.07 vs 0.9; p < .001).
Secondary Endpoint(s)
Mean time from the start of study medication administration to the time the patient was ready for discharge from the operating room was 14.7 minutes (95% CI, 13.1 to 16.4) for sugammadex compared with 18.6 minutes (95% CI, 16.6 to 20.8) for neostigmine/glycopyrrolate (p = .02).
Endpoint(s)
Mean time from study medication administration to extubation was 11 minutes with sugammadex compared with 15.2 minutes with neostigmine/glycopyrrolate (p = .003).
Mean time from study medication administration to actual operating room discharge was 19.9 minutes with sugammadex compared with 24.1 minutes with neostigmine/glycopyrrolate (p = .005).
Mean times from first incision to extubation or actual operating discharge did not differ between groups, nor did times from last stitch to extubation or actual operating room discharge or times from postanesthesia care unit admission to postanesthesia care unit discharge readiness or actual postanesthesia care unit discharge.
Comments: In a similar study enrolling 140 patients undergoing laparoscopic surgery, sugammadex administered at deep blockade was compared with neostigmine plus atropine administered at moderate blockade. Recovery of TOF to 0.9 occurred in a mean of 2.4 minutes (95% CI, 2.1 to 2.7) with sugammadex compared with 8.4 minutes (95% CI, 2.7 to 9.8 minutes) with neostigmine plus atropine.26
Limitations: This study was not powered to identify differences in postoperative respiratory complications. The neostigmine/glycopyrrolate combination used in this study is not available in the United States.
Drug: Sugammadex vs Edrophonium and Neostigmine
Reference: Sacan O, et al, 200727
Study Design: Open-label, single-center study
Study Funding: Organon USA, Inc.
Patients: 60 patients undergoing elective surgery with a standardized desflurane-remifentanil-rocuronium anesthetic regimen
Intervention: Patients received sugammadex 4 mg/kg (n = 20), edrophonium 1 mg/kg plus atropine 10 mcg/kg (n = 20), or neostigmine 70 mcg/kg plus glycopyrrolate 15 mcg/kg (n = 20) at 15 minutes or longer after the last dose of rocuronium.
Results
Primary Endpoint(s)
T1 heights were similar at the time of reversal, but the time to achieve TOF ratios of 0.7 and 0.9 were much shorter with sugammadex (71 and 107 seconds, respectively; p < .05) than with edrophonium plus atropine (202 and 331 seconds, respectively) or neostigmine plus glycopyrrolate (625 and 1,044 seconds, respectively).
Secondary Endpoint(s)
All patients in the sugammadex group achieved a TOF ratio of 0.9 within 5 minutes after reversal administration, compared with no patients in the edrophonium plus atropine group and 5% of patients in the neostigmine plus glycopyrrolate group (p < .05). A TOF ratio of 0.7 within 30 minutes was achieved only in 7 patients in the edrophonium plus atropine group and in 9 patients in the neostigmine plus glycopyrrolate group, and a TOF ratio of 0.9 within 30 minutes was achieved only in 2 and 5 patients, respectively.
Endpoint(s)
Heart rate was increased in the neostigmine group. Dry mouth was reduced in the sugammadex group (5% vs 85% with neostigmine plus glycopyrrolate and 95% with edrophonium plus atropine).
Limitations: This was not a randomized study with concealed allocation; patients who preferred not to receive sugammadex were randomly assigned to one of the other 2 study groups.
Drug: Rocuronium plus Sugammadex vs Succinylcholine
Reference: Lee C, et al, 2009; Bridion Prescribing Information, 20151,28
Study Design: Randomized, safety-assessor blinded, multicenter study
Study Funding: Schering-Plough
Patients: 110 adult patients (64 women and 46 men) who underwent elective surgical procedures under general anesthesia that required endotracheal intubation and a short duration of neuromuscular relaxation. Median age was 43 years, 78% of patients were White, and body mass index (BMI) was less than 30 kg/m2.
Intervention: Rocuronium 1.2 mg/kg followed in 3 minutes by sugammadex 16 mg/kg (n = 55) was compared with spontaneous recovery from succinylcholine 1 mg/kg (n = 55).
Results
Primary Endpoint(s)
Mean time from neuromuscular blocker administration to recovery of T1 to 10% was 4.4 minutes with rocuronium plus sugammadex compared with 7.1 minutes with succinylcholine (p < .001), for a treatment difference of −2.7 minutes (95% CI, −3.1 to −2.2).
Secondary Endpoint(s)
Mean time to recovery of T1 to 90% was 6.2 minutes with rocuronium plus sugammadex compared with 10.9 minutes with succinylcholine (p < .001), for a treatment difference of −4.6 minutes (95% CI, −5.5 to −3.8).
Clinical signs of anesthetic and neuromuscular recovery before transfer to and discharge from the recovery room were comparable between groups.
Endpoint(s)
Time from start of sugammadex administration to recovery of T4:T1 ratio to 0.9 was 2.2 minutes.
Comments: In another study comparing rocuronium followed by reversal with sugammadex 16 mg/kg with recovery after succinylcholine, the time from placement of the tracheal tube to spontaneous ventilation was 216 seconds with rocuronium plus sugammadex compared with 406 seconds with succinylcholine (p = .002). The median time from intubation to TOF recovery to 0.9 was 168 seconds with rocuronium plus sugammadex compared with 518 seconds with succinylcholine (p < .001).29 The sugammadex 16 mg/kg dose was selected from the results of phase 2 studies comparing sugammadex with placebo administered shortly after rocuronium. In a randomized, assessor-blinded, placebo-controlled, dose-finding study enrolling 45 patients undergoing elective surgery requiring intubation, sugammadex 2, 4, 8, 12, or 16 mg/kg or placebo was administered 5 minutes after the administration of rocuronium 1.2 mg/kg. A dose-dependent reduction in mean recovery time was observed, declining from 122 minutes (spontaneous recovery) to less than 2 minutes (12 and 16 mg/kg groups). The mean recovery time to TOF ratio of 0.9 was 122 minutes with placebo, compared with 56.5 minutes with sugammadex 2 mg/kg, 15.8 minutes with 4 mg/kg, 2.8 minutes with 8 mg/kg, 1.4 minutes with 12 mg/kg, and 1.9 minutes with 16 mg/kg.30 Similarly, a phase 2 study compared sugammadex doses of 2, 4, 8, 12, or 16 mg/kg with placebo administered 3 or 15 minutes after rocuronium. When given at 3 minutes after rocuronium, mean recovery time to TOF of 0.9 was 108.4 minutes with placebo, and 44.7 minutes with sugammadex 2 mg/kg, 6.9 minutes with 4 mg/kg, 2.4 minutes with 8 mg/kg, 2.4 minutes with 12 mg/kg, and 1.8 minutes with 16 mg/kg.31 Sugammadex was also evaluated in a randomized, assessor-blinded, placebo-controlled, dose-finding study enrolling 98 male patients undergoing elective surgery requiring muscle relaxation only to facilitate tracheal intubation. Patients received sugammadex 1, 2, 4, 6, or 8 mg/kg or placebo at 3, 5, or 15 minutes after a 0.6 mg/kg dose of rocuronium. All patients were anesthetized with propofol and fentanyl. The mean time to recovery of the TOF ratio to 0.9 after dosing at 3, 5, and 15 minutes declined from 52.1, 51.7, and 35.6 minutes, respectively, after administration of placebo, and to 1.8, 1.5, and 1.4 minutes, respectively, after administration of sugammadex 8 mg/kg. A dose-dependent reduction in time to recovery of the TOF ratio was observed. Following sugammadex administration, 20.4% of patients showed signs of inadequate anesthesia.8
CONTRAINDICATIONS, WARNINGS, AND PRECAUTIONS
Contraindications
Sugammadex is contraindicated in patients with known hypersensitivity to sugammadex or any of the product ingredients.1
Warnings and Precautions
Hypersensitivity reactions to sugammadex, varying from isolated skin reactions to serious systemic reactions (including anaphylactic reactions), have occurred, including in patients with no prior exposure to sugammadex. In a study evaluating the frequency of hypersensitivity to sugammadex, anaphylaxis occurred in 0.3% of patients (n = 1) after receiving a single dose of sugammadex 16 mg/kg. The most commonly reported hypersensitivity reactions were nausea, pruritus, and urticaria; all showed a dose-response relationship, occurring more frequently after a sugammadex 16 mg/kg dose compared with 4 mg/kg or placebo. Anaphylaxis has also been reported in the postmarketing setting, including at doses lower than 16 mg/kg.1
Marked bradycardia, including cases resulting in cardiac arrest, has been observed within minutes of sugammadex administration. Patients should be closely monitored for hemodynamic changes during and after reversal. Treatment with anticholinergics such as atropine is advised for clinically significant bradycardia.1
Ventilatory support is required for all patients receiving sugammadex until adequate spontaneous respiration is restored and the ability to maintain a patent airway is ensured. Measures to ensure adequate ventilation should be taken if neuromuscular blockade persists after sugammadex administration or recurs following extubation.1
A delayed or minimal response to sugammadex occurred in a small number of patients in clinical trials.1
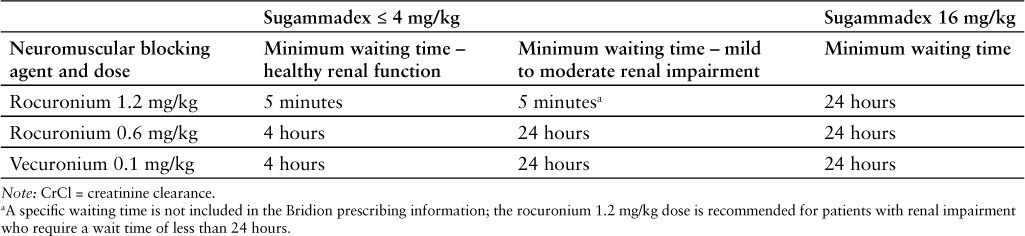
Following administration of sugammadex, a minimum waiting time is necessary before administration of a steroidal neuromuscular blocking agent, as the effectiveness of readministration of rocuronium or vecuronium following administration of sugammadex is expected to be reduced. Table 1 lists the recommended waiting times. When rocuronium 1.2 mg/kg is administrated within 30 minutes after reversal with sugammadex, the onset of neuromuscular blockade may be delayed up to 4 minutes and the duration of neuromuscular blockade may be reduced to 15 minutes. If neuromuscular blockade is required before the wait time has elapsed, a nonsteroidal neuromuscular blocking agent should be used. The onset of a depolarizing neuromuscular blocking agent may be slower than expected because postjunctional nicotinic receptors may still be occupied by the neuromuscular blocking agent.1
Table 1.
Recommended waiting times for readministration of rocuronium or vecuronium after reversal with sugammadex in patients with healthy renal function (CrCl ≥ 80 mL/min)1
Recurrence of neuromuscular blockade may occur as a result of displacement of rocuronium or vecuronium from sugammadex by other drugs. The risk of displacement reactions is greatest during the time period equivalent to 3 times the sugammadex half-life.1 The risk of neuromuscular blockade recurrence may also be increased with the use of lower than recommended doses of sugammadex or with the concomitant use of drugs that potentiate neuromuscular blockade.1
Sugammadex doses up to 16 mg/kg were associated with increases in activated partial thromboplastin time (aPTT) and prothrombin time/international normalized ratio (INR) of up to 25% for up to 1 hour in healthy volunteers.1 Lesser increases were observed in patients undergoing major orthopedic surgery who were concomitantly treated with heparin or low-molecular-weight heparin (LMWH) and sugammadex 4 mg/kg in clinical trials, with no increase in bleeding events, postoperative anemia, transfusion, or mean 24-hour drainage volume compared with usual care.32 Coagulation parameters should be carefully monitored in patients with known coagulopathies and in patients being treated with therapeutic anticoagulation, thromboprophylaxis drugs other than heparin or LMWH, or thromboprophylaxis drugs and a 16 mg/kg dose of sugammadex.1,32
Sugammadex use is not recommended in patients with severe renal function impairment (CrCl less than 30 mL/min), including those requiring hemodialysis.1 Although sugammadex has been observed to reverse neuromuscular blockade with rocuronium in patients with severe renal impairment, reversal was slowed and the rocuronium-sugammadex complex was detectable in plasma for 7 days. Safety data are insufficient to recommend the use of sugammadex in this population.11,12
There are no data regarding the use of sugammadex in pregnant women. In animal reproductive studies, no evidence of teratogenicity was found; however, there was an increase in incidences of incomplete ossification and reduced fetal body weight at a dose associated with maternal toxicity.1
There are no data regarding sugammadex excretion in human milk or its effects on the breast-feeding infant or on milk production. Sugammadex is excreted in rat milk. The benefits of breast-feeding should be considered, along with the importance of treatment to the mother and the potential for adverse effects in the breast-feeding infant.1
Sugammadex has a high affinity for bone and is believed to bind to hydroxyapatite in skeletal bone and teeth. In animal studies, deposition was greater in juvenile animals, with a prolonged retention in the long bones. Animal studies also revealed interference with enamelization of teeth from repeated administration of sugammadex and possibly from a single dose at a developmental stage when tooth enamel is forming.2
The safety and efficacy of sugammadex have not been established in pediatric patients.1 Juvenile animal studies have demonstrated effects on bone and tooth enamel.1 Studies assessing sugammadex for pediatric use are ongoing.33
ADVERSE REACTIONS
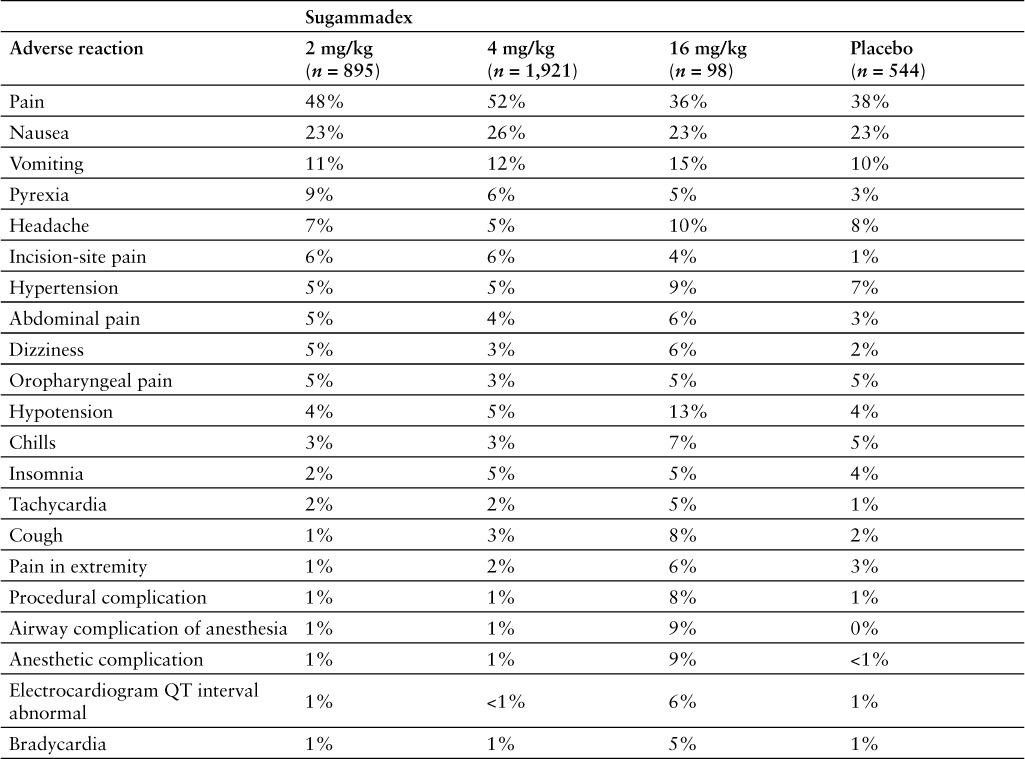
The most common adverse reactions reported with sugammadex are vomiting, pain, nausea, hypotension, and headache.1 Adverse reactions possibly related to sugammadex include allergic reactions, reduced anesthesia levels, headache, tiredness, cold feeling at the application site, dry mouth, oral discomfort, nausea, diarrhea, taste perversion, parosmia, paresthesia, increased AST and gamma-glutamyltransferase levels, and moderate injection-site irritation.4,7,8,10,30 Pooled adverse reaction rates from phase 1 to 3 studies comparing sugammadex and placebo are summarized in Table 2.1 In meta-analyses, no difference in drug-related adverse effects was observed between sugammadex and placebo.34 In a systematic review, sugammadex reduced drug-related adverse effects compared with neostigmine, but this difference was not significant.13
Table 2.
Adverse reactions from pooled phase 1 to 3 studies (incidence ≥ 5% and more common with sugammadex than with placebo)1
DRUG INTERACTIONS
Certain drugs, including hormonal contraceptives, could become less effective when coadministered with sugammadex due to a lowering of (free) plasma concentrations. Sugammadex may bind progesterone; therefore, administration of a sugammadex bolus dose is considered equivalent to missing a dose or doses of oral contraceptives containing an estrogen or progesterone. If an oral contraceptive is taken on the same day as sugammadex or if a patient is using an non-oral hormonal contraceptive, the patient must use an additional, nonhormonal contraceptive method or a back-up method of contraception for the next 7 days.1
Displacement of rocuronium or vecuronium from sugammadex by other drugs could result in recurrence of neuromuscular blockade. The risk of displacement reactions is greatest in the time period equivalent to 3 times the half-life of sugammadex. Toremifene, which has a relatively high binding affinity for sugammadex and is present at relatively high plasma concentrations, may be expected to displace rocuronium or vecuronium from sugammadex. Reversal of neuromuscular blockade may be delayed in patients who have received toremifene on the day of surgery.1 Flucloxacillin, another drug with a high potential for a displacement interaction, was not observed to reverse the effects of sugammadex when administered to subjects after administration of sugammadex for reversal of rocuronium or vecuronium blockade.35
The risk of recurrence of neuromuscular blockade may also be increased with the administration of drugs that potentiate neuromuscular blockade.1
Coagulation parameters should be carefully monitored in patients receiving thromboprophylaxis drugs other than heparin or LMWH or in patients receiving thromboprophylaxis drugs and a 16 mg/kg dose of sugammadex.1 Treatment with sugammadex did not change anti-Xa activity or aPTT to a clinically meaningful extent following pretreatment with LMWH or unfractionated heparin.36 No further change in platelet aggregation was observed when sugammadex was administered to patients receiving daily low-dose aspirin.37
RECOMMENDED MONITORING
Patients should be closely monitored from the time of administration until complete recovery of neuromuscular function to ensure adequate ventilation and maintenance of a patent airway. Recovery of neuromuscular function should be determined by assessment of skeletal muscle tone and respiratory measurements in addition to the response to peripheral nerve stimulation.1
Patients should also be closely monitored for signs of hypersensitivity reactions and hemodynamic changes.
DOSING
Sugammadex is administered intravenously (IV) as a single bolus injection over 10 seconds into an existing IV line. Doses and timing of sugammadex administration should be based on twitch responses and the extent of spontaneous recovery. Dosing is based on actual body weight.1
For reversal of rocuronium- and vecuronium-induced neuromuscular blockade, a sugammadex dose of 4 mg/kg is recommended if spontaneous recovery of the twitch response has reached 1 to 2 PTCs and there are no twitch responses to TOF stimulation. A sugammadex dose of 2 mg/kg is recommended if spontaneous recovery has reached the reappearance of T2 in response to TOF stimulation.1
A sugammadex dose of 16 mg/kg is recommended if there is a clinical need to reverse rocuronium-induced neuromuscular blockade within 3 minutes after administration of a single dose of rocuronium 1.2 mg/kg. The efficacy of sugammadex for immediate reversal of vecuronium-induced blockade has not been studied.1
Sugammadex may be injected into a running infusion with the following IV solutions: sodium chloride 0.9%, dextrose 5%, sodium chloride 0.45% and dextrose 2.5%, dextrose 5% in sodium chloride 0.9%, Isolyte P with dextrose 5%, lactated Ringer's solution, and Ringer's solution. The infusion line should be flushed (eg, with sodium chloride 0.9%) between administration of sugammadex and other drugs. Sugammadex is physically incompatible with verapamil, ondansetron, and ranitidine.1
PRODUCT AVAILABILITY
Sugammadex was originally submitted for FDA approval in October 2007 and granted priority review. The FDA Advisory Committee on Anesthetics and Life Support recommended sugammadex for approval in March 2008; however, the FDA did not approve sugammadex, citing concerns over hypersensitivity and allergic reactions.38,39 Following the submission of amendments to the application, sugammadex received FDA approval on December 15, 2015.33 It is available as a 100 mg/mL injection solution in single-dose vials containing sugammadex 200 mg per 2 mL and 500 mg per 5 mL.1 Sugammadex should be stored at 25°C (77°F), with excursions permitted to 15°C to 30°C (59°F to 86°F). It should be protected from light; if not protected from light, the vial should be used within 5 days.1
DRUG SAFETY/RISK EVALUATION AND MITIGATION STRATEGY (REMS)
No REMS is required for sugammadex.33
Required postmarketing studies include a pediatric study, a study analyzing characteristics that may identify “nonresponders,” and studies comparing sugammadex to placebo or to other reversal agents in American Society of Anesthesiologist's class III or IV patients and in morbidly obese patients.33
CONCLUSION
Sugammadex rapidly reverses rocuronium- and vecuronium-induced neuromuscular blockade, with few adverse reactions; however, cost is likely to be a major factor limiting use of this agent. Sugammadex has demonstrated faster reversal times than neostigmine; however, additional data are necessary to establish an advantage of faster reversal in a broad clinical setting. Alternative agents are inexpensive, generally well tolerated (with a primary adverse reaction of dry mouth), and effective at reversing neuromuscular blockade induced by a wider range of agents.
Footnotes
*Founder and Contributing Editor, The Formulary
†Clinical Professor, College of Pharmacy, Washington State University Spokane
‡Director, Drug Information Center, and Professor of Pharmacy Practice, College of Pharmacy, Washington State University Spokane.
The authors indicate no relationships that could be perceived as a conflict of interest.
REFERENCES
- 1.Bridion (sugammadex) [prescribing information] Whitehouse Station, NJ: Merck & Co; December 2015. [Google Scholar]
- 2.US Food and Drug Administration Anesthetic and Life Support Drugs Advisory Committee briefing material. FDA website. http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4346b1-00-index.htm. Published March 11, 2008. Accessed April 1, 2016.
- 3.Nigrovic V, Bhatt SB, Amann A. Simulation of the reversal of neuromuscular block by sequestration of the free molecules of the muscle relaxant. J Pharmacokinet Pharmacodyn. 2007;34(6):771–788. doi: 10.1007/s10928-007-9068-y. [DOI] [PubMed] [Google Scholar]
- 4.Cammu G, De K, am PJ, Demeyer I et al. Safety and tolerability of single intravenous doses of sugammadex administered simultaneously with rocuronium or vecuronium in healthy volunteers. Br J Anaesth. 2008;100(3):373–379. doi: 10.1093/bja/aem402. [DOI] [PubMed] [Google Scholar]
- 5.de Boer HD, van Egmond J, van de Pol F, Bom A, Driessen JJ, Booij LH. Time course of action of sugammadex (Org 25969) on rocuronium-induced block in the Rhesus monkey, using a simple model of equilibration of complex formation. Br J Anaesth. 2006;97(5):681–686. doi: 10.1093/bja/ael240. [DOI] [PubMed] [Google Scholar]
- 6.Kopman AF. Sugammadex: A revolutionary approach to neuromuscular antagonism. Anesthesiology. 2006;104(4):631–633. doi: 10.1097/00000542-200604000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Gijsenbergh F, Ramael S, Houwing N, van Iersel T. First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology. 2005;103(4):695–703. doi: 10.1097/00000542-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Sparr HJ, Vermeyen KM, Beaufort AM et al. Early reversal of profound rocuronium-induced neuromuscular blockade by sugammadex in a randomized multicenter study: Efficacy, safety, and pharmacokinetics. Anesthesiology. 2007;106(5):935–943. doi: 10.1097/01.anes.0000265152.78943.74. [DOI] [PubMed] [Google Scholar]
- 9.Peeters P, Passier P, Smeets J et al. Sugammadex is cleared rapidly and primarily unchanged via renal excretion. Biopharm Drug Dispos. 2011;32(3):159–167. doi: 10.1002/bdd.747. [DOI] [PubMed] [Google Scholar]
- 10.Sorgenfrei IF, Norrild K, Larsen PB et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: A dose-finding and safety study. Anesthesiology. 2006;104(4):667–674. doi: 10.1097/00000542-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Panhuizen IF, Gold SJ, Buerkle C et al. Efficacy, safety and pharmacokinetics of sugammadex 4 mg kg-1 for reversal of deep neuromuscular blockade in patients with severe renal impairment. Br J Anaesth. 2015;114(5):777–784. doi: 10.1093/bja/aet586. [DOI] [PubMed] [Google Scholar]
- 12.Cammu G, Van Vlem B, van den Heuvel M. Dialysability of sugammadex and its complex with rocuronium in intensive care patients with severe renal impairment. Br J Anaesth. 2012;109(3):382–390. doi: 10.1093/bja/aes207. et al. [DOI] [PubMed] [Google Scholar]
- 13.Abad-Gurumeta A, Ripollés-Melchor J, Casans-Francés R. A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia. 2015;70(12):1441–1452. doi: 10.1111/anae.13277. et al; Evidence Anaesthesia Review Group. [DOI] [PubMed] [Google Scholar]
- 14.Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: Results of a randomized, controlled trial. Eur J Anaesthesiol. 2010;27(10):874–881. doi: 10.1097/EJA.0b013e32833d56b7. [DOI] [PubMed] [Google Scholar]
- 15.Khuenl-Brady KS, Wattwil M, Vanacker BF, Lora-Tamayo JI, Rietbergen H, Alvarez-Gómez JA. Sugammadex provides faster reversal of vecuronium-induced neuromuscular blockade compared with neostigmine: A multicenter, randomized, controlled trial. Anesth Analg. 2010;110(1):64–73. doi: 10.1213/ane.0b013e3181ac53c3. [DOI] [PubMed] [Google Scholar]
- 16.Suy K, Morias K, Cammu G et al. Effective reversal of moderate rocuronium- or vecuronium-induced neuromuscular block with sugammadex, a selective relaxant binding agent. Anesthesiology. 2007;106(2):283–288. doi: 10.1097/00000542-200702000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Shields M, Giovannelli M, Mirakhur RK, Moppett I, Adams J, Hermens Y. Org 25969 (sugammadex), a selective relaxant binding agent for antagonism of prolonged rocuronium-induced neuromuscular block. Br J Anaesth. 2006;96(1):36–43. doi: 10.1093/bja/aei314. [DOI] [PubMed] [Google Scholar]
- 18.Vanacker BF, Vermeyen KM, Struys MM et al. Reversal of rocuronium-induced neuromuscular block with the novel drug sugammadex is equally effective under maintenance anesthesia with propofol or sevoflurane. Anesth Analg. 2007;104(3):563–568. doi: 10.1213/01.ane.0000231829.29177.8e. [DOI] [PubMed] [Google Scholar]
- 19.Gaszynski T, Szewczyk T, Gaszynski W. Randomized comparison of sugammadex and neostigmine for reversal of rocuronium-induced muscle relaxation in morbidly obese undergoing general anaesthesia. Br J Anaesth. 2012;108(2):236–239. doi: 10.1093/bja/aer330. [DOI] [PubMed] [Google Scholar]
- 20.Flockton EA, Mastronardi P, Hunter JM et al. Reversal of rocuronium-induced neuromuscular block with sugammadex is faster than reversal of cisatracurium-induced block with neostigmine. Br J Anaesth. 2008;100(5):622–630. doi: 10.1093/bja/aen037. [DOI] [PubMed] [Google Scholar]
- 21.Tassonyi E, Pongrácz A, Nemes R, Asztalos L, Lengyel S, Fülesdi B. Reversal of pipecuronium-induced moderate neuromuscular block with sugammadex in the presence of a sevoflurane anesthetic: A randomized trial. Anesth Analg. 2015;121(2):373–380. doi: 10.1213/ANE.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 22.Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: A randomized comparison with neostigmine. Anesthesiology. 2008;109(5):816–824. doi: 10.1097/ALN.0b013e31818a3fee. [DOI] [PubMed] [Google Scholar]
- 23.Lemmens HJ, El-Orbany MI, Berry J, Morte JB, Jr, Martin G. Reversal of profound vecuronium-induced neuromuscular block under sevoflurane anesthesia: Sugammadex versus neostigmine. BMC Anesthesiol. 2010;10:15. doi: 10.1186/1471-2253-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groudine SB, Soto R, Lien C, Drover D, Roberts K. A randomized, dose-finding, phase II study of the selective relaxant binding drug, sugammadex, capable of safely reversing profound rocuronium-induced neuromuscular block. Anesth Analg. 2007;104(3):555–562. doi: 10.1213/01.ane.0000260135.46070.c3. [DOI] [PubMed] [Google Scholar]
- 25.Brueckmann B, Sasaki N, Grobara P et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: A randomized, controlled study. Br J Anaesth. 2015;115(5):743–751. doi: 10.1093/bja/aev104. [DOI] [PubMed] [Google Scholar]
- 26.Geldner G, Niskanen M, Laurila P et al. A randomized controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia. 2012;67(9):991–998. doi: 10.1111/j.1365-2044.2012.07197.x. [DOI] [PubMed] [Google Scholar]
- 27.Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: A comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth Analg. 2007;104(3):569–574. doi: 10.1213/01.ane.0000248224.42707.48. [DOI] [PubMed] [Google Scholar]
- 28.Lee C, Jahr JS, Candiotti KA, Warriner B, Zornow MH, Naguib M. Reversal of profound neuromuscular block by sugammadex administered three minutes after rocuronium: A comparison with spontaneous recovery from succinylcholine. Anesthesiology. 2009;110(5):1020–1025. doi: 10.1097/ALN.0b013e31819dabb0. [DOI] [PubMed] [Google Scholar]
- 29.Sørensen MK, Bretlau C, Gätke MR, Sørensen AM, Rasmussen LS. Rapid sequence induction and intubation with rocuronium-sugammadex compared with succinylcholine: A randomized trial. Br J Anaesth. 2012;108(4):682–689. doi: 10.1093/bja/aer503. [DOI] [PubMed] [Google Scholar]
- 30.de Boer HD, Driessen JJ, Marcus MA, Kerkkamp H, Heeringa M, Klimek M. Reversal of rocuronium-induced (1.2 mg/kg) profound neuromuscular block by sugammadex: A multicenter, dose-finding and safety study. Anesthesiology. 2007;107(2):239–244. doi: 10.1097/01.anes.0000270722.95764.37. [DOI] [PubMed] [Google Scholar]
- 31.Pühringer FK, Rex C, Sielenkämper AW et al. Reversal of profound, high-dose rocuronium-induced neuromuscular blockade by sugammadex at two different time points: An international, multicenter, randomized, dose-finding, safety assessor-blinded, phase II trial. Anesthesiology. 2008;109(2):188–197. doi: 10.1097/ALN.0b013e31817f5bc7. [DOI] [PubMed] [Google Scholar]
- 32.Rahe-Meyer N, Fennema H, Schulman S et al. Effect of reversal of neuromuscular blockade with sugammadex versus usual care on bleeding risk in a randomized study of surgical patients. Anesthesiology. 2014;121(5):969–977. doi: 10.1097/ALN.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 33.Rosebraugh C. NDA approval letter: Bridion (sugammadex NDA 022225). US Food and Drug Administration website. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/022225Orig1s000ltr.pdf. Published December 15, 2015. Accessed December 21, 2015.
- 34.Abrishami A, Ho J, Wong J, Yin L, Chung F. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database Syst Rev. 2009. p. CD007362. [DOI] [PubMed]
- 35.Kam PJ, Heuvel MW, Grobara P et al. Flucloxacillin and diclofenac do not cause recurrence of neuromuscular blockade after reversal with sugammadex. Clin Drug Investig. 2012;32(3):203–212. doi: 10.2165/11598980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.De Kam PJ, Kruithof AC, van Lierop MJ. Lack of a clinically relevant effect of sugammadex on anti-Xa activity or activated partial thromboplastin time following pretreatment with either unfractionated or low-molecular-weight heparin in healthy subjects. Int J Clin Pharmacol Ther. 2014;52(8):631–641. doi: 10.5414/CP202091. et al. [DOI] [PubMed] [Google Scholar]
- 37.de Kam PJ, El Galta R, Kruithof AC et al. No clinically relevant interaction between sugammadex and aspirin on platelet aggregation and coagulation parameters. Int J Clin Pharmacol Ther. 2013;51(12):976–985. doi: 10.5414/CP201970. [DOI] [PubMed] [Google Scholar]
- 38.FDA Advisory Committee unanimously recommends U.S. approval of sugammadex, the first and only selective relaxant binding agent [press release] Kenilworth, NJ: Schering-Plough; March 11, 2008. http://www.prnewswire.com/news-releases/fda-advisory-committee-unanimously-recommends-us-approval-of-sugammadex-the-first-and-only-selective-relaxant-binding-agent-56892052.html. Accessed April 1, 2016. [Google Scholar]
- 39.Loftus P. Schering-Plough postsurgery drug rejected by FDA. Wall Street Journal. website. http://www.wsj.com/articles/SB121759239776004387. Published August 2, 2008. Accessed April 1, 2016.


