Supplemental Digital Content is Available in the Text.
Key Words: viremia copy-years, seroconverters, when to start, cART initiation, CD4 cell count, HIV-RNA
Abstract
Background:
Viremia copy-years (VCY), a time-updated measure of cumulative HIV exposure, predicts AIDS/death; although its utility in deciding when to start combination antiretroviral therapy (cART) remains unclear. We aimed to assess the impact of initiating versus deferring cART on risk of AIDS/death by levels of VCY both independent of and within CD4 cell count strata ≥500 cells per cubic millimeter.
Methods:
Using Concerted Action on Seroconversion to AIDS and Death in Europe (CASCADE) data, we created a series of nested “trials” corresponding to consecutive months for individuals ≥16 years at seroconversion after 1995 who were cART-naive and AIDS-free. Pooling across all trials, time to AIDS/death by CD4, and VCY strata was compared in those initiating vs. deferring cART using Cox models adjusted for: country, sex, risk group, seroconversion year, age, time since last HIV-RNA, and current CD4, VCY, HIV-RNA, and mean number of previous CD4/HIV-RNA measurements/year.
Results:
Of 9353 individuals, 5312 (57%) initiated cART and 486 (5%) acquired AIDS/died. Pooling CD4 strata, risk of AIDS/death associated with initiating vs. deferring cART reduced as VCY increased. In patients with high CD4 cell counts, ≥500 cells per cubic millimeter, there was a trend for a greater reduction for those initiating vs. deferring with increasing VCY (P = 0.09), with the largest benefit in the VCY ≥100,000 copy-years/mL group [hazard ratio (95% CI) = 0.41 (0.19 to 0.87)].
Conclusions:
For individuals with CD4 ≥500 cells per cubic millimeter, limiting the cumulative HIV burden to <100,000 copy-years/mL through cART may reduce the risk of AIDS/death.
INTRODUCTION
Although CD4 cell counts are used routinely to monitor adults with HIV infection, viral loads also have an important role in the monitoring and staging of adults with HIV.1,2 One or 2 values of an individual's viral load are often used to determine combination antiretroviral therapy (cART) failure, their risk of transmitting HIV to others, and to tailor first-line cART regimens.3–5 However, assessment of an individual's viral load at a single point in time fails to capture cumulative exposure to HIV replication which may have been over a period of 10 years or more. Several investigators have proposed that a measure of cumulative viral burden might provide useful additional information and, in particular, a measurement of viremia copy-years (VCY) has been proposed.6 VCY is akin to cigarette pack-years when assessing exposure to tobacco; A VCY of 1000 copy-years/mL is the equivalent to an individual having a viral load of 1000 copies per milliliter for an entire year or a viral load of 500 copies per milliliter for 2 years. The measurement of VCY has been shown to predict death and AIDS in both the absence6 and presence7,8 of cART, independently of the individual's most recent CD4 count and viral load. This independent association suggests that cumulative HIV burden is associated with an increased risk of development of clinical events through mechanisms other than immunodeficiency.
United States guidelines recommend immediate cART initiation, regardless of CD4 cell count5,9 due to evidence that exposure to uncontrolled viremia is associated with an increased risk of death, AIDS, and non-AIDS events.5,10–12 The START (Starting Antiretroviral Treatment Early Improves Outcomes for HIV-Infected Individuals) trial has recently reported that waiting to initiate cART until CD4 <350 cells per cubic millimeter increases the likelihood of serious illness or death compared with immediate initiation.13 VCY serves as a measurement of cumulative exposure to HIV, and so it is important to determine whether VCY contributes to the likelihood of illness and death and whether cART initiation before the accrual of VCY could help optimize clinical and public health HIV outcomes.
Randomized trials are unlikely to be conducted to determine whether accrual of viremia VCY before cART initiation increases mortality because of the difficulty and expertise in enrolling participants soon after seroconversion, and because cART is now recommended in many asymptomatic populations. In addition, there is substantial potential for lead-time bias in analyses using VCY because of variability in the extent of HIV replication an individual will have been exposed to previous enrollment into care. One way to limit this bias is to restrict analyses to participants with serial viral load measurements since a known seroconversion date; such data are available from the Concerted Action on Seroconversion to AIDS and Death in Europe (CASCADE) Collaboration, an international multicenter collaboration of data from persons with well-estimated dates of HIV seroconversion. Previous analyses of CASCADE data have shown a protective effect of initiating cART on AIDS/death at CD4 <500 cells per cubic millimeter [hazard ratio (HR) 0.59 (95% CI: 0.43 to 0.81) and HR 0.75 (0.49–1.14) in CD4 cell strata 200–349 and 350–499, respectively], but no evidence for a reduction in risk at CD4 ≥500 cells per cubic millimeter [HR 1.10 (0.67–1.79)].14 Here we examine the effect of initiating or deferring cART at different levels of VCY on HIV disease progression. We investigate whether or not individuals with CD4 ≥500 cells per cubic millimeter but high VCY would benefit from starting cART.
METHODS
Study Population
Data from CASCADE in EuroCoord (www.EuroCoord.net) 2013 data update were used for this analysis.15 Briefly, CASCADE is a cohort collaboration of 29 cohorts of individuals with well-estimated dates of HIV seroconversion from Europe (94%), Australia (2%), Canada (0.5%), and Sub-Saharan Africa (3%). Date of seroconversion is estimated as the midpoint between the last negative and first positive HIV antibody test results with a maximum of 3 years between the test dates (85%), laboratory evidence of acute seroconversion (real-time polymerase chain reaction positivity of incomplete Western blot) (13%), the date of seroconversion illness with a negative and positive test no more than 3 years apart (2%), or by a probability distribution to determine the most likely date of transmission for men with hemophilia infected with HIV after transfusion with clotting factor concentrates (<1% of the sample).
All cohorts contributing to CASCADE received ethical approval from their individual ethics review boards.
Adults (≥16 years old) seroconverting in the cART era (post 1995) were included provided they had at least 1 HIV-RNA measurement between 4 and 12 months after seroconversion. Two Sub-Saharan African cohorts were excluded from this analysis as their CD4 cell count and cART initiation patterns are different from those in industrialized country cohorts.16
Study Design
We created a series of sequential nested “trials” corresponding to consecutive months of follow-up beginning 4 months after seroconversion, where each month represents the baseline month for a new trial (Fig. 1). As described previously, this approach allows appropriate adjustment for time-dependent confounding.17,18 We created new trials with all eligible individuals for each month between January 1996 and May 2013. Individuals were eligible for a trial if they were cART-naive before the baseline month, had a CD4 or HIV-RNA measurement 12 months before the baseline month, and were AIDS-free until the end of the baseline month. Time to AIDS/death was compared in those who initiated cART in each baseline month versus those who deferred, pooling across all trials.
FIGURE 1.
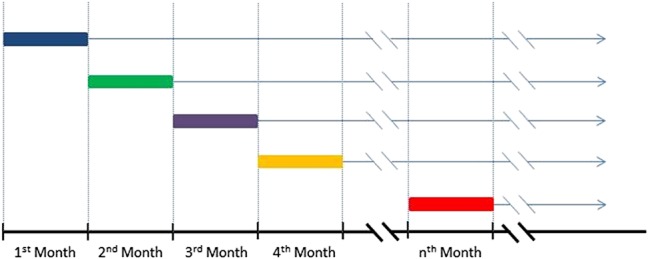
A diagram of the “trials” construction. Individuals are assessed for eligibility at the beginning of each month (respective trial baseline). Each eligible individual is classified as having initiated or deferred cART in the baseline month. Time is measured from the beginning of the following month until AIDS, death, or censoring for each eligible individual (excluding any with an outcome during the baseline month). Cox proportional hazards models are used to assess the effect of initiating compared with deferring cART on time to AIDS/death, pooled across all trials.
AIDS events in the first year of seroconversion were not considered as disease progression outcomes, but rather as severe seroconversion illness. In addition, invasive candidiasis was not considered an outcome in this analysis as it is typically less severe and associated with longer survival compared with other AIDS-defining conditions.19–22
Viremia Measurements
If HIV-RNA could be continuously measured within an individual from seroconversion [with the viral load distribution at any time t called as V(t)], then VCY would be calculated as the area under the HIV-RNA curve, or the integral of HIV-RNA from seroconversion to time t = T.
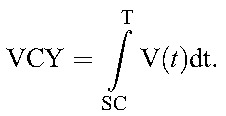 |
However, in practice, we do not have continuously measured viral loads, but rather snapshots of HIV-RNA measurements for each individual at irregularly spaced intervals (usually approximately 3 monthly). The best approximation to the integral with the data available can be obtained through use of the trapezoidal rule, which is how we approximated VCY for the remainder of this analysis. At any given time point, J, say, VCY(J) is given by: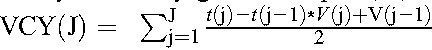 . We examined HIV-RNA data for implausible values and identified 3 individuals whose HIV-RNA dropped by factors of 4, 26, and 87 between consecutive measurements and without apparently starting cART. These are far greater drops than would be expected based on the known biological variation of HIV-RNA.23,24 As all 3 individuals were recorded as having started cART in the following month, we assumed that the date of cART initiation had been incorrectly recorded, and reset the cART start dates for these individuals to 1 month before that recorded.
. We examined HIV-RNA data for implausible values and identified 3 individuals whose HIV-RNA dropped by factors of 4, 26, and 87 between consecutive measurements and without apparently starting cART. These are far greater drops than would be expected based on the known biological variation of HIV-RNA.23,24 As all 3 individuals were recorded as having started cART in the following month, we assumed that the date of cART initiation had been incorrectly recorded, and reset the cART start dates for these individuals to 1 month before that recorded.
To estimate HIV exposure equally for all individuals, we removed HIV-RNA measurements taken in the first 3 months of seroconversion, as we were unlikely to capture the well-documented peak in viremia shortly after seroconversion25 for all individuals. In addition, we assumed that HIV-RNA measurements remained relatively stable over the period 4–12 months (consistent with findings of the viral load stabilizing after the initial peak in viremia), allowing us to make the assumption that an individual's first available HIV-RNA over the period 4–12 months was equal to their HIV-RNA at month 4.
Data Analysis
We describe baseline characteristics between those who initiated or deferred cART during the study period. We estimated the HRs for initiating versus deferring cART by levels of VCY (<10,000 copy-years/mL, ≥10,000–19,999 copy-years/mL, ≥20,000–49,999 copy-years/mL, ≥50,000–99,999 copy-years/mL, ≥100,000 copy-year/mL) pooled across and stratified by CD4 cell count strata (initiate at CD4 <350 cells/mm3 compared with initiate at higher values, ≥350 cells/mm3, and initiate at <500 cells/mm3 compared with initiate at higher values, ≥500 cells/mm3) using Cox proportional hazards models. We adjusted for trial-independent factors including country of care, sex, HIV transmission risk group, seroconversion year, and trial-dependent factors of current age, time since last HIV-RNA measurement, CD4, VCY, HIV-RNA, and mean number of previous CD4/HIV-RNA measurements per year. Trial-dependent factors were ascertained before the baseline month to ensure they were measured before the decision to initiate or defer cART in the current month. Continuous variables were modeled using restricted cubic splines, all with 3 knots with the exception of current CD4 which was modeled with 5 knots.26 Most individuals contributed to more than 1 trial, so we used a robust variance estimator to account for within-person correlation. To investigate whether a threshold existed where cART initiation showed the most benefit, we fitted interactions between initiating cART and VCY as a continuous variable with a 3-knot spline.
Furthermore, we investigated whether there was a benefit of incorporating other measures of viremia into the decision about when to initiate cART, namely, current HIV-RNA (most recent measurement), average HIV-RNA (mean of all previous measurements), and maximum HIV-RNA (maximum of all previous measurements). To compare results between all HIV-RNA measurements with VCY, we used the same inclusion criteria for all analyses. We used the Akaike27 information criteria (AIC), a measure of the relative quality of statistical models which evaluates trade-off between model complexity and goodness of fit, to determine which measure of viremia best fits the data.
RESULTS
Baseline Characteristics
The CASCADE 2013 update contains information on 30,006 individuals, of whom 21,082 seroconverted in the cART era, during or after 1996. Of those, we excluded 916 individuals from African cohorts and 10,813 individuals without at least 1 cART-naive HIV-RNA measurement within 4–12 months of seroconversion, leaving 9353 individuals in the analysis.
Among those seroconverting in the cART era (n = 21,082), men who have sex between men were slightly overrepresented in this analysis compared with those excluded (80% vs. 62%) and those who likely acquired HIV through sex between men and women were slightly underrepresented (9% vs. 29%). Date of seroconversion was later in those included in this analysis [November 2005 (July 2002–August 2008)] than in those excluded [July 2004 (July 2000–March 2008)] explained by availability of routine HIV-RNA measurements within the cohorts. All other baseline characteristics were similar among those included and excluded from this analysis (data not shown).
Of 9353 individuals, 5312 (57%) initiated cART, 326 (3%) acquired AIDS, and 160 (2%) died. Median [interquartile range (IQR) [25th–75th percentile] follow-up was 4.1 (1.8, 7.2) years. Most individuals were men (85%), and modes of HIV transmission included sex between men (71%), sex between men and women (21%), injection drug use (4%), and unknown (4%). Median (IQR) CD4 at cART initiation was 342 (265, 450) cells per cubic millimeter and did not vary by VCY category. Median (IQR) seroconversion age was 33 (27, 40) years between 1996 and 2013. Individuals contributed to a median (IQR) of 21 (13, 36) trials.
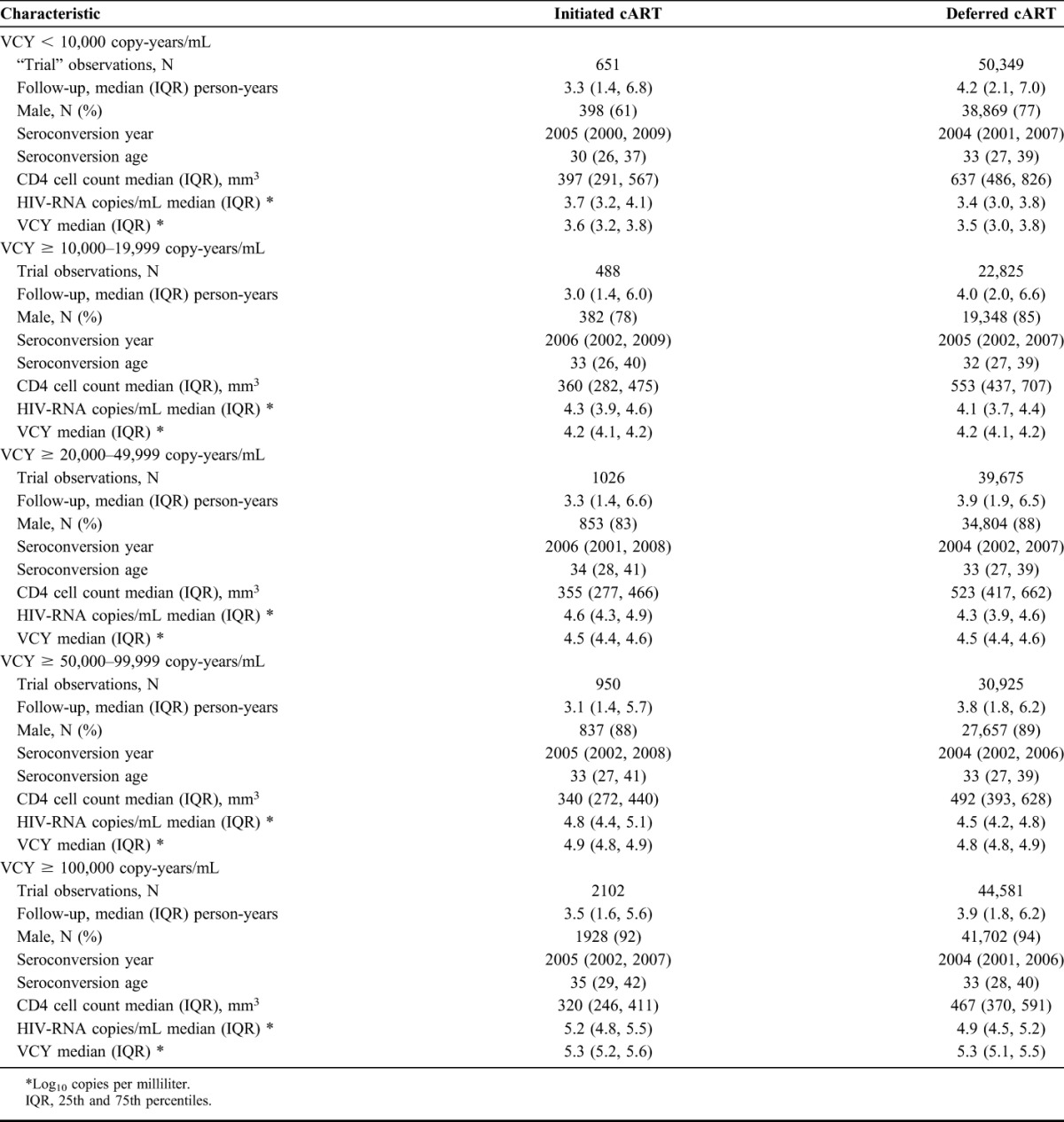
Individuals who initiated cART typically had much lower CD4 cell counts and higher HIV-RNA values than those deferring cART. Men were also more likely to defer in the lower viral copy-years strata (Table 1).
TABLE 1.
Baseline Characteristic for Individuals Who Initiated or Deferred cART by Levels of VCY
Viremia Copy-Years
Pooling across CD4 cell count strata, HRs for the effect of initiating cART compared with deferring on time to AIDS/death significantly decreased as VCY increased (P-trend < 0.001). For example, at times when the VCY was in the range 10,000–20,000 copy-years/mL, there was only a modest 9% reduction in the hazard of AIDS/death associated with immediate initiation of cART compared with deferral [HR = 0.91 (95% CI: 0.57 to 1.46)], whereas at times when the VCY was >100,000 copy-years/mL, the estimated reduction in risk of AIDS/death associated with immediate versus deferred initiation was 56% [HR = 0.44 (95% CI: 0.35 to 0.55)], Table 2. Among individuals initiating with CD4 ≥350 cells per cubic millimeter, there was a modest trend (P = 0.11) toward a greater benefit of immediate initiation of cART (vs. deferral), although the results continued to suggest some benefit of earlier initiation among the group with VCY >100,000 copy-years/mL [HR = 0.68 (95% CI: 0.49 to 0.94)], Table 2. As expected among individuals initiating with CD4 <350 cells per cubic millimeter, immediate initiation was beneficial in all VCY categories (all HR < 1) (see Table, Supplemental Digital Content, http://links.lww.com/QAI/A817).
TABLE 2.
The Effect of Initiation Compared With Deferring cART on Time to AIDS/Death by VCY Alone by CD4 Cell Count Strata (≥350, ≥500 Cells/mm3)
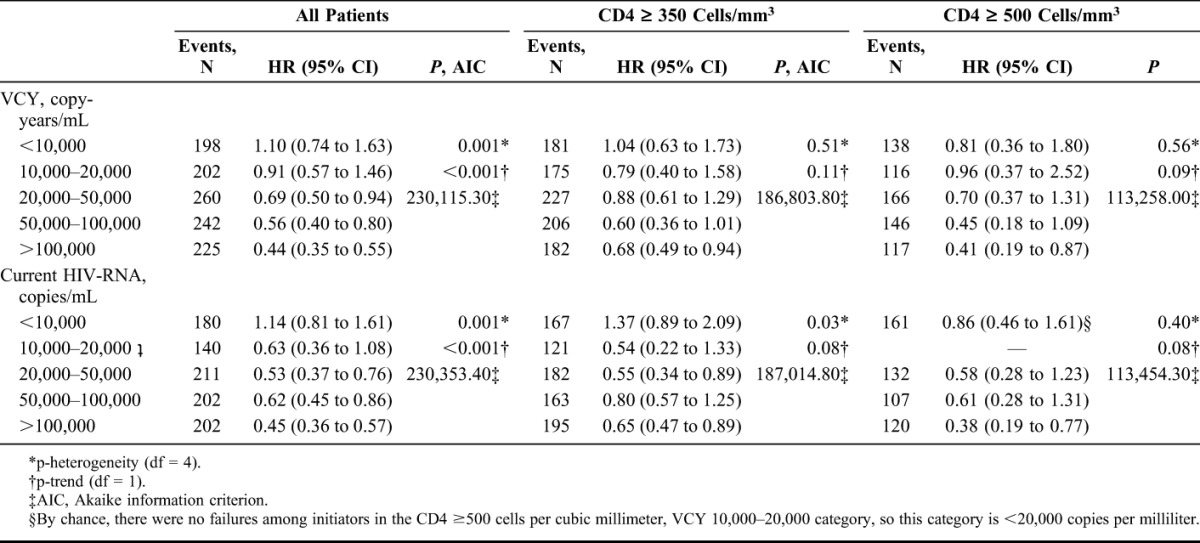
Modeling initiation of cART by VCY as a continuous variable showed the same trends as the categorical analysis, Figure 2. No obvious threshold of copy-years was found; however, pooling CD4 cell count categories, the upper bound of the 95% CI first fell below one when VCY passed 17,343 copies-years/mL, suggesting that among individuals with VCY values above this threshold, immediate initiation of cART may result in a reduction in the risk of AIDS/death. Stratifying by CD4 cell count, in those with CD4 ≥350 cells per cubic millimeter, the upper bound of the 95% CI fell below one when VCY surpassed 52,826 copy-years/mL, again suggesting that among individuals with high CD4 cell counts and VCY values above this threshold, immediate initiation of cART may result in a reduction in the risk of AIDS/death.
FIGURE 2.
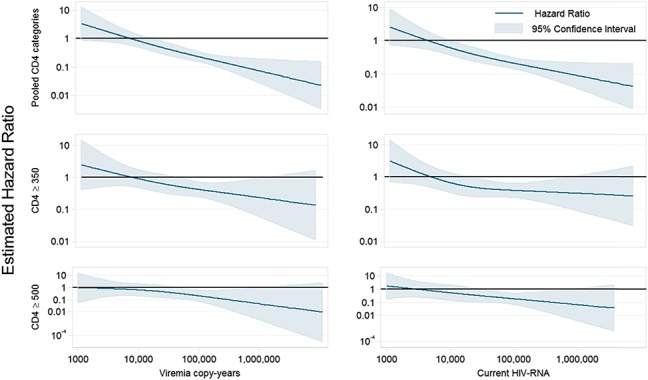
The effect of initiating compared with deferring cART on time to AIDS/death by VCY and CD4 cell count modeled continuously with 3 knot splines using the CASCADE data set.
Using a CD4 count threshold of 500 cells per cubic millimeter showed similar results. For those with CD4 ≥500 cells per cubic millimeter, the greatest benefit of initiation was seen when VCY >100,000 copy-years/mL [HR = 0.41 (0.19, 0.87), P-trend = 0.09], Table 2. Modeling VCY continuously, the upper bound of the 95% CI in those with CD4 ≥500 cells per cubic millimeter fell below one when VCY surpassed 38,152 copy-years/mL. In those with a CD4 count <500 cells per cubic millimeter, there was an overall benefit of treatment initiation in VCY categories >10,000 copy-years/mL (see Table, Supplemental Digital Content, http://links.lww.com/QAI/A817).
Other Measures of Viremia
Pooling CD4 strata, the HRs for the effect of initiating cART on time to AIDS/death decreased as most recent HIV-RNA increased (P-trend < 0.001) with the largest benefit of initiation seen when current HIV-RNA exceeded 100,000 copies/mL [HR = 0.45 (0.36, 0.57)]. Among individuals with a CD4 count ≥350 cells per cubic millimeter, there was a modest trend (P-trend = 0.08) for an increased benefit of immediate initiation (vs. deferral) as the current HIV-RNA increased, with the largest benefit of immediate initiation seen if the current HIV-RNA was >100,000 copies/mL [HR = 0.65 (0.47, 0.89)], Table 2. Stratifying by CD4, there was a benefit of initiating versus deferring for all individuals with CD4 <350 cells per cubic millimeter regardless of current HIV-RNA level, as expected from the VCY analysis. The same trends were seen when modeling VCY and current HIV-RNA continuously, Figure 2, and when considering average and maximum viremia (data not shown).
Using a CD4 threshold of 500 cells per cubic millimeter, similar results were obtained for the average and maximum viremia (data not shown).
Pooling CD4 strata, model fit was best for VCY (minimum AIC, 230115) compared with current (increase in AIC = 238), average (increase in AIC = 124), and maximum HIV-RNA (increase in AIC = 163). Maximum HIV-RNA fits the model best in the CD4 <500 cells per cubic millimeter strata (minimum AIC, 1024345; increase in AIC = 32, 128, 15 for VCY, current, and average HIV-RNA, respectively, for copy-years, current, average, and maximum HIV-RNA, respectively). In the CD4 ≥500 cells per cubic millimeter strata, VCY gave the best model fit (minimum AIC = 113,258.00, increase in AIC = 196, 84, for current, average, and maximum HIV-RNA, respectively).
DISCUSSION
Pooling CD4 cell count strata, there is a benefit of initiating cART as the cumulative and absolute HIV-RNA increases, with benefits observed as the total VCY exceeds approximately 17,500 copy-years/mL. What is of clinical interest, however, is whether there is benefit of immediate cART initiation in individuals with healthy immune systems (CD4 ≥500 cells per cubic millimeter) and high levels of viremia. Among individuals with CD4 ≥500 cells per cubic millimeter, we found a modest benefit of earlier cART initiation for those with high cumulative and absolute HIV-RNA >100,000 copy-years/mL and copies per milliliter, associated with reducing risk of AIDS/death by 59% (13%–81%) and 62% (23%–81%). Our results support the recent evidence from the START trial28 which found serious illness or death was reduced by 53% among those treated immediately vs. waiting to initiate until CD4 cell count dropped below 350 cells per cubic millimeter.13
All measures of viremia showed consistent and similar results with an increased benefit of cART initiation with increasing VCY. Among the pooled and separate CD4 cell count strata, there was not a single viremia measure that consistently showed best model fit using AIC. VCY fits best when pooling CD4 and in the CD4 ≥350 cells per cubic millimeter strata, whereas average viremia fits best in the CD4 <350 cells per cubic millimeter strata. Although VCY incorporates cumulative HIV burden, it requires frequent HIV-RNA measurements from the start of infection, which are not available in most HIV-positive individuals. Even if such measurements are available, cumulative viremia is difficult and time-consuming to calculate. Average and maximum HIV-RNA also require frequent measurements from seroconversion, so too are not relevant for most HIV-positive individuals. Current HIV-RNA, however, is a measure that is easily obtained from all HIV-positive individuals and is therefore of greatest clinical relevance.
Although observational studies are not designed to inform the “when to start” question, we provide evidence that cART initiation is beneficial when CD4 cell counts fall below 350 cells per cubic millimeter, supporting other observational studies.14,29–31 The START trial has recently reported a modest absolute risk reduction of AIDS, other serious illnesses, and death for cART initiation at CD4 cell counts above 500 cells per cubic millimeter11 compared with deferring initiation to CD4 below 350 cells per cubic millimeter.13 Our analysis, using data before guidelines recommending immediate cART initiation, suggests that benefit is likely to be greatest in those with highest viremia burden and adds to the body of evidence which informs clinical guidelines.32
We reflected the dynamic process of initiating cART by allowing individuals to contribute information to multiple trials rather than just considering a single point in time. This provided estimates of the average benefit of initiating cART compared with deferring cART at particular levels of CD4 cell counts and cumulative exposure to HIV-RNA. Our estimates can therefore be used to inform trade-offs between initiating treatment at varying points in disease progression compared with the lifelong challenges of initiating therapy, such as adherence and adverse effects.
The availability of HIV-RNA data from HIV seroconversion allowed us to investigate when to start treatment based on a variety of measures of viremia captured during the life course of HIV infection. Of particular importance, there is potential for lead-time bias33 when measuring cumulative exposure to viremia in sero-prevalent cohorts which is essentially eliminated in this sero-converter study as we have serial HIV-RNA measurements taken from the date of seroconversion. This is, therefore, the first study, to our knowledge, that has compared the benefit of cART initiation by these levels of viremia in combination with CD4 cell count. Nevertheless, despite nearly 10,000 seroconverters being included, we were not able to assess the impact of initiating versus deferring within the CD4 strata where decisions on whether cART should be initiated have previously been most controversial (CD4 >350 cells/mm3).
In addition to AIDS and death, there are several other non–AIDS defining conditions that can affect morbidity and mortality. Increased exposure to viremia has been shown to be associated with cardiovascular disease,34 multimorbidity,35 and AIDS and non-AIDS malignancies,5,36,37 so had these data been available, our estimates could have shown a stronger benefit of cART initiation. CASCADE does not currently collect data on non–AIDS conditions.
Like all observational studies, our estimates rely on the assumption of no unmeasured confounding. We adjusted for some of the most important factors in deciding when to initiate therapy, but it is possible that other unmeasured factors, such as comorbidities or likelihood of adherence, played a role in the initiation of cART in our population. The HRs above one for cART initiation versus deferred treatment, albeit with wide confidence intervals, in the group with low current HIV-RNA suggest we may lack information on some confounders; this could be a particular concern among those with a CD4 count ≥350 cells per cubic millimeter, a group for which not all treatment guidelines recommended initiation of cART during the study period.
It is unlikely that randomized evidence will ever be available on when to initiate cART by these measures of viremia, so applying robust statistical methods to large observational data sets presented here will likely provide the best evidence that will ever be available. Our data suggest that deferring cART in an individual unwilling or unable to start treatment immediately may not impact the risk of AIDS/death provided a healthy CD4 cell count (≥350, 500 cells/mm3) and low VCY (<50,000 copy-years/mL) are maintained. However, we found consistently that AIDS and death were delayed among those who initiated treatment with CD4 cell counts ≥350 cells per cubic millimeter and VCY >100,000 copy-years/mL.
Supplementary Material
ACKNOWLEDGMENTS
CASCADE Steering Committee: Julia Del Amo (Chair), Laurence Meyer (Vice Chair), Heiner C. Bucher, Geneviève Chêne, Osamah Hamouda, Deenan Pillay, Maria Prins, Magda Rosinska, Caroline Sabin, Giota Touloumi.
CASCADE Co-ordinating Centre: Kholoud Porter (Project Leader), Ashley Olson, Andrea Cartier, Lorraine Fradette, Sarah Walker, Abdel Babiker.
CASCADE Clinical Advisory Board: Heiner C. Bucher, Andrea De Luca, Martin Fisher, Roberto Muga.
CASCADE Collaborators: Australia PHAEDRA cohort (Tony Kelleher, David Cooper, Pat Grey, Robert Finlayson, Mark Bloch) Sydney AIDS Prospective Study and Sydney Primary HIV Infection cohort (Tony Kelleher, Tim Ramacciotti, Linda Gelgor, David Cooper, Don Smith); Austria Austrian HIV Cohort Study (Robert Zangerle); Canada South Alberta clinic (John Gill); Estonia Tartu Ülikool (Irja Lutsar); France ANRS CO3 Aquitaine cohort (Geneviève Chêne, Francois Dabis, Rodolphe Thiebaut), ANRS CO4 French Hospital Database (Dominique Costagliola, Marguerite Guiguet), Lyon Primary Infection cohort (Philippe Vanhems), French ANRS CO6 PRIMO cohort (Marie-Laure Chaix, Jade Ghosn), ANRS CO2 SEROCO cohort (Laurence Meyer, Faroudy Boufassa); Germany German HIV-1 seroconverter cohort (Osamah Hamouda, Claudia Kücherer, Barbara Bartmeyer); Greece AMACS (Anastasia Antoniadou, Georgios Chrysos, Georgios L. Daikos); Greek Haemophilia cohort (Giota Touloumi, Nikos Pantazis, Olga Katsarou); Italy Italian Seroconversion Study (Giovanni Rezza, Maria Dorrucci), ICONA cohort (Antonella d'Arminio Monforte, Andrea De Luca.) Netherlands Amsterdam Cohort Studies among homosexual men and drug users (Maria Prins, Ronald Geskus, Jannie van der Helm, Hanneke Schuitemaker); Norway Oslo and Ulleval Hospital cohorts (Mette Sannes, Oddbjorn Brubakk, Anne-Marte Bakken Kran); Poland National Institute of Hygiene (Magdalena Rosinska); Spain Badalona IDU hospital cohort (Roberto Muga, Jordi Tor), Barcelona IDU Cohort (Patricia Garcia de Olalla, Joan Cayla), CoRIS-scv (Julia del Amo, Santiago Moreno, Susana Monge); Madrid cohort (Julia Del Amo, Jorge del Romero), Valencia IDU cohort (Santiago Pérez-Hoyos); Sweden Swedish InfCare HIV Cohort, Sweden (Anders Sönnerborg); Switzerland Swiss HIV Cohort Study (Heiner C. Bucher, Huldrych Günthard, Martin Rickenbach); Ukraine Perinatal Prevention of AIDS Initiative (Ruslan Malyuta); United Kingdom Public Health England (Gary Murphy), UK Register of HIV Seroconverters (Kholoud Porter, Anne Johnson, Andrew Phillips, Abdel Babiker), University College London (Deenan Pillay); African cohorts: Genital Shedding Study (US: Charles Morrison; Family Health International, Robert Salata, Case Western Reserve University, Uganda: Roy Mugerwa, Makerere University, Zimbabwe: Tsungai Chipato, University of Zimbabwe); International AIDS Vaccine Initiative (IAVI) Early Infections Cohort (Kenya, Rwanda, South Africa, Uganda, Zambia: Pauli N. Amornkul, IAVI, USA; Jill Gilmour, IAVI, UK; Anatoli Kamali, Uganda Virus Research Institute/Medical Research Council Uganda; Etienne Karita, Projet San Francisco, Rwanda).
EuroCoord Executive Board: Fiona Burns, University College London, UK; Geneviève Chêne, University of Bordeaux, France; Dominique Costagliola (Scientific Coordinator), Institut National de la Santé et de la Recherche Médicale, France; Carlo Giaquinto, Fondazione PENTA, Italy; Jesper Grarup, Region Hovedstaden, Denmark; Ole Kirk, Region Hovedstaden, Denmark; Laurence Meyer, Institut National de la Santé et de la Recherche Médicale, France; Heather Bailey, University College London, UK; Alain Volny Anne, European AIDS Treatment Group, France; Alex Panteleev, St. Petersburg City AIDS Centre, Russian Federation; Andrew Phillips, University College London, UK, Kholoud Porter, University College London, UK; Claire Thorne, University College London, UK.
EuroCoord Council of Partners: Jean-Pierre Aboulker, Institut National de la Santé et de la Recherche Médicale, France; Jan Albert, Karolinska Institute, Sweden; Silvia Asandi, Romanian Angel Appeal Foundation, Romania; Geneviève Chêne, University of Bordeaux, France; Dominique Costagliola (chair), INSERM, France; Antonella d'Arminio Monforte, ICoNA Foundation, Italy; Stéphane De Wit, St. Pierre University Hospital, Belgium; Peter Reiss, Stichting HIV Monitoring, Netherlands; Julia Del Amo, Instituto de Salud Carlos III, Spain; José Gatell, Fundació Privada Clínic per a la Recerca Bíomèdica, Spain; Carlo Giaquinto, Fondazione PENTA, Italy; Osamah Hamouda, Robert Koch Institut, Germany; Igor Karpov, University of Minsk, Belarus; Bruno Ledergerber, University of Zurich, Switzerland; Jens Lundgren, Region Hovedstaden, Denmark; Ruslan Malyuta, Perinatal Prevention of AIDS Initiative, Ukraine; Claus Møller, Cadpeople A/S, Denmark; Kholoud Porter, University College London, United Kingdom; Maria Prins, Academic Medical Centre, Netherlands; Aza Rakhmanova, St. Petersburg City AIDS Centre, Russian Federation; Jürgen Rockstroh, University of Bonn, Germany; Magda Rosinska, National Institute of Public Health, National Institute of Hygiene, Poland; Manjinder Sandhu, Genome Research Limited; Claire Thorne, University College London, UK; Giota Touloumi, National and Kapodistrian University of Athens, Greece; Alain Volny Anne, European AIDS Treatment Group, France.
EuroCoord External Advisory Board: David Cooper, University of New South Wales, Australia; Nikos Dedes, Positive Voice, Greece; Kevin Fenton, Public Health England, USA; David Pizzuti, Gilead Sciences, USA; Marco Vitoria, World Health Organisation, Switzerland.
EuroCoord Secretariat: Silvia Faggion, Fondazione PENTA, Italy; Lorraine Fradette, University College London, UK; Richard Frost, University College London, UK; Andrea Cartier, University College London, UK; Dorthe Raben, Region Hovedstaden, Denmark; Christine Schwimmer, University of Bordeaux, France; Martin Scott, UCL European Research & Innovation Office, UK.
Footnotes
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under EuroCoord grant agreement n° 260694 and Medical Research Council UK.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Mellors JW, Margolick JB, Phair JP, et al. Prognostic value of HIV-1 RNA, CD4 cell count, and CD4 Cell count slope for progression to AIDS and death in untreated HIV-1 infection. JAMA. 2007;297:2349–2350. [DOI] [PubMed] [Google Scholar]
- 2.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. [DOI] [PubMed] [Google Scholar]
- 3.Attia S, Egger M, Muller M, et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. [DOI] [PubMed] [Google Scholar]
- 4.Murray JS, Elashoff MR, Iacono-Connors LC, et al. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS. 1999;13:797–804. [DOI] [PubMed] [Google Scholar]
- 5.Zoufaly A, Stellbrink HJ, Heiden MA, et al. Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis. 2009;200:79–87. [DOI] [PubMed] [Google Scholar]
- 6.Cole SR, Napravnik S, Mugavero MJ, et al. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. 2010;171:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mugavero MJ, Napravnik S, Cole SR, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53:927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright ST, Hoy J, Mulhall B, et al. Determinants of viremia copy-years in people with HIV/AIDS after initiation of antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;66:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the international Antiviral Society-USA Panel. JAMA. 2014;312:410–425. [DOI] [PubMed] [Google Scholar]
- 10.El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 11.Lundgren JD, Babiker A, El-Sadr W, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–1155. [DOI] [PubMed] [Google Scholar]
- 12.Reekie J, Gatell JM, Yust I, et al. Fatal and nonfatal AIDS and non-AIDS events in HIV-1-positive individuals with high CD4 cell counts according to viral load strata. AIDS. 2011;25:2259–2268. [DOI] [PubMed] [Google Scholar]
- 13.National Institute of Allergy and Infectious Diseases (NIAID). Starting antiretroviral treatment early improves outcomes for HIV-infected individuals. 2015. Available at: https://www.niaid.nih.gov/news/newsreleases/2015/Pages/START.aspx. Accessed June 18, 2015. [Google Scholar]
- 14.Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Wolf F, Sabin C, Kirk O, et al. Developing a multidisciplinary network for clinical research on HIV infection: the EuroCoord experience. Clin Invest. 2012;2:255–264. [Google Scholar]
- 16.Pantazis N, Morrison C, Amornkul PN, et al. Differences in HIV natural history among African and non-African seroconverters in Europe and seroconverters in sub-Saharan Africa. PLoS One. 2012;7:e32369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Writing Committee for the CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocroft A, Oancea C, van Lunzen J, et al. Decline in esophageal candidiasis and use of antimycotics in European patients with HIV. Am J Gastroenterol. 2005;100:1446–1454. [DOI] [PubMed] [Google Scholar]
- 20.Lundgren JD, Pedersen C, Clumeck N, et al. Survival differences in European patients with AIDS, 1979-89. The AIDS in Europe study group. BMJ. 1994;308:1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo K, Law M, Kaldor JM, et al. The role of initial AIDS-defining illness in survival following AIDS. AIDS. 1995;9:57–63. [DOI] [PubMed] [Google Scholar]
- 22.Mocroft AJ, Lundgren JD, d'Armino Monforte A, et al. Survival of AIDS patients according to type of AIDS-defining event. The AIDS in Europe Study Group. Int J Epidemiol. 1997;26:400–407. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett JA, DeMasi R, Dawson D, et al. Variability in repeated consecutive measurements of plasma human immunodeficiency virus RNA in persons receiving stable nucleoside reverse transcriptase inhibitor therapy or no treatment. J Infect Dis. 1998;178:1803–1805. [DOI] [PubMed] [Google Scholar]
- 24.Brambilla D, Reichelderfer PS, Bremer JW, et al. The contribution of assay variation and biological variation to the total variability of plasma HIV-1 RNA measurements. The Women Infant Transmission Study Clinics. Virology Quality Assurance Program. AIDS. 1999;13:2269–2279. [DOI] [PubMed] [Google Scholar]
- 25.Pantaleo G, Graziosi C, Fauci AS. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327–335. [DOI] [PubMed] [Google Scholar]
- 26.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. [DOI] [PubMed] [Google Scholar]
- 27.Akaike H. A new look at the statistical model identification. Automatic Control IEEE Trans. 1974;19:716–723. [Google Scholar]
- 28.University of Minnesota—Clinical and Translational Science Institute Strategic. Timing of Antiretroviral Treatment (START). Available at: https://clinicaltrials.gov/ct2/show/results/NCT00867048. [Google Scholar]
- 29.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabin CA, Cooper DA, Collins S, et al. Rating evidence in treatment guidelines: a case example of when to initiate combination antiretroviral therapy (cART) in HIV-positive asymptomatic persons. AIDS. 2013;27:1839–1846. [DOI] [PubMed] [Google Scholar]
- 33.Cole SR, Li R, Anastos K, et al. Accounting for leadtime in cohort studies: evaluating when to initiate HIV therapies. Stat Med. 2004;23:3351–3363. [DOI] [PubMed] [Google Scholar]
- 34.Calmy A, Gayet-Ageron A, Montecucco F, et al. HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial. AIDS. 2009;23:929–939. [DOI] [PubMed] [Google Scholar]
- 35.Salter ML, Lau B, Go VF, et al. HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clin Infect Dis. 2011;53:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruyand M, Thiebaut R, Lawson-Ayayi S, et al. Role of uncontrolled HIV RNA level and immunodeficiency in the occurrence of malignancy in HIV-infected patients during the combination antiretroviral therapy era: Agence Nationale de Recherche sur le Sida (ANRS) CO3 aquitaine cohort. Clin Infect Dis. 2009;49:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guiguet M, Boue F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–1159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


