Abstract
Leishmaniasis is a vector-borne disease that is transmitted by sandflies and caused by obligate intracellular protozoa of the genus Leishmania. In the present study, we carried out a screening on the experimental infection of Phlebotomus pernioucus by bioluminescent Leishmania infantum using murine model and artificial feeder. We developed a real-time polymerase chain reaction (RT-PCR)-based method to determine individually the number of Leishmania promastigotes fed by infected flies. Among 1840 new emerged female sand flies, 428 were fed on the infected mice. After their death, they were analysed individually by RT-PCR. Our results demonstrated just a single Leishmania positive female at sixth day post meal. A total of 1070 female sand flies were exposed in contact with artificial feeder containing the human blood with two different quantities of Leishmania parasites: 2.106/mL and 1.107/mL. A blood meal including 1.107/mL LUC-promastigotes was proposed to 270 females and 75 (28%) flies were engorged. Among them, 44 (59%) were positive by RT-PCR analysis, with a relative average of 50551 Leishmania parasites. In case of blood feeding of females with 2.106/mL promastigotes, 57 out of 800 (7%) females succeed to feed from artificial feeder which 22 (39%) were positive with a relative average of 6487 parasites.
Keywords: experimental infection, bioluminescence, Leishmania infantum, Phlebotomus perniciosus
Leishmaniases are vector-borne diseases caused by obligate protozoan parasites from the genus Leishmania (Trypanosomatida: Trypanosomatidae). Leishmaniases are endemic in large areas of the tropics, subtropics and the Mediterranean basin, including more than 98 countries, where there are a total of 350 million people at risk and 12 million cases infected. Canine leishmaniasis is a serious problem and it is estimated that 2.5 million dogs are infected in the Mediterranean basin only (Moreno & Alvar 2002). Among the endemic regions on five continents, there is an estimated incidence of 0.7-1.2 million cases of cutaneous leishmaniasis (CL) and 0.2-0.4 million cases of visceral leishmaniasis (VL) in these countries (Alvar et al. 2012).
Leishmaniasis is transmitted by the bite of infected female sand flies (Diptera: Psychodidae: Phlebotominae) whose hosts are mammals such as canids, rodents, marsupials, hyraxes, or human beings. Among more than 800 sand fly species described, approximately 166 species have been reported to be proven (49 species) or potential (118 spp.) vectors for different pathogenic Leishmania parasites in the Old (six Leishmania spp.) and New World (14 Leishmania spp.) (Akhoundi et al. 2016).
Leishmania infantum is the causative agent of infantile visceral leishmaniasis in the Old World e.g. Mediterranean region (Marty et al. 2007, Pomares et al. 2016), Middle-East (Hotez et al. 2012), central and south Asia (Strelkova et al. 2015) as well as New World e.g. south America (Kuhls et al. 2011) where it has been called Leishmania chagasi. It also causes rare cases of CL and mucocutaneous leishmaniasis (MCL) throughout the Mediterranean basin. This etiologic agent of VL has a high prevalence in Europe particularly in the southern regions leading to a latent public health threat (Ready 2010). It has been reported in numerous publications targeting Leishmania infection in the symptomatic and asymptomatic dogs (Canis familiaris) (Solano-Gallego et al. 2011, Laurenti et al. 2013), rabbit (García et al. 2014), Hare (Molina et al. 2012), etc. as the reservoir. The dogs (C. familiaris) are the principal domestic reservoir in southern Europe with an average seroprevalence up to 25% (Maia & Cardoso 2015).
There are numerous sand fly species belonging to genus Phlebotomus; subgenera Larroussius and Adlerius which have been reported as the proven or potential vector of L. infantum in the Mediterranean region (Depaquit et al. 2013, Akhoundi et al. 2016). Among them, Phlebotomus pernicioucus is one of the known and important proven vectors particularly in south Europe (Prudhomme et al. 2015).
To simulate the leishmaniasis biological cycle in the laboratory conditions, several investigations were conducted explaining the experimental infection of sand fly vectors by Leishmania parasites using alive animal hosts (e.g. dog, mice, hamster and etc.), and/or artificial feeder (Volf & Volfova 2011, Aslan et al. 2013, Martín-Martín et al. 2015). The most of mentioned studies were concentrated on the cutaneous Leishmania species (Belkaid et al. 1998, Mears et al. 2015). Beside these studies, there are some investigations which have been focused on the viscerotropic species (Martín-Martín et al. 2015, Sadlova et al. 2015).
For the sand fly species used for experimental infection, P. orientalis (Sadlova et al. 2015), P. pernicioucus (Guarga et al. 2000), P. longipalpis (Maia et al. 2011) have been selected as the suitable candidates for simulation of Old World leishmaniasis in the laboratory condition.
Some investigations have applied the labelled transfected Leishmania species for improving the knowledge on the biology of parasites in sand fly and animal hosts in in vivo and in vitro experiments.
In the present study, we coupled for the first time, an experimental infection of P. pernioucus by bioluminescent L. infantum using BALB/c mice and artificial feeder in order to monitor experimental infection and to quantify the relative sand fly infection rate analysing by real-time polymerase chain reaction (RT-PCR).
MATERIALS AND METHODS
Sand fly colony - In the present study, we colonised P. perniciosus coming from Dr R Molina insectarium (Instituto de Salud Carlos III, Spain). We maintained different biological stages of egg, larvae, pupae and adult under controlled conditions of incubator including 26-27ºC temperature, 10/14 h light/dark photoperiod and > 70% relative humidity.
The freshly emerged adults were transferred by a mouth aspirator in the insect rearing cages (BugDorm-1, 30x30x30cm and 24 x 24 mesh/square inch). Cotton soaked with a sterile sugar solution (30%) was offered them permanently.
The BALB/c mice anesthetised intraperitoneally with ketamine/xylazine (150 mg/kg and 15 mg/kg) were used for female nutrition once per week. Moreover, the glasses artificial feeders were served using 10 mL of human blood purchased freshly from Etablissement Français du Sang (EFS). For simulation of the host skin, the porcine intestinal membrane-previously disinfected by 70% ethanol and sterile physiological saline-were used. In order to prevent any coagulation, the blood was kept at 37ºC using water flowing system. The female sand flies were fed twice per week for one hour. The blood-fed females were transferred from the original cage into a new plastic oviposition pot with an equal number of males for mating and egg laying. The larvae were fed with a mixture of rabbit feces and pellets as previously described (Volf & Volfova 2011).
Parasites culture – L. infantum strain MON-1 (MHOM/FR/94/LPN101) with transgenic modification-expressing luciferase (LUC-parasites) was routinely maintained and passaged (Michel et al. 2011). L. infantum promastigotes were cultivated in incubator at 26ºC in M199 medium supplemented with adenosine 0.1 mM, biotin 1 µg/mL, bovine hemin 5 µg/mL, streptomycin 100 µg/mL, penicillin 100 U/mL, 2 µg/mL biopterin, L-glutamine 2 mM, folic acid 10 µg/mL and 10% fetal calf serum (culture medium) (Michel et al. 2011). Exponential growth phases were anticipated before parasite inoculations into mice (2.108 metacyclic promastigotes) or experimental infection with artificial feeder (2.106 and 1.107 metacyclic promastigotes/mL of human blood).
Mice, inoculation of LUC-parasites and ethics statement - Five BALB/c mice with seven weeks old were purchased from Charles River (France) and maintained under specific pathogen-free, dietary and stable climatic conditions. They were kept under surveillance according to the regulations of the European Union, the French Ministry of Agriculture and to Federation of Laboratory Animal Science Associations (FELASA) recommendations. The experiments were approved by the ethics committee of the Nice School of Medicine, France (Protocol number: NCE/2014-189). BALB/c mice were inoculated by 2.108 luciferase-transgenic promastigotes via intravenous route (IV) in the tail base.
Bioluminescence imaging - Mice infected with LUC-parasites were imaged using the Photon Imager (Biospace Lab, France). Luciferin solution (300 mg/kg) was injected to mice via intraperitoneal (IP) route in order to permit observing and monitoring the emission of Leishmania bioluminescence. Ten minutes after the Luciferin injection, the mice were anesthetised by 5% isoflurane/1L, O2. Min-1 atmosphere and put then directly in the imaging chamber of the Photon Imager with 2% isoflurane/0.2L O2. per mouse min-1 atmosphere.
Acquisition of emitted photons radiated by infected mice, with a charge-coupled device camera, were monitored for 20 min in previously defined regions of interest (ROI) that delimited the surface of analysis.
Sand fly xenodiagnoses with infected mice - The LUC-parasite infected mice previously confirmed by imaging were selected for sand fly infection.
BALB/c mice with different post-infection dates were anesthetised intraperitoneally with ketamine/xylazine (150 mg/kg and 15 mg/kg) and placed individually for 45 min in the cage directly in contact with 120 to 200 female P. perniciosus (four to seven days old) and equal number of males for each test.
The blood-fed females were then separated after 24 h and transferred to oviposition pots containing equal male numbers and maintained in incubator under controlled conditions as mentioned above (Hlavacova et al. 2013).
Sand fly infections with artificial feeder - For each feeding, 10 mL human blood containing 2.106/mL or 1.107/mL LUC-metacyclic promastigotes were proposed directly via the cage mesh to 120 to 200 female sand flies during one hour. The blood-engorged females were separated and kept as described above. The dead females were conserved at -20ºC for the further molecular analyses.
DNA extraction and RT-PCR - Each blood-fed female sample was put in a sterile tube of Lysing Kits (Precellys®) containing 100 µL sterile water and 20 µL Chelex 5% and then homogenised by Precellys® (2 x 30 sec, with a break of 15 sec).
After centrifuging at 8000 rpm for five min, the whole solution was transferred into a new 1.5 mL microtube and incubated at 90ºC for 40 min. The second centrifugation was carried out at 15000 rpm for 10 min. Then the supernatant of diphasic solution was transferred into a new 1.5 mL microtube and kept at -20ºC.
RT-PCR was implemented for detection and quantification of L. infantum targeting minicircle kinetoplast DNA (kDNA). Primers and probe previously described by Mary et al. (2004) comprising 20 pmol of each forward (5’-CTTTTCTGGTCCTCCGGGTAGG-3’) and reverse (5’-CCACCCGGCCCTATTTTACACCAA-3’) primers and 3.33 pmol of TaqMan probe (FAM-TTTTCGCAGAACGCCCCTACCCGC-TAMRA) were used for Leishmania screening and quantification. The assays were performed with a final volume of 10 µL including 2.5 µL DNA sample.
The standard curve was obtained from the primary DNA extraction source of 2.5.107 parasites and diluted serially (six times) with 1/10 rate which corresponding to the 50000 to 0.05 parasites in 2.5 µL. RT-PCR program was implemented in two steps temperature of 95ºC and 60ºC for 40 cycles. A pair of positive and negative controls was used for each assay.
RESULTS
In vivo screening of LUC-L. infantum in infected mice - BALB/c mice were inoculated by 2.108 LUC-Leishmania promastigotes via IV route according to the protocol, described above. The infected mice were imaged and monitored for the presence of the luminescence (expressed as photons/s/cm2) particularly in ROI of the target organs such as the liver or spleen. The mice infection was verified from two to four weeks post IV inoculation (Fig. 1).
Fig. 1. : in vivo monitoring of LUC - Leishmania infantum in the region of interest of the mice’ target organs.
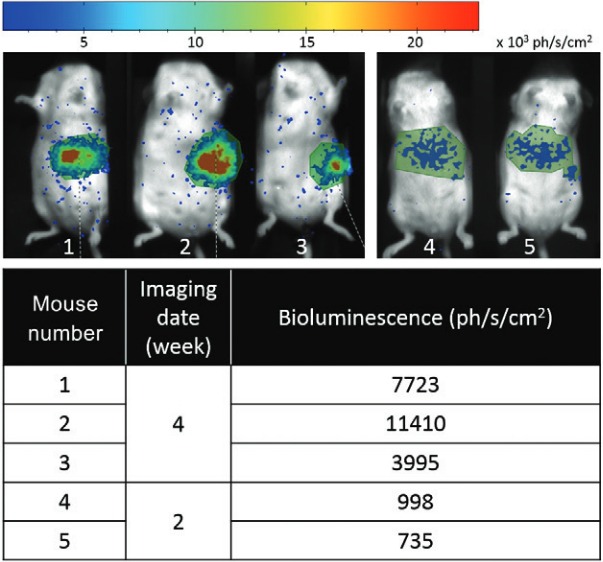
Sand fly xenodiagnoses with infected mice - Five infected mice were anesthetised and proposed in contact to a total of 1840 new emerged female sand flies at different dates of mice post infection. Among them, 428 females were fed blood meal and after their death, they were individually analysed by RT-PCR. Our results demonstrated just a single female Leishmania positive at sixth day after blood meal (Table I).
TABLE I. The characteristics of different experimental infections of Phlebotomus perniciosus by LUC - Leishmania infantum .
| Experimental model | Promastigote load | Experimentation | Fed sand flies(%) | Leishmania positive sand flies/Fed sand flies | Average Leishmania number (extremes) | |||
|---|---|---|---|---|---|---|---|---|
| Artificial feeder | 1.107/mL of blood | 1 | 57/150 (38%) | 75/270 (28%) | 32/57 (56%) | 44/75 (59%) | 32015 (11-287573) | 50551 (11-481978) |
| 2 | 18/120 (15%) | 12/18 (66%) | 99982 (125-481978) | |||||
| 2.106/mL of blood | 1 | 10/200 (5%) | 57/800 (7%) | 4/10 (40%) | 22/57 (39%) | 134 (10-458) | 6487 (10-65825) | |
| 2 | 12/200 (6%) | 4/12 (33%) | 129 (18-341) | |||||
| 3 | 20/200 (10%) | 9/20 (45%) | 6322 (20-65825) | |||||
| 4 | 15/200 (7.5%) | 5/15 (33%) | 11300 (256-34801) | |||||
| Murin model | 2.108 (IV route) | Mouse 1 (8 week post infection) | 17/120 (14%) | 428/ 1840 (23%) | / | 1/428 (0.2%) | / | 11 |
| Mouse 1 (10 week p.i) | 15/200 (7.5%) | / | / | |||||
| Mouse 1 (11 week p.i) | 10/200 (5%) | / | / | |||||
| Mouse 2 (8 week p.i) | 58/120 (48%) | / | / | |||||
| Mouse 2 (11 week p.i) | 21/200 (10.5%) | / | / | |||||
| Mouse 3 (21 week p.i) | 16/200 (8%) | / | / | |||||
| Mouse 3 (30 week p.i) | 29/200 (14.5%) | / | / | |||||
| Mouse 4 (2 week p.i) | 147/200 (78%) | 1/147 (0.7%) | 11 | |||||
| Mouse 4 (2 week p.i) | 67/200 (33.5%) | / | / | |||||
| Mouse 5 (2 week p.i) | 48/200 (24%) | / | / | |||||
Sand fly infection with artificial feeder - A total of 1070 new emerged female sand flies were exposed in contact with artificial feeder containing the human blood with two different quantities of Leishmania parasites: 2.106/mL and 1.107/mL.
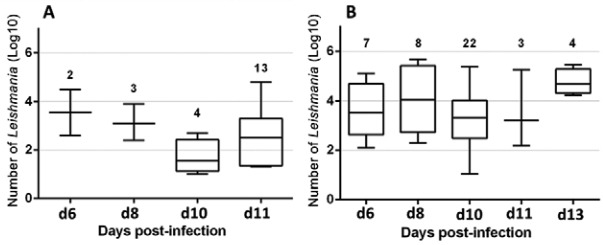
A blood meal including 1.107/mL LUC-promastigotes was proposed to 270 females and 75 (28%) flies were engorged. Among them, 44 (59%) were positive by RT-PCR analysis, with a relative average of 50551 Leishmania parasites (Table I). The mean of Leishmana number in infected P. perniciosus at different days of p. i. are presented in the Table II. The maximum mean (130164) of parasites, obtained by fed flies were seen at d8 p.i. (Table II).
TABLE II. The average of Leishmania number in the infected Phlebotomus perniciosus in different days of post infection.
| Experimental model | Promastigote load | Days post infection | Infected sand fly number | Leishmania number/day p.i. (mean/median) |
|---|---|---|---|---|
| Artificial feeder | 1.107/mL of blood | d6 | 7 | 28665/3310 |
| d8 | 8 | 130164/36080 | ||
| d10 | 22 | 17762/2091 | ||
| d11 | 3 | 62434/1598 | ||
| d13 | 4 | 101075/49778 | ||
| 2.106/mL of blood | d6 | 2 | 17580/17580 | |
| d8 | 3 | 3431/1407 | ||
| d10 | 4 | 134/35 | ||
| d11 | 13 | 7440/341 |
Among 800 female sand flies prospected by 2.106/mL promastigotes, 57 (7%) female flies succeed to feed from the artificial feeder which 22 (39%) were positive with a relative average of 6487 Leishmania parasites (Table I). The details of Leishmania number at various post infection dates are given in the Table II.
The post infection intensities of female P. perniciosus in different dates are shown in the Figs 2-3. With considering the whole infected females in each condition of 2.106/mL and 1.107/mL, we found 27% and 68% of heavy infections (> 1000 promastigotes) respectively (Figs 2-3).
Fig. 2. : infection intensity of female Phlebotomus perniciosus (light: < 100, moderate: 100-1000, heavy: 1000) in different dates of post infection analysed by a real-time polymerase chain reaction). (A) 2.106/mL LUC-promastigotes; (B) 1.107/mL LUC- promastigotes.
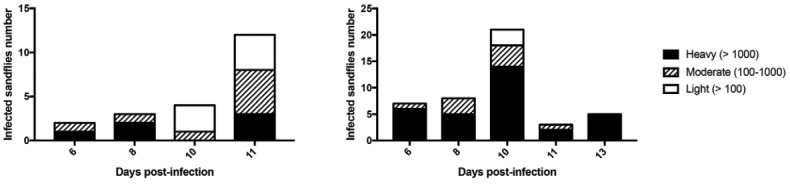
Fig. 3. : the Leishmania infantum promastigote loads at different days of post infection of female Phlebotomus perniciosus, prospected by real-time polymerase chain reaction. (A) 2.106/mL LUC-promastigotes; (B) 1.107/mL LUC-promastigotes. The number of infected flies is indicated in the above of each day.
DISCUSSION
In vivo imaging techniques are the valuable tools which are used in the recent decade. These methods have been applied for real time screening of labelled invasive microorganisms in living animals. In the recent years, they have been served for in vitro and in vivo monitoring of Leishmania spread in the invertebrate sand fly vectors and vertebrate animals (Guevara et al. 2001, Sadlova et al. 2011). For this purpose, Leishmania parasites have transfected with the genes of fluorescent proteins such as GFP, RFP and luciferase and used for the studies targeting experimental infection (Kimblin et al. 2008, Sadlova et al. 2011, Calvo-Álvarez 2012, Taheri et al. 2015). These methods have some advantages including the (i) monitoring diseases along the time without sacrificing the living animal, (ii) visualising the Leishmania infection in the target organs, (iii) allowing repetitive observation of metabolically active cells due to non-destructive and non-invasive characteristics, (iv) screening in real time the drug efficacy in qualitative assessment of an infection.
In the present study, we used for the first time, luciferase transfected L. infantum for experimental infection of P. pernicious - as the proven vector - by murine model and artificial feeder. Lang et al. (2005) reported the successful utilisation of L. amazonensis recombinants stably expressing the firefly luciferase gene for their experiments including (i) in vitro drug screening on the clinically relevant stage of the parasites (i.e. amastigote-loaded mouse bone marrow derived macrophages), and (ii) the monitoring of the parasitic process in living animals. Moreover, they indicated that both control and luciferase-expressing parasites are driving the same clinical processes in BALB/c mice. Hutchens and Luker (2007) in their review paper reported several cases of application of bioluminescence imaging for studying not only on the infectious diseases, but also, on the bacterial infections e.g. Salmonella typhimurium. Based on their reports, pathogens, insects and mammalian cells can be engineered to express one or more luciferase enzymes as reporters for in vivo imaging. In the other study conducted in same year, Lecoeur et al. (2007) with drug treatment using aminoglycoside ointment (a topical treatment of CL) against luciferase transgenic L. major, explained in details the advantage of this technique as a robust method to rapidly assess efficacy of drugs/compounds, to screen treatment modalities and to allow standardised comparison of different therapeutic agents. After these studies, different engineered viscero - and dermotropic Leishmania species to express luciferase were widely used with mice models for screening the Leishmania infection, quantifying the parasites, drug therapies (Thalhofer et al. 2010, Talmi-Frank et al. 2012, Reguera et al. 2014, Reimão et al. 2015). Taking advantages of mentioned studies, we used the bioluminescent L. infantum. The murine model prospected in the present study was a VL model without cutaneous clinical lesion. Despite 428 females flies fed on the infected mice, we found only one female with a low load relative number of Leishmania equivalent to 11 parasites. Sadlova et al. (2015) studied the same model by injecting L. donovani parasites derived from P. orientalis into the ear of BALB/c mice. According to their analysis, those female sand flies which were fed on the infected (17% Leishmania positive from nine-15 weeks post infection) and collateral (positive pool of sand flies) ears were the Leishmania positive samples.
Maia et al. (2011) compared the experimental transmission of two strains of dermotropic and viscerotropic L. infantum by two vectors of Lutzomyia longipalpis and P. perniciosus. They used an artificial feeder contained rabbit blood with 1.107/mL promastigotes. They obtained an average of 65768 parasites with viscerotropic strain. We had a similar experimental condition with them e.g. the same temperature (26ºC) and relative humidity (70%) and the number of viscerotropic promastigotes/mL blood (1.107/mL). Our findings showed the average of 50551 promastigotes which were close to the results of Maia et al. (2011).
In the other study carried out by Aslan et al. (2013), they suggested infected blood containing 5.106 L. infantum /mL to L. longipalpis. They found, at 8th days post infection, an average of 2.104, 8.104 and 4.104 parasites in the seven, nine and 13 artificially infected sand flies respectively. Based on our results with a blood meal containing 1.107 parasites/mL, we obtained an average of 30164 parasites at d8 p.i. which is in the range of 2,0000 and 8,0000 parasites reported by Aslan et al. (2013) for the same day.
Based on the results obtained in the present study, there was a wide variability in infection intensity of P. perniciosus and their acquired Leishmania parasite burden. The artificial feeder seems to be an efficient method to obtain high rate of infectivity. Several factors can play the role in this phenomenon such as the type of Leishmania (viscerotropic or dermotropic) and sand fly species, their maintenance condition e.g. temperature, the proposed number of parasite detected by RT-PCR and bioluminescence imaging.
ACKNOWLEDGEMENTS
To Dr Ricardo Molina, Instituto de Salud Carlos III, Madrid, Spain; Dr Emmanuel Lemichez and his research team 6, Inserm 1065, Nice, France; Dr Véronique Corcelle and the C3M animal facilities, UMR-UNS/Inserm 1065, Nice, France; Dr Denis Sereno IRD-Mivegec Montpellier, France; Dr Anne-Marie Cuisinier and Virbac for the grant CIFRE, Carros, France.
REFERENCES
- Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, et al. A historical overview of the classification, evolution and dispersion of Leishmania parasites and sand flies. Plos Negl Trop Dis. 2016;10(3): doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5): doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan H, Dey R, Meneses C, Castrovinci P, Jeronimo SMB, et al. A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies. J Infect Dis. 2013;207:1328–1338. doi: 10.1093/infdis/jis932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188(10):1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Álvarez E, Guerrero NA, Álvarez-Velilla R, Prada CF, Requena JM, Punzón C, et al. Appraisal of a Leishmania major strain stably expressing mCherry fluorescent protein for both in vitro and in vivo studies of potential drugs and vaccine against cutaneous leishmaniasis. PLoS Negl Trop Dis. 2012;6(11): doi: 10.1371/journal.pntd.0001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaquit J, Bounamous A, Akhoundi M, Augot D, Sauvage F, Dvorak V, et al. A taxonomic study of Phlebotomus (Larroussius) perfiliewi s. l. Infect Genet Evol. 2013;20:500–508. doi: 10.1016/j.meegid.2013.10.006. [DOI] [PubMed] [Google Scholar]
- García N, Moreno I, Alvarez J, Cruz ML, Navarro A, Pérez-Sancho M, et al. Evidence of Leishmania infantum infection in rabbits (Oryctolagus cuniculus) in a natural area in Madrid, Spain. 5BioMed Res Int. 2014;2014 doi: 10.1155/2014/318254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarga JL, Lucientes J, Peribáñez MA, Molina R, Gracia MJ, Castillo JA. Experimental infection of Phlebotomus perniciosus and determination of the natural infection rates of Leishmania infantum in dogs. Acta Trop. 2000;77(2):203–207. doi: 10.1016/s0001-706x(00)00141-8. [DOI] [PubMed] [Google Scholar]
- Guevara P, Pinto-Santíni D, Rojas A, Crisante G, Añez N, Ramírez JL. Green fluorescent protein-tagged Leishmania in Phlebotomine sand flies. J Med Entomol. 2001;38(1):39–43. doi: 10.1603/0022-2585-38.1.39. [DOI] [PubMed] [Google Scholar]
- Hlavacova J, Votypka J, Volf P. The effect of temperature on Leishmania (Kinetoplastida: Trypanosomatidae) development in sand flies. J Med Entomol. 2013;50(5):955–958. [PubMed] [Google Scholar]
- Hotez PJ, Savioli L, Fenwick A. Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis. 2012;6(2): doi: 10.1371/journal.pntd.0001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens M, Luker GD. Applications of bioluminescence imaging to the study of infectious diseases. Cell Microbiol. 2007;9(10):2315–2322. doi: 10.1111/j.1462-5822.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- Kimblin N, Peters N, Debrabant A, Secundino N, Egen J, Lawyer P, et al. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc Natl Acad Sci USA. 2008;105(29):10125–10130. doi: 10.1073/pnas.0802331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhls K, Alam MZ, Cupolillo E, Ferreira GEM, Mauricio IL, et al. Comparative microsatellite typing of New World Leishmania infantum reveals low heterogeneity among populations and its recent Old World origin. PLoS Negl Trop Dis. 2011;5(6): doi: 10.1371/journal.pntd.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Goyard S, Lebastard M, Milon G. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cell Microbiol. 2005;7(3):383–392. doi: 10.1111/j.1462-5822.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- Laurenti MD, Rossi CN, Matta VLR, Tomokane TY, Corbett CE, Secundino NFC, et al. Symptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Vet Parasitol. 2013;196(3-4):296–300. doi: 10.1016/j.vetpar.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Lecoeur H, Buffet P, Morizot G, Goyard S, Guigon G, Milon G, et al. Optimization of topical therapy for Leishmania major localized cutaneous leishmaniasis using a reliable C57BL/6 Model. PLoS Negl Trop Dis. 2007;1(2): doi: 10.1371/journal.pntd.0000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia C, Cardoso L. Spread of Leishmania infantum in Europe with dog travelling. Vet Parasitol. 2015;213(1-2):2–11. doi: 10.1016/j.vetpar.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Maia C, Seblova V, Sadlova J, Votypka J, Volf P. Experimental transmission of Leishmania infantum by two major vectors: a comparison between a viscerotropic and a dermotropic strain. PLoS Negl Trop Dis. 2011;5: doi: 10.1371/journal.pntd.0001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Martín I, Jiménez M, González E, Eguiluz C, Molina R. Natural transmission of Leishmania infantum through experimentally infected Phlebotomus perniciosus highlights the virulence of Leishmania parasites circulating in the human visceral leishmaniasis outbreak in Madrid, Spain. 138Vet Res. 2015;46 doi: 10.1186/s13567-015-0281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty P, Izri A, Ozon C, Haas P, Rosenthal E, del Giudice P, et al. A century of leishmaniasis in Alpes-Maritimes, France. Ann Trop Med Parasitol. 2007;101(7):563–574. doi: 10.1179/136485907X229121. [DOI] [PubMed] [Google Scholar]
- Mary C, Faraut F, Lascombe L, Dumon H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J Clin Microbiol. 2004;42(11):5249–5255. doi: 10.1128/JCM.42.11.5249-5255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears ER, Modabber F, Don R, Johnson GE. A Review: the current in vivo models for the discovery and utility of new anti-leishmanial drugs targeting cutaneous leishmaniasis. PLoS Negl Trop Dis. 2015;9(9): doi: 10.1371/journal.pntd.0003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel G, Ferrua B, Lang T, Maddugoda MP, Munro P, Pomares C, et al. Luciferase-expressing Leishmania infantum allows the monitoring of amastigote population size, in vivo, ex vivo and in vitro. PLoS Negl Trop Dis. 2011;5(9): doi: 10.1371/journal.pntd.0001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina R, Jiménez MI, Cruz I, Iriso A, Martín-Martín I, Sevillano O, et al. The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Vet Parasitol. 2012;190(1-2):268–271. doi: 10.1016/j.vetpar.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002;18(9):399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- Pomares C, Marty P, Bañuls AL, Lemichez E, Pratlong F, Faucher B, et al. Genetic diversity and population structure of Leishmania infantum from southeastern France: evaluation using multi-locus microsatellite typing. PLoS Negl Trop Dis. 2016;10(1): doi: 10.1371/journal.pntd.0004303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudhomme J, Toty C, Kasap OE, Rahola N, Vergnes B, Maia C, et al. New microsatellite markers for multi-scale genetic studies on Phlebotomus ariasi Tonnoir, vector of Leishmania infantum in the Mediterranean area. Acta Trop. 2015;142:79–85. doi: 10.1016/j.actatropica.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Ready PD. Leishmaniasis emergence in Europe. 19505Euro Surveill. 2010;15(10) [PubMed] [Google Scholar]
- Reguera RM, Calvo-Álvarez E, Álvarez-Velilla R, Balaña-Fouce R. Target-based vs. phenotypic screenings in Leishmania drug discovery: a marriage of convenience or a dialogue of the deaf? Int J Parasitol. 2014;4(3):355–357. doi: 10.1016/j.ijpddr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimão JQ, Oliveira JC, Trinconi CT, Cotrim PC, Coelho AC, Uliana SRB. Generation of luciferase-expressing Leishmania infantum chagasi and assessment of miltefosine efficacy in infected hamsters through bioimaging. PLoS Negl Trop Dis. 2015;9(2): doi: 10.1371/journal.pntd.0003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlova J, Seblova V, Votypka J, Warburg A, Volf P. Xenodiagnosis of Leishmania donovani in BALB/c mice using Phlebotomus orientalis: a new laboratory model. 158Parasit Vectors. 2015;8 doi: 10.1186/s13071-015-0765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlova J, Yeo M, Seblova V, Lewis MD, Mauricio I, Volf P, et al. Visualisation of Leishmania donovani fluorescent hybrids during early stage development in the sand fly vector. PLoS ONE. 2011;6(5): doi: 10.1371/journal.pone.0019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. 86Parasit Vectors. 2011;4 doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkova MV, Ponirovsky EN, Morozov EN, Zhirenkina EN, Razakov SA, Kovalenko DA, et al. A narrative review of visceral leishmaniasis in Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, the Crimean Peninsula and Southern Russia. 330Parasit Vectors. 2015;8 doi: 10.1186/s13071-015-0925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri T, Nik HS, Seyed N, Doustdari F, Etemadzadeh MH, Torkashvand F, et al. Generation of stable L. major (+EGFP-LUC) and simultaneous comparison between EGFP and luciferase sensitivity. Exp Parasitol. 2015;150:44–55. doi: 10.1016/j.exppara.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Talmi-Frank D, Jaffe CL, Nasereddin A, Baneth G. Leishmania tropica experimental infection in the rat using luciferase-transfected parasites. Vet Parasitol. 2012;187(1-2):57–62. doi: 10.1016/j.vetpar.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Thalhofer CJ, Graff JW, Love-Homan L, Hickerson SM, Craft N, Beverley SM, et al. In vivo imaging of transgenic Leishmania parasites in a live host. 1980J Vis Exp. 2010;41 doi: 10.3791/1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volf P, Volfova V. Establishment and maintenance of sand fly colonies. J Vector Ecol. 2011;36(1):S1–S9. doi: 10.1111/j.1948-7134.2011.00106.x. [DOI] [PubMed] [Google Scholar]


