ABSTRACT
Among different types of protein aggregation, amyloids are a biochemically well characterized state of protein aggregation that are associated with a large number of neurodegenerative diseases including Parkinson's disease, Alzheimer and Creutzfeldt-Jakob disease. Yeast, Saccharomyces cerevisiae is an insightful model to understand the underlying mechanism of protein aggregation. Many yeast molecular chaperones can modulate aggregation and misfolding of proteins including α-Syn and the Sup35 prion. Hsp31 is a homodimeric protein structurally similar to human DJ-1, a Parkinson's disease-linked protein, and both are members of the DJ-1/ThiJ/PfpI superfamily. An emerging view is that Hsp31 and its associated superfamily members each have divergent multitasking functions that have the common theme of responding and managing various types of cellular stress. Hsp31 has several biochemical activities including chaperone and detoxifying enzyme activities that modulate at various points of a stress pathway such as toxicity associated with protein misfolding. However, we have shown the protective role of Hsp31's chaperone activity can operate independent of detoxifying enzyme activities in preventing the early stages of protein aggregate formation and associated cellular toxicities. We provide additional data that collectively supports the multiple functional roles that can be accomplished independent of each other. We present data indicating Hsp31 purified from yeast is more active compared to expression and purification from E. coli suggesting that posttranslational modifications could be important for Hsp31 to be fully active. We also compare the similarities and differences in activities among paralogs of Hsp31 supporting a model in which this protein family has overlapping but diverging roles in responding to various sources of cellular stresses.
Keywords: Hsp31, DJ-1 superfamily; stress response; chaperone; methylglyoxalase; degylcase; prion, α-synuclein
INTRODUCTION
Parkinson's disease is associated with progressive deterioration of dopaminergic neurons in the substantia nigra and the second most common age-related neurodegenerative disease.1 Accumulation of high level of reactive oxygen species (ROS), mitochondrial dysfunction and α-Syn aggregation are forms of cellular stress that lead to toxicity and neuronal cell death.2 Mutations in certain genes are involved in the development of familial form of PD including the PARK7 gene encoding DJ-1. DJ-1 is a member of ThiJ/DJ-1/PfpI protein superfamily that are a quintessential multitasking or moonlighting protein family as evidenced by their involvement in multiple cellular functions including oxidative stress sensing, protein folding, proteasome degradation, mitochondrial complex stabilization, methylglyoxalase and deglycation enzyme activities.3-7 The E. coli protein, hchA, has been bioinformatically identified as a moonlighting protein.8 The members of the ThiJ/DJ-1/Pfp1 superfamily appear to have evolved to numerous mechanisms to manage cellular stress. The protein superfamily members are present across the evolutionary spectrum including prokaryotes and the budding yeast, Saccharomyces cerevisiae, that has four paralogs named Hsp31, Hsp32, Hsp33 and Hsp34.9,10 Hsp31 consists of 237 amino acids with a molecular weight of 25.5 kDa and forms a homodimer in solution.9 It possesses the Cys-His-Glu catalytic triad common to ThiJ/DJ-1/PfpI superfamily proteins.9,10 This short review will discuss recent studies demonstrating Hsp31 exhibits diverse cellular functions to assist cellular survival under stress conditions.
ROLE OF HSP31 IN CELLULAR STRESS RESPONSE AND PROTEOTOXIC STRESS
Consistent with the main role of cellular stress response, Hsp31 expression is strongly induced during late phases of growth and required for survival under conditions of nutrient limitation.11 Hsp31 expression is also induced when yeast cells are treated with H2O2 to produce oxidative stress and this up-regulation of Hsp31 is under the control of stress responsive transcription factor, Yap1.12,13 In addition, Hsp31 possesses robust glutathione independent glyoxalase activity that converts the toxic metabolite, glyoxal (MG), into D-lactate.12,14,15 The catalytic triad Cys-His-Glu of Hsp31 is vital for this enzymatic activity and critical for suppressing the elevated level of ROS by MG.12,15 An overall view has emerged that indicates Hsp31 has important metabolic and regulatory roles in cytoprotective pathways.16
In addition to the enzyme activity, we also demonstrated that Hsp31 has broad chaperone activity in several classic protein aggregation assays indicating its ability to manage misfolded proteins that initiate proteotoxic stress in the cell.12 Intriguingly, Hsp31 purified directly from a yeast expression system, the GAL promoter induced movable ORF tag system (MORF),17 had increased enzymatic activity compared to Hsp31 purified from recombinant E. coli (Fig. 1). We also demonstrated that the yeast-purified protein was more active in preventing aggregation of several substrate proteins including α-Syn. The increased activity may be due to the difference in the affinity tags used but may be a result of posttranslational modification(s) that occur in the cell. Several reports have indicated that post-translational modifications or differing levels of oxidation of the cysteine residue can alter activity of DJ-1.18,19 We also show that Hsp31 is a more potent methylglyoxalase compared to DJ-1 consistent with several other studies (Fig. 1).14,15 In addition, we showed that yeast Hsp31 is more active in preventing protein aggregation compared to DJ-1. Interestingly, it has also been observed that Hsp31 rescues α-Syn toxicity to a greater extent than DJ-1 in vivo.5 These results raise the intriguing possibility that Hsp31 is constitutively active whereas DJ-1 must undergo an activation event to increase its activity.
FIGURE 1.
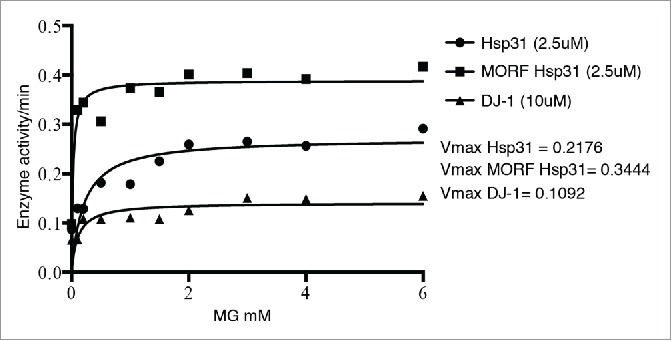
The hyperbolic plot of substrate concentration versus rate of D-lactate production by Hsp31 (circles), MORF Hsp31 (squares) and DJ-1 (triangles) are shown and the solid line represents the Michaelis-Menten best-fit model. The Vmax parameters were determined based on this model.
Hsp31 inhibits the formation of aggregates of a wide range of proteins including insulin, citrate synthase, α-Syn and the Sup35 prion.12 The recombinant soluble α-Syn and Sup35 proteins can readily polymerize into amyloidogenic fibrils in vitro.20 Hsp31 inhibits protein aggregation formation in vitro and foci formation of GFP-tagged proteins in vivo. In addition, over-expression of Hsp31 can rescue cells from toxicity associated with overexpression of α-Syn.12 The rescue phenotype could be mediated by several different mechanisms including Hsp31's methylglyoxalase or deglycation activities. However, a Hsp31 mutant deficient in methylglyoxalase activity is still active in preventing α-Syn in vitro aggregation and prevention of toxicity from overexpressed α-Syn. Another possible rescue mechanism could be the autophagy pathway, particularly because deletion of HSP31 impairs autophagy under carbon starvation conditions.11 Autophagy does alleviate toxicity from α-Syn overexpression because we show a synthetic lethal relationship between α-Syn overexpression and deletion of ATG8. However, we demonstrated that overexpression of Hsp31 in the atg8Δ strain can rescue α-Syn-mediated toxicity.12 These data show that despite the multitasking abilities and roles of Hsp31, the chaperone activity appears to have the ability to prevent α-Syn independent of other activities. In support of our finding, another study found the autophagy pathway was not crucial in preventing the Hsp31 chaperone activity against a cytoplasmic aggregation prone protein.21 The same study also demonstrated that Hsp31 chaperone activity overlaps with the Ubr-dependent degradation pathway but is independent of its function oxidative stress response.21 Further exploration of this model would need to utilize mutants that abrogate chaperone function without affecting enzyme activity and other biological functions.
The typical models of protein aggregation propose unfolded monomers as an initiating event that progresses to unstable oligomeric intermediates, and finally elongates to larger oligomers. The observed anti-aggregation activity of Hsp31 raises the question of what stage Hsp31 intervenes in the protein aggregation process. On the bases of our in vitro studies, Hsp31 likely interacts with early oligomeric intermediates of α-Syn and therefore prevents higher oligomer formation. For example, when soluble α-Syn was mixed with Hsp31, formation of precipitated SDS-resistant oligomers was markedly reduced. Likewise, the soluble fraction of α-Syn, which included SDS-resistant oligomers in the size range of 25-50 KDa, was almost completely abolished in the presence of Hsp31 indicating that Hsp31 likely interacts with the monomeric or early oligomers in preventing the formation of higher order oligomers. We observed similar results with the in vitro ThioT assay, in which incubating the Hsp31 with α-Syn completely prevented the increase in fluorescence intensity associated with increasing fibril formation again supporting our model that Hsp31 acts at early stages of protein aggregation.12 Of particular interest is that DJ-1 has been shown to interact with monomeric and oligomeric forms of α-Syn as determined by co-IP.5 This same study also showed that DJ-1 interacts with α-Syn in vivo as well. Along with α-Syn aggregation formation, we also demonstrated the inhibitory effect of Hsp31 on the Sup35 prion based on reduction of aggregates observed in cell lysates. These results support the notion that Hsp31 acts at the early stages of oligomerization to prevent further protein oligomerization.12 Interestingly, we previously showed that the overexpression of Hsp31 reduces the level of Sup35 aggregation but we also show that it is unable to cure prions from a [PSI+] strain (Fig. 2). [PSI+] strains contain Sup35 prion aggregates that can be cured by chaperones such as Hsp104, but the lack of curing by Hsp31 suggests that it lacks disaggregase activity and cannot intervene in an established prion cycle. Moreover, our study showed a lack of co-localization between Hsp31 and Sup35 aggregates with fluorescence microscopy, rather Hsp31 is occluded from Sup35 prion aggregates, indicating that Hsp31 acts on its substrates prior to the formation of large aggregates.12 The strong chaperone activity of Hsp31 suggests that it may modulate prion aggregates but might need to cooperate with other chaperones similar to what has been demonstrated with other small HSPs such as Hsp26 and Hsp42.22 Further studies investigating the role of Hsp31 in modulating prionogenesis are ongoing in our lab.
FIGURE 2.
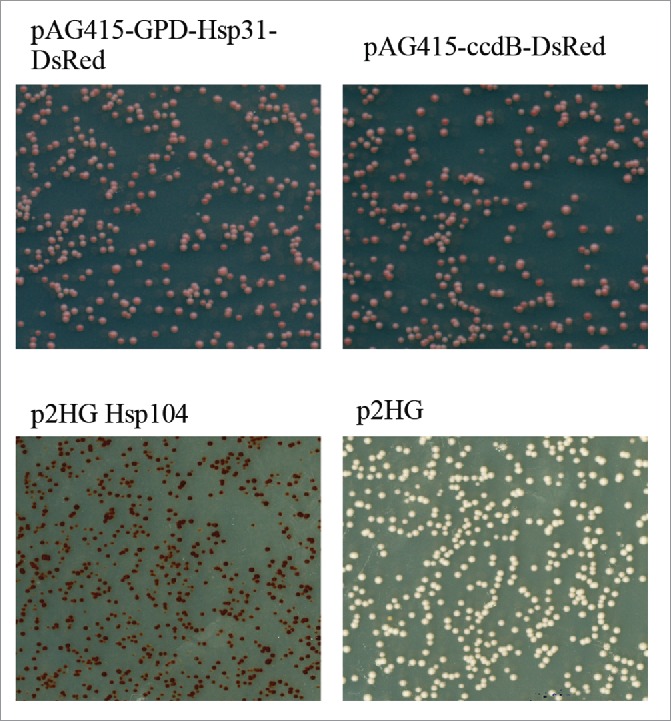
Hsp31 cannot resolve the Sup35 amyloids. [PSI+] cells were transferred with pAG415-GPD-Hsp31-DsRed, p2HG Hsp104 and their corresponding empty vectors. Cultures were incubated at 30°C for 16 hrs before plating on ¼ YPD medium to develop colony color. Change from white to red color phenotype demonstrated curing. Hsp104 is able to cure the [PSI+] phenotype as seen by the red colonies. Hsp31 expression under these conditions can transiently reduce prion aggregation as shown by semi-denaturing gels and microscopy but is not able to cure prions.
COMPARISON OF HSP31 PARALOGS
The Hsp31 mini-family is composed of four paralogs; Hsp31 (YDR533C), Hsp32 (YMR322C), Hsp33 (YOR391C), and Hsp34 (YPL280W).9,10 Genes of the Hsp31 mini family are located at the subtelomeric region of the genome in Saccharomyces cerevisiae. HSP31 is considered the parental gene with HSP32, HSP33 and HSP34 originating from it during gene duplication events. Among all the members of this mini-family, Hsp31 is most divergent and it shares approximately 70% homology with the other members of the family those possess more than 90% homology between them.9 All the members of Hsp31 family contain the same Cys-His-Glu catalytic triad as present in the E. coli ortholog but interestingly no protease activity has been detected so far for Hsp31 or other paralogs. Previously, it was shown that mutation in the catalytic triad largely abolishes glyoxalase activity12,15 but this catalytic triad is not required for chaperone activity of Hsp31.12 These results indicate that the anti-aggregation activity of Hsp31 is not under the influence of its enzymatic activity rather, it has a direct chaperone activity against misfolded proteins. Intriguingly, all the paralogs of the Hsp31 minifamily possess comparable activity against α-Syn aggregation and toxicity when they are overexpressed from the GAL promoter.5 Furthermore, the chaperone activity of Hsp31, Hsp32 and Hsp33 against a cytoplasmic aggregation-prone protein is independent of their role in oxidative stress response and the vacuolar degradation pathway.21 However, unlike Hsp31, the other paralogs possess very little methylglyoxalase activity and are unable to protect the cells from glyoxal toxicity.15 These results again support the notion that anti-aggregation activity of Hsp31 mini-family is independent of its enzymatic activity. In addition, the lack of methylglyoxalase activity in the paralogs is evidence that the paralogs are diverging but additional studies dissecting the roles within this paralog group are needed to further uncover these diverging functions. The Hsp31 protein family are broadly spread across fungal species with varying levels of paralog duplications and additional evidence of divergence including differences in localization in the Schizosaccharomyces pombe Hsp31 family members.23 A functional comparison of Hsp31 and its paralogs are summarized in Table 1 highlighting the similarities and differences among these proteins.
TABLE 1.
Functional summary of Hsp31 and paralogs.
| Function/Attribute | Hsp31 | Hsp32 | Hsp33 | Hsp34 |
|---|---|---|---|---|
| Catalytic triad | Yes | Yes | Yes | Yes |
| Chromosome Position | Interstitial | Telomeric | Telomeric | Telomeric |
| Sequence homology | ˜70% | >90% | >90% | >90% |
| Chaperone activity | +++ | +++ | +++ | +++ |
| Methylglyoxalase | +++ | +/− | +/− | +/− |
| Deglycase | +++ | ND | ND | ND |
| Role in Autophagy | + | + | + | + |
| Peak mRNA level | Early SP | DS | Early SP | ND |
| Peak steady state protein level | SP | ND | ND | ND |
| Stress granule and P body localization | Yes | Yes | ND | ND |
| Mitochondrial localization | Yes | ND | ND | ND |
ND = Not determined
SP = Stationary Phase
DS = Diauxic shift
HSP31 PLAYS AN IMPORTANT ROLE IN MAINTAINING REDOX HOMEOSTASIS
Oxidative stress occurs when intra-cellular reactive oxygen species (ROS) overwhelms the anti-oxidative defense system in the cell present during normal aerobic metabolism or by exposure to external radical generating agents. ROS triggers damage to macromolecules in the form of oxidative modifications and misfolding of proteins, associated with the development of diseases and pathological conditions such as PD and prions.2,24,25 Hsp31 has an important role in maintenance of redox homeostasis in yeast under oxidative stress generated by methylglyoxal or H2O2.13,15 Like many other heat-shock genes HSP26, HSP12, HSP82, and SSA3, expression of HSP31 is strongly induced at diauxic shift when the cells are stressed by nutrient limitation and by accumulation of oxidative metabolites.11-13 Others and we also reported an elevated level of Hsp31 under oxidative stress when cells were treated with H2O2.12,13 Similarly, Hsp31 also plays a role in the survival of cells during stationary phase and protects cells from oxidative stress caused by methylglyoxal and H2O2 accumulation.13,15 In addition, we demonstrated that Hsp31 expression was induced under proteotoxic stress such as overexpression of α-Syn. In support of the role of Hsp31 in managing this proteotoxic stress, we found that deletion of HSP31 synergizes with α-Syn expression to increasing toxicity. We reported an increase in ROS level in the hsp31Δ strain that correlates with increased toxicity by α-Syn expression compared to wild type strains, indicating that the presence of Hsp31 is important in reducing ROS to basal levels.12 In agreement with our study, overexpression of Hsp31 robustly suppresses both cytosolic and mitochondrial ROS levels instigated by MG and H2O2 and therefore provides cytoprotection.15 In addition, Hsp31 localizes to mitochondria and preserves mitochondrial integrity by redistributing glutathione to the cytoplasm under oxidative stress.15 Another study examined the deglycase activity of Hsp31 and showed that it efficiently deglycates proteins with glycated Cys, Arg and Lys amino acid residues.26 Taken together, these results suggest that Hsp31 is an integral part of the heat shock protein system and plays a vital role in maintaining cellular homeostasis.
CONCLUSION
Once again, our results together with other recent findings demonstrate the multitasking ability of Hsp31, which is particularly important during stressful situations. It functions as a stress response chaperone, glutathione independent methylglyoxalase, has a role in the autophagy pathway and acts as a deglycase. Other possible functions have been observed for this superfamily including a report of RNA binding for DJ-1 and protease activity for other family members.27-30 These multiple functions can modulate the protein misfolding and stress pathways at various points in the cellular network but our results also highlight that Hsp31 has the ability to inhibit protein aggregation distinct of its enzymatic activity (Fig. 3). We believe that Hsp31 acts at the initial phases of protein misfolding process and prevents the formation of larger aggregates but does not possess disaggregase activity.
FIGURE 3.
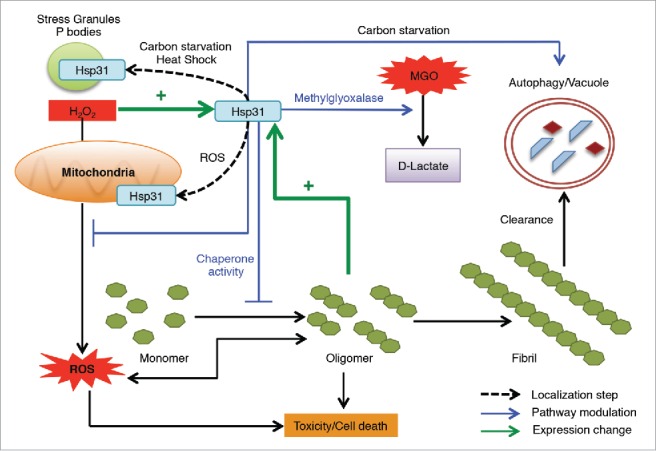
The homeostatic functions of Hsp31 associated with protecting cells from stress. Hsp31 is a methylglyoxalase that converts MGO into D-lactate independent of glutathione. Proteotoxic stress induced the expression of Hsp31, which exerts a protective function against toxic effect of oligomers in yeast cells. Oxidative stress induces the expression of Hsp31, re-localizes it to mitochondria resulting in reduced levels of ROS. Response to other stresses leads to Hsp31 localization to P bodies and stress granules. HSP31 deletion under carbon starvation compromises the autophagy pathway, which is a pathway used to clear oligomerized or aggregated proteins. Despite the role of Hsp31 in autophagy, it has a protective effect against α-Syn oligomerization independent of its role in autophagy because of its inhibitory effect early in the oligomerization process.
FUTURE DIRECTIONS
Given the apparent functional diversity of Hsp31 revealed so far, it is likely that there might be other chaperone dependent and independent functions of this protein that may exist. Many heat shock proteins (HSP) work in collaboration with other chaperone in order to be fully active. The exploration and identification of protein-protein interaction partners of Hsp31 would provide insight on mechanism and roles of Hsp31. There is dearth of protein-protein interaction information for Hsp31 although it was reported to interact with other chaperones according to a large-scale proteomic study.31 Deletion of HSP31 down-regulates the Ssa3, a Hsp70 paralogn, mRNA level at stationary phase suggesting a correlation between Hsp31 and Hsp70 activity.11 Human homolog DJ-1 is known to interact with many chaperones including Hsp70 and mitochondrial Hsp70 indicating that translocation of DJ-1 to mitochondria depends on these chaperones.4 A distinct possibility is that relocation of Hsp31 to mitochondria, P bodies or stress granules under oxidative stress is dependent on interactions with other chaperones. Our data support that Hsp31 acts at early stages of protein aggregation but the precise mechanism is not clear. Hsp31 may interact with unfolded monomers to sequester them from progressing to oligomers or alternatively, it might become active only after smaller oligomers are formed. The experiments we performed do not differentiate between these two scenarios and therefore further investigation is needed. Although we have shown that Hsp31 cannot cure [PSI+] prions, its ability to reduce the Sup35 prion aggregation raises the possibility that Hsp31 has a potential role in modulating prion induction.
Abbreviations
- α-Syn
α-Synuclein
- PD
Parkinson's disease
- HSP
Heat shock protein
- ROS
Reactive oxygen species
- MGO
Methylglyoxal
- MORF
Yeast movable ORF
- DsRed
Red fluorescence protein from Discosoma sp
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Funding
K.A. was supported by the Fulbright Foundation – Pakistan. This publication was made possible, in part, with support from the Indiana Clinical and Translational Sciences Institute funded, in part by grant # UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award and grant # R01 GM087461 from National Institutes of Health - NIGMS.
REFERENCES
- [1].Ross CA, Poirier MA. Opinion: What is the role of protein aggregation in neurodegeneration? Nat Rev Mol Cell Biol 2005; 6:891-8; PMID:16167052; http://dx.doi.org/ 10.1038/nrm1742 [DOI] [PubMed] [Google Scholar]
- [2].Witt SN, Flower TR. α-Synuclein, oxidative stress and apoptosis from the perspective of a yeast model of Parkinson's disease. FEMS Yeast Res 2006; 6:1107-16; PMID:17156009; http://dx.doi.org/ 10.1111/j.1567-1364.2006.00135.x [DOI] [PubMed] [Google Scholar]
- [3].Ariga H, Takahashi-Niki K, Kato I, Maita H, Niki T, Iguchi-Ariga SM. Neuroprotective function of DJ-1 in Parkinson's disease. Oxid Med Cell Longev 2013; 2013:683920; PMID:23766857; http://dx.doi.org/ 10.1155/2013/683920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Batelli S, Albani D, Rametta R, Polito L, Prato F, Pesaresi M, Negro A, Forloni G. DJ-1 modulates α-Synuclein aggregation state in a cellular model of oxidative stress: relevance for Parkinson's disease and involvement of HSP70. PLoS One 2008; 3:e1884; PMID:18382667; http://dx.doi.org/ 10.1371/journal.pone.0001884 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [5].Zondler L, Miller-Fleming L, Repici M, Goncalves S, Tenreiro S, Rosado-Ramos R, Betzer C, Straatman KR, Jensen PH, Giorgini F, et al.. DJ-1 interactions with α-synuclein attenuate aggregation and cellular toxicity in models of Parkinson's disease. Cell Death Dis 2014; 5:e1350; PMID:25058424; http://dx.doi.org/ 10.1038/cddis.2014.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee JY, Song J, Kwon K, Jang S, Kim C, Baek K, Kim J, Park C. Human DJ-1 and its homologs are novel glyoxalases. Hum Mol Genet 2012; 21:3215-25; PMID:22523093; http://dx.doi.org/ 10.1093/hmg/dds155 [DOI] [PubMed] [Google Scholar]
- [7].Toyoda Y, Erkut C, Pan-Montojo F, Boland S, Stewart MP, Muller DJ, Wurst W, Hyman AA, Kurzchalia TV. Products of the Parkinson's disease-related glyoxalase DJ-1, D-lactate and glycolate, support mitochondrial membrane potential and neuronal survival. Biol Open 2014; 3:777-84; PMID:25063200; http://dx.doi.org/ 10.1242/bio.20149399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khan I, Chen Y, Dong T, Hong X, Takeuchi R, Mori H, Kihara D. Genome-scale identification and characterization of moonlighting proteins. Biol Direct 2014; 9:30; PMID:25497125; http://dx.doi.org/ 10.1186/s13062-014-0030-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Graille M, Quevillon-Cheruel S, Leulliot N, Zhou CZ, Li de la Sierra Gallay I, Jacquamet L, Ferrer JL, Liger D, Poupon A, Janin J, et al.. Crystal structure of the YDR533c S. cerevisiae protein, a class II member of the Hsp31 family. Structure 2004; 12:839-47; PMID:15130476; http://dx.doi.org/ 10.1016/j.str.2004.02.030 [DOI] [PubMed] [Google Scholar]
- [10].Wilson MA, St Amour CV, Collins JL, Ringe D, Petsko GA. The 1.8-A resolution crystal structure of YDR533Cp from Saccharomyces cerevisiae: a member of the DJ-1/ThiJ/PfpI superfamily. Proc Natl Acad Sci U S A 2004; 101:1531-6; PMID:14745011; http://dx.doi.org/ 10.1073/pnas.0308089100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Miller-Fleming L, Antas P, Pais TF, Smalley JL, Giorgini F, Outeiro TF. Yeast DJ-1 superfamily members are required for diauxic-shift reprogramming and cell survival in stationary phase. Proc Natl Acad Sci U S A 2014; 111:7012-7; PMID:24706893; http://dx.doi.org/ 10.1073/pnas.1319221111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tsai CJ, Aslam K, Drendel HM, Asiago JM, Goode KM, Paul LN, Rochet JC, Hazbun TR. Hsp31 Is a stress response chaperone that intervenes in the protein misfolding process. J Biol Chem 2015; 290:24816-34; PMID:26306045; http://dx.doi.org/ 10.1074/jbc.M115.678367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Skoneczna A, Micialkiewicz A, Skoneczny M. Saccharomyces cerevisiae Hsp31p, a stress response protein conferring protection against reactive oxygen species. Free Radic Biol Med 2007; 42:1409-20; PMID:17395014; http://dx.doi.org/ 10.1016/j.freeradbiomed.2007.01.042 [DOI] [PubMed] [Google Scholar]
- [14].Hasim S, Hussin NA, Alomar F, Bidasee KR, Nickerson KW, Wilson MA. A glutathione-independent glyoxalase of the DJ-1 superfamily plays an important role in managing metabolically generated methylglyoxal in Candida albicans. J Biol Chem 2014; 289:1662-74; PMID:24302734; http://dx.doi.org/ 10.1074/jbc.M113.505784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bankapalli K, Saladi S, Awadia SS, Goswami AV, Samaddar M, D'Silva P. Robust glyoxalase activity of Hsp31, a ThiJ/DJ-1/PfpI family member protein, Is critical for oxidative stress resistance in Saccharomyces cerevisiae. J Biol Chem 2015; 290:26491-507; PMID:26370081; http://dx.doi.org/ 10.1074/jbc.M115.673624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wilson MA. Metabolic role for yeast DJ-1 superfamily proteins. Proc Natl Acad Sci U S A 2014; 111:6858-9; PMID:24785296; http://dx.doi.org/ 10.1073/pnas.1405511111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gelperin DM, White MA, Wilkinson ML, Kon Y, Kung LA, Wise KJ, Lopez-Hoyo N, Jiang L, Piccirillo S, Yu H, et al.. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev 2005; 19:2816-26; PMID:16322557; http://dx.doi.org/ 10.1101/gad.1362105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Madian AG, Hindupur J, Hulleman JD, Diaz-Maldonado N, Mishra VR, Guigard E, Kay CM, Rochet JC, Regnier FE. Effect of single amino acid substitution on oxidative modifications of the Parkinson's disease-related protein, DJ-1. Mol Cell Proteomics 2012; 11:M111 010892; PMID:22104028; http://dx.doi.org/ 10.1074/mcp.M111.010892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hulleman JD, Mirzaei H, Guigard E, Taylor KL, Ray SS, Kay CM, Regnier FE, Rochet JC. Destabilization of DJ-1 by familial substitution and oxidative modifications: implications for Parkinson's disease. Biochem 2007; 46:5776-89; http://dx.doi.org/ 10.1021/bi7001778 [DOI] [PubMed] [Google Scholar]
- [20].Krzewska J, Tanaka M, Burston SG, Melki R. Biochemical and functional analysis of the assembly of full-length Sup35p and its prion-forming domain. J Biol Chem 2007; 282:1679-86; PMID:17121860; http://dx.doi.org/ 10.1074/jbc.M608110200 [DOI] [PubMed] [Google Scholar]
- [21].Amm I, Norell D, Wolf DH. Absence of the yeast Hsp31 chaperones of the DJ-1 superfamily perturbs cytoplasmic protein quality control in late growth phase. PLoS One 2015; 10:e0140363; PMID:26466368; http://dx.doi.org/ 10.1371/journal.pone.0140363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duennwald ML, Echeverria A, Shorter J. Small heat shock proteins potentiate amyloid dissolution by protein disaggregases from yeast and humans. PLoS Biol 2012; 10:e1001346; PMID:22723742; http://dx.doi.org/ 10.1371/journal.pbio.1001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhao Q, Su Y, Wang Z, Chen C, Wu T, Huang Y. Identification of glutathione (GSH)-independent glyoxalase III from Schizosaccharomyces pombe. BMC Evolutionary Biol 2014; 14:86; http://dx.doi.org/ 10.1186/1471-2148-14-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Grant CM. Sup35 methionine oxidation is a trigger for de novo [PSI(+)] prion formation. Prion 2015; 9:257-65; PMID:26267336; http://dx.doi.org/ 10.1080/19336896.2015.1065372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson's disease. J Parkinsons Dis 2013; 3:461-91; PMID:24252804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mihoub M, Abdallah J, Gontero B, Dairou J, Richarme G. The DJ-1 superfamily member Hsp31 repairs proteins from glycation by methylglyoxal and glyoxal. Biochem Biophys Res Commun 2015; 463:1305-10; PMID:26102038; http://dx.doi.org/ 10.1016/j.bbrc.2015.06.111 [DOI] [PubMed] [Google Scholar]
- [27].Chen J, Li L, Chin LS. Parkinson disease protein DJ-1 converts from a zymogen to a protease by carboxyl-terminal cleavage. Hum Mol Genet 2010; 19:2395-408; PMID:20304780; http://dx.doi.org/ 10.1093/hmg/ddq113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Koide-Yoshida S, Niki T, Ueda M, Himeno S, Taira T, Iguchi-Ariga SM, Ando Y, Ariga H. DJ-1 degrades transthyretin and an inactive form of DJ-1 is secreted in familial amyloidotic polyneuropathy. Int J Mol Med 2007; 19:885-93; PMID:17487420 [PubMed] [Google Scholar]
- [29].Olzmann JA, Brown K, Wilkinson KD, Rees HD, Huai Q, Ke H, Levey AI, Li L, Chin LS. Familial Parkinson's disease-associated L166P mutation disrupts DJ-1 protein folding and function. J Biol Chem 2004; 279:8506-15; PMID:14665635; http://dx.doi.org/ 10.1074/jbc.M311017200 [DOI] [PubMed] [Google Scholar]
- [30].van der Brug MP, Blackinton J, Chandran J, Hao LY, Lal A, Mazan-Mamczarz K, Martindale J, Xie C, Ahmad R, Thomas KJ, et al.. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci U S A 2008; 105:10244-9; PMID:18626009; http://dx.doi.org/ 10.1073/pnas.0708518105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gong Y, Kakihara Y, Krogan N, Greenblatt J, Emili A, Zhang Z, Houry WA. An atlas of chaperone-protein interactions in Saccharomyces cerevisiae: implications to protein folding pathways in the cell. Mol Sys Biol 2009; 5:275. [DOI] [PMC free article] [PubMed] [Google Scholar]


