Abstract
Throughout its 30-year history, the right gastroepiploic artery (GEA) has been useful for in situ grafts in coronary artery bypass grafting (CABG). The early graft patency rate is high, and the late patency rate has improved by using the skeletonized GEA graft and proper target selection, which involves having a target coronary artery with a tight >90% stenosis. Total arterial revascularization with the internal thoracic artery and GEA grafts is an option for achieving better outcomes from CABG procedures.
Keywords: Coronary artery disease, Ischemic heart disease, Coronary artery bypass graft, Myocardial revascularization, Gastroepiploic artery
Introduction
The history of coronary artery bypass grafting (CABG) exceeds 50 years. The saphenous vein and various arterial grafts have been used and evaluated for their functional efficacy; however, the choice of conduit has been a major concern for cardiac surgeons desiring a good surgical outcome [1]. It is clear that the internal thoracic artery (ITA) is superior to the saphenous vein in terms of the patency rate and patients’ long-term outcomes [2]. Consequently, intensive investigations were undertaken to find a new arterial conduit for CABG.
Before CABG, a procedure in which the graft is directly anastomosed to the coronary artery, was instituted, the gastroepiploic artery (GEA) was used for indirect myocardial revascularization (Vineberg procedure) for the posterior or inferior wall of the heart [3]. The aorta-coronary bypass using the saphenous vein graft was successfully introduced by Favaloro [4] in the Cleveland Clinic in 1967. Afterwards, anastomosis of the GEA to the right coronary artery was attempted by Edwards in the early 1970s [5]. The GEA graft then emerged as an alternative arterial conduit for CABG, and pioneering articles were published in 1987 with successful clinical results by Pym et al. [6] in Canada and Suma et al. [7] in Japan. Since that time, the GEA graft gained attention as an attractive arterial conduit for CABG.
Anatomy, Histology, and Physiology
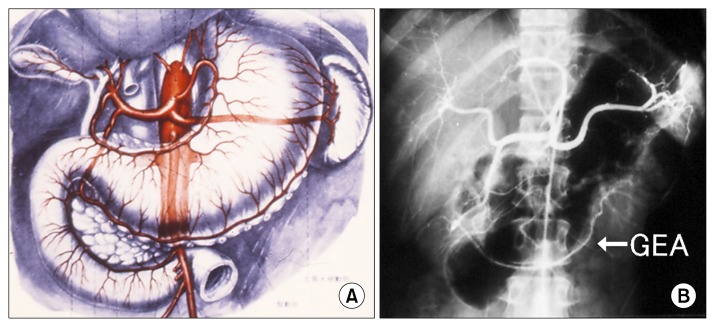
The right GEA is one of four arteries that supply blood to the stomach. It is the largest terminal branch of the gastroduodenal artery, which originates from the common hepatic artery (Fig. 1).
Fig. 1.
(A) Schematic and (B) angiogram of the gastroepiploic artery (GEA).
It is important to know that the GEA arises from the superior mesenteric artery in rare occasions [8]. If a cardiologist was not aware of this, hepatic or selective gastroduodenal angiography to seek the GEA graft patency after the operation can fail to find a patent GEA graft. The right GEA reaches more than half of the greater curvature of the stomach in about 90% of people [7,9], and terminates in a varying fashion with or without communication to the left GEA [10].
Histologically, the wall thickness of the GEA is similar to the ITA [11], but has different characteristics from the ITA in terms of spasmogeneity. The GEA contains many smooth muscle cells in the media, whereas the ITA has rich elastic fibers in media. Therefore, the GEA is considered a muscular artery and the ITA is an elastic artery. This difference is quite important in clinical situations during and after surgery, because GEA spasm readily arises from surgical manipulation whereas the ITA hardly becomes spastic.
Arteriosclerosis of the GEA was found to be less frequent than in the coronary artery [12]. In our previous study, the GEA and ITA used in CABG were examined pathologically and moderate to severe arteriosclerosis was noted in 8% of GEA grafts and 1% of ITA grafts [13]. Although the GEA had arteriosclerosis more frequently than the ITA, the GEA may be an arteriosclerosis-resistant artery, as all patients included in the study had severe coronary artery diseases.
Various functional studies have been published as the GEA has been increasingly used for CABG. In response to vasoactive agents, the GEA contracts with ergonovine, serotonin, and phenylephrine, and the ITA reacted similarly [14]. Another study demonstrated that the GEA contractions by potassium chloride, serotonin, and norepinephrine were stronger than the ITA contractions [15]. These results suggest that prevention of spasm provoked by platelet aggregators, adrenergic stimulation, or depolarizing agents is important in GEA grafting [16].
Interestingly, the responses of the GEA to histamine were different than in the ITA. Histamine dilates the GEA and contracts the ITA [17,18]. Our group introduced an implantable ultrasonic Doppler miniprobe for the patients undergoing CABG, which was attached to the GEA and other grafts at the end of the operation. Then the skin was closed and the patient started postoperative rehabilitation. During exercise, drug administration, and meals, the graft blood flow was monitored. A week postoperatively, the probe was removed without injury. We found that the GEA flow increased after meals [19]. This finding is compatible with the previously mentioned GEA dilatation by histamine. We also found that the GEA was more capable of prostacyclin production than the saphenous vein [20].
Surgical Procedure
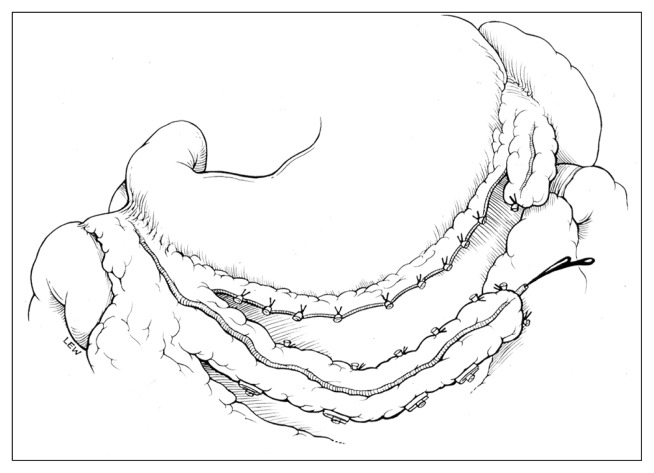
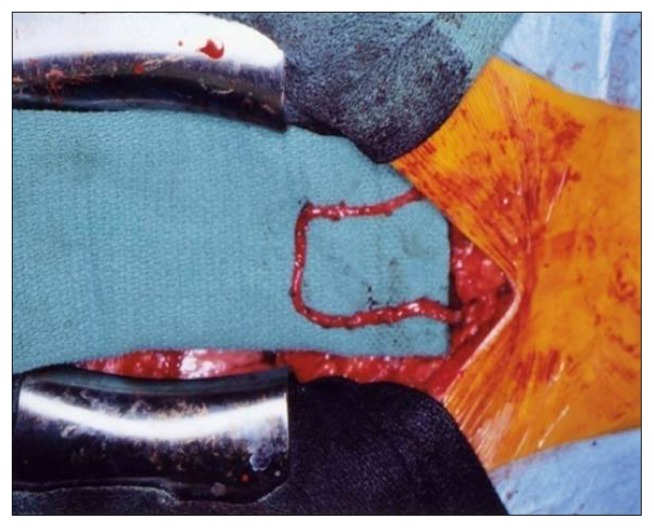
The midline incision was about 5 cm longer than the usual sternal splitting incision of ordinary CABG. Following a pericardiotomy, the peritoneum was open and the stomach was pulled out to the surgical field. The GEA was detected by palpation to determine size and length. It is important to touch the GEA softly because it readily contracts under mechanical stimulation. Then the GEA was mobilized from the greater curvature of the stomach using electric cautery or an ultrasonic scalpel as a pedicle (Fig. 2), or in a skeletonized fashion without any surrounding tissue (Fig. 3).
Fig. 2.
Detachment of the gastroepiploic artery from the stomach.
Fig. 3.
Skeletonized gastroepiploic artery graft.
The skeletonized GEA can be used as a free graft if necessary [21]. The skeletonized GEA produces a longer graft length and is easier to use for sequential anastomoses. Also, Y- or I-composite grafts using a free GEA graft in combination with the in situ left or right ITA graft further increase the possibility of total arterial complete myocardial revascularization.
There was a concern about gastric ischemia due to GEA detachment from the stomach in the early period when GEA grafting was first instituted. Therefore, we measured gastric mucosal blood flow during surgery. The endoscopic Doppler flow study clearly demonstrated that no gastric ischemia occurred in any part of the stomach following GEA detachment [22].
After mobilizing the GEA from the pylorus to more than half of the greater curvature, its distal end was cut and a diluted papaverine solution was slowly injected into the GEA lumen to relieve spasm. The intraluminal papaverine procedure is effective to obtain a good caliber and maximum flow of the GEA for successful anastomosis. Accordingly, perioperative use of diltiazem is essential.
The GEA and the coronary artery anastomosis are performed under a cardioplegic arrest or an off-pump beating heart. Finally, the GEA is fixed to the epicardium properly to avoid kinking or twisting. Fig. 4 shows a complete arterial CABG procedure with bilateral ITA and GEA in situ grafts.
Fig. 4.
(A, B) Coronary artery bypass grafting with in situ bilateral internal thoracic artery and gastroepiploic artery graft.
Indication For Gastroepiploic Artery Grafting
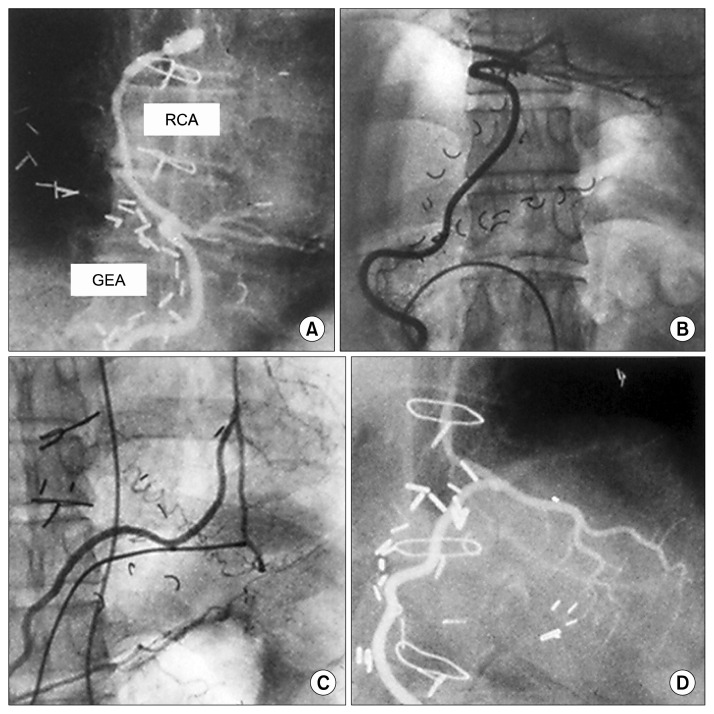
The GEA is most suitable for grafting the distal right coronary artery and the posterior descending artery because this site is the nearest for the in situ GEA graft and the most distant from the in situ right ITA graft.
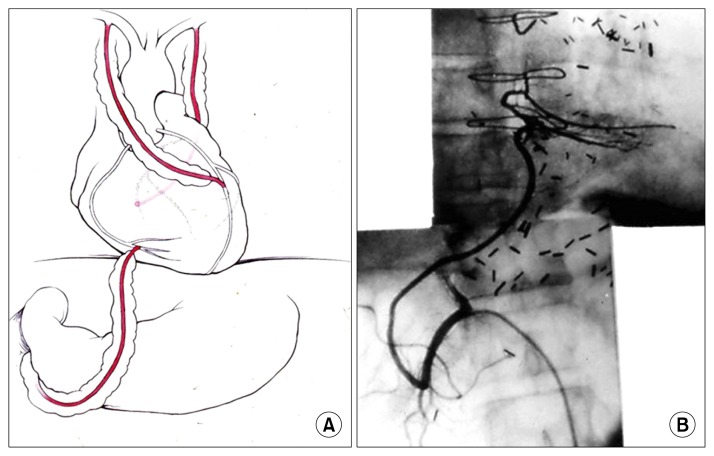
The distal circumflex artery is also a possible target for the GEA graft. The anterior descending artery is a common site for the left ITA, but it is an option for the GEA grafting target when the ITA is unavailable (Fig. 5).
Fig. 5.
The in situ GEA graft anastomosed to various sites of the coronary artery. (A) Main RCA. (B) Posterior descending artery. (C) Left anterior descending artery. (D) Circumflex. RCA, right coronary artery; GEA, gastroepiploic artery.
The GEA is advantageous for the patient with atherosclerotic ascending aorta because the in situ arterial grafts are necessary for the aortic no-touch technique [23]. In cases of redo CABG, the GEA is useful because the abdomen is a virgin area, which makes GEA preparation easy before re-sternotomy.
GEA grafts are not recommended in very elderly patients or those with extreme obesity. In addition, unstable hemodynamics during emergency surgery or proposed future abdominal operations are a contraindication for the in situ GEA graft.
Clinical Results
Since 1987 when Pym et al. [6] and Suma et al. [7] reported their successful clinical applications of GEA grafting, international investigators have started using the GEA graft, and clinical and basic research on the graft has drawn special attention [24–28].
In general, early postoperative clinical results and GEA graft patency have been reported to be satisfactory. Unique reports have revealed that the GEA was successfully used in children who had coronary artery aneurysm and stenosis due to Kawasaki disease and required CABG [29,30].
In regard to the risks of GEA grafting, we compared the surgical results between patients with and without a GEA graft in CABG. There was no increase in postoperative comorbidity, particularly abdominal complications, with use of the GEA graft [22]. At 5 years after our first GEA graft case, we presented the midterm results in 200 patients [31]. The operative mortality and early GEA patency rate were identical in the two groups. Concerning the flow capacity of the GEA graft, pre- and postoperative stress myocardial scintigraphy showed that the washout ratio of the GEA grafted area was significantly increased after surgery.
Following the successful initial period, the GEA graft was used more extensively and its long-term results have been reported. For the investigators who performed CABG combined with bilateral ITA and GEA grafts, 10- to 15-year postoperative survival rates were demonstrated to be excellent [32–36]. In our 20-year experience, 5-, 10- and 15-year actuarial survival rates were 91.7%, 81.4%, and 71.3%, respectively, in 1,118 follow-up patients [36].
Table 1 shows the patients’ characteristics and surgical results of our 30-year experience with GEA grafting. In patients with triple vessel disease having bilateral ITA to the left coronary artery system, the patients with a GEA graft to the right coronary artery resulted in significantly better late survival than patients who received the saphenous vein graft [37,38]. In a comparison between free GEA and free right ITA grafts, Hwang et al. [39] demonstrated that the 5- and 10-year event-free survival rates were similar in those two grafts used for a composite Y graft with the left ITA.
Table 1.
Outcomes of coronary artery bypass grafting using the gastroepiploic artery graft over 30 years (N=1,500)
| Characteristic | Value |
|---|---|
| Male/female | 1,212/288 |
|
| |
| Age (yr) | 63 (6–82) |
|
| |
| Coronary lesion | |
| 1-Vessel | 9 |
| 2-Vessel | 218 |
| 3-Vessel | 1,010 |
| Left main trunk | 263 |
|
| |
| Ejection fraction (%) | 51.6 |
|
| |
| No. of grafts | 3.4 |
|
| |
| Concomitant grafts | |
| Internal thoracic artery | 1,458 (97) |
| Radial artery | 133 (9) |
| Saphenous vein | 823 (55) |
|
| |
| Site of GEA anastomosis | |
| Left anterior descending | 70 |
| Diagonal | 10 |
| Left circumflex | 319 |
| Right coronary artery | 1,238 |
|
| |
| Mode of GEA graft | |
| In situ single | 1,346 |
| Free single | 52 |
| Free composite | 56 |
| Sequential | 76 |
|
| |
| Operative death | 18 (1.2) |
Values are presented as number, mean (range), or number (%), unless otherwise stated.
GEA, gastroepiploic artery.
Gastroepiploic Artery Graft Patency
Regarding the patency rate of the GEA graft, a tight stenosis of the target coronary artery is important to maximize the potential for patency. The American College of Cardiology Foundation/American Heart Association guideline for CABG stated that arterial grafting to the right coronary artery is contraindicated for patients with <90% stenosis of the native vessel [40].
In our experience, the cumulative patency rate of the GEA graft was 97.1% at 1 month, 92.3% at 1 year, 85.5% at 5 years, 80.9% at 7 years, and 66.5% at 10 years following surgery [36]. This relatively low 10-year patency rate has been improved by using a skeletonized GEA graft only to the coronary artery with a tight >90% stenosis. Using this approach, Suzuki et al. [41] reported 97.8%, 94.7%, and 90.2% cumulative patency rates immediately, and 5 and 8 years after surgery, respectively.
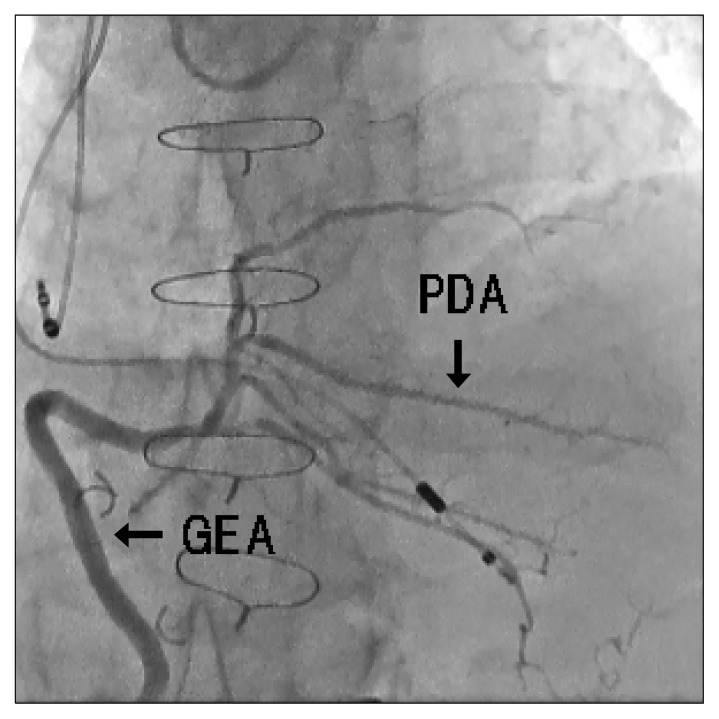
On the other hand, a network meta-analysis of randomized controlled trials reported that the GEA graft has a higher risk of graft occlusion compared with other types of conduits [42]. While heavy GEA users and non-users have not agreed on the exact decision tree for choosing the conduit to the right coronary artery, those who apply GEA grafts with skeletonization and proper target selection will find the GEA to be reliable. Fig. 6 demonstrates a widely patent GEA graft anastomosed to the posterior descending artery at 20 years after surgery.
Fig. 6.
The GEA graft anastomosed to the PDA at 20 years after coronary artery bypass grafting. GEA, gastroepiploic artery; PDA, posterior descending artery.
Conclusion
In conclusion, the GEA is a great, elegant, and attractive conduit for CABG.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Gaudino M, Taggart D, Suma H, Puskas JD, Crea F, Massetti M. The choice of conduits in coronary artery bypass surgery. J Am Coll Cardiol. 2015;66:1729–37. doi: 10.1016/j.jacc.2015.08.395. [DOI] [PubMed] [Google Scholar]
- 2.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CP, Hirose T, Brancato R, Aventura A, Yamamoto N. Revascularization of the posterior (diaphragmatic) portion of the heart. Ann Thorac Surg. 1966;2:791–805. doi: 10.1016/S0003-4975(10)66658-1. [DOI] [Google Scholar]
- 4.Favaloro RG. Saphenous vein autograft replacement of severe segmental coronary artery occlusion: operative technique. Ann Thorac Surg. 1968;5:334–9. doi: 10.1016/S0003-4975(10)66351-5. [DOI] [PubMed] [Google Scholar]
- 5.Mills NL, Everson CT. Right gastroepiploic artery: a third arterial conduit for coronary artery bypass. Ann Thorac Surg. 1989;47:706–11. doi: 10.1016/0003-4975(89)90122-7. [DOI] [PubMed] [Google Scholar]
- 6.Pym J, Brown PM, Charrette EJ, Parker JO, West RO. Gastroepiploic-coronary anastomosis: a viable alternative bypass graft. J Thorac Cardiovasc Surg. 1987;94:256–9. [PubMed] [Google Scholar]
- 7.Suma H, Fukumoto H, Takeuchi A. Coronary artery bypass grafting by utilizing in situ right gastroepiploic artery: basic study and clinical application. Ann Thorac Surg. 1987;44:394–7. doi: 10.1016/S0003-4975(10)63799-X. [DOI] [PubMed] [Google Scholar]
- 8.Suma H, Sato H. The in situ right gastroepiploic artery graft via the superior mesenteric artery. J Thorac Cardiovasc Surg. 1989;98:1150. [PubMed] [Google Scholar]
- 9.Hannoun L, Le Breton C, Bors V, Helenon C, Bigot JM, Parc R. Radiological anatomy of the right gastroepiploic artery. Anat Clin. 1984;5:265–71. doi: 10.1007/BF01798750. [DOI] [PubMed] [Google Scholar]
- 10.Yamato T, Hamanaka Y, Hirata S, Sakai K. Esophagoplasty with an autogenous tubed gastric flap. Am J Surg. 1979;137:597–602. doi: 10.1016/0002-9610(79)90030-8. [DOI] [PubMed] [Google Scholar]
- 11.Van Son JA, Smedts F, Vincent JG, van Lier HJ, Kubat K. Comparative anatomic studies of various arterial conduits for myocardial revascularization. J Thorac Cardiovasc Surg. 1990;99:703–7. [PubMed] [Google Scholar]
- 12.Larsen E, Johansen A, Andersen D. Gastric arteriosclerosis in elderly people. Scand J Gastroenterol. 1969;4:387–9. doi: 10.3109/00365526909180620. [DOI] [PubMed] [Google Scholar]
- 13.Suma H, Wanibuchi Y, Furuta S, Isshiki T, Yamaguchi T, Takanashi R. Comparative study between the gastroepiploic and the internal thoracic artery as a coronary bypass graft. Size, flow, patency, histology. Eur J Cardiothorac Surg. 1991;5:244–7. doi: 10.1016/1010-7940(91)90171-F. [DOI] [PubMed] [Google Scholar]
- 14.Koike R, Suma H, Kondo K, et al. Pharmacological response of internal mammary artery and gastroepiploic artery. Ann Thorac Surg. 1990;50:384–6. doi: 10.1016/0003-4975(90)90479-P. [DOI] [PubMed] [Google Scholar]
- 15.Dignan RJ, Yeh T, Jr, Dyke CM, et al. Reactivity of gastroepiploic and internal mammary arteries: relevance to coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1992;103:116–22. [PubMed] [Google Scholar]
- 16.Suma H. Spasm of the gastroepiploic artery graft. Ann Thorac Surg. 1990;49:168–9. doi: 10.1016/0003-4975(90)90390-R. [DOI] [PubMed] [Google Scholar]
- 17.O’Neil GS, Chester AH, Schyns CJ, Tadjkarimi S, Pepper JR, Yacoub MH. Vascular reactivity of human internal mammary and gastroepiploic arteries. Ann Thorac Surg. 1991;52:1310–4. doi: 10.1016/0003-4975(91)90019-M. [DOI] [PubMed] [Google Scholar]
- 18.Favaloro RG, Effler DB, Sheldon WC. Successful pulmonary embolectomy: report of a case. Cleve Clin Q. 1967;34:119–25. doi: 10.3949/ccjm.34.2.119. [DOI] [PubMed] [Google Scholar]
- 19.Takayama T, Suma H, Wanibuchi Y, Tohda E, Matsunaka T, Yamashita S. Physiological and pharmacological responses of arterial graft flow after coronary artery bypass grafting measured with an implantable ultrasonic Doppler miniprobe. Circulation. 1992;86(5 Suppl):II217–23. [PubMed] [Google Scholar]
- 20.Oku T, Yamane S, Suma H, et al. Comparison of prostacyclin production of human gastroepiploic artery and saphenous vein. Ann Thorac Surg. 1990;49:767–70. doi: 10.1016/0003-4975(90)90018-2. [DOI] [PubMed] [Google Scholar]
- 21.Suma H, Tanabe H, Yamada J, Mikuriya A, Horii T, Isomura T. Midterm results for use of the skeletonized gastroepiploic artery graft in coronary artery bypass. Circ J. 2007;71:1503–5. doi: 10.1253/circj.71.1503. [DOI] [PubMed] [Google Scholar]
- 22.Suma H, Wanibuchi Y, Furuta S, Takeuchi A. Does use of gastroepiploic artery graft increase surgical risk? J Thorac Cardiovasc Surg. 1991;101:121–5. [PubMed] [Google Scholar]
- 23.Suma H. Coronary artery bypass grafting in patients with calcified ascending aorta: aortic no-touch technique. Ann Thorac Surg. 1989;48:728–30. doi: 10.1016/0003-4975(89)90807-2. [DOI] [PubMed] [Google Scholar]
- 24.Carter MJ. The use of the right gastro-epiploic artery in coronary artery bypass grafting. Aust N Z J Surg. 1987;57:317–21. doi: 10.1111/j.1445-2197.1987.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 25.Lytle BW, Cosgrove DM, Ratliff NB, Loop FD. Coronary artery bypass grafting with the right gastroepiploic artery. J Thorac Cardiovasc Surg. 1989;97:826–31. [PubMed] [Google Scholar]
- 26.Suma H, Takeuchi A, Hirota Y. Myocardial revascularization with combined arterial grafts utilizing the internal mammary and the gastroepiploic arteries. Ann Thorac Surg. 1989;47:712–5. doi: 10.1016/0003-4975(89)90123-9. [DOI] [PubMed] [Google Scholar]
- 27.Kusukawa J, Hirota Y, Kawamura K, et al. Efficacy of coronary artery bypass surgery with gastroepiploic artery: assessment with thallium 201 myocardial scintigraphy. Circulation. 1989;80(3 Pt 1):I135–40. [PubMed] [Google Scholar]
- 28.Beretta L, Lemma M, Vanelli P, et al. Gastroepiploic artery free graft for coronary bypass. Eur J Cardiothorac Surg. 1990;4:323–7. doi: 10.1016/1010-7940(90)90210-Q. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi Y, Gomi A, Okamura Y, Mori H, Nagashima M. Coronary revascularization in a child with Kawasaki disease: use of right gastroepiploic artery. Ann Thorac Surg. 1990;50:294–6. doi: 10.1016/0003-4975(90)90754-T. [DOI] [PubMed] [Google Scholar]
- 30.Isomura T, Hisatomi K, Asoh S, et al. Revascularization with the right gastroepiploic artery in Kawasaki’s disease. J Thorac Cardiovasc Surg. 1990;100:796–8. [PubMed] [Google Scholar]
- 31.Suma H, Wanibuchi Y, Terada Y, Fukuda S, Takayama T, Furuta S. The right gastroepiploic artery graft: clinical and angiographic midterm results in 200 patients. J Thorac Cardiovasc Surg. 1993;105:615–22. [PubMed] [Google Scholar]
- 32.Nishida H, Tomizawa Y, Endo M, Koyanagi H, Kasanuki H. Coronary artery bypass with only in situ bilateral internal thoracic arteries and right gastroepiploic artery. Circulation. 2001;104(12 Suppl 1):I76–80. doi: 10.1161/hc37t1.094812. [DOI] [PubMed] [Google Scholar]
- 33.Hirose H, Amano A, Takanashi S, Takahashi A. Coronary artery bypass grafting using the gastroepiploic artery in 1,000 patients. Ann Thorac Surg. 2002;73:1371–9. doi: 10.1016/S0003-4975(02)03416-1. [DOI] [PubMed] [Google Scholar]
- 34.Formica F, Ferro O, Greco P, Martino A, Gastaldi D, Paolini G. Long-term follow-up of total arterial myocardial revascularization using exclusively pedicle bilateral internal thoracic artery and right gastroepiploic artery. Eur J Cardiothorac Surg. 2004;26:1141–8. doi: 10.1016/j.ejcts.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Tavilla G, Kappetein AP, Braun J, Gopie J, Tjien AT, Dion RA. Long-term follow-up of coronary artery bypass grafting in three-vessel disease using exclusively pedicled bilateral internal thoracic and right gastroepiploic arteries. Ann Thorac Surg. 2004;77:794–9. doi: 10.1016/S0003-4975(03)01659-X. [DOI] [PubMed] [Google Scholar]
- 36.Suma H, Tanabe H, Takahashi A, et al. Twenty years experience with the gastroepiploic artery graft for CABG. Circulation. 2007;116(11 Suppl):I188–91. doi: 10.1161/CIRCULATIONAHA.106.678813. [DOI] [PubMed] [Google Scholar]
- 37.Glineur D, D’hoore W, Price J, et al. Survival benefit of multiple arterial grafting in a 25-year single-institutional experience: the importance of the third arterial graft. Eur J Cardiothorac Surg. 2012;42:284–90. doi: 10.1093/ejcts/ezr302. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T, Asai T, Matsubayashi K, et al. In off-pump surgery, skeletonized gastroepiploic artery is superior to saphenous vein in patients with bilateral internal thoracic arterial grafts. Ann Thorac Surg. 2011;91:1159–64. doi: 10.1016/j.athoracsur.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Hwang HY, Cho KR, Kim KB. Equivalency of right internal thoracic artery and right gastroepiploic artery composite grafts: five-year outcomes. Ann Thorac Surg. 2013;96:2061–8. doi: 10.1016/j.athoracsur.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–210. doi: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T, Asai T, Nota H, et al. Early and long-term patency of in situ skeletonized gastroepiploic artery after off-pump coronary artery bypass graft surgery. Ann Thorac Surg. 2013;96:90–5. doi: 10.1016/j.athoracsur.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 42.Benedetto U, Raja SG, Albanese A, Amrani M, Biondi-Zoccai G, Frati G. Searching for the second best graft for coronary artery bypass surgery: a network meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg. 2015;47:59–65. doi: 10.1093/ejcts/ezu111. [DOI] [PubMed] [Google Scholar]