Abstract
Background
The advantages of using a homograft in valve replacement surgery are the excellent hemodynamic profile, low risk of thromboembolism, and low risk of prosthetic valve infection. The aim of this study was to evaluate the long-term outcomes of homograft implantation in the aortic valve position.
Methods
This is a retrospective study of 33 patients (>20 years old) who underwent aortic valve replacement or root replacement with homografts between April 1995 and May 2015. Valves were collected within 24 hours from explanted hearts of heart transplant recipients (<60 years) and organ donors who were not suitable for heart transplantation. The median follow-up duration was 35.6 months (range, 0 to 168 months).
Results
Aortic homografts were used in all patients. The 30-day mortality rate was 9.1%. The 1- and 5-year survival rates were 80.0%±7.3% and 60.8%±10.1%, respectively. The 1-, 5-, and 10-year freedom from reoperation rates were 92.3%±5.2%, 68.9%±10.2%, and 50.3%±13.6%, respectively. The 1-, 5-, and 10-year freedom from significant aortic dysfunction rates were 91.7%±8.0%, 41.7%±14.2%, and 25.0%±12.5%, respectively.
Conclusion
Homografts had the advantages of a good hemodynamic profile and low risk of thromboembolic events, and with good outcomes in cases of aortitis.
Keywords: Aortic root, Aortic valve, Homograft, Allograft
Introduction
Homograft implantation has been used for a variety of aortic root and aortic valve diseases. The advantages of using homografts in valve replacement surgery include the excellent hemodynamic profile, good hemostasis, low risk of thromboembolism, and low risk of prosthetic valve infection [1–4]. In particular, the aortic homograft has proven its value in complex aortic root pathology such as endocarditis with abscess formation [5].
However, the disadvantages are homograft tissue degeneration and destruction—durability is the primary problem. Reoperation on the aortic root is also difficult because reimplantation of the coronary arteries can be complicated. Due to a shortage of the homograft bank reserves, access to the proper size of homograft can be limited.
Since the 1990s, only a few centers have used homografts and only a few studies have been published in Korea, in part due to the scarcity of homografts. The main concern has been durability and the quality of the homograft after thawing. The aims of this study were to evaluate long-term outcomes using homografts in the aortic valve position, and to determine the durability of homografts and the mode of failure.
Methods
1) Patient characteristics
This is a retrospective study of 33 patients (>20 years old) who underwent aortic valve replacement or root replacement with a homograft in the Asan Medical Center between April 1995 and May 2015. The mean age at operation was 47.2±2.6 years. Twenty-five patients were male (75.8%). Five patients had hypertension (15.2%) (Table 1). The underlying reasons for aortic valve surgery were native valve endocarditis (n=15, 45.5%), prosthetic valve endocarditis (n=5, 15.2%), and aortitis (n=6, 18.2%) (Table 2). The indication for use of a homograft was not confirmed. We considered the use of a homograft based on the surgeon’s preference, available homograft size, and degree of infection or aortitis. The primary endpoint was the day of death or explantation of the homograft. The median follow-up duration was 35.6 months (range, 0 to 168 months).
Table 1.
Baseline characteristics (N=33)
| Variable | Value |
|---|---|
| Mean age (yr) | 47.2±2.6 |
| Female | 8 (24.2) |
| Hypertension | 5 (15.2) |
| Diabetes mellitus | 3 (10.1) |
| Cerebrovascular accident history | 2 (6.1) |
| Behcet’s disease | 5 |
| Infective endocarditis | 21 |
| Complicated infection | 18 |
Values are presented as mean±standard deviation or number (%).
Table 2.
Preoperative diagnosis (N=33)
| Diagnosis | No. of patients |
|---|---|
| Native valve endocarditis | 15 |
| Prosthetic valve endocarditis | 5 |
| Failure of prosthetic valve (paravalvular leakage) | 3 |
| AAE | 1 |
| AAE, ascending aortic aneurysm | 1 |
| Aortitis | 6 |
| Ventricular septal defect patch site abscess | 1 |
| Aortic regurgitation, mitral regurgitation | 1 |
AAE, aortoannular ectasia.
2) Homograft preservation technique
Valves were collected within 24 hours from explanted hearts of heart transplant recipients (<60 years old). Another source was from organ donors who were not suitable for heart transplantation. Valves that had a congenital anomaly, large fenestrations in the cusps, or calcification in the cusps or aortic wall were excluded. Valves were extracted and placed in Roswell Park Memorial Institute (RPMI) medium with addition of low-dose antibiotics (50 IU/mL penicillin and 50 μg/mL streptomycin). The valves were held for 6 hours at 37°C. After culture, freezing solutions were prepared with 90 mL RPMI medium plus 10 mL dimethyl sulfoxide (DMSO) solution (i.e., 90 mL RPMI medium plus 10 mL DMSO solution). The valves were frozen using a controlled-rate freezing machine, with the tissue temperature decreased at a rate of 1°C per minute to −40°C. The life-span of the homograft was 10 years. The homograft was used after confirming that cultures of the solutions and valves were negative.
3) Homograft thawing and dilution technique
For implantation, the homograft should be transported at a temperature below −100°C. Just before being used, the homograft was thawed rapidly at 37°C to 42°C to prevent crystallization. The bag containing the homograft was soaked in 40°C saline for 6 to 7 minutes before the ice had melted completely. The homograft was taken out of the bag, and sequentially placed for 5 minutes in each of four dilute solutions of RPMI plus DMSO at 5%, 2.5%, 0%, and 0%, respectively.
4) Implantation technique
Twenty-nine patients underwent the root replacement technique, 2 underwent an intact noncoronary sinus scalloped technique, and 1 underwent an inclusion technique. Every procedure was performed under bicaval cannulation via median sternotomy. The mean cardiopulmonary bypass time was 234.4±15.6 minutes; aortic cross-clamp time was 175.88±9.32 minutes. Mitral valve replacement was performed in 3 patients (10.1%), and other concomitant procedures are summarized in Table 3.
Table 3.
Concomitant procedures (N=33)
| Procedures | No. of patients |
|---|---|
| Mitral valve replacement | 3 |
| Ascending aorta replacement | 1 |
| MVP | 1 |
| MVP, tricuspid annuloplasty | 2 |
| Ascending aorta replacement, ventricular septal defect closure | 1 |
| Ascending aorta replacement, MVP | 1 |
| Ascending aorta and total arch replacement, descending aorta replacement | 1 |
| Pulmonary valve replacement, right ventricular outflow tract reconstruction | 1 |
| Left ventricular outflow tract reconstruction | 1 |
| Permanent pace-maker insertion | 1 |
MVP, mitral valvuloplasty.
5) Statistical analysis
Continuous variable data are expressed as mean± standard deviation, and categorical variable data are expressed as percentages (%). Survival and freedom from reoperation rates were estimated using the Kaplan-Meier method. To determine differences in echocardiography results before and after surgery, the Wilcoxon signed rank test was used. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed using PASW SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Results
1) Echocardiography
Postoperative echocardiography was performed in all patients, excluding immediate postoperative deaths. All patients had aortic regurgitation (AR) before surgery. Immediate postoperative echocardiography was repeated 6.6±2.1 days later. The mean preoperative AR grade was 3.5±1.1; the immediate postoperative AR grade was 0.9±0.5, which was downgraded significantly (p<0.001). The aortic valve systolic pressure gradient was downgraded significantly from 59.1±28.8 to 15.8±7.9 (p=0.021); the aortic valve mean pressure gradient was also downgraded significantly from 35.1±17.7 to 8.4±4.7 (p=0.021).
2) Early outcomes
The 30-day mortality rate was 9.1%. Three patients died within 30 days postoperatively; the cause in each case was sepsis with multi-organ failure. Two patients had postoperative bleeding and underwent surgery to control the bleeding (6.1%). One patient required an intra-aortic balloon pump, and another required a biventricular assist device due to low cardiac output. Another patient was diagnosed with right ventricular failure due to right coronary artery ostium stenosis; coronary artery bypass grafting was performed in this patient.
3) Late outcomes
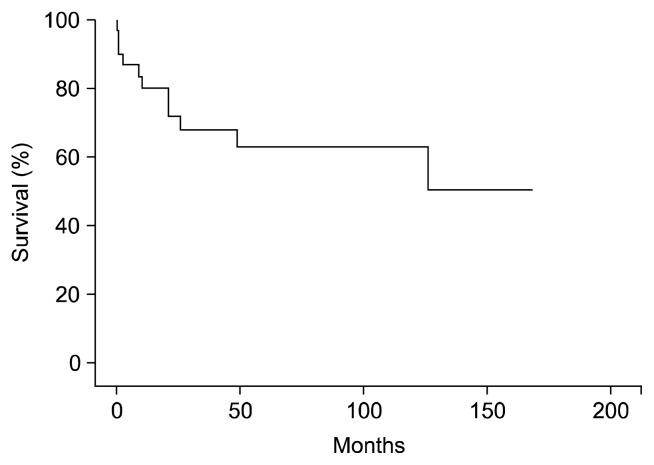
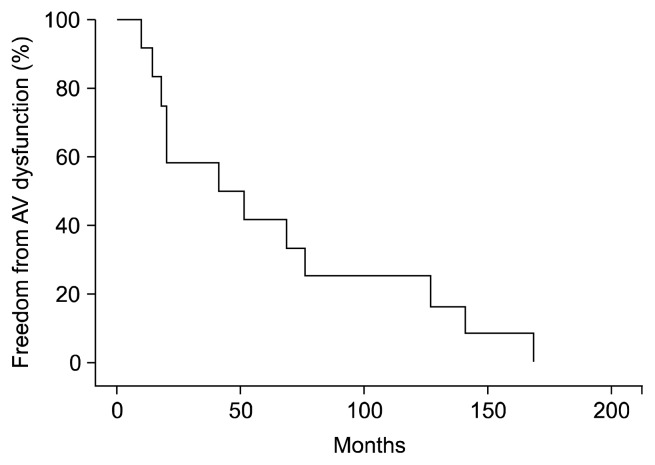
The 1- and 10-year survival rates were 80.3%±7.2% and 63.4%±9.5%, respectively (Fig. 1). The 1-, 5-, and 10-year freedom from significant aortic valve dysfunction rates, defined as moderate to severe AR and aortic stenosis that required medication and treatment, were 91.7%±8.0%, 41.7%±14.2%, and 25.0%±12.5%, respectively (Fig. 2).
Fig. 1.
Survival rate after homograft implantation in aortic valve and root position.
Fig. 2.
Freedom from AV dysfunction: moderate to severe aortic regurgitation or aortic stenosis. AV, aortic valve.
One death 2.3 months after surgery was due to low cardiac output. One patient died following 3 more operations after the first homograft root replacement surgery. Another patient died of gallbladder cancer. For five patients, the duration of follow-up was unknown; we confirmed the day of death using end-day of social insurance.
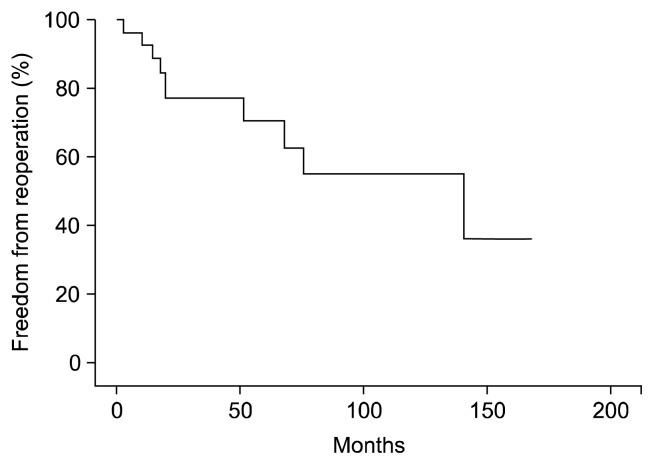
The freedom from reoperation rates at 1, 5, and 10 years were 92.4%±5.1%, 70.6%±9.7%, and 54.9%± 12.4%, respectively (Fig. 3). The main reason for reoperation was structural valve failure causing AR. Four patients had cusp prolapse, 1 had a cusp tear, and 2 had a cusp perforation. Two patients had abscess formation near the homograft valve. One patient had a history of infective endocarditis (Table 4). Of 21 patients who had preoperative infections, 2 had relapsed infective endocarditis (9.5%). One relapsed 2 months after the homograft root replacement surgery and the other relapsed 12 months after the surgery. Six patients had aortitis, and 1 of these was a recurrence (16.7%). Four patients had aortic valve replacement with a mechanical valve, and the other 3 patients had a Bentall operation.
Fig. 3.
Freedom from reoperation.
Table 4.
Mode of homograft failure (N=33)
| Etiology | No. of patients |
|---|---|
| LCC prolapse | 1 |
| Right coronary cusp prolapse | 1 |
| NCC perforation | 1 |
| LCC cusp tear | 1 |
| LCC motion limitation | 1 |
| NCC prolapse with vegetation | 1 |
| Abscess formation at aortic wall | 2 |
| LCC prolapse with ascending aorta pseudoaneurysm | 1 |
| Behcet’s disease (dissection of left ventricle myocardium) | 1 |
LCC, left coronary cusp; NCC, non-coronary cusp.
4) Behcet’s disease
In our study, 5 patients were diagnosed with Behcet disease before surgery; all had homograft failure after aortic root replacement with a homograft. Three patients had structural failure with cusp prolapse or perforation. One patient had a complication of Behcet aortitis extending to the left ventricle. The median duration of homograft failure in Behcet disease was 51.2 months (range, 10.1 to 75.7 months).
Discussion
Park et al. [4] used homografts for aortic root replacement in 15 patients and for aortic graft interposition in 8. These results were compatible with ours, but use of the homograft was different. In addition, we considered the high degree of early mortality to be due to uncontrolled infection and aortitis. We selected homografts for more severely ill, high-risk patients. The 30-day mortality rate was 9.1% (n=3) in our patients. Park et al. [4] reported an early mortality rate of 8.7%. The 10-year overall survival rate in our patients was 63.4%±9.5%, and was 59.6% for Park et al. [4].
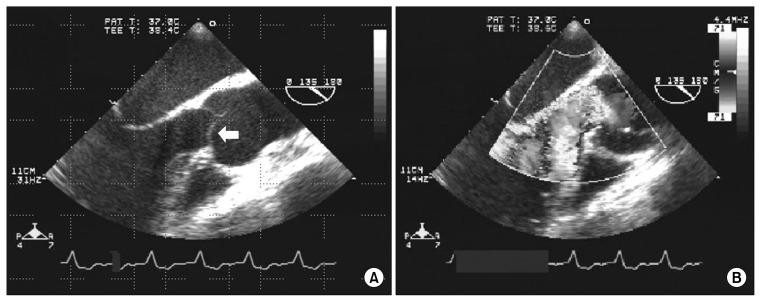
The major cause of failure of homografts has been AR [2]. Early postoperative AR is due to technical factors. Late AR seems to be due to commissural malalignment, cusp distortion, and cusp prolapse (Fig. 4). The homograft aortic wall shows a tendency toward calcification. Fukushima et al. [6] reported that structural deterioration was related to age at operation. They also reported that full-root and inclusion-cylinder implantation techniques had better results, with less structural failure, than subcoronary techniques. We only had 1 case of early postoperative homograft failure, which was due to uncontrolled infection.
Fig. 4.
(A, B) Trans-esophageal echocardiography of the structural failure of homograft; right coronary cusp prolapse (arrow) with accelerated aortic regurgitation.
Previous studies reported that some risk factors, such as young recipient age, older donor age, recipient body mass index, and blood transfusion history were predictors of structural deterioration. Deterioration was caused by necrosis or apoptosis of the interstitial cells, possibly due to the host immune response [7]. Multiple inflammatory cytokines, which are upregulated in patients with obesity, might aggravate inflammation in the homograft. Blood transfusion activated the immune system, and accelerated inflammatory reactions in the homograft [6]. However, many studies suggest that homografts rarely deteriorate within 5 years, and function well without immunosuppressive drugs. Similar to homografts, biological prosthetic valves structurally deteriorated over 10 to 15 years. However, patient-prosthesis mismatch occurred less after homograft implantation than after biological prosthetic valve implantation [8].
Some studies reported that homografts offer no benefits as aortic valve substitutes, except for patients with complicated endocarditis or aortitis [5,9]. Patients with prosthetic valve endocarditis have high surgical mortality rates of 10% to 20%. Debridement of infected tissue and targeted use of antibiotics are more important than the choice of valve. Nonetheless, other studies and many hospitals have used homografts for active infective endocarditis at the aortic root with periannular abscess formation [10].
Behcet disease is a multi-systemic inflammatory disease. Cardiac involvement in Behcet disease has a poor prognosis. Surgical treatment for AR in Behcet disease has been challenging because of the high risk of postoperative morbidity and mortality, with a high incidence of valve dehiscence after simple aortic valve replacement. Fragility of aortic structures necessitates of second or third operation. In our study, the prognosis was poor in aortic root replacement with a homograft in Behcet disease cases, but many studies showed that a homograft is better than other prosthetic valves. Jeong et al. [11] reported that aortic root replacement with a homograft and concomitant annular reinforcement reduces the risk of dehiscence and thromboembolism, which are prominent in Behcet disease [12].
1) Limitations
This was a retrospective study with a small sample size representing a single-center experience. We were unable to identify specific risk factors for homograft failure. The preservation and thawing of the homograft were based on a protocol. However, many physicians were involved in the preparation of the homograft, and it was not easy to maintain homograft quality. We were unable to determine the time of occurrence of AR because these data represent a long time period (April 1995 and May 2015), and outpatient visits to the hospital occurred irregularly for the few years following surgery.
2) Conclusions
Homografts have several advantages of a good hemodynamic profile and low risk of thromboembolic events. In particular, anticoagulation therapy was not used in this study, and thromboembolic events were not observed. The major cause of failure of homografts has been AR because of structural disruption. The control of homograft quality by careful management of preservation and thawing techniques is important. Further follow-up and studies are necessary for evaluation of the causes and risks of homograft failure.
Acknowledgments
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund No.KTCS04-052).
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.O’Brien MF, Harrocks S, Stafford EG, et al. The homograft aortic valve: a 29-year, 99.3% follow up of 1,022 valve replacements. J Heart Valve Dis. 2001;10:334–44. [PubMed] [Google Scholar]
- 2.Staab ME, Nishimura RA, Dearani JA, Orszulak TA. Aortic valve homografts in adults: a clinical perspective. Mayo Clin Proc. 1998;73:231–8. doi: 10.4065/73.3.231. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Na CY, Oh SS, Lee CH, Baek MJ, Kim CW. Homograft aortic root replacement. Korean J Thorac Cardiovasc Surg. 2005;38:197–203. [Google Scholar]
- 4.Park S, Hwang HY, Kim KH, Kim KB, Ahn H. Midterm follow-up after cryopreserved homograft replacement in the aortic position. Korean J Thorac Cardiovasc Surg. 2012;45:30–4. doi: 10.5090/kjtcs.2012.45.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preventza O, Mohamed AS, Cooley DA, et al. Homograft use in reoperative aortic root and proximal aortic surgery for endocarditis: a 12-year experience in high-risk patients. J Thorac Cardiovasc Surg. 2014;148:989–94. doi: 10.1016/j.jtcvs.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima S, Tesar PJ, Pearse B, et al. Long-term clinical outcomes after aortic valve replacement using cryopreserved aortic allograft. J Thorac Cardiovasc Surg. 2014;148:65–72.e2. doi: 10.1016/j.jtcvs.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 7.Koolbergen DR, Hazekamp MG, de Heer E, et al. The pathology of fresh and cryopreserved homograft heart valves: an analysis of forty explanted homograft valves. J Thorac Cardiovasc Surg. 2002;124:689–97. doi: 10.1067/mtc.2002.124514. [DOI] [PubMed] [Google Scholar]
- 8.Flameng W, Herregods MC, Vercalsteren M, Herijgers P, Bogaerts K, Meuris B. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation. 2010;121:2123–9. doi: 10.1161/CIRCULATIONAHA.109.901272. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen DT, Delahaye F, Obadia JF, et al. Aortic valve replacement for active infective endocarditis: 5-year survival comparison of bioprostheses, homografts and mechanical prostheses. Eur J Cardiothorac Surg. 2010;37:1025–32. doi: 10.1016/j.ejcts.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 10.Yankah AC, Klose H, Petzina R, Musci M, Siniawski H, Hetzer R. Surgical management of acute aortic root endocarditis with viable homograft: 13-year experience. Eur J Cardiothorac Surg. 2002;21:260–7. doi: 10.1016/S1010-7940(01)01084-3. [DOI] [PubMed] [Google Scholar]
- 11.Jeong DS, Kim KH, Kim JS, Ahn H. Long-term experience of surgical treatment for aortic regurgitation attributable to Behcet’s disease. Ann Thorac Surg. 2009;87:1775–82. doi: 10.1016/j.athoracsur.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Ma WG, Zheng J, Zhu JM, Liu YM, Li M, Sun LZ. Aortic regurgitation caused by Behcet’s disease: surgical experience during an 11-year period. J Card Surg. 2012;27:39–44. doi: 10.1111/j.1540-8191.2011.01392.x. [DOI] [PubMed] [Google Scholar]