Abstract
Tryptophan (Trp) metabolism via the kynurenine pathway (KP) was assessed in normal healthy US volunteers at baseline and after acute Trp depletion (ATD) and acute Trp loading (ATL) using amino acid formulations. The hepatic KP accounts for ~90% of overall Trp degradation. Liver Trp 2,3-dioxygenase (TDO) contributes ~70% toward Trp oxidation, with the remainder achieved by subsequent rate-limiting enzymes in the KP. TDO is not influenced by a 1.15 g Trp load, but is maximally activated by a 5.15 g dose. We recommend a 30 mg/kg dose for future ATL studies. ATD activates TDO and enhances the Trp flux down the KP via its leucine component. Higher plasma free [Trp] and lower total [Trp] are observed in women, with no gender differences in kynurenines. Kynurenic acid is lower in female Caucasians, which may explain their lower incidence of schizophrenia. African-American and Hispanic women have a lower TDO and Trp oxidation relative to free Trp than the corresponding men. African-American women have a potentially higher 3-hydroxyanthranilic acid/anthranilic acid ratio, which may protect them against osteoporosis. Future studies of the KP in relation to health and disease should focus on gender and ethnic differences.
Keywords: acute tryptophan loading and depletion; ethnicity and gender; kynurenine aminotransferase; kynurenic acid; kynureninase; tryptophan 2,3-dioxygenase; tryptophan oxidation
Introduction
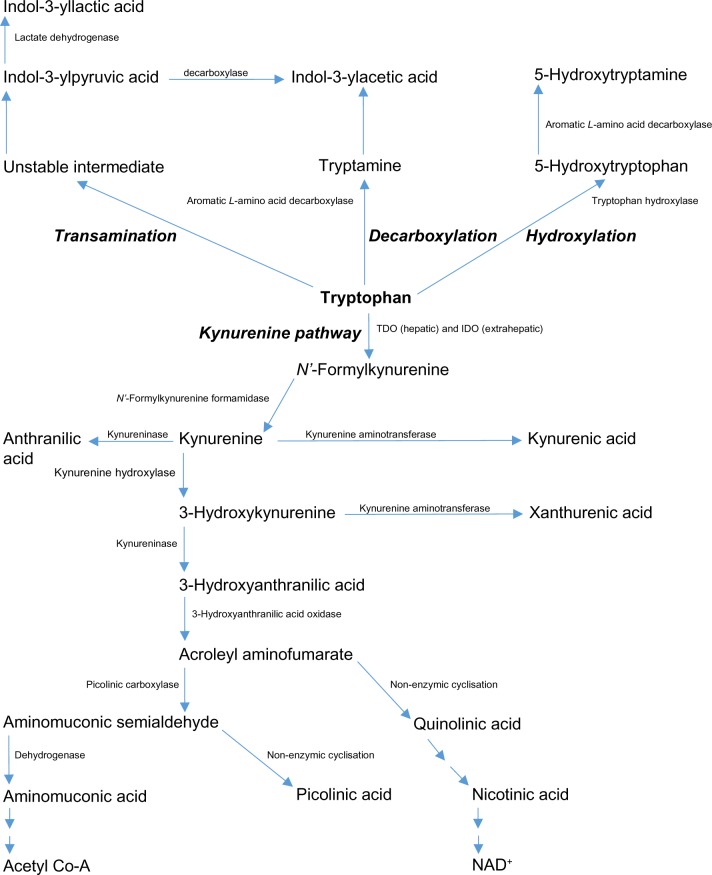
The essential amino acid L-tryptophan (Trp) is the precursor of a number of biologically active metabolites, including the pellagra-preventing factor nicotinic acid (vitamin B3), the important redox cofactors NAD+ (P+) H, the zinc-binding compound picolinic acid, the neuroactive kynurenine (K) and its metabolites kynurenic acid (KA) and quinolinic acid (QA), the mood-enhancing indolylamine 5-hydroxytryptamine (5-HT or serotonin), and the pineal hormone melatonin. At most, only 1% of dietary Trp is utilized for protein synthesis, because, in a person in nitrogen equilibrium, the amount of protein degraded is matched exactly by that synthesized, with the bulk of Trp being available for metabolism.1 Of the four known Trp-metabolic pathways (Fig. 1), the hepatic kynurenine pathway (KP) accounts for >90% of total body Trp disposal,1,2 though the other three minor pathways also produce biologically important metabolites. The hepatic KP is controlled by the first enzyme, Trp 2,3-dioxygenase (TDO, EC 1.13.11.11, formerly Trp pyrrolase).1,2 The importance of TDO in Trp degradation and hence its availability under normal physiological conditions is best exemplified by the finding that deletion of the mouse TDO gene elevates plasma [Trp] by 9.3-fold.3 In extrahepatic tissues, Trp is oxidized by another hemoprotein indoleamine 2,3-dioxygenase (IDO: EC 1.13.11.17), though its activity is negligible under normal conditions, but can be dramatically enhanced after immune activation to astoundingly high levels, rendering it the major player in control of Trp availability under immune-related pathological conditions.4 A comprehensive review of the role of IDO in health and disease has recently been published.5
Figure 1.
Tryptophan metabolic pathways. Reproduced from Ref. 72, and adapted from Figure 1 in Badawy AA-B. Pellagra and alcoholism: a biochemical perspective. Alcohol Alcohol. 2014;49:238–250.
Trp has been used in a variety of clinical conditions, notably behavioral, cognitive, and mood disorders, eg, aggression, anxiety, bipolar disorder, major depressive disorder, premenstrual syndrome, seasonal affective disorder, and sleep disorders.6 The common denominator in all these conditions is the presumed 5-HT dysfunction. In major depressive disorder, wherein Trp has been most commonly used, its antidepressant efficacy is modest and variable, most likely because of its excessive hepatic degradation by TDO, whose activity can be enhanced by the raised levels of glucocorticoids and possibly also catecholamines, hence the suggestion that efficacy can be enhanced by joint administration with antidepressant drugs or other TDO inhibitors.7 Whereas emphasis in Trp research has in the past been serotonin-related, more recently metabolites along the KP have become the focus of interest in view of their neuronal activities in a variety of clinical conditions involving immune dysfunction, including schizophrenia.8–11 In particular, the kynurenine metabolites 3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA), and QA have been shown to possess immunosuppressive properties and have thus been implicated in the immune response to bacterial, parasitic, and viral infections,12 as well as in fetal tolerance.4
The functional capacity of the KP in health and disease has been assessed from the early 1960s by measuring urinary K metabolite excretion and plasma [Trp] and [K] following oral administration of a Trp load.13–16 With the advent of more sensitive detection techniques, eg, high-performance liquid chromatography (HPLC) and mass spectrometry, it has become possible to quantify the much lower plasma levels of kynurenines (Ks). Quantification was initially limited to K and occasionally 3-HK,17 but now includes many other K metabolites.18 Acute Trp loading (ATL) in the past involved oral administration of Trp itself, but is currently performed using a mixture of ~15 amino acids enriched with Trp. A similar mixture from which Trp is omitted is used to induce acute Trp depletion (ATD). Both ATD and ATL are powerful research and diagnostic tools for assessing the role of brain 5-HT in behavior in normal subjects and those with various disorders.6 Whereas both ATD and ATL continue to be used to investigate 5-HT function, they are being increasingly used to assess the KP, eg, in relation to oxidative stress in chronic brain injury and Huntington’s disease.19,20 The ATD test21,22 is based on depletion of plasma Trp and hence its availability to the brain by several mechanisms: (1) stimulation of protein synthesis by Leu and other essential amino acids; (2) increased hepatic Trp oxidation by Leu; and (3) competition with Trp for cerebral uptake by competing amino acids (CAAs), mainly the three branched-chain amino acids (BCAAs) Leu, Ile, and Val and the aromatic amino acids Phe and Tyr. In the above ATL studies,19,20 only one dose of Trp (6 g in a 100 g formulation) was used. This is a relatively large Trp dose (~86 mg/kg for a 70 kg adult), which could activate liver TDO, thereby contributing to kynurenine metabolite formation on top of that due to the flux of Trp down the KP. Whereas a 50 mg/kg Trp dose does not activate liver TDO in humans16 or rats,23 a 75 mg/kg dose does in rats.24 As will be shown in the present work, liver TDO is indeed activated (maximally) in humans by a 5.15 g dose of Trp (~74 mg/kg for a 70 kg adult). The standard amino acid mixture for ATL contains 5.15 g of Trp in a 50 g dose and 10.3 g in the traditional 100 g dose, with the control formulation containing 1.15 or 2.3 g, respectively, for these two doses.21 No Trp dose–response study of plasma kynurenine metabolites has been undertaken with ATL using amino acid mixtures; only after administration of Trp alone (see the “Results and discussion” section). Also, the presence of Leu among the BCAAs in the above mixtures enhances the flux of Trp down the KP,22 thus further complicating interpretation of the kynurenine metabolite data. One aim of the present study was to establish a suitable dose of Trp for future ATL with minimal interference from TDO activation or the Leu component of the formulation. Another aim was to assess gender differences in Trp disposition and KP activity after Trp loading, as cerebral serotonin synthesis has been suggested to be lower in women,25 possibly related to plasma Trp availability to the brain following ATD. As the effect of gender on plasma kynurenine metabolites has not been studied, we considered it important to assess any likely differences. Thirdly, as the subjects studied were of various US ethnic groups, we have attempted to assess any likely ethnic differences in Trp disposition and KP activity, though in view of the small numbers of subjects studied, our conclusions regarding ethnic differences must be considered preliminary and in need of replication in larger numbers. Thus, in the present study with 114 healthy US volunteers of both genders and different ethnic backgrounds, we measured fasting plasma Trp and its K metabolites at baseline and after oral intake of various doses of Trp, and addressed the potential effects of Leu in the formulations used, including that for ATD, and gender, and ethnic differences at baseline and following ATL.
Subjects and Methods
Subjects
Subjects of this study undergoing ATL and ATD had previously been assessed for plasma Trp disposition and availability to the brain following oral administration of amino acid mixtures at two dose levels: 50 and 100 g26 and for plasma Ks using 50 g formulations with different BCAA content to assess the role of Leu in Trp oxidation.22 In this latter study, the standard doses of Leu and other BCAAs were decreased by 20%, 30%, and 40%. Trp flux down the KP was lowest with the 40% less BCAA formulation. This latter formulation is therefore considered in the present study as broadly representative of a Trp-only formulation (ie, with a minimal contribution of Leu to the Trp flux; see “Design” below).
In the present study, healthy volunteers of both genders (age range: 18–40 years) were recruited by one of the authors (DMD) during his tenure at the Health Sciences Centers of both Texas (Houston) and Wake Forest (Winston-Salem, North Carolina) Universities. Subjects enrolled in the two studies were deemed healthy after vigorous screening and gave written informed consent to participate. The two studies were conducted in accordance with the Declaration of Helsinki. Details of recruitment, inclusion, and exclusion criteria and ethical approval by the Institutional Review Boards of the above Universities have all been described.22,26
Design
This was a randomized, double-blind between-group rather than within-group study to reduce participant burden and attrition. The study is best presented in three parts: (1) baseline comparisons; (2) separate and combined effects of Leu and a small Trp dose (1.15 g) on the flux of Trp down the KP in 50 g doses of control formulations and in comparison with effects of a similar dose of the ATD formulation; and (3) effects of larger Trp doses (5.15 and 10.3 g) in the ATL formulations. The compositions of all formulations are given in Table 1. As stated above, because Trp was not used alone in this study, the control group with the least Leu content was used as an approximate substitute. This is the first control group in Table 1 (Control 1) with the low (40% less) [Leu] dose used in our recent study22 of the effects of Leu on Trp oxidation. Because of the relatively large content of BCAAs in the original ATD, ATL, and control formulations,21 specificity for 5-HT was impaired. This was addressed27 in the control formulation by reducing the BCAA content by 40%. The lower content of BCAA in Control 1 is compensated for by proportionate increases in the content of the non-CAAs. We also assessed kynurenine metabolite levels in the present study in an ATD group to establish the effect of Leu on Trp oxidation in the absence of exogenously administered Trp by comparing the Control 2 and ATD groups. We hope that a comparison of the data obtained with the first three groups in Table 1 will help dissociate the roles of Leu and Trp in influencing the Trp flux. The ATL group at a 50 g dose can be compared directly with the Control 2 group. Finally, the 100 g ATL group can be reasonably compared with the 50 g ATL group, as we did not have a 100 g control group. The formulations were not administered on a body weight basis, but subjects had an average body weight of 69.1 ± 9.9 kg (mean ± SD; n = 114) and there were no statistically significant differences between men (70.7 ± 9.6; n = 54) and women (67.5 ± 10.2; n = 60).
Table 1.
Composition of the amino acid formulations.
| FORMULATION | CONTROL 1 | CONTROL 2 | ATD | ATL | ATL |
|---|---|---|---|---|---|
| Number of subjects (n) | 12 | 12 | 10 | 25 | 20 |
| Dose of formulation | 51.25 | 51.15 | 50.00 | 55.15 | 110.30 |
| Trp added | Moderate | Moderate | Nil | High | Higher |
| Leucine level | Low | High | High | High | Higher |
| Trp | 1.15 | 1.15 | 0.00 | 5.15 | 10.30 |
| Leu | 4.05 | 6.75 | 6.75 | 6.75 | 13.50 |
| Ile | 2.40 | 4.00 | 4.00 | 4.00 | 8.00 |
| Val | 2.72 | 4.45 | 4.45 | 4.45 | 8.90 |
| Phe | 2.85 | 2.85 | 2.85 | 2.85 | 5.70 |
| Tyr | 3.45 | 3.45 | 3.45 | 3.45 | 6.90 |
| Ala | 3.34 | 2.75 | 2.75 | 2.75 | 5.50 |
| Arg | 2.98 | 2.45 | 2.45 | 2.45 | 4.90 |
| Cys | 1.64 | 1.35 | 1.35 | 1.35 | 2.70 |
| Gly | 1.94 | 1.60 | 1.60 | 1.60 | 3.20 |
| His | 1.94 | 1.60 | 1.60 | 1.60 | 3.20 |
| Lys | 5.41 | 4.45 | 4.45 | 4.45 | 8.90 |
| Met | 1.82 | 1.50 | 1.50 | 1.50 | 3.00 |
| Pro | 7.41 | 6.10 | 6.10 | 6.10 | 12.20 |
| Ser | 4.19 | 3.45 | 3.45 | 3.45 | 6.90 |
| Thro | 3.95 | 3.25 | 3.25 | 3.25 | 6.50 |
Note: Values are in g.
Abbreviations: ATD, acute tryptophan depletion; ATL, acute tryptophan loading. (All others are standard amino acid abbreviations).
Procedure
Full details of the procedure and experimental environments have been described previously.22,26,27 Briefly, after overnight fasting, venous blood samples (10 mL each) were withdrawn for baseline parameters. Subjects then received one of the five formulations detailed in Table 1 over 20 minutes, and hourly blood samples were then withdrawn up to 7 hours after consumption of drinks. Subjects remained fasting during the entire procedure, following which they received a meal, which, for the ATD and control groups, was rich in Trp.
Laboratory procedures
Fasting plasma was isolated from the blood samples in ethylenediamine tetraacetate (EDTA) tubes and was frozen at −70 °C until transported under frozen conditions to Cardiff, UK, for analysis. Plasma ultrafiltrates for free Trp determination were prepared from fresh plasma (before freezing) as described,28 as frozen storage increases albumin binding of Trp.28 Both free and total (free + albumin-bound) [Trp], and K and its metabolites (KA, 3-HK, xanthurenic acid [XA], 3-HAA, and anthranilic acid [AA]) were determined by HPLC isocratically, with ultraviolet (UV) and fluorimetric detection.18 Limits of detection varied between 22 (3-HK) and 72 (AA) nM (UV) and 5 (Trp) and 32 (3-HAA) nM (fluorimetry). Between-day coefficients of variation varied between 1.9% (AA) and 7.7% (3-HK).18
With our HPLC method,18 baseline fasting [3-HK] values are considerably higher than those reported in the literature (which are on average ~0.31 μM), most likely due to the presence of a co-eluting substance as yet unidentified. However, as will be described below, the increases in [3-HK] from baseline (calculated by subtracting the baseline values) are similar to values in the Trp loading literature using other procedures showing the low baseline fasting [3-HK].19,20 Also, in one subject with a baseline [3-HK] of 0.31 μM, the subsequent increases also matched those in the literature. We therefore considered it appropriate to make this subtraction throughout and to insert a historical baseline [3-HK] value of 0.31 μM for all groups.
Expressions of results
Concentrations of all analytes are expressed in absolute units (μM). Plasma Trp binding is expressed as the percentage free Trp (100 × [free Trp]/[total Trp]. Enzyme activities are expressed in the Trp literature as ratios (or ratio percentages) of products to substrates. This has been the case for some time for TDO, but was extended more recently to subsequent enzymes of the KP.29 Table 2 gives the definitions of these enzyme expressions. TDO activity is also expressed relative to free Trp, in view of the finding30 that free Trp determines to a large degree the flux of Trp down the KP. Another expression, also given in Table 2, is that of total Trp oxidation, which is useful in expressing the flux of Trp down the KP, especially in the absence of changes in TDO activity, eg, after loading with small amounts of Trp.22 With total tryptophan oxidation (TTOX), extrahepatic tissues contribute significantly to kynurenine aminotransferase (KAT) and kynureninase (kynase), but minimally to IDO under normal physiological conditions.4 Thus, TDO expressed in this study is likely to include a small (<5%) contribution from IDO.
Table 2.
Expressions of kynurenine pathway enzyme activities and tryptophan oxidation.
| ABBREVIATION | DEFINITION | EXPRESSION |
|---|---|---|
| TDO | Trp dioxygenase relative to total Trp | 100 × [K]/[total Trp] |
| TDOF | Trp dioxygenase relative to free Trp | 100 × [K]/[free Trp] |
| KOHase | Kynurenine hydroxylase | 100 × [3-HK]/[K] |
| KAT A | Kynurenine aminotransferase (K → KA) | 100 × [KA]/[K] |
| KAT B | Kynurenine aminotransferase (3-HK → XA) | 100 × [XA]/[3-HK] |
| Total KAT | Sum of KAT A + KAT B | |
| Kynase A | Kynureninase (K → AA) | 100 × [AA]/[K] |
| Kynase B | Kynureninase (3-HK → 3-HAA) | 100 × [3-HAA]/[3-HK] |
| Total Kynase | Sum of Kynase A + Kynase B | |
| TTOX | Total Trp oxidation relative to total Trp | 100 × [total kynurenines]/[total Trp] |
| TTOXF | Total Trp oxidation relative to free Trp | 100 × [total kynurenines]/[free Trp] |
Statistical analysis
Results were analyzed statistically by one-way analysis of variance (ANOVA) for repeated measures for within-group and between-group differences using Sigma Plot version 11 (Systat, UK). For multiple group comparisons using this program, the Holm–Sidak test is recommended as the first-line procedure, as it is more powerful than the Tukey or Bonferroni tests and can be used for pairwise comparisons and those versus a control group. When data failed the normality (Shapiro–Wilk) test, Friedman repeated measures ANOVA on ranks was performed followed by the appropriate test. A 0.05 probability (P) level was taken as indicative of the least significant differences.
Results and Discussion
Baseline plasma tryptophan and kynurenine metabolites and gender and ethnic differences
We had previously reported18 baseline plasma Trp and kynurenine metabolite concentrations in a healthy US control group of 114 subjects of both genders. We now report (Table 3) that gender differences among these 114 subjects were observed only in free and total [Trp], but not in kynurenine or five of its metabolites (KA, 3-HK, XA, 3-HAA, and AA). Plasma free [Trp] was 31% higher in women, whereas total [Trp] was 15% lower. A 17% lower total [Trp] in women had been reported previously.31 Accordingly, the percent free Trp (an expression of Trp binding to albumin) in our study was 53% higher in women. This suggests that circulating Trp availability is higher in women. The decreased Trp binding in women is not due to a decrease in albumin or an increase in non-esterified fatty acids (NEFAs), as both showed no significant gender differences. A female-specific substance (or substances) may therefore be responsible, the nature of which requires investigation. That Trp availability to the brain is also higher in women is suggested by the 42% higher [free Trp]/[CAA] ratio due to the higher free Trp and the 8% lower [CAA] in women. These results suggest that Trp availability to the brain for both the cerebral KP and 5-HT synthesis is moderately higher in women than in men and are thus at odds with the reported25 lower serotonin biosynthetic rate in women (see also below).
Table 3.
Gender comparison of baseline fasting plasmatryptophan and kynurenine metabolites in healthy volunteers.
| PARAMETER | TOTAL GROUP (n = 114) | MALES (n = 54) | FEMALES (n = 60) AND SIGNIFICANCE OF DIFFERENCES FROM MALES | |
|---|---|---|---|---|
| Free tryptophan (Trp) (μM) | 5.29 ± 0.24 | 4.54 ± 0.28 | 5.96 ± 0.34* | P = 0.010 |
| Total tryptophan (Trp) (μM) | 63 ± 2 | 69 ± 3 | 59 ± 2* | P = 0.010 |
| % Free Trp | 8.40 ± 0.47 | 6.58 ± 0.75 | 10.10 ± 0.52* | P = 0.002 |
| Nonesterified fatty acids (NEFA) (mM) | 0.296 ± 0.012 | 0.299 ± 0.015 | 0.293 ± 0.018 | |
| Albumin (g/L) | 49.6 ± 0.30 | 49.7 ± 0.42 | 49.6 ± 0.44 | |
| Competing amino acids (CAA) (μM) | 552 ± 11 | 575 ± 17 | 530 ± 13* | P = 0.047 |
| [Free [Trp]/[CAA] ratio | 0.0096 ± 0.0006 | 0.0079 ± 0.0008 | 0.0112 ± 0.0008* | P = 0.001 |
| [Total Trp]/[CAA] ratio | 0.114 ± 0.003 | 0.120 ± 0.031 | 0.111 ± 0.000 | |
| 3-Hydroxykynurenine (3-HK), μM, (revised) | 9.20 ± 0.36 (0.31) | 9.37 ± 0.58 (0.31) | 9.06 ± 0.43 (0.31) | |
| Kynurenine (K) (μM) | 2.15 ± 0.12 | 2.17 ± 0.18 | 2.14 ± 0.17 | |
| 3-Hydroxyanthranilic acid (3-HAA) (μM) | 0.28 ± 0.03 | 0.25 ± 0.03 | 0.31 ± 0.03 | |
| Xanthurenic acid (XA) (μM) | 0.16 ± 0.03 | 0.15 ± 0.03 | 0.17 ± 0.03 | |
| Kynurenic acid (KA) (μM) | 0.078 ± 0.019 | 0.099 ± 0.029 | 0.059 ± 0.023 | |
| Anthranilic acid (AA) (μM) | 0.069 ± 0.01 | 0.090 ± 0.023 | 0.053 ± 0.01 | |
| Total kynurenines (Ks) (μM) | 3.09 ± 0.14 | 3.12 ± 0.21 | 3.08 ± 0.19 | |
| TDO | 3.41 ± 0.26 | 3.14 ± 0.32 | 3.63 + 0.40 | |
| TDOF | 41 ± 3 | 48 ± 6 | 36 ± 4* | P = 0.024 |
| TTOX | 4.90 ± 0.33 | 4.49 ± 0.48 | 5.22 ± 0.47 | |
| TTOXF | 58 ± 4 | 68 ± 7 | 52 ± 4* | P = 0.010 |
| K OHase | 14.4 ± 0.8 | 14.3 ± 1.2 | 14.5 ± 1.1 | |
| KAT A | 3.63 ± 0.40 | 4.55 ± 0.64 | 2.78 ± 0.48 | |
| KAT B | 52 ± 7 | 48 ± 9 | 54 ± 10 | |
| Total KAT | 56 ± 7 | 53 ± 9 | 57 ± 11 | |
| Kynase A | 3.21 ± 0.65 | 4.15 ± 1.14 | 2.48 ± 0.67 | |
| Kynase B | 90 ± 6 | 81 ± 9 | 100 ± 9 | |
| Total kynase | 93 ± 6 | 85 ± 9 | 102 ± 9 | |
| [3-HAA]/[AA] ratio (n = 88,46,42) | 5.76 ± 1.09 | 6.92 ± 1.08 | 5.65 ± 1.01 | |
Notes: Values are means ± SEM for the numbers indicated above. The 3-HK values are those observed experimentally, but a (0.31) value based on the smaller literature values (see the text) has been applied for total kynurenines and the relevant ratios.
The results in Table 3 also give data on Ks and enzyme activities expressed as products to substrate ratio percentages. There were no significant gender differences in Ks, and the 33% lower [KA] in women did not reach statistical significance (P = 0.079). [KA] will be shown below to be significantly lower in Caucasian women, relative to men. Female subjects also had a significantly lower TDO activity and total Trp oxidation, but only relative to plasma free Trp [ie, tryptophan 2,3-dioxygenase relative to free tryptophan (TDOF) and total tryptophan oxidation relative to free tryptophan (TTOXF)]. On this basis, it could be argued that Trp oxidation by women may be moderately lower than that by men, which could also explain the higher free Trp availability to the brain of women. As will be shown below, the lower TDOF and TTOXF expressions are limited to African-American and Hispanic women, but not Caucasians.
The plasma [K]/[Trp] ratio is widely used to express TDO or IDO activity, and its use is both valid and informative under conditions when induction of either enzyme and the resulting enhanced flux of Trp down the KP are assured and robust, eg, with cortisol (TDO) or interferon-γ (IFN-γ) (IDO). However, under basal conditions, this ratio does not reflect totally the normal flux of Trp down the KP. Accordingly, we previously proposed22 new expressions using free Trp and total Ks. Free Trp plays a major role in the Trp flux,30 and the sum of Ks better reflects this flux. As the TDOF and TTOXF data in Table 3 show, women exhibit significantly lower rates of Trp oxidation. The flux of Trp down the rat hepatic KP is controlled mainly by TDO.30 In the present work with human plasma, it appears that this may also be the case, as the six ratios of TDO/TTOX and TDOF/TTOXF in men and women, and the combined total in Table 3 is ~0.70 (range: 0.6923–0.7069). A similar value of 0.70 is also obtained for the ratio of [K]/[Ks]. Linear regression showed highly significant (P < 0.001) correlations between each of the above three ratio pairs (r = 0.942–0.968). Because other Ks, in particular QA, were not measured, the above ratio of 0.7 is not a maximum value. Although QA is the largest K metabolite excreted in urine under basal conditions (45%–52%),1,32 its plasma concentration is on average 0.348 μM (range: 0.253–0.451; calculated from data in Refs. 9,34). By adding 11% for QA contribution to the total Ks (3.09) in Table 3, the TTOX and TTOXF values can be upgraded to 5.44 and 65, respectively. The TDO/TTOX and TDOF/TTOXF ratios can now be revised to 0.627 and 0.631, respectively. Thus, it may be provisionally concluded that the contribution of TDO (plus a minimal one from IDO under normal basal conditions) to Trp oxidation via the KP is 63%, and the remaining 37% may be due to other rate-limiting enzymes, possibly kynureninase and picolinate carboxylase.
Human KP enzyme activities can be approximated from plasma K metabolite ratios. Three studies by a Norwegian group29,33,34 were performed in this regard. We compared a number of parameters from the total group in Table 3 here with the corresponding pooled calculated data from the above three studies as follows: TDO (3.41 vs 2.31), TTOX (4.90 vs 2.50), K hydroxylase (14.4 vs 1.8), KAT A (K → KA) (5.58 vs 2.56), KAT B (3-HK → XA) (52 vs 43), total KAT (58 vs 46), kynase A (K → AA) (3.21 vs 0.95), kynase B (3-HK → 3-HAA) (90 vs 98), and total kynase (93 vs 99). As plasma [K] is lower in nonfasting subjects,35 the higher values for TDO, TTOX, KAT A, and kynase A in our study may due to our higher [K] (2.15 vs 1.49 μM), and other differences may be due to food intake by the Norwegian subjects. Further assessment of KP enzymes will be made in the following sections. From our results and those by the above group, it is clear that KAT and kynase are more active with 3-HK as substrate than with K. This is consistent with the higher [3-HAA] compared with [AA] in Table 3 and the above three Norwegian studies, and the much lower km of kynase for 3-HK than for K.36,37 KP enzyme activities have been studied in rat liver and/or four other tissues.38–40 In the study in several tissues,40 direct enzyme assays did not correlate with product/substrate ratios. This is to be expected given the in vitro nature of enzyme assays. However, the comparable ratios for TDO and kynase A (K→AA) in Table 3 of the present work at least reflect the comparable rates of Trp flux through these two enzymes in isolated rat hepatocytes.41
It has been suggested that the ratio of plasma [3-HAA]/[AA] may contribute to disorders with an inflammatory component, and may represent a novel marker for the assessment of inflammation and its progression.42 Under such conditions, the ratio is decreased because of a large increase in [AA], while [3-HAA] remains generally unaltered. As stated above and also shown in Table 3, in our normal subjects [3-HAA] is higher than [AA], and the above ratio does not exhibit gender differences in the whole group. Ethnic differences in this ratio and the possible role of KA in this ratio will be discussed below.
When the above data were analyzed by ethnicity, new gender differences emerged (Table 4). Thus, Trp binding to albumin was significantly lower in women of all three ethnic groups, due to a higher free, but not total, [Trp], though only the higher free Trp in the Caucasian women was significant. In the absence of significant gender differences in NEFA or albumin, the lower Trp binding in women may, as stated above, be due to the presence of substance(s) in the blood of women capable of displacing Trp.
Table 4.
Baseline plasma tryptophan disposition and kynurenine metabolites by gender and ethnicity.
| PARAMETER | CAUCASIANS | AFRICAN AMERICANS | HISPANICS | ASIAN AMERICANS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ALL (n = 40) | MALES (21) | FEMALES (19) | ALL (N = 50) | MALES (19) | FEMALES (31) | ALL (n = 18) | MALES (7) | FEMALES (11) | MALES (n = 6) | |
| Free Trp | 5.07 ± 0.42 | 4.34 ± 0.34 | 5.96 ± 0.79* | 5.05 ± 0.29 | 4.14 ± 0.41 | 5.61 ± 0.36 | 5.86 ± 0.66 | 4.53 ± 0.89 | 6.71 ± 0.85 | 6.30 ± 1.35 |
| Total Trp | 65 ± 3 | 70 ± 3 | 59 ± 5 | 64 ± 3 | 69 ± 5 | 60 ± 3 | 56 ± 5 | 61 ± 12 | 53 ± 3 | 67 ± 12 |
| % Free Trp | 7.80 ± 0.54 | 6.20 ± 0.55 | 10.10 ± 0.78* | 7.89 ± 0.55 | 6.00 ± 0.71 | 9.35 ± 0.69* | 10.46 ± 1.37 | 7.43 ± 2.46 | 12.66 ± 1.48* | 9.40 ± 5.11 |
| NEFA | 0.28 ± 0.02 | 0.27 ± 0.02 | 0.28 ± 0.04 | 0.31 ± 0.02 | 0.34 ± 0.03 | 0.29 ± 0.02 | 0.29 ± 0.12 | 0.31 ± 0.03 | 0.28 ± 0.04 | 0.28 ± 0.03 |
| Albumin | 49.9 ± 0.34 | 50.1 ± 0.5 | 49.6 ± 0.7 | 49.6 ± 0.5 | 49.1 ± 0.8 | 49.9 ± 0.6 | 48.7 ± 0.8 | 49.1 ± 1.4 | 48.5 ± 1.0 | 51.5 ± 0.8 |
| CAA | 537 ± 17 | 570 ± 22 | 496 ± 25* | 565 ± 16 | 598 ± 29 | 545 ± 19 | 518 ± 21 | 488 ± 38 | 537 ± 25 | 603 ± 65 |
| Free Ratio | 0.0094 ± 0.0012 | 0.0076 ± 0.0010 | 0.0120 ± 0.002* | 0.0089 ± 0.0081 | 0.0069 ± 0.0016 | 0.0103 ± 0.0008 | 0.0113 ± 0.0014 | 0.0093 ± 0.002 | 0.0125 ± 0.0018 | 0.0104 ± 0.0030 |
| Total Ratio | 0.121 ± 0.006 | 0.123 ± 0.006 | 0.119 ± 0.011 | 0.113 ± 0.004 | 0.115 ± 0.007 | 0.110 ± 0.005 | 0.108 ± 0.010 | 0.125 ± 0.023 | 0.099 ± 0.008 | 0.111 ± 0.021 |
| 3-HK | (0.31) | (0.31) | (0.31) | (0.31) | (0.31) | (0.31) | (0.31) | (0.31) | (0.31) | (0.31) |
| K | 2.40 ± 0.21 | 2.31 ± 0.31 | 2.51 ± 0.27 | 1.97 ± 0.19 | 1.86 ± 0.25 | 2.04 ± 0.26 | 2.14 ± 0.27 | 2.92 ± 0.51 | 1.65 ± 0.20 | 1.74 ± 0.34 |
| 3-HAA | 0.302 ± 0.037 | 0.229 ± 0.038 | 0.390 ± 0.06* | 0.273 ± 0.031 | 0.274 ± 0.055 | 0.273 ± 0.037 | 0.245 ± 0.039 | 0.227 ± 0.054 | 0.257 ± 0.055 | 0.223 ± 0.087 |
| XA | 0.182 ± 0.241 | 0.171 ± 0.047 | 0.195 ± 0.060 | 0.138 ± 0.030 | 0.138 ± 0.044 | 0.137 ± 0.040 | 0.161 ± 0.058 | 0.093 ± 0.049 | 0.205 ± 0.090 | 0.181 ± 0.093 |
| KA | 0.127 ± 0.029 | 0.197 ± 0.047 | 0.042 ± 0.012* | 0.122 ± 0.030 | 0.083 ± 0.038 | 0.146 ± 0.043 | 0.068 ± 0.022 | 0.112 ± 0.052 | 0.040 ± 0.010 | 0.232 ± 0.130 |
| AA | 0.097 ± 0.025 | 0.138 ± 0.044 | 0.048 ± 0.011* | 0.045 ± 0.010 | 0.026 ± 0.007 | 0.057 ± 0.016 | 0.064 ± 0.022 | 0.122 ± 0.085 | 0.045 ± 0.019 | 0.065 ± 0.023 |
| Total Ks | 3.42 ± 0.25 | 3.35 ± 0.38 | 3.49 ± 0.32 | 2.86 ± 0.20 | 2.69 ± 0.26 | 2.96 ± 0.29 | 3.00 ± 0.31 | 3.78 ± 0.63 | 2.31 ± 0.23* | 2.75 ± 0.49 |
| TDO | 3.69 ± 0.34 | 3.30 ± 0.50 | 4.25 ± 0.42 | 3.08 ± 0.47 | 2.70 ± 0.42 | 3.40 ± 0.71 | 3.82 ± 0.63 | 4.79 ± 1.31 | 3.11 ± 0.50 | 2.60 ± 0.40 |
| TDOF | 47 ± 5 | 53 ± 9 | 42 ± 4 | 39 ± 6 | 45 ± 11 | 36 ± 6* | 36 ± 8 | 64 ± 14 | 25 ± 4* | 28 ± 10 |
| TTOX | 5.26 ± 0.53 | 4.79 ± 0.83 | 5.91 ± 0.60 | 4.47 ± 0.54 | 3.90 ± 0.51 | 4.93 ± 0.81 | 5.36 ± 0.80 | 6.20 ± 1.75 | 4.36 ± 0.60 | 4.10 ± 1.11 |
| TTOXF | 67 ± 7 | 76 ± 11 | 59 ± 6 | 57 ± 7 | 65 ± 14 | 53 ± 7* | 51 ± 9 | 65 ± 14 | 34 ± 4* | 44 ± 15 |
| KOHase | 12.9 ± 1.4 | 13.4 ± 2.2 | 12.3 ± 1.7 | 15.7 ± 1.3 | 16.7 ± 1.8 | 15.2 ± 1.8 | 14.5 ± 2.2 | 10.6 ± 2.8 | 18.8 ± 2.8 | 17.8 ± 2.8 |
| KAT A | 5.29 ± 1.01 | 8.53 ± 1.56 | 1.67 ± 0.56* | 6.19 ± 1.65 | 4.46 ± 2.25 | 7.16 ± 2.30 | 3.21 ± 0.74 | 3.84 ± 1.48 | 2.42 ± 0.81 | 13.33 ± 3.83 |
| KAT B | 58 ± 12 | 55 ± 15 | 63 ± 19 | 44 ± 10 | 45 ± 14 | 43 ± 13 | 52 ± 19 | 30 ± 15 | 66 ± 29 | 58 ± 30 |
| Total KAT | 63 ± 12 | 64 ± 15 | 65 ± 19 | 50 ± 10 | 49 ± 14 | 50 ± 14 | 55 ± 19 | 34 ± 17 | 68 ± 29 | 71 ± 32 |
| Kynase A | 4.04 ± 1.42 | 5.97 ± 0.24 | 1.91 ± 0.49* | 2.28 ± 0.68 | 1.40 ± 0.40 | 2.79 ± 1.06 | 3.50 ± 1.38 | 4.18 ± 1.72 | 2.73 ± 2.03 | 3.74 ± 1.69 |
| Kynase B | 97 ± 11 | 74 ± 12 | 126 ± 19 | 88 ± 10 | 88 ± 18 | 88 ± 12 | 79 ± 13 | 74 ± 17 | 83 ± 2 | 72 ± 28 |
| Total kynase | 101 ± 11 | 80 ± 13 | 128 ± 19 | 90 ± 10 | 89 ± 18 | 91 ± 12 | 82 ± 13 | 78 ± 18 | 86 ± 19 | 76 ± 29 |
| 3-HAA/AA | 5.65 ± 1.01 | 3.68 ± 0.67 | 8.55 ± 2.11* | 8.43 ± 1.55 | 11.93 ± 3.10 | 6.16 ± 1.44 | 4.09 ± 1.45 | 1.98 ± 0.43 | 5.60 ± 2.38 | 3.21 ± 1.29 |
Notes: Values (in μM or ratio percentage) are means ± SEM for the numbers of subjects in parentheses. The *denotes a significant difference between females and males with each ethnic group (by repeated measures ANOVA) with P values between 0.05 and 0.001.
The increase in free Trp availability to the brain, expressed as the free [Trp]/[CAA] ratio, is confined to Caucasian women. There were no significant gender differences among Caucasians in TDO activities or TTOX whether expressed relative to total or free Trp. However, compared to men, Caucasian women showed significantly higher [3-HAA], but lower [KA], [AA], KAT A, and kynase A. This suggests that transamination of K to KA and K hydrolysis to AA by kynureninase are impaired in Caucasian women. This may be induced by estrogens, which are known to inhibit both enzymes by binding their pyridoxal 5′-phosphate cofactor.43,44 Estrogens thus mimic the effects of B6 deficiency on both enzymes.33,45 Compared to men, African-American and Hispanic women exhibited lower TDO activity and Trp oxidation when expressed relative to free Trp, with 20% and 19% lower values in African-Americans and 61% and 48% in Hispanics. These greater decreases in Hispanic women also reflected a significant decrease (39%) in total Ks.
The [3-HAA]/[AA] ratio was comparable between Caucasians, Hispanics, and Asian Americans, and about one-half of that of African-Americans (Table 4). Only the higher value in Caucasian women approached significance (P = 0.059) compared with that of Caucasian men. The similarly higher ratio in Hispanic women and the lower ratio in African-American women did not achieve statistical significance, because of the large individual variations. The differences in this ratio are not due to differences in [3-HAA], whose values were gender comparable, but are caused mainly by the lower [AA] in women. The 49% lower [AA] in Caucasian women was significant (P = 0.011), whereas the 63% lower (Hispanic women) and the 146% higher (African-American women) values were not. Statistical significance in these parameters and their ratio could be expected in larger samples.
The above data in Tables 3 and 4 could be of clinical significance in relation to schizophrenia (SZ) and immune-related disorders. The lower KAT A in Caucasian women could explain the lower incidence of schizophrenia in women,46,47 which has been suggested to involve protection by estrogens48 through their interaction with brain-derived neurotrophic factor or a lower lipid peroxidation level in women.49 We propose here an additional mechanism, namely inhibition of transamination of K to KA by estrogen metabolites binding and thereby inactivating the KAT cofactor, pyridoxal 5′-phosphate (PLP). The rationale is as follows. (1) The KA theory of SZ involves decreased functions of the NMDA (N-methyl-D-aspartate) receptors of the excitatory amino acid glutamate and the α7 nicotinic acetylcholine receptors.10 (2) Cerebrospinal levels of KA, the physiological antagonist of these receptors,50 are increased in SZ.10 (3) By binding the PLP cofactor, estrogens inhibit KAT (and also another PLP-dependent enzyme, kynase).43,44 (4) Rat kidney mitochondrial and cytosolic KAT activity is 33% lower in females, and the higher activity in males is decreased to female levels by estrogens.44 The rat kidney has the highest tissue [KA], and its KAT is the second most active after the hepatic enzyme.40 (5) Elevation of brain KA in SZ results from increased transamination of K to KA due to the accumulation of K following inhibition of cortical K hydroxylase (K mono-oxygenase) activity.51 The increased formation of KA reflects increased substrate availability for KAT, and not activation of the enzyme, as its activity is unaltered in SZ.51 (5) Accordingly, inhibitors of KAT have been proposed as potential SZ therapeutic agents.52
The higher [KA] and KAT approximated activity in African-American women is surprising, but could be attributed to the lower use of estrogen contraceptives by women of this ethnicity (and also Hispanic women),53,54 resulting in less inhibition of KAT. It is possible that individual variations in [KA] within any ethnic group may be a function of the levels of endogenous estrogens and those used as contraceptives. This study is the first attempt to examine human gender differences in KAT, and future studies should include a parallel assessment of the estrogen status as well as that of vitamin B6. Current literature does not permit a clear conclusion regarding gender differences in incidence of SZ in African-Americans or Hispanic Americans. SZ incidence is greater in African-Americans than Caucasians, even after adjusting for the socioeconomic status,55 though diagnostic bias is an obstacle to a clear-cut conclusion.56 In a study of psychosis across ethnic groups in the UK,57 a higher incidence of SZ was reported in Afro-Caribbean and Black African subjects compared to White subjects. However, the incidence of psychosis in the first two ethnic groups was higher in women. Whether a similar pattern emerges in African-American women remains to be seen and, if so, the higher [KA]/[K] ratio reported here for this ethnic group may be of diagnostic relevance.
The effects of estrogens on the KP are not limited to KAT inhibition, but inhibition extends to TDO4 and kynureninase.1 The overall effect of estrogens is increased excretion of kynurenine metabolites in a pattern resembling that observed in vitamin B6 deficiency, the mechanism of which involves competition with the pyridoxal 5′-phosphate cofactor of kynureninase by estrogen metabolites1 and possibly also the KAT inhibition reported here.
The gender differences among ethnic groups in the [3-HAA]/[AA] ratio described above (Table 4) may be relevant to immune-related conditions. A lower ratio has been suggested to be associated with a range of neurological and other disorders, including osteoporosis, chronic brain injury, Huntington’s disease, coronary heart disease, thoracic disease, stroke, and depression.42 In most of these conditions, [3-HAA] is decreased whereas [AA] is increased. The higher [3-HAA]/[AA] ratio is African-Americans, compared with the other three ethnic groups (Table 4) is consistent with, and may explain, the lower incidence of osteoporosis in this ethnic group. Protective factors for African-Americans against fracture include their higher peak bone mass, increased obesity rates, greater muscle mass, lower bone turnover rates, and advantageous femur geometry.58 In the present study, the age range of our subjects was 18–40 years. Whether the higher [3-HAA]/[AA] ratio is also present in older subjects remains to be established. Elderly African-Americans, however, lose the advantages accrued by younger ones, which include increased calcium absorption, superior renal calcium conservation, and the absence of increased bone loss because of skeletal resistance to parathyroid hormone.58 A potential relationship between the above ratio and neurological diseases cannot be suggested from the present results, as the latter apply to normal subjects aged 40 years or less.
In the accompanying paper,59 we showed that KA administration to rats increases liver [AA] and kynase A activity and observed a highly significant correlation between liver [KA] and [AA], but not between [KA] and [3-HAA]. We suggest that KA enhances kynase A activity. Linear regression analysis of the KA, AA, and kynase A data in Table 3 of this paper revealed significant positive correlations between plasma KA and AA (r = 0.669; P = 0.049), KA and kynase A (r = 0.679; P = 0.044) and AA and kynase A (r = 0.941; P < 0.001). Additionally, a highly significant correlation was observed between kynase A activity and the [3-HAA]/[AA] ratio (r = 0.813; P = 0.008). It thus appears that baseline physiological levels of KA can influence kynase activity and the above ratio, and an increase in [KA] could therefore have potential clinical consequences additional to NMDA receptor antagonism.
Previous studies of plasma tryptophan disposition after various tryptophan doses
It is helpful to review at this stage previously published data on the maximum increases in plasma Trp and kynurenine concentrations after oral administration of various doses of Trp to establish differences in Trp disposition between ATL using Trp alone versus Trp in amino acid formulations. All studies listed in Table 515–17,20–22,27,60–62 involved oral Trp administration, except one60 in which Trp was given intravenously. From the data summarized, the following conclusions could be drawn: (1) Plasma [Trp] is highest and maximally increased earlier (at 0.5 hours) after intravenous, compared with oral, administration. (2) Dose-dependent increases in plasma free and total [Trp] and [K] are observed irrespective of the route of administration or the Trp formulation. (3) Elevation of plasma free or total [Trp] is less efficient after Trp administration in amino acid formulations than with Trp alone. As plasma [Trp] returns to baseline levels after five to seven hours in all the studies listed in Table 5, impaired Trp absorption from ATL amino acid preparations versus Trp only can be ruled out, leaving enhanced Trp disposal as the more likely mechanism. As will be shown below, the presence of Leu in Trp-containing amino acid formulations increases the Trp flux down the KP, and the experiments in the next section will attempt to dissociate the effects of Leu and Trp on this flux.
Table 5.
Plasma tryptophan and kynurenine(s) in normal subjects after loading with various doses of tryptophan: previous studies.
| REFERENCE | TRP FORMULATION/DOSAGE | FREE TRP | TOTAL TRP | % FREE TRP | K | 3-HK | KA | XA | 3-HAA | QA |
|---|---|---|---|---|---|---|---|---|---|---|
| 15 | Trp only (10 females): | 1 h | 3 h | |||||||
| 50 mg/kg | 511 | 21.4 | ||||||||
|
| ||||||||||
| 16 | Trp only in males: | 1 h | 1 h | 1 h | ||||||
| 10 mg/kg (4) | 25 | 125 | 20% | 5.1 (2 h) | ||||||
| 25 mg/kg (5) | 73 | 276 | 26% | 12.7 (2 h) | ||||||
| 50 mg/kg (7) | 117 | 490 | 24% | 27.4 (3 h) | ||||||
|
| ||||||||||
| 17 | Trp only (1/5): | 1.52 h | 1.52 h | 1.52 h | 4.05 h | 4.23 h | ||||
| 100 mg/kg | 328 | 717 | 46% | 68.0 | 10 | |||||
|
| ||||||||||
| 61 | Trp only (5 males): | 1.5–2 h | 1.5–2h | 1.5–2 h | 4 h | |||||
| 0 mg/kg | 5 | 61 | 8% | 2.4 | ||||||
| 50 mg/kg | 176 | 612 | 29% | 32.7 | ||||||
| 100 mg/kg | 328 | 832 | 39% | 71.1 | ||||||
|
| ||||||||||
| 60 | Trp only iv (14 males): | 0.5 h | 3 h | |||||||
| 0 g (0 mg/kg) 1 g (~14 mg/kg) | 70 | 4 | ||||||||
| 3 g (~43 mg/kg) 5 g (~71 mg/kg) | 340 | 12.5 | ||||||||
| 980 | 22.5 | |||||||||
| 1320 | 37.5 | |||||||||
|
| ||||||||||
| 62 | ATD control (8/13)*: 0 g (0 mg/kg) | 2 h | 2 h | 2 h | ||||||
| 2.3 g (~33 mg/kg) | 12.5 | 56 | 22% | |||||||
| 37.5 | 123 | 30% | ||||||||
|
| ||||||||||
| 26 | ATL: | 3 h | 3 h | 3 h | ||||||
| 1.15 g (~16 mg/kg) (12/12) | 8 | 143 | 6% | |||||||
| 5.15 g (~74 mg/kg) (12/12) | 76 | 491 | 15% | |||||||
| 10.3 g (~147 mg/kg) (10/10) | 93 | 528 | 18% | |||||||
|
| ||||||||||
| 27 | ATD control (5/7): | 2 h | 2 h | 2 h | ||||||
| 1.15 g (~16 mg/kg) | 16.1 | 100 | 16% | |||||||
|
| ||||||||||
| 22 | ATD control (6/6): | 2 h | 2 h | 2 h | 3 h | |||||
| 1.15 g (~16 mg/kg) | 16 | 106 | 15% | 2.9 | ||||||
|
| ||||||||||
| 20 | ATL (4/11): | 5 h | 5 h | 5 h | 5 h | 5 h | 5 h | 5 h | ||
| 6 g (~86 mg/kg) | 310 | 64 | 12.3 | 5.3 | 8.6 | 1.7 | 5.8 | |||
Note:
Depressed patients in remission.
Separate and combined effects of leucine and a small tryptophan dose on plasma tryptophan disposition and kynurenine metabolites
Leu enhances the flux of Trp down the KP in both hepatocytes isolated from high-Leu-fed rats63 and human volunteers receiving the control formulation of the ATD test.22 In this latter study, the flux of Trp, expressed as the sum of six Ks in plasma was decreased dose dependently when [Leu] was decreased from the traditional 13.2% (6.75 g in a total amount of 51.25 g of the amino acid formulation, or ~96 mg of Leu/kg body weight) by 20%, 30%, and 40%. TDO activation in rats is the most likely mechanism of the increased flux,63 and the Leu component of the amino acid formulation is the mediator of the increased flux in the human study.22 As flux was minimal with the low Leu formulation (~58 mg of Leu/kg), we considered this latter formulation the nearest to a Trp-only control (referred to in Table 1 as Control 1 group). With this assumption, we were able to dissociate the effects of Leu from those of the small Trp dose by comparing data in the first three amino acid formulations in Table 1.
The time course of effects of oral administration of these three formulations on plasma Trp, K, and five of its metabolites is shown in Table 6. The effects of Leu in the absence of added Trp are those observed in the ATD group. Here, as expected, there were progressive decreases in free and total [Trp] and [K], reaching a maximum of 85%, 92%, and 45%, respectively, at 5, 4, and 5 hours. The decreases in these three parameters at five hours have previously been reported.19,20 Also not surprisingly, the K transamination product KA was not elevated, and KAT A activity was accordingly not altered. KAT A activity has also been shown to be unaltered by ATD, despite a reported decrease in [KA], presumably because of a greater decrease in [K],19,20 compared with that in the present work. By contrast, significant increases in K metabolites and their sum were induced by the ATD formulation (Table 6), suggesting that the flux of Trp down the KP was enhanced by the Leu component. In our previous study,22 we proposed that this increased flux is caused by TDO enhancement by Leu. In the present study (Table 6), TDO activity and TTOX were both significantly increased when expressed relative to either total or free Trp. The observed increase in [3-HK] suggests the activation of K hydroxylase (Table 6), and it could be argued that the lower than expected decrease in [K] (45%) after Trp depletion is the result of TDO activation. Our reported increases in 3-HK and 3-HAA are at odds with previous work,19,20 in which they were undetectable at 5 hours after ATD. No explanation can be advanced at present. Differences in sample storage and/or processing are possible explanations. A brief review of the literature shows that, of the Trp metabolites in the KP, only plasma [K] has been measured after ATD and was found to be decreased, as was the case in the present study and the above papers.19,20 The absence of a universal decrease in K metabolites after ATD in the present work is consistent with Leu promoting the flux of Trp down the KP,22 and this should be taken into consideration in ATD studies addressing the KP.
Table 6.
Time-course of changes in plasma tryptophan and kynurenine metabolites after oral administration of amino acid formulations containing or lacking Trp.
| PARAMETER | 0 h | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | 7 h |
|---|---|---|---|---|---|---|---|---|
| Free [Trp] | ||||||||
| ATD (high Leu) n = 10 | 5.02 ± 0.46 | *4.01 ± 0.36* | *2.25 ± 0.22* | *1.47 ± 0.21* | *0.97 ± 0.14* | *0.77 ± 0.09* | *0.95 ± 0.14* | *0.94 ± 0.14* |
| C1 (F3) (low Leu) n = 12 | 6.09 ± 0.44 | *13.90 ± 1.15 | *16.57 ± 1.54 | *14.65 ± 0.68* | *10.97 ± 1.06 | *8.44 ± 0.87 | 6.71 ± 0.46 | 5.96 ± 0.43 |
| C2 (FO) (high Leu) n = 12 | 4.78 ± 0.22 | *14.68 ± 1.77 | *16.12 ± 1.85 | *11.28 ± 0.97 | *8.82 ± 0.63 | *6.59 ± 0.32 | *5.97 ± 0.36 | *5.68 ± 0.30 |
|
| ||||||||
| Total [Trp] | ||||||||
| ATD | 73 ± 4* | *58 ± 5* | *38 ± 4* | *21 ± 2* | *6 ± 2* | *14 ± 2* | *14 ± 3* | *17 ± 3* |
| C1 | 40 ± 4 | *100 ± 15 | *97 ± 13 | *79 ± 8 | *60 ± 7 | 46 ± 4 | 42 ± 4 | 37 ± 3 |
| C2 | 44 ± 3 | *103 ± 9 | *106 ± 9 | *85 ± 7 | *63 ± 5 | 46 ± 3 | 39 ± 3 | *37 ± 2 |
|
| ||||||||
| % Free Trp | ||||||||
| ATD | 6.88 ± 1.09* | 6.91 ± 0.88* | 5.92 ± 1.27* | 7.00 ± 1.26* | 6.06 ± 1.12* | 5.50 ± 0.87* | 6.79 ± 1.26* | 5.53 ± 1.31* |
| C1 | 15.22 ± 1.75 | 13.90 ± 2.36 | 17.08 ± 2.88 | 18.54 ± 1.89 | 18.28 ± 3.00* | 18.35 ± 2.60* | 15.98 ± 3.12 | 16.11 ± 3.72 |
| C2 | 10.86 ± 0.75 | 14.25 ± 1.70 | *15.21 ± 1.26 | 13.27 ± 1.44 | *14.02 ± 1.34 | *14.33 ± 1.06 | *15.33 ± 1.13 | *15.35 ± 1.05 |
|
| ||||||||
| K | ||||||||
| ATD | 1.88 ± 0.15* | 1.81 ± 0.21* | 1.45 ± 0.26* | 1.25 ± 0.30* | *1.24 ± 0.23* | *1.04 ± 0.17* | *1.19 ± 0.19* | *1.17 ± 0.23* |
| C1 | 1.72 ± 0.21 | 2.08 ± 0.33 | *2.49 ± 0.33 | *2.52 ± 0.28 | *2.28 ± 0.27 | 2.02 ± 0.26 | 1.74 ± 0.20 | 1.90 ± 0.30 |
| C2 | 1.38 ± 0.09 | *2.51 ± 0.22 | *3.06 ± 0.27 | *3.22 ± 0.40 | *2.68 ± 0.28 | *2.64 ± 0.30 | *2.12 ± 0.28 | *2.47 ± 0.27 |
|
| ||||||||
| KA | ||||||||
| ATD | 0.36 ± 0.16 | 0.43 ± 0.18 | 0.52 ± 0.19* | 0.43 ± 0.18* | 0.28 + 0.10* | 0.42 ± 0.13* | 0.34 ± 0.13* | 0.32 ± 0.11* |
| C1 | 0.33 ± 0.14 | 0.48 ± 0.11* | *0.57 ± 0.15* | 0.39 ± 0.12* | *1.02 ± 0.12* | *0.58 ± 0.16* | 0.47 ± 0.06* | 0.45 ± 0.13* |
| C2 | 0.45 ± 0.11 | *0.96 ± 0.20 | *1.34 ± 0.18 | *1.47 ± 0.19 | *1.76 ±± 0.26 | *1.36 ± 0.14 | *0.97 ± 0.19 | *0.92 ± 0.17 |
|
| ||||||||
| 3-HK | ||||||||
| ATD | 0.30 ± 0.00 | *2.96 ± 0.27* | *3.46 ± 0.47* | *4.31 ± 0.90 | *4.84 ± 1.27 | *4.27 ± 1.04* | *3.48 + 0.72* | *2.98 ± 0.68 |
| C1 | 0.30 ± 0.00 | *5.07 ± 0.97 | *4.89 ± 0.92 | *6.31 ± 1.50 | *8.41 ± 1.02 | *8.50 ± 1.12 | *6.55 ± 0.86 | *4.85 ± 0.77 |
| C2 | 0.31 ± 0.006 | *5.95 ± 1.23 | *6.22 ± 1.00 | *5.55 ± 0.88 | *7.78 ± 1.83 | *10.01 ± 1.71 | *7.39 ± 1.56 | *5.53 ± 1.10 |
|
| ||||||||
| XA | ||||||||
| ATD | 0.47 ± 0.04* | 0.60 ± 0.29* | 0.30 ± 0.08* | *0.20 ± 0.07* | *0.60 ± 0.11* | 0.55 ± 0.16* | *0.92 ± 0.14* | 043 ± 0.08* |
| C1 | 0.16 ± 0.07 | 0.19 ± 0.06* | 0.14 ± 0.03* | 0.10 ± 0.03* | 0.08 ± 0.007* | *0.05 ± 0.01* | 0.16 ± 0.04* | *0.44 ± 0.09* |
| C2 | 0.21 ± 0.08 | *1.87 ± 0.19 | *2.00 ± 0.16 | *1.88 ± 0.16 | *2.21 ± 0.26 | *1.86 ± 0.25± | *1.65 ± 0.24 | *1.59 ± 0.27± |
|
| ||||||||
| 3-HAA | ||||||||
| ATD | 0.59 ± 0.08 | *1.21 ± 0.35 | *1.42 ± 0.26* | *1.68 ± 0.24 | *1.53 ± 0.28 | 1.34 ± 0.51 | 1.13 ± 0.46 | 0.89 ± 0.38 |
| C1 | 0.24 ± 0.07 | *0.57 ± 0.18* | *0.50 ± 0.12* | 0.32 ± 0.10* | 0.32 ± 0.07* | 0.68 ± 0.18* | 0.34 ± 0.10* | *0.89 ± 0.19* |
| C2 | 0.42 ± 0.06 | *1.61 ± 0.15 | *2.15 ± 0.12 | *1.94 ± 0.13 | *1.52 ± 0.16 | *1.92 ± 0.18 | *1.47 ± 0.14 | *1.29 ± 0.10 |
|
| ||||||||
| AA | ||||||||
| ATD | 0.13 ± 0.02* | 0.15 ± 0.03* | *0.39 ± 0.08* | 0.12 ± 0.02* | 0.14 ± 0.02* | 0.12 ± 0.05* | 0.14 ± 0.04* | *0.06 ± 0.01* |
| C1 | 0.63 ± 0.12 | *1.14 ± 0.21 | *1.14 ± 0.27 | *1.05 ± 0.18 | *1.19 ± 0.19 | 0.56 ± 0.14 | 0.92 ± 0.19 | *1.34 ± 0.26* |
| C2 | 0.41 ± 0.10 | *0.89 ± 0.24 | *1.22 ± 0.29 | 0.98 ± 0.25 | *1.28 ± 0.47 | 1.02 ± 0.27 | *1.26 ± 0.43 | 0.70 ± 0.14 |
|
| ||||||||
| Total Kynurenines | ||||||||
| ATD | 3.73 ± 0.15 | *7.16 ± 0.80* | *7.54 ± 0.58* | *8.99 ± 1.03* | *8.63 ± 1.30* | *7.74 ± 1.05* | *7.20 ± 0.58* | 5.85 ± 1.04* |
| C1 | 3.12 ± 0.29 | *9.55 ± 1.23 | *9.67 ± 0.98* | *10.69 ± 1.58* | *13.64 ± 1.20 | *12.45 ± 1.32* | *10.18 ± 1.12* | *10.58 ± 1.07 |
| C2 | 3.26 ± 0.27 | *13.76 ± 1.28 | *15.38 ± 1.20 | *14.66 ± 1.02 | *16.98 ± 2.01 | *18.723 ± 1.82 | *19.11 ± 5.43 | *12.73 ± 1.31 |
|
| ||||||||
| TDO | ||||||||
| ATD | 2.57 ± 0.30 | 3.12 ± 0.80 | 3.82 ± 1.05 | *5.95 ± 1.57* | *20.67 ± 4.41 | *7.43 ± 4.04 | *8.50 ± 1.96* | *6.88 ± 1.96 |
| C1 | 4.30 ± 0.41 | *2.08 ± 0.29 | *2.57 ± 0.29 | *3.19 ± 0.30 | 3.80 ± 0.44 | 4.39 ± 0.47 | 4.14 ± 0.53 | 5.14 ± 0.75 |
| C2 | 3.14 ± 0.24 | 2.44 ± 0.43 | 2.89 ± 0.44 | 3.79 ± 0.65 | *4.25 ± 0.85 | *5.74 ± 1.05 | *5.44 ± 1.07 | *6.68 ± 0.89 |
|
| ||||||||
| TDOF | ||||||||
| ATD | 36 ± 5 | 45 ± 6* | 64 ± 18* | *85 ± 35* | *128 ± 51* | *135 ± 45* | *125 ± 43* | *124 ± 28* |
| C1 | 28 ± 4 | *15 ± 2 | *15 ± 3* | *17 ± 2* | 21 ± 5 | 24 ± 5 | 26 ± 5 | 32 ± 5 |
| C2 | 29 ± 2 | *17 ± 2 | 19 ± 3 | 29 ± 5 | 30 ± 5 | *40 ± 7 | *35 ± 7 | *43 ± 6 |
|
| ||||||||
| TTOX | ||||||||
| ATD | 5.11 ± 0.37 | *12.34 ± 2.77 | *19.84 ± 2.70* | *42.81 ± 2.98* | *143.80 ± 17.47 | *55.29 ± 16.34 | *51.43 ± 19.08 | *34.41 ± 11.04 |
| C1 | 7.82 ± 0.74 | 9.55 ± 1.19 | 9.97 ± 1.02* | *13.53 ± 1.60 | *22.73 ± 2.67 | *27.06 ± 2.70* | *24.04 ± 2.74* | *28.59 ± 2.41 |
| C2 | 7.41 ± 0.67 | *13.36 ± 2.32 | *14.51 ± 1.98 | *17.25 ± 3.06 | *26.95 ± 5.43 | *40.70 ± 6.00 | *49.00 ± 6.16 | *34.4 ± 4.86 |
|
| ||||||||
| TTOXF | ||||||||
| ATD | 74 ± 8 | 179 ± 19* | *335 ± 50* | *612 ± 119* | *890 ± 185* | *1005 ± 201* | *758 ± 189* | *622 ± 134* |
| C1 | 51 ± 6* | 69 ± 10* | 58 ± 11* | 73 ± 10* | *124 ± 24* | *147 ± 26* | *152 ± 24* | *177 ± 19* |
| C2 | 68 ± 6 | *94 ± 11 | *95 ±15 | *130 ± 22 | *192 ± 36 | *285 ± 45 | *320 ± 45 | *224 ± 34 |
|
| ||||||||
| KOHase | ||||||||
| ATD | 16 ± 2 | *163 ± 28 | *239 ± 71 | *345 ± 85* | *390 ± 104 | *410 ± 103 | *292 ± 68 | *254 ± 57 |
| C1 | 21 ± 3 | *308 ± 72 | *212 ± 34 | *273 ± 57 | *446 ± 78 | *473 ± 82 | *434 ± 85 | *246 ± 50 |
| C2 | 22 ± 2 | *237 ± 33 | *203 ± 34 | *172 ± 42 | *290 ± 49 | *379 ± 72 | *348 ± 69 | *224 ± 35 |
|
| ||||||||
| KATA (K→KA) | ||||||||
| ATD | 10 ± 1* | 8 ± 2* | 12 ± 6* | 16 ± 7* | 11 ± 5* | 15 ± 4* | 7 ± 1* | 27 ± 9 |
| C1 | 19 ± 14 | 23 ± 10 | 23 ± 11 | 15 ± 9* | *45 ± 9 | 29 ± 13* | 27 ± 11 | 24 ± 5 |
| C2 | 33 ± 7 | 38 ± 9 | 44 ± 6 | *46 ± 8 | *66 ± 123 | *51 ± 9 | 46 ± 12 | 37 ± 5 |
|
| ||||||||
| KATB (3-HK→XA) | ||||||||
| ATD | 156 ± 4 | *20 ± 16 | *9 ± 6* | *5 ± 2* | *12 ± 10 | *24 ± 10 | *26 ± 17 | *14 ± 4 |
| C1 | 52 ± 5 | *4 ± 1* | *3 ± 24* | *2 ± 1* | *1 ± 2* | *1 ± 1* | *2 ± 4* | *9 ± 3* |
| C2 | 44 ± 8 | 60 ± 17 | 34 ± 6 | 35 ± 6 | 33 ± 5 | 38 ± 16 | 40 ± 11 | 34 ± 9 |
|
| ||||||||
| Total KAT | ||||||||
| ATD | 166 ± 9 | *28 ± 17 | *21 ± 16 | *21 ± 7* | *23 ± 20* | *39 ± 2 | *33 ± 12 | *41 ± 3* |
| C1 | 71 ± 15 | *27 ± 10* | *26 ± 29* | *17 ± 9* | *47 ± 8* | *30 ± 13* | *29 ± 13* | *33 ± 7* |
| C2 | 77 ± 10 | 98 ± 23 | 78 ± 8 | 81 ± 9 | 99 ± 15 | 89 ± 18 | 86 ± 17 | 71 ± 10 |
|
| ||||||||
| Kynase A (K→AA) | ||||||||
| ATD | 7 ± 2* | 8 ± 2 | *27 ± 14 | 10 ± 2 | 11 ± 3* | 12 ± 2* | 12 ± 2* | 5 ± 2* |
| C1 | 37 ± 12 | 55 ± 14 | 46 ± 15 | 42 ± 11 | 52 ± 13 | 28 ± 8 | 53 ± 11 | 70 ± 15* |
| C2 | 30 ± 7 | 35 ± 6 | 40 ± 7 | 30 ± 10 | 48 ± 10 | 39 ± 13 | 59 ± 36 | 28 ± 5 |
|
| ||||||||
| Kynase B (3-HK→3-HAA) | ||||||||
| ATD | 197 ± 20* | *41 ± 11 | *41 ± 13 | *39 ± 15 | *32 ± 16 | *31 ± 25 | *32 ± 2* | *30 ± 6 |
| C1 | 80 ± 23 | *11 ± 4* | *10 ± 5* | *5 ± 3* | *4 ± 1* | *8 ± 2* | *5 ± 2* | *18 ± 7 |
| C2 | 135 ± 15 | *27 ± 3 | *35 ± 7 | *35 ± 10 | *20 ± 5 | *19 ± 3 | *20 ± 9 | *24 ± 7 |
|
| ||||||||
| Total Kynase | ||||||||
| ATD | 204 ± 7* | *49 ± 12 | *68 ± 15 | *49 ± 14 | *43 ± 16 | *43 ± 24 | *44 ± 16* | *35 ± 11 |
| C1 | 117 ± 25 | 66 ± 13 | *56 ± 15 | *47 ± 4 | 56 ± 13 | *36 ± 8 | 58 ± 11 | 88 ± 15 |
| C2 | 165 ± 24 | *62 ± 20 | *75 ± 71 | *65 ± 18 | *68 ± 19 | *58 ± 22 | *79 ± 20 | *52 ± 13 |
Notes: Values are means ± SEM for the numbers in each group stated above. Values for each parameter within each group have been compared with those at zero-time and the significance of the differences is indicated by an asterisk on the left-hand side of the values. Values in the acute Trp depletion (ATD) group and in the Control C1 group have been compared with those in the Control C2 group and the significance of the differences is indicated by an asterisk on the right-hand side of the values. Significance (P) was 0.05–0.001.
The pellagragenic effect of Leu is thought to also involve the inhibition of kynase activity,22 and the results in Table 6 indeed demonstrate inhibition of kynase B activity at one to seven hours by ATD. Kynase A activity, however, was not inhibited by ATD. Similarly, whereas KAT A activity was not decreased by ATD, KAT B activity was. It therefore appears that metabolism of 3-HK is preferentially undermined by Leu. A potential mechanism of kynase inhibition by Leu is that of decreased availability of the PLP cofactor, which could result from its depletion by BCAA aminotransferase acting on its Leu substrate.22 The baseline (zero-time) data in Table 6 show that kynase B activity represents 68%–97% of the total enzyme activity. This is consistent with purified kynase from human liver exhibiting Km values for 3-HK and K of 7.7 × 10−5 and 1 × 10−3 M, respectively, and an activity ratio of these two substrates of 15:1.37 In vitamin B6 deficiency, KAT in mitochondria is thought to be protected against PLP depletion. Thus, whereas kynase activity (~92% of which exists in rat liver or kidney cytosols) is strongly inhibited by B6 deficiency, KAT activity is inhibited in kidney cytosols (which represents 54% of total tissue activity) but not in mitochondria (43% of total KAT activity).64 Whether this compartmental protection plays a role in K conversion to AA or KA in humans requires investigation.
The results in Table 6 with the C1 control group could be considered the nearest to a Trp control group with minimal interference from Leu. As expected, the flux of Trp down the KP is reflected by increases over time in the plasma concentrations of free and total Trp, K, and five of its metabolites, and total Ks. Compared to the ATD group, the C1 group induced greater elevations in all parameters, except XA and 3-HAA. Unlike the elevation of TDO by the Leu component of the ATD formulation, TDO activity in the C1 group was not significantly altered whether expressed relative to total of free Trp. This suggests that a 1.15 g Trp dose (~16.4 mg/kg body weight for a 70 kg adult) does not activate TDO (or the combined TDO/IDO) activity. As stated above, a single 50 mg/kg dose of Trp does not activate TDO in human16 or rats.24 However, flux of Trp is enhanced in C1, as suggested by the increased TTOX and TTOXF, and the apparent increase in K hydroxylase. As was the case with ATD, KAT A and kynase A activities were not altered by C1, whereas KAT B and kynase B activities were strongly decreased, suggesting that K conversion to KA or AA is also protected, as is the case after ATD. The preferential impairment of 3-HK conversion to 3-HAA may be explained by a potential inhibition of kynase by high substrate (3-HK) concentrations, as has been demonstrated with the recombinant human enzyme.65 It could therefore be suggested that kynase inhibition by Leu (in ATD) and Trp (in C1) is caused by two different mechanisms: PLP depletion and substrate inhibition.
The increases in plasma free and total Trp and K concentrations observed in the C1 control group (small Trp dose; Table 6) correspond to those previously published by us using amino acid mixtures.22,26,27 When Trp (10 mg/kg) was given alone,16 the increases in total Trp and free Trp at 1 hour and in K at 2 hours were 25%, 50%, and 105%, respectively, greater than in the present work, suggesting that absorption and subsequent metabolism of Trp are delayed by the presence of other amino acids.
Trp binding (expressed as the percent free Trp) was not influenced by ATD or C1. The absence of a change after ATD is not surprising given that no Trp was added. With C1, moderate but insignificant increases (of up to 22%) in the percent free Trp were observed. Greater increases occurred in C2. It may therefore be concluded that a 1.15 g dose of Trp causes only a partial saturation of the Trp binding sites on albumin.
The results in Table 6 with the C2 control group represent the combined effects of the larger Leu dose and the small Trp dose. Not surprisingly, there were no differences in plasma free or total [Trp] between the two control groups C2 and C1 with the same Trp content. [K] and [3-HK] were also not different. However, the levels of KA, XA, 3-HAA, and total Ks were significantly greater in C2 than in C1. To establish whether the combined effects of Leu + Trp equal the sum of the two separate effects, comparisons were made of the sum of the separate effects (in ADT and C1) and those of the combined effects (in C2). As Leu (ATD) and Trp (C1) acted differently on a number of parameters, such as free and total Trp, K, and XA, it was considered more appropriate to conduct these comparisons first on total Ks and then on the remaining parameters. In data not shown here, but can be calculated from Table 6, total Ks, AA, 3-HAA, and KA showed reasonable concordance between the sums and the combined effects. With total Ks, the differences between the combined effects and sums at 1, 2, 3, 4, 5, 6, and 7 hours were in all but one case moderate (+9%, +17%, −11%, −16%, +16%, +56%, and −1%, respectively). We therefore believe that we have reasonably dissociated the effects of Leu from those of Trp.
Effects of tryptophan loading at various doses on plasma tryptophan disposition and kynurenine metabolite concentrations
In this section, the effects of the 5.15 g Trp load (in a 50 g dose of the amino acid formulation) are compared with those observed in the C2 control group in Table 1 (1.15 g of Trp in the 50 g dose with the same Leu content). The effects of the 10.3 g Trp load are compared with those of the 5.15 g load, as we did not have a control formulation for the 100 g dose. These comparisons are given in Table 7.
Table 7.
Time-course of changes in plasma tryptophan and kynurenine metabolites after oral administration of various doses of tryptophan in the amino acid formulation.
| PARAMETER AND TRP DOSE | 0 h | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | 7 h |
|---|---|---|---|---|---|---|---|---|
| Free [Trp] | ||||||||
| C2 (FO) (1.15 g) n = 12 | 4.78 ± 0.22 | *14.7 ± 1.8 | *16.1 ± 1.85 | *11.3 ± 1.0 | *8.8 ± 0.6 | *6.6 ± 0.3 | *6.0 ± 0.4 | *5.7 ± 0.3 |
| 5.15 g (n = 25) | 4.80 ± 0.66 | *37.3 ± 2.5* | *66.6 ± 4.6* | *74.5 ± 7.0* | *62.2 ± 6.2* | *48.3 ± 5.4* | *32.6 ± 4.6* | *22.4 ± 3.9* |
| 10.30 g (n = 20) | 7.04 ± 0.62 | *37.6 ± 3.4 | *73.4 ± 6.9 | *93.5 ± 9.8* | *103.0 ± 11.8* | *95.8 ± 12.6* | *85.0 ± 12.2* | *68.8 ± 11.3* |
|
| ||||||||
| Total [Trp] | ||||||||
| 1.15 g | 44 ± 3 | *103 ± 9 | *106 ± 9 | *85 ± 7 | *63 ± 5 | 46 ± 3 | 39 ± 3 | *37 ± 2 |
| 5.15 | 54 ± 3 | *203 ± 9* | *298 ± 11* | *301 ± 13* | *261 ± 12* | *209 ± 15* | *162 ± 14* | *119 ± 12* |
| 10.30 | 61 ± 4 | *236 ± 13 | *354 ± 16* | *399 ± 17* | *410 ± 24* | *411 ± 30* | *371 ± 32* | *318 ± 33* |
|
| ||||||||
| % Free Trp | ||||||||
| 1.15 | 6.9 ± 1.1 | 6.9 ± 0.9 | 5.9 ± 1.3 | 7.0 ± 1.3 | 6.1 ± 1.1 | 5.5 ± 0.8 | 6.8 ± 1.3 | 5.5 ± 1.3 |
| 5.15 | 8.9 ± 0.7* | *18.3 ± 1.2* | *22.3 ± 1.5* | *24.7 ± 2.0* | *23.8 ± 1.7* | *23.1 ± 2.0* | 15.6 ± 2.0* | *18.8 ± 1.7* |
| 10.30 | 11.5 ± 1.3* | *15.9 ± 0.9 | *20.7 ± 1.6 | *23.4 ± 1.7 | *25.1 ± 2.1 | *23.3 ± 2.1 | *22.9 ± 2.1 | *21.6 ± 2.1 |
|
| ||||||||
| K | ||||||||
| 1.15 | 1.72 ± 0.21 | 2.08 ± 0.33 | *2.49 ± 0.33 | *2.52 ± 0.28 | *2.28 ± 0.27 | 2.02 ± 0.26 | *2.12 ± 0.28 | *2.47 ± 0.27 |
| 5.15 | 2.70 ± 0.31 | *4.54 ± 0.54* | *8.80 ± 0.83* | *15.22 ± 1.55* | *19.13 ± 1.89* | *19.16 ± 2.14* | *17.06 ± 2.03* | *14.27 ± 1.95* |
| 10.30 | 2.74 ± 0.32 | 3.53 ± 0.35 | *8.51 ± 1.43 | *16.63 ± 2.58 | *27.18 ± 3.77* | *32.71 ± 4.10* | *35.56 ± 4.67* | *35.39 ± 4.48* |
|
| ||||||||
| KA | ||||||||
| 1.15 | 0.45 ± 0.11 | *0.96 ± 0.20 | *1.34 ± 0.18 | *1.47 ± 0.19 | *1.76 ± 0.26 | *1.36 ± 0.14 | *0.97 ± 0.19 | *0.92 ± 0.17 |
| 5.15 | 0.06 ± 0.02* | *0.23 ± 0.03* | *0.59 ± 0.08* | *1.48 ± 0.18 | *1.99 + 0.28* | *2.00 ± 0.28* | *1.50 ± 0.26* | *0.86 ± 0.17 |
| 10.30 | 0.34 ± 0.07* | *0.87 ± 0.13* | *0.96 ± 0.17 | *2.47 ± 0.40 | *3.57 ± 0.64* | *3.88 ± 0.70* | *4.55 ± 0.71* | *4.35 ± 0.64* |
|
| ||||||||
| 3-HK | ||||||||
| 1.15 | 0.31 ± 0.006 | *5.95 ± 1.23 | *6.22 ± 1.00 | *5.55 ± 0.88 | *7.78 ± 1.83 | *10.01 ± 1.71 | *7.39 ± 1.56 | *5.53 ± 1.10 |
| 5.15 | 0.31 ± 0.00 | *5.57 ± 0.63 | *7.92 ± 1.18* | *11.51 ± 2.36 | *8.90 ± 1.27 | *9.27 ± 1.20 | *9.31 ± 1.39 | *8.54 ± 1.65 |
| 10.30 | 0.31 ± 0.00 | *9.14 ± 1.11* | *11.99 ± 1.19* | *13.19 ± 1.46* | *14.03 ± 1.70* | *13.00 ± 1.99* | *13.43 ± 1.96* | *12.19 ± 2.07* |
|
| ||||||||
| XA | ||||||||
| 1.15 | 0.21 ± 0.08 | *1.87 ± 0.19 | *2.00 ± 0.16 | *1.88 ± 0.16 | *2.21 ± 0.26 | *1.86 ± 0.25 | *1.65 ± 0.24 | *1.59 ± 0.27 |
| 5.15 | 0.37 ± 0.05 | *0.91 ± 0.11* | *1.18 ± 0.22* | *1.53 ± 0.22 | *2.02 ± 0.39 | *2.37 ± 0.52 | *1.63 ± 0.32 | *1.07 ± 0.19* |
| 10.30 | 0.30 ± 0.05 | *0.80 ± 0.24 | *1.02 ± 0.25 | *1.18 ± 0.24 | *2.65 ± 0.54 | *3.26 ± 0.60 | *3.21 ± 0.62 | *6.22 ± 1.80* |
|
| ||||||||
| 3-HAA | ||||||||
| 1.15 | 0.42 ± 0.06 | *1.61 ± 0.15 | *2.15 ± 0.12 | *1.94 ± 0.13 | *1.52 ± 0.16 | *1.92 ± 0.18 | *1.47 ± 0.14 | *1.29 ± 0.10 |
| 5.15 | 0.37 ± 0.03 | *3.33 ± 0.49* | *3.69 ± 0.50* | *3.77 ± 0.52* | *3.16 ± 0.47* | *3.30 ± 0.45 | *3.20 ± 0.48* | *2.81 ± 0.48* |
| 10.30 | 0.30 ± 0.05 | *2.16 ± 0.37 | *2.47 ± 0.58* | *3.64 ± 0.67 | *2.75 ± 0.62 | *3.04 ± 0.65 | *4.45 ± 0.70 | *3.81 ± 0.51 |
|
| ||||||||
| AA | ||||||||
| 1.15 | 0.41 ± 0.10 | *0.89 ± 0.24 | *1.22 ± 0.29 | 0.98 ± 0.25 | *1.28 ± 0.47 | 1.02 ± 0.27 | *1.26 ± 0.43 | 0.70 ± 0.14 |
| 5.15 | 0.043 ± 0.012 | 0.09 ± 0.03* | *0.112 ± 0.04* | 0.14 ± 0.06* | 0.13 ± 0.04* | 0.07 ± 0.02* | 0.06 ± 0.02* | 0.07 ± 0.02* |
| 13.30 | 0.043 ± 0.009 | 0.22 ± 0.07 | *0.33 ± 0.08* | *0.84 ± 0.19* | *0.97 ± 0.24* | *1.30 ± 0.27* | *1.30 ± 0.31* | *1.02 ± 0.25* |
|
| ||||||||
| Total Kynurenines | ||||||||
| 1.15 | 3.26 ± 0.27 | *13.76 ± 1.28 | *15.38 ± 1.20 | *14.66 ± 1.02 | *16.98 ± 2.01 | *18.72 ± 1.82 | *19.11 ± 5.43 | *12.73 ± 1.31 |
| 5.15 | 3.85 ± 0.35* | *14.40 ± 1.07* | *22.02 ± 1.45* | *32.31 ± 2.69* | *34.98 ± 2.76* | *35.41 ± 2.91* | *32.53 ± 2.74* | *27.33 ± 3.27* |
| 10.30 | 4.03 ± 0.34 | *16.72 ± 1.20 | *25.28 ± 1.94 | *37.95 ± 3.17 | *51.15 ± 4.47* | *57.19 ± 5.17* | *62.50 ± 6.62* | *62.98 ± 6.61* |
|
| ||||||||
| TDO | ||||||||
| 1.15 | 3.14 ± 0.24 | 2.44 ± 0.43 | 2.89 ± 0.44 | 3.79 ± 0.65 | *4.25 ± 0.85 | *5.74 ± 1.05 | *5.44 ± 1.07 | *6.68 ± 0.89 |
| 5.15 | 5.00 ± 0.43 | *2.24 ± 0.31 | *2.95 ± 0.22 | 5.06 ± 0.71 | *7.33 ± 0.63* | *9.17 ± 0.87* | *10.53 ± 0.85* | *11.99 ± 0.85* |
| 10.30 | 4.49 ± 0.54 | *1.50 ± 0.20 | *2.40 ± 0.44 | 4.17 ± 0.81 | *6.63 ± 1.37 | *7.96 ± 1.72 | *9.58 ± 1.57 | *11.13 ± 1.87 |
|
| ||||||||
| TDOF | ||||||||
| 1.15 | 29 ± 2 | *17 ± 2 | 19 ± 3 | 29 ± 5 | 30 ± 5 | *40 ± 7 | *35 ± 7 | *43 ± 6 |
| 5.15 | 56 ± 12* | *12 ± 13* | *13 ± 14* | *20 ± 3 | *31 ± 5 | 40 ± 5 | 52 ± 8* | 64 ± 10* |
| 10.30 | 39 ± 5 | *9 ± 1 | *12 ± 3 | *18 ± 4 | 26 ± 8 | 34 ± 10 | 42 ± 12 | 51 ± 15 |
|
| ||||||||
| TTOX | ||||||||
| 1.15 | 7.41 ± 0.67 | *13.36 ± 2.32 | *14.51 ± 1.98 | *17.25 ± 3.06 | *26.95 ± 5.43 | *40.70 ± 6.00 | *49.00 ± 6.16 | *34.4 ± 4.86 |
| 5.15 | 7.58 ± 0.93 | 7.51 ± 0.70* | 7.64 ± 0.58* | *11.41 ± 1.18 | *14.24 ± 1.45* | *18.27 ± 2.22* | *22.32 ± 2.50 | *26.04 ± 3.59 |
| 10.30 | 6.60 ± 0.62 | 7.08 ± 0.59 | 7.14 ± 0.48 | *9.51 ± 0.90 | *12.48 ± 1.85 | *13.91 ± 2.05 | *16.85 ± 2.22 | *19.80 ± 2.78 |
|
| ||||||||
| TTOXF | ||||||||
| 1.15 | 68 ± 6 | *94 ± 11 | *95 ± 15 | *130 ± 22 | *192 ± 36 | *285 ± 45 | *320 ± 45 | *224 ± 34 |
| 5.15 | 118 ± 18 | *44 ± 5 | *37 ± 4* | *51 ± 6* | *66 ± 6* | 95 ± 11* | 134 ± 17 | *166 ± 26 |
| 10.30 | 57 ± 6 | 44 ± 4 | *34 ± 4 | *41 ± 6 | 50 ± 14 | 60 ± 15 | 74 ± 19 | *92 ± 23 |
|
| ||||||||
| KOHase | ||||||||
| 1.15 | 22 ± 2 | *237 ± 33 | *203 ± 34 | *172 ± 42 | *290 ± 49 | *379 ± 72 | *348 ± 69 | *224 ± 35 |
| 5.15 | 11 ± 2 | *123 ± 31 | *90 ± 18* | *76 ± 15* | *46 ± 8* | *48 ± 11* | *55 ± 16* | *60 ± 17* |
| 10.30 | 11 ± 2 | *259 ± 38 | *141 ± 22 | *79 ± 12 | *52 ± 8 | *40 ± 6* | *38 ± 6* | *34 ± 6* |
|
| ||||||||
| KATA (K→KA) | ||||||||
| 1.15 | 33 ± 7 | 38 ± 9 | 44 ± 6 | *46 ± 8 | *66 ± 123 | *51 ± 9 | 46 ± 12 | 37 ± 5 |
| 5.15 | 2.22 ± 0.9* | *5.1 ± 1.2* | *6.7 ± 0.9* | *9.7 ± 1.0* | *10.4 ± 1.0* | *10.4 ± 1.0* | *8.8 ± 1.2* | *6.0 ± 1.* |
| 10.30 | 12.4 ± 3.6* | *24.6 ± 4.6* | 11.3 ± 1.4* | 14.8 ± 1.8 | 13.1 ± 1.1 | 11.9 ± 1.2 | 12.8 ± 1.5* | 12.3 ± 2.0* |
|
| ||||||||
| KATB (3-HK→XA) | ||||||||
| 1.15 | 44 ± 8 | 60 ± 17 | 34 ± 6 | 35 ± 6 | 33 ± 5 | 38 ± 16 | 40 ± 11 | 34 ± 9 |
| 5.15 | 119 ± 16 | *16 ± 2* | *15 ± 3* | *13 ± 5 | *23 ± 4 | *26 ± 5 | *18 ± 4 | *12 ± 8 |
| 10.30 | 97 ± 16 | *9 ± 3 | *9 ± 1* | *9 ± 3 | *19 ± 8 | *25 ± 10 | *24 ± 5* | *51 ± 16* |
|
| ||||||||
| Total KAT | ||||||||
| 1.15 | 77 ± 10 | 98 ± 23 | 78 ± 8 | 81 ± 9 | 99 ± 15 | 89 ± 18 | 86 ± 17 | 71 ± 10 |
| 5.15 | 121 ± 15 | *21 ± 4* | *22 ± 3* | *23 ± 5* | *33 ± 4* | *36 ± 4* | *27 ± 4* | *18 ± 8 |
| 10.30 | 109 ± 16 | *34 ± 6 | *20 ± 2 | *24 ± 4 | *32 ± 8 | *37 ± 10 | *37 ± 6 | *63 ± 18* |
|
| ||||||||
| Kynase A (K→AA) | ||||||||
| 1.15 | 30 ± 7 | 35 ± 6 | 40 ± 7 | 30 ± 10 | 48 ± 10 | 39 ± 13 | 59 ± 36 | 28 ± 5 |
| 5.15 | 1.60 ± 0.32* | 1.83 ± 0.42* | 1.27 ± 0.37* | *1.23 ± 0.53* | *1.03 ± 0.42* | *0.66 ± 0.43 | *0.47 ± 0.17* | *0.87 ± 0.33* |
| 10.30 | 1.57 ± 0.34 | *6.23 ± 0.30* | *3.88 ± 0.56* | *5.05 ± 0.75* | *3.57 ± 0.67* | *3.97 ± 0.57* | *3.66 ± 0.63* | *2.88 ± 0.46* |
|
| ||||||||
| Kynase B (3-HK→3-HAA) | ||||||||
| 1.15 | 135 ± 15 | *27 ± 3 | *35 ± 7 | *35 ± 10 | *20 ± 5 | *19 ± 3 | *20 ± 9 | *24 ± 7 |
| 5.15 | 119 ± 10 | *60 ± 13 | *46 ± 9 | *33 ± 9 | *36 ± 8 | *36 ± 9 | *34 ± 8 | *33 ± 7 |
| 10.34 | 97 ± 16 | *24 ± 11 | *20 ± 3* | *28 ± 5 | *20 ± 3 | *23 ± 5 | *33 ± 10 | *31 ± 10 |
|
| ||||||||
| Total Kynase | ||||||||
| 1.15 | 165 ± 24 | *62 ± 20 | *75 ± 71 | *65 ± 18 | *68 ± 19 | *58 ± 22 | *79 ± 20 | *52 ± 13 |
| 5.15 | 121 ± 11* | *62 ± 14 | *47 ± 9 | *34 ± 9 | *37 ± 8 | *37 ± 7 | *34 ± 8 | *33 ± 13 |
| 10.30 | 99 ± 17 | *30 ± 12 | *24 ± 5* | *33 ± 5 | *24 ± 5 | *27 ± 6 | *37 ± 9 | *34 ± 11 |
Notes: Values are means ± SEM for the numbers in each group indicated above and the significance of the differences from baseline (0 h) is indicated by an asterisk on the left-hand side of the values. Values in the 5.15 g Trp group were compared with those in the 1.15 g group, whereas those in the 10.3 g group were compared with those in the 5.15 g group and the significance of the differences is indicated by an asterisk on the right-hand side of the values. P-values in all cases were 0.049–0.001.
Free and total [Trp] were dose-dependently and significantly elevated by Trp loading. The percentage free Trp (expressing Trp binding) was increased by the two larger Trp doses, but not by the small (1.15 g) dose in the C2 control group. The similar increases in this parameter caused by the larger Trp doses suggest that the Trp-binding sites on albumin are maximally occupied by the 5.15 g dose and that the extra Trp derived from the 10.3 g dose is reflected in the higher free [Trp] in this group.
Concentrations of plasma K, its metabolites, and their sum were also increased dose-dependently. Because TDO activity in the three groups was not elevated until the four-hour time point, it may be concluded that the increased formation of K metabolites is initially caused by the flux of Trp down the KP. The absence of differences in TDO activity or TTOX between the two larger Trp groups suggests that the 5.15 g dose induces the maximum activation of TDO and enhancement of Trp oxidation. A similar dose in rats (75 mg/kg) also activates TDO.24 We therefore recommend that, for maximum TDO activation in humans, a ~75 mg/kg Trp dose should be used. By contrast, a 1.15 g (~16 mg/kg) dose of Trp with a minimal effect of Leu (as in the C1 control group in Table 6) will not influence TDO activity. A 2 g oral Trp dose (~28.6 mg/kg) has traditionally been used in ATL studies.14 It has been suggested that a 50 mg/kg dose (~3.5 g for a 70 kg adult) should be used for ATL.66 This latter dose does not activate TDO in rats (measured by enzyme assay) or humans,16,23 but causes a temporary significant increase in the serum [K]/[Trp] ratio in rats.59 We therefore recommend the smaller dose of 2 g, or, more accurately, a 30 mg/kg dose, as a means of correcting for body weight differences among study subjects.
Enzyme activities expressed by product/substrate ratio percentages (Table 7) show that Trp loading with the two large doses preferentially decreases KAT and kynase activities with 3-HK as substrate. With KAT B, competitive inhibition by Trp may be a possible mechanism,67 whereas with kynase B a potential inhibition by both 3-HK and K64 is likely. It will be noted that KAT A and kynase A activities in the 1.15 g Trp group are considerably higher than in the other two groups. This is due to [KA] and [AA] being higher and [K] being lower in this group.
Gender differences in the 21 parameters studied in Table 7 were examined in the three Trp-treatment groups (data not shown). Only a few gender differences were detected, none of which was in the 1.15 g Trp-treated group. In the 5.15 g group, significantly lower KAT A activity at 4 hours and KAT B activity at 2, 6, and 7 hours after the Trp dose was observed in women, compared to men. In the 10.3 g group, more differences were observed: a higher free [Trp] in women at 4–7 hours; a lower [K] in women at 3–7 hours; a lower total Ks in women at 4–7 hours; a lower TDO and TTOX in women at 4–7 hours whether expressed relative to total or free Trp. These findings in conjunction with the data in Table 7 suggest that: (1) whereas TDO is activated maximally equally in men and women, an excessive Trp dose decreases TDO activation only in women; (2) the decreased overall Trp oxidation in women with the 10.3 g Trp dose is consistent with the decreases in TDO and the subsequent flux of Trp down the KP; (3) the lower KAT activity in women observed with the 5.15 g Trp dose is overcome by the larger dose possibly because of increased substrate availability.
Because the data in Table 4 show that ethnicity influences some Trp-related parameters, an attempt was made to examine ethnic responses to Trp loading by combining the data from both the 5.15 and 10.3 g Trp-treated groups to obtain sufficient (if still small) numbers for statistical purposes. However, numbers were still too small (not exceeding 8 in subgroups) with large individual variations. In Caucasians, for example, whereas baseline gender differences exist for [3-HAA], [KA], [AA], and KAT A and Kynase A (Table 4), the same differences were also observed during ATL, but did not reach statistical significance. Ethnic responses to ATL for the combined genders were also compared for the 21 Trp parameters. Little differences were observed (data not shown), thus suggesting that no ethnic differences in kynurenine metabolite formation can be expected in future ATL studies.
General Discussion and Conclusions
We have assessed Trp disposition and metabolism along the KP in a sample of healthy US volunteers and identified important features. We suggest that liver TDO contributes some 70% toward Trp oxidation along the hepatic KP, with most of the remainder possibly achieved by the subsequent rate-limiting enzymes kynureninase and picolinate carboxylase. We assume that a small contribution is likely from the extrahepatic Trp-degrading enzyme IDO under the normal physiological conditions of the present work. Dose–response experiments with ATL demonstrated that maximum activation of TDO is caused by a 5.15 g dose of Trp, with higher dosage impairing Trp oxidation. To standardize dosage for future ATL studies and to avoid TDO activation, we recommend a 30 mg/kg body weight dose. Acute Trp loading is generally believed to induce the so-called functional vitamin B6 deficiency, defined as a decrease in the pyridoxal 5′-phosphate cofactor for kynureninase,1 as a result of increased transamination of K to KA and 3-HK to XA, and explaining the increased urinary excretion of XA and also KA. However, as the results in Table 6 with the control C1 group (the nearest to a Trp-only control) show, KAT A is not influenced and KAT B is actually inhibited. Also, our data in rats treated with a 50 mg/kg dose of Trp (detailed in the accompanying paper)59 show that KAT A and B and also kynureninase A and B are all enhanced by Trp. There may be a species difference here, but another potential mechanism of this B6 deficiency that has not previously been suggested is that of increased transamination of Trp to indolepyruvic acid (IPA) and further metabolites. Trp administered in a large dose to rats increases liver Trp aminotransferase activity by 4.7-fold,68 and intravenous Trp administration to humans dose-dependently elevates the plasma concentrations of indol-3-lactic and indol-3-acetic acids.60 Conversion of IPA to Trp has been suggested when a healthy woman on a Trp-free diet given IPA achieved nitrogen balance but then lost it when the latter was withdrawn from the Trp-free diet.69 Transamination of Trp to IPA is common in plants and bacteria, including intestinal flora, but has not been studied in humans. However, the rapid increase in plasma concentrations of the above IPA metabolites following intravenous Trp administration60 suggests that tissues and not intestinal flora play an important role. In rat liver, 60% of Trp transamination is achieved by tyrosine aminotransferase.68 It is therefore likely that a significant level of Trp transamination to IPA occurs in humans especially after Trp loading. IPA is thought to possess many properties, including anxiolysis, sedation, and sleep promotion, and is also known to be metabolized to kynurenic acid.70 Aminotransferases play important roles in brain physiology as catalysts in the formation of keto acids as energy sources. Significant Trp transamination to IPA in the human brain is also very likely, in view of the wide substrate specificity of aminotransferases. For example, the recombinant human brain KAT II isoenzyme shows a similarly high catalytic efficiency for Trp as for K.71 These considerations may be relevant to the pharmacological actions of Trp and are worthy of investigation.
Baseline assessments are important, as they can reveal important gender and ethnic differences, notably (1) the higher plasma free [Trp] in women and consequently the increased availability of Trp for the cerebral kynurenine and serotonin pathways; (2) the lower kynurenic acid in Caucasian women, which may explain the lower incidence of schizophrenia in this ethnic group, possibly because of their greater use of estrogen contraception; (3) the potentially higher [3-HAA]/[AA] ratio in African-American women, which may explain their protection against osteoporosis. Other ethnic differences in KP activity could not be suggested here with certainty because of the small numbers studied. This is an important limitation in the present study, and future studies examining gender and ethnic differences in KP activity in larger numbers of subjects could yield important information for further understanding of the important role of this pathway in health and disease. Another limitation of the present study is the younger age group of subjects (18–40 years), and future studies will require comparisons with older subjects. Also, we suggest that future studies linking KP activity to schizophrenia and other behavioral disorders should assess the role of potential determinants and/or confounders: in particular (1) differences in diagnostic criteria and instruments used within a single population or across different countries with varied cultural and ethnic backgrounds, which could lead to different diagnostic conclusions, and (2) the need to establish the endogenous and pharmacological estrogen status as a function of age in women.
Acknowledgments
AA-BB holds an honorary professorial position at Cardiff Metropolitan University and thanks S. Khatun and A. Steptoe for skillful technical assistance.
Abbreviations
- AA
anthranilic acid
- ATD
acute tryptophan depletion
- ATL
acute tryptophan loading
- HPLC
high-performance liquid chromatography
- 3-HAA
3-hydroxyanthranilic acid
- 3-HK
3-hydroykynurenine
- 5-HT
5-hydroxytryptamine or serotonin
- IDO
indoleamine 2,3-dioxygenase
- Kynase
kynureninase
- Kynase A
(K → AA)
- Kynase B
(3-HK → 3-HAA)
- KA
kynurenic acid
- K
kynurenine
- KAT
kynurenine aminotransferase
- KAT A
(K → KA)
- KAT B
(3-HK → XA)
- KP
kynurenine pathway
- Ks
kynurenines
- NEFAs
non-esterified fatty acids
- QA
quinolinic acid
- Trp
tryptophan
- TDO
tryptophan 2,3-dioxygenase
- TDOF
tryptophan 23-dioxygenase relative to free tryptophan
- TTOX
total tryptophan oxidation
- TTOXF
total tryptophan oxidation relative to free tryptophan
- XA
xanthurenic acid
Footnotes
ACADEMIC EDITOR: Gilles Guillemin, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1242 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (R01AA14988 and R01AA018124). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: AA-BB, DMD. Directed the recruitment, screening and enrollment of the study subjects, their intake of amino acid mixtures, blood sampling, and processing of plasmas: DMD. Supervised the analytical procedures, analyzed the data, developed the structure and arguments for the paper, and wrote the first draft of the manuscript: AA-BB. Both authors jointly agreed with the manuscript results and conclusions, made critical revisions, and reviewed and approved the final version of the manuscript.
REFERENCES
- 1.Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6:1–97. doi: 10.1016/0098-2997(83)90005-5. [DOI] [PubMed] [Google Scholar]
- 2.Badawy AA-B. Tryptophan metabolism in alcoholism. Nutr Res Rev. 2002;1:123–52. doi: 10.1079/NRR200133. [DOI] [PubMed] [Google Scholar]
- 3.Kanai M, Funakoshi H, Takahashi H, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behaviour in mice. Mol Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badawy AA-B. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep. 2015;35:e00261. doi: 10.1042/BSR20150197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung AWS, Terentis AC, King NC, Thomas SR. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci. 2015;129:601–72. doi: 10.1042/CS20140392. [DOI] [PubMed] [Google Scholar]
- 6.Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res. 2009;2:45–60. doi: 10.4137/ijtr.s2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badawy AA-B. Tryptophan: the key to boosting brain serotonin synthesis in depressive illness. J Psychopharmacol. 2013;27:878–93. doi: 10.1177/0269881113499209. [DOI] [PubMed] [Google Scholar]
- 8.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:1–13. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2009;2:1–19. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–32. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mondelli V, Howes O. Editorial: inflammation: its role in schizophrenia and the potential of anti-inflammatory effects of antipsychotics. Psychopharmacol. 2014;231:317–8. doi: 10.1007/s00213-013-3383-3. [DOI] [PubMed] [Google Scholar]
- 12.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–65. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 13.Michael AF, Drummond KW, Doeden D, Anderson JA, Good RA. Tryptophan metabolism in man. J Clin Invest. 1964;43:1730–46. doi: 10.1172/JCI105048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price JM, Brown RR, Yess N. Testing the functional capacity of the tryptophan-niacin pathway in man by analysis of urinary metabolites. Adv Metab Disord. 1965;2:159–225. doi: 10.1016/b978-1-4831-6750-3.50009-3. [DOI] [PubMed] [Google Scholar]
- 15.Green AR, Bloomfield MR, Woods HF, Seed M. Metabolism of an oral tryptophan load by women and evidence against the induction of tryptophan pyrrolase by oral contraceptives. Br J Clin Pharmacol. 1978;5:233–41. doi: 10.1111/j.1365-2125.1978.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green AR, Aronson JK, Curzon G, Woods HF. Metabolism of an oral tryptophan load. I: effects of dose and pretreatment with tryptophan. Br J Clin Pharmacol. 1980;10:603–10. doi: 10.1111/j.1365-2125.1980.tb00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Møller SE. Pharmacokinetics of tryptophan, renal handling of kynurenine and the effect of nicotinamide on its appearance in plasma and urine following L-tryptophan loading of healthy subjects. Eur J Clin Pharmacol. 1981;21:137–42. doi: 10.1007/BF00637514. [DOI] [PubMed] [Google Scholar]
- 18.Badawy AA-B, Morgan CJ. Rapid isocratic liquid chromatographic separation and quantification of tryptophan and six kynurenine metabolites in biological samples with ultraviolet and fluorimetric detection. Int J Tryptophan Res. 2010;3:175–86. doi: 10.4137/IJTR.S6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay GM, Forrest CM, Stoy N, Christofides J, Egerton M, Stone TW. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur J Neurol. 2005;12:1–13. doi: 10.1111/j.1468-1331.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- 20.Stoy N, Mackay G, Forrest CM, et al. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J Neurochem. 2005;9:611–23. doi: 10.1111/j.1471-4159.2005.03070.x. [DOI] [PubMed] [Google Scholar]
- 21.Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacol. 1985;87:173–7. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- 22.Badawy AA-B, Lake SL, Dougherty DM. Mechanisms of the pellagragenic effect of leucine: stimulation of hepatic tryptophan oxidation by administration of branched-chain amino acids to healthy human volunteers and the role of plasma free tryptophan and total kynurenines. Int J Tryptophan Res. 2014;7:23–32. doi: 10.4137/IJTR.S18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badawy AA-B, Evans M. The regulation of rat liver tryptophan pyrrolase by its cofactor haem – experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem J. 1975;150:511–20. doi: 10.1042/bj1500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badawy AA-B, Welch AN, Morgan CJ. Tryptophan pyrrolase in haem regulation: the mechanism of the opposite effects of tryptophan on rat liver 5- aminolaevulinate synthase activity and the haem saturation of tryptophan pyrrolase. Biochem J. 1981;198:309–14. doi: 10.1042/bj1980309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishiziwa S, Benkelfat C, Young SN, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94:5308–13. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dougherty DM, Marsh-Richard DM, Mathias W, et al. Comparison of 50- and 100-g L-tryptophan depletion and loading formulations for altering 5-HT synthesis: pharmacokinetics, side-effects and mood states. Psychopharmacol. 2008;198:431–45. doi: 10.1007/s00213-008-1163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badawy AA-B, Dougherty DM, Richard DM. Specificity of the acute tryptophan and tyrosine plus phenylalanine depletion and loading tests II. Normalisation of the tryptophan and the tyrosine plus phenylalanine to competing amino acid ratios in a new control formulation. Int J Tryptophan Res. 2010;3:35–47. doi: 10.4137/ijtr.s5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badawy AA-B. Plasma free tryptophan revisited: what you need to know and do before measuring it. J Psychopharmacol. 2010;24:809–15. doi: 10.1177/0269881108098965. [DOI] [PubMed] [Google Scholar]
- 29.Midttun Ø, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:1371–9. doi: 10.1002/rcm.4013. [DOI] [PubMed] [Google Scholar]
- 30.Smith SA, Pogson CI. The metabolism of L-tryptophan by isolated rat liver cells: effect of albumin binding and amino acid competition on oxidation of tryptophan by tryptophan 2,3-dioxygenase. Biochem J. 1980;186:977–86. doi: 10.1042/bj1860977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohashi H, Iizuka H, Yoshihara S, et al. Determination of L-tryptophan and L-kynurenine in human serum by using LC-MS after derivatization with (R)-DBD-PyNCS. Int J Tryptophan Res. 2013;3(Suppl 1):9–14. doi: 10.4137/IJTR.S11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rios-Avila L, Nijhout HF, Reed MC, Sitren HS, Gregory JF., III A mathematical model of tryptophan metabolism via the kynurenine pathway provides insights into effects of vitamin B-6 deficiency, tryptophan loading, and induction of tryptophan 2,3-dioxygenase on tryptophan metabolites. J Nutr. 2013;143:1509–19. doi: 10.3945/jn.113.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Silva VR, Rios-Avila L, Lamers Y, et al. Metabolite profile analysis reveals functional effects of 28-day vitamin B-6 restriction on one-carbon metabolism and tryptophan catabolic pathways in healthy men and women. J Nutr. 2013;143:1719–27. doi: 10.3945/jn.113.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulvik A, Theofylaktopoulou D, Midttun Ø, Nygård O, Eusson SJ, Ueland PM. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr. 2013;98:934–40. doi: 10.3945/ajcn.113.064998. [DOI] [PubMed] [Google Scholar]
- 35.Badawy AA-B, Dougherty DM, Marsh-Richard DM, Steptoe A. Activation of liver tryptophan pyrrolase mediates the decrease in tryptophan availability to the brain after acute alcohol consumption by normal subjects. Alcohol Alcohol. 2009;4:267–71. doi: 10.1093/alcalc/agp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inada J, Okuno E, Kimura M, Kido R. Intracellular localization and characterization of 3-hydroxykynureninase in human liver. Int J Biochem. 1984;16:623–8. doi: 10.1016/0020-711x(84)90031-4. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi F, Shibata Y. Kynurenine metabolism in vitamin-B-6-deficient rat liver after tryptophan injection. Biochem J. 1984;220:693–9. doi: 10.1042/bj2200693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bender DA. Inhibition in vitro of the enzymes of the oxidative pathway of tryptophan metabolism and of nicotinamide nucleotide synthesis by benserazide, cabidopa and isoniazid. Biochem Pharmacol. 1980;29:707–12. doi: 10.1016/0006-2952(80)90544-4. [DOI] [PubMed] [Google Scholar]
- 39.Shibata K, Marugami M, Kondo T. In vivo inhibition of kynurenine aminotransferase activity by isonicotinic acid hydrazide in rats. Biosci Biotechnol Biochem. 1996;60:874–6. doi: 10.1271/bbb.60.874. [DOI] [PubMed] [Google Scholar]
- 40.Pawlak D, Tankiewicz A, Matys T, Buczko W. Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J Physiol Pharmacol. 2003;5:175–89. [PubMed] [Google Scholar]
- 41.Salter M, Bender DA, Pogson CI. Leucine and tryptophan metabolism in rats. Biochem J. 1985;225:277–81. doi: 10.1042/bj2250277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darlington LG, Forrest CM, Mackay GM, Smith RA, Smith AJ, Stone TW. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptophan Res. 2010;3:51–9. doi: 10.4137/ijtr.s4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bender DA, Tagoe CE, Vale JA. Effects of oestrogen administration on vitamin B6 and tryptophan metabolism in the rat. Br J Nutr. 1982;47:609–14. doi: 10.1079/bjn19820072. [DOI] [PubMed] [Google Scholar]
- 44.Mason M, Manning B. Effects of steroid conjugates on availability of pyridoxal phosphate for kynureninase and kynurenine aminotransferase. Am J Clin Nutr. 1971;24:786–91. doi: 10.1093/ajcn/24.7.786. [DOI] [PubMed] [Google Scholar]
- 45.Knox WE. The relation of kynureninase to tryptophan metabolism in pyridoxine deficiency. Biochem J. 1953;5:379–85. doi: 10.1042/bj0530379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia. Arch Gen Psychiat. 2003;60:565–71. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- 47.Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis. Schizophr Res Treatment. 2012;2012:916198. doi: 10.1155/2012/916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu YC, Hill RA, Gogos A, Van den Buse M. Sex differences and the role of estrogen in animal models of schizophrenia: interaction with BDNF. Neuroscience. 2013;23:67–83. doi: 10.1016/j.neuroscience.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 49.Ramos-Loyo J, Medina-Hernández V, Estarrón-Espinosa M, Canales-Aguirre A, Gómez-Pinedo U, Cerdán-Sánchez LF. Sex differences in lipid peroxidation and fatty acid levels in recent onset schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:S154–61. doi: 10.1016/j.pnpbp.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Stone W. The neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–85. [PubMed] [Google Scholar]
- 51.Sathyasaikumar KV, Stachowski EK, Wonodi I, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–56. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu H-Q, Okuyama M, Kajii Y, Pocivavsek A, Bruno JP, Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effect of an orally active enzyme inhibitor. Schizophr Bull. 2014;40(suppl 2):S2152–8. doi: 10.1093/schbul/sbt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dehlendorf C, Park SY, Emeremni AA, Comer D, Vincent K, Borrero S. Racial/ethnic disparities in contraceptive use: variation by age and women’s reproductive experiences. Am J Obstet Gynecol. 2014;210(6):526.e1–526.e9. doi: 10.1016/j.ajog.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borrero S, Zhao X, Mor MK, Schwarz EB, Good CB, Gellad WF. Adherence to hormonal contraception among women veterans: differences by race/ethnicity and contraceptive supply. Am J Obstet Gynecol. 2013;20:103e111. doi: 10.1016/j.ajog.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 55.Bresnahan M, Begg MD, Brown A, et al. Race and risk of schizophrenia in a US birth cohort: another example of health disparity? Int J Epidemiol. 2007;36:751–8. doi: 10.1093/ije/dym041. [DOI] [PubMed] [Google Scholar]
- 56.Neighbors HW, Trierweiler SJ, Ford B, Muroff JR. Racial differences in DSM diagnosis using a semi-structured instrument: the importance of clinical judgment in the diagnosis of African Americans. J Health Soc Behav. 2003;43:237–56. [PubMed] [Google Scholar]
- 57.Fearon P, Kirkbridge JB, Morgan C, et al. AESOP Study Group Incidence of schizophrenia and other psychoses in ethnic minority groups: results from the MRC AESOP Study. Psychol Med. 2006;36:1541–50. doi: 10.1017/S0033291706008774. [DOI] [PubMed] [Google Scholar]
- 58.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88(Suppl):545S–50S. doi: 10.1093/ajcn/88.2.545S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Badawy AA-B, Bano S. Tryptophan metabolism in rat liver after administration of tryptophan, kynurenine metabolites and kynureninase inhibitors. Int J Tryptophan Res. 2016:9. doi: 10.4137/IJTR.S38190. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huether G, Hajak G, Reimer A, et al. The metabolic fate of infused L-tryptophan in men: possible clinical implications of the accumulation of circulating tryptophan and tryptophan metabolites. Psychopharmacology (Berl) 1992;109:422–32. doi: 10.1007/BF02247718. [DOI] [PubMed] [Google Scholar]
- 61.Yuwiler A, Brammer GL, Morley JE, Raleigh MJ, Flannery JW, Geller E. Short-term and repetitive administration of oral tryptophan in normal men. Arch Gen Psychiatry. 1981;38:619–26. doi: 10.1001/archpsyc.1981.01780310019001. [DOI] [PubMed] [Google Scholar]
- 62.Delgado P, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action: reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47:411–4. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- 63.Salter M, Pogson CI, Bender DA. Leucine and tryptophan metabolism in rats. Biochem J. 1985;225:277–81. doi: 10.1042/bj2250277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osagawara N, Hagino Y, Kotake Y. Kynurenine aminotransferase, kynureninase and the increase in xanthurenic acid excretion. J Biochem. 1962;52:162–6. doi: 10.1093/oxfordjournals.jbchem.a127591. [DOI] [PubMed] [Google Scholar]
- 65.Walsh HA, Botting NP. Purification and biochemical characterization of some of the properties of recombinant human kynureninase. Eur J Biochem. 2002;269:2069–74. doi: 10.1046/j.1432-1033.2002.02854.x. [DOI] [PubMed] [Google Scholar]
- 66.Allegri G, Costa C, De Antoni A. A further contribution to the choice of the dose for tryptophan load test. Acta Vitaminol Enzymol. 1978;32:163–6. [PubMed] [Google Scholar]
- 67.Han Q, Robinson H, Cai T, Tagle DA, Li J. Structural insight into the inhibition of human kynurenine aminotransferase I/glutamine transaminase K. J Med Chem. 2009;52:2786–93. doi: 10.1021/jm9000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stanley JC, Nicholas AR, Dickson AJ, Thompson IN, Pogson CI. Tryptophan aminotransferase activity in rat liver. Biochem J. 1984;220:341–4. doi: 10.1042/bj2200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richards P, Brown CL, Lowe SM. Synthesis of tryptophan from 3- indolepyruvic acid by a healthy woman. J Nutr. 1972;102:1547–50. doi: 10.1093/jn/102.11.1547. [DOI] [PubMed] [Google Scholar]
- 70.Politi V, D’Alessioi S, Stazio G, De Luca G. Antioxidant properties of indole-3-pyruvic acid. Adv Exp Med Biol. 1996;398:291–8. doi: 10.1007/978-1-4613-0381-7_46. [DOI] [PubMed] [Google Scholar]
- 71.Han Q, Cai T, Tagle DA, Robinson H, Li J. Substrate specificity and structure of human aminoadipate aminotransferase/kynurenine aminotransferase II. Biosci Rep. 2008;28:205–15. doi: 10.1042/BSR20080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badawy AA-B. Pellagra and alcoholism: a biochemical perspective. Alcohol Alcohol. 2014;49:238–50. doi: 10.1093/alcalc/agu010. [DOI] [PubMed] [Google Scholar]