Abstract
Background
Paraplegia is a devastating complication following operations on the thoracoabdominal aorta. We investigated whether histidine-tryptophan-ketoglutarate (HTK) solution could reduce the extent of ischemia/reperfusion (IR) spinal cord injuries in a rat model using a direct delivery method.
Methods
Twenty-four Sprague-Dawley male rats were randomly divided into four groups. The sham group (n=6) underwent a sham operation, the IR group (n=6) underwent only an aortic occlusion, the saline infusion group (saline group, n=6) underwent an aortic occlusion and direct infusion of cold saline into the occluded aortic segment, and the HTK infusion group (HTK group, n=6) underwent an aortic occlusion and direct infusion of cold HTK solution into the occluded aortic segment. An IR spinal cord injury was induced by transabdominal clamping of the aorta distally to the left renal artery and proximally to the aortic bifurcation for 60 minutes. A neurological evaluation of locomotor function was performed using the modified Tarlov score after 48 hours of reperfusion. The spinal cord was harvested for histopathological and immunohistochemical examinations.
Results
The spinal cord IR model using direct drug delivery in rats was highly reproducible. The Tarlov score was 4.0 in the sham group, 1.17±0.75 in the IR group, 1.33±1.03 in the saline group, and 2.67±0.81 in the HTK group (p=0.04). The histopathological analysis of the HTK group showed reduced neuronal cell death.
Conclusion
Direct infusion of cold HTK solution into the occluded aortic segment may reduce the extent of spinal cord injuries in an IR model in rats.
Keywords: Spinal cord, Reperfusion injury
Introduction
Paraplegia has been reported to account for 5% to 20% of devastating complications following operations on the thoracoabdominal aorta [1–4]. Several methods exist to prevent this postoperative complication, such as (1) distal perfusion with shunts or a partial bypass, (2) hypothermia, (3) drainage of the cerebrospinal fluid, (4) reattachment of the intercostal vessels, (5) evoked-potential monitoring, and (6) the development of pharmacological agents to increase tolerance against ischemic insults. However, it is necessary to develop a better prevention method, and many studies have been conducted with the goal of developing a mechanism to replicate spinal cord ischemia/reperfusion (IR) injuries in animal models [1,5–9].
Drugs reducing the impact of spinal cord injuries can be categorized into blood-flow maintenance agents and metabolic regulation agents [10]. Examples of the former type of drug include papaverine, nitroglycerine, and nitroprusside. The latter type of drug is subdivided into neuronal metabolic rate reducers (barbiturates and opiates), cell membrane stabilizers (calcium channel blockers and sodium-channel blockers), removers of toxic metabolites (superoxide dismutase and allopurinol), alleviators of metabolite-induced inflammation (steroids, prostaglandin, and adenosine), and excitotoxicity reducers (riluzole, memantine, and magnesium) [10–12].
Bretschneider [13] first developed histidine-tryptophan-ketoglutarate (HTK) solution (Bretschneider’s solution, Custodiol) in the 1970s, and it is still widely used in organ preservation for transplantation (e.g., the kidney, liver, and pancreas) and myocardial protection during open-heart surgery [14]. HTK solution is an effective organ preservation solution due to the powerful buffer action of histidine, the cell membrane stabilization and antioxidant effects of tryptophan, and the role of ketoglutarate as a substrate [13,15].
Despite its use as a preservation solution for various organs, the effect of HTK on the peripheral nervous system has only been studied by Lassner et al. [16] in 1995. Moreover, its protective effect on the central nervous system and its potential role in IR injuries have not been studied. Recent studies have reported neuroprotective effects of histidine analogs (a principal component of HTK), which were enhanced by the excitotoxicity-reducing effect of HTK solution [17,18]. The principal aim of the present study was to confirm the role of HTK solution as a protective solution for spinoplegia. Therefore, an animal model involving direct drug infusion into the occluded aortic segment was developed to determine the effect of HTK in spinal cord nervous tissue.
Methods
1) Experimental animals
Male Sprague-Dawley (SD) rats weighing approximately 250 to 300 g were used in this study. Approval from the Animal Care and Use Committee of Chungnam National University was obtained. All experimental animals were not fed for twelve hours prior to the experiment, and they were confirmed to be neurologically sound.
2) Experimental groups
Twenty-four male SD rats were randomly divided into 4 groups. The sham group (n=6) underwent a sham operation. The IR group (n=6) underwent 60 minutes of aortic occlusion and 48 hours of reperfusion. The saline infusion group (n=6) was infused with 10 mL/kg of 4°C saline into the occluded aortic segment, as well as undergoing 60 minutes of aortic occlusion and 48 hours of reperfusion. The HTK infusion group (n=6) was infused with 10 mL/kg of HTK solution into the occluded aortic segment, as well as undergoing 60 minutes of aortic occlusion and 48 hours of reperfusion (Table 1).
Table 1.
Experimental group (n=6)
| Group | Body weight (g) | Infusion | Ischemia (min) | Reperfusion (hr) |
|---|---|---|---|---|
| Sham | 262.5±5.2 | No | 0 | No |
| Ischemia/reperfusion | 263.3±5.2 | No | 60 | 48 |
| Saline | 266.7±7.5 | Cold saline 10 mL/kg | 60 | 48 |
| HTK | 264.4±5.8 | Cold HTK solution 10 mL/kg | 60 | 48 |
HTK, histidine-tryptophan-ketoglutarate.
3) Surgical method
(1) Development of the aortic occlusion and spinal cord ischemia/reperfusion model
In order to assess the reproducibility of the spinal cord IR model using direct drug delivery in rats, dye was injected and hemodynamic changes were measured in 3 model rats.
The model rats were anesthetized through an intraperitoneal injection of 50 mg/kg of pentobarbital. Subsequently, an intraperitoneal injection of 100 IU/kg of heparin was performed, and then the model rats were placed in the supine position on the operating table. The body temperature of the model rats was maintained at 37°C by periodically checking the intrarectal temperature using a temperature probe and applying heating pads as needed (CMA 150 temperature controller; CMA, North Chelmsford, MA, USA). Real-time hemodynamic index measurements were recorded at 3 different time points: before, during, and after aortic occlusion. An electrocardiogram was performed, and the heart rate, blood pressure, pulse pressure, and intrarectal temperature were measured using a Model 7400 physiological recorder (Astro-Med, West Warwick, RI, USA). The aforementioned parameters were analyzed using Chart and Scope (ver. 3.0; ADInstruments, New South Wales, Australia).
The IR spinal cord injury operation was performed under ×16 magnification. The region of operation was aseptically disinfected with betadine. The left common carotid artery was cannulated with a polyethylene (PE)-50 tube (external diameter of 0.97 mm) to continuously monitor real-time hemodynamic changes. The PE-50 tube was connected with a PE-10 tube (external diameter of 0.60 mm) to the dissected left inguinal area. The left femoral artery was cannulated with a PE-10 tube, through which the drug was injected and the blood pressure of the model rat was measured. The same procedure was performed in the dissected right inguinal area, and the right femoral artery was cannulated to measure the distal blood pressure. A median thoracoabdominal incision was made, and the abdominal organs were moved towards the right side of the model rats. The aorta was carefully dissected distally to the left renal artery and tied with 5-0 silk sutures. The proximal portion of the aortic bifurcation was dissected together with the inferior vena cava. The intubated cannula inside the left femoral artery was palpably advanced to the location between the left renal artery and the aortic bifurcation. The loops of both ends were pulled to occlude the aorta for 60 minutes to induce an ischemic spinal cord injury (Fig. 1).
Fig. 1.
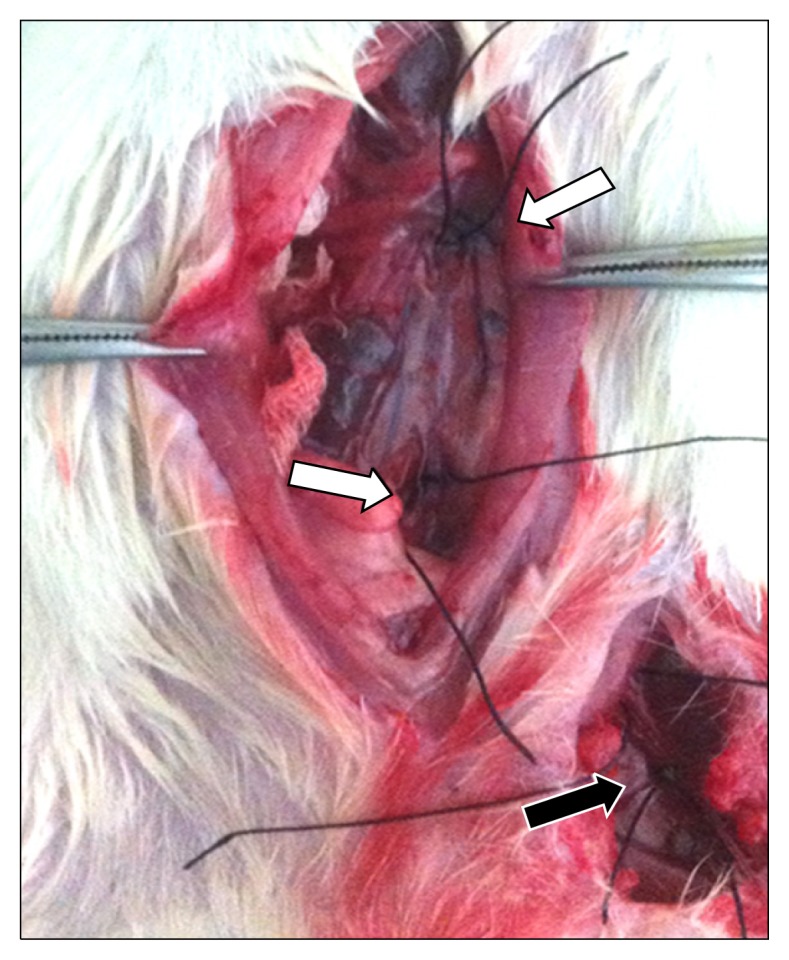
Silk string loops were applied at the aorta distally to the left renal artery and proximally to the aortic bifurcation (white arrow). The left common femoral artery was cannulated with a polyethylene-10 tube (black arrow).
Reperfusion of the aorta was induced after 60 minutes of aortic occlusion, and the femoral artery cannulas were removed. The femoral arteries were ligated for hemostasis. The operative wound was closed with continuous 4-0 nylon sutures, and 50 mg/kg of first-generation cephalosporin and antibiotic cream were applied. The model rats were then moved back to a cage, and were verified to have regained consciousness. The rats were supplied with food and water starting 6 hours after the operation. Urine was drained twice per day by compressing the hypogastrium of the model rats. Tryphan blue dye (2 mL) was injected in the region around the occluded aorta to confirm that the drug was delivered solely to the site of occlusion (Fig. 2).
Fig. 2.
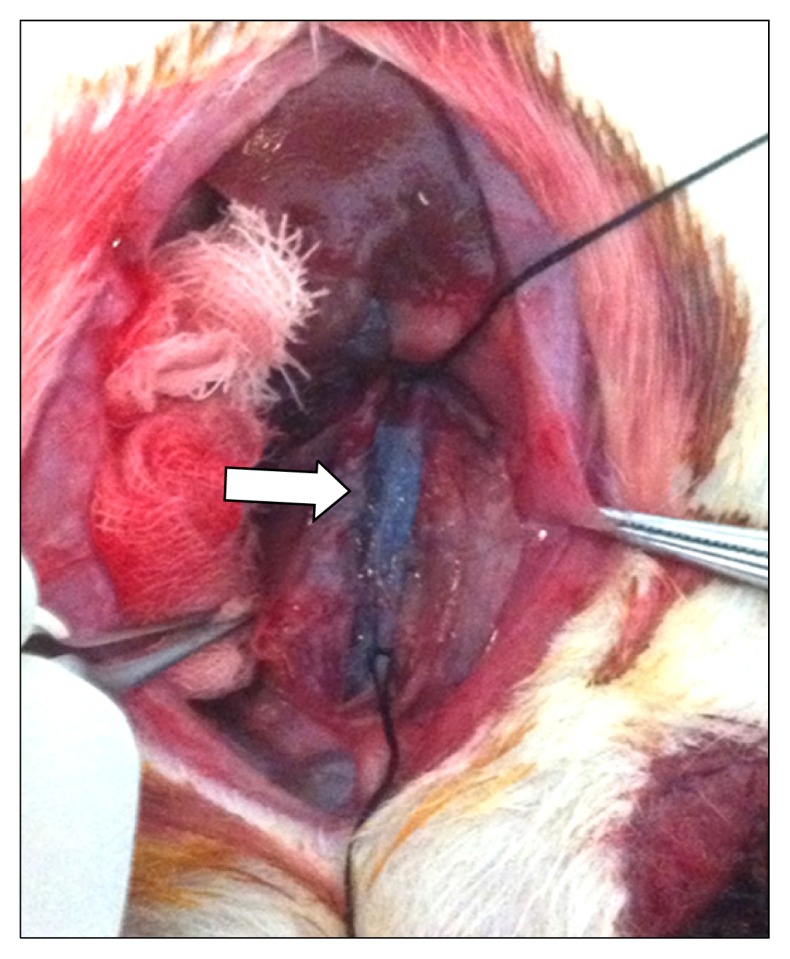
The cross-clamp was applied at the aorta distally to the left renal artery and proximally to the aortic bifurcation. When dye was infused, it was visible only in the occluded segment of the aorta (arrow).
(2) Drug delivery in the ischemia/reperfusion spinal cord injury model
The IR spinal cord injury models were prepared as described above, but the left common carotid artery and right femoral artery were not intubated. Instead, the left femoral artery was cannulated with a PE-10 tube and a median thoracoabdominal incision was made to occlude the aorta. The IR group was injected with no drug, the saline group was injected with 10 mL/kg of 4°C cold saline for 5 minutes, and the HTK group was injected with 10 mL/kg of 4°C HTK solution for 5 minutes. The surgical method described above was then followed.
4) Locomotor function test
All experimental animals were tested for locomotor function using the modified Tarlov scoring system [19,20]. In a small portion of the experimental animals, the locomotor function scores of the ipsilateral limb were lower than were observed for the contralateral limb. In such animals, the score of the contralateral limb was used as a reference value. Grade 0 was given to animals with spastic paralysis and no movement of the hind limb. Grade 1 was given to animals with spastic paralysis and slight movement of the hind limb. Grade 2 was given to animals that exhibited good movement of the hind limb but were unable to stand. Grade 3 was given to animals that could stand but were unable to walk normally, and grade 4 was given to animals that made a complete recovery.
5) Postoperative tissue collection
All experimental animals were sacrificed after the locomotor function test, which was performed 48 hours after surgery. Anesthesia was induced through an intramuscular injection of 50 mg/kg of ketamine hydrochloride and 50 mg/kg of xylazine hydrochloride. The animals were put to death by cervical vertebra dislocation. The lower thoracic vertebrae and lumbar vertebrae were extracted. The extracted vertebrae were stored in 10% neutral formalin for histopathological and immunohistochemical tests.
6) Histopathological tests
(1) Hematoxylin-eosin staining
The extracted tissues were fixated in 10% neutral formalin for 3 days. Four slices 2 mm in thickness in the transverse plane of the second and third vertebrae of the lumbar spinal cord were obtained and microscope slides were prepared. The slides were fixed in 10% neutral formalin and paraffin, and sliced to be 5 μm in thickness. The slides were then stained with hematoxylin and eosin dye to study the nuclei and cytoplasm of the cells.
(2) Assessment of pathological grade
A pathologist conducted a blind pathological assessment of each experimental group. The pathological grade was given based on the proportion of red neurons with ischemic injury and the area of infarction [19,20]. The red neurons exhibited cell shrinkage, pyknosis, degradation of the nucleus, Nissl body disappearance, and eosinophilic staining.
The images of the slides were acquired using a scanner at ×400 magnification (Aperio Imagescope ver. 11.0; Leica, Rockleigh, NJ, USA). The number of red neurons was measured using an image analysis program (ImageJ; DeNovo software, Los Angeles, CA, USA). The number of red neurons was measured at the anterior horn of the spinal cord at ×200 magnification, and a grade was assigned according to the ratio of red neurons to total neurons. Grade 0 was assigned when red neurons were not detected. Grade 1 was given for a ratio of red neurons to total neurons of <15%. Grade 2 corresponded to a ratio of red neurons to total neurons of >15%. Grade 3 corresponded to focal and mild infarction affecting <25% of the gray matter, and grade 4 was given to samples with diffuse and severe infarction affecting > 25% of the gray matter.
7) Statistics
PASW SPSS ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used to analyze the data and to perform statistical analyses. Continuous variables are expressed as means±standard deviations, and categorical variables are expressed as median values. Analysis of variance was used to compare continuous variables across the experimental groups. The Kruskal-Wallis test was conducted to compare pathological findings and Tarlov scores. Significant results were evaluated using the post hoc test with the Mann-Whitney rank sum law. For hemodynamic variables, the bootstrap-t method or the paired t-test using a bootstrap was used to compensate for the low number of samples. All p-values <0.05 were considered to indicate statistical significance.
Results
1) Overall surgical data
Unexpected cases of hemorrhage that occurred during the intubation of the catheter into femoral artery or aortic dissection were excluded from the total of experimental cases. The average operation time was approximately 90 minutes for non-problematic operations. Every experimental animal regained consciousness from anesthesia without any problems, and no instances of mortality were noted. The average length of PE-10 tubing that was inserted was approximately 3.5–4.0 cm.
2) Changes in hemodynamic indices
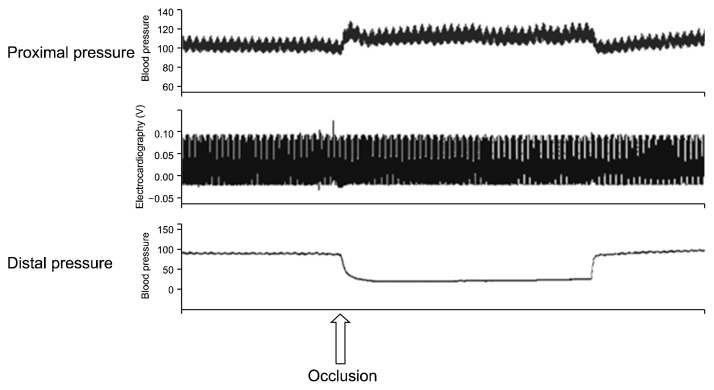
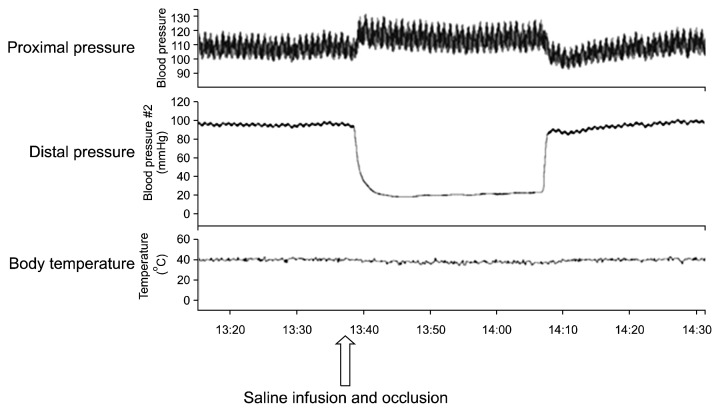
When occlusions were made in the aorta distally to the left renal artery and in the aorta at the aortic bifurcation, the proximal pressure increased from 102.7±3.1 mmHg to 113.3±4.2 mmHg (p=0.04) and the distal pressure decreased from 14.1±4.5 mmHg to 22.2±0.8 mmHg (p=0.05). The pulse pressure increased from 12.2±3.5 mmHg to 15.7±8.9 mmHg (p=0.31) and the heart rate decreased from 343.3±29.6 beats/min to 336.7±22.5 beats/min (p=0.25), but no significant change in body temperature was observed (Table 2, Fig. 3). When cold saline was injected after occluding the aorta, a transient decrease of 1.5°C in the body temperature was noted, but the body temperature returned to normal (Fig. 4).
Table 2.
Hemodynamic profile before and after aortic occlusion (n=3)
| Parameter | Proximal | p-value | Distal | p-value | ||
|---|---|---|---|---|---|---|
| Resting | Occlusion | Resting | Occlusion | |||
| Systolic blood pressure | 108.3±4.5 | 120.9±5.6 | 0.004 | 121.7±5.9 | 22.7±0.9 | <0.01 |
| Diastolic blood pressure | 96.2±2.1 | 105.1±3.2 | 0.005 | 105.3±3.2 | 21.4±0.9 | <0.01 |
| Mean blood pressure | 102.7±3.1 | 113.3±4.2 | 0.006 | 114.1±4.5 | 22.2±0.8 | <0.01 |
| Pulse pressure | 12.2±3.5 | 15.7±8.9 | 0.031 | 15.9±4.6 | 1.0±0.4 | <0.01 |
| Heart rate | 343.3±29.6 | 336.7±22.5 | 0.252 | 343.3±29.6 | 336.7±22.5 | 0.252 |
Values are presented as mean±standard deviation.
Fig. 3.
Hemodynamic changes before, during, and after occlusion of the aorta in the ischemic model.
Fig. 4.
Hemodynamic changes before, during, and after occlusion of the aorta with infusion of cold saline in the saline infusion model.
3) Results of motor neuron tests
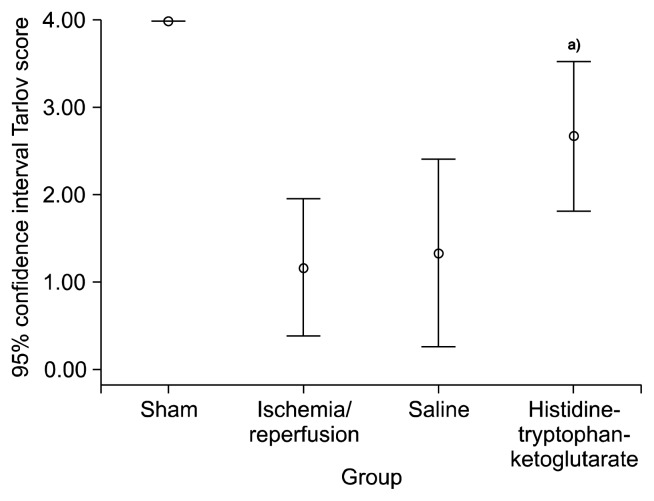
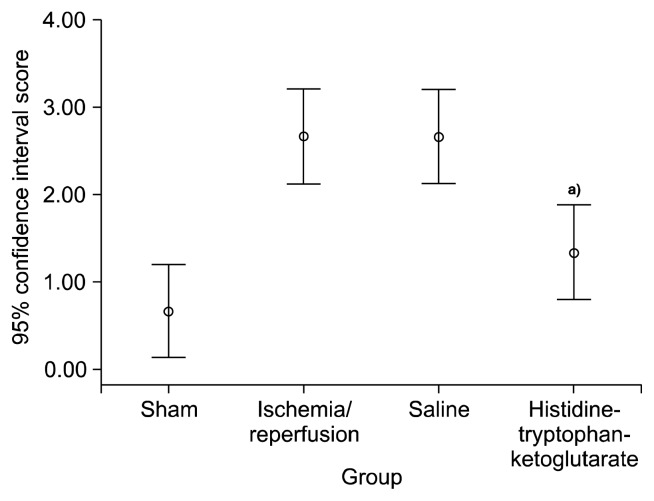
The sham group exhibited normal lower limb locomotor function in all cases. The IR group showed a significant difference in comparison to the sham group (1.17±0.75, p=0.01). The result of the motor neuron test for the saline group was 1.33±1.03, whereas it was 2.67±0.81 for the HTK group, which was a significant difference (p=0.04) (Fig. 5).
Fig. 5.
The Tarlov score of each group. a)Versus saline group (p=0.04).
4) Histopathological assessment
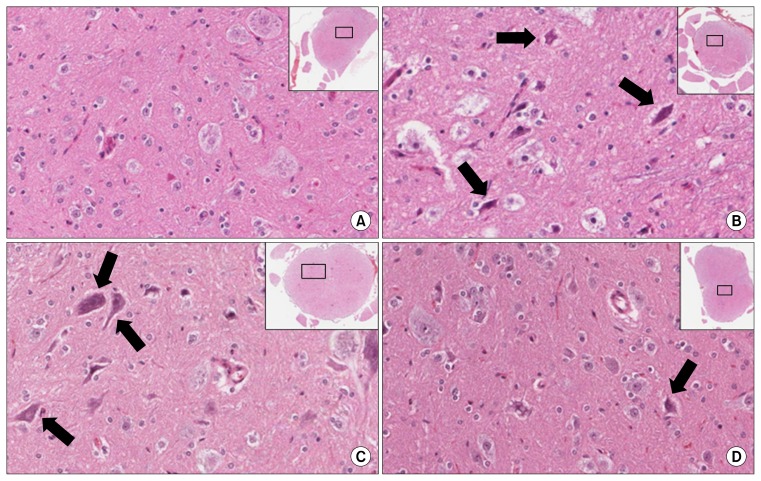
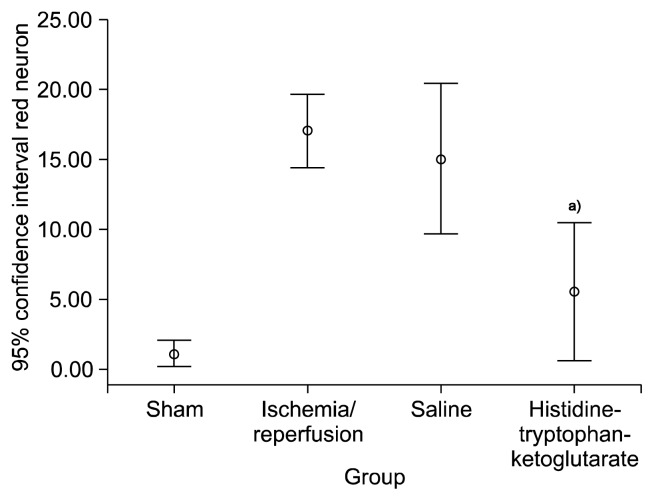
Red neurons were rarely found in the sham group, numerous red neurons were observed in the IR group and the saline group, while few red neurons were observed in the HTK group (Fig. 6). Using image analysis software, the number of red neurons was determined to be 1.0±0.81 for the sham group, 17.0±2.53 for the IR group, 15.0±5.18 for the saline group, and 5.5±4.73 for HTK group (p=0.02). The histopathological scores were 0.67±0.52 for the sham group, 2.67±0.52 for the IR group, 2.67±0.52 for the saline group, and 1.17±0.41 for the HTK group (p=0.04) (Figs. 7, 8).
Fig. 6.
(A) No red neurons were found in the sham group. Many red neurons (arrows) were present in (B) the ischemia/reperfusion group and (C) the saline group, and (D) few red neurons were present in the histidine-tryptophan-ketoglutarate group (H&E, ×200).
Fig. 7.
Red neuron count of each group. a)Versus saline group (p=0.001).
Fig. 8.
Histopathologic score of each group. IR, ischemia/reperfusion; HTK, histidine-tryptophan-ketoglutarate. a)Versus saline group (p=0.02).
Discussion
When blood flow to an organ is blocked and the oxygen supply cannot meet the demand for oxygen, the normal phosphorylation process does not occur in mitochondria, resulting in ATP depletion [10]. The shortage of ATP causes abnormalities in the membrane potential and imbalances in intracellular electrolytes. Influxed calcium ions induce the secretion of various enzymes, denature nucleic acids and proteins, and cause reactive oxygen production and cytotoxicity. An increase in intracellular sodium ions induces cellular swelling [10,18], and the increase of lactic acid in anaerobic conditions causes intracellular acidification [10,12]. An inflammatory reaction is caused by the accumulation of toxic substances, increasing the amount of nitric oxide synthase, which in turn increases free radicals [9,10,20].
Excitotoxicity is a damage process unique to neurons in which N-methyl-D-aspartate (NMDA) or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors are overexpressed due to glutamate and its analogs [10]. Excitotoxicity interrupts an increase of intracellular sodium ions, exacerbates edema, and inhibits the maintenance of a normal membrane potential. As a consequence, the amount of free reactive oxygen species increases, which in turn increases the recruitment of inflammatory cells to the damaged area [10]. Apoptosis occurs in damaged cells because they continuously secrete inflammatory mediators and activate neutrophils, macrophages, and T-cells [20].
HTK solution is an organ preservation solution principally composed of histidine, tryptophan, and ketoglutarate, which are substances that can freely pass through the blood-brain barrier [13]. The strong buffering action of histidine prevents a pH drop inside the cell and inhibits anaerobic processes, thereby blocking the accumulation of lactic acid [10]. Tryptophan has a protective effect on the cellular membrane, ketoglutarate acts as a substrate in anaerobic conditions [13], and mannitol blocks cellular swelling [21]. HTK solution has a low viscosity (310 osm) and contains a low amount of K+ (9 mmol) and Na+ (15 mmol). In order to achieve equilibrium of the intracellular electrolytes at the initiation of perfusion, HTK solution is recommended to be administered at a dosage of 1 mL/min/g (per weight of organ) at 4°C to 8°C for more than 5 minutes [13]. However, the allowable dose of HTK solution for SD rats is limited, so the maximum amount (10 mL/kg for 5 minutes) was chosen.
In addition to the widely known organ preservation effect of HTK solution, recent studies have reported that histidine analogs are capable of reducing ischemic injury by decreasing excitotoxicity. Shen et al. [17] verified a significant decrease in NMDA levels mediated by L-carnosine (beta-alanyl-L-histidine) in a spinal cord IR rat model, and Tang et al. [18] reported that macrophages could be modified to display neuroprotective effects. Stvolinsky et al. [22] confirmed that the antioxidant effect and cellular membrane stabilization effect of histidine allow it to have a neuroprotective effect. Research on each component of HTK solution has yet be conducted, but the neuroprotective effect of HTK solution via the buffering action of histidine and various other mechanisms has been elucidated [22].
Although many drug injection pathways exist, oral administration is not an efficient method for simulating actual clinical methods used in emergency settings [10]. The simplest method for drug delivery is via intravenous infusion. An advantage of intravenous infusion is that additional handling of the blood vessels is not needed, but it also has disadvantages. For instance, selective injection to a targeted organ is impossible, and adverse reactions and toxicity due to the systemic circulation of the drug are possible. Another method of drug delivery is retrograde intravenous infusion via the inferior vena cava or the hemiazygos vein. This method requires additional manipulation of the blood vessels, and is also disadvantageous because drug metabolism may be altered when the drug passes through the hepatic circulation and because toxicity due to the systemic circulation of the drug remains possible. A third method is direct injection into the spinal artery, which was performed in the present study. This is considered the ideal method because it causes the least amount of systemic toxicity. Moreover, intrathecal injections are advantageous because they do not require drugs to pass through the blood-brain barrier and the drugs administered in this manner act directly on the spinal cord. The disadvantages of this procedure include the need for the additional manipulation of blood vessels, the possibility of serious adverse effects involving hemorrhage, the risk of nerve injury, and the increase of cerebrospinal pressure induced by the drug injection [10].
Spinal cord IR models have already been developed in various types of experimental animals. Various methods are used to induce spinal cord IR injuries, such as occluding the thoracic aorta using a Fogarty catheter via the femoral artery, occluding the aorta by direct thoracic incision, and occluding the aorta through laparotomy [19,20,23–25]. Another method involves occluding the aorta distally from the left renal artery. This method was used in rabbits during the early stages of development of the spinal cord IR model, but it can now be used in SD rats as well [26].
Different amounts of time for inducing an IR injury have been reported in previous studies [26–28]. In the present study, we determined that inducing an ischemic injury for 30 to 45 minutes caused lower limb paralysis immediately after the experiment, but an almost complete recovery was observed after 48 hours. However, when an ischemic injury was introduced for 60 minutes, lower limb paralysis was maintained in the model rats, and therefore a method involving 60 minutes of ischemic injury was chosen for the spinal cord IR model.
During the early stage of formulating hypotheses and experimental design, systemic adverse effects of HTK solution were a major concern; therefore, a model was developed in which the drug was only delivered to the aortic segment. Using large animals presents problems involving experimental space and financial concerns. With regard to rabbits, the lack of experimental reagents and methods for conducting various molecular biological studies is an issue. In light of factors such as size, safety, reproducibility, and suitability for diverse molecular biological studies, SD rats were chosen as the experimental animal. The model of creating an occlusion distally to the left renal artery was modified for our purposes. The common femoral artery was cannulated, the aorta proximal to aortic bifurcation was occluded simultaneously, and the drug was selectively delivered to the aortic segment only. Although drug delivery by puncturing the aorta is possible in rabbits, using this method in SD rats leads to problems with thrombosis and hemostasis before and after the operation because the internal membrane of the aorta is thinner than 1 mm. The degree of lower limb movement deterioration due to ligation of the common femoral artery in SD rats has been found to be almost negligible, indicating that a novel animal model could therefore be developed in SD rats [29,30]. In the present study, we measured the locomotor ability of the contralateral limb because our goal was to conduct histological and molecular biological research solely on the spinal cord and to evaluate the contralateral limb.
As preclinical research on the protective effect of HTK solution on the spinal cord in which the drug was delivered to the occluded aorta segment in an SD rat spinal cord IR model, the present research is subject to multiple limitations. First, functional or histopathologically based research to identify the component of HTK that is responsible for its neuroprotective effect has not been conducted. Additionally, the proper dose of HTK solution has not yet been determined. Second, the possibility of reciprocal interactions of HTK with the neuroprotective effects of anesthetic agents such as pentobarbital and ketamine has not been studied. Third, the results of this study cannot be generalized due to the small number of experimental animals used in our research. Given these limitations, in order to confirm the neuroprotective effect of HTK solution, it is necessary to conduct large-scale animal experiments incorporating molecular biological research methodologies.
In conclusion, the results of the present study, in which we examined the protective effect of HTK solution on the spinal cord by delivering the drug specifically into the occluded aorta segment in a spinal cord IR SD rat model, allow the following conclusions to be made. First, this new method of producing a spinal cord IR model in SD rats was verified to be reliable. Second, the protective effect of HTK solution on the spinal cord was confirmed, and it is therefore necessary to ascertain the proper injection method and dosage, as well as to conduct further molecular biological research on this topic.
Acknowledgments
This work was supported by research fund of Chungnam National University and Chungnam National University Hospital.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Estrera AL, Sheinbaum R, Miller CC, 3rd, Harrison R, Safi HJ. Neuromonitor-guided repair of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S131–5. doi: 10.1016/j.jtcvs.2010.07.058. [DOI] [PubMed] [Google Scholar]
- 2.Estrera AL, Miller CC, 3rd, Huynh TT, Porat E, Safi HJ. Neurologic outcome after thoracic and thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2001;72:1225–30. doi: 10.1016/S0003-4975(01)02971-X. [DOI] [PubMed] [Google Scholar]
- 3.Wong DR, Parenti JL, Green SY, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg. 2011;212:569–79. doi: 10.1016/j.jamcollsurg.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 4.Isaka M, Kumagai H, Sugawara Y, et al. Cold spinoplegia and transvertebral cooling pad reduce spinal cord injury during thoracoabdominal aortic surgery. J Vasc Surg. 2006;43:1257–62. doi: 10.1016/j.jvs.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Mauney MC, Blackbourne LH, Langenburg SE, Buchanan SA, Kron IL, Tribble CG. Prevention of spinal cord injury after repair of the thoracic or thoracoabdominal aorta. Ann Thorac Surg. 1995;59:245–52. doi: 10.1016/0003-4975(94)00815-O. [DOI] [PubMed] [Google Scholar]
- 6.Coselli JS, LeMaire SA, Koksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002;35:631–9. doi: 10.1067/mva.2002.122024. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs MJ, Mess W, Mochtar B, Nijenhuis RJ, Statius van Eps RG, Schurink GW. The value of motor evoked potentials in reducing paraplegia during thoracoabdominal aneurysm repair. J Vasc Surg. 2006;43:239–46. doi: 10.1016/j.jvs.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Kakinohana M, Nakamura S, Fuchigami T, Sugahara K. Transcranial motor-evoked potentials monitoring can detect spinal cord ischemia more rapidly than spinal cord-evoked potentials monitoring during aortic occlusion in rats. Eur Spine J. 2007;16:787–93. doi: 10.1007/s00586-006-0165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumagai H, Isaka M, Sugawara Y, et al. Intra-aortic injection of propofol prevents spinal cord injury during aortic surgery. Eur J Cardiothorac Surg. 2006;29:714–9. doi: 10.1016/j.ejcts.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 10.Reece TB, Kern JA, Tribble CG, Cassada DC. The role of pharmacology in spinal cord protection during thoracic aortic reconstruction. Semin Thorac Cardiovasc Surg. 2003;15:365–77. doi: 10.1053/S1043-0679(03)00088-1. [DOI] [PubMed] [Google Scholar]
- 11.Lazaris AM, Maheras AN, Vasdekis SN, et al. Protective effect of remote ischemic preconditioning in renal ischemia/reperfusion injury, in a model of thoracoabdominal aorta approach. J Surg Res. 2009;154:267–73. doi: 10.1016/j.jss.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 12.Reece TB, Kron IL, Okonkwo DO, et al. Functional and cytoarchitectural spinal cord protection by ATL-146e after ischemia/reperfusion is mediated by adenosine receptor agonism. J Vasc Surg. 2006;44:392–7. doi: 10.1016/j.jvs.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Bretschneider HJ. Myocardial protection. Thorac Cardiovasc Surg. 1980;28:295–302. doi: 10.1055/s-2007-1022099. [DOI] [PubMed] [Google Scholar]
- 14.Bretschneider HJ, Hubner G, Knoll D, Lohr B, Nordbeck H, Spieckermann PG. Myocardial resistance and tolerance to ischemia: physiological and biochemical basis. J Cardiovasc Surg (Torino) 1975;16:241–60. [PubMed] [Google Scholar]
- 15.Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83:1289–98. doi: 10.1097/01.tp.0000265586.66475.cc. [DOI] [PubMed] [Google Scholar]
- 16.Lassner F, Becker M, Fansa H, Walter GF, Berger A. Preservation of peripheral nerve grafts: a comparison of normal saline, HTK organ preservation solution, and DMEM Schwann cell culture medium. J Reconstr Microsurg. 1995;11:447–53. doi: 10.1055/s-2007-1006559. [DOI] [PubMed] [Google Scholar]
- 17.Shen Y, He P, Fan YY, et al. Carnosine protects against permanent cerebral ischemia in histidine decarboxylase knockout mice by reducing glutamate excitotoxicity. Free Radic Biol Med. 2010;48:727–35. doi: 10.1016/j.freeradbiomed.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Tang SC, Arumugam TV, Cutler RG, et al. Neuroprotective actions of a histidine analogue in models of ischemic stroke. J Neurochem. 2007;101:729–36. doi: 10.1111/j.1471-4159.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- 19.Oh SS, Choe G, Kim WG. Neuroprotective effect of phenytoin and hypothermia on a spinal cord ischemic injury model in rabbits. Korean J Thorac Cardiovasc Surg. 2008;41:405–16. [Google Scholar]
- 20.Shin YC, Choe GY, Kim WG. Neuroprotective effect of cyclosporin A on spinal cord ischemic injury in rabbits. Korean J Thorac Cardiovasc Surg. 2006;39:739–48. [Google Scholar]
- 21.Stone TW, Forrest CM, Mackay GM, Stoy N, Darlington LG. Tryptophan, adenosine, neurodegeneration and neuroprotection. Metab Brain Dis. 2007;22:337–52. doi: 10.1007/s11011-007-9064-3. [DOI] [PubMed] [Google Scholar]
- 22.Stvolinsky S, Kukley M, Dobrota D, Mezesova V, Boldyrev A. Carnosine protects rats under global ischemia. Brain Res Bull. 2000;53:445–8. doi: 10.1016/S0361-9230(00)00366-X. [DOI] [PubMed] [Google Scholar]
- 23.Lang-Lazdunski L, Matsushita K, Hirt L, et al. Spinal cord ischemia: development of a model in the mouse. Stroke. 2000;31:208–13. doi: 10.1161/01.STR.31.1.208. [DOI] [PubMed] [Google Scholar]
- 24.Marsala M, Galik J, Ishikawa T, Yaksh TL. Technique of selective spinal cord cooling in rat: methodology and application. J Neurosci Methods. 1997;74:97–106. doi: 10.1016/S0165-0270(97)02270-X. [DOI] [PubMed] [Google Scholar]
- 25.Awad H, Ankeny DP, Guan Z, Wei P, McTigue DM, Popovich PG. A mouse model of ischemic spinal cord injury with delayed paralysis caused by aortic cross-clamping. Anesthesiology. 2010;113:880–91. doi: 10.1097/ALN.0b013e3181ec61ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Usul H, Cakir E, Cobanoglu U, et al. The effects of tyrphostine Ag 556 on experimental spinal cord ischemia reperfusion injury. Surg Neurol. 2004;61:45–54. doi: 10.1016/S0090-3019(03)00539-1. [DOI] [PubMed] [Google Scholar]
- 27.Tian F, Xu LH, Zhao W, Tian LJ, Ji XL. The optimal therapeutic timing and mechanism of puerarin treatment of spinal cord ischemia-reperfusion injury in rats. J Ethnopharmacol. 2011;134:892–6. doi: 10.1016/j.jep.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 28.Isbir CS, Ak K, Kurtkaya O, et al. Ischemic preconditioning and nicotinamide in spinal cord protection in an experimental model of transient aortic occlusion. Eur J Cardiothorac Surg. 2003;23:1028–33. doi: 10.1016/S1010-7940(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 29.Hellingman AA, Bastiaansen AJ, de Vries MR, et al. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur J Vasc Endovasc Surg. 2010;40:796–803. doi: 10.1016/j.ejvs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Sirmali M, Uz E, Sirmali R, et al. Protective effects of erdosteine and vitamins C and E combination on ischemia-reperfusion-induced lung oxidative stress and plasma copper and zinc levels in a rat hind limb model. Biol Trace Elem Res. 2007;118:43–52. doi: 10.1007/s12011-007-0010-3. [DOI] [PubMed] [Google Scholar]