Abstract
Background
We evaluated early and long-term results after heart transplantation (HTPL).
Methods
One hundred five consecutive patients (male:female=80:25) who underwent HTPL between 1994 and 2013 were enrolled. Based on the changes in immunosuppressive regimen, the study patients were divided into two groups. Early and long-term clinical outcomes were evaluated and compared between the patients who underwent HTPL before (group E, n=41) and after July 2009 (group L, n=64). The group L patients were older (p<0.001), had higher incidence of hypertension (p=0.001) and chronic kidney disease (p<0.001), and more frequently needed preoperative mechanical ventilation (p=0.027) and mechanical circulatory support (p=0.014) than the group E patients.
Results
Overall operative mortality was 3.8%, and postoperative morbidities included acute kidney injury (n=31), respiratory complications (n=16), reoperation for bleeding (n=15) and wound complications (n=10). There were no significant differences in early results except acute kidney injury between group E and group L patients. Overall survival rates at 1, 5, and 10 years were 83.8%, 67.7%, and 54.9%, respectively, with no significant difference between the two patient groups. Rejection-free rates at 1 and 5 years were 63.0% and 59.7%, respectively; rates were significantly higher in group L than in group E (p<0.001).
Conclusion
Despite increased preoperative comorbidities, group L patients showed similar early and long-term outcomes and significantly higher rejection-free rates when compared with group E patients.
Keywords: Transplantation, Heart, Outcome assessment
Introduction
Since the first report in 1967, more than 100,000 patients have undergone heart transplantation (HTPL) worldwide, and it has become a gold standard treatment for patients with end-stage heart disease [1,2]. The current report from the International Society for Heart and Lung Transplantation (ISHLT) demonstrated that 1- and 5-year survival rates were 81% and 69%, respectively [3,4]. The aims of this study were to evaluate early and long-term results of HTPL and to analyze changes in patient characteristics and clinical outcomes of HTPL in the early and late periods.
Methods
The study protocol was reviewed by the institutional review board of the Seoul National University Hospital and approved as a minimal risk retrospective study (approval number: H-1512-068-727) that did not require individual consent based on the institutional guidelines for waiving consent.
1) Patient characteristics
One hundred five patients (male:female=80:25) underwent HTPL between March 1994 and December 2013. There were 4 patients who underwent combined heart-kidney transplantation. Mean recipient age at time of operation was 50.8±14.3 years, and mean donor age was 33.1±12.0 years. Dilated cardiomyopathy (n=57, 54.3%) and ischemic cardiomyopathy (n=27, 25.7%) were the two most common causes of end-stage heart disease. Twenty-two patients (21.0%) had a history of previous cardiac surgery. Based on the changes in immunosuppressive regimen in our institute, the study patients were divided into group E (patients who underwent HTPL before July 2009; n=41), and group L (patients who underwent HTPL after July 2009; n=64) (Table 1). Group L patients were significantly older, and had more comorbidities such as hypertension, dyslipidemia, coronary artery disease (CAD), and chronic kidney disease (CKD) than group E patients. Patients from group L more often needed mechanical ventilation or mechanical circulatory support preoperatively. Recipient aortic cross-clamp and cardiopulmonary bypass times were significantly longer in group L than in group E. Donor age was also older in group L than in group E (Table 2).
Table 1.
Preoperative characteristics of the study patients
| Variable | Total (n=105) | Group E (n=41) | Group L (n=64) | p-value |
|---|---|---|---|---|
| Age (yr) | 50.8±14.3 | 43.6±11.5 | 55.4±14.1 | <0.001 |
| Sex (female) | 25 (23.8) | 6 (14.6) | 19 (29.7) | 0.077 |
| Diagnosis | 0.214 | |||
| Dilated cardiomyopathy | 57 (54.3) | 27 (65.9) | 30 (46.9) | |
| Ischemic cardiomyopathy | 27 (25.7) | 7 (17.1) | 20 (31.3) | |
| Others | 21 (20.0) | 7 (17.1) | 14 (21.9) | |
| Comorbidities | ||||
| Diabetes mellitus | 25 (23.8) | 6 (14.6) | 19 (29.7) | 0.077 |
| Hypertension | 38 (36.2) | 7 (17.1) | 31 (48.4) | 0.001 |
| Dyslipidemia | 17 (16.2) | 2 (4.9) | 15 (23.4) | 0.012 |
| Coronary artery disease | 27 (25.7) | 6 (14.6) | 21 (32.8) | 0.038 |
| Chronic kidney disease | 24 (22.9) | 1 (2.4) | 23 (35.9) | <0.001 |
| History of cerebrovascular accident | 12 (11.4) | 4 (9.8) | 8 (12.5) | 0.666 |
| Preoperative mechanical ventilation | 15 (14.3) | 2 (4.9) | 13 (20.3) | 0.027 |
| Preoperative mechanical circulatory support | 20 (19.0) | 3 (7.3) | 17 (26.6) | 0.014 |
| Follow-up duration (mo) | 57.4±62.3 | 101.9±79.3 | 28.9±17.5 | <0.001 |
Values are presented as mean±standard deviation or number (%).
Group E, patients who underwent HTPL before July 2009; group L, patients who underwent HTPL after July 2009.
HTPL, heart transplantation.
Table 2.
Operative data of the study patients
| Variable | Total (n=105) | Group E (n=41) | Group L (n=64) | p-value |
|---|---|---|---|---|
| History of cardiac surgery | 22 (21.0) | 8 (19.5) | 14 (21.9) | 0.772 |
| Donor ischemic time (min) | 155±52 | 145±43 | 162±57 | 0.083 |
| Recipient aortic cross clamp time (min) | 93±27 | 77±27 | 102±23 | <0.001 |
| Recipient cardiopulmonary bypass time (min) | 247±74 | 194±49 | 281±68 | <0.001 |
| Kidney co-transplantation | 4 (3.8) | 0 | 4 (6.3) | 0.154 |
| Donor | ||||
| Age (yr) | 33.1±12.0 | 30.3±11.6 | 35.0±11.9 | 0.050 |
| Sex (female) | 25 (23.8) | 7 (17.1) | 18 (28.1) | 0.195 |
Values are presented as number (%) or mean±standard deviation.
Group E, patients who underwent HTPL before July 2009; group L, patients who underwent HTPL after July 2009.
HTPL, heart transplantation.
2) Surgical techniques and operative data
The bicaval and single left atrial anastomosis technique was used in most patients (n=102, 97.1%). The biatrial anastomosis technique was used in 3 patients who underwent HTPL in the early period. Before implantation of the donor heart, an additional dose of cold cardioplegic solution was infused through the aortic root or retrograde coronary sinus cannula in most of the patients. The mean donor ischemic, recipient aortic cross-clamp, and cardiopulmonary bypass times were 155±52, 93±27, and 247±74 minutes, respectively (Table 2).
3) Immunosuppressive therapy
Our immunosuppressive regimens for HTPL were: (1) a calcineurin inhibitor such as cyclosporine or tacrolimus, (2) an antiproliferative agent such as azathioprine (AZA, Imuran) or mycophenolate mofetil (MMF), and (3) corticosteroids such as prednisone or prednisolone, as previously reported [5]. Cyclosporine, AZA, and prednisolone were used in the early period until June 1999, when MMF replaced AZA as the antiproliferative agent. Cyclosporine was changed to tacrolimus with an addition of interleukin-2 receptor antagonists after July 2009. Intravenous methylprednisolone (500 mg) was administered intraoperatively, followed by 3 doses (150 mg every 8 hours) postoperatively. Then, prednisone medication was given at a daily dose (1 mg/kg) and tapered over six months to 0.1 mg/kg per day.
4) Evaluation of clinical outcomes
All patients underwent surveillance endomyocardial biopsy after HTPL for the monitoring of rejection. In group E, endomyocardial biopsy was performed weekly for the first 4 weeks, once every 4 weeks until the third month, and then every 3 months until the second year. In group L, endomyocardial biopsy was less frequently performed at the discretion of the cardiologists: monthly for the first 3 months, and every 3 months during the first year. Rejection severity was graded from 0 to 3R based on the ISHLT grading system, and significant rejection was defined as rejection grade 2R or higher [6]. Chronic kidney disease was defined as decreased kidney function (decreased glomerular filtration rate) for 3 or more months. Postoperative acute kidney injury was defined as an increase of more than 50% in serum creatinine level from the preoperative value or a need for renal replacement therapy irrespective of serum creatinine level.
All patients underwent regular postoperative follow-up through the outpatient clinic at 3- or 4-month intervals. The patients were also contacted by telephone for confirmation of their condition if they were not present on their last scheduled visit. Clinical follow-up was completed on August 31, 2014. Follow-up was completed in all patients with a median follow-up duration of 36.9 months. Operative mortality was defined as any death within 30 days after surgery.
5) Statistical analysis
Statistical analysis was performed using the IBM SPSS software ver. 19.0 (IBM Co., Armonk, NY, USA). Data were expressed as mean±standard deviation, median with ranges, or proportions. Comparisons between the two groups were performed using the chi-square or Fisher exact test for categorical variables and Student t-test for continuous variables. Survival rates were estimated using the Kaplan-Meier method and comparisons between 2 groups were performed using the log-rank test. The Cox proportional hazard model was adopted for multivariable analysis of risk factors for time related events. To identify significant predictors of overall survival and freedom from rejection, variables with p<0.1 on univariate analysis and clinically important factors were included in the multivariate model. A p-value <0.05 was considered as statistically significant.
Results
1) Early results
Operative mortality (any death within 30 days) was 3.8% (4/105; 1 in group E and 3 in group L). There were no patients who showed the suggested diagnostic criteria of primary graft failure. In-hospital mortality (any death before hospital discharge, including operative mortality) was 9.5% (10/105); this included 3 patients in group E and 7 patients in group L. Postoperative complications included acute kidney injury (n=31, 29.5%), respiratory complications (n=16, 15.4%), reoperation for bleeding (n=15, 14.4%), wound complication (n=10, 9.5%), cerebrovascular accident (n=6, 5.8%), and arrhythmia (n=4, 3.8%). Use of postoperative mechanical circulatory support such as an intra-aortic balloon pump and extracorporeal membrane oxygenation was considered if patients showed difficulties in weaning from cardiopulmonary bypass for >30 minutes after completion of all anastomoses. Postoperative mechanical circulatory support was needed in 22 patients (21.0%); intra-aortic balloon pump in 12 patients and extracorporeal membrane oxygenation in 14 patients. Four patients were supported by both intra-aortic balloon pump and extracorporeal membrane oxygenation. There were no significant differences in operative mortality (p=0.557) and in postoperative complications between the two patient groups, except for a higher incidence of acute kidney injury in group L compared to group E (p=0.025) (Table 3).
Table 3.
Early clinical results
| Variable | Total (n=105) | Group E (n=41) | Group L (n=64) | p-value |
|---|---|---|---|---|
| Operative mortality (≤30 day) | 4 (3.8) | 1 (2.4) | 3 (4.7) | 0.557 |
| Early morbidities | ||||
| Acute kidney injury | 31 (29.5) | 7 (17.1) | 24 (37.5) | 0.025 |
| Arrhythmia | 4 (3.8) | 1 (2.4) | 3 (4.7) | 0.557 |
| Wound complication | 10 (9.5) | 3 (7.5) | 7 (10.9) | 0.563 |
| Respiratory complication | 16 (15.4) | 6 (15.0) | 10 (15.6) | 0.932 |
| Cerebrovascular accident | 6 (5.8) | 1 (2.5) | 5 (7.8) | 0.258 |
| Reoperation for bleeding | 15 (14.4) | 3 (7.5) | 12 (18.8) | 0.112 |
| Postoperative mechanical circulatory support | 22 (21.0) | 8 (19.5) | 14 (21.9) | 0.772 |
| Intensive care unit stay (day) | 16±13 | 14±15 | 18±12 | 0.162 |
| Discharge (day) | 42±32 | 35±19 | 46±38 | 0.063 |
Values are presented as number (%) or mean±standard deviation.
Group E, patients who underwent HTPL before July 2009; group L, patients who underwent HTPL after July 2009.
HTPL, heart transplantation.
2) Long-term survival and event-free rates
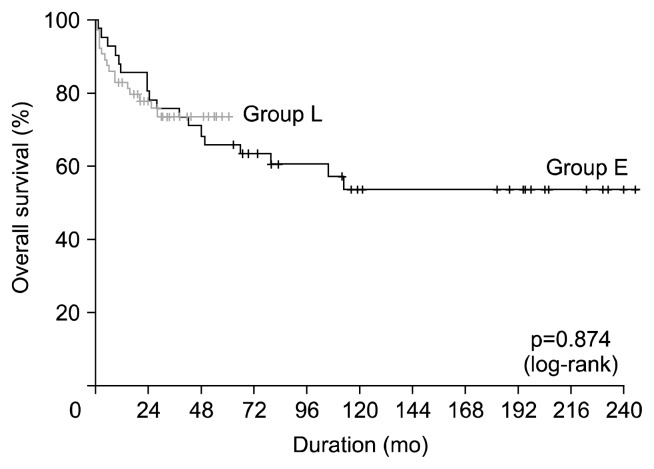
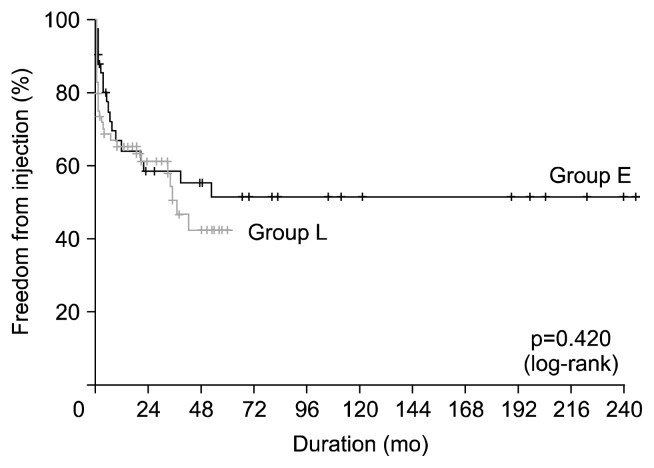
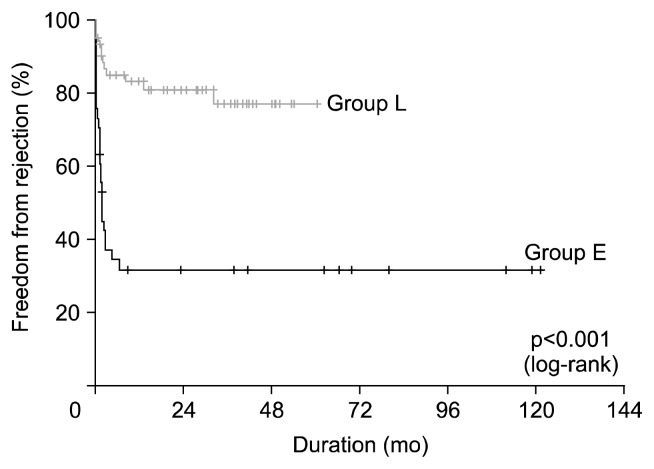
Late mortality (any death >30 days after surgery) was 29.7% (30/101). The common causes of late mortality were infection (n=14), rejection (n=7), and malignancy (n=3). The 1-, 5-, and 10-year survival rates were 83.8%, 67.7%, and 54.9%, respectively; there were no significant differences in overall survival between the two patient groups (p=0.874) (Fig. 1). Multivariable analysis demonstrated that preoperative mechanical ventilation (p=0.034), CKD (p=0.007), recipient aortic cross-clamp (p=0.037), and cardiopulmonary bypass times (p=0.001) were significant risk factors for overall survival. Change in immunosuppressive regimen (p=0.020) was the significant protective factor for overall survival (Table 4). Freedom from infection rates at 1, 5, and 10 years were 64.7%, 47.0%, and 47.0%, respectively; there were no significant differences between the two patient groups (p=0.420) (Fig. 2). Freedom from rejection rates at 1, 5, and 10 years were 69.9%, 61.6%, and 61.6%, respectively; freedom from rejection rates were significantly higher in group L than in group E (p<0.001) (Fig. 3). Multivariable analysis demonstrated that change in immunosuppressive regimen was the significant protective factor for freedom from rejection rates (p<0.001) (Table 5).
Fig. 1.
Kaplan-Meier curve of overall survival.
Table 4.
Analysis of risk factors for overall survival using a Cox proportional hazard model
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Categorical variables | ||||
|
| ||||
| Sex (male) | 0.58 (0.27–1.21) | 0.145 | ||
|
| ||||
| Donor sex (male) | 0.83 (0.39–1.78) | 0.632 | ||
|
| ||||
| Diagnosis | ||||
|
| ||||
| Non-CMP (ref=CMP) | 1.29 (0.53–3.13) | 0.570 | ||
|
| ||||
| Preoperative mechanical ventilation | 2.42 (1.04–5.62) | 0.040 | 2.89 (1.08–7.71) | 0.034 |
|
| ||||
| Preoperative mechanical circulatory support | 1.50 (0.65–3.48) | 0.341 | ||
|
| ||||
| Diabetes mellitus | 0.69 (0.29–1.67) | 0.412 | ||
|
| ||||
| Hypertension | 1.04 (0.51–2.15) | 0.910 | ||
|
| ||||
| History of cerebrovascular accident | 1.04 (0.37–2.95) | 0.944 | ||
|
| ||||
| Dyslipidemia | 0.37 (0.09–1.55) | 0.174 | ||
|
| ||||
| Chronic kidney disease | 2.82 (1.28–6.21) | 0.010 | 4.39 (1.49–12.95) | 0.007 |
|
| ||||
| Coronary artery disease | 0.87 (0.38–2.03) | 0.755 | ||
|
| ||||
| History of cardiac surgery | 1.16 (0.53–2.57) | 0.707 | ||
|
| ||||
| Immunosuppressive regimen | 0.990 | 0.020 | ||
|
| ||||
| Cyclosporine+MMF+Pd (ref: cyclosporine+AZA+Pd) | 1.07 (0.41–2.81) | 0.891 | 0.62 (0.21–1.84) | 0.385 |
|
| ||||
| Tacrolimus+MMF+Pd (ref: cyclosporine+AZA+Pd) | 1.02 (0.44–2.36) | 0.969 | 0.16 (0.05–0.58) | 0.005 |
|
| ||||
| BMI (kg/m2) | 0.294 | |||
|
| ||||
| <18.5 (ref: 18.5≤BMI<23) | 0.48 (0.11–2.09) | 0.328 | ||
|
| ||||
| ≥23 (ref: 18.5≤BMI<23) | 1.41 (0.71–2.83) | 0.329 | ||
|
| ||||
| Continuous variables | ||||
|
| ||||
| Age (yr) | 1.02 (0.99–1.04) | 0.194 | ||
|
| ||||
| Donor age (yr) | 1.02 (0.99–1.05) | 0.143 | ||
|
| ||||
| Donor ischemic time (min) | 1.00 (1.00–1.01) | 0.512 | ||
|
| ||||
| Recipient aortic cross-clamp time (min) | 1.02 (1.00–1.03) | 0.017 | 1.02 (1.00–1.03) | 0.037 |
|
| ||||
| Recipient cardiopulmonary bypass time (min) | 1.01 (1.00–1.01) | 0.024 | 1.01 (1.00–1.01) | 0.007 |
HR, hazard ratio; CI, confidence interval; CMP, cardiomyopathy; ref, reference; MMF, mycophenolate mofetil; Pd, prednisone; AZA, azathioprine; BMI, body mass index.
Fig. 2.
Kaplan-Meier curve of freedom from infection.
Fig. 3.
Kaplan-Meier curve of freedom from rejection.
Table 5.
Analysis of risk factors for freedom from rejection using a Cox proportional hazard model
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Categorical variables | ||||
|
| ||||
| Sex (male) | 1.16 (0.54–2.54) | 0.701 | ||
|
| ||||
| Donor sex (male) | 0.88 (0.43–1.81) | 0.735 | ||
|
| ||||
| Diagnosis | ||||
|
| ||||
| Non-CMP (ref: CMP) | 0.51 (0.18–1.44) | 0.204 | ||
|
| ||||
| Preoperative mechanical ventilation | 0.29 (0.07–1.21) | 0.090 | ||
|
| ||||
| Preoperative mechanical circulatory support | 0.45 (0.16–1.27) | 0.130 | ||
|
| ||||
| Diabetes mellitus | 1.05 (0.51–2.15) | 0.905 | ||
|
| ||||
| Hypertension | 0.63 (0.31–1.27) | 0.198 | ||
|
| ||||
| History of cerebrovascular accident | 1.22 (0.48–3.11) | 0.682 | ||
|
| ||||
| Dyslipidemia | 0.87 (0.36–2.07) | 0.751 | ||
|
| ||||
| Chronic kidney disease | 0.25 (0.08–0.81) | 0.021 | ||
|
| ||||
| Coronary artery disease | 0.57 (0.25–1.29) | 0.175 | ||
|
| ||||
| History of cardiac surgery | 0.89 (0.39–2.02) | 0.784 | ||
|
| ||||
| Immunosuppressive regimen | <0.001 | <0.001 | ||
|
| ||||
| Cyclosporine+MMF+Pd (ref: cyclosporine+AZA+Pd) | 0.17 (0.07–0.43) | <0.001 | 0.12 (0.97–1.00) | <0.001 |
|
| ||||
| Tacrolimus+MMF+Pd (ref: cyclosporine+AZA+Pd) | 0.09 (0.05–0.20) | <0.001 | 0.12 (0.06–0.27) | <0.001 |
|
| ||||
| BMI (kg/m2) | 0.355 | |||
|
| ||||
| <18.5 (ref: 18.5≤BMI<23) | 0.43 (0.13–1.42) | 0.165 | ||
|
| ||||
| ≥23 (ref: 18.5≤BMI<23) | 1.02 (0.52–2.00) | 0.948 | ||
|
| ||||
| Continuous variables | ||||
|
| ||||
| Age (yr) | 0.97 (0.95–0.99) | 0.009 | ||
|
| ||||
| Donor age (yr) | 0.97 (0.94–1.00) | 0.034 | ||
|
| ||||
| Donor ischemic time (min) | 0.99 (0.99–1.00) | 0.074 | ||
|
| ||||
| Recipient aortic cross-clamp time (min) | 0.98 (0.96–0.99) | 0.002 | ||
|
| ||||
| Recipient cardiopulmonary bypass time (min) | 0.99 (0.98–0.99) | <0.001 | ||
HR, hazard ratio; CI, confidence interval; CMP, cardiomyopathy; ref, reference; MMF, mycophenolate mofetil; Pd, prednisone; AZA, azathioprine; BMI, body mass index.
Discussion
The present study demonstrated two main findings. First, HTPL patients of the late period had more comorbidities compared to the early HTPL patients; they were older, more of them had hypertension and chronic kidney disease, and needed preoperative mechanical ventilation and mechanical circulatory support more frequently than the earlier patients. Second, the late period HTPL group showed similar early and long-term outcomes and a lower rejection rate in spite of increased preoperative comorbidities when compared with early period HTPL patients.
Since the first procedure in 1967, HTPL has become a gold standard treatment for patients with end-stage heart failure and more than 100,000 patients have undergone HTPL worldwide [1,2]. During the past half-century, there have been advances in donor and recipient selection, perioperative care, and immunosuppression strategies of HTPL [1]. The ISHLT reported that there have been changes in adult HTPL recipients’ profiles over time. The changes included increased comorbidities and high-risk characteristics of recipients, which might result from a combination of changing demographics of the general population as well as the willingness of clinicians to transplant higher risk patients. The age and comorbidity of donors have also been increasing [3,4,7]. With increasing organ shortages, most centers are currently accepting higher risk donors, particularly older donors [3,4,7,8]. The present study also showed similar changes in HTPL recipients’ profiles. Over the period of our study, recipients who underwent HTPL at our institution became older, and had more comorbidities such as hypertension, dyslipidemia, CAD, and CKD. In addition, the number of recipients requiring preoperative mechanical ventilation or mechanical circulatory support increased. Donor age also increased from 30.3±11.6 years in group E patients to 35.0± 11.9 years in group L patients.
The ISHLT reported that survival rates after HTPL were 81% and 69% at 1 and 5 years respectively, with a median survival of 11 years, and that risk factors such as preoperative mechanical circulatory support, preoperative mechanical ventilator, the cause of end-stage heart disease, recipient age, recipient height, donor age, donor heart ischemic time, retransplantation, transplant center volume, previous transfusion, recipient pre-transplant bilirubin level, and recipient pre-transplant creatinine level significantly influenced overall survival rates [3,4,7]. Additional factors such as previous sternotomy, body mass index, donor sex, race, smoking, and hypertension or diabetes mellitus of recipient were also known to significantly influence overall survival [9–13].
The present study demonstrated similar overall survival rates as those reported by the ISHLT. Despite higher risk factors of recipients and donors in the later period, early results and overall survival of HT PL patients were similar to those of the earlier period. In the multivariable analysis, preoperative mechanical ventilation, CKD, recipient aortic crossclamp, and cardiopulmonary bypass times were significant risk factors affecting overall survival. In addition, change in immunosuppressive regimen was a significant protective factor affecting overall survival. However, previously reported factors, such as donor ischemic time, preoperative use of mechanical circulatory support, the cause of end-stage heart disease, and age of recipient or donor, were not risk factors for overall survival rates according to the present study. This might be due to the relatively small number of enrolled patients. Similar to the findings of this present study, one previous study demonstrated that increased warm ischemic time was related to a reduced survival in HTPL [14]. Warm ischemic time was defined as the time the donor organ arrived in the recipient operating room until reperfusion, which was approximately similar to recipient aortic cross clamp time. The previous study suggested that the finding of reduced survival in recipients with increased warm ischemic time warranted further investigation with analysis of a possible mechanism [14].
Risk factors for death from infection were demonstrated to include old recipient age, female sex, preoperative mechanical ventilator support, or mechanical circulatory support [15,16]. In the present study, the freedom-from-infection rate was similar between the two patient groups. One previous study developed and validated a novel 13-point risk score to predict acute rejection based on 4 variables (age, race, sex, human leukocyte antigen matching); younger age, race other than Asian, female sex, and increased degree of human leukocyte antigen mismatch were associated with increased rejection rate [17]. The tacrolimus/MMF combination therapy was also associated with greater freedom from rejection rates, compared with cyclosporine/MMF therapy [18]. The present study demonstrated a higher freedom from rejection rate in the later period, when tacrolimus replaced cyclosporine. In multivariable analysis, the change in immunosuppressive regimen was the only significant protective factor for freedom from rejection. Although older recipient age was a risk factor for freedom from rejection in univariate analysis, it became insignificant in the multivariable analysis. We assumed that the relatively small number of enrolled patients affected the results.
There are limitations to the present study that must be recognized. First, it is a non-randomized, retrospective study with observational data in a single institution. Second, the number of enrolled patients was relatively small to make a definite conclusion. Third, the follow-up duration for the group L patients was relatively short.
In conclusion, early and long-term results after HTPL showed a 30-day mortality rate of 3.8%, a 5-year survival rate of 71.2%, and a 10-year survival rate of 54.9%. Despite increased morbidities of HT PL recipients and donors, early and long-term clinical outcomes were similar between the earlier and later group patients. Rejection free survival rate increased significantly over a period of time, probably resulting from the change in immunosuppressive regimen.
Acknowledgments
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund No.KTCS04-048).
Footnotes
This article was presented at the 46th Annual Meeting of the Korean Society for Thoracic and Cardiovascular Surgery, Yeosu, Korea, October 23th–25th, 2014.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.DePasquale EC, Schweiger M, Ross HJ. A contemporary review of adult heart transplantation: 2012 to 2013. J Heart Lung Transplant. 2014;33:775–84. doi: 10.1016/j.healun.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez Cetina Biefer H, Sundermann SH, Emmert MY, et al. Surviving 20 years after heart transplantation: a success story. Ann Thorac Surg. 2014;97:499–504. doi: 10.1016/j.athoracsur.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 3.Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:996–1008. doi: 10.1016/j.healun.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report--2013; focus theme: age. J Heart Lung Transplant. 2013;32:951–64. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Yeom SY, Hwang HY, Oh SJ, Cho HJ, Lee HY, Kim KB. Heart transplantation in the elderly patients: midterm results. Korean J Thorac Cardiovasc Surg. 2013;46:111–6. doi: 10.5090/kjtcs.2013.46.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report--2012. J Heart Lung Transplant. 2012;31:1052–64. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Goldsmith KA, Sharples LD, Sudarshan C, et al. Twenty-five years of heart transplantation at Papworth Hospital: changes in factors influencing short-and long-term patient survival over time. Open Transplant J. 2008;2:13–20. doi: 10.2174/1874418400802010013. [DOI] [Google Scholar]
- 9.Tallaj JA, Pamboukian SV, George JF, et al. Have risk factors for mortality after heart transplantation changed over time?: insights from 19 years of Cardiac Transplant Research Database study. J Heart Lung Transplant. 2014;33:1304–11. doi: 10.1016/j.healun.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Weiss ES, Allen JG, Arnaoutakis GJ, et al. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT) Ann Thorac Surg. 2011;92:914–21. doi: 10.1016/j.athoracsur.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Kilic A, Weiss ES, Yuh DD, Shah AS, Conte JV. Factors associated with 5-year survival in older heart transplant recipients. J Thorac Cardiovasc Surg. 2012;143:468–74. doi: 10.1016/j.jtcvs.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Kilic A, Weiss ES, George TJ, et al. What predicts long-term survival after heart transplantation?: an analysis of 9,400 ten-year survivors. Ann Thorac Surg. 2012;93:699–704. doi: 10.1016/j.athoracsur.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Hong KN, Iribarne A, Worku B, et al. Who is the high-risk recipient?: predicting mortality after heart transplant using pretransplant donor and recipient risk factors. Ann Thorac Surg. 2011;92:520–7. doi: 10.1016/j.athoracsur.2011.02.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marasco SF, Kras A, Schulberg E, Vale M, Lee GA. Impact of warm ischemia time on survival after heart transplantation. Transplant Proc. 2012;44:1385–9. doi: 10.1016/j.transproceed.2011.12.075. [DOI] [PubMed] [Google Scholar]
- 15.George JF, Pamboukian SV, Tallaj JA, et al. Balancing rejection and infection with respect to age, race, and gender: clues acquired from 17 years of cardiac transplantation data. J Heart Lung Transplant. 2010;29:966–72. doi: 10.1016/j.healun.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Smart FW, Naftel DC, Costanzo MR, et al. Risk factors for early, cumulative, and fatal infections after heart transplantation: a multiinstitutional study. J Heart Lung Transplant. 1996;15:329–41. [PubMed] [Google Scholar]
- 17.Kilic A, Weiss ES, Allen JG, et al. Simple score to assess the risk of rejection after orthotopic heart transplantation. Circulation. 2012;125:3013–21. doi: 10.1161/CIRCULATIONAHA.111.066431. [DOI] [PubMed] [Google Scholar]
- 18.Guethoff S, Meiser BM, Groetzner J, et al. Ten-year results of a randomized trial comparing tacrolimus versus cyclosporine a in combination with mycophenolate mofetil after heart transplantation. Transplantation. 2013;95:629–34. doi: 10.1097/TP.0b013e318277e378. [DOI] [PubMed] [Google Scholar]