Abstract
Individuals with genotypes that code for reduced dopaminergic brain activity often exhibit a predisposition towards aggression. However, it remains largely unknown how dopaminergic genotypes may increase aggression. Lower-functioning dopamine systems motivate individuals to seek reward from external sources such as illicit drugs and other risky experiences. Based on emerging evidence that aggression is a rewarding experience, we predicted that the effect of lower-functioning dopaminergic functioning on aggression would be mediated by tendencies to seek the environment for rewards. Caucasian female and male undergraduates (N = 277) were genotyped for five polymorphisms of the Dopamine D2 Receptor (DRD2) gene, reported their previous history of aggression, and their dispositional reward-seeking. Lower-functioning DRD2 profiles were associated with greater sensation-seeking, which then predicted greater aggression. Our findings suggest that lower-functioning dopaminergic activity puts individuals at risk for violence because it motivates them to experience aggression’s hedonically rewarding qualities.
Keywords: dopamine, aggression, reward, DRD2, sensation-seeking
Introduction
Impulsive aggression is a vestige of our more violent, evolutionary history. Understanding how genetic influences perpetuate these ancient tendencies is crucial to fully comprehending these behaviors. Key questions include which genes are linked to violence and through what psychological phenotypes might they manifest their effects? In what follows, we test the hypothesis that genotypes that disrupt dopaminergic brain functioning motivate individuals to seek external sources of reward, which in turn, is associated with aggression.
Dopamine and Reward-Seeking
Dopamine is perhaps one of the most researched monoamine neurotransmitters. This neuromodulatory substrate is critical to the human experience of reward and reinforcement learning (Ikemoto, 2007). The dopamine D2 receptor (DRD2) gene encodes the post-synaptic D2 receptor in the brain, with particular effect in regions of the mesolimbocortical dopaminergic pathway (Ikemoto, 2007). DRD2 genotypes that code for reduced numbers of D2 dopamine binding sites result in reduced dopaminergic brain functioning in the striatum and prefrontal cortex (Blum et al., 1996; Comings & Blum, 2000). This reduced dopaminergic functioning in the brain translates directly to an impaired subjective experience of reward. According to the reward deficiency hypothesis, such a blunted sense of internally generated reward and positive affect motivates individuals to seek external sources of reward from their environment (Blum et al., 1996; Comings & Blum, 2000). This reward-seeking behavior often becomes a risk factor as it leads individuals to risky behaviors that deliver intense, though short-lived, experiences of dopaminergically-mediated reward. Specifically, lower-functioning dopaminergic systems often translate to impulsive, rewarding behavioral tendencies such as substance abuse (Comings et al., 1996), risky sexual behavior (Guo & Tong, 2006), and possibly aggression.
Dopamine and Aggression
Reduced dopaminergic activity is reliably linked to impulsive violence (for a review see Seo, Patrick, & Kennealy, 2008). Pharmacological manipulations of striatal dopamine functioning have produced aggression in mice (Couppis & Kennedy, 2008; Rodriguiz, Chu, Caron, & Wetsel, 2004). In humans, these same dopamine manipulations can also alter aggression (e.g., Rocca, Marchiaro, Cucuzza, & Bogetto, 2002). Among children, DRD2 polymorphisms that coded for reduced dopamine function were linked to aggressive behaviors such as bullying, anger expression, and cruelty (Zai et al., 2012). Following the developmental trajectory, individuals with such DRD2 genotypes showed a more violent path from adolescence to adulthood than their counterparts (Guo, Roettget, & Shih, 2007) and greater antisocial behavior if they had criminal fathers (DeLisi, Beaver, Vaughn, & Wright, 2009) or were raised in disadvantaged neighborhoods (Beaver, Gibson, DeLisi, Vaughn, & Wright, 2012). DRD2 genotypes are also associated with psychopathy (Wu & Barnes, 2013) and violent victimization among criminal offenders (Vaske, Wright, & Beaver, 2011). However, the link between DRD2 genotype and such criminality has previously failed to replicate (Kasiakogia-Worlley et al., 2011). Taken together, it appears that individuals whose DRD2 genotype codes for reduced, aberrant dopaminergic activity are also at risk for greater violence. Yet why would such a biological disposition towards reward-seeking behavior also predispose one towards aggression?
Aggression and Reward
Conventionally, aggression is thought to arise from negative feelings such as anger and pain (Berkowitz, 1989). However, people often perceive aggressive behavior as potentially cathartic and mood-improving (Bushman, Baumeister, & Phillips, 2001). Indeed, when aggression is in retaliation to a provocation it is reported as pleasant (Ramírez, Bonniot-Cabanac, & Cabanc, 2005). Further, such retaliatory aggression is associated with reward activity in the dopaminergic reward network of the brain: the dorsal (Krämer, Jansma, Tempelmann, & Münte, 2007) and ventral striatum (Chester & DeWall, in press). If aggression is truly a rewarding experience then it should show greater prevalence among individuals high in sensation-seeking, the facet of impulsivity that is the tendency to seek rewarding experiences in the environment (Whiteside & Lynam, 2001). Just so, there is a robust association between sensation-seeking and greater aggression (Derefinko, DeWall, Metze, Walsh, & Lynam, 2011; Joireman, Anderson, & Strathman, 2003). Converging evidence from both behavioral and neural science suggest that aggression is rewarding, thus rendering violence an appetitive option to individuals who tend to pursue rewarding experiences such as those with high functioning DRD2 genotypes.
An alternative prediction might be that DRD2 genotypes might exert their influence not through changes in reward-seeking tendencies, but through self-control. Dopaminergic circuitry in the brain extends into regions of the prefrontal cortex that subserve self-control processes (Baler & Volkow, 2006; Posner, Rothbart, Sheese, & Tang, 2007). As such, lower-functioning DRD2 genotypes are likely to also impair self-control by blunting the activity in the brain areas. This prediction meshes well with previous research suggesting that self-control, and its neural bases, has a substantial genetic component (Beaver, Connolly, Schwartz, Al-Ghamdi, & Kobeisy, 2013; Yancey, Venables, Hicks, & Patrick, 2013). Aggression often arises from impaired self-control (DeLisi & Vaughn, 2014; Denson, DeWall, & Finkel, 2012; Finkel, 2013; Gottfredson & Hirschi, 1990). Therefore the effect of low-functioning DRD2 genotype on greater aggression may occur through impaired self-control.
Present Study
Overview
Aggression’s rewarding nature and the DRD2 genotype’s ability to promote reward-seeking tendencies formed the basis for the central hypothesis of our study: that DRD2 genotypes which code for reduced dopaminergic functioning are linked to greater aggression through increased reward-seeking tendencies. To test this mediational hypothesis, we genotyped female and male undergraduates on five single nucleotide polymorphisms (SNPs) of the DRD2 gene that had previously been linked to aggression, reduced dopaminergic brain functioning, and/or sensation-seeking tendencies. After genotyping, participants reported their history of aggressive behavior and their dispositional tendency to seek rewarding experiences via the sensation-seeking facet of impulsivity (Whiteside & Lynam, 2001). We also measured trait self-control to test the alternative hypothesis that impaired self-control would mediate the DRD2-aggression link. These procedures were part of a larger, longitudinal project that aimed to understand the genetic basis for impulsivity and rash behavior1.
SNP selection
As part of the larger project, participants were genotyped for a number of SNPs from a number of genes. For the DRD2 and neighboring ANKK1 genes located on chromosome 11, participants were genotyped for 11 SNPs. Among these polymorphisms, five had been previously linked to aggression, reduced dopaminergic brain functioning, and/or sensation-seeking tendencies and thus were considered suitable for inclusion in our study. T allele carriers of the Taq1A rs1800497 SNP (located at position 112776038) show greater childhood aggression (Zai et al., 2012), aggressive side-effects of epilepsy treatments (Helmstaedter et al., 2013), exacerbated externalizing problems (Esposito-Smythers, Spirito, Rizzo, McGeary, & Knopik, 2009), greater endorsement of sensation-seeking behaviors and personality (Davis & Loxton, 2013), and reduced dopamine signaling in the striatum (Noble, Blum, Ritchie, Montgomery, & Sheridan, 1991). T allele carriers of the Taq1D rs1800498 SNP (located at position 112796798) exhibit greater features of Antisocial Personality Disorder (Nemoda et al., 2010) and childhood aggression (Zai et al., 2012). G allele carriers of the rs1799978 SNP (located at position 112851561) demonstrated greater childhood aggression (Zai et al., 2012). T allele carriers of the rs12364283 SNP (located at position 112776038) that exists on the promoter region of the DRD2 gene, demonstrate greater sensation-seeking behaviors and personality (Davis & Loxton, 2013), Finally, A allele carriers of the rs4581480 SNP (located at position 113453752) have shown reduced dopaminergic brain functioning in response to rewarding stimuli (Peciña et al., 2013). Together these five SNPs were used to test our proposed mediation model.
Methods
Participants
Participants were originally 376 female and male undergraduates recruited from introductory psychology courses and received both course credit and monetary incentives for participation. “High risk” participants were over-recruited to ensure sufficient variability in personal conduct issues (e.g., aggression). Participants were determined to be “high risk” if they fell within the upper quartile of a 12-item composite measure of conduct issues administered in a screening session prior to recruitment (quartiles determined separately for males and females). Due to the relatively small numbers of racial minorities in this sample and the variance in DRD2 allelic frequency among these groups, racial minorities were excluded from the sample to avoid population stratification. Participants were 277 Caucasian undergraduates (50.9% female; Age: M = 18.88, SD = 0.47) of whom approximately 25% were categorized as “high risk”. This sample size is consistent with previous research linking DRD2 genotypes with aggressive behavior (Zai et al. 2012) and is sufficient to conduct the bootstrapped mediation test that we propose below.
Materials
Physical aggression composite score
We focused our aggression measure on the form of physical aggression, as this is the form most associated with DRD2 genotypes (e.g., Zai et al., 2012). Items from two different measures were aggregated to form a composite measure of physical aggression. Items included those from the screening measure that assessed physical aggression (e.g., Before the age of 18, did you ever pick on smaller peers or threaten or tease those who were too scared to fight you?; Before the age of 18, did you ever take part in a fight where a group of your friends were against another group?), and three additional physical aggression items from the Crime and Analogous Behavior Scale (CAB; Lynam, Whiteside, & Jones, 1999), including: Ever been in a physical fight?; Ever hurt someone intentionally to the extent that they needed bandages or a doctor?; and Ever attacked someone with intent of seriously hurting or killing them? All five items from the aggression composite were scored ‘yes’ or ‘no’ (1 and 0, respectively). Values were then averaged across the five items to create a physical aggression index that could range from 0 to 1.
Self-Control Scale
The Self- Control Scale is a 36-item self-report questionnaire developed by Tangney, Baumeister, & Boone (2004) to assess individual differences in multiple aspects of self-control. Items are rated on a 5-point scale, from ‘Not At All Like Me’ to ‘Very Much Like Me’ (sample items: I am good at resisting temptation; I have a hard time breaking bad habits). Internal consistency on this measure was adequate in the present study (α = .91).
UPPS-P Impulsivity Scale
The UPPS-P (Lynam, Smith, Whiteside, & Cyders, 2006; Whiteside & Lynam, 2001) includes 59 items, scored on a 4-point Likert-style scale, that assess five distinct personality pathways to impulsive behavior: negative urgency (the tendency to behave rashly when distressed), lack of premeditation (failure to think about consequences of behavior before acting), lack of perseverance (failure to persist in tasks or obligations), sensation seeking (preference for stimulation and excitement), and positive urgency (tendency to act rashly when feeling positive emotion). Internal consistency is good to excellent for all of the subscales as shown in previous research (Whiteside, Lynam, Miller, & Reynolds, 2005) and in the present study (α = .82–.93).
Procedure
This study represents data from the first year of a 3-year longitudinal data collection in which data were collected annually. All data for the present study were obtained from the first year precluding any longitudinal analyses. All study procedures were reviewed and approved by the institution’s Office of Research Integrity and a federal Certificate of Confidentiality was acquired. After providing informed consent, participants were asked to voluntarily provide a saliva sample for genotyping. Then, participants completed a battery of computerized questionnaires that included a demographics questionnaire, the aggression items, the Self-Control Scale, and the UPPS-P impulsivity scale. Saliva samples were collected by using Oragene saliva kits (DNA Genotek) from the participants who signed additional consent forms for genotyping at the time of the experiment. The subjects were de-identified for genetic analysis, and only identification numbers were used to link genetic data with questionnaire data.
Genotyping
Five DRD2 gene SNPs were identified because of their associations with aggressive tendencies: rs1800497, rs1800498, rs1799978, rs12364283, and rs4581480. Linkage disequilibrium (LD) among these SNPs is low, with a maximum r2=0.19 between rs1800498 and rs4581480, with LD for most of the SNP pairs being less than 0.10. DNA was purified from saliva according to the manufacturer’s directions (DNA Genotek). DNA was quantified by UV absorbance at 260 nm, diluted to 10 ng/μl and the SNPs were genotyped by Sequenom MassARRAY iPLEX technology (W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University; http://ycga.yale.edu/).
Results
Descriptives
For genotyping results see Table 1. Genotypes were coded in an additive fashion for each of the five SNPs, in which 0 indicated the presence of no risk alleles, 1 indicated the presence of a single risk allele, and 2 indicated the presence of two risk alleles. All five SNPs were within Hardy-Weinberg equilibrium, ps = .07–.44. We then averaged across all five DRD2 genotypes to yield a multilocus dopamine profile for each participant that could range from 0 – 2 (as in Davis & Loxton, 2013). Averaging was used instead of summing as 13 participants were missing genotype data from one SNP, which would have artificially deflated their multilocus dopamine profile. The resulting multilocus dopamine profile exhibited substantial variance, M = 1.09, SD = 0.21. Across all participants, physical aggression levels (which could range from 0 to 1) showed substantial variability, M = 0.24, SD = 0.29, observed range = 0 – 1. Participants reported a relatively large amount of sensation-seeking, possible range = 1 – 4; M = 3.04, SD = 0.51, observed range = 1.58 – 4.00. Self-Control Scale data were missing from two participants. Participants reported a relatively large amount of self-control, possible range = 1 – 5; M = 3.23, SD = 0.56, observed range = 1.47 – 5.00.
Table 1.
List of DRD2 SNPs tested. Percentages represent the amount of participants (N = 277) of each genotype.
| SNP | Risk Alleles | Homozygous Risk | Heterozygous | Homozygous Non-Risk |
|---|---|---|---|---|
| rs1800497 | T > C | 3.2% | 32.5% | 64.3% |
| rs1800498 | T > C | 34.7% | 51.6% | 13.7% |
| rs1799978* | G > A | 90.2% | 9.8% | 0.0% |
| rs12364283 | C > T | 1.8% | 15.2% | 83.0% |
| rs4581480 | A > G | 84.1% | 14.8% | 1.1% |
Genotype data for this SNP were missing from 13 participants.
Mediation Model
A bias-corrected, bootstrapped mediation model (Preacher & Hayes, 2008) was fit to the data using the INDIRECT macro for SPSS and 1,000 bootstrap samples in which the multilocus dopamine profile was the independent variable, sensation-seeking was the mediator, and the aggression measure was the dependent variable. Gender was included as a covariate of the indirect effect.
The mediation model explained 17.0% of the variance in physical aggression, F(3,273) = 19,92, p < .001. Multilocus dopamine profiles were unassociated with physical aggression, B = .05, t(273) = 0.70, p = .482. Supporting our mediation hypotheses, multilocus dopamine profiles exhibited an indirect effect on physical aggression through greater levels of sensation-seeking (95% confidence interval: .003, .060; Figure 1). Specifically, multilocus dopamine profiles were marginally associated with greater sensation-seeking, B = .27, t(273) = 1.93, p = .054, which was in turn associated with greater physical aggression, B = .10, t(273) = 2.94, p = .004. Controlling for this indirect effect reduced the effect of multilocus dopamine profiles on physical aggression, B = .03, t(273) = 0.37, p = .714. As a covariate, being female was significantly associated with substantially less physical aggression, B = −.19, t(273) = −5.65, p < .001.
Figure 1.
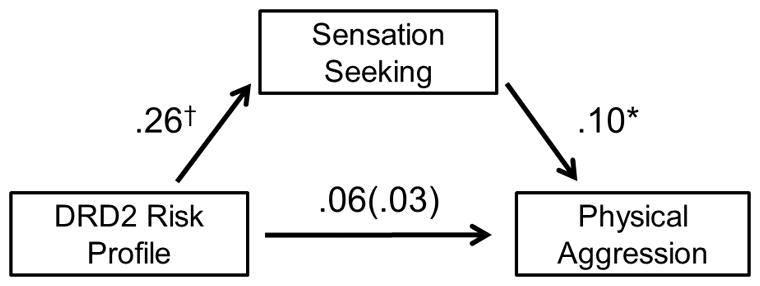
Bootstrapped mediation model whereby greater sensation-seeking mediated the positive association between multilocus DRD2 risk profiles and physical aggression (controlling for gender). Values represent partial, unstandardized regression coefficients. The parenthesized value represents the direct effect after controlling for the indirect path. †p < .055, *p < .005.
This mediation model was also fit with trait self-control as the mediator. Higher self-control scores were negatively associated with participants’ histories of aggression, B = −.12, t(271) = −4.20, p < .001. However, Self-Control Scale scores were unassociated with scores on the DRD2 risk profile, B = −.05, t(271) = −0.30, p = .763, nor did they mediate the effect of the DRD2 profile on aggression (95% C.I. −.028, .061.
Discussion
Genetic effects explain approximately half of the variance in human aggression (Miles & Carey, 1997). Understanding the genetic contributions to aggression is crucial for a full understanding of this behavior (Barnes, Boutwell, Beaver, Gibson, & Wright, 2014). Much research has exposed the genes that are linked to aggressive behavior, as well as the environmental cues that moderate their effects and the biological pathways through which they operate (Raine, 2008). Less research has focused on the psychological phenotypes (i.e., personality characteristics) that may be the mechanisms through which genes influence aggressive behavior (e.g., Chester et al., 2015). While we did not directly replicate the finding that DRD2 genotypes that coded for reduced dopamine functioning was directly associated with greater aggression (e.g., Zai et al., 2012), we observed data consistent with the prediction that this effect may occur through increased sensation-seeking. This finding adds to the growing literature that implicates the DRD2, DRD4, and DAT1 dopamine genes as potent predictors of antisocial behavior (e.g., Guo et al., 2007). Whereas previous research has examined the association between DRD2 functioning and these constructs among non-human animals, violent offenders, adolescents, and children, our study is one of the first to examine them among healthy, well-adjusted, young adults. These results suggest the need for further inquiry into the nature of the relationship between DRD2 and aggression.
Sensation-seeking, and not self-control, was the key factor in how dopamine functioning was connected to aggression. This model suggests that genetic predispositions for greater reward-seeking not only dispose individuals to seek out risky experiences such as illicit drug use (Comings et al., 1996) but to occasionally meet this goal in the form of violent altercations. Moreover, these results support the conceptualization of aggression as a rewarding behavior. The role of reward in aggression remains a nascent area of inquiry, but the ability of dopaminergic activity to predict this behavior suggests that, like substance abuse and risky sex, belligerence is reinforced by endogenous reward activity in the brain (e.g., Chester & DeWall, in press). Aggression interventions may benefit greatly from this putative role for positive affect and reward as motivational factors. Although some treatments recognize the reinforcing qualities of aggressive reactions and behaviors (e.g., McKay & Rogers, 2000), few deal directly with the role positive emotions may play in its inception. The behavioral and pharmacological treatments typically applied to reinforcing behaviors such as substance abuse may be useful to integrate into interventions for interpersonal violence and perhaps even tailored to those with genotypes that put them at such a risk.
Our study was limited in that our sample was comprised of psychology undergraduate students who are unlikely to possess substantially violent tendencies or to be at considerable risk to commit violent crimes. Another limitation was that our aggression measures used a dichotomous response scale in which participants were asked whether they had committed a given act, yes or no. Future research should include measures of the frequency and severity of aggression in order to measure aggression in a more sensitive way. It is possible that both of these limitations resulted in reduced variability in aggression and therefore served as a conservative test of our hypothesis, and our effects may be larger in more violent samples. Therefore, it is crucial to replicate and extend these findings among diverse, non-student, high-risk, and non-Caucasian populations and use more multidimensional measures of aggression (e.g., unprovoked vs. provoked; displaced vs. direct) and measures beyond self-report.
Previous research linking dopamine genotypes to antisocial behavior has shown substantial gene x environment interactions (e.g., DeLisi et al., 2009). Our study was limited in that we did not measure environmental aspects of our participants’ early lives, such as socioeconomic status or the aggressiveness of their parents. These factors likely moderate our observed effects. Future research will benefit greatly from establishing how early environmental influences impact not only the expression of genes in the form of aggression, but also the psychological phenotypes that mediate these effects. Such a moderated-mediation approach to behavioral genetics is likely to be a promising avenue.
Conclusions
Aggression, oft characterized as stemming from negative affect, may also be motivated by positive affect and hedonic reward (Chester & DeWall, in press). This novel concept suggests that genotypes that modulate the activity of the brain’s dopaminergic reward circuit might also impact sensation-seeking and thus aggressive behavior. We found support for this prediction, in that greater sensation-seeking was observed among individuals who possessed DRD2 genotypes that code for reduced dopaminergic functioning, and this heightened sensation-seeking predicted higher levels of aggression. Our findings reify the role of positive affect and reward in aggression and suggest that interventions target the potentially reinforcing nature of violence, which may serve to mollify this societal ill.
Acknowledgments
This research was supported by funding from the National Institutes of Health (grant # P50-DA05312) to University of Kentucky’s Center for Drug Abuse Research Translation and from the University of Kentucky’s Department of Behavioral Science. The authors also gratefully acknowledge research support from the National Institutes on Drug Abuse (grant # DA007304 and grant # T32DA035200) and National Center for Advancing Translational Sciences (grant # UL1TR000117) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. We also thank Richard Milich for his assistance in developing the study and in data collection.
Footnotes
The sensation-seeking and aggression data from this sample appear in another manuscript on MAOA genotypes and aggression (Chester et al., 2015). However, these data have not previously been analyzed in their relation to DRD2 genotypes.
References
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends in Molecular Medicine. 2006;12(12):559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Barnes JC, Boutwell BB, Beaver KM, Gibson CL, Wright JP. On the consequences of ignoring genetic influences in criminological research. Journal of Criminal Justice. 2014;42(6):471–482. [Google Scholar]
- Beaver KM, Connolly EJ, Schwartz JA, Al-Ghamdi MS, Kobeisy AN. Genetic and environmental contributions to stability and change in levels of self-control. Journal of Criminal Justice. 2013;41(5):300–308. [Google Scholar]
- Beaver KM, Gibson CL, DeLisi M, Vaughn MG, Wright JP. The Interaction Between Neighborhood Disadvantage and Genetic Factors in the Prediction of Antisocial Outcomes. Youth Violence and Juvenile Justice. 2012;10(1):25–40. [Google Scholar]
- Berkowitz L. Affective aggression: The role of stress, pain, and negative affect. In: Geen RG, Donnerstein E, editors. Human aggression: Theories, research, and implications for social policy. San Diego: Academic Press; 1989. pp. 49–72. [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJH, Cull JG, Comings DE. The D2 Dopamine Receptor Gene as a Determinant of Reward Deficiency Syndrome. Journal of the Royal Society of Medicine. 1996;89(7):396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman BJ, Baumeister RF, Phillips CM. Do people aggress to improve their mood? Catharsis beliefs, affect regulation opportunity, and aggressive responding. Journal of Personality and Social Psychology. 2001;81(1):17–32. [PubMed] [Google Scholar]
- Chester DS, DeWall CN. The pleasure of revenge: Retaliatory aggression arises from a neural imbalance towards reward. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsv082. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester DS, DeWall CN, Derefinko KJ, Estus S, Peters JR, Lynam DR, Jiang Y. Monoamine oxidase A (MAOA) genotype predicts greater aggression through impulsive reactivity to negative affect. Behavioural Brain Research. 2015;283:97–101. doi: 10.1016/j.bbr.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. In: van GGE, de Bruin JPC, Feenstra MGP, Pennartz CMA, Uylings HBM, editors. Progress in Brain Research. Vol. 126. Elsevier; 2000. pp. 325–341. [DOI] [PubMed] [Google Scholar]
- Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D. The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics. 1996;6(1):73–79. doi: 10.1097/00008571-199602000-00006. [DOI] [PubMed] [Google Scholar]
- Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology. 2008;197(3):449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- Davis C, Loxton NJ. Addictive behaviors and addiction-prone personality traits: Associations with a dopamine multilocus genetic profile. Addictive Behaviors. 2013;38(7):2306–2312. doi: 10.1016/j.addbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- DeLisi M, Beaver KM, Vaughn MG, Wright JP. All in the Family Gene × Environment Interaction Between DRD2 and Criminal Father Is Associated With Five Antisocial Phenotypes. Criminal Justice and Behavior. 2009;36(11):1187–1197. [Google Scholar]
- DeLisi M, Vaughn MG. Foundation for a temperament-based theory of antisocial behavior and criminal justice system involvement. Journal of Criminal Justice. 2014;42(1):10–25. [Google Scholar]
- Denson TF, DeWall CN, Finkel EJ. Self-Control and Aggression. Current Directions in Psychological Science. 2012;21(1):20–25. [Google Scholar]
- Derefinko KJ, DeWall CN, Metze AV, Walsh EC, Lynam DR. Do different facets of impulsivity predict different types of aggression? Aggressive Behavior. 2011;37(3):223–233. doi: 10.1002/ab.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito-Smythers C, Spirito A, Rizzo C, McGeary JE, Knopik VS. Associations of the DRD2 TaqIA polymorphism with impulsivity and substance use: Preliminary results from a clinical sample of adolescents. Pharmacology Biochemistry and Behavior. 2009;93(3):306–312. doi: 10.1016/j.pbb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel EJ. The I3 model: Metatheory, theory, and evidence. Advances in experimental social psychology. 2013;49:1. [Google Scholar]
- Gottfredson MR, Hirschi T. A general theory of crime. xvi. Stanford University Press; 1990. [Google Scholar]
- Guo G, Roettger ME, Shih JC. Contributions of the DAT1 and DRD2 genes to serious and violent delinquency among adolescents and young adults. Human Genetics. 2007;121(1):125–136. doi: 10.1007/s00439-006-0244-8. [DOI] [PubMed] [Google Scholar]
- Guo G, Tong Y. Age at first sexual intercourse, genes, and social context: Evidence from twins and the dopamine D4 receptor gene. Demography. 2006;43(4):747–769. doi: 10.1353/dem.2006.0029. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Mihov Y, Toliat MR, Thiele H, Nuernberg P, Schoch S, … Hurlemann R. Genetic variation in dopaminergic activity is associated with the risk for psychiatric side effects of levetiracetam. Epilepsia. 2013;54(1):36–44. doi: 10.1111/j.1528-1167.2012.03603.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Research Reviews. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joireman J, Anderson J, Strathman A. The aggression paradox: Understanding links among aggression, sensation seeking, and the consideration of future consequences. Journal of Personality and Social Psychology. 2003;84(6):1287–1302. doi: 10.1037/0022-3514.84.6.1287. [DOI] [PubMed] [Google Scholar]
- Kasiakogia-Worlley K, McQuillin A, Lydall GJ, Patel S, Kottalgi G, Gunwardena P, … Gurling HMD. Lack of allelic association between markers at the DRD2 and ANKK1 gene loci with the alcohol-dependence syndrome and criminal activity. Psychiatric Genetics. 2011;21(6):323–324. doi: 10.1097/YPG.0b013e3283458a68. [DOI] [PubMed] [Google Scholar]
- Krämer UM, Jansma H, Tempelmann C, Münte TF. Tit-for-tat: The neural basis of reactive aggression. NeuroImage. 2007;38(1):203–211. doi: 10.1016/j.neuroimage.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, Cyders MA. The UPPS-P: Assessing five personality pathways to impulsive behavior (Technical Report) West Lafayette, IN: Purdue University; 2006. [Google Scholar]
- Lynam DR, Whiteside S, Jones S. Self-reported psychopathy: A validation study. Journal of Personality Assessment. 1999;73(1):110–132. doi: 10.1207/S15327752JPA730108. [DOI] [PubMed] [Google Scholar]
- McKay M, Rogers PD. The Anger Control Workbook. New Harbinger Publications, Inc; Oakland, CA: 2000. [Google Scholar]
- Miles DR, Carey G. Genetic and environmental architecture on human aggression. Journal of Personality and Social Psychology. 1997;72(1):207–217. doi: 10.1037//0022-3514.72.1.207. [DOI] [PubMed] [Google Scholar]
- Nemoda Z, Lyons-Ruth K, Szekely A, Bertha E, Faludi G, Sasvari-Szekely M. Association between dopaminergic polymorphisms and borderline personality traits among at-risk young adults and psychiatric inpatients. Behavioral and Brain Functions. 2010;6:4. doi: 10.1186/1744-9081-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. ALlelic association of the d2 dopamine receptor gene with receptor-binding characteristics in alcoholism or gene ism. Archives of General Psychiatry. 1991;48(7):648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Peciña M, Mickey BJ, Love T, Wang H, Langenecker SA, Hodgkinson C, … Zubieta J-K. DRD2 polymorphisms modulate reward and emotion processing, dopamine neurotransmission and openness to experience. Cortex. 2013;49(3):877–890. doi: 10.1016/j.cortex.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Raine A. From Genes to Brain to Antisocial Behavior. Current Directions in Psychological Science. 2008;17(5):323–328. [Google Scholar]
- Ramírez JM, Bonniot-Cabanac MC, Cabanac M. Can Aggression Provide Pleasure? European Psychologist. 2005;10(2):136–145. [Google Scholar]
- Rocca P, Marchiaro L, Cocuzza E, Bogetto F. Treatment of borderline personality disorder with risperidone. The Journal of Clinical Psychiatry. 2002;63(3):241–244. doi: 10.4088/jcp.v63n0311. [DOI] [PubMed] [Google Scholar]
- Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behavioural Brain Research. 2004;148(1–2):185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Seo D, Patrick CJ, Kennealy PJ. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggression and Violent Behavior. 2008;13(5):383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High Self-Control Predicts Good Adjustment, Less Pathology, Better Grades, and Interpersonal Success. Journal of Personality. 2004;72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Vaske J, Wright JP, Beaver KM. A Dopamine Gene (DRD2) Distinguishes Between Offenders Who Have and Have Not Been Violently Victimized. International Journal of Offender Therapy and Comparative Criminology. 2011;55(2):251–267. doi: 10.1177/0306624X10361583. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. [Google Scholar]
- Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: A four-factor model of impulsivity. European Journal of Personality. 2005;19(7):559–574. [Google Scholar]
- Wu T, Barnes JC. Two dopamine receptor genes (DRD2 and DRD4) predict psychopathic personality traits in a sample of American adults. Journal of Criminal Justice. 2013;41(3):188–195. [Google Scholar]
- Yancey JR, Venables NC, Hicks BM, Patrick CJ. Evidence for a heritable brain basis to deviance-promoting deficits in self-control. Journal of Criminal Justice. 2013;41(5):309–317. doi: 10.1016/j.jcrimjus.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai CC, Ehtesham S, Choi E, Nowrouzi B, de Luca V, Stankovich L, … Beitchman JH. Dopaminergic system genes in childhood aggression: Possible role for DRD2. The World Journal of Biological Psychiatry. 2012;13(1):65–74. doi: 10.3109/15622975.2010.543431. [DOI] [PubMed] [Google Scholar]


