The strigolactone signaling target, SMXL7, signals from the nucleus to regulate diverse aspects of shoot development through mechanisms that in part do not require its conserved EAR motif.
Abstract
Strigolactones (SLs) are hormonal signals that regulate multiple aspects of shoot architecture, including shoot branching. Like many plant hormonal signaling systems, SLs act by promoting ubiquitination of target proteins and their subsequent proteasome-mediated degradation. Recently, SMXL6, SMXL7, and SMXL8, members of the SMAX1-LIKE (SMXL) family of chaperonin-like proteins, have been identified as proteolytic targets of SL signaling in Arabidopsis thaliana. However, the mechanisms by which these proteins regulate downstream events remain largely unclear. Here, we show that SMXL7 functions in the nucleus, as does the SL receptor, DWARF14 (D14). We show that nucleus-localized D14 can physically interact with both SMXL7 and the MAX2 F-box protein in a SL-dependent manner and that disruption of specific conserved domains in SMXL7 affects its localization, SL-induced degradation, and activity. By expressing and overexpressing these SMXL7 protein variants, we show that shoot tissues are broadly sensitive to SMXL7 activity, but degradation normally buffers the effect of increasing SMXL7 expression. SMXL7 contains a well-conserved EAR (ETHYLENE-RESPONSE FACTOR Amphiphilic Repression) motif, which contributes to, but is not essential for, SMXL7 functionality. Intriguingly, different developmental processes show differential sensitivity to the loss of the EAR motif, raising the possibility that there may be several distinct mechanisms at play downstream of SMXL7.
INTRODUCTION
Shoot system architectural characteristics strongly influence the productivity of many crop species, and architectural traits have been selected in both historical and contemporary breeding schemes. Understanding the mechanisms that regulate shoot architecture, and its environmental responsiveness, is therefore an important goal for plant research. It is well established that long-distance hormonal signals, including auxin, cytokinin, and strigolactone (SL), are key regulators of shoot architecture and allow communication both within the shoot system and between the shoot and root (Domagalska and Leyser, 2011). For instance, cytokinin produced in the root system in response to the availability of nitrate ions is systemically transported to the shoot, where it promotes branching (Kiba et al., 2011; Müller et al., 2015). Similarly, root-derived SL plays a key role in negatively regulating branching in response to low phosphate availability in the rhizosphere (Kohlen et al., 2011). However, our understanding of the molecular mechanisms that act downstream of these hormones to alter developmental processes in the shoot is currently limited.
This is particularly true of SLs. Analysis of the phenotypes of SL biosynthesis and signaling mutants has revealed roles for SLs in the regulation of shoot branching, branching angle, plant height, stem thickness, and leaf blade elongation (Smith and Waters, 2012). The role of SLs in regulating shoot branching has been intensively studied, resulting in two contrasting, nonexclusive models for their mode of action. In the first, SLs are proposed to act locally in axillary buds by upregulating the expression of the BRANCHED1 (BRC1) gene, which encodes a TEOSINTE BRANCHED1/CYCLOIDEA/PCNA domain transcription factor (Braun et al., 2012). BRC1 is well-established as a regulator of bud outgrowth, and brc1 mutants have strongly increased, SL-resistant branching (Aguilar-Martínez et al., 2007; Brewer et al., 2009). In the second model, SLs are proposed to act throughout the shoot to trigger clathrin-mediated endocytosis of the PIN1 auxin efflux carrier (Crawford et al., 2010; Shinohara et al., 2013). This hinders the establishment of positive feedback-driven, canalized auxin export from buds into the stem, which has been proposed to be required for the outgrowth of buds (Li and Bangerth, 1999; Prusinkiewicz et al., 2009; Crawford et al., 2010; Shinohara et al., 2013). For the other aspects of shoot architecture affected in SL mutants, very little is known about the downstream mechanisms involved. It is therefore an open question as to whether there is a single downstream target or class of targets for SL signaling or whether different aspects of shoot responses to SL are mediated by distinct downstream targets.
While the downstream targets of SL signaling remain poorly defined, in recent years there has been rapid progress in our understanding of proximal events in SL signaling. Multiple studies have demonstrated that the DWARF14 (D14) α/β fold protein is very likely to act as a SL receptor, since it binds and hydrolyses the synthetic SL analog GR24 and is required for sensitivity to SL (Liu et al., 2009; Hamiaux et al., 2012; Waters et al., 2012; Kagiyama et al., 2013; Zhao et al., 2013; Chevalier et al., 2014). D14 acts in concert with the MAX2 F-box protein, which forms part of an SCF-type ubiquitin ligase complex (Stirnberg et al., 2007; Hamiaux et al., 2012). Thus, as is the case in many other hormonal signaling pathways in plants, the regulated degradation of target proteins by the 26S proteasome appears to be a key feature of SL signaling. The principle proteolytic targets of SL signaling appear to be a clade of proteins in the SMAX1-LIKE (SMXL) family, typified by SMXL7 in Arabidopsis thaliana and D53 in rice (Oryza sativa) (Jiang et al., 2013; Zhou et al., 2013; Soundappan et al., 2015; Wang et al., 2015). SMXL proteins are weakly homologous to HEAT SHOCK PROTEIN101 ClpB chaperonins and are named for the founding member, SUPPRESSOR OF MAX2 1 (SMAX1) (Stanga et al., 2013). SMAX1 is also a probable target of SCFMAX2, although in this case in response to signaling mediated by the KAI2 receptor protein, itself a close relative of D14, for which the endogenous ligand is currently unknown (Waters et al., 2012; Stanga et al., 2013; Soundappan et al., 2015). In Arabidopsis, SMXL7 is rapidly degraded in response to treatment with rac-GR24 in a D14- and MAX2-dependent manner (Soundappan et al., 2015; Wang et al., 2015). Furthermore, loss of SMXL7 function, in combination with that of its close relatives SMXL6 and SMXL8, is sufficient to suppress completely the SL-related shoot and root phenotypes of max2, suggesting that these proteins are the primary and probably only direct targets of MAX2-mediated SL signaling (Soundappan et al., 2015; Wang et al., 2015). In line with both models of the regulation of shoot branching, the smxl6 smxl7 smxl8 max2 quadruple mutant has both strongly upregulated BRC1 expression and strongly downregulated PIN1 levels relative to max2 (Soundappan et al., 2015).
SMXL proteins contain a well-conserved ETHYLENE-RESPONSE FACTOR Amphiphilic Repression (EAR) motif (Ohta et al., 2001), which has been widely viewed as an important contributor to SMXL function (Jiang et al., 2013; Zhou et al., 2013; Smith and Li, 2014; Soundappan et al., 2015; Wang et al., 2015), although this is based only on circumstantial evidence. EAR motifs allow proteins to interact with partners containing corresponding C-Terminal to Lissencephaly Homology (CTLH) domains (Szemenyei et al., 2008). One of the best-characterized of these interactions is between the EAR motif in Aux/IAA proteins (repressors of auxin signaling) and the TOPLESS RELATED (TPR) family of chromatin remodeling factors (Szemenyei et al., 2008). The EAR motif in SMXL7 permits relatively weak interaction with TPR proteins, particularly TPR2 (Soundappan et al., 2015; Wang et al., 2015). On this basis, it has been suggested that SMXL proteins act as regulators of transcription (Smith and Li, 2014), but the functional relevance of this interaction, and indeed the EAR motif in general, has not been established. Furthermore, even if the EAR motif is functionally important, as suggested by its evolutionary conservation, it must be noted that there are other CTLH-domain proteins in plants, and these may be functionally relevant partners for SMXL/D53 family members in addition to, or instead of, TPR proteins (Bennett and Leyser, 2014).
Detailed characterization of the function of relevant SMXL/D53 family proteins thus represents an opportunity to dissect the downstream events in SL signaling. In this report, we assess the function of SMXL7 in SL signaling and shoot development in Arabidopsis. We demonstrate that SMXL7 colocalizes with SL signaling components D14 and MAX2 in the nucleus and that this localization is necessary for both SMXL7 function and degradation. We demonstrate the SL-dependent physical interaction of SMXL7 with D14, but not MAX2, in planta. We show that SMXL7 is expressed, and shows SL-induced degradation, throughout the shoot system in vascular-associated tissues and that the shoot is well buffered against changes in SMXL7 transcription, but not SMXL7 stability. Deleting or modifying the EAR motif in SMXL7 does not affect its localization or degradation, and although this reduces SMXL7 activity, it does not abolish it. We find that different aspects of development are differentially sensitive to the loss of the EAR motif. This raises the intriguing possibility that there are distinct mechanisms downstream of SMXL7.
RESULTS
Strigolactone Signaling Components Are Localized to and Function in the Nucleus
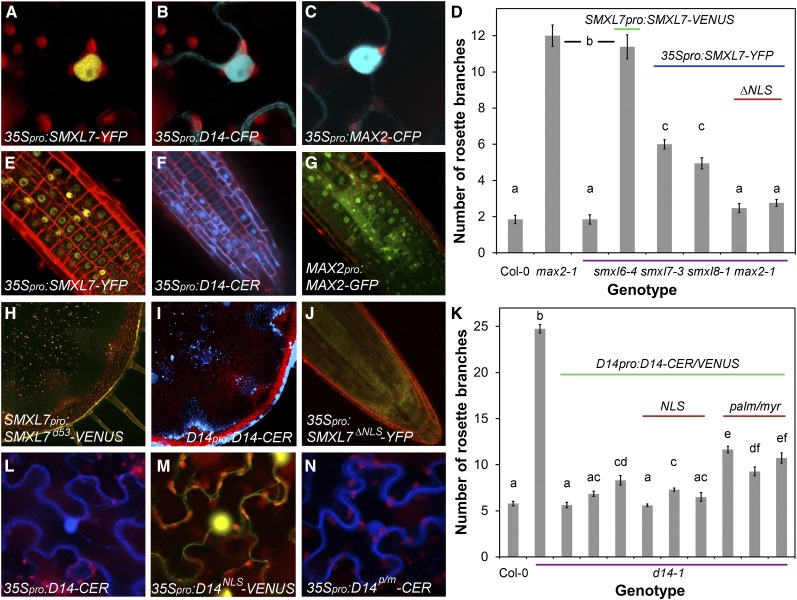
To understand the mechanisms by which SMXL7 activity regulates shoot architecture, we assessed its subcellular localization using a translational fusion to YFP (SMXL7-YFP) (Figure 1). Nuclear localization of SMXL7 has previously been demonstrated (Soundappan et al., 2015), and consistent with this, transient expression of 35Spro:SMXL7-YFP in leaf epidermal cells of Nicotiana benthamiana resulted in a signal highly localized to the nucleus (Figure 1A; Supplemental Figure 1) (Soundappan et al., 2015). We observed the same highly enriched nuclear localization in root cells when we introduced the 35Spro:SMXL7-YFP construct into Arabidopsis (Figure 1E). However, this construct only partially restored the shoot branching phenotype of the smxl6-4 smxl7-3 smxl8-1 max2-1 mutant (hereafter smxl678 max2) toward the expected max2-like branch number (Figure 1D). This could be because the protein fusion is not fully functional or because the 35S promoter does not recapitulate endogenous SMXL7 promoter activity. We thus transformed Arabidopsis with a similar fusion driven by the native SMXL7 promoter (SMXL7pro:SMXL7-VENUS). This fusion protein completely restored the branching phenotype of the smxl678 max2 mutant to max2 levels (Figure 1D), demonstrating that the fusion protein is functional.
Figure 1.
SL Signaling Components Function in the Nucleus.
(A) to (C) Subcellular localization of SMXL7-YFP (A), D14-CFP (B), and MAX2-CFP (C) in N. benthamiana epidermal cells, transiently expressed from the 35S promoter.
(D) Primary rosette branching levels in Col-0, max2-1, and smxl6-4 smxl7-3 smxl8-1 max2-1 transformed with nothing (3rd bar), SMXL7pro:SMXL7-VENUS (4th bar), 35Spro:SMXL-YFP (two independent homozygous lines, 5th and 6th bars), and 35Spro:SMXL7ΔNLS-YFP, in which the nuclear localization signal has been deleted (two independent homozygous lines, 7th and 8th bars). Plants were grown in long days for 7 weeks. n = 13 to 22; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
(E) to (G) Subcellular localization of SMXL7-YFP (E), D14-CERULEAN (F), and MAX2-GFP in Arabidopsis root meristems, in homozygous transgenic lines expressing the fusion proteins from the 35S promoter ([E] and [F]) or MAX2 promoter (G).
(H) and (I) Expression of SMXL7d53-VENUS (in which the protein has been stabilized by replacing amino acids 812RGKTVV817 with T) (H) and D14-CERULEAN (I) in transverse hand sections through Arabidopsis inflorescence stems, in homozygous transgenic lines expressing the fusion proteins from their native promoters.
(J) Subcellular localization in an Arabidopsis root meristem of SMXL7ΔNLS-YFP in homozygous transgenic lines expressing the fusion protein from the 35S promoter.
(K) Rosette branching levels in Col-0 and d14-1 transformed with nothing (2nd bar) and D14pro:D14-CERULEAN (three independent lines, 3rd to 5th bars), D14pro:D14NLS-VENUS in which a strong nuclear localization signal has been added (three independent lines, 6th to 8th bars), and D14pro:D14palm/myr-CERULEAN in which a palmitoylation/myristoylation motif has been added (three independent lines, 9th to 11th bars). The data are taken from a larger data set and show the most, median, and least rescued lines for each construct. The full data set is shown in Supplemental Figure 1. Plants were grown in short days for 4 weeks, grown in long days until the inflorescence stem was 10 cm long, and then decapitated. Primary rosette branches were counted 10 d later (Greb et al., 2003). n = 13 to 20; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
(L) to (N) Subcellular localization of D14-CERULEAN (L), D14NLS-VENUS (M), and D14palm/myr-CERULEAN (N) in N. benthamiana epidermal cells, transiently expressed from the 35S promoter.
Despite the partially and fully rescued phenotypes in these lines, it proved difficult to detect the protein in wild-type shoots, presumably due to its instability, especially in the presence of SL. In rice, the orthologous D53 protein is stabilized by deletion of five amino acids in the first NTPase domain (Jiang et al., 2013; Zhou et al., 2013), and it has previously been shown that a slightly larger deletion has a similar stabilizing effect on SMXL7 in Arabidopsis (Soundappan et al., 2015). We reasoned that a stabilized version of SMXL7 might be more easily visualized, and we thus utilized a version of the protein (SMXL7d53) containing an equivalent mutation to that seen in d53 (812Arg-Gly-Lys-Thr-Val-Val817 → Thr). We found that, indeed, SMXL7d53-VENUS or YFP fusions could now be clearly detected in stems when expressed from the native promoter (Figure 1H).
We identified a predicted nuclear localization signal (NLS) in the SMXL7 protein (amino acid position 85 to 117; RLPSSKSTPTTTVEEDPPVSNSLMAAIKRSQAT). Consistent with its predicted function, specific deletion of this motif (SMXL7ΔNLS) reduced the nuclear localization of SMXL7-YFP (Figure 1J; Supplemental Figure 1). It has previously been shown that treatment with 5 µM rac-GR24 results in the rapid degradation of SMXL7-YFP, such that little fusion protein is detectable within 20 min (Soundappan et al., 2015). Similar treatments of SMXL7ΔNLS-YFP had little effect, suggesting that the NLS, and presumably therefore nuclear localization, is required for its SL-mediated degradation (Supplemental Figure 1). The deletion of the NLS also compromised the ability of the fusion protein expressed from the 35S promoter to restore increased branching levels to the smxl678 max2 mutant (Figure 1K). These data suggest that the nuclear localization of SMXL7 is required for its function.
Previous reports show that the other known components of SL signaling, D14 and MAX2, are expressed in the same vascular-associated tissues as SMXL7 and are at least partially nuclear (Chevalier et al., 2014; Stirnberg et al., 2007). To explore the colocation of these proteins in more detail, we used both transient expression assays in N. benthamiana and stable expression in Arabidopsis. Transient expression of a D14-CFP fusion protein in N. benthamiana leaf epidermal cells driven by the 35S promoter revealed localization to both the nucleus and cytoplasm (Figure 1B; Supplemental Figure 1), as previously observed by Chevalier et al. (2014). We observed the same subcellular localization in the root when we transformed Arabidopsis with a 35Spro:D14-CERULEAN FLUORESCENT PROTEIN (CER) construct (Figure 1F), including colocalization with SMXL7 (Supplemental Figure 1). We also transformed Arabidopsis with a D14pro:D14-CER construct. Like SMXL7, this fusion protein was difficult to detect in the shoot but could be observed with high laser intensities (Figure 1I).
To assess where in the cell D14 functions, we created variants of fluorescently tagged D14 with either a strong NLS (D14NLS-VENUS), predicted to increase its nuclear localization, or a palmitoylation/myristoylation motif (D14palm/myr-CER), which should sequester D14 at the plasma membrane. Transient expression in N. benthamiana confirmed the expected changes in protein distribution (Figures 1L to 1N). We then assessed the ability of these proteins to restore wild-type levels of shoot branching to the d14-1 mutant when driven from the D14 promoter. We found that D14-CER and D14NLS-VENUS, but not D14palm/myr-CER, could completely restore wild-type branching levels to d14-1 (Figure 1K; Supplemental Figure 1). These data suggest that D14 is also predominantly functional in the nucleus. We observed a similar localization pattern for MAX2-CFP fusions as for D14 in N. benthamiana cells (Figure 1C). We confirmed this subcellular localization pattern in Arabidopsis roots (Figure 1G) but were unable to detect reliably MAX2-fluorescent protein fusions in Arabidopsis stems, despite the well-documented activity of the MAX2 promoter in these tissues (Stirnberg et al., 2007) as well as MAX2-dependent SL response (Shinohara et al., 2013).
SMXL7, D14, and MAX2 Interact in the Nucleus
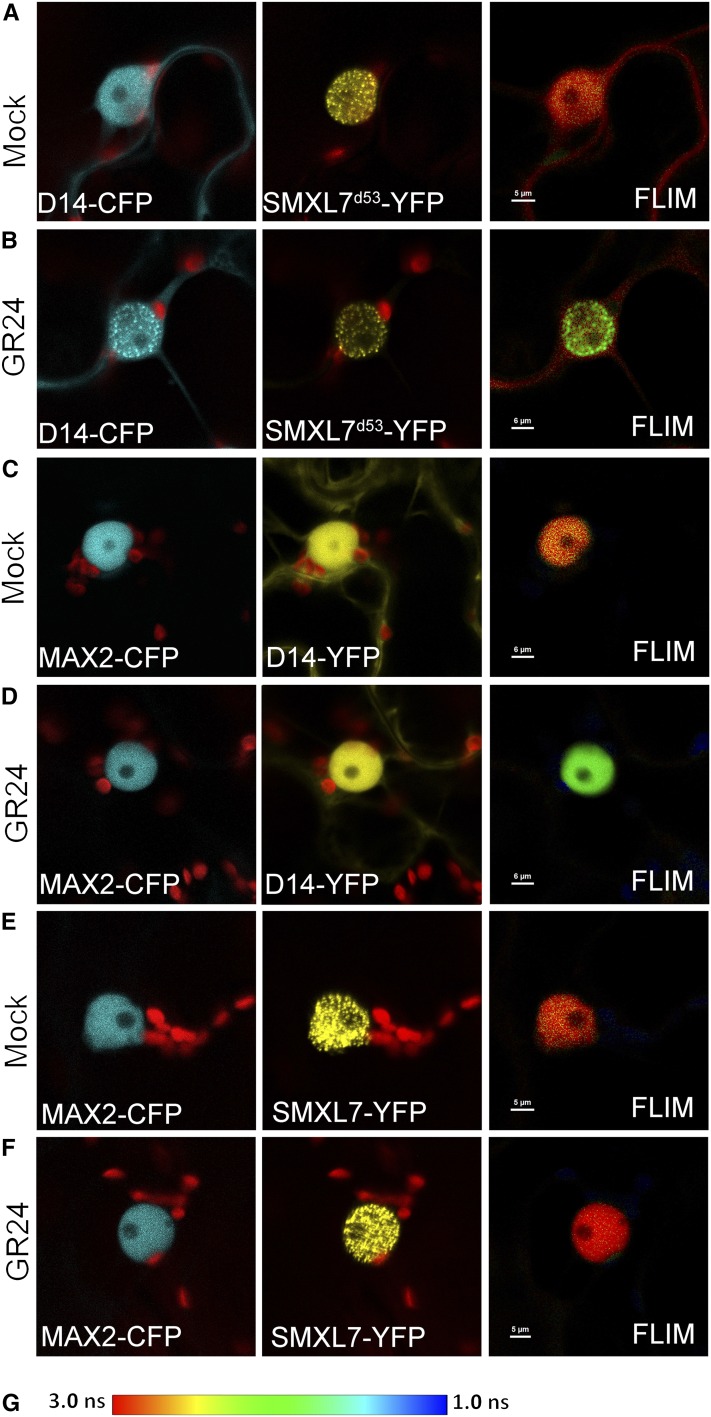
Several recent studies have shown that SMXL7/D53 proteins are ubiquitinated and targeted for degradation in response to D14-mediated SL perception in a MAX2-dependent manner (Jiang et al., 2013; Zhou et al., 2013; Umehara et al., 2015; Soundappan et al., 2015; Wang et al., 2015). Consistent with this degradation, SL-triggered interactions between D14 and SMXLs, and between D14 and MAX2, have been detected using various assays in several species, with evidence of a complex involving all three proteins (Hamiaux et al., 2012; Jiang et al., 2013; Zhou et al., 2013; Umehara et al., 2015; Wang et al., 2015). To assess these interactions further, we tested for protein-protein interactions between these components in N. benthamiana leaf epidermal cells using Förster resonance energy transfer (FRET) with fluorescence lifetime imaging microscopy (FLIM).
For the D14-CFP/SMXL7-YFP pair, the SMXL7 signal was rapidly lost upon GR24 treatment, so we again used the stabilized SMXL7d53 version of the protein, which allowed us to assess the interaction more easily. As expected based on previous reports (Jiang et al., 2013; Zhou et al., 2013; Wang et al., 2015), we observed FRET between cotransfected D14-CFP and SMXL7d53-YFP only upon rac-GR24 treatment (Figures 2A and 2B). The SMXL7 protein is clearly localized to nuclear speckles (Soundappan et al., 2015), and it primarily in these speckles that we detected FRET. D14 appears to be recruited to the speckles in response to GR24 treatment.
Figure 2.
SMXL7 Physically Interacts with D14 in a SL-Dependent Manner.
Detection of FRET in N. benthamiana epidermal cells doubly transfected with D14-CFP/SMXL7d53-YFP, MAX2-CFP/D14-CFP, and MAX2-CFP/SMXL7-YFP pairs, expressed from the 35S promoter. Colocalization of the acceptor and donor molecules is shown in the first and second panel in each row. The CFP donor was excited with using a 405-nm laser line, and emission from the YFP acceptor (indicating the occurrence of FRET) was monitored by FLIM (third panel in each row). The fluorescence lifetime of YFP (green) is shorter than CFP (red).
(A) and (B) FRET-FLIM analysis of D14-CFP and SMXL7d53-YFP in mock treated cells (A) or cells treated with 5 μM rac-GR24 (B)
(C) and (D) FRET-FLIM analysis of MAX2-CFP and D14-YFP in mock-treated cells (C) or cells treated with 5 μM rac-GR24 (D).
(E) and (F) FRET-FLIM analysis of MAX2-CFP and SMXL7-YFP in mock-treated cells (E) or cells treated with 5 μM rac-GR24 (F).
(G) Color scheme for FLIM analysis, indicating fluorescence lifetimes between 3 and 1 ns.
We also observed rac-GR24-dependent FRET between MAX2-CFP and D14-YFP. This occurred across the whole nucleus (Figures 2C and 2D). We tested the interaction between MAX2-CFP and SMXL7-VENUS and observed no interaction in the absence or presence of rac-GR24 (Figures 2E and 2F). In this case SMXL7 signal remained stable upon GR24 treatment, so we did not need to use SMXL7d53. These results suggest that cotransfected D14, but not MAX2, is needed for efficient SMXL7 degradation. We confirmed that using SMXL7d53 in this assay does not affect the lack of interaction with MAX2 (Supplemental Figure 2). Taken together, these data suggest that SMXL7 interacts only indirectly with MAX2, with D14 acting as a bridge to bring these components together. Our in planta data thus confirm the previously reported interactions among the Arabidopsis SL signaling proteins. Interestingly, we also detected FRET between SMXL7-YFP and SMXL6-CFP in N. benthamiana leaf cells, suggesting that there is dimerization among the SMXL proteins themselves (Supplemental Figure 2). Consistent with these observations, we observed colocalization of SMXL6-mCherry and SMXL7-VENUS fusion proteins in Arabidopsis roots and their simultaneous degradation in response to treatment with rac-GR24 (Supplemental Figure 2).
SMXL7 Variants Affect Protein Stability
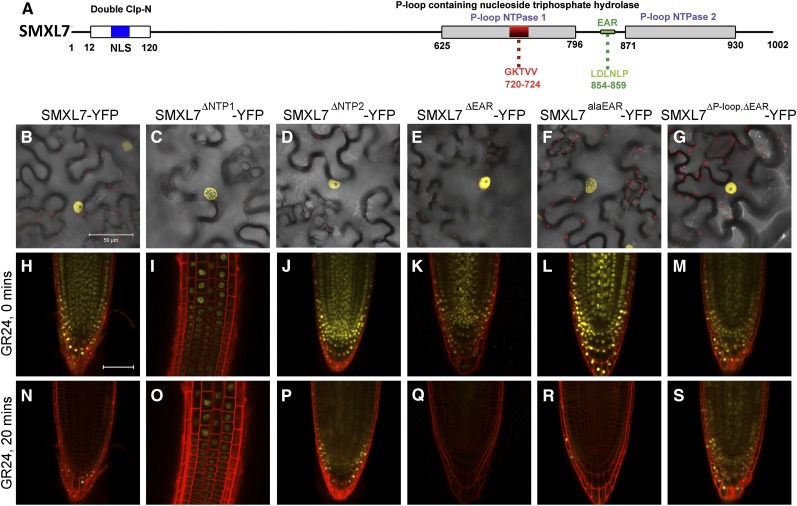
To gain further insights into mechanisms of SMXL7 action, we created several variants of the SMXL7 protein, lacking defined protein motifs (Figure 3; Supplemental Table 1). First, we mutated the first NTPase domain, deleting either the whole domain (SMXL7NTP1) or using the existing SMXL7ΔP-loop (Soundappan et al., 2015) or SMXL7d53 (see above) variants to specifically delete the P-loop motif. Second, we created a protein deletion that removed the entire second NTPase domain (SMXL7NTP2). Third, we modified the EAR motif (Leu-Asp-Leu-Asn-Leu-Pro) in SMXL7, either by deleting it (SMXL7ΔEAR) or by replacing the conserved leucine residues with alanines (Ala-Asp-Ala-Asn-Ala-Pro; SMXL7alaEAR), which has previously been shown to abolish its interaction with TPR2 in yeast two-hybrid assays (Soundappan et al., 2015). We also created two double deletions of both the P-loop and the EAR motif (SMXL7ΔP-loop,ΔEAR and SMXL7d53,alaEAR). We then tested how these SMXL7 variants affected nuclear localization by transiently expressing them in N. benthamiana epidermal cells. We observed that nuclear targeting is not obviously affected by any of these alterations (Figures 3B to 3G). We then assessed the SL response of these proteins in the roots of homozygous transgenic Arabidopsis lines expressing these fusion proteins from the 35S promoter. Consistent with the results in N. benthamiana, all of these variants showed strong nuclear localization (Figures 3H to 3M). As expected, we observed that the two new variants affecting the first NTPase domain resulted in severely compromised rac-GR24-induced degradation of the proteins (Figures 3I and 3O). Deletion of the entire NTP1 domain also seemed to reduce the accumulation of this protein in root tips, but it was possible to detect the protein in the transition zone (Figures 3I and 3O). In contrast, the SMXL7NTP2 variant retained the GR24-inducible degradation of the protein, while both EAR motif variants were both rapidly degraded in response to GR24 treatment (Figures 3J to 3L and 3P to 3R).
Figure 3.
SMXL7 Variants Affect Protein Stability.
(A) Structure of SMXL7 protein identifying conserved domains targeted in this study.
(B) to (G) Subcellular localization of SMXL-YFP (B), SMXLΔNTP1-YFP (C), SMXLΔNTP2-YFP (D), SMXLΔEAR-YFP (E), SMXLalaEAR-YFP (F), and SMXLΔP-loop,ΔEAR-YFP (G) in N. benthamiana epidermal cells, transiently expressed from the 35S promoter. Bar = 50 μm.
(H) to (S) Subcellular localization of SMXL-YFP ([H] and [N]), SMXLΔNTP1-YFP ([I] and [O]), SMXLΔNTP2-YFP ([J] and [P]), SMXLΔEAR-YFP ([K] and [Q]), SMXLalaEAR-YFP ([L] and [R]), and SMXLΔP-loop,ΔEAR-YFP ([M] and [S]) in the root tips of homozygous transgenic Arabidopsis lines expressing the fusion proteins from the 35S promoter, treated with 5 μM rac-GR24, at 0 min ([H] to [M]) and 20 min ([N] to [S]) after treatment. Bar = 100 μm.
SMXL7 Expression Levels Do Not Have Dose-Dependent Effects in the Wild Type
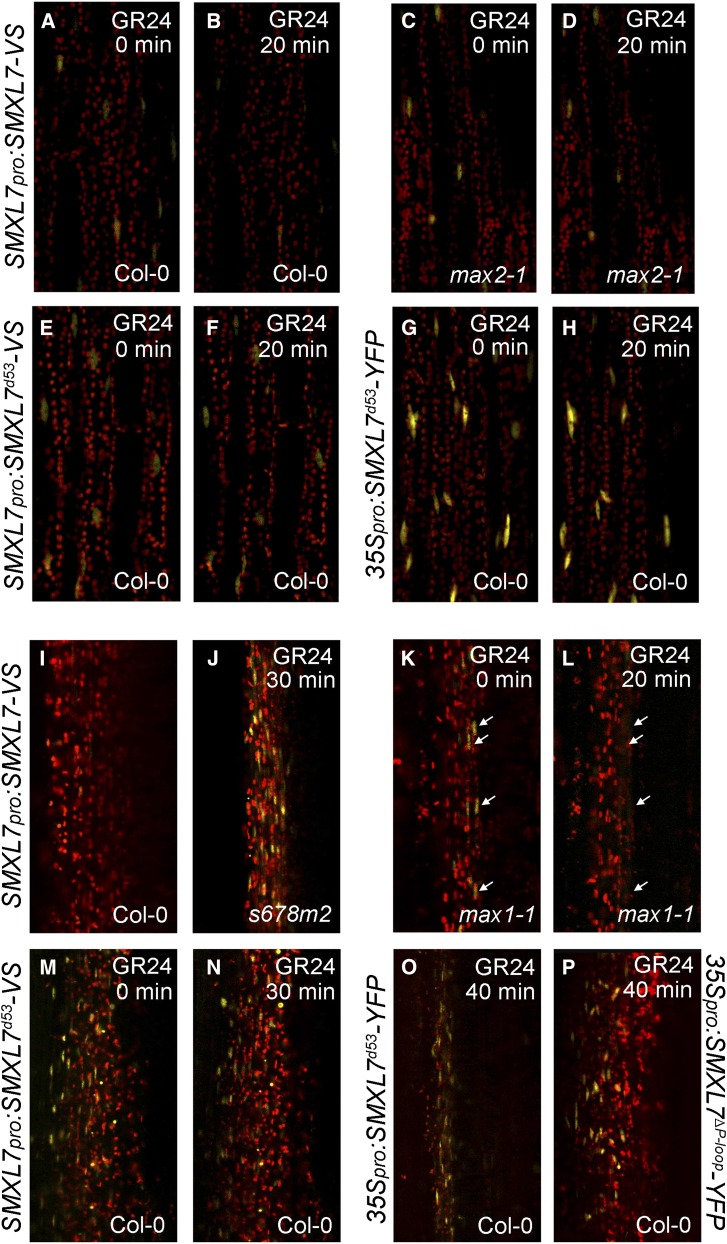
To test the function of these SMXL7 protein variants on different aspects of shoot architecture, we created homozygous transgenic lines in relevant genetic backgrounds (Table 1). We first assessed the effect of SMXL7 expression levels on shoot development using transgenic lines with altered SMXL7 copy numbers and expression levels (Table 1). For each line in each background, we took representative lines to homozygosity in the T3 generation. A suite of phenotypes affected by SL signaling in the shoot has previously been described, including leaf blade and petiole length, degree of branching, branch angle, height, and stem thickness (Smith and Waters, 2012; Soundappan et al., 2015), and we assessed these characteristics in our lines (Figure 4). We first confirmed that our constructs were expressed in leaves and stems and that the fusion proteins respond to rac-GR24 as expected. In leaves, we were able to observe robust expression of SMXL7 in petioles (Figure 4A). As expected, the SMXL7-VENUS signal was rapidly reduced in response to 5 μM rac-GR24 treatment in a wild-type background, but not in max2-1 (Figures 4A to 4D). In wild-type stems, SMXL7-VENUS was very difficult to detect (Figure 4I). However, in a max1-1 mutant background, which is defective in SL biosynthesis, we detected reliable expression, which was strongest in the vascular cambium (Figure 4K) and was greatly reduced by 5 μM GR24 treatment (Figure 4L). Again, SMXL7 was stabilized in a background containing the max2-1 mutation (Figure 4J).
Table 1. Arabidopsis Transgenic Lines.
| Background | Construct | Copy No. | GR24 Response | Total T1 Lines | T1 Lines with Representative Phenotype |
|---|---|---|---|---|---|
| Col-0 | SMXL7pro:SMXL7-VENUS | 4 | Y | 4 | 4 |
| s678 max2 | SMXL7pro:SMXL7-VENUS | 2 | Y | 5 | 5 |
| max2-1 | SMXL7pro:SMXL7-VENUS | 4 | N | 5 | 5 |
| max1-1 | SMXL7pro:SMXL7-VENUS | 4 | Y | Crossed | |
| Col-0 | SMXL7pro:SMXLd53-VENUS | 4 | N | 6 | 6 |
| s678 max2 | SMXL7pro:SMXL7d53-VENUS | 2 | N | 3 | 3 |
| s678 | SMXL7pro:SMXL7d53-VENUS | 2 | N | Crossed | |
| Col-0 | SMXL7pro:SMXL7ΔP-loop-VENUS | 4 | N | 4 | 3 |
| s678 max2 | SMXL7pro:SMXL7ΔEAR-VENUS | 2 | Y | 6 | 5 |
| s678 max2 | SMXL7pro:SMXL7alaEAR-VENUS | 2 | N | 4 | 4 |
| s678 max2 | SMXL7pro:SMXL7ΔP-loop,ΔEAR-VENUS | 2 | N | 4 | 3 |
| Col-0 | SMXL7pro:SMXL7d53,alaEAR-VENUS | 4 | N | 3 | 3 |
| s678 | SMXL7pro:SMXL7d53,alaEAR-VENUS | 2 | N | 2 | 2 |
| Col-0 | 35Spro:SMXL7-YFP | 4 | Y | 2 | 2 |
| s678 max2 | 35Spro:SMXL7ΔNLS-YFP | 2 | N | 5 | 5 |
| Col-0 | 35Spro:SMXL7d53-YFP | 4 | N | 6 | 2 |
| Col-0 | 35Spro:SMXL7ΔP-loop-YFP | 4 | N | 5 | 2 |
| s678 max2 | 35Spro:SMXL7ΔEAR-YFP | 2 | N | 5 | 3 |
| s678 max2 | 35Spro:SMXL7alaEAR-YFP | 2 | N | 5 | 2 |
| Col-0 | 35Spro:SMXL7ΔP-loop,ΔEAR-YFP | 4 | N | 3 | 3 |
List of stably transformed SMXL7 protein fusion lines in Arabidopsis used in this study. Constructs were transformed into the indicated genetic backgrounds; the total number of T1 lines analyzed is indicated, along with the number of lines showing the phenotypes discussed here. The copy number of SMXL7 in these lines and whether the transgenic whether the transgenic SMXL7 protein can be degraded in response to rac-GR24 are also indicated.
Figure 4.
SMXL7 Responds to SL Treatment in Leaves and Stems.
Red signal is chloroplast fluorescence. Yellow signal is SMXL7-YFP/VENUS.
(A) to (H) Response of SMXL7 variants in leaf petioles to 20 min treatment with 5 μM rac-GR24: SMXL7-VENUS in the Col-0 ([A] and [B]) and max2-1 ([C] and [D]) backgrounds; SMXL7d53-VENUS in Col-0 ([E] and [F]) and SMXL7d53-YFP in Col-0 ([G] and [H]). Expression from the SMXL7 promoter ([A] to [F]) or 35S promoter ([G] and [H]).
(I) Very low levels of SMXL7-VENUS in longitudinal hand sections through Col-0 stems.
(J) to (P) Response of SMXL7 variants expressed in longitudinal hand sections of stems to treatment with 5 μM rac-GR24 (treatment time indicated in panel); SMXL7-VENUS in smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2) (J) and max1-1 ([K] and [L]) backgrounds; SMXL7d53-VENUS in Col-0 ([M] and [N]); SMXL7d53-YFP in Col-0 (O) and SMXL7ΔP-loop-YFP in Col-0 (P). Expression from the SMXL7 promoter ([I] to [N]) or 35S promoter ([O] and [P]). White arrows indicate SMXL7 expressing nuclei before and after treatment with rac-GR24.
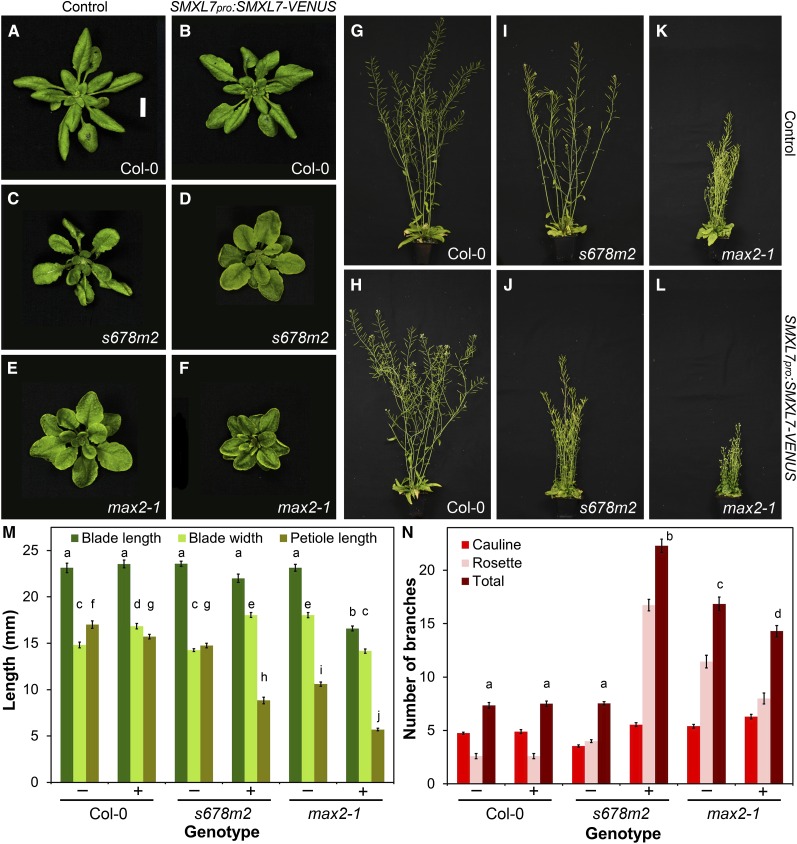
We quantified a range of shoot phenotypes in Col-0, smxl678 max2 (no SMXL7 activity), and max2 (overactive SMXL7) backgrounds as a baseline for assessing the function of SMXL7 variants (Figure 5) (Supplemental Figure 3). We found that expression of SMXL7pro:SMXL7-VENUS from its native promoter is sufficient to rescue all of the assessed smxl678 max2 phenotypes to an approximately max2 phenotype (Figures 5C, 5D, 5I, 5J, 5M, and 5N; Supplemental Figure 3). When this construct was expressed in a wild-type background, thereby increasing SMXL7 copy number, we found only minor effects on the assessed characteristics (Figures 5A, 5B, 5G, 5H, 5M, and 5N; Supplemental Figure 3). However, we found that introducing SMXL7pro:SMXL7-VENUS into a max2-1 SL signaling-deficient background produced clear additional effects on leaf development, in general exaggerating the effects of max2-1. In leaves, petiole length and blade length were reduced further compared with max2, but blade width was reduced and an additional phenotype of upward rolling of the leaf margins was observed (Figures 5E, 5F, and 5M). Plant height was reduced below that of max2 mutants, as was branch angle and stem diameter (Figures 5K and 5L; Supplemental Figure 3). However, we did not detect any further increase in primary shoot branching, probably because this is near-maximal in max2-1 (Figures 5K, 5L, and 5N). Furthermore, the plants showed a reduced fertility phenotype not observed in max2-1. Very similar phenotypes were observed in d14-1 SMXL7pro:SMXL7-VENUS and max1-1 SMXL7pro:SMXL7-VENUS (Supplemental Figure 3). In summary, unlike wild-type plants, genotypes deficient in SMXL7 degradation showed a clear dose-dependent response to increasing SMXL7 copy number in most tissues (e.g., smxl678 max2 < max2 < max2 SMXL7pro:SMXL-VENUS).
Figure 5.
Effect of SMXL7 Dose on Development.
(A) to (F) Rosette leaf morphology in Col-0 ([A] and [B]), smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2) ([C] and [D]), and max2-1 ([E] and [F]) untransformed ([A], [C], and [E]) or homozygous for SMXL7pro:SMXL7-VENUS ([B], [D], and [F]).
(G) to (L) Adult shoot morphology in Col-0 ([G] and [H]), smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2) ([I] and [J]), and max2-1 ([K] and [L]) untransformed ([G], [I], and [K]) or homozygous for SMXL7pro:SMXL7-VENUS ([H], [J], and [L]).
(M) Leaf blade length, blade width, and petiole length in the 7th leaf of Col-0, smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2), and max2-1 untransformed (−) or homozygous for SMXL7pro:SMXL7-VENUS (+). n = 11 to 16; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
(N) Number of cauline, rosette and total primary branches in Col-0, smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2), and max2-1 untransformed (−) or transformed with SMXL7pro:SMXL7-VENUS (+), measured at proliferative arrest. n = 20 to 24; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
Shoot Tissues Are Widely Sensitive to SMXL7 Activity
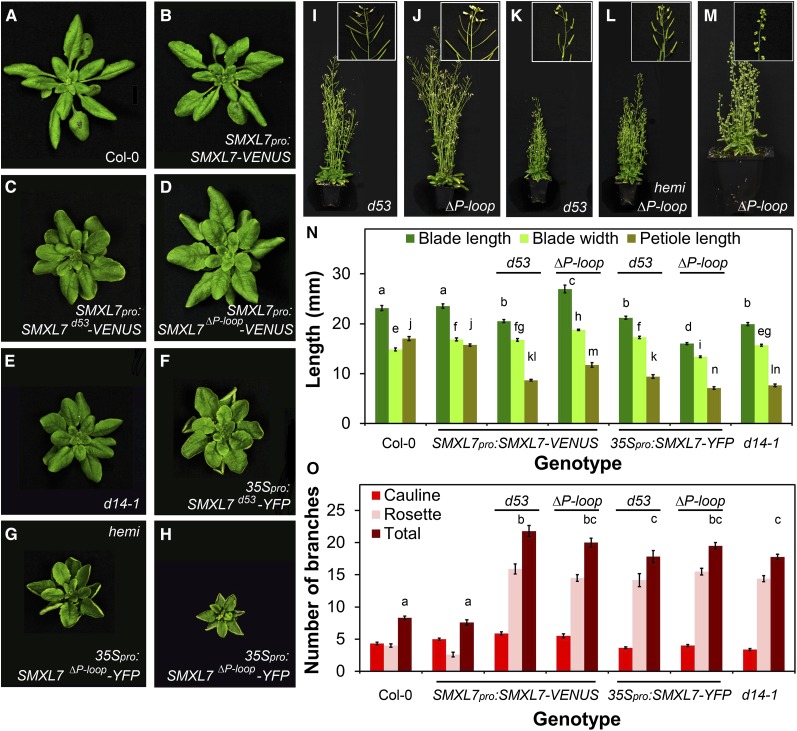
The results described above suggest that, in a wild-type context, MAX2-mediated degradation can buffer against increases in SMXL7 expression in the shoot; that is, wild-type plants are resistant to alterations in SMXL7 expression level. To probe the relationship between SMXL7 degradation and shoot architecture further, we examined the effects of introducing stabilized SMXL7 driven from the native SMXL7 promoter into relevant genetic backgrounds (Table 1). We first confirmed that these constructs resulted in SMXL7 accumulation in leaves and stems and that the protein was resistant to rac-GR24-induced degradation in these tissues (Figures 4E to 4H and 4M to 4P). We then quantified the phenotypic effects of expressing stabilized SMXL7 (Figure 6) (Supplemental Figure 4). In the wild type, this generally resulted in a clear phenocopy of SL-deficient or SL signaling mutants. The plants typically resembled d14-1 in leaf morphology, shoot morphology, and shoot branching levels (Figures 6A to 6D and 6I to 6J; Supplemental Figure 4). Expression of stabilized SMXL7 from the 35S promoter resulted in leaf morphology resembling max2-1 leaves expressing SMXL7pro:SMXL7-VENUS, with leaf margins that rolled upward, and exaggerated petiole and blade length phenotypes (compare Figures 6F to 6H with Figures 5E and 5F).
Figure 6.
Effect of SMXL7 Stabilization on Development.
(A) to (H) Rosette leaf morphology in Col-0 untransformed (A) or transformed with SMXL7pro:SMXL7-VENUS (B), SMXL7pro:SMXL7d53-VENUS (C), SMXL7pro:SMXL7ΔP-loop-VENUS (D), 35Spro:SMXL7d53-YFP (F), 35Spro:SMXL7ΔP-loop-YFP ([G], hemizygous; [H], homozygous), and in d14-1 (E).
(I) to (M) Adult shoot morphology (main picture) and reproductive morphology (inset) in Col-0 transformed with SMXL7pro:SMXL7d53-VENUS (I), SMXL7pro:SMXL7ΔP-loop-VENUS (J), 35Spro:SMXL7d53-YFP (K), and 35Spro:SMXL7ΔP-loop-YFP ([L], hemizygous; [M], homozygous).
(N) Leaf blade length, blade width and petiole length in the 7th leaf of untransformed Col-0, Col-0 transformed with SMXL7pro:SMXL7-VENUS, SMXL7pro:SMXL7d53-VENUS, SMXL7pro:SMXL7ΔP-loop-VENUS, 35Spro:SMXL7d53-YFP, 35Spro:SMXL7ΔP-loop-YFP (hemizygous), and in d14-1. n = 9 to 16; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
(O) Number of cauline, rosette, and total primary branches of untransformed Col-0, Col-0 transformed with SMXL7pro:SMXL7-VENUS, SMXL7pro:SMXL7d53-VENUS, SMXL7pro:SMXL7ΔP-loop-VENUS, 35Spro:SMXL7d53-YFP, 35Spro:SMXL7ΔP-loop-YFP (hemizygous), and in d14-1. n = 10 to 13; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
Overexpression of stabilized versions similarly resulted in qualitatively similar phenotypes to max2-1 SMXL7pro:SMXL7-VENUS, with reduced height and decreased branching angle relative to max2-1 (Figures 6K to 6M; Supplemental Figure 4). Furthermore, there was poor fertility in these lines, and in more extreme cases, a failure of reproductive organs to develop properly (Figures 6K to 6M). However, shoot branching was no greater than in max2-1 or d14-1 (Figure 6N). For all of the assessed phenotypes, the effect of overexpressing SMXL7d53 was often weaker than that of SMXL7Δp-loop. Furthermore, plants hemizygous for 35Spro:SMXL7Δp-loop-YFP had a less extreme phenotype than homozygous plants (Figures 6G, 6H, 6L, and 6M).
These results show that stabilization of SMXL7 in its endogenous expression domain results in a close phenocopy of SL mutants, similar to the d53 mutant in rice (Jiang et al., 2013; Zhou et al., 2013), but higher expression levels can give additional phenotypes. Some of these are only observed when the 35S promoter is used, and thus they may result from ectopic expression of SMXL7.
SMXL7 Activity Is Sufficient to Alter PIN1 Protein and Auxin Transport Levels
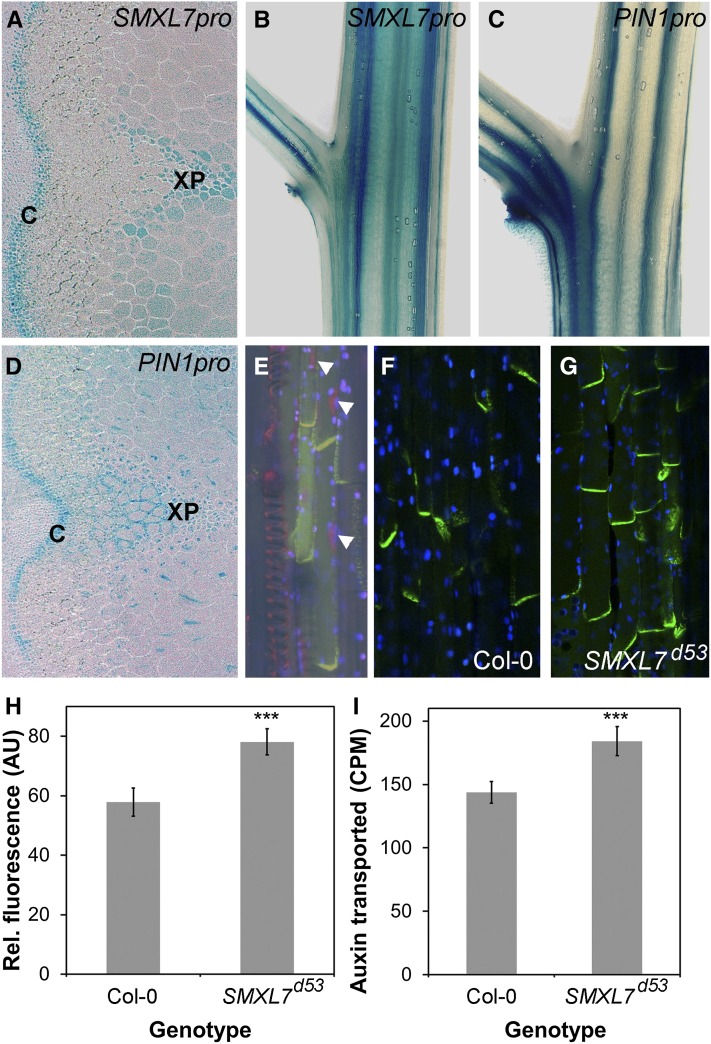
We previously demonstrated that SL signaling negatively regulates PIN1 protein accumulation at the basal plasma membrane of xylem parenchyma cells in the stem, which is associated with reduced stem auxin transport (Crawford et al., 2010; Shinohara et al., 2013). The smxl678 max2 quadruple mutant has a corresponding reduction in PIN1 relative to max2, as assessed by fluorescence of a PIN1-GFP fusion protein at the basal plasma membranes of xylem parenchyma cells in the stem (Soundappan et al., 2015). We therefore tested whether altering SMXL7 activity was sufficient to cause changes in PIN1 protein levels (Figure 7). We first tested whether the PIN1 and SMXL7 expression patterns show significant overlap in the stem using GUS reporter lines and found that there is indeed clear overlap in their expression patterns in vascular-associated tissues (Figures 7A to 7D), particularly in the cambium and xylem parenchyma, which are major sites of PIN1 expression in the stem (Bennett et al., 2006). Furthermore, when we crossed SMXL7pro:SMXL7-VENUS into a PIN1pro:PIN1-GFP reporter line, we found that the proteins appeared to be expressed in the same cells in the xylem parenchyma, in the nucleus and at the plasma membrane, respectively (Figure 7E). We then tested whether PIN1 protein levels are increased if SMXL7 activity is increased by crossing PIN1pro:PIN1-GFP into the SMXL7pro:SMXL7d53-VENUS background. Compared with control plants, PIN1-GFP protein levels at the basal plasma membrane of xylem parenchyma cells in the stem were indeed increased in this line (Figures 7F to 7H). Similarly, auxin transport through stem segments was increased in the SMXL7pro:SMXL7d53-VENUS line relative to the wild type (Figure 7I).
Figure 7.
SMXL7 Influences PIN1 and Auxin Transport.
(A) to (D) Expression of SMXL7pro:GUS ([A] and [B]) and PIN1pro:GUS ([C] and [D]) in transverse ([A] and [D]) and longitudinal ([B] and [C]) sections of Arabidopsis inflorescence stems. Small bold letters indicate tissues within the stem: C, cambium; XP, xylem parenchyma.
(E) Coexpression of SMXL7-VENUS (red, arrowheads) and PIN1-GFP (green) in xylem parenchyma cells of the Arabidopsis inflorescence stem in a line doubly homozygous for the fusion proteins expressed from their respective native promoters. The bright-field image is superimposed, showing cell boundaries and spiral thickening on the adjacent xylem. Blue indicates chloroplast autofluorescence.
(F) and (G) Expression of PIN1pro:PIN1-GFP in Col-0 and Col-0 homozygous for SMXL7pro:SMXL7d53-VENUS.
(H) Quantification of PIN1-GFP levels at the basal plasma membrane in xylem parenchyma cells of Arabidopsis inflorescence stems in Col-0 and Col-0 homozygous for SMXL7pro:SMXL7d53-VENUS. n = 8 plants per genotype, five membranes measured in each plant. Error bars indicate se; asterisks indicate significant difference from the wild type at the P < 0.005 level (t test).
(I) Transport of radiolabeled IAA through 18-mm stem segments of Col-0 and Col-0 homozygous for SMXL7pro:SMXL7d53-VENUS over a 6-h timeframe. n = 17 to 19; error bars indicate se. Asterisks indicate significant difference from the wild type at the P < 0.005 level (t test).
The EAR Motif Contributes to but Is Not Essential for SMXL7 Function
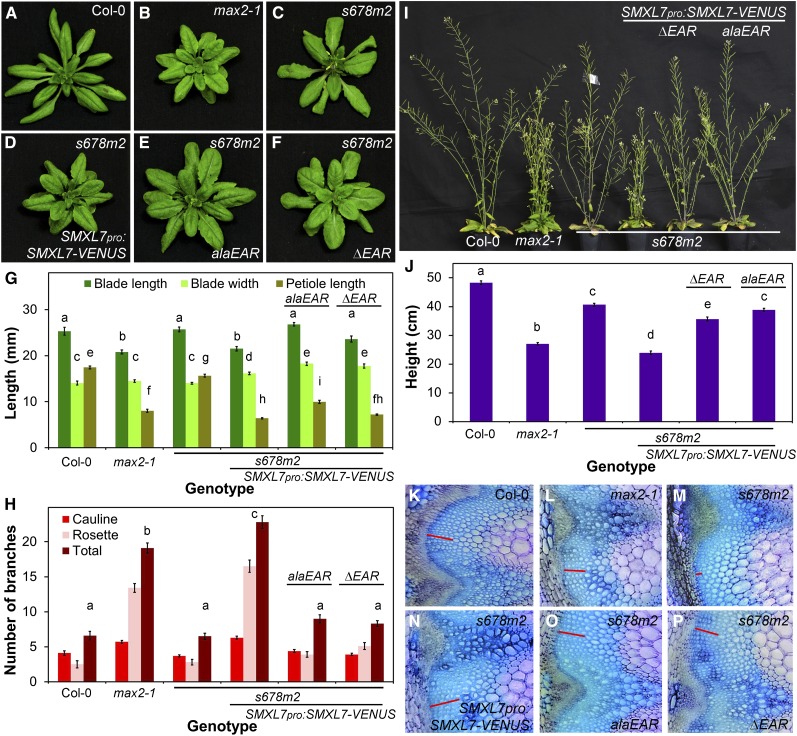
The presence of the EAR motif in SMXL7 and related proteins has led to speculation about a potential role for SMXL proteins in regulating transcription via interaction with TPL transcriptional repressor proteins (Smith and Li, 2014), but the contribution of the EAR motif to the function of SMXL proteins has been unclear. We thus used our constructs to assess the role of the EAR motif in SMXL7-mediated regulation of shoot architecture by comparing the ability of SMXL7pro:SMXL7alaEAR-VENUS and SMXL7pro:SMXL7ΔEAR-VENUS to restore the smxl678 max2 mutant phenotype toward the max2 phenotype, relative to control SMXL7pro:SMXL7-VENUS lines (Figure 8). With respect to leaf morphology, both SMXL7alaEAR and SMXL7ΔEAR cause a decrease in petiole length and increase in blade length, similar to wild-type SMXL7, resulting in max2-like leaves (Figures 8A to 8G). Expression of both SMXL7alaEAR and SMXL7ΔEAR causes a large decrease in branching angle, though not to the same extent as intact SMXL7 (Supplemental Figure 5). Expression of both these proteins is also associated with a slight, though not significant, increase in shoot branching relative to the smxl678 max2 and Col-0 backgrounds (Figure 8H). The effect is much less than that caused by intact SMXL7, which can restore branching to max2-like levels. Similarly, expression of both SMXL7alaEAR and SMXL7ΔEAR is associated with only a very small reduction in plant height, which is not significant for SMXL7alaEAR (Figure 8J). Here again, this contrasts with the effect of intact SMXL7 expression, which can fully restore the max2 height phenotype to smxl678 max2 plants (Figure 8J). In combination, these effects result in shoots in the EAR mutant lines intermediate in appearance between max2 and smxl678 max2 (Figures 8A to 8F and 8I).
Figure 8.
Phenotypic Effects of SMXL7 Are Partially EAR-Independent.
(A) to (F) Rosette leaf morphology in Col-0 (A), max2-1 (B), untransformed smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2) (C), or s678m2 homozygous for SMXL7pro:SMXL7-VENUS (D), SMXL7pro:SMXL7alaEAR-VENUS (E), and SMXL7pro:SMXL7ΔEAR-VENUS (F).
(G) Leaf blade length, blade width, and petiole length in the 7th leaf of Col-0, max2-1, untransformed smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2), or s678m2 homozygous for SMXL7pro:SMXL7-VENUS, SMXL7pro:SMXL7alaEAR-VENUS, and SMXL7pro:SMXL7ΔEAR-VENUS. n = 10; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
(H) Number of cauline, rosette, and total primary branches of Col-0, max2-1, untransformed smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2), or s678m2 homozygous for SMXL7pro:SMXL7-VENUS, SMXL7pro:SMXL7alaEAR-VENUS, and SMXL7pro:SMXL7ΔEAR-VENUS. n = 10; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
(I) Adult shoot morphology in (left to right) Col-0, max2-1, untransformed smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2), or s678m2 homozygous for SMXL7pro:SMXL7-VENUS, SMXL7pro:SMXL7ΔEAR–VENUS, and SMXL7pro:SMXL7alaEAR-VENUS.
(J) Height of the primary inflorescence stem in Col-0, max2-1, untransformed smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2), or s678m2 homozygous for SMXL7pro:SMXL7-VENUS, SMXL7pro:SMXL7alaEAR-VENUS, and SMXL7pro:SMXL7ΔEAR-VENUS, measured at proliferative arrest. n = 10; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
(K) to (P) Stem anatomy in Col-0 (K), max2-1 (L), untransformed smxl6-4 smxl7-3 smxl8-1 max2-1 (s678m2) (M), or s678m2 homozygous for SMXL7pro:SMXL7-VENUS (N), SMXL7pro:SMXL7alaEAR-VENUS (O), and SMXL7pro:SMXL7ΔEAR-VENUS (P) plants stained with toluidine blue. Red bar illustrates the depth of the interfascicular cambium layer. Images are representative of multiple independent samples.
The smxl678 max2 mutant (and smxl678) has a sprawling growth habit with increased branching angle and stems that often appear unable to bear their own weight (Figure 8I) (Soundappan et al., 2015). Since load-bearing in stems is typically associated with thickening of the interfascicular cambium (Ko et al., 2004), we investigated whether there was a defect in cambial formation in smxl678 max2. We observed that, indeed, there is very little interfascicular cambium present in smxl678 max2, a phenotype that can be rescued by expression of intact SMXL7-VENUS (Figures 8K to 8N). Interestingly, max2 and SL biosynthetic mutants also have reduced interfascicular cambium compared with Col-0, and treatment with SL can promote interfascicular cambium development (Agusti et al., 2011). It is thus very difficult to assess the ability of the SMXL variants to restore the smxl678 max2 phenotype toward the max2 phenotype. We therefore did not attempt detailed quantification. Nevertheless, it is clear that both SMXL7alaEAR and SMXL7ΔEAR are able to promote the development of cambium in a smxl678 max2 background (Figures 8O and 8P). A similar result is observed for the diameter of the primary inflorescence, which likely reflects cambium activity. Both max2 and smxl678 max2 have thinner stems than the wild type (Supplemental Figure 5). Here again, the increase in stem thickness in smxl678 max2 plants expressing SMXL7alaEAR and SMXL7ΔEAR demonstrates that these proteins are able to increase stem diameter.
We also tested the ability of 35Spro:SMXL7alaEAR-YFP to restore the smxl678 max2 mutant phenotype toward the max2 phenotype relative to a control 35Spro:SMXL7-YFP line (Supplemental Figure 6). We found no significant differences between SMXL7alaEAR and intact SMXL7 in their modest but statistically significant ability to reduce blade length, petiole length, or branch angle and to increase shoot branching toward max2 branch numbers (ANOVA + Tukey HSD, P > 0.05) (Supplemental Figure 6). Neither line shows a clear reduction in plant height relative to smxl678 max2 (Supplemental Figure 6). These data demonstrate that in general, SMXL7alaEAR-YFP is as active as SMXL7-YFP when expressed from the 35S promoter. Although these data seem somewhat inconsistent with those presented for expression from the SMXL7 promoter, the rescue by 35Spro:SMXL7-YFP is not complete, and so when expressed from the 35S promoter, SMXL7alaEAR is not being tested across the full range of SMXL7 activity.
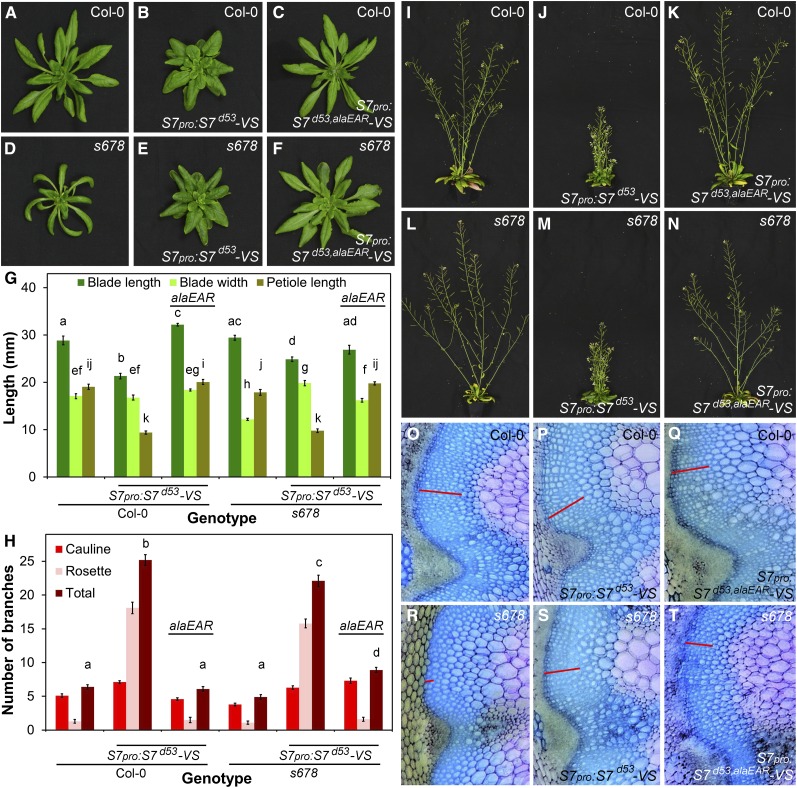
Loss of the EAR Motif Counteracts SMXL7 Stabilization
We next tested whether the EAR motif is required for the more extreme phenotypes associated with expression of stabilized SMXL7 (Figure 6) by comparing the effects of SMXL7pro:SMXL7d53,alaEAR-VENUS to SMXL7pro:SMXL7d53-VENUS in both Col-0 and smxl6-4 smxl7-3 smxl8-1 (smxl678) backgrounds (Figure 9; Supplemental Figure 7). Strikingly, mutation of the EAR motif completely abolishes the d14-like phenotypes caused by stabilized SMXL7d53 in a wild-type background, resulting in plants with essentially wild-type leaf shape, branch angle, branch numbers, and primary stem height and thickness (Figures 9A to 9C and 9G to 9K; Supplemental Figure 7). In isolation, this result could be interpreted as demonstrating that the protein lacking both a functional degron and EAR motif is simply nonfunctional, resulting in no phenotypic effects. However, analysis of the same constructs in the smxl678 background shows that this is not the case, and the protein is still functional, albeit with reduced activity. Although SMXL7d53,alaEAR does not induce strong d14-like phenotypes in a smxl678 background, it is still able to rescue the smxl678 phenotype toward a wild-type phenotype, or in some cases beyond wild-type and toward a d14-like phenotype (Figures 9D to 9F and 9L to 9N). The leaves of smxl678 SMXL7pro:SMXL7d53,alaEAR-VENUS have wild-type dimensions and full rescue of the reduced width of smxl678 leaves (Figure 9G). Both the increased branch angle and reduced stem width of smxl678 are restored to wild-type (Supplemental Figure 7). The number of branches is slightly increased (Figure 9H) and height of the primary stem is slightly decreased (Supplemental Figure 7) relative to both smx678 and the wild type, such that the plants have a more d14-like phenotype. The lack of interfascicular cambium in smxl678 is also at least partially rescued by SMXL7d53,alaEAR (Figures 9R to 9T). These data show that the SMXL7d53,alaEAR protein is active and functional with respect to all the phenotypes assessed, but the protein does not appear to have the hypermorphic activity required for gain-of-function phenotypes. Indeed, based on its ability to restore the smxl678 mutant to wild-type, the activity of this protein seems to be approximately equivalent to wild-type SMXL7. We also observed similar effects in 35Spro:SMXL7Δp-loop,ΔEAR-YFP relative to 35Spro:SMXL7Δp-loop-YFP, where the strong gain-of-function phenotypes caused by stabilization were negated by the loss of the EAR motif (Supplemental Figure 8).
Figure 9.
Loss of the EAR Motif Counteracts SMXL7 Stabilization.
(A) to (C) Rosette leaf morphology in untransformed Col-0 (A) or Col-0 transformed with SMXL7pro:SMXL7d53-VENUS (B) or with SMXL7pro:SMXL7d53,alaEAR-VENUS (C).
(D) to (F) Rosette leaf morphology in untransformed smxl6-4 smxl7-3 smxl8-1 (smxl678) (D) or smxl678 homozygous for SMXL7pro:SMXL7d53-VENUS (E) or SMXL7pro:SMXL7d53,alaEAR-VENUS (F).
(G) Leaf blade length, blade width, and petiole length in the 7th leaf of Col-0 and smxl678, either untransformed or homozygous for SMXL7pro:SMXL7d53-VENUS or SMXL7pro:SMXL7d53,alaEAR-VENUS. n = 10; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
(H) Number of cauline, rosette and total primary branches of Col-0 and smxl678, either untransformed or homozygous for SMXL7pro:SMXL7d53-VENUS or SMXL7pro:SMXL7d53,alaEAR-VENUS. n = 10; error bars indicate se; bars with the same letter are not significantly different from one another (ANOVA + Tukey HSD, P < 0.05).
(I) to (K) Adult shoot morphology in untransformed Col-0 (I) or Col-0 homozygous for SMXL7pro:SMXL7d53-VENUS (J) or with SMXL7pro:SMXL7d53,alaEAR-VENUS (K).
(L) to (N) Adult shoot morphology in untransformed smxl678 (L) or smxl678 homozygous for SMXL7pro:SMXL7d53-VENUS (M) or with SMXL7pro:SMXL7d53,alaEAR-VENUS (N).
(O) to (Q) Stem anatomy in untransformed Col-0 (O) or Col-0 homozygous for SMXL7pro:SMXL7d53-VENUS (P) or SMXL7pro:SMXL7d53,alaEAR-VENUS (Q) plants stained with toluidine blue. Red bar illustrates the depth of the interfascicular cambium layer. Images are representative of multiple independent samples.
(R) to (T) Stem anatomy in untransformed smxl678 (R) or smxl678 homozygous for SMXL7pro:SMXL7d53-VENUS (S) or SMXL7pro:SMXL7d53,alaEAR-VENUS (T) plants stained with toluidine blue. Red bar illustrates the depth of the interfascicular cambium layer.
DISCUSSION
Strigolactone Signaling Perception Is Predominantly Nuclear
The past few years have seen a rapid increase in our understanding of SL signaling, including the definition of a core pathway mediated by D14 (reviewed in Bennett and Leyser, 2014). The results presented here refine and elaborate upon the essential details previously established and lend support to key concepts in planta. We show SL-dependent physical association of D14 and SMXL7 in planta, as predicted by previous analyses (Jiang et al., 2013; Zhou et al., 2013; Umehara et al., 2015; Wang et al., 2015). Furthermore, we confirm that D14 also interacts with MAX2, but that there is no evidence of direct interaction between SMXL7 and MAX2. Previous reports have provided evidence for the nuclear localization for SMXL7-family proteins (Jiang et al., 2013; Wang et al., 2015; Soundappan et al., 2015), and we have demonstrated not only that this is the case in a wide variety of tissues, but also that this localization is required for both full SMXL7 function and its efficient degradation. While SMXL7 localization is difficult to detect outside the nucleus, we find that D14 and MAX2 are localized to both the nucleus and cytoplasm. However, our data suggest that, at least in the case of D14, it is the nuclear pool that is responsible for D14 function. The relevance of the cytoplasmic pool is unclear.
Our results also suggest that SMXL proteins may act as heterodimers, though again, whether this if of functional relevance is currently unclear. Another intriguing aspect suggested by our data is the apparent subnuclear localization of SMXL7 in distinct bodies or “speckles.” The SL-dependent association of D14 with SMXL7 involves recruitment of D14 to the speckles, as indicated by a clear FRET signal for the D14-SMXL7 interaction focused in these sites. In contrast, the association of MAX2 and D14 does not alter their subnuclear localization and occurs throughout the nucleus, suggesting that this interaction may occur independently of SMXL7. Nuclear speckles are generally viewed as storage and assembly areas for splicing factors, which are associated with transcriptionally active sites (Reddy et al., 2012). This could be interpreted as an association between SMXL7 and transcriptional regulation; however, evidence for this is currently lacking, and current models for transcriptional regulation by SMXL7 involve repression rather than activation. The molecular mode of action of SMXL7 is unclear. SMXL7 is a large protein with no obvious homology to transcription factors, yet it can bind to TPR proteins through its EAR motif (Soundappan et al., 2015; Wang et al., 2015), and so could potentially act as scaffold protein in chromatin assemblies. Further investigation is required in this respect, although as discussed further below, it is clear that the EAR motif is not absolutely required for SMXL7 activity.
Strigolactone Triggers SMXL7 Degradation across the Plant Body
The principal components of SL signaling are strongly expressed in vascular-associated tissues in both the shoot and root system (Stirnberg et al., 2007; Chevalier et al., 2014; Soundappan et al., 2015). However, results presented here also suggest that MAX2 and D14 are more broadly active than these expression patterns superficially suggest. For instance, we see rapid turnover of SMXL7 in all cells of the Arabidopsis root meristem in 35Spro:SMXL7-YFP lines in response to rac-GR24 treatment. This turnover is demonstrably D14- and MAX2-dependent (Soundappan et al., 2015), but expression of D14 and MAX2 is much higher in the stele than elsewhere (Stirnberg et al., 2007; Chevalier et al., 2014). The weak expression of MAX2 and D14 detected in other root tissues in transcriptomic studies (Brady et al., 2007) is apparently sufficient to mediate SMXL7 degradation. There may also be non-cell-autonomous accumulation of SL signaling proteins (Chevalier et al., 2014), but the addition of a strong nuclear localization signal to D14, which may limit its cell-to-cell movement, has apparently no effect on its function.
The results presented here show that SL-triggered MAX2-dependent SMXL7 degradation is also detectable in the shoot in petioles and stems. We have previously shown that SL signaling regulates the abundance of the PIN1 protein at the basal plasma membrane of xylem parenchyma cells (Bennett et al., 2006; Crawford et al., 2010; Shinohara et al., 2013). Here, we show that SMXL7 and PIN1 are expressed in the same vascular cambium and xylem parenchyma cells in the stem; furthermore, we have observed direct coexpression of SMXL7 and PIN1 within the same xylem parenchyma cell. The tools we have developed in this study will be useful in dissecting the relationship between SMXL7 degradation, PIN1 depletion from the plasma membrane, and the diverse SL-regulated shoot morphological phenotypes in more detail.
Plant Tissues Respond to a Wide Range of SMXL7 Doses
Our results suggest that there is broad competence to respond to SMXL7 in the shoot, even in tissues where SMXL7 is not normally highly expressed. For instance, overexpression of stabilized SMXL7 results in dramatic phenotypes, such as defective flower development, that do not occur when the same protein is expressed from its native promoter. It is not currently clear whether this is due to off-target effects or because the cellular activities targeted by SMXL7 occur outside the normal range of SMXL7 expression and are thus ectopically affected when SMXL7 is overexpressed.
Apart from these responses to ectopic expression of SMXL7, our data suggest that tissues are sensitive to SMXL7 protein levels over a very wide concentration range that extends both above and below the typical wild-type range. Expression of the wild-type SMXL7 protein is unable to take plants out of the wild-type phenotypic range, presumably because of the rapid degradation of the protein by the D14-MAX2 system. This idea is supported by the very low levels of detectable SMXL7-VENUS in SMXL7pro:SMXL7-VENUS lines in all tissues examined. In contrast, dramatic phenotypes result from expression of SMXL7 when it is stabilized, either by expression in a SL-insensitive or SL-deficient mutant background, or by deletion of the P-loop of its first NTPase domain. In these situations, there is a clear SMXL7 dose response for the shoot phenotypes we analyzed. For example, expressing SMXL7pro:SMXL7-VENUS in the max2-1, d14-1, or max1-1 backgrounds results in strong phenotypic effects that exceed those seen in SMXL7pro:SMXL7d53-VENUS. In both cases, the transgenically expressed SMX7 is stabilized, but max2-1 SMXL7pro:SMXL7-VENUS effectively has at least four stabilized SMXL proteins, SMXL6, SXML8, and endogenous and transgenic SMXL7, while SMXLpro:SMXL7d53-VENUS has only one stabilized protein, plus three labile SMXL proteins. Consistent with this dose sensitivity, the effect of SMXLpro7:SMXL7-VENUS in smxl678 max2 is much weaker than in max2, because in the former background, the SMXL7 transgene is the only copy of a SMXL6/7/8-type gene present. One interpretation of these data is that SMXL6 and SMXL8 act in a qualitatively similar manner to SMXL7 in the regulation of development, although they may make quantitatively smaller contributions, consistent with their respective loss-of-function phenotypes (Soundappan et al., 2015). Certainly, overexpression of stabilized SMXL6 generates at least some of the same phenotypic effects as we show for SMXL7 (Wang et al., 2015). Thus, the opposite end of this dose–response range occurs with the complete loss of SMXL6, SMXL7, and SMXL8, which also causes dramatic phenotypes that are in several cases quantitatively opposite to the effect of increasing SMXL7 activity.
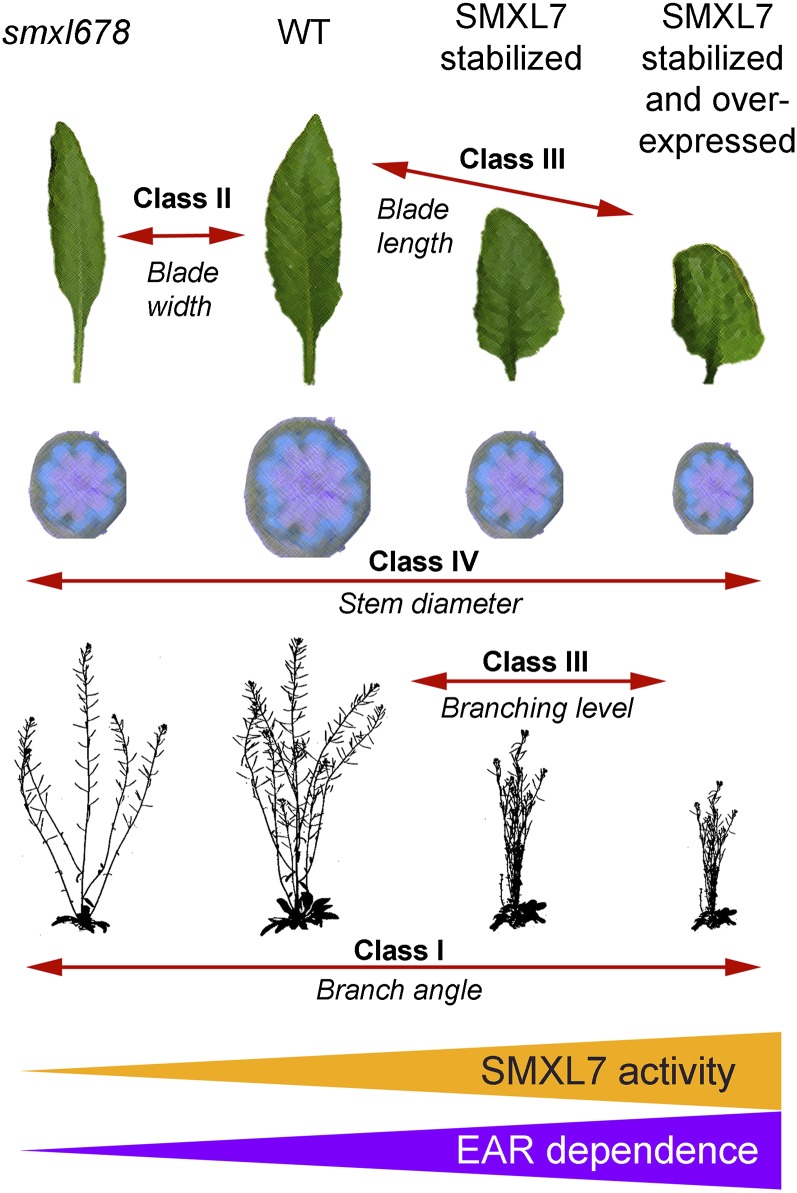
Altering SMXL7 activity can thus be conceived as shifting development along various phenotypic continua, as illustrated in Figure 10. These phenotypes can be categorized into four broad classes. In class I, branching angle appears to be sensitive across the full range of SMXL levels, with smxl678 mutants having the widest branch angles, while very high accumulation of SMXLs results in the lowest branch angles. In class II, blade width is dose-sensitive across the lower end of the range, but the response hits a maximum, with very high SMXL levels unable to increase blade width further. In class III, branch numbers, primary stem height, petiole length, and blade length are dose-sensitive at the higher end of the SMXL range. Reductions in SMXL level do not strongly affect these phenotypes, but effects are progressively stronger with increasing SMXL levels above those of wild-type plants. For shoot branching, maximum levels are reached at lower SMXL levels than, for example, for petiole length, but this is because all nodes have an actively growing branch, so further increases in primary branching levels are not possible. Phenotypes only observed at very high SMXL levels, such as leaf curling, can also be included in this category. Lastly, in class IV, stem width and interfascicular cambium development show a bell-shaped relationship with SMXL7 dose, with both low and high doses resulting in reductions compared with the wild type.
Figure 10.
SMXL7 Activity and EAR Dependency.
Summary diagram illustrating the effects of modulating SMXL7 activity on different shoot architectural traits. The smxl678 triple mutant represents a very low level of SMXL7-like activity relative to the wild type, while stabilized SMXL7 represents much higher activity than the wild type, further enhanced when SMXL7 is also overexpressed (orange triangle). Different phenotypes show different sensitivity to modulation of SMXL7 activity. Class I traits, for instance branch angle, are sensitive to SMXL7 across the whole range of activity. Class II traits, for instance blade width, are especially sensitive to the lower range of SMX7 activity, but not to the higher range. Class III traits, including shoot branching and leaf blade length and height, are especially sensitive to the higher range of SMXL7 activity, but not to the lower end of the range. Class IV traits do not show a linear relationship with SMXL7 activity. Phenotypic effects that occur at the higher range of SMXL7 activity are dependent on the presence of the EAR motif, but those at the lower end of the range do not require the EAR motif (purple triangle).
There are many possible explanations for the different dose–response curves for these phenotypes. It could be that all the phenotypes are mediated by the same targets downstream of SMXL7, but the resulting morphological effects are differently buffered against, or sensitive to, variations in the activity of targets. For example, it is possible that changes in branch angle and changes in plant height are both caused by SMXL7-mediated changes in PIN1 accumulation and, therefore, changes in auxin distribution, but that branch angle is more sensitive to these changes than height. Alternatively, there may be multiple downstream SMXL7 targets that are themselves differentially sensitive to SMXL7 levels. Our analysis of the EAR sensitivity of these phenotypes provides some insight on these alternatives.
Signaling Downstream of SMXL7: One or Many Targets?
SLs have very clearly defined effects on multiple shoot developmental traits. An intriguing question is whether these traits are all regulated through the same immediate target of SMXLs or whether there are multiple SMXL targets. There is good evidence that both quantitatively and qualitatively, most SL signaling in the shoot involves targeted degradation of SMXL6, SMXL7, and SMXL8 (Soundappan et al., 2015; Wang et al., 2015). However, events downstream of SMXL7 are currently much less clear.
Our analysis of the role of the EAR motif in different shoot phenotypes reveals clear differential sensitivity to the loss of the EAR motif among SL-regulated shoot phenotypes. For example, SMXL7 effects on leaf morphology and branch angle are relatively unaffected by removal of the EAR motif. In contrast, the effects on branch number and plant height are particularly sensitive to EAR mutation. These observations suggest the attractive hypothesis that branching and height are regulated by an EAR-dependent SMXL7 interaction, such as a transcriptional response mediated by TPL (or homologs). In contrast, SMXL7 effects on leaf morphology and branch angle could be mediated by interaction between SMLX7 and a partner other than TPL, which could result in nontranscriptional responses. However, despite differential sensitivity to EAR mutation, the EAR-mutated SMXL7 variants can still affect all the phenotypes tested to at least some extent. Furthermore, there is a strong correlation between those phenotypes that can be rescued by EAR-mutated SMXL7 and those phenotypes that are sensitive to low SMXL doses (Figure 10). For instance, the two most EAR-dependent phenotypes, branch number and primary stem height, are also the least affected by complete loss of SMXL6, SMXL7, and SMXL8. Conversely, the least EAR-dependent phenotypes, such as leaf width and branch angle, are sensitive to variation in SMXL levels at the low end of the range. This suggests that SMXL7 with a mutant EAR motif simply has low activity, and so is unable to induce phenotypes that depend on high levels of SMXL7 activity, such as increased shoot branch number, but they can affect phenotypes that can respond to low levels of SMXL7 activity, such as branch angle.
In this context, petiole length provides an interesting exception to the pattern described above. Like shoot branch number and plant height, petiole length is wild-type in the smxl678 background, but increasing levels of SMXL7 activity above wild-type result in progressively shorter petioles. However, unlike shoot branching and plant height, the short petiole length phenotypes associated with high SMXL7 activity in the max2 background are almost completely insensitive to EAR mutation. This suggests that not all the variation in the sensitivity of shoot phenotypes to EAR deletion can be explained simply by quantitative variation in the requirement for a single unified SMXL7 activity. It also argues against the possibility that deletion of the EAR motif has some rather nonspecific effect on SMXL7 abundance or activity. Rather, it supports the hypothesis that SMXL7 might regulate different downstream processes through different immediate targets. In this respect, SMXL7 and homologs can be considered as conceptually similar to DELLA proteins, which can act through various targets to regulate growth (Hauvermale et al., 2012). Indeed, this is a useful comparison in more general contexts. Although DELLAs were originally discovered as targets for gibberellin signaling, it is now clear that they act as growth regulatory hubs, and it is perhaps more useful to conceptualize them as the end point of the GA signaling pathway, rather than an intermediary in it. In a similar way, the emerging picture for SMXL7 and homologs is that these proteins have functions that are independent of SL. This might explain why both the smxl678 triple mutant and lines with stabilized SMXL7 have reduced interfascicular cambium development and stem width. It is possible that cambium development requires SMXL7 expression in tissues in which SL signaling does not occur but is inhibited by SMXL7 activity in tissues where there is SL signaling.
While the core signaling mechanism for SL is increasingly well characterized, we are only just beginning to understand the downstream pathways that lead to the diverse effects of SL on plant development. A clear priority will be to test the ability of SMXL7 variants to regulate specific immediate downstream targets such as PIN1 or potential transcriptional targets in the shoot.
METHODS
Plant Materials
The Arabidopsis thaliana max1-1, max2-1 (Stirnberg et al., 2002), max4-5, PIN1:GUS (Bennett et al., 2006), d14-1 (Waters et al., 2012), smxl6-4 smxl7-3 smxl8-1 max2-1, 35Spro:SMXL7-YFP, 35Spro:SMXL7ΔP-loop-YFP (Soundappan et al., 2015), and PIN1pro:PIN1-GFP (Xu et al., 2006) lines have been described previously. Where new genotypes were assembled by crossing relevant existing genotypes, the required homozygous lines were identified using visible, fluorescent, or selectable markers or using PCR genotyping.
Cloning
Supplemental Table 2 shows all of the constructs made in this study. The SMXL7, SMXL6, D14, and MAX2 coding sequences were amplified from cDNA and cloned into pDONR 221 using Gateway reactions using the primers listed in Supplemental Table 3. Variants of SMXL7 and D14 were created from pENTR 221 SMXL7/D14 with the Q5 Site-Directed Mutagenesis Kit (New England Biolabs), as described in Supplemental Table 1. The SMXL7, D14, and MAX2 promoters were amplified from genomic DNA and cloned into pDONR 1R4 using Gateway reactions, using the primers listed in Supplemental Table 3. CERULEAN and CITRINE tags were amplified from non-Gateway vectors and cloned in pDONR 2R3, using the primers listed in Supplemental Table 3. Existing entry vectors were used for 35Spro, GFP, CFP, YFP, VENUS, N7-VENUS (=NLS), mCherry, and GUS. Final assembly of constructs was performed using multisite Gateway reactions. Binary destination vectors for these reactions are listed in Supplemental Table 2.
MAX2:MAX2-GFP was assembled using conventional cloning. pBI101.3 was digested with XmaI/SacI to drop out GUS, which was replaced with GFP. The MAX2 promoter was amplified and cloned into the pBI101.3 GFP vector via SalI/BamHI digest. The MAX2 cDNA was then amplified and cloned into the pBI101.3 MAX2:GFP vector via a BamHI/XmaI digest. Primers are listed in Supplemental Table 3.
To express constructs transiently in Nicotiana benthamiana epidermal cells, Agrobacterium tumefaciens strain EHA105 carrying the constructs of interest, grown to an OD600 of 0.5, was infiltrated into leaves of 4- to 5-week-old plants (Bozkurt et al., 2011). Constructs were stably transformed into various Arabidopsis genetic backgrounds using the Agrobacterium floral dip method (Clough and Bent, 1998).
Plant Growth Conditions
Mature Arabidopsis plants for analysis were grown on Levington’s F2 compost, under a standard 16-h/8-h light/dark cycle (22°C/18°C) in controlled environment rooms with light provided by white fluorescent tubes (intensity ∼150 µmol m−2 s−1). Mature N. benthamiana plants for transient transformation were grown on Levington’s F2 compost, under a standard 16-h/8-h light/dark cycle in a greenhouse for 4 to 5 weeks. For axenic growth of seedlings, seeds were sterilized and stratified at 4°C for several days. Seedlings were grown on Arabidopsis thaliana salts (ATS) medium (Wilson et al., 1990) with 1% sucrose, solidified with 0.8% agar. Plates were oriented vertically in growth chambers with a 16-h/8-h light/dark cycle (22°C/18°C), with light provided by white fluorescent tubes (intensity ∼150 µmol m−2 s−1).
Leaf Measurements
The 7th leaf of each plant was marked with indelible marker at ∼4 weeks after germination. These leaves were provisionally measured at 35 d after germination and then measured again at 37 d after germination to confirm that growth of these leaves was arrested. The length of the petiole and the blade, and the maximum width of the blade were measured.
Branching, Height, and Stem Measurements
Branch angle was measured as the angle formed between branch and primary stem at the axil by imaging branches, and the angles in the images were quantified using ImageJ (Schneider et al., 2012). Plant height and the number of primary cauline and rosette inflorescences were measured at global proliferative arrest (∼7 weeks after germination). The diameter of the basal internode of the primary inflorescence was also measured at global proliferative arrest using a Keyence digital microscope.
Imaging of Reporter Fusions
Fluorescent reporter proteins in leaves and roots were imaged using confocal laser scanning microscopy with a Zeiss LSM780 imaging system. Excitation was performed using a 514-nm laser for YFP and VENUS, and a 405-nm laser for CFP and CER. For Arabidopsis leaves, samples were mounted on glass slides with or without 5 μM GR24 in liquid ATS solution, then imaged with a 20× lens. For N. benthamiana, leaves were infiltrated with Agrobacterium carrying the relevant reporter line (see above), along with 5 μM rac-GR24 (LeadGen Labs) or mock solution. After 2 h, leaf pieces were immobilized on Petri dishes with high-strength adhesive and imaged using a 20× water-immersion lens. For Arabidopsis roots, 3- to 5-d-old seedlings were mounted on slides with 10 μM propidium iodide (±5 μM rac-GR24) and then imaged using a 20× lens.
For imaging of fluorescent reporters in stems, hand sections were made through the vascular bundles of basal internodal stem segments of 6-week-old plants, and the slices were then embedded in agar plates. The samples were then covered with ATS medium (±5 μM GR24) and imaged by confocal laser scanning microscopy using a Zeiss LSM700 imaging system with 20× water immersion lenses. Excitation was performed using a 514-nm laser for YFP, 488-nm laser for GFP, and 405-nm laser for CFP and CER. PIN1-GFP quantification was performed on nonsaturated images, using Zeiss ZEN software. Fluorescence intensity in the GFP channel was measured in four or five basal plasma membranes per sample, in at least eight independent samples.
Staining for GUS activity was performed by incubating tissue in staining solution (0.1 M Na2HPO4, 5 μM K3[Fe(CN)6], 5 μM K4[Fe(CN)6], 0.05% Triton-X-100, and 0.5 mg/mL X-gluc [5-bromo-4-chloro-3-indolyl-β-d-glucuronide]) overnight at 37°C. The tissue was the destained in 70% ethanol and imaged using a Keyence digital microscope.
FRET Detection
To detect the interaction between SMXL7, D14, and MAX2, FRET was measured by FLIM using a Leica TCS SP8 confocal laser scanning microscope equipped with a time-correlated single-photon counting module. A pulsed diode laser (405 nm) tuned at 20 MHz was used to excite the donor alone (CFP) and the donor in the presence of acceptor (CFP +YFP). The emission from 450 to 490 nm was collected by a HyD detector in 256 × 256 pixel format. The acquired FLIM decay curve from regions of interest was fitted by two-exponential theoretical models using SymPhoTime software. The mean CFP lifetimes were calculated as the mean values of the fit function and analyzed using SymPhoTime software. At least 10 cells were tested for each FRET experiment.
The heterodimerization of SMXL7-YFP/SMXL6-CFP was tested in FRET assays by spectral imaging using a Zeiss LSM 780 confocal microscope with a 20× water immersion lens. To detect the fluorescence of the CFP donor and the YFP acceptor, excitation with a 405-nm argon laser line was performed, and lambda series were collected with the Zeiss META detector. The lambda stack of images was collected in an emission range of 415 to 620 nm with a 24-channel (9-nm interval) detector. The regions of interest in the nucleus were selected randomly. The normalized fluorescence intensity in each channel from different regions of interest was collected and processed using Zeiss LSM image Examiner software to create the spectral profiles.
Histology
Staining for GUS activity was performed as described above. Resin sections were then made of GUS stained tissues by fixing in FAA solution and dehydrating through an ethanol series prior to infiltration and subsequent embedding with embedding media (Leica HistoResin Standard Kit). The tissues were sliced into 7-μm sections with a Leica RM2255 microtome. For analysis of cambial activity, hand sections through the basal internode of the primary inflorescence were stained with toluidine blue O. Images were taken with a Keyence digital microscope.
Auxin Transport Assays
Auxin transport assays were modified from those described by Crawford et al. (2010). The 18-mm stem segments from basal internodes were excised, and the apical end was submerged in 30 μL ATS without sucrose (pH = 5.6), containing 1 μM 14C-IAA (American Radiolabeled Chemicals) in an Eppendorf tube. Stems were incubated for 6 h, and the basal 5 mm of the segment was then excised, placed in 200 μL scintillation liquid, and shaken overnight at 400 rpm prior to scintillation counting.
Accession Numbers
Sequence data from this article can be found in TAIR under the following accession numbers: D14 (At3g03990), MAX2 (At2g42620), PIN1 (At1g73590), SMXL6 (At1g07200), SMXL7 (At2g29970), and SMXL8 (At2g40130).
Supplemental Data
Supplemental Figure 1. SL signaling components function in the nucleus.
Supplemental Figure 2. SMXL7 physically interacts with D14 in a SL-dependent manner.
Supplemental Figure 3. Effect of SMXL7 dose on development.
Supplemental Figure 4. Effect of SMXL7 stabilization on development.
Supplemental Figure 5. Effects of SMXL7 are partially EAR-independent.
Supplemental Figure 6. Effects of SMXL7 are partially EAR-independent.
Supplemental Figure 7. Loss of the EAR motif counteracts SMXL7 stabilization.
Supplemental Figure 8. Loss of the EAR motif counteracts SMXL7 stabilization.
Supplemental Table 1. Constructs made in this study.
Supplemental Table 2. Cloning primers used in this study.
Supplemental Table 3. Protein variants made in this study.
Supplementary Material
Acknowledgments
This work was funded by the European Research Council (No. 294514–EnCoDe), by the Gatsby Foundation (GAT3272C), and by the Chinese Government Scholarship PhD Program (Sichuan Agriculture University) to Y.L. We thank David Nelson for very helpful discussions.
AUTHOR CONTRIBUTIONS
T.B., P.L., and O.L. designed the research. Y.L., S.W., and T.B. performed research. Y.L., S.W., and T.B. analyzed data. T.B. and O.L. wrote the article.
Glossary
- SL
strigolactone
- FRET
Förster resonance energy transfer
- FLIM
fluorescence lifetime imaging microscopy
- ATS
Arabidopsis thaliana salts
Footnotes
Articles can be viewed without a subscription.
References
- Aguilar-Martínez J.A., Poza-Carrión C., Cubas P. (2007). Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J., Herold S., Schwarz M., Sanchez P., Ljung K., Dun E.A., Brewer P.B., Beveridge C.A., Sieberer T., Sehr E.M., Greb T. (2011). Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. USA 108: 20242–20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T., Leyser O. (2014). Strigolactone signalling: standing on the shoulders of DWARFs. Curr. Opin. Plant Biol. 22: 7–13. [DOI] [PubMed] [Google Scholar]
- Bennett T., Sieberer T., Willett B., Booker J., Luschnig C., Leyser O. (2006). The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr. Biol. 16: 553–563. [DOI] [PubMed] [Google Scholar]
- Bozkurt T.O., Schornack S., Win J., Shindo T., Oliva R., Cano L.M., Jones A.M.E., Huitema E., van der Hoorn R.A.L., Kamoun S. (2011). Phytophthora infestans effector AVRblb2 prevents focal secretion of a plant immune protease. Proc. Natl. Acad. Sci. USA 108: 20832–20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S.M., Orlando D.A., Lee J.Y., Wang J.Y., Koch J., Dinneny J.R., Mace D., Ohler U., Benfey P.N. (2007). A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318: 801–806. [DOI] [PubMed] [Google Scholar]
- Braun N., et al. (2012). The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol. 158: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer P.B., Dun E.A., Ferguson B.J., Rameau C., Beveridge C.A. (2009). Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 150: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F., Nieminen K., Sánchez-Ferrero J.C., Rodríguez M.L., Chagoyen M., Hardtke C.S., Cubas P. (2014). Strigolactone promotes degradation of DWARF14, an α/β hydrolase essential for strigolactone signaling in Arabidopsis. Plant Cell 26: 1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Crawford S., Shinohara N., Sieberer T., Williamson L., George G., Hepworth J., Müller D., Domagalska M.A., Leyser O. (2010). Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913. [DOI] [PubMed] [Google Scholar]
- Domagalska M.A., Leyser O. (2011). Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 12: 211–221. [DOI] [PubMed] [Google Scholar]
- Greb T., Clarenz O., Schafer E., Müller D., Herrero R., Schmitz G., Theres K. (2003). Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 17: 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamiaux C., Drummond R.S., Janssen B.J., Ledger S.E., Cooney J.M., Newcomb R.D., Snowden K.C. (2012). DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 22: 2032–2036. [DOI] [PubMed] [Google Scholar]
- Hauvermale A.L., Ariizumi T., Steber C.M. (2012). Gibberellin signaling: a theme and variations on DELLA repression. Plant Physiol. 160: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagiyama M., Hirano Y., Mori T., Kim S.Y., Kyozuka J., Seto Y., Yamaguchi S., Hakoshima T. (2013). Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells 18: 147–160. [DOI] [PubMed] [Google Scholar]
- Kiba T., Kudo T., Kojima M., Sakakibara H. (2011). Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 62: 1399–1409. [DOI] [PubMed] [Google Scholar]
- Ko J.-H., Han K.-H., Park S., Yang J. (2004). Plant body weight-induced secondary growth in Arabidopsis and its transcription phenotype revealed by whole-transcriptome profiling. Plant Physiol. 135: 1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlen W., Charnikhova T., Liu Q., Bours R., Domagalska M.A., Beguerie S., Verstappen F., Leyser O., Bouwmeester H., Ruyter-Spira C. (2011). Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 155: 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., et al. (2013). DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.J., Bangerth F. (1999). Autoinhibition of indoleacetic acid transport in the shoots of two-branched peas (Pisum sativum) plants and its relationship to correlative dominance. Physiol. Plant. 106: 415–420. [Google Scholar]
- Liu W., Wu C., Fu Y., Hu G., Si H., Zhu L., Luan W., He Z., Sun Z. (2009). Identification and characterization of HTD2: a novel gene negatively regulating tiller bud outgrowth in rice. Planta 230: 649–658. [DOI] [PubMed] [Google Scholar]
- Müller D., Waldie T., Miyawaki K., To J.P., Melnyk C.W., Kieber J.J., Kakimoto T., Leyser O. (2014). Cytokinin is required for escape but not release from auxin mediated apical dominance. Plant J. 82: 874–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Matsui K., Hiratsu K., Shinshi H., Ohme-Takagi M. (2001). Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinkiewicz P., Crawford S., Smith R.S., Ljung K., Bennett T., Ongaro V., Leyser O. (2009). Control of bud activation by an auxin transport switch. Proc. Natl. Acad. Sci. USA 106: 17431–17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A.S., Day I.S., Göhring J., Barta A. (2012). Localization and dynamics of nuclear speckles in plants. Plant Physiol. 158: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N., Taylor C., Leyser O. (2013). Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 11: e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Li J. (2014). Signalling and responses to strigolactones and karrikins. Curr. Opin. Plant Biol. 21: 23–29. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Waters M.T. (2012). Strigolactones: destruction-dependent perception? Curr. Biol. 22: R924–R927. [DOI] [PubMed] [Google Scholar]
- Soundappan I., Bennett T., Morffy N., Liang Y., Stanga J.P., Abbas A., Leyser O., Nelson D.C. (2015). SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell 27: 3143–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanga J.P., Smith S.M., Briggs W.R., Nelson D.C. (2013). SUPPRESSOR OF MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiol. 163: 318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P., Furner I.J., Ottoline Leyser H.M. (2007). MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 50: 80–94. [DOI] [PubMed] [Google Scholar]
- Stirnberg P., van De Sande K., Leyser H.M. (2002). MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141. [DOI] [PubMed] [Google Scholar]
- Szemenyei H., Hannon M., Long J.A. (2008). TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386. [DOI] [PubMed] [Google Scholar]
- Umehara M., Cao M., Akiyama K., Akatsu T., Seto Y., Hanada A., Li W., Takeda-Kamiya N., Morimoto Y., Yamaguchi S. (2015). Structural requirements of strigolactones for shoot branching inhibition in rice and Arabidopsis. Plant Cell Physiol. 56: 1059–1072. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang B., Jiang L., Liu X., Li X., Lu Z., Meng X., Wang Y., Smith S.M., Li J. (2015). Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-like SMXL repressor proteins for ubiquitination and degradation. Plant Cell 27: 3128–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M.T., Nelson D.C., Scaffidi A., Flematti G.R., Sun Y.K., Dixon K.W., Smith S.M. (2012). Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 139: 1285–1295. [DOI] [PubMed] [Google Scholar]
- Wilson A.K., Pickett F.B., Turner J.C., Estelle M. (1990). A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol. Gen. Genet. 222: 377–383. [DOI] [PubMed] [Google Scholar]
- Xu J., Hofhuis H., Heidstra R., Sauer M., Friml J., Scheres B. (2006). A molecular framework for plant regeneration. Science 311: 385–388. [DOI] [PubMed] [Google Scholar]
- Zhao L.H., et al. (2013). Crystal structures of two phytohormone signal-transducing α/β hydrolases: karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res. 23: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., et al. (2013). D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 504: 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.