Abstract
Background
Autism spectrum disorder (ASD) represents a wide range of neurodevelopmental disorders characterized by impairments in social interaction, language, communication and range of interests. Autism is usually diagnosed in children 3–5 years of age using behavioral characteristics; thus, diagnosis shortly after birth would be beneficial for early initiation of treatment.
Aim
This retrospective study sought to identify newborns at risk for ASD utilizing bloodspot specimens in an immunoassay.
Materials & methods
The present study utilized stored frozen specimens from ASD children already diagnosed at 15–36 months of age. The newborn specimens and controls were analyzed by immunoassay in a multiplex system that included 90 serum biomarkers and subjected to statisical analysis.
Results
Three sets of five biomarkers associated with ASD were found that differed from control groups. The 15 candidate biomarkers were then discussed regarding their association with ASD.
Conclusion
This study determined that a statistically selected panel of 15 biomarkers successfully discriminated presumptive newborns at risk for ASD from those of nonaffected controls.
Keywords: autism, biomarkers, bloodspots, newborn, screening, spectrum disorder
Autism spectrum disorder (ASD) encompasses a cluster of pervasive neurodevelopmental disabilities associated with alterations or deficits in three behavior-related areas, namely: societal interactions; language, communication, and speech; and range of interests and activities [1]. ASD encompasses a heterogeneous complex of multiple genes acting in various combinations and interactions, justifying the need for medical genetics consultation [2,3]. The prevalence of ASD has increased almost 20-fold since the 1970s, and reports published since 2007 indicate that ASD ranges in estimates from one per 91 to one per 130 individuals [4,5]. Studies in the UK, USA and South Korea have reported that ASD affects 1–2% of children under the age of 8 years [5,6]. Presently, no newborn biomarker screening programs exist for ASD despite accounting analyses revealing a lifetime per capita incremental cost of US$3.2 million [7]. Thus, newborn screening for ASD represents a major unmet public health need in the USA and worldwide.
Symptoms of ASD occur as early as 2 years of age and diagnoses are frequently made by the age of 4 years. However, not all children develop the symptoms of ASD at the same time, and it appears there are two phases in the onset of autism. First, an early-onset phase occurs at birth, and second, a later regressive-onset form develops in early childhood at 2–4 years of age [8]. Although the early-onset phase of ASD is present at birth, it is not visibly apparent in newborns. Except for subtle physical appearances such as head size, intra-ocular distance and facial asymetrics, the traits representative of metabolic, immunologic and inflammatory disturbances in internal organ systems remain undetected. The six proposed causes of autism involve both metabolic and immunologic dysfunctions and include: increased oxidative stress; decreased methionine metabolism and trans-sulfuration: aberrant free and bound metal burden; gastro-intestinal (GI) disturbances; immune/inflammation dysregulation; and autoimmune targeting [9–11]. By contrast, the later regressive onset of ASD exhibits unremarkable development for 15 months or so, during which time the child gradually begins to show deficits in speech, language, social play and interaction, eye contact and vocalized expressions. Moreover, the regressive-onset phase of ASD is characterized by brain chemistry changes in early childhood due to abnormal levels of neuropeptides, hormones and neurotrophins involved in establishing neuronal networks, patterns, outgrowths and synapses.
A newborn screening program for early-onset ASD should be capable of utilizing a combination of ASD-associated biomarkers representative of the six proposed causes of autism in order to identify newborns at risk. If ASD remains undetected, newborns could be deprived of available clinical/social services that address speech and behavior modification therapies. Parents of ‘at-risk’ infants, if detected at birth, may choose to enroll and participate in treatments and interventions that encompass neurological examinations, language and speech training, cognitive evaluations and applied behavioral ana lysis. Such clinical interventions soon after birth might serve to mitigate and ameliorate the effects and manifestations of the regressive-onset phase of ASD that is already manifesting at 15–24 months of age.
The objectives of the present pilot study were threefold: first, a search was conducted for ASD-associated biomarkers from a pool of 90 candidate biomarkers to serve as a screening panel for early-onset ASD in newborns. Second, following accumulation of the immunoassay results, the data were subjected to multiple statistical group modeling analyses to identify groups or sets of the best-fit biomarkers. Finally, the best-fit biomarkers, which were found to be distinct from the controls, were subjected to searches in the global biomedical literature to determine their association, or lack thereof, with ASD. Thus, the present paper describes the results and findings of a retrospective study in search of candidate biomarkers intended to screen newborns for autism.
Materials & methods
Specimens
In collaboration with the Center for Disability Services (NY, USA), 40 families with autistic children between the ages of 3 and 5 years born in New York State who had been diagnosed with autism at the center, were selected from among a group of 200 cases. A single developmental pediatrician made all the diagnoses using the DSM-IV-TR criteria [12], thus assuring that diagnosis was consistent from child to child. These families were contacted by the center, provided with a description of the study and its goals and invited to participate in the study. A brief questionnaire and consent form were included in the mailing (CFDS IRB 07-010) and 20 positive responses were returned. Using the information provided by the parents regarding date of birth, 16 of these infants were identified in the repository of residual frozen newborn screening specimens at the Wadsworth Center (NYS IRB 07-044). The child’s gender and date of birth were transferred to a code sheet together with the coded identity and newborn screening accession number. The electronic connection of the accession number and all identifiers were severed leaving only the single bloodspot card with a new accession number and gender. Using the accession number to locate the stored frozen specimen, a single 3-mm punch from the Guthrie card specimen was placed in a vial identified only by code. Furthermore, two age-matched control specimens collected at time of retrieval were punched from the specimen immediately before and after the target specimen in the pack of dried bloodspot cards. The connection between the accession number and the demographic information was eliminated by severing the laboratory accession number from the identifying card.
The dried bloodspot specimen has been employed in New York State and worldwide newborn screening for the last 45 years. With over 12 million babies screened, the contributions of the cell lysates in the specimen are considered insignificant, as the hematocrit of each baby is approximately 45–48% and the leukocyte and platelet contribution is negligible. Based on such criteria, comparisons of the autism cases versus control cases can be considered valid. Specimens are accepted for testing upon visual inspection of the bloodspot. Specimens are rejected if the bloodspot lacks sufficient surface area, as encircled on the filter paper, to punch a suitable 3-mm spot. In accordance with internal review board (human use permission) regulations, all patient identifiers attached to the bloodspot specimen were removed. Thus, clinical information of the newborns regarding obstetric complications/outcomes prior to term delivery were lost to further follow-up.
Immunoassay
The specimens were submitted to Rules-Based Medicine (TX, USA) for analysis in their multiplex immunoassay system. Details of the immunoassay procedure have been previously published [13,14]. The Rules-Based Medicine platform is a multiplex commercial product (kit) already validated for precision, sensitivity, specificity, accuracy and assay limitations. Assays are deemed ‘acceptable’ if results fall within the working range of the assay, that is, results are rejected if the values are less than or greater than the range of the standard curve. The immunoassay probed for 90 commercially available biomarkers in serum eluted from the 3-mm punch. Reaction results from the 90 nanobeads/tube (for each biomarker) and data thereof were collected and recorded by the computer system. The 90 biomarkers employed in this study are listed in Table 1. Biomarker concentrations were expressed as ng/ml for proteins and pg/ml for cytokines with minimal concentrations detected being 0.17 μg/ml and 3.5 pg/ml, respectively. Although the immunoassay produced biomarker concentrations, results are reported as increased or decreased levels, along with whether the modeled groups were significantly different from the control group. Also, due to the small sample size, no attempts were made to distinguish gender differences.
Table 1.
The alphabetically arranged 90-analyte panel of biomarkers and best-fit models used in the present study.
| Analyte biomarker name | Best model | Second best model | Third best model | Analyte biomarker name | First best model | Second best model | Third best model |
|---|---|---|---|---|---|---|---|
| β-2 microglobulin | IL-6 | ||||||
| α-2 macroglobulin | IL-7 | O | |||||
| α-fetoprotein | ≠ | IL-8 | ≠ | ||||
| Adiponectin | IL-10 | ||||||
| ApoA1 | IL-12p40 | ||||||
| ApoCIII | IL-12p70 | ||||||
| ApoH | IL-13 | ||||||
| β-2 microglobulin | IL-15 | ||||||
| Basic FGF | IL-16 | ||||||
| Brain-derived neurotrophin | IL-18 | ||||||
| Calcitonin | X | Insulin | |||||
| Carcinoembryonic antigen | Leptin | ||||||
| CD40 | KLK3 | X | |||||
| CD40 ligand | Lp(a) | O | |||||
| CK-MB | Lymphotactin | ||||||
| C-reactive protein | MCP-1 | ||||||
| Complement 3 | MDC | ||||||
| EGF | MIP-1α | ||||||
| ENA-78 | MIP-1β | ||||||
| Endothelin-1 | MMP-2 | ||||||
| EN-RAGE | MMP-3 | ||||||
| Eotaxin | MMP-9 | ||||||
| Erythropoietin | MUC1 | ||||||
| Factor VII | MUC16 | ≠ | |||||
| Fatty acid-binding protein | Myeloperoxidase | ||||||
| Ferritin | X | Myoglobin | |||||
| Fibrinogen | PAI-1 | ||||||
| G-CSF | PAPP-A | ||||||
| GM-CSF | Prostatic acid phosphatase | ||||||
| Growth hormone 0 | RANTES | ||||||
| GST | O | Serum amyloid-P | |||||
| Haptoglobin | SGOT | ||||||
| ICAM-1 | SHBG | ||||||
| IFN-γ | Stem cell factor | ||||||
| IgA | Throxine-binding globulin | ||||||
| IgE | Tissue factor | ||||||
| IGF-1 | TIMP-1 | ≠ | |||||
| IgM | TNF-α | ||||||
| IL-1α | TNF-β | O | |||||
| IL-1β | TNFRII | ≠ | |||||
| IL-1ra | Thrombopoietin | ||||||
| IL-2 | Thyroid-stimulating hormone | X | |||||
| IL-3 | VCAM-1 | ||||||
| IL-4 | X | VEGF | |||||
| IL-5 | O |
The best, second best and third best selected modeled biomarkers are indicated with O, X and ≠ symbols, respectively.
Statistical methods
The 90 analytes exhibited a variety of distributional forms. Biomarkers were removed if missing too many values, and retained biomarkers were selectively log transformed in an attempt to achieve normality. Missing data points were handled according to standard practices in the handbook ‘Statistical Analysis with Missing Data’ [15]. Imputations (substitution for a data point) were performed among retained biomarkers before initiating model selection. Model selection was accomplished in two steps. First, stepwise matched logistic regression and its regularized variants were performed on all retained biomarkers to obtain subsets of candidate biomarkers [16]. Then, all possible regressions (32,768) were performed and compared on the basis of their Bayesian Information Criteria (BIC). Seventy-six of the 90 possible biomarkers yielded sufficient data for ana lysis. Ranked combination sets of the contending biomarkers were sought that could discriminate affected ASD cases from controls.
The present biomarker statistical analyses constitute nonparametric data that do not fit a normal distribution and are ranked and ordinal. The differences between case and control populations were compared resulting in p-values derived from group distribution analysis, BIC and matched logistic regression.
Results
Data analysis
The standard immunoassay method of analysis presently provided by the methodology of the Rules-Based Medicine biotech company clearly demonstrated that multiple biomarkers were either elevated or reduced in the 76 member marker panel. However, their software lacked the discriminatory statistical power to identify clusters or groups of biomarkers that were specifically associated with autism, but not with controls. For this reason, the biomarker immunoassay results were subsequently subjected to logistic regression and assessed by BIC to distinguish affected newborns from controls. Logistic regression measured the relationship between one categorical dependent variable (biomarker) and a continuous independent variable (or several) by converting the dependent variable to probability scores. The probabilities describing the possible outcome of single trials were then modeled, as a function of the explanatory variables using a logistic function.
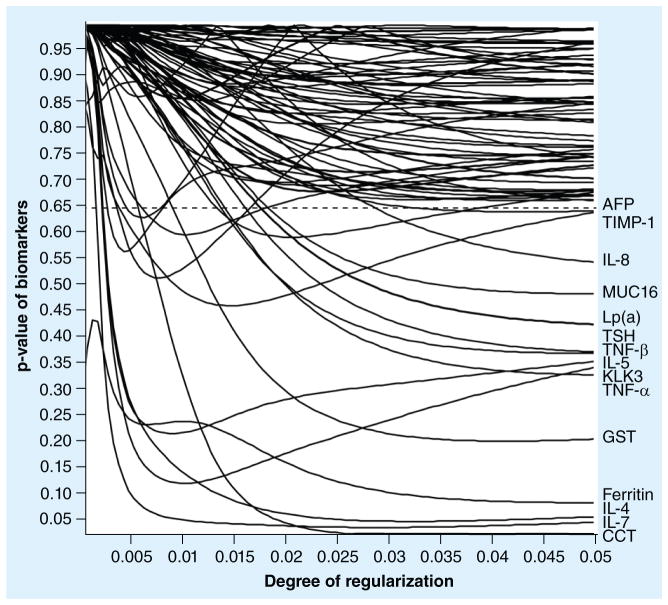
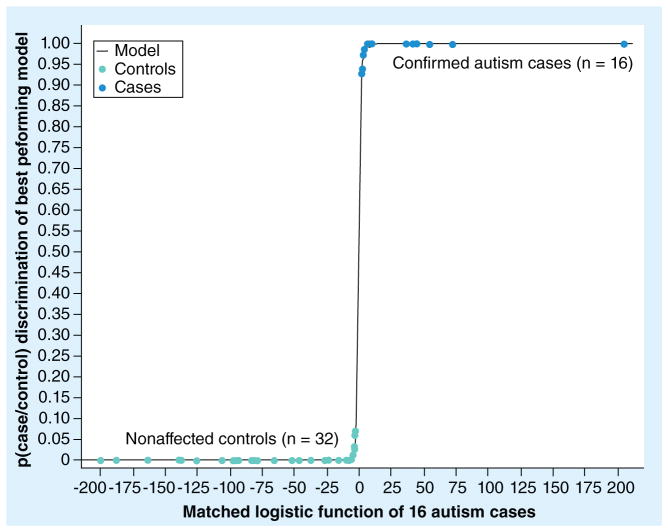
Model selection was accomplished in two steps. First, regularized matched logistic regression was performed on all obtained immunoassays resulting in data identification of 15 candidate biomarkers (Figure 1 & Table 2). Then, all possible (215) regressions were performed and compared on the basis of their BIC. The biomarker combinations (sets) were then ranked by the BIC and a minimum BIC model of 15 (three sets of five) was selected as the most promising panel of biomarkers (Table 2). As shown in Figure 2, the complete separation of the 16 autism cases from the 32 nonaffected control groups was demonstrated. Figure 1 demonstrates a natural break in the group distribution below the 0.65–0.95 region. The discriminatory function of logistic regression is observed to lie in its power to separate the two groups. It can be seen that 15 of the 76 biomarkers were readily distinguished from the remaining markers, which clustered above the dotted line in the upper quadrant of Figure 1. Changing the set of 15 biomarkers by deletion, addition or substitution of biomarkers decreased its effectiveness in discriminating cases from controls.
Figure 1. The regularized matched logistic regression of 76 exploratory biomarkers in 16 matched autism spectrum disorder cases.
The increasing degree of regularization along the right side of the figure suggests that only 15 biomarkers could contribute to a screening panel for autism. The remaining biomarkers are clustered at the top of the figure and are separated by a natural break in the two populations at p-values exceeding 0.65 (above dotted line).
Table 2.
The top 15 (three sets of five) ranked biomarkers regarding their direct or indirect relationship, biomedical and biochemical linkage and serum levels in presumptive autistic newborns.
| Top 15 modeled biomarkers | Relationship with ASD | Ref. | ||
|---|---|---|---|---|
| Direct/indirect | Biomedical/biochemical linkage | Levels | ||
| Best predictive group of biomarkers | ||||
| GST | Direct | Oxidative stress-related; abnormal methionine, glutathione and cysteine metabolism | Decreased | [17–21] |
| Lp(a) | Indirect | A marker for oxidative stress and abnormal homocysteine metabolism | Increased | [22–26] |
| IL-7 | Direct | Involved in β- and T-cell development, brain differentiation and structure | Increased | [39,40,42] |
| IL-5 | Direct | Increased levels during ASD pregnancy; involved in CNS perinatal growth | Increased | [30–32,35,42] |
| TNF-β | Direct | Enhances brain inflammation and dysfunction; promotes autoimmunity and inflammation | Increased | [43–46] |
| Second best predictive group of biomarkers | ||||
| TSH | Direct | Mean basal TSH levels are lower in ASD individuals; promotes CNS function | Decreased | [51–53,55] |
| KLK3 | Direct | Linked to proinflammatory cytokines in ASD neuroinflammation of the brain | Decreased | [56–59] |
| Calcitonin | Indirect | Inversely related to TSH blood levels; decreases blood calcium levels | Increased | [61,62,64,65] |
| IL-4 | Direct | Increased levels during ASD pregnancy; found in amniotic fluids | Increased | [33–35,42] |
| Ferritin | Direct | Binds and transports iron; ASD patients show iron deficiency with and without anemia | Decreased | [66–69] |
| Third best predictive group of biomarkers | ||||
| IL-8 | Direct | Abnormal immune responses related to brain inflammation; promotes autoimmunity | Increased | [36–38] |
| TNF-α | Direct | Increased levels in brain inflammation and GI tract dysfunction in ASD patients | Increased | [73–76] |
| MUC16 | Indirect | Acts with TNF-α and cytokines in GI tract; found in tears of ASD patients | Decreased | [79–82] |
| TIMP-1 | Indirect | Involved in degradation of extracellular matrix; glial cell secretions in brain | Decreased | [49,84–86] |
| AFP | Direct | An antioxidant agent found to be elevated in ASD pregnancies; regulates apoptosis | Increased | [89–93] |
ASD: Autism spectrum disorder; GI: Gastrointestinal; TSH: Thyroid-stimulating hormone.
Figure 2. The case-to-control discrimination of the best performing statistical model.
The p-value of the case-to-control model (p[case/control]) is shown as a matched logistic function of the 16 model-selected autism cases..
Among the 15 biomarkers, the best set of five ranked in order from the highest BIC score included GST, Lp(a), IL-7, IL-5 and TNF-α (Table 2). The second best set included thyroid-stimulating hormone (TSH), KLK3, calcitonin (CCT) and IL-4 in addition to ferritin. The third best set included IL-8, TNF-α, MUC16, TIMP-1 and AFP. Thus, one could utilize various combinations of newborn screening biomarker panels for ASD that could include: the first set of five alone; the first and second sets combined; or a combined set of all three sets totaling 15 biomarkers. Only further studies will determine the optimal biomarker combinations to be used for newborn screening of ASD in the future.
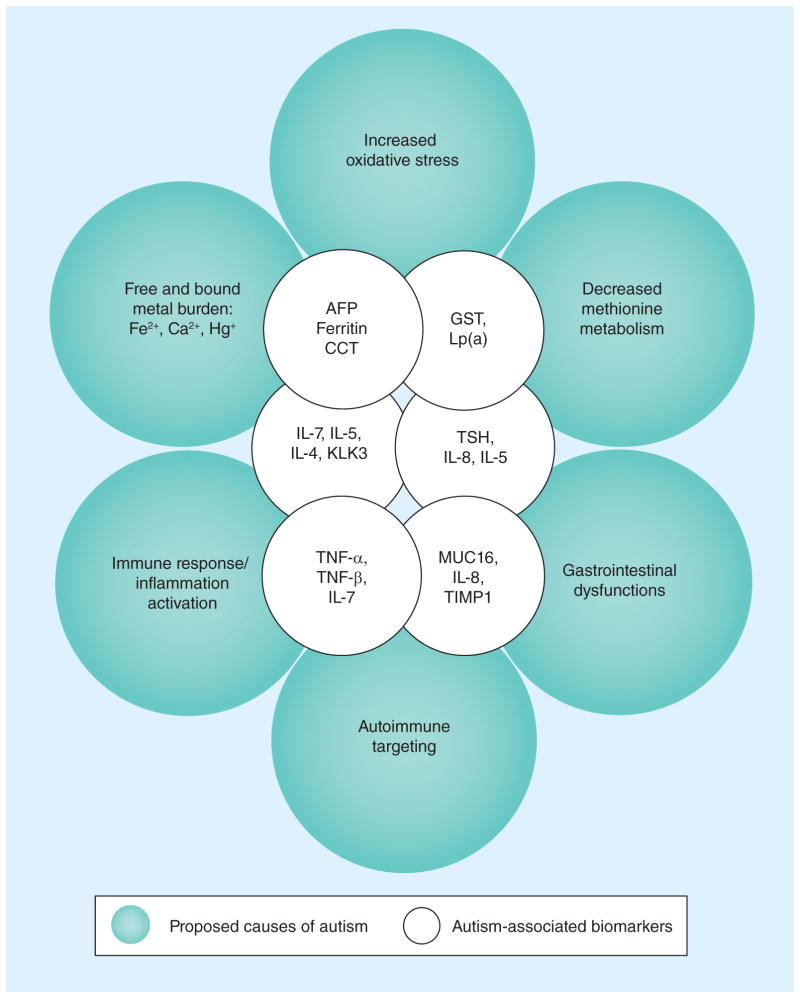
The 15 statistically modeled biomarkers (ranked in three sets of five) are displayed in Table 2 to indicate their direct or indirect relationship to ASD, biomedical/biological importance and whether the biomarker serum levels were increased or decreased. In Figure 3, the 15 selected biomarkers were positioned in overlapping inner circles in jutaxposition to overlapping outer circles depicting the six proposed causes of ASD [9,10]. The amount and position of overlap demonstrates the relationship of the biomarker to one or more causative events of ASD. It is readily apparent that the biomarkers associated with the metabolic deficits of autism overlap with both CCT, ferritin and AFP and with GST and Lp(a); while the immune/inflammation, autoimmune targeting and gastrointestinal dysfunctions overlap with the proinflammatory cytokines (PICs) and neuronal death, injury and repair agents. Interestingly, some of the intermediate positioned biomarkers, which include TSH, MUC16 and KLK3, overlap with the metabolic, immune/inflammation and gastrointestinal proposed causes of ASD.
Figure 3. A circle model of proposed causes of autism versus screen-selected biomarkers.
A circle model of the proposed six causes of autism is shown on the outer circles, while the inner overlapping circles indicate the biomarkers associated with the causative metabolic or immune response/inflammation events. The overlap of the outer and inner circles indicates the degree of association of the two agents.
CCT: Calcitonin; TSH: Thyroid-stimulating hormone.
Discussion
The results reported here have identified a putative set of 15 biomarkers (three ranked sets of five) for autism that, when combined or analyzed as sets, could be used in newborn screening laboratories to identify newborns at increased risk of developing autism. Each of the 15 biomarkers selected from the present analysis are individually discussed below concerning their direct or indirect association with ASD. It should be noted that each of the biomarkers discussed below are related and/or associative of ASD, but have not been confirmed to be causative with autism. Finally, it should be understood that the newborn autism specimens samples were diagnosed in the regressive-onset stage of ASD, that is, early childhood (3–5 years of age). The biomarkers in the present report, by definition, are representative of early-onset markers obtained at time of birth. Since the early-onset stage of autism has only recently been recognized [8], most of the literature references discussed below were obtained from studies representing the regressive-onset in children and those from juvenile/adult stages of ASD.
GST is a metabolic biomarker directly associated with ASD. The human gene product for GST constitutes a candidate susceptibility protein due to its tissue distribution and role in oxidative stress and methionine metabolism, which results in neuronal injury and death [17]. Some teratogenic alleles, such as the GST haplotype, act in mothers during pregnancy to contribute to neurodevelopmental disorders in their offspring. Placental overtransmission of the GSP1*A haplotype has indicated that reduced GST levels and activities in the mother during pregnancy increases the likelihood of ASD in her fetus [18]. Results of a recent study further demonstrated that glutathione, total glutathione and activity levels of GST were significantly lower in autistic patients as compared with control subjects; however, homocysteine, thioredoxin reductase and perioxidoxin levels were remarkably higher [19]. Furthermore, novel dataset analyses have demonstrated that a genotype risk for the homozygous GST deletion genotype is highly associated with autism [20]. Autistic children with metabolic disturbances are known to display reduced metabolic activities of GST, cysteine, glutathione and methionine, which are associated with methionine transmethylation and trans-sulfation [21].
The Lp(a) complex consists of a low-density lipoprotein (LDL) bound to ApoA that, when present at high levels, constitutes a risk factor for cardiovascular and atherosclerotic disease. Lp(a) is a biomarker that is indirectly associated with autism. It is considered to be a marker for oxidative stress and abnormal homocysteine metabolism associated with total cholesterol, high-density lipoprotein (HDL), LDL and apo-lipoproteins [22]. These lipid-associated factors have been directly linked to coronary artery heart disease, cardiovascular disorders and atherosclerosis [23,24]. Lp(a) is currently utilized to assess oxidative stress that causes red blood cell rigidity, hemolysis and increased blood viscosity in patients at risk for heart disease [25,26]. Oxidized Lp(a) can also induce elevated levels of homocysteine in conjunction with oxidative stress resulting in cell death and injury [27]. In this regard, ASD patients have been found to be at considerable risk of developing coronary heart disease by midlife [28]. Elevated levels of homocysteine, an indicator of an impaired folate-dependent methionine cycle, have been observed in autistic children, but not in reference control groups [29].
PICs are small cell-signaling proteins secreted by lymphoreticular cells that serve as intracellular communication agents; these include interleukins, interferons, chemokines and TNF. PICs are directly associated with neuroinflammation in the brain of ASD individuals. Accumulating evidence has confirmed that abnormal immune responses of PICs (IL-5 and IL-8) in the brain and GI tract may serve as clinical and biological trait markers for ASD [30–32]. Increased levels of other PICs (IL-4 and IL-5) have been detected in the amniotic fluid of women who had borne a fetus later diagnosed with ASD [33,34]. Furthermore, increased levels of IL-4 and IL-5 were found in the maternal serum of women in midgestation pregnancies [35]. ASD patients also display increased innate and adaptive immune responses expressed through the chemokines (IL-8) and the Th1 pathways suggesting that localized brain inflammations and autoimmune disorders may be involved in the pathogenesis of ASD [36–38]. IL-7, first described as a hemopoietic chemokine, has crucial functions involving both B- and T-cell lymphocyte development [39,40]. Other studies have determined that IL-7 is involved in neural cell differentiation in brain structuring through glial cell activities [41]. Finally, IL-4, IL-5 and IL-7 cytokines are known to be involved in development of the CNS during fetal growth and following birth [42].
TNF-β, also known as lymphotoxin-α, is a cytolytic cytokine involved in brain autoimmunity (multiple sclerosis [MS], experimental allergic encephalomyelitis), B-cell development and apoptosis following cell injury. TNF-β is directly associated with ASD due to its involvement with neurological dysfunction resulting from neuronal cell death [43]. Astroglial cells of MS patients are known to produce high levels of TNF-β that exacerbates inflammation and demyelination in brain tissues [44]. In experimental allergic encephalomyelitis states, TNF-β has been found to maintain lymphoid environments crucial for sustaining late-phase T-cell immune responses [45]. TNF-β also contributes an enhancing role in the etiology of ASD in that brain autoantibodies were found to be present in 45% of the sera collected from children with ASD [46]. The autoantibodies found in the ASD children were directed against brain-derived neurotrophic peptides/proteins, dietary peptides, bacterial toxins and xenobiotics [47–50].
TSH is a hypothalamic-derived hormone that stimulates the thyroid gland production of thyroxine to regulate overall body metabolism. TSH is a biomarker directly related to autism in clinical patients. Mean TSH basal and peak levels have long been known to be significantly lower in autistic patients than in control groups [51]. It has been suggested that hypothalamic dysfunction may be the basis for the reduced TSH levels; moreover, there exists a parallel between thyroid function and impairment in verbal communication [52]. Thyroxine is also known to be critical for post-natal development and function of the CNS [53]. TSH production has been further demonstrated in T cells of the intestinal mucosal immune system that was induced by IL-7 cytokines [54]. In thyroid disease, dyslipidemia coexists with metabolic abnormalities, oxidative stress and hemodynamic alterations, which supports studies reporting an increased risk of cardiovascular disease associated with autism. In this regard, subclinical thyroid disease in ASD has been linked to lipid metabolic alterations of LDL/HDL cholesterol, triglycerides, apolipoproteins and Lp(a) as discussed above in the paragraph on lipoproteins [55].
KLK3 is a glycoprotein trypsin-like serine pro-tease belonging to the kallikrein-related peptidase enzyme family. KLK3, which displays an epitope commonly known as PSA, is produced by salivary, breast, lung, uterus and prostate epithelial cells and is detectable in serum, urine, amniotic fluid and breast milk. KLK3 is not directly associated with autism, but is linked to PICs involved in ASD neuroinflammation (see cytokine paragraph above). An association has been shown to exist between the cytokines IL-4 and TNF-α and circulating levels of KLK3 [56,57]. KLK3 can also induce proliferation and expression of cytokines from peripheral blood lymphocytes [58,59]. High levels of KLK3 can induce expression of IL-6 and TNF-α and activate an immune response involving both IL-4 and IL-5 in ASD patients [60].
CCT is a hormone produced by the parafollicular cells of the thyroid that acts to decrease blood calcium levels by reducing calcium resorption in bone. CCT has an indirect association with ASD in that it is inversely related to circulating blood levels of TSH (see above) and is directly correlated with CGPP in the brain [61,62]. CCT can also act to inhibit TSH hypothalamic cell secretion [63]. Similar to CCT, CGRP is a member of the CCT family of peptides, is expressed in brain neurons and is elevated in the blood of ASD infants [64]. Elevated CGRP levels have been reported when assayed in newborn dried bloodspots and CGRP is known to be a neurotransmitter, neuromodulator and a trophic nerve factor [65]. Finally, it is germane to this discussion that CGRP is regulated by cytokines such as TNF-α.
Ferritin is a protein that binds, transports and stores iron and is directly correlated with iron deficiency, anemia and sleep disturbances in ASD and Asberger children [66–68]. Iron deficiency with and without anemia can impair cognition, reduce brain development and cause mood changes and loss of mental concentration in ASD children [68,69]. Autism is associated with altered bound and free metal body burden (storage) of both mercury and iron [70]. Metal levels of iron have been indirectly monitored using urinary total porphyrin in ASD patients as a measure of hepatic detoxification and oxidative stress [71]. Moreover, aberrant levels of nonprotein-bound iron can result in impairment of the redox status in ASD patients in the balance between oxidative stress and antioxidant defense; such factors can further contribute to disturbances in lipid metabolism and peroxidation [72].
TNF-α is a monocyte-derived cytotoxic protein involved in systemic inflammation and cell death. TNF-α has a direct association with ASD in both neuroinflammation of the brain and increased cytokine production associated with GI tract dysfunctions [73]. Blood monocytes from ASD patients displayed marked increases in cytokine and TNF-α activities in response to stimulation of immune cells [74]. Evidence has accumulated showing abnormalities in the inflammatory response system of ASD patients as evidenced by increased production of TNF-α [75]. Thus, autism appears to be accompanied by an activation of the monocyte arm of the body’s inflammatory response system. Moreover, TNF-α has been reported to be a marker for inflammatory damage and apoptosis and is elevated in the cerebrospinal fluid of ASD children [34,76]. Thus, it has been proposed that TNF-α may be involved in the pathogenesis of autism. Another study has determined that TNF-α showed increased expression and regulatory roles in cytokine-induced inflammation and apoptosis in lymphoblasts of autistic individuals [77]. It was further demonstrated that TNF-α levels were significantly increased compared with controls in histologic autopsied preparations of ASD brain tissues [36]. Thus, elevations of proinflammatory TNF-α levels in early brain ontogenesis can be associated with disturbances of mental functions and neurologic insults in the later life of ASD individuals [78].
MUC16, which displays an epitope commonly known as CA125, is a highly glycosylated glycoprotein that has an indirect relationship with ASD. It is located on the lining of intestinal mucosa and lymphoid nodes are frequently positioned adjacent to this lining. Inflammation in the mucosal layers of the GI tract has been shown to be indirectly associated with ASD patients [79]. Inflammatory cytokine activators such as TNF-α can induce and modulate the expression of secretary gene products such as MUC16, among others, and these cell surface mucins protect against intestinal inflammations by the inhibition of toxin-induced cell death in epithelial cells [80,81]. MUC16 is also expressed and released from tears in the ocular membrane surface of epithelial cells in dry eye syndrome as observed in ASD patients [82]. Finally, MUC16 has been reported to have an inverse correlation with metabolic syndrome involving LDL and HDL, lipoprotein cholesterol and elevated triglycerides as shown above for Lp(a) [83].
TIMP is a glycoprotein that serves as a natural inhibitor of the matrix metalloproteinases (MMPs), a family of peptidase enzymes involved in the degradation of tissue extracellular matrix and in apoptotic functions. TIMPs have an indirect association with ASD through their interaction with PICs [49]. In the brain and throughout the nervous system, TIMPs are produced and secreted by astrocytes and microglial cells involved in processes of neural injury, inflammation and tissue repair [84,85]. The TIMPs are found in both blood and brain tissue following neuronal injury and can influence the levels of PICs produced in the brain [86,87]. Neuronal injury induces activation of microglial cells that induce MMPs to produce increased levels of TNF-α and other cytokines and chemokines. In brain inflammatory diseases such as MS and acute disseminated encephalomyelitis, MMP levels are increased while TIMP levels are decreased [88].
AFP is a tumor-associated fetal glycoprotein that has served as a biomarker for both fetal defects and malignancies. AFP has a direct relationship with ASD in that it is elevated in the maternal serum of mothers giving birth to children later diagnosed with ASD [89]. Mothers with ASD children exhibited maternal serum AFP levels that were significantly elevated compared with control subjects. Mothers with presumptive ASD fetuses showed an increased risk of elevated maternal serum AFP levels regardless of their congenital malformations and comorbidities. Previous studies have also indicated a potential role of AFP as an antioxidant and a growth regulator via apoptosis, signal transduction and cytostatic/cytotoxic mechanisms [90]. These potential roles of AFP fit well into theories explaining the pathophysiology of autism where oxidative stress, aberrent methionine/homocysteine metabolism (neural tube defects) and immunologic deficiencies have been proposed [91]. Overall, AFP may act in cellular defense against oxidative stress both as a heavy metal transporter (copper/zinc) and as a superoxide radical scavenger [92]. Finally, AFP may also play a role in autism causation through defective retinoic acid signal transduction pathways and in folic acid dietary supplementation [93]. Overall, AFP may act in cellular defense by means of antioxidative stress activity, heavy metal transportation/delivery and in oxygen radical scavenging.
While the above screening results are encouraging, caution must be taken regarding the limitations of drawing conclusions from only 16 confirmed ASD cases and 32 nonaffected control cases. It is obvious that larger studies using similarly confirmed ASD cases must be undertaken. Although ASD is known to have a wide spectrum of phenotypic expression and progress, the correct subclassification of affected children in the wide spectrum of autism is still in the preliminary stages [94]. The fact that the diagnosis assigned to each child in this study was by a single clinician using DSM-IV-TR criteria strengthens their designation as a uniform population with closely similar phenotypes. The statistical model selection procedure presently employed was clearly influenced by the distribution of biomarkers; it may be that the attempts of transformations to normality employed, aimed at making the analyte distributions more Gaussian-like, tempered the influence of extreme observations that might have otherwise contributed to the regression data.
In future studies, taking advantage of the experience gained in developing multiplex immunoassays, biomarkers from the best performing models could be converted into multiplex assays for use in larger studies on newborns selected as described here [14]. Additional biomarkers could also be added to the panel simply through addition of appropriate beads. Thus, one could utilize various combinations of newborn screening biomarker panels for ASD that could include: the first set of five alone; the first and second sets combined; or combined sets of all three totaling 15 markers. Only future studies will determine the optimal biomarker combinations to be used for the newborn screening for ASD in the future. If this newborn panel of biomarkers could be confirmed in larger studies, states such as California, Texas and New York would be more likely to consider its adoption since speech, language, and applied behavioral therapies and treatments are already available. Governmental institutions do not screen for diseases/disorders that cannot be treated. In addition, studies employing the later regressed onset of ASD using neuropeptide testing have already been reported and would seem feasible to employ [36,95]. Later onset screening for ASD would be the natural follow-up to test presumptive positive ASD infants that were previously detected in newborn screening programs. The authors believe that further investigations using these sets of biomarkers (and possibly others) should be encouraged, which might eventually lead to the ability to screen for early-onset newborn autism using Guthrie dried bloodspot specimens.
Future perspective
The pace of autism research and gained knowledge has increased exponentially in the last decade. This is true not only in the clinic, but also at the research bench. In the next 5–10 years, we can expect the autism field to expand and broaden its present base of knowledge in the areas of toxic metals, nutrition, GI biochemistry, genetic loci, medical imaging, autoimmunity and inflammation of the brain. In genetics, increased discoveries of gene clustering and autism susceptability gene loci will be at the forefront. The measurement of heavy metals will lend itself to measurements in the hair of autistic patients, in children and adults, to identify both deficiencies and excessive amounts. The development of new diets and dietary supplements will improve the everyday well-being of those with autism. The suspected linkage of GI peptides to autism will be pursued in light of the discovery that GI peptides have the same gene transcript and mRNA as the neuropeptides of the brain. In the field of medical imaging, disruptions in the motor and sensory areas of the brain will be visualized and defined in regions such as the amygdala and orbital–frontal cortex. The autoimmune association with autism via the human leukocyte HLA system will be clarified especially in the HLA-DRB1*011 and the HLA-DRB1*3 regions. The role of perinatal testosterone exposure in the ‘extreme male brain’ syndrome in autistic patients will be more fully elucidated. Finally, as stated in the present report, procedures to screen newborns at the onset of autism is currently underway and will be further exploited.
Executive summary.
Context
Autism is diagnosed in children 15–36 months of age using behavioral characteristics. Since all agree that earlier diagnosis would benefit the child, the current study sought a way by which a child could be identified at birth for risk of developing autism.
Objective
This study assesses the use of newborn screening specimens (Guthrie bloodspot card) to identify newborns at increased risk of developing autism based on a subset of patterns obtained from a large panel of biomarkers.
Design
Residual specimens from the New York Newborn Screening program were used in the study. Children (3–5 years of age) with autism as identified at a local patient care program became possible study participants. Their parents were contacted and asked permission to retrieve the stored Guthrie specimen card for analysis. These specimens, along with controls, were analyzed by immunoassay in a multiplex system that included 90 blood and serum biomarkers. These results were then analyzed to look for subsets of patterns found in the autistic children but not present, as a pattern, in controls.
Participants
Subjects were selected as described above. Twenty candidate subjects were identified by medical record examination.
Main outcome measure
This retrospective study was designed to investigate the question ‘Are there sets of biomarkers obtainable from Guthrie specimens with the potential to identify newborns at increased risk for autism?’.
Statistical methods
The 90 analytes had different values missing, varying between 0 and 100%, and exhibited a variety of distributional forms. Variables were removed from further analysis if too many values were missing, and retained variables were selectively log transformed if it improved their normality. Missing values among the retained variables were imputed before model selection began.
Model selection was accomplished in two steps. First, regularized matched logistic regression was performed on all retained variables to obtain a subset of 13 candidate variables. Then, all possible 213 regressions were performed and compared on the basis of their Bayesian Information Criteria.
Results
Seventy-six of the 90 possible biomarkers yielded sufficient data for analysis. The top 15 variable combinations were assessed by the Bayesian Information Criteria and were selected as the most promising panel of biomarkers.
Conclusion
This set of model-selected biomarkers merits further investigation into its ability to discriminate infants at risk for developing autism in a population of newborns.
Acknowledgments
The authors would like to thank M Nordhauser, DJ Silverman and the staff at the Center for Disability Services (NY, USA) for their efforts in communicating with the parents. They thank the NYS Newborn Screening Program for making residual specimens available and Rules-Based Medicine for their immunoassay contributions. Most of all, they thank the parents of these children who made their child’s newborn specimen available. The authors further acknowledge the assistance of AA Reilly in the Wadsworth Center’s Bioinformatics Core for his biostatistical expertise.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This work was supported by NIH Contract ADB-NO1-DK-6-3430 (HHSN267200603430), Novel Technologies in Newborn Screening to KA Pass (principal investigator). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪ ▪ of considerable interest
- 1.McPartland J, Volkmar FR. Autism and related disorders. Handb Clin Neurol. 2012;106:407–418. doi: 10.1016/B978-0-444-52002-9.00023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 3.Weiss LA. Autism genetics: emerging data from genome-wide copy-number and single nucleotide polymorphism scans. Expert Rev Mol Diagn. 2009;9:795–803. doi: 10.1586/erm.09.59. [DOI] [PubMed] [Google Scholar]
- 4.Kogan MD, Blumberg SJ, Schieve LA, et al. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124:1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- 5.Baron-Cohen S, Scott FJ, Allison C, et al. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry. 2009;194:500–509. doi: 10.1192/bjp.bp.108.059345. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Leventhal BL, Koh YJ, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168:904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- 7.Ganz ML. The lifetime distribution of the incremental societal costs of autism. Arch Pediatr Adolesc Med. 2007;161:343–349. doi: 10.1001/archpedi.161.4.343. [DOI] [PubMed] [Google Scholar]
- 8.Miller VM, Racine R, Zalcman A. Neuroimmune mechanisms in autism. In: Kusnecov A, Anisman H, editors. The Handbook of Psychoneuroimmunology. John Wiley & Sons; 2012. pp. 1–32. [Google Scholar]
- 9▪.Bradstreet JJ, Smith S, Baral M, Rossignol DA. Biomarker-guided interventions of clinically relevant conditions associated with autism spectrum disorders and attention deficit hyperactivity disorder. Altern Med Rev. 2012;15:15–32. Describes the use of biomarkers as ‘reporter proteins’. [PubMed] [Google Scholar]
- 10▪ ▪.Geier DA, Geier MR. Autism spectrum disorder-associated biomarkers for case evaluation and management by clinical geneticists. Expert Rev Mol Diagn. 2008;8:671–674. doi: 10.1586/14737159.8.6.671. Describes plausible causal events of autism. [DOI] [PubMed] [Google Scholar]
- 11.Goines P, Van de Water J. The immune system’s role in the biology of autism. Curr Opin Neurol. 2010;23:111–117. doi: 10.1097/WCO.0b013e3283373514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattila ML, Kielinen M, Linna SL, et al. Autism spectrum disorders according to DSM-IV-TR and comparison with DSM-5 draft criteria: an epidemiological study. J Am Acad Child Adolesc Psychiatry. 2011;50:583–592.e11. doi: 10.1016/j.jaac.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 13▪.Skogstrand K, Thorsen P, Norgaard-Pedersen B, Schendel DE, Sorensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51:1854–1866. doi: 10.1373/clinchem.2005.052241. One of the first reports to use dried bloodspot assays for autism. [DOI] [PubMed] [Google Scholar]
- 14.Lindau-Shepard BA, Pass KA. Newborn screening for cystic fibrosis by use of a multiplex immunoassay. Clin Chem. 2010;56:445–450. doi: 10.1373/clinchem.2009.132480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2. Wiley-Interscience; NJ, USA: 2002. [Google Scholar]
- 16.Tian GL, Lang HB, Liu Z, Tan MT. Regularized (bridge) logistic regression for variable selection based on ROC criterion. Stat Interface. 2009;2:493–502. [Google Scholar]
- 17.James SJ, Cutler P, Melnyk S, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- 18.Williams TA, Mars AE, Buyske SG, et al. Risk of autistic disorder in affected offspring of mothers with a glutathione S-transferase P1 haplotype. Arch Pediatr Adolesc Med. 2007;161:356–361. doi: 10.1001/archpedi.161.4.356. [DOI] [PubMed] [Google Scholar]
- 19.Al-Yafee YA, Al-Ayadhi LY, Haq SH, El-Ansary AK. Novel metabolic biomarkers related to sulfur-dependent detoxification pathways in autistic patients of Saudi Arabia. BMC Neurol. 2011;11:139. doi: 10.1186/1471-2377-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buyske S, Williams TA, Mars AE, et al. Analysis of case-parent trios at a locus with a deletion allele: association of GSTM1 with autism. BMC Genet. 2006;7:8. doi: 10.1186/1471-2156-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James SJ, Melnyk S, Jernigan S, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2001;141B:947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsarouhas K, Tsitsimpikou C, Haliassos A, et al. Study of insulin resistance, TNF-α, total antioxidant capacity and lipid profile in patients with chronic heart failure under exercise. In Vivo. 2012;25:1031–1037. [PubMed] [Google Scholar]
- 23.Dong J, Guo H, Yang R, et al. A novel and precise method for simultaneous measurement of serum HDL and LDL subfractions and lipoprotein (a) cholesterol by ultracentrifugation and high-performance liquid chromatography. Clin Chim Acta. 2012;413:1071–1076. doi: 10.1016/j.cca.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Momiyama Y, Ohmori R, Fayad ZA, et al. Associations between serum lipoprotein(a) levels and the severity of coronary and aortic atherosclerosis. Atherosclerosis. 2012;222:241–244. doi: 10.1016/j.atherosclerosis.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Nwose EU, Richards RS, Bwititi P, Butkowski E. Serum bilirubin and lipoproteina: how are these associated with whole blood viscosity? Redox Rep. 2012;17:8–13. doi: 10.1179/1351000211Y.0000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh Wu J. Lipoprotein(a) in vascular disease, cancer and longevity. Chang Gung Med J. 2011;34:555–564. [PubMed] [Google Scholar]
- 27.Heermeier K, Schneider R, Heinloth A, Wanner C, Dimmeler S, Galle J. Oxidative stress mediates apoptosis induced by oxidized low-density lipoprotein and oxidized lipoprotein(a) Kidney Int. 1999;56:1310–1312. doi: 10.1046/j.1523-1755.1999.00685.x. [DOI] [PubMed] [Google Scholar]
- 28.Tyler CV, Schramm SC, Karafa M, Tang AS, Jain AK. Chronic disease risks in young adults with autism spectrum disorder: forewarned is forearmed. Am J Intellect Dev Disabil. 2011;116:371–380. doi: 10.1352/1944-7558-116.5.371. [DOI] [PubMed] [Google Scholar]
- 29.Pasca SP, Nemes B, Vlase L, et al. High levels of homocysteine and low serum paraoxonase 1 arylesterase activity in children with autism. Life Sci. 2006;78:2244–2248. doi: 10.1016/j.lfs.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 30▪ ▪.DeFelice ML, Ruchelli ED, Markowitz JE, et al. Intestinal cytokines in children with pervasive developmental disorders. Am J Gastroenterol. 2003;98:1777–1782. doi: 10.1111/j.1572-0241.2003.07593.x. Relates the importance of cytokines in autism. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki K, Matsuzaki H, Iwata K, et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS ONE. 2011;6:e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- 33.Abdallah MW, Larsen N, Grove J, et al. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J Biol Psychiatry. 2011 doi: 10.3109/15622975.2011.639803. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman AW, Jyonouchi H, Comi AM, et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol. 2005;33:195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Goines PE, Croen LA, Braunschweig D, et al. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: a case–control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Chauhan A, Sheikh AM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson PG, Kuddo T, Song EY, et al. Selected neurotrophins, neuropeptides, and cytokines: developmental trajectory and concentrations in neonatal blood of children with autism or Down syndrome. Int J Dev Neurosci. 2006;24:73–80. doi: 10.1016/j.ijdevneu.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Molloy CA, Morrow AL, Meinzen-Derr J, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2001;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Michaelson MD, Mehler MF, Xu H, Gross RE, Kessler JA. Interleukin-7 is trophic for embryonic neurons and is expressed in developing brain. Dev Biol. 1996;179:251–263. doi: 10.1006/dbio.1996.0255. [DOI] [PubMed] [Google Scholar]
- 41.Moors M, Vudattu NK, Abel J, et al. Interleukin-7 (IL-7) and IL-7 splice variants affect differentiation of human neural progenitor cells. Genes Immun. 2010;11:11–20. doi: 10.1038/gene.2009.77. [DOI] [PubMed] [Google Scholar]
- 42.Chang Y, Albright S, Lee F. Cytokines in the central nervous system: expression of macrophage colony stimulating factor and its receptor during development. J Neuroimmunol. 1994;52:9–17. doi: 10.1016/0165-5728(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 43.Grosjean MB, Lenzlinger PM, Stahel PF, et al. Immunohistochemical characterization of Fas (CD95) and Fas Ligand (FasL/CD95L) expression in the injured brain: relationship with neuronal cell death and inflammatory mediators. Histol Histopathol. 2007;22:235–250. doi: 10.14670/HH-22.235. [DOI] [PubMed] [Google Scholar]
- 44.Plant SR, Arnett HA, Ting JP. Astroglial-derived lymphotoxin-α exacerbates inflammation and demyelination, but not remyelination. Glia. 2001;49:1–14. doi: 10.1002/glia.20089. [DOI] [PubMed] [Google Scholar]
- 45.Suen WE, Bergman CM, Hjelmstrom P, Ruddle NH. A critical role for lymphotoxin in experimental allergic encephalomyelitis. J Exp Med. 1997;186:1233–1240. doi: 10.1084/jem.186.8.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connolly AM, Chez MG, Pestronk A, Arnold ST, Mehta S, Deuel RK. Serum autoantibodies to brain in Landau–Kleffner variant, autism, and other neurologic disorders. J Pediatr. 1991;134:607–613. doi: 10.1016/s0022-3476(99)70248-9. [DOI] [PubMed] [Google Scholar]
- 47▪ ▪.Singh VK, Warren RP, Odell JD, Warren WL, Cole P. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun. 1991;7:97–103. doi: 10.1006/brbi.1993.1010. Brain autoantibodies were localized in autistic patients. [DOI] [PubMed] [Google Scholar]
- 48.Vojdani A, Pangborn JB, Vojdani E, Cooper EL. Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int J Immunopathol Pharmacol. 2003;16:189–199. doi: 10.1177/039463200301600302. [DOI] [PubMed] [Google Scholar]
- 49.Vojdani A, Bazargan M, Vojdani E, et al. Heat shock protein and gliadin peptide promote development of peptidase antibodies in children with autism and patients with autoimmune disease. Clin Diagn Lab Immunol. 2004;11:515–524. doi: 10.1128/CDLI.11.3.515-524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Connolly AM, Chez M, Streif EM, et al. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau–Kleffner syndrome, and epilepsy. Biol Psychiatry. 2006;59:354–363. doi: 10.1016/j.biopsych.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 51▪ ▪.Hashimoto T, Aihara R, Tayama M, Miyazaki M, Shirakawa Y, Kuroda Y. Reduced thyroid-stimulating hormone response to thyrotropin-releasing hormone in autistic boys. Dev Med Child Neurol. 1991;33:313–319. doi: 10.1111/j.1469-8749.1991.tb14882.x. Demonstrates a thyroid link to autism in early childhood. [DOI] [PubMed] [Google Scholar]
- 52.Nir I, Meir D, Zilber N, Knobler H, Hadjez J, Lerner Y. Brief report: circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. J Autism Dev Disord. 1995;25:641–654. doi: 10.1007/BF02178193. [DOI] [PubMed] [Google Scholar]
- 53.Cohen DJ, Young JG, Lowe TL, Harcherik D. Thyroid hormone in autistic children. J Autism Dev Disord. 1980;10:445–450. doi: 10.1007/BF02414820. [DOI] [PubMed] [Google Scholar]
- 54.Scofield VL, Montufar-Solis D, Cheng E, Estes MK, Klein JR. Intestinal TSH production is localized in crypt enterocytes and in villus ‘hotblocks’ and is coupled to IL-7 production: evidence for involvement of TSH during acute enteric virus infection. Immunol Lett. 2005;99:36–44. doi: 10.1016/j.imlet.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids. 2011;2011:575840. doi: 10.1155/2011/575840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SO, Lou W, Hou M, Onate SA, Gao AC. Interleukin-4 enhances prostate-specific antigen expression by activation of the androgen receptor and Akt pathway. Oncogene. 2003;22:7981–7988. doi: 10.1038/sj.onc.1206735. [DOI] [PubMed] [Google Scholar]
- 57.Bouraoui Y, Ricote M, Garcia-Tunon I, et al. Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer. Cancer Detect Prev. 2008;32:23–32. doi: 10.1016/j.cdp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Zisman A, Lindner A, Zisman E, Lindner U, Mozes E. Prostate-specific antigen induces proliferation of peripheral blood lymphocytes and cytokine secretion in benign prostate hypertrophy patients. Eur Urol. 1999;36:258–265. doi: 10.1159/000068008. [DOI] [PubMed] [Google Scholar]
- 59.Cansino Alcaide JR, Vera San Martin R, Rodriguez de Bethencourt Codes F, et al. Prostatic specific antigen (PS), pro-inflammatory cytokines, and prostatic pathology (benign prostatic hyperplasia and cancer). Relationship with malignancy. Arch Esp Urol. 2009;62:359–366. doi: 10.4321/s0004-06142009000500005. [DOI] [PubMed] [Google Scholar]
- 60.Zabransky DJ, Smith HA, Thoburn CJ, et al. Lenalidomide modulates IL-8 and antiprostate antibody levels in men with biochemically recurrent prostate cancer. Prostate. 2012;72:487–498. doi: 10.1002/pros.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zofkova I, Bednar J. Effects of calcitonin, 1,25(OH)2 vitamin D3 and trifluoperazine on thyrotropic secretion and their mutual interactions. Exp Clin Endocrinol. 1988;92:268–274. [PubMed] [Google Scholar]
- 62.Ma R, Morshed S, Latif R, Zaidi M, Davies TF. The influence of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on osteoclastogenesis. Thyroid. 2011;21:897–906. doi: 10.1089/thy.2010.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahren B. Effects of calcitonin, katacalcin, and calcitonin gene-related peptide on basal and TSH-stimulated thyroid hormone secretion in the mouse. Acta Physiol Scand. 1989;135:133–137. doi: 10.1111/j.1748-1716.1989.tb08560.x. [DOI] [PubMed] [Google Scholar]
- 64.Anderson LE, Seybold VS. Calcitonin gene-related peptide regulates gene transcription in primary afferent neurons. J Neurochem. 2004;91:1417–1429. doi: 10.1111/j.1471-4159.2004.02833.x. [DOI] [PubMed] [Google Scholar]
- 65.Song EY, Vandunk C, Kuddo T, Nelson PG. Measurement of CGRP in dried blood spots using a modified sandwich enzyme immunoassay. J Neurosci Methods. 2006;155:92–97. doi: 10.1016/j.jneumeth.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 66.Dosman CF, Drmic IE, Brian JA, et al. Ferritin as an indicator of suspected iron deficiency in children with autism spectrum disorder: prevalence of low serum ferritin concentration. Dev Med Child Neurol. 2006;48:1008–1009. doi: 10.1017/S0012162206232225. [DOI] [PubMed] [Google Scholar]
- 67▪ ▪.Dosman CF, Brian JA, Drmic IE, et al. Children with autism: effect of iron supplementation on sleep and ferritin. Pediatr Neurol. 2007;36:152–158. doi: 10.1016/j.pediatrneurol.2006.11.004. Importance of iron supplementation shown in autistic children. [DOI] [PubMed] [Google Scholar]
- 68.Herguner S, Kelesoglu FM, Tanidir C, Copur M. Ferritin and iron levels in children with autistic disorder. Eur J Pediatr. 2012;171:143–146. doi: 10.1007/s00431-011-1506-6. [DOI] [PubMed] [Google Scholar]
- 69.Latif A, Heinz P, Cook R. Iron deficiency in autism and Asperger syndrome. Autism. 2002;6:103–114. doi: 10.1177/1362361302006001008. [DOI] [PubMed] [Google Scholar]
- 70.Obrenovich ME, Shamberger RJ, Lonsdale D. Altered heavy metals and transketolase found in autistic spectrum disorder. Biol Trace Elem Res. 2011;144:475–486. doi: 10.1007/s12011-011-9146-2. [DOI] [PubMed] [Google Scholar]
- 71.Youn SI, Jin SH, Kim SH, Lim S. Porphyrinuria in Korean children with autism: correlation with oxidative stress. J Toxicol Environ Health A. 2010;73:701–710. doi: 10.1080/15287391003614000. [DOI] [PubMed] [Google Scholar]
- 72.Pecorelli A, Leoncini S, De Felice C, et al. Nonprotein-bound iron and 4-hydroxynonenal protein adducts in classic autism. Brain Dev. 2012;35(2):146–154. doi: 10.1016/j.braindev.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 73▪ ▪.Jyonouchi H, Sun S, Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology. 2002;46:76–84. doi: 10.1159/000065416. Inflammation and cytokines linked to autistic patients in the clinic. [DOI] [PubMed] [Google Scholar]
- 74.Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2012;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45:1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- 76.Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. Elevation of tumor necrosis factor-α in cerebrospinal fluid of autistic children. Pediatr Neurol. 2007;36:361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 77.Malik M, Sheikh AM, Wen G, Spivack W, Brown WT, Li X. Expression of inflammatory cytokines, Bcl2 and cathepsin D are altered in lymphoblasts of autistic subjects. Immunobiology. 2011;216:80–85. doi: 10.1016/j.imbio.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Zubarev OE, Klimenko VM. Elevation of proinflammatory cytokines level at early age as the risk factor of neurological and mental pathology development. Ross Fiziol Zh Im I M Sechenova. 2011;97:1048–1059. [PubMed] [Google Scholar]
- 79.Wakefield AJ, Ashwood P, Limb K, Anthony A. The significance of ileo-colonic lymphoid nodular hyperplasia in children with autistic spectrum disorder. Eur J Gastroenterol Hepatol. 2005;17:827–836. doi: 10.1097/00042737-200508000-00009. [DOI] [PubMed] [Google Scholar]
- 80.Sheng YH, Lourie R, Linden SK, et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut. 2011;60:1661–1670. doi: 10.1136/gut.2011.239194. [DOI] [PubMed] [Google Scholar]
- 81.Croix JA, Bhatia S, Gaskins HR. Inflammatory cues modulate the expression of secretory product genes, Golgi sulfotransferases and sulfomucin production in LS174T cells. Exp Biol Med (Maywood) 2011;236:1402–1412. doi: 10.1258/ebm.2011.011186. [DOI] [PubMed] [Google Scholar]
- 82.Albertsmeyer AC, Kakkassery V, Spurr-Michaud S, Beeks O, Gipson IK. Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells. Exp Eye Res. 2010;90:444–451. doi: 10.1016/j.exer.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Joo NS, Kim KN, Kim KS. Serum CA125 concentration has inverse correlation with metabolic syndrome. J Korean Med Sci. 2011;26:1328–1332. doi: 10.3346/jkms.2011.26.10.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J Neurosci Res. 2003;74:801–806. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindberg RL, De Groot CJ, Montagne L, et al. The expression profile of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in lesions and normal appearing white matter of multiple sclerosis. Brain. 2001;124:1743–1753. doi: 10.1093/brain/124.9.1743. [DOI] [PubMed] [Google Scholar]
- 86.Dafnis I, Tzinia AK, Tsilibary EC, Zannis VI, Chroni A. An apolipoprotein E4 fragment affects matrix metalloproteinase 9, tissue inhibitor of metalloproteinase 1 and cytokine levels in brain cell lines. Neuroscience. 2012;210:21–32. doi: 10.1016/j.neuroscience.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nuttall RK, Silva C, Hader W, et al. Metalloproteinases are enriched in microglia compared with leukocytes and they regulate cytokine levels in activated microglia. Glia. 2007;55:516–526. doi: 10.1002/glia.20478. [DOI] [PubMed] [Google Scholar]
- 88.Ichiyama T, Kajimoto M, Suenaga N, Maeba S, Matsubara T, Furukawa S. Serum levels of matrix metalloproteinase-9 and its tissue inhibitor (TIMP-1) in acute disseminated encephalomyelitis. J Neuroimmunol. 2006;172:182–186. doi: 10.1016/j.jneuroim.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 89.Abdallah MW, Grove J, Hougaard DM, Norgaard-Pedersen B, Ibrahimov F, Mortensen EL. Autism spectrum disorders and maternal serum α-fetoprotein levels during pregnancy. Can J Psychiatry. 2011;56:727–734. doi: 10.1177/070674371105601204. [DOI] [PubMed] [Google Scholar]
- 90.Pressman EK, Thornburg LL, Glantz JC, et al. Inflammatory cytokines and antioxidants in midtrimester amniotic fluid: correlation with pregnancy outcome. Am J Obstet Gynecol. 2011;204:155.e1–e7. doi: 10.1016/j.ajog.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 91.Newschaffer CJ, Croen LA, Daniels J, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 92.Carlini P, Ferranti P, Polizio F, Ciriolo MR, Rotilio G. Purification and characterization of α-Fetoprotein from the human hepatoblastoma HepG2 cell line in serum-free medium. Biometals. 2007;20:869–878. doi: 10.1007/s10534-006-9080-5. [DOI] [PubMed] [Google Scholar]
- 93.King CR. A novel embryological theory of autism causation involving endogenous biochemicals capable of initiating cellular gene transcription: a possible link between twelve autism risk factors and the autism ‘epidemic’. Med Hypotheses. 2011;76:653–660. doi: 10.1016/j.mehy.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 94.Hu VW, Steinberg ME. Novel clustering of items from the autism diagnostic interview-revised to define phenotypes within autism spectrum disorders. Autism Res. 2009;2:67–77. doi: 10.1002/aur.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mizejewski GJ. Biomarker testing for suspected autism spectrum disorder in early children: is such testing now feasible? Biomarkers Med. 2012;6(4):1–4. doi: 10.2217/bmm.12.34. [DOI] [PubMed] [Google Scholar]