Abstract
Chlamydia trachomatis is the most common preventable cause of tubal infertility in women. In high-income countries, despite public health control efforts, C. trachomatis case rates continue to rise. Most medium and low-income countries lack any Chlamydia control program; therefore, a vaccine is essential for the control of Chlamydia infections. A rationally designed Chlamydia vaccine requires understanding of the immunological correlates of protective immunity, pathological responses to this mucosal pathogen, identification of optimal vaccine antigens and selection of suitable adjuvant delivery systems that engender protective immunity. Fortunately, Chlamydia vaccinology is facilitated by genomic knowledge and by murine models that reproduce many of the features of human C. trachomatis infection. This article reviews recent progress in these areas with a focus on subunit vaccine development.
Keywords: Chlamydia, vaccine, antigen, adjuvant, tissue-resident memory T cells, immunoproteomics
Chlamydia trachomatis genital tract infection is the most prevalent bacterial sexually transmitted infection in the United States and likely globally. The World Health Organization (WHO) estimates that over 130 million new cases of C. trachomatis infection occur each year including 68 million new infections in women, of whom up to one million become infertile following the development of pelvic inflammatory disease (PID) [1]. In 2013, there were 1.4 million cases of Chlamydia infection reported in the United States to the Centers for Disease Control and Prevention (CDC) [2]. Reported cases are thought to represent only 50% of the actual number of cases. The highest age-specific rate was found among persons aged 14–24 years where the prevalence was nearly three times that found in persons aged 25–39 years. A recent study estimated that the direct lifetime medical cost in United States from the estimated annual 2.8 million cases of C. trachomatis infections was greater than 500 million US dollars [3].
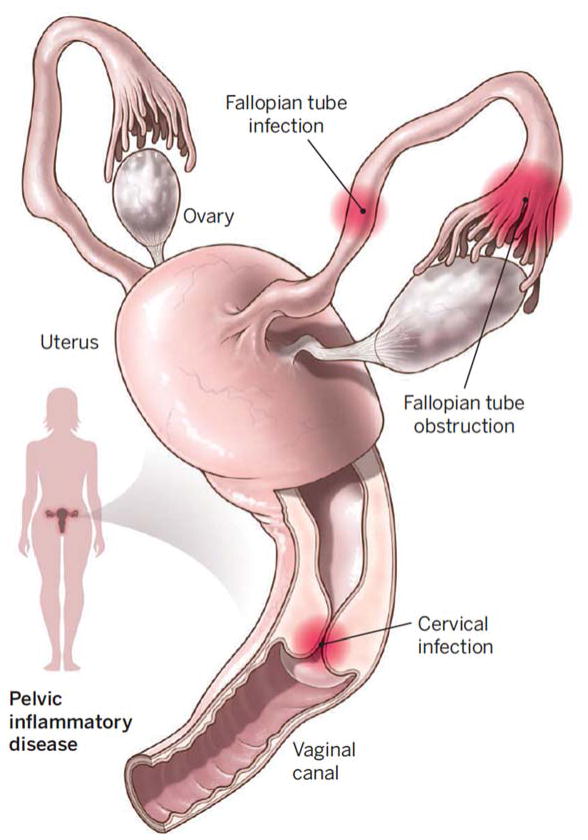
C. trachomatis is a Gram-negative obligate intracellular bacterium. This bacterium most commonly infects columnar epithelial cells of the endocervix of women and urethra of men to initiate local inflammation that causes mucopurulent cervicitis in women and nongonococal urethritis in men, respectively. Between 70–90% of women and 30–50% of men with infection are asymptomatic and consequently most people with C. trachomatis infection do not seek testing and treatment. Untreated infection is the source of onward transmission and in women can spread from the cervix to the upper reproductive tract (i.e., uterus, fallopian tubes) and cause PID, tubal factor infertility and ectopic pregnancy [4] (Fig. 1). It is estimated that about 15% of untreated Chlamydial infections lead to PID and 10 to 15% of PID results in infertility. A C. trachomatis vaccine that prevents cervical infection, PID and its sequelae has tremendous potential to improve women’s health worldwide.
Figure 1. Reproductive damage.
Pelvic inflammatory disease in women caused by C. trachomatis (sites of infection shown) can result in tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. Reproduced with permission from [37].
Public health measures, including national screening recommendations, partner identification, and treatment to control C. trachomatis infection, have been ongoing for over two decades in many developed countries. However, despite these public health efforts, case rates of C. trachomatis infection have been rising over the past two decades. The failure of public health control program may be due to early antibiotic treatment blunting the development of individual protective immunity resulting in reduction of herd immunity to C. trachomatis [5]. This phenomenon has been termed as the arrested immunity hypothesis. Experimental studies that used the mouse model of Chlamydia genital tract infection have demonstrated that early antibiotic treatment interrupts the establishment of protective immunity [6]. As antibiotics are not the ultimate solution, development of an effective Chlamydia vaccine is the essential next step to control this persistent health problem [7].
Chlamydia vaccine research started in 1959 when C. trachomatis was first isolated in egg yolk in China [8]. Shortly after human Chlamydia vaccine trials were conducted using chemically inactivated whole organism formulated with a variety of oil in water adjuvants. The best results of these trials showed that up to 70% of vaccinees were protected against the ocular disease, trachoma. However, immunity quickly waned over time and was no longer detectable three years after vaccination [9]. Vaccine studies in non-human primates demonstrated that the best protection required the highest amount of organism indicating that the inactivated C. trachomatis vaccine was of limited immunogenicity. Furthermore the best protection was seen when the same C. trachomatis strain was used for vaccine and challenge infection. Of concern, C. trachomatis infection in some primates resulted in more severe disease with worse inflammation post-vaccination demonstrating a role for incomplete immunity in enhanced inflammatory pathology [9,10]. However a critical analysis of human trachoma vaccine trials failed to demonstrate similar vaccine immunopathology in humans [11]. Nonetheless concern about exacerbated inflammatory pathology in previously vaccinated non-human primates has continued to be a barrier that impedes human Chlamydia vaccine trial research.
Contemporary C. trachomatis vaccine research has focused on the development of subunit vaccines, live attenuated C. trachomatis vaccines or whole inactivated organism vaccines. Among these three options, the whole inactivated organism vaccines are least attractive as they were the vaccine vehicles that elicited inflammatory pathology in earlier primate models of trachoma. Many promising studies have evaluated the use of plasmid-deficient C. muridarum as live attenuated vaccine in mouse models and recent studies have shown the efficacy of a plasmid-deficient C. trachomatis serovar A vaccine in the macaque trachoma model. Surprisingly, the rhesus macaque genital tract model revealed that plasmid-deficient C. trachomatis serovar D vaccine induced inflammation equivalent to wild-type and failed to prevent challenge infection. This observation raises concerns that plasmid-deficient genital strains may not be sufficiently attenuated to use as a live attenuated vaccine. This review therefore focuses on subunit vaccine research. Subunit vaccines hold the promise of a chemically defined product capable of eliciting a predefined protective immune response. Furthermore subunit vaccine development is based on the solid foundation of Chlamydia genomics [12,13]. A rational Chlamydia subunit vaccine design requires: 1) an understanding of the immunological correlates of protective immunity and pathological responses to this mucosal pathogen, 2) identification of effective vaccine antigens, 3) selection of a suitable adjuvant and delivery system to induce long lasting immunity, and, 4) appropriate animal models to test vaccine efficacy. This review summarizes recent progress in these four areas with a focus on concepts and approaches undertaken in our laboratory.
Immune correlates of protective immunity against C. trachomatis
Complete characterization of immunity to C. trachomatis genital tract infection and identification of immune correlates of protective immunity is important for the development of an effective subunit Chlamydia vaccine. However, since it is not ethically acceptable to withhold treatment for known infections in humans, it is impossible to directly define the natural course of C. trachomatis infection in humans and the risk of reinfection after primary infection that has naturally resolved. Experimental challenge with C. trachomatis in humans is fraught with ethical concerns regarding immunopathology. Therefore available human data of protective immunity to C. trachomatis is mostly indirect and inferred from epidemiological studies. Overall, the data from human studies reveal that a degree of partial protective immunity against reinfection is established after human genital infection of C. trachomatis [14]. Th1-type immune mechanism including CD4 lymphocytes and IFN-γ are correlates of acquired immunity in human C. trachomatis infections [15]. In 2005 we published a prospective study involving sex workers at high risk of C. trachomatis infection which showed that IFN-γ and IL-13 production by peripheral blood mononuclear cells (PBMCs) in response to C. trachomatis antigens was associated with reduced risk of incident infection, and thus could represent a phenotypic marker of human protective immunity to C. trachomatis [16]. Studies on women with C. trachomatis infection showed that Chlamydia-specific IgA in cervical secretions reduced the intensity of shedding [17]. However, anti-Chlamydia IgA in cervical mucus did not correlate with the risk of reinfection [16]. Direct evidence for protective mechanisms to Chlamydia genital infection has come from animal models including mouse and non-human primate models. The role of different immune components and their interactions in protective immunity to Chlamydia are described below.
CD4 T cells
Results from the mouse demonstrate that CD4 T cells play a dominant protective role in Chlamydia genital infection, which support results from human studies. The species-matched Chlamydia muridarum mouse model has identified two CD4 T cell-mediated mechanisms that are sufficient for clearing Chlamydia from the genital tract.
The first CD4 mechanism, discovered in the mid-1990s, is dependent on IFN-γ and inducible nitric oxide synthetase (iNOS) [18,19]. In this mechanism, Chlamydia specific CD4 T cells recognize infected epithelial cells and produce IFN-γ. IFN-γ and T cell-epithelial cell contact via ICAM-1 then induces high expression of epithelial iNOS that generates chlamydiacidal levels of nitric oxide [20]. This mechanism has been shown to be functional in both mouse and human epithelial cells. In recent murine vaccine studies our lab demonstrated that multi-functional CD4 T cells that co-secrete IFN-γ and TNF-α was a better correlate of immunity against C. muridarum infection than CD4 T cells that secreted IFN-γ alone [21]. Although the requirement for multifunctional Th1 is not well understood, it is possible that IFN-γ and TNF-α have a synergistic effect in the induction of iNOS and/or that multifunctional Th1 T cells possess a more robust degranulation phenotype compared to Th1 T cells producing only IFN-γ. Consistent with the importance of multiple cytokines, Johnson et al. [22] showed that treatment of murine epithelial cells with IFN-γ alone was not sufficient to terminate C. muridarum replication, while treatment of murine epithelial cells with activated T cell supernatants was a potent terminator of C. muridarum replication via an iNOS dependent mechanism
The second CD4-mediated Chlamydia replication termination mechanism was identified by Johnson’s group in 2010 [23] and is dependent on a subset of CD4 T cells that express a granule associated protein called Plac8. This mechanism like iNOS dependent immunity relied on T cell-epithelial cell contact followed by T cell degranulation and was only seen in Chlamydia-specific CD4 T cells that expressed Plac8 [22]. The discovery of the Plac8-dependent mechanism resolved a longstanding mystery in the Chlamydia immunobiology that iNOS knockout mice were not significantly compromised in their ability to clear C. muridarum genital tract infections [24]. This may be because iNOS knockout mice still had the Plac8 clearance mechanism in place to clear C. muridarum genital tract infections. This was supported by the observation that Plac8 knockout mice were modestly compromised in their ability to clear C. muridarum genital tract infection at late points (> 3 weeks) after infection [22]. When Plac8 knockout mice were treated with an iNOS inhibitor (functionally a dual Plac8 and iNOS knockout) they were profoundly unable to clear C. muridarum genital infections over an 8 week period. All Chlamydia specific Th1 cells seem to possess iNOS-dependent clearance mechanism. However, only a subset of Chlamydia Th1 cells express Plac8 (CD4Plac8) capable of utilizing both degranulation-dependent and iNOS-dependent clearance mechanisms. Based on these data, CD4Plac8 cells that secrete IFN-γ and TNF-α may be the long sought biomarker for protective immunity. Further investigation on the role of this novel specific CD4 T cell subpopulation in natural and vaccine-generated immunity needs to be undertaken. Importantly the Plac8 mediated Chlamydia inhibition has been demonstrated only in the mouse model and needs to be identified in humans as well.
CD8 T cells
Although Chlamydia infections induce both CD4 and CD8 immune responses in humans and mice, the role of CD8 T cells in protective immunity is not clear. In the murine model, it is generally observed that CD8 T cells are not necessary for clearing genital tract infection or protecting against reinfection [25,26]. In contrast, they seem to contribute significantly to upper genital tract pathology [27,28] and infertility [29] at least in the murine model. There is a possibility that the protective role mediated by a subset CD8 T cells is redundant in the presence of CD4 T cell protective immunity. An early study reported that adoptive transfer of a multifunctional Chlamydia-specific CD8 T cell clone into chronically infected nude mice cleared C. muridarum infection from the genital tract [30]. A recent non-human primate study reported that CD8 T cells play an important role in live-attenuated trachoma vaccine-mediated protective immunity [31]. This study showed that CD8 T cells from protected macaques exhibited proliferation against Chlamydia antigen and that depletion of CD8 T cells completely abrogated protective immunity. Polyfunctional CD8 T cells have also been shown to better predict protection against HIV infection [32] and the HIV-specific responses are associated with certain HLA class I [33].
Tissue-resident memory T cells (Trm)
Protective T cells need to be at the site of infectious challenge in order to provide immunity. Given that Chlamydia is a mucosal pathogen, this implies that such cells need to be resident in tissue beneath the mucosa. During infection or immunization, a subpopulation of effector T cells seed both lymphoid tissue and non-lymphoid tissue where these cells differentiate and develop into tissue-resident memory T cells with distinct phenotype and function for long-term residency and survival. Such tissue-resident memory T cells are on standby and capable of immediately recognizing pathogens that enter through the local tissues and mount robust local immune responses to limit the spread of infection. An understanding of the biology of Trms should provide important insights into the protective immune mechanisms at the site of pathogen entry, which will be a basis for designing better vaccines against many mucosal pathogens including Chlamydia.
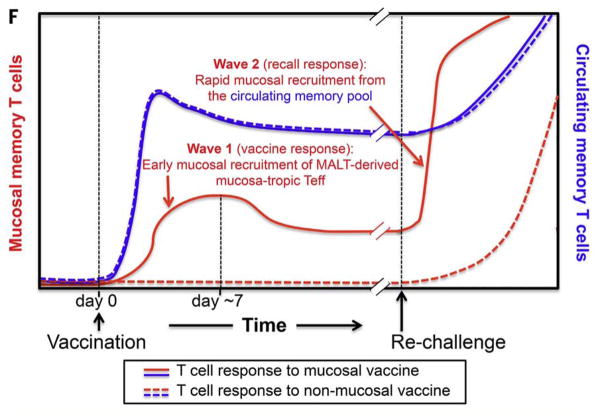
Trm was first described in the Chlamydia field in 1990 when Kiviat et al. [34] observed lymphoid follicles with T cells and plasma cells in endometrial biopsies of women with C. trachomatis infections. Morrison et al. [35] conducted an in situ immunohistochemistry study in the C. muridarum model and saw predominantly clustered CD4 T cells with few CD8 T cells and B cells present in the genital tract long after infection had resolved (day 70). These observations provide the best histopathological correlate of protective immunity. Recently, Stary et al. [36,37] demonstrated that two waves of protective CD4 Th1 cells develop after mucosal immunization (either intrauterine or intranasal application) with UV-inactivated C. trachomatis organism formulated with a cationic nanocarrier adjuvant incorporating the TLR-7/8 agonist, resiquimod. Mucosal vaccination induced a wave of effector T cells that seeded uterine mucosa and established resident memory T cells (first wave) in addition to circulating memory T cells. Upon genital C. trachomatis infection, local reactivation of uterine Trm cells efficiently triggered the recruitment of circulating memory T cells (second wave). Optimal pathogen clearance was shown to be dependent on both waves of memory cells. Importantly, systemic immunization did not induce Trm cells and only induced the second wave, and mice were suboptimally protected, even when circulating memory cells were abundant (Fig. 2). Thus, the protective effect of this mucosal vaccine depended on the synergistic action of two memory T cell subsets: tissue resident memory T cells and circulating memory T cells. Early mucosal seeding with Trm CD4 T cells was the key to a successful C. trachomatis vaccine in this model system. This finding constitutes a major step forward in understanding C. trachomatis immunology and provides a mechanistic basis for mucosal immunization against C. trachomatis.
Figure 2. Two waves of vaccine-induced C. trachomatis-specific memory T cells.
The first wave generated mucosal T cells which provided early protection and a second wave generated systemic T cells which augmented early and late protection. Mucosal immunization with adjuvant induced both the first and second waves of T cells. Systemic immunization induced only the second wave and generated incomplete protection. Reproduced with permission from [36].
B cells and antibody
B cell and antibody-mediated immunity against Chlamydia infection is not completely understood [38]. In 1997, Su et al. demonstrated that B cell-deficient mice (μMT) cleared C. muridarium primary infections with normal kinetics of bacterial shedding from the genital tract but knockout mice were more susceptible to reinfection compared to wild type control mice [39]. Further studies conducted by Morrison and colleagues showed that immune wild type mice that were depleted of either circulating CD4 and/or CD8 T cells by parenteral antibody treatment were able to clear the secondary Chlamydia infection [26]. Strikingly immune B cell-deficient mice were unable to resolve secondary infection in the absence of T cells [40]. However adoptive transfer of immune serum into immune B cell deficient mice in the absence of T cells reconstituted their ability to clear secondary infection [41]. Surprisingly, passive transfer of immune serum into naïve wild type mice did not provide protection from primary infection. While these data show that B cells and antibody have a role in clearing secondary infection, direct antibody-dependent neutralization or complement-mediated killing is unlikely to account for antibody-mediated protection during secondary infection since passive transfer of immune serum only protected antigen-experienced hosts rather than naïve mice. It may be that parenternal anti CD4 antibody treatment to deplete CD4 T cells failed to completely deplete tissue resident CD4 T cells. An alternative protection mechanism mediated by antibody may be enhanced antigen presentation mediated by Fc receptors at the mucosal tissue level that expanded mucosal Trm cells. It is known that professional antigen presenting cells bear Fc receptors and are 1,000 times more efficient at presenting antibody coated antigen [42]. In support of this conjecture Moore et al. [43] reported that Fc receptor-mediated antibody effector mechanisms are indeed important in anti-Chlamydia secondary immune responses. These results reflect infection-induced immunity and parallel studies by Farris et al [44] used recombinant MOMP as vaccine. They observed that MOMP immunity is more strongly antibody dependent than is infection immunity, although both also required CD4 T cells.
Recently, Li and McSorley demonstrated that local Chlamydia-specific CD4 T cell priming was also significantly reduced in B cell-deficient mice. This reduced local response was accompanied by spread of Chlamydia throughout the peritoneal cavity, resembling the pathology of women with the Fitz-Hugh Curtis syndrome associated with C. trachomatis PID. These findings reveal a role for B cells in limiting the intra peritoneal spread of Chlamydia during primary infection [45].
Animal models for vaccine research
Various animal models have been developed to evaluate Chlamydia vaccine efficacy which include mouse, guinea pig and nonhuman primate models [46].
C. muridarum mouse model
Mice are the most widely used animals for the study of genital Chlamydia infection and evaluation of candidate vaccines. The mouse models have advantages because of their small size, ease of handling, a wealth of immunologic reagents and well-characterized inbred and knockout strains. C. muridarum genital infection model is established by intravaginal infection. Of note C. muridarum is a natural mouse pathogen that causes pneumonitis, was originally isolated from the lung of mice and may spontaneously spread via the respiratory route. Even though the genital tract model is somewhat artificial in its route of infection, it does recapitulate many significant features of human C. trachomatis pelvic infections including ascending infection that results in salpingitis, hydrosalpinx and infertility [47]. Macrophages and lymphocytes including B cells, CD4 T cells and CD8 T cells infiltrate the genital tract tissues and CD4 T cells predominate throughout the course of infection and are essential for protective immunity. Mice that clear genital infection with C. muridarum are significantly resistant to reinfection manifested as low shedding of short duration. Immunity to Chlamydia genital infection and vaccine development using the C. muridarum murine model was comprehensively and elegantly summarized by Farris and Morrison [48].
There are also some notable differences in immunity and pathogenesis between the mouse model and human infection, which need to be considered before translating mouse data into human pathobiology. C. trachomatis possess different ompA genotypes that are specifically associated with conjunctival, genital and LGV infections. However, C. muridarum has a nonvariant ompA genotype that does not reflect the antigenic diversity of human C. trachomatis infection. Protective immunity in the C. muridarum genital infection mouse model develops very quickly and infection is resolved within 4 weeks while it can take several months for humans to develop immunity and clear infection. The failure to develop immunity can result in long-term chronic infection after C. trachomatis primary infection in humans, which does not seem to occur with C. muridarum infection [49]. In addition, primary C. muridarum genital infection is enough to cause most mice to develop tubal dilatation followed by hydrosalpinx that leads to infertility, while the risk of fallopian tube pathology in women with C. trachomatis PID occurs in only 10 to 15% of women whose increases with repeated infection [50].
C. trachomatis transcervical mouse model
Mice can also be genitally infected with human C. trachomatis serovars. However intravaginal inoculation with C. trachomatis generally produces mild genital infection that is unable to ascend to the upper genital tract and is cleared mainly by innate immune responses [51]. Gondek et al. established the C. trachomatis serovar L2 transcervical infection model where the organism is directly inoculated into the uterine cavity to establish productive infection. Immune CD4 T cells were demonstrated to be essential for clearance in this model [52]. Transcervical inoculation of C. trachomatis is performed using a Non-Surgical Embryo Transfer (NSET) device for mice. Mice previously transcervically infected with C. trachomatis serovar L2 were shown to be resistant to challenge infection, confirming that this model can be used to evaluate C. trachomatis vaccine candidates. Recently transcervical C. trachomatis serovar D infection was also shown to be an efficient and reliable model to evaluate C. trachomatis vaccine candidates [53]. An important caveat is the C. trachomatis transcervical model requires a challenge inoculum several orders of magnitude greater than the C. muridarum model or natural human C. trachomatis infection.
C. caviae guinea pig model
Another rodent model for Chlamydia genital infection is the guinea pig infected with C. caviae. Although the C. caviae guinea pig model is constrained by availability of reagent and lack of knockout animals, an important advantage of this model is that the pathology following Chlamydia infection, effects of reproductive hormones on infection, and sexual transmission of infection more closely resemble human Chlamydia pathobiology than does the mouse model. The model remains under utilized in Chlamydia vaccine research and may be especially useful for trachoma vaccine research.
C. trachomatis non-human primate models
Because of the closer evolutionary relatedness between human and non-human primates (NHP), primates have been valuable in the study of pelvic inflammatory disease caused by C. trachomatis [54]. However, the use of non-human primates to evaluate vaccine efficacy against C. trachomatis genital tract infection has had limited success. Although several studies have shown that the NHP model is a promising platform to evaluate trachoma vaccine, there is only one vaccine study that has targeted genital tract infection [55]. In this study, a rhesus monkey model of Chlamydial genital tract infection was used to investigate the efficacy of a plasmid-deficient C. trachomatis strain (CTD153) as a live attenuated vaccine. The CTD153 strain has an attachment/uptake defect and induces lower levels of cytokine production in vitro and in the murine genital tract [56]. Unfortunately, resistance to reinfection developed in the macaque genital tract only after multiple challenge infections irrespective of the presence or absence of the plasmid. Since there is no known natural NHP strain of Chlamydia, high doses of C. trachomatis inoculation were also required. In addition, differences in immune responses and disease states were found when using different C. trachomatis serovars and among outbred NHPs, which make the NHP model much more complicated to standardize for vaccinology studies. Therefore, the general consensus is that NHP model is not feasible nor necessary for use as a model to evaluate vaccines against C. trachomatis genital infection before proceeding to human trials.
Discovery of novel Chlamydia antigens
Much contemporary C. trachomatis vaccine research has focused on the production of subunit vaccines based on individual protective C. trachomatis proteins. To date, the Chlamydia major outer membrane protein (MOMP) has been the most widely investigated subunit vaccine candidate in multiple animal models. However studies indicate that vaccines based on MOMP alone afford only incomplete protection and efficacy is highly dependent on conformational structure. Other candidate antigens including outer membrane protein 2 (OMP2), heat shock protein 60 (HSP60), polymorphic membrane protein D (PmpD), cysteine rich protein A (CrpA), homolog of Yersinia pseudotuberculosis YopD (YopD), enolase and Chlamydia protease-like activity factor (CPAF) have been identified as vaccine antigens [48,57]), but none of these candidates is demonstrably better than MOMP. With the Chlamydia genome having been entirely deciphered [12,13], theoretically all potential vaccine antigens are now known. Recently novel strategies based on genomics to discover T cell antigens and antibody-inducing antigens for the development of Chlamydia vaccines have been reported resulting in a significant expansion in the number of antigens evaluated.
Reverse vaccinology approach
Reverse vaccinology is a term coined by Rappuoli et al [58]. It is based on unbiased bioinformatics analysis of the whole genome to predict and select novel antigens for vaccine development. Finco et al. [59] reported a strategy for discovery of Chlamydia vaccine candidates involving four major steps. (1) the C. trachomatis serovar D genome was bioinformatically analysed to identify proteins associated with the outer and inner membrane, secreted proteins and proteins predicted to be involved in virulence or pathogenicity; (2) 120 Chlamydia proteins were selected for expression and purification in an Escherichia coli expression system; (3) recombinant proteins were used to generate protein arrays for recognition by sera from C. trachomatis-infected humans and for their ability to stimulate IFN-γ producing CD4 T cells from C. trachomatis-infected mice; and (4) for the protective activity of combinations of selected antigens (C. muridarum homologs) as evaluated using the C. muridarum lung infection model. The study led to the discovery of 21 pure B cell antigens (antibody-inducing antigens), 16 pure T cell antigens (CD4/IFN-γ-inducing antigens) and 5 antigens inducing both T cell responses and antibody. Seven proteins were finally identified as protective antigens in the animal model and a combination of 4 antigens provided robust additive protection. The protection was mainly due to cellular immunity mediated by CD4 T cells. A second study [60] used a whole genome scale proteome array consisting of 908 C. trachomatis proteins to profile human antibody responses in 99 women with C. trachomatis infection. This study showed that 719 Chlamydia proteins were recognized by sera from at least one subject; only 27 proteins were recognized by 50% or more of the subject sera and were suggested as potential vaccine candidates. However the study by Finco et al. [59] showed that proteins with B cell epitopes do not substantially overlap with proteins containing T cell epitopes. Since only a minority of proteins have both T and B cell epitopes, this approach may not efficiently identify T cell antigens which are central for Chlamydia immunity.
Immunoproteomics approach
The immunoproteomic approach uses genomic information to guide the delineation of the T cell immunoproteome of a pathogen based on peptide binding by MHC molecules [61,62]. This approach became feasible due to advancements in tandem mass spectrometry MS/MS technology that provide improved sensitivity limits at, or below, one femtomole. The immunoproteomic approach identifies MHC class I and II bound pathogen-derived peptides that are directly eluted from infected primary dendritic cells (DCs). This approach has a false discovery rate of < 2% and creates a vast improvement for the positive validation rate compared to reverse vaccinology, presumably because its findings are empiric and result from physiological antigen processing and presentation. Since the identified peptides depend on both the affinity for the MHC molecule as well as the frequency of their presentation, such peptides may only represent the most “fit” peptides rather than the entire antigenic repertoire. This approach involves the following steps; (i) generation of DCs from mouse bone marrow, (ii) infection of bone marrow derived DCs (BM-DCs) with the pathogen for12 or more hours, (iii) lysis and isolation of MHC class I and II molecules from pulsed BM-DCs using allele-specific anti-MHC monoclonal antibody affinity columns, (iv) elution of peptides from purified MHC molecules, (v) analysis of purified MHC-bound peptides by MS/MS and (vi) validation of the identified peptides, and their cloned parent proteins in vitro and in vivo to identify potential vaccine candidates.
The immunoproteomics approach is capable of identifying antigens able to stimulate CD4 T cells via MHC class II. With this technology 27 C. muridarum CD4 T cell antigens were identified in C57BL/6 mice, which represents about 3% of the Chlamydia proteome [53,61]. Excluding those Chlamydia proteins with significant sequence homology to human and other bacterial proteins,13 proteins were selected for evaluation as vaccine antigens. Eleven of these engendered significant protection against C. muridarum infection in mice and seven proteins (PmpG, PmpE, PmpF, TC0420, Aasf, TC0825, and RplF) induced protection better than or equal to the MOMP [63]. Further studies of DCs from C57BL/6 mice infected with the human strain, C. trachomatis serovar D revealed nine antigenic proteins that were orthologs between the C. muridarum and C. trachomatis proteome [53]. Of particular interest, from both Chlamydia species the polymorphic membrane family of proteins generated MHC class II binding epitopes at multiple sites within the protein sequence and on different MHC class II molecules. The C. trachomatis and C. muridarum genomes encode nine different Pmps (PmpA to PmpI) [12,13]. All nine C. trachomatis Pmps have been reported to mediate adhesion to human epithelial cells [64]. In aggregate five outer membrane proteins [four Pmps (PmpE, -F, -G, and -H) and MOMP] were identified as T cell antigens via the immunoproteomic approach suggesting that outer membrane proteins may have advantages over other groups of proteins in presenting to the immune system. It was previously reported that the three Pmps (PmpE, PmpG and PmpH) constitute 61% of the total Pmp protein abundance [65] and MOMP is already known to constitute over 60% of the total outer membrane protein mass [66]. Therefore high abundance proteins as well as outer membrane localization may play a role in favoring antigen presentation.
PmpG is the most protective C. muridarum T cell antigen yet discovered via the immunoproteomic approach. Vaccination with PmpG resulted in 20 times and 1,000 times reduction in day 6 and day 13 shedding post C. muridarum challenge, respectively compared to that of mice with no vaccination [67]. Two subsequent studies validated the immunodominance of PmpG in the murine model [34,68].
A single-component subunit vaccine however may not provide optimal protection against infection due to MHC variation in the human population, or because the antigen may be antigenically or phase variable. A successful Chlamydia vaccine will likely need to be composed of multiple Chlamydia recombinant proteins in order to provide a broad coverage in an outbred population and cross-protect against multiple variants of C. trachomatis. A shortcoming of MOMP is allelic variation. A shortcoming of Pmps is phase variation. Both forms of antigenic variation are likely the evolutionary result of immune selection because these proteins are the targets of immunity. However combination of both antigens may be an ideal Chlamydia vaccine. Our studies demonstrate that a recombinant protein vaccine consisting of four Pmps (PmpEFGH) with MOMP formulated with a Th1 polarizing adjuvant significantly accelerated clearance in the C57BL/6 mouse C. trachomatis transcervical infection model [53] and in the C. muridarum genital infection mouse model using mice of different MHC backgrounds [69]. Overall we suggest that Chlamydia outer membrane proteins are likely to be the most important T cell antigens useful in the development of a C. trachomatis subunit vaccine.
Recombinant MOMP protein with VD4 multimers to engender protective B cell responses
MOMP has been studied as a leading vaccine candidate for 3 decades in multiple animal model systems and is capable of inducing both strong T and B cell immune responses. MOMP is an integral membrane protein that is very difficult to prepare in its native conformation. Recombinant MOMP immunization has provided only partial and variable protection most likely due to lack of the native structure of the protein [70]. Given that B cells and antibody play a role in T cell priming and limiting spread during initial infection as well as accelerating clearance during reinfection, a universally appealing approach in Chlamydia vaccine development is to select antigens such as the MOMP that are able to elicit antibody capable of binding to the surface of the native organism. However, the expense and technical difficulty related to the production of MOMP in its native conformation make it difficult to scale for vaccine development. Recently Olsen et al. [71] developed a novel recombinant MOMP vaccine construct based on engineering variable domain 4 (VD4) multimers within the MOMP sequence. The VD4 region from MOMP protein contains the highly conserved species-specific neutralizing B cell epitopes (LNPTIAG) that appears relevant to antibody-mediated protection against genital infection. Interestingly, the LNPTIAG epitope was also previously identified as a MOMP T cell epitope via the immunoproteomic approach [53]. They engineered MOMP with multimers of VD4 and this novel recombinant MOMP vaccine raised high titered antibody responses that neutralized most C. trachomatis serovars in vitro. Besides broadly neutralizing antibody responses, the construct also induced robust T cell responses, which conferred protection against vaginal infection and upper genital tract pathological changes in the mouse intravaginal C. trachomatis model. The inclusion of this newly developed recombinant MOMP with VD4 multimers may represent a novel solution for Chlamydia MOMP-based vaccine formulations. Determining the mechanism by which MOMP-VD4 multimers elicit functional antibodies is now an important research goal. Perhaps multimerization of the B-cell epitope allows this novel recombinant protein to directly activate antigen specific B cells.
Adjuvants and delivery systems for efficient vaccines against Chlamydia
Since recombinant proteins generally demonstrate poor immunogenicity, a significant challenge in developing an effective Chlamydia subunit vaccine is to discover an optimal adjuvant that delivers antigens in an appropriate way to elicit potent protective immunity in vivo. Immunological advances have revealed a much higher degree of complexity and exquisite specificity of the innate immune system than previous thought, which has inspired a new generation of novel adjuvant formulations that tailor the induction of defined immune responses against specific pathogens [72]. Pattern recognition receptors (PRRs) are expressed in DCs either on cell surfaces or intracellular compartments. The binding of pathogen-derived molecules to the PRR receptors to detect invading pathogens is crucial for initiating innate immunity and consequently activating adaptive immune responses. To date, various families of PRRs have been identified including Toll Like Receptors, C-type lectin receptors (CLR), NOD-like receptors (NLR) and RIG-I-like receptors (RLR). The discovery of the prominent roles played by these receptors has clarified the mechanisms behind novel adjuvant technologies. Characteristics of innate immune receptors and their ligands were comprehensively reviewed [73]. In the past ten years, the range of adjuvants and delivery system used in experimental and clinical vaccines against infectious diseases, including C. trachomatis, has rapidly increased [48,74,75]. Below we highlight several studies that applied novel adjuvants and delivery systems for Chlamydia vaccines that have shown promise.
Liposome DDA formulated with TDB or MPL (DDA/TDB or DDA/MPL)
In the past several years, our laboratory screened a series of adjuvants using PmpG as a model antigen to evaluate vaccine protection in the C. muridarum genital tract infection model [63,67]. These adjuvants include three cationic liposome formulations (dimethyldioctadecylammonium bromide -trehalose 6,6=-dibehenate [DDA-TDB], DDA-monophosphoryl lipid A [DDA-MPL], and DDA-monomycolylglycerol [DDA-MMG]), ISCOM (AbISCO-100), CpG-ODN1826 and Montanide ISA720–CpG-ODN1826. We found that vaccination with DDA-MPL or DDA-TDB (also named CAF01) formulation conferred the greatest level of protection that generated the highest frequency of T cells coexpressing IFN-γ and TNF-α. The frequency of multifunctional CD4 T cells induced by different adjuvant formulations accurately tracked the corresponding pattern of protection against C. muridarum genital tract infection, supporting the hypothesis that IFN-γ and TNF-α-secreting CD4 T cells are a correlate of protective immunity [21].
The small cationic molecule DDA forms liposomes that act as antigen “depot” to ensure the long-term release of antigen. DDA does not have direct effects on the maturation of DCs, and the combination of DDA with TDB or MPL delivers the PAMPs to DCs via the liposomal surface charge, thereby potentiating immunostimulation. TDB selectively activates the FcR-Syk-Card9 pathway in antigen-presenting cells to induce a unique innate immune activation program that directs protective Th1 and Th17 immunity [76]. Ishikawa et al. [77] reported that the monocyte-inducible C-type lectin (Mincle) is the essential cell receptor for TDB. DDA-TDB (CAF01) promotes both strong humoral and cell mediated immune responses and has been found to be a very useful adjuvant for a number of different vaccines [78,79]. DDA/TDB is currently undergoing clinical trials in humans for both a tuberculosis [80] and HIV subunit vaccine [81]. MPL is a derivative of LPS but more than 100 times less toxic. MPL activates via TLR4 and triggers the Trif-dependent pathway [82]. MPL is licensed for vaccines against human papillomavirus types 16 and 18 (Cervarix GSK) and hepatitis B virus (Fendrix GSK) and thus may be a feasible adjuvant component for a human C. trachomatis vaccine.
Mucosal adjuvants
Mucosa are the major entry ports for most human pathogens including Chlamydia. Therefore mucosal adjuvants should be ideal to generate local immunity in mucosal compartments to prevent infections. However, most licensed vaccines are adminisitered parenterally and most are ineffective in generating immune responses that localize to the mucosa. Currently the most commonly used experimental mucosal adjuvants can be divided into toxin-based adjuvants (e.g. LT, CT), immunostimulatory adjuvants (e.g. MPL, CpG, QS21) and particulate adjuvants serving as delivery vehicles (e.g. virus-like particles, liposomes and emulsions). Newsted et al. [83] recently reviewed advances and challenges in mucosal adjuvant technology and the mucosal administration routes, targets, and human clinical testing.
LT and CT are potent, but also toxic, mucosal adjuvants. New generation of LT and CT adjuvants using site-directed mutagenesis can mitigate toxicity. LTK63 is one mutant that preserves adjuvant activity in the absence of enzymatic activity and toxicity. LTK63 combined with CpG was shown to be effective in both mucosal and systemic immunization [84]. The study by Finco et al. [59] used LTK63/CpG adjuvant co-administrated systematically with newly discovered T and B cell antigens to evaluate vaccine efficacy against Chlamydia. That study demonstrated that LTK/CpG vaccine formulations generated strong Th1 immune responses characterized by induction of antigen-specific CD4/IFN-γ co-expressing by TNF-α or IL-2. The impact on tissue resident T cells was not studied.
Charge switching synthetic adjuvant particles (cSAPs) recently reported by Stary et al. [36] is an exciting advancement in Chlamydia vaccine development. A triblock copolymer, poly(D,L-lactic-co-glycolic acid)-b-poly(L-histidine)-b-poly(ethylene glycol) (PLGA-PLH-PEG) is a biodegrdable nanocarrier that was developed recently to target encapsulated antibiotics to the bacterial surface [85]. The adjuvant cSAPs was created by incorporating a second polymer PLA that was covalently coupled to R848 (resiquimod), a potent TLR 7/8 intracellualr agonist (PLA-R484) into PLGA-PLH-PEG polymer. The cSAPs form a hydrophobic core PLGA with R484 and a hydrophilic surface consisting of PLH and PEG. At physiologic pH 7.4, cSAPs carry a slight negative surface charge but acidification to below pH 6.5 makes cSAPs cationic and thus able to conjugate with negatively charged bacteria such as C. trachomatis. The study demonstrated that mucosal immunization (either via an intrauterine or intranasal route) with UV inactivated C. trachomatis (UV-Ct) organism complexed with the adjuvant cSAPs elicited an excellent long-lived protection comparable to that conferred by live C. trachomatis immunization, whereas immunization with UV-Ct alone induced tolerogenic effect that rendered mice hypersusceptible to subsequent C. trachomatis challenge. This study showed that activation of naïve CD4 cells in the mucosal environment, in the presence or absence of the adjuvant cSAPs, differentially recruited CD103 negative or positive DCs that induced Th1 or Treg cells respectively. The mucosal immunization of UV-Ct with cSAP generates Trm that seeded the uterine mucosa and strongly correlated with protective immunity. Systemic immunization with cSAP and UV-Ct failed to elicit Trm and protection.
Expert commentary and five-year view
Although there is no licensed vaccine for human C. trachomatis infection, recent research is getting us closer to this goal. The findings on tissue-resident memory T cells have provided insight into protective immunity mechanisms to C. trachomatis infection [86]. A vaccine to prevent sexually transmitted C. trachomatis needs to develop tissue-resident memory T cells that are capable of detecting incoming C. trachomatis and mount a robust local immune response to limit the spread of infection. Since the frequency of circulating multifunctional CD4 T cells coexpressing IFN-γ and TNF-α most accurately correlates with the pattern of protection against C. muridarum genital tract infection, IFN-γ producing CD4 T cells that highly coexpress TNF-α could be used as a systemic marker for the evaluation of candidate vaccines. Immunoproteomics and reverse vaccinology approaches have identified several novel protective antigens and recombinant outer membrane proteins, such as Pmps and MOMP are particularly promising subunit vaccine candidates. Adjuvants and delivery systems are critical for subunit vaccine formulations. For example, the protective antigen PmpG formulated with various adjuvants showed different levels of protective immunity depending on the adjuvant chosen. PmpG formulated with the adjuvant DDA/TDB induced potent IFN-γ/TNFα/IL-17 CD4 immune responses and conferred substantial protection against Chlamydia challenge. However when PmpG was formulated with adjuvant CpG or alum, low immune responses were detected and no protection was observed. The discovery of pattern recognition receptors as part of the innate immune system is spurring many novel adjuvant strategies for vaccine delivery. The optimal Chlamydia vaccine adjuvant needs to induce multifunctional Trm in genital tissues and maintain them for long duration. The mucosal adjuvant cSAP formulated with a TLR 7/8 ligand and UV inactivated C. trachomatis generated Trm that seeded the genital tissue and provided excellent protection against infection and pathology. Since whole organism vaccines previously induced inflammatory pathology, a subunit Chlamydia recombinant protein vaccine formulated with cSAP should be attempted. While the mucosal adjuvant (charge switching synthetic adjuvant particles, cSAPs) is an exciting breakthrough for Chlamydia vaccine development its manufacture remains complex and its delivery to the human genital mucosa problematic.
Developing a Chlamydia vaccine is scientifically tractable due to progress in understanding protective immune mechanisms, genome based antigen identification, adjuvant and vaccine delivery technologies and animal models. Research efforts in the next five years will build on these four areas to optimize vaccine formulations and delivery systems in suitable animal models and generate a suitable Chlamydia vaccine(s) to be tested in human clinical trial. The era of Chlamydia vaccine discovery is nearing an end. It is imperative that the best vaccine candidate now be taken in human trials. Computer modeling suggests that even a partially protective vaccine would rapidly reduce the prevalence of genital infection [87]. Health economic analysis suggests that a C. trachomatis vaccine is cost-effective [88]. The current major obstacle for Chlamydia vaccine trials is the failure of public health leaders to explicitly identify C. trachomatis vaccines as a high priority. Collaborations among academic, public health and the private sector are necessary to overcome this tragic inertia given that up to 1 million women per year become infertile from this preventable infection.
Key issues.
Optimal protection conferred by mucosal vaccine requires the synergistic action of two memory T cell subsets: tissue resident memory T cells and circulating memory T cells, and early mucosal seeding with Trm CD4 T cells is the key to a successful C. trachomatis vaccine.
Multifunctional CD4 T cells secreting IFN-γ/TNFα are the most efficient cells to terminate Chlamydia replication and will likely lead to a potential biomarker for protective immunity.
Immunoproteomic approaches resulted in a significant expansion in the number of T cell antigens for the development of a Chlamydia vaccine.
Chlamydial outer membrane proteins (Pmps and MOMP) are among the major T cell antigens useful in the development of a C. trachomatis subunit vaccine and should be used in combination to enhance coverage. MOMP is the most important antigen for the development of functional antibodies and a novel VD4 multimeric MOMP offers a practical solution to using recombinant MOMP able to stimulate functional antibodies as a vaccine antigen.
Adjuvants play important roles in modern vaccine formulations by acting as bridge between innate and adaptive immune responses.
Transcervical C. trachomatis infection mouse model is useful for the evaluation of C. trachomatis vaccine candidates. Non-human primate models need to be standardized and are not currently ready for evaluation of vaccine against Chlamydia genital infection. Robust vaccine protection in the C. muridarum and transcervical C. trachomatis murine models is sufficient to advance a Chlamydia vaccine into human trial.
Recent remarkable progress renders Chlamydia vaccine scientifically tractable, and effective collaborations of academic, public health and private sectors are required for successful development of a C. trachomatis vaccine, a major infertility prevention strategy.
Footnotes
Declaration of interest
Some of the work described was supported by the National Institute of Allergy and Infectious Diseases (Award Numbers R01AI076483 and R01AI076483), Genome BC and Western Economic Diversification. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy And Infectious Diseases or the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.World Health Organization. 2015. Progress report of the implementation of the global strategy for prevention and control of sexually transmitted infections: 2006–2015. [Google Scholar]
- 2.US Center for disease control, Division of STD prevention. 2014. Sexually transmitted disease surveillance 2013. [Google Scholar]
- 3.Owusu-Edusei K, Jr, Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sexually transmitted diseases. 2013;40(3):197–201. doi: 10.1097/OLQ.0b013e318285c6d2. [DOI] [PubMed] [Google Scholar]
- 4.Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. The New England journal of medicine. 2015;372(21):2039–2048. doi: 10.1056/NEJMra1411426. [DOI] [PubMed] [Google Scholar]
- 5.Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. The Journal of infectious diseases. 2005;192(10):1836–1844. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 6.Su H, Morrison R, Messer R, Whitmire W, Hughes S, Caldwell HD. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. The Journal of infectious diseases. 1999;180(4):1252–1258. doi: 10.1086/315046. [DOI] [PubMed] [Google Scholar]
- 7.Brunham RC, Rappuoli R. Chlamydia trachomatis control requires a vaccine. Vaccine. 2013;31(15):1892–1897. doi: 10.1016/j.vaccine.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longbottom D. Chlamydial vaccine development. Journal of medical microbiology. 2003;52(Pt 7):537–540. doi: 10.1099/jmm.0.05093-0. [DOI] [PubMed] [Google Scholar]
- 9.Grayston JT, Wang SP. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sexually transmitted diseases. 1978;5(2):73–77. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Wang SP, Grayston JT, Alexander ER. Trachoma vaccine studies in monkeys. American journal of ophthalmology. 1967;63(5 Suppl):1615–1630. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- 11.Mabey DC, Hu V, Bailey RL, Burton MJ, Holland MJ. Towards a safe and effective chlamydial vaccine: lessons from the eye. Vaccine. 2014;32(14):1572–1578. doi: 10.1016/j.vaccine.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens RS, Kalman S, Lammel C, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282(5389):754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 13.Read TD, Brunham RC, Shen C, et al. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic acids research. 2000;28(6):1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geisler WM. Duration of untreated, uncomplicated Chlamydia trachomatis genital infection and factors associated with chlamydia resolution: a review of human studies. The Journal of infectious diseases. 2010;201(Suppl 2):S104–113. doi: 10.1086/652402. [DOI] [PubMed] [Google Scholar]
- 15.Batteiger BE, Xu F, Johnson RE, Rekart ML. Protective immunity to Chlamydia trachomatis genital infection: evidence from human studies. The Journal of infectious diseases. 2010;201(Suppl 2):S178–189. doi: 10.1086/652400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen CR, Koochesfahani KM, Meier AS, et al. Immunoepidemiologic profile of Chlamydia trachomatis infection: importance of heat-shock protein 60 and interferon-gamma. The Journal of infectious diseases. 2005;192(4):591–599. doi: 10.1086/432070. [DOI] [PubMed] [Google Scholar]
- 17.Brunham RC, Kuo CC, Cles L, Holmes KK. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infection and immunity. 1983;39(3):1491–1494. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igietseme JU. Molecular mechanism of T-cell control of Chlamydia in mice: role of nitric oxide in vivo. Immunology. 1996;88(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Igietseme JU. The molecular mechanism of T-cell control of Chlamydia in mice: role of nitric oxide. Immunology. 1996;87(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Igietseme JU, Uriri IM, Hawkins R, Rank RG. Integrin-mediated epithelial-T cell interaction enhances nitric oxide production and increased intracellular inhibition of Chlamydia. Journal of leukocyte biology. 1996;59(5):656–662. doi: 10.1002/jlb.59.5.656. [DOI] [PubMed] [Google Scholar]
- 21**.Yu H, Karunakaran KP, Kelly I, et al. Immunization with live and dead Chlamydia muridarum induces different levels of protective immunity in a murine genital tract model: correlation with MHC class II peptide presentation and multifunctional Th1 cells. Journal of immunology. 2011;186(6):3615–3621. doi: 10.4049/jimmunol.1002952. A report showing that multiple functional CD4 T cells that coexpress IFN-γ and TNF-a were the most reliable correlate of immunity against C. muridaum infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RM, Kerr MS, Slaven JE. Plac8-dependent and inducible NO synthase-dependent mechanisms clear Chlamydia muridarum infections from the genital tract. Journal of immunology. 2012;188(4):1896–1904. doi: 10.4049/jimmunol.1102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayarapu K, Kerr M, Ofner S, Johnson RM. Chlamydia-specific CD4 T cell clones control Chlamydia muridarum replication in epithelial cells by nitric oxide-dependent and -independent mechanisms. Journal of immunology. 2010;185(11):6911–6920. doi: 10.4049/jimmunol.1002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igietseme JU, Perry LL, Ananaba GA, et al. Chlamydial infection in inducible nitric oxide synthase knockout mice. Infection and immunity. 1998;66(4):1282–1286. doi: 10.1128/iai.66.4.1282-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infection and immunity. 1995;63(12):4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infection and immunity. 2001;69(4):2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vlcek KR, Li W, Manam S, et al. The contribution of Chlamydia-specific CD8 T cells to upper genital tract pathology. Immunology and cell biology. 2015 doi: 10.1038/icb.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murthy AK, Li W, Chaganty BK, et al. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infection and immunity. 2011;79(7):2928–2935. doi: 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igietseme JU, He Q, Joseph K, et al. Role of T lymphocytes in the pathogenesis of Chlamydia disease. The Journal of infectious diseases. 2009;200(6):926–934. doi: 10.1086/605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Igietseme JU, Magee DM, Williams DM, Rank RG. Role for CD8+ T cells in antichlamydial immunity defined by Chlamydia-specific T-lymphocyte clones. Infection and immunity. 1994;62(11):5195–5197. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivares-Zavaleta N, Whitmire WM, Kari L, Sturdevant GL, Caldwell HD. CD8+ T cells define an unexpected role in live-attenuated vaccine protective immunity against Chlamydia trachomatis infection in macaques. Journal of immunology. 2014;192(10):4648–4654. doi: 10.4049/jimmunol.1400120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International HIVCS. Pereyra F, Jia X, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiviat NB, Wolner-Hanssen P, Eschenbach DA, et al. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. The American journal of surgical pathology. 1990;14(2):167–175. doi: 10.1097/00000478-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 35**.Morrison SG, Morrison RP. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infection and immunity. 2000;68(5):2870–2879. doi: 10.1128/iai.68.5.2870-2879.2000. A virtual case study of tissue-resident memory T cells using C. muridarium genital infection model via in situ immunohistochemistry analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Stary G, Olive A, Radovic-Moreno AF, et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348(6241):aaa8205. doi: 10.1126/science.aaa8205. A breakthough study demonstrates that early mucosal seeding with CD4 resident memory T cells (Trm) is the key to a successful C. trachomatis vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunham RC. IMMUNOLOGY. A Chlamydia vaccine on the horizon. Science. 2015;348(6241):1322–1323. doi: 10.1126/science.aac6528. [DOI] [PubMed] [Google Scholar]
- 38.Li LX, McSorley SJ. A re-evaluation of the role of B cells in protective immunity to Chlamydia infection. Immunology letters. 2015;164(2):88–93. doi: 10.1016/j.imlet.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infection and immunity. 1997;65(6):1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infection and immunity. 2000;68(12):6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. Journal of immunology. 2005;175(11):7536–7542. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. The Journal of experimental medicine. 1984;160(4):1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore T, Ananaba GA, Bolier J, et al. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology. 2002;105(2):213–221. doi: 10.1046/j.0019-2805.2001.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farris CM, Morrison SG, Morrison RP. CD4+ T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infection and immunity. 2010;78(10):4374–4383. doi: 10.1128/IAI.00622-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li LX, McSorley SJ. B cells enhance antigen-specific CD4 T cell priming and prevent bacteria dissemination following Chlamydia muridarum genital tract infection. PLoS pathogens. 2013;9(10):e1003707. doi: 10.1371/journal.ppat.1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.De Clercq E, Kalmar I, Vanrompay D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infection and immunity. 2013;81(9):3060–3067. doi: 10.1128/IAI.00357-13. A recent review of various animal models for the study of genital Chlamydia infection and evaluation of candidate vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infection and immunity. 2002;70(6):2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Farris CM, Morrison RP. Vaccination against Chlamydia genital infection utilizing the murine C. muridarum model. Infection and immunity. 2011;79(3):986–996. doi: 10.1128/IAI.00881-10. A review summarizing immunity to Chlamydia genital infection and vaccine development using the C. muridaum model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molano M, Meijer CJ, Weiderpass E, et al. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. The Journal of infectious diseases. 2005;191(6):907–916. doi: 10.1086/428287. [DOI] [PubMed] [Google Scholar]
- 50.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. The Journal of infectious diseases. 2010;201(Suppl 2):S134–155. doi: 10.1086/652395. [DOI] [PubMed] [Google Scholar]
- 51.Sturdevant GL, Caldwell HD. Innate immunity is sufficient for the clearance of Chlamydia trachomatis from the female mouse genital tract. Pathog Dis. 2014;72(1):70–73. doi: 10.1111/2049-632X.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gondek DC, Olive AJ, Stary G, Starnbach MN. CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract. Journal of immunology. 2012;189(5):2441–2449. doi: 10.4049/jimmunol.1103032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karunakaran KP, Yu H, Jiang X, et al. Outer membrane proteins preferentially load MHC class II peptides: implications for a Chlamydia trachomatis T cell vaccine. Vaccine. 2015;33(18):2159–2166. doi: 10.1016/j.vaccine.2015.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bell JD, Bergin IL, Schmidt K, Zochowski MK, Aronoff DM, Patton DL. Nonhuman primate models used to study pelvic inflammatory disease caused by Chlamydia trachomatis. Infect Dis Obstet Gynecol. 2011;2011:675360. doi: 10.1155/2011/675360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qu Y, Frazer LC, O’Connell CM, et al. Comparable Genital Tract Infection, Pathology, and Immunity in Rhesus Macaques Inoculated with Wild-Type or Plasmid-Deficient Chlamydia trachomatis Serovar D. Infection and immunity. 2015;83(10):4056–4067. doi: 10.1128/IAI.00841-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Connell CM, AbdelRahman YM, Green E, et al. Toll-like receptor 2 activation by Chlamydia trachomatis is plasmid dependent, and plasmid-responsive chromosomal loci are coordinately regulated in response to glucose limitation by C. trachomatis but not by C. muridarum. Infection and immunity. 2011;79(3):1044–1056. doi: 10.1128/IAI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nature reviews Immunology. 2005;5(2):149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 58.Rappuoli R. Reverse vaccinology. Current opinion in microbiology. 2000;3(5):445–450. doi: 10.1016/s1369-5274(00)00119-3. [DOI] [PubMed] [Google Scholar]
- 59**.Finco O, Frigimelica E, Buricchi F, et al. Approach to discover T- and B-cell antigens of intracellular pathogens applied to the design of Chlamydia trachomatis vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(24):9969–9974. doi: 10.1073/pnas.1101756108. A report on the discovery of Chlamydia vaccine candidates using reverse vaccinology approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Zhang Y, Lu C, Lei L, Yu P, Zhong G. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. Journal of immunology. 2010;185(3):1670–1680. doi: 10.4049/jimmunol.1001240. [DOI] [PubMed] [Google Scholar]
- 61**.Karunakaran KP, Rey-Ladino J, Stoynov N, et al. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. Journal of immunology. 2008;180(4):2459–2465. doi: 10.4049/jimmunol.180.4.2459. A report on the discovery of Chlamydia vaccine candidates using Immunoproteomics approach. [DOI] [PubMed] [Google Scholar]
- 62.Karunakaran KP, Yu H, Foster LJ, Brunham RC. Development of a Chlamydia trachomatis T cell Vaccine. Human vaccines. 2010;6(8):676–680. doi: 10.4161/hv.6.8.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu H, Jiang X, Shen C, et al. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infection and immunity. 2010;78(5):2272–2282. doi: 10.1128/IAI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Becker E, Hegemann JH. All subtypes of the Pmp adhesin family are implicated in chlamydial virulence and show species-specific function. MicrobiologyOpen. 2014;3(4):544–556. doi: 10.1002/mbo3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saka HA, Thompson JW, Chen YS, et al. Quantitative proteomics reveals metabolic and pathogenic properties of Chlamydia trachomatis developmental forms. Molecular microbiology. 2011;82(5):1185–1203. doi: 10.1111/j.1365-2958.2011.07877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infection and immunity. 1981;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu H, Karunakaran KP, Jiang X, Shen C, Andersen P, Brunham RC. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infection and immunity. 2012;80(4):1510–1518. doi: 10.1128/IAI.06338-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson RM, Yu H, Kerr MS, Slaven JE, Karunakaran KP, Brunham RC. PmpG303-311, a protective vaccine epitope that elicits persistent cellular immune responses in Chlamydia muridarum-immune mice. Infection and immunity. 2012;80(6):2204–2211. doi: 10.1128/IAI.06339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu H, Karunakaran KP, Jiang X, Brunham RC. Evaluation of a multisubunit recombinant polymorphic membrane protein and major outer membrane protein T cell vaccine against Chlamydia muridarum genital infection in three strains of mice. Vaccine. 2014;32(36):4672–4680. doi: 10.1016/j.vaccine.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infection and immunity. 2001;69(10):6240–6247. doi: 10.1128/IAI.69.10.6240-6247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71**.Olsen AW, Follmann F, Erneholm K, Rosenkrands I, Andersen P. Protection Against Chlamydia trachomatis Infection and Upper Genital Tract Pathological Changes by Vaccine-Promoted Neutralizing Antibodies Directed to the VD4 of the Major Outer Membrane Protein. The Journal of infectious diseases. 2015;212(6):978–989. doi: 10.1093/infdis/jiv137. A report showing a novel recombinant MOMP protein with a multivalent VD4 region raises a high titered antibody response that neutralizes the most prevalent serovars. [DOI] [PubMed] [Google Scholar]
- 72.Knudsen NP, Olsen A, Buonsanti C, et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Scientific reports. 2016;6:19570. doi: 10.1038/srep19570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Veer M, Meeusen E. New developments in vaccine research--unveiling the secret of vaccine adjuvants. Discovery medicine. 2011;12(64):195–204. [PubMed] [Google Scholar]
- 74.Lee S, Nguyen MT. Recent advances of vaccine adjuvants for infectious diseases. Immune network. 2015;15(2):51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Igietseme JU, Eko FO, Black CM. Chlamydia vaccines: recent developments and the role of adjuvants in future formulations. Expert review of vaccines. 2011;10(11):1585–1596. doi: 10.1586/erv.11.139. [DOI] [PubMed] [Google Scholar]
- 76.Werninghaus K, Babiak A, Gross O, et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. The Journal of experimental medicine. 2009;206(1):89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishikawa E, Ishikawa T, Morita YS, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. The Journal of experimental medicine. 2009;206(13):2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aagaard C, Hoang T, Dietrich J, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nature medicine. 2011;17(2):189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 79.Agger EM, Rosenkrands I, Hansen J, et al. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PloS one. 2008;3(9):e3116. doi: 10.1371/journal.pone.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Dissel JT, Joosten SA, Hoff ST, et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32(52):7098–7107. doi: 10.1016/j.vaccine.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 81.Karlsson I, Brandt L, Vinner L, et al. Adjuvanted HLA-supertype restricted subdominant peptides induce new T-cell immunity during untreated HIV-1-infection. Clinical immunology. 2013;146(2):120–130. doi: 10.1016/j.clim.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 82.Nicholls EF, Madera L, Hancock RE. Immunomodulators as adjuvants for vaccines and antimicrobial therapy. Annals of the New York Academy of Sciences. 2010;1213:46–61. doi: 10.1111/j.1749-6632.2010.05787.x. [DOI] [PubMed] [Google Scholar]
- 83.Newsted D, Fallahi F, Golshani A, Azizi A. Advances and challenges in mucosal adjuvant technology. Vaccine. 2015;33(21):2399–2405. doi: 10.1016/j.vaccine.2015.03.096. [DOI] [PubMed] [Google Scholar]
- 84.McCluskie MJ, Weeratna RD, Davis HL. Intranasal immunization of mice with CpG DNA induces strong systemic and mucosal responses that are influenced by other mucosal adjuvants and antigen distribution. Molecular medicine. 2000;6(10):867–877. [PMC free article] [PubMed] [Google Scholar]
- 85.Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS nano. 2012;6(5):4279–4287. doi: 10.1021/nn3008383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson RM, Brunham RC. Tissue resident T cells as the central paradigm of Chlamydia immunity. Infection and immunity. 2016 doi: 10.1128/IAI.01378-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gray RT, Beagley KW, Timms P, Wilson DP. Modeling the impact of potential vaccines on epidemics of sexually transmitted Chlamydia trachomatis infection. The Journal of infectious diseases. 2009;199(11):1680–1688. doi: 10.1086/598983. [DOI] [PubMed] [Google Scholar]
- 88.Owusu-Edusei K, Jr, Chesson HW, Gift TL, Brunham RC, Bolan G. Cost-effectiveness of Chlamydia vaccination programs for young women. Emerging infectious diseases. 2015;21(6):960–968. doi: 10.3201/eid2106.141270. [DOI] [PMC free article] [PubMed] [Google Scholar]