Abstract
Nervous system development and circuit formation requires neurons to migrate from their birthplaces to specific destinations.Migrating neurons detect extracellular cues that provide guidance information. In Caenorhabditis elegans, the Q right (QR) and Q left (QL) neuroblast descendants migrate long distances in opposite directions. The Hox gene lin-39 cell autonomously promotes anterior QR descendant migration, and mab-5/Hox cell autonomously promotes posterior QL descendant migration. Here we describe a nonautonomous role of mab-5 in regulating both QR and QL descendant migrations, a role masked by redundancy with lin-39. A third Hox gene, egl-5/Abdominal-B, also likely nonautonomously regulates Q descendant migrations. In the lin-39mab-5egl-5 triple mutant, little if any QR and QL descendant migration occurs. In addition to well-described roles of lin-39 and mab-5 in the Q descendants, our results suggest that lin-39, mab-5, and egl-5 might also pattern the posterior region of the animal for Q descendant migration. Previous studies showed that the spon-1 gene might be a target of MAB-5 in Q descendant migration. spon-1 encodes a secreted basement membrane molecule similar to vertebrate F-spondin. Here we show that spon-1 acts nonautonomously to control Q descendant migration, and might function as a permissive rather than instructive signal for cell migration. We find that increased levels of MAB-5 in body wall muscle (BWM) can drive the spon-1 promoter adjacent to the Q cells, and loss of spon-1 suppresses mab-5 gain of function. Thus, MAB-5 might nonautonomously control Q descendant migrations by patterning the posterior region of the animal to which Q cells respond. spon-1 expression from BWMs might be part of the posterior patterning necessary for directed Q descendant migration.
Keywords: Hox, mab-5, egl-5, F-spondin, cell migration
HOX transcription factors are principal regulators of cell fate and control major aspects of development, including nervous system development. Hox factors regulate nervous system development in part through cell-autonomous specification of cell fate, axon guidance, and regulation of migratory neural progenitors (Studer et al. 1996; Gavalas et al. 1997; Arenkiel et al. 2004). Additionally there is evidence that Hox genes in the developing brain can cell nonautonomously control axon guidance (Gavalas et al. 1997). Neuron migration is an important aspect of nervous system development and many neurons and neuroblasts migrate from their initial birthplace to specific regions of the periphery (neural crest) or cortex. The Q neuroblasts of Caenorhabditis elegans represent a tractable and well-studied model for Hox gene-controlled neuroblast migration (Chapman et al. 2008; Middelkoop and Korswagen 2014). The Q neuroblasts Q right (QR) and Q left (QL) are bilaterally symmetric cells that undergo identical patterns of division, migration, and apoptosis to produce three neurons each (Sulston and Horvitz 1977). QR on the right side of the animal migrates anteriorly, undergoing cell division and migration, giving rise to three neurons, with AQR migrating the farthest residing near the posterior pharyngeal bulb (Sulston and Horvitz 1977; Chapman et al. 2008). QL migrates posteriorly undergoing identical cell divisions, giving rise to three neurons, of which PQR migrates the farthest to reside posterior to the anus, near the phasmid ganglion (Chalfie et al. 1983; Kenyon 1986; Salser and Kenyon 1992; Whangbo and Kenyon 1999; Korswagen et al. 2000; Chapman et al. 2008).
Hox transcription factors govern QR and QL descendant migrations. Canonical Wnt signaling through detection of extracellular EGL-20/Wnt induces transcription of antennapedia-like Hox gene mab-5 in the Q cells (Maloof et al. 1999). MAB-5 is necessary in the QL lineage for posterior migration, and in the absence of MAB-5 all QL daughters migrate anteriorly (Harris et al. 1996). MAB-5 is also sufficient for posterior Q cell migrations, as expression of MAB-5 in QR causes posterior migration of the entire QR lineage (Salser and Kenyon 1992). Levels of MAB-5 appear to be tightly controlled, as MAB-5 is able to both activate and inhibit its own expression to ensure a specific level of MAB-5 (Mentink et al. 2014). There is strong evidence that the directional control of MAB-5 is cell autonomous. Q cell-specific knockdown of mab-5 causes complete anterior migration of PQR, and specific induction of MAB-5 in anterior migrating Q descendants causes them to reverse direction of migration (Cowing and Kenyon 1992; Harris et al. 1996; Shen et al. 2014). The genes that MAB-5 controls to drive posterior migration are largely unknown. A recent study used whole organism RNA sequencing (RNA-seq) of mab-5 mutants paired with functional analysis to determine genes regulated by MAB-5 in migration (Tamayo et al. 2013). This study found enrichment for secreted and transmembrane molecules regulated by MAB-5, suggesting that MAB-5 might directly regulate a cell’s interaction with its environment.
The Deformed-like Hox gene lin-39 is required in QR descendants for anterior migration. Both QL and QR initially express lin-39, but mab-5 inhibits lin-39 expression in QL when it is expressed in response to Wnt signaling (Clark et al. 1993; Salser et al. 1993; Wang et al. 2013). lin-39 drives expression of the MIG-13 transmembrane receptor molecule in QL descendants, which, along with SDN-1/syndecan, mediates anterior migration (Wang et al. 2013; Sundararajan et al. 2015).
The C. elegans genome contains an abbreviated Hox cluster on linkage group III containing, among other Hox genes, lin-39, mab-5, and the Abdominal-B-like Hox gene egl-5. These genes control posterior body regions that represent their order on the chromosome (Kenyon 1986; Chisholm 1991; Clark et al. 1993; Van Auken et al. 2000). The most anterior gene lin-39 is expressed in QR and transiently in QL, but also in the P3–P8 cells, hyp7 hypodermis, ventral cord neurons, and sex myoblasts (Clandinin et al. 1997; Maloof et al. 1999; Yang et al. 2005; Wagmaister et al. 2006a,b; Kalis et al. 2014). lin-39 mutants show improper development of P3–8.p cells, which results in vulvaless animals (Clark et al. 1993; Salser et al. 1993). Next, mab-5 is expressed in QL, but also in posterior body wall muscles (BWMs), P7–P12, and the V5 and V6 hypodermal seam cells (Kenyon 1986; Salser and Kenyon 1996; Ji et al. 2013). MAB-5 and LIN-39 can act in parallel in the P cells, where they are both expressed, or play opposing roles in Q neuroblast migration where MAB-5 directs posterior migration, and LIN-39 promotes anterior migration (Salser and Kenyon 1992; Clark et al. 1993; Wang et al. 2013). MAB-5 also interacts genetically with the more posteriorly expressed Hox gene egl-5 (Chisholm 1991; Ferreira et al. 1999). egl-5 is expressed in the tail region of the animal, including BWMs, P11–P12, V6 descendants, HSN, PVM, PVC, and rectal epithelial cells, but not the Q cells (Ferreira et al. 1999). mab-5 and egl-5 interact in different ways depending on the cell. In P10–P11, MAB-5 inhibits egl-5 expression, and in V6 descendants MAB-5 promotes expression of egl-5 (Chisholm 1991; Ferreira et al. 1999; Li et al. 2009). These three C. elegans Hox genes have complex cell-specific interactions controlling development of midbody and posterior regions. All functions of these Hox genes described to date appear to be cell-autonomous roles, including the roles of LIN-39 and MAB-5 in Q descendant migration.
Previous studies show that MAB-5 acts in the Q descendants themselves to control direction of migration. In this work, we describe a new, nonautonomous role of mab-5 in the ability of Q descendants to migrate, but not direction. We show that lin-39 and mab-5 act in parallel in both AQR and PQR migration, and that transgenic expression of mab-5 in posterior BWMs rescues AQR and PQR defects in lin-39mab-5 double mutants. Further, we describe AQR and PQR migration defects in egl-5 mutants, expression of which is not detectable in Q lineages. Together, these results point to a nonautonomous role of mab-5 and possibly lin-39 and egl-5 in Q migrations. Expression of these genes might pattern the posterior of the animal, providing migration information to the Q descendants. Thus, MAB-5 and LIN-39 might both establish an anterior–posterior Q descendant guidance system (nonautonomous role) and control how the Q descendants respond to this guidance system (autonomous role).
A previous RNA-seq study identified spon-1 as a potential transcriptional target of MAB-5 (Tamayo et al. 2013). The mab-5(e1751) gain-of-function (gof) mutation causes ectopic expression of mab-5 in many cells, including QR, and drives posterior migration of the QR descendant AQR (Salser et al. 1993; Chapman et al. 2008). RNA-mediated interference (RNAi) knockdown of spon-1 partially suppressed posterior AQR migration in mab-5(gof), suggesting that spon-1 mediates the effects of mab-5(gof) in posterior migration (Tamayo et al. 2013). spon-1 encodes a secreted basement membrane molecule similar to vertebrate F-spondin (Woo et al. 2008). In vertebrates, F-spondin is secreted by the floor plate of the neural tube and has multiple roles in neural adhesion, neural crest migration, and axon guidance (Klar et al. 1992; Burstyn-Cohen et al. 1999; Debby-Brafman et al. 1999; Zisman et al. 2007). F-spondin becomes processed into three peptides that can both attract and repel developing axons (Tzarfaty-Majar et al. 2001; Zisman et al. 2007). F-spondin has been implicated in Alzheimer’s disease (AD) as a binding partner to amyloid precursor protein, with F-spondin treatment improving memory and β-amyloid levels in AD model mice (Ho and Sudhof 2004; Hoe et al. 2005; Hafez et al. 2012). In addition to its role in disease, F-spondin is conserved in many species, including and has domain similarity to the established nervous system development molecule Reeler (Klar et al. 1992; Higashijima et al. 1997; Burstyn-Cohen et al. 1999; Hu et al. 2016). In C. elegans, SPON-1/F-spondin plays a role in neural adhesion and development (Woo et al. 2008). spon-1 is required for muscle cell adhesion, and null or strong loss-of-function mutants are embryonic lethal (Woo et al. 2008). Despite being an important nervous system development molecule, little is known about the regulation of F-spondin expression. Here we show that spon-1 itself is required for AQR and PQR migration. Furthermore, we show that spon-1 promoter activity in BWMs can be driven by MAB-5, and that spon-1 phenotypes are rescued by BWM-derived SPON-1. Finally, we present evidence that spon-1 acts in BWM to mediate the effects of mab-5(gof). Taken together, our results suggest that mab-5 has a nonautonomous role in Q descendant migration, possibly by patterning the posterior region of the animal for proper Q migration. Further, they suggest that the spon-1/F-spondin gene might be a target of MAB-5 in posterior BWMs, which in part provides information for Q descendant migration.
Materials and Methods
Genetics
All experiments were carried out using standard C. elegans technique at 20°C (Brenner, 1974). Mutations used were: LGX: lqIs2[Posm-6::gfp]; LGI: lrp-1(ku156). LGII: spon-1(e2623, ju430ts, ju402), hlh-1(cc561), muIs16[mab-5::gfp]. LGIII: mab-5(e1239, e2088, and e1751), lin-39(n1760), egl-5(n945), rdvIs1[Pegl-17::mCherry]. LGIV: lqIs80 [Pscm::gfp::caax]. LGV: sid-1(pk3321), lqIs58[Pgcy-32::cfp], wgIs54 [egl-5::TY1::egfp::3xFLAG, UNC-119+]. Unknown chromosomal location, lqIs227 and lqIs228 [Pspon-1::gfp], and lqIs271[Pmyo-3::mab-5]. lqIs227 and lqIs228 were created by integration of juEx592 (Woo et al., 2008), and lqIs271 by integration of lqEx808. Extrachromosomal arrays were generated using standard gonadal injection (Mello and Fire, 1995) and include: lqEx708 and lqEx709 [Pscm::spon-1(RNAi), Pgcy-32::cfp]; lqEx732 and lqEx937 [Pegl-17::spon-1(RNAi), Pgcy-32::cfp]; lqEx808 [Pmyo-3::mab-5, Pgcy-32::cfp]; lqEx834 [Pegl-17::myr-mCherry, Pegl-17::mCherry::HIS-24]; lqEx849 [Pegl-17::REELER, Pgcy-32::yfp]; lqEx854 [Pegl-17::REELER::gfp, Pgcy-32::cfp]; lqEx855, lqEx856 and lqEx858 [Pegl-17::TSR1-5, Pgcy-32::cfp]; lqEx859 and lqEx860 [Pmyo-3::spon-1, Pgcy-32::cfp]; lqEx897 and lqEx898 [Pspon-1::mab-5::cfp, Pgcy-32::yfp]; lqEx759 [Pegl-17::spon-1]; lqEx938, lqEx940 and lqEx941 [Pmyo-3::spon-1(RNAi), Pscm::gfp, Pgcy-32::mCherry]; lqEx942 [Pgcy-32::yfp], into lin-39(n1760) egl-5(n945); lqEx930 and lqEx943 [Pspon-1::EGL-5::cfp, Pgcy-32::yfp].
Transgene construction
Details about transgene construction are available by request. The entire spon-1 genomic region was amplified by PCR and placed behind myo-3 and egl-17 promoters. Pegl-17::SP::TSR1-5 contained the spon-1 endogenous signal peptide, first 29 residues, followed by the five thrombospondin repeats, residues 431–819. Pegl-17::Reeler was made by using the first 430 residues, which contain both the Reeler and Spondin domains. Pmyo-3::mab-5 was using a mab-5 complementary DNA with the first endogenous mab-5 intron (the mab-5 minigene). Pspon-1::mab-5::cfp was made fusing the mab-5 minigene to cfp at the C terminus. Pspon-1::egl-5::cfp was made using the entire egl-5 genomic region.
Scoring AQR and PQR migration
Scoring was done using Pgcy-32, which is expressed exclusively in AQR, PQR, and URXl/r (Chapman et al. 2008). The position of AQR and PQR was scored as previously described using a compound fluorescent microscope (Chapman et al. 2008; Dyer et al. 2010). ju430ts animals were allowed to lay eggs for 3 hr at 15°, then plates were shifted to 20°. Some animals exhibited pat phenotype; viable animals were scored for AQR and PQR as described above. The triple mutant lin-39mab-5egl-5 was maintained over the hT2 balancer, and scored progeny had wild-type maternal contribution for each gene. Some Hox single and double mutant combinations were balanced by hT2. Positions 4a and 4b were separated by the PDE neuron marked by Posm-6::gfp, which represents the region of Q cell birth. Neurons directly over the PDE were marked as position 4a. Significance of difference was determined by Fisher’s exact test.
Line scan analysis of Pspon-1::gfp expression
Animals were synchronized to 4–4.5 hr posthatching using previous published techniques (Honigberg and Kenyon 2000; Chapman et al. 2008). Animals were mounted on a 2% (w/v) agarose pad in M9 containing 5 mM sodium azide. Fluorescent micrographs were acquired for 100 ms at ×100 using a Qimaging Rolera EM CCD camera and Metamorph software. Intensity of GFP was measured using ImageJ. Lines were drawn 1 pixel wide from the center of the posterior pharyngeal bulb to the posterior end of the anus on both dorsal and ventral BWM segments. Segmented lines were drawn through BWM nuclei, with pixels set at the anterior, posterior, and center of each muscle cell. Only animals that had both left and right muscle quadrants aligned were scored. To account for animal curvature, we averaged pixel intensity over each percentage of each line measured. This gives a percentage referring to the percentage of distance from anterior to posterior of the animal. This gave similar results to artificial straightening using ImageJ and was less cumbersome. Twenty animals were imaged on both dorsal and ventral segments for each genotype, yielding 40 scans per genotype. In our analysis, dorsal and ventral data were combined for each animal, and any dorsal–ventral differences were not included. Standard deviations were calculated for each percentage position (error bars), and a two-tailed Student’s t-test with unequal variance was used to determine significance of difference at each percentage position using a Bonferroni correction for multiple comparisons (100 in each genotype, Q < 0.05, P < 0.0005). Transgenes lqIs227 and lqIs228 showed similar posterior bias and lqIs227 was chosen for subsequent analysis due to chromosomal location (lqIs227 is on the region of LGI balanced by hT2). For lin-39mab-5egl-5 triple hox line scan analysis, lqIs228 was used for comparison, due to linkage of the lqIs227 transgene.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Hox genes mab-5, lin-39, and egl-5 control QR and QL descendant migrations
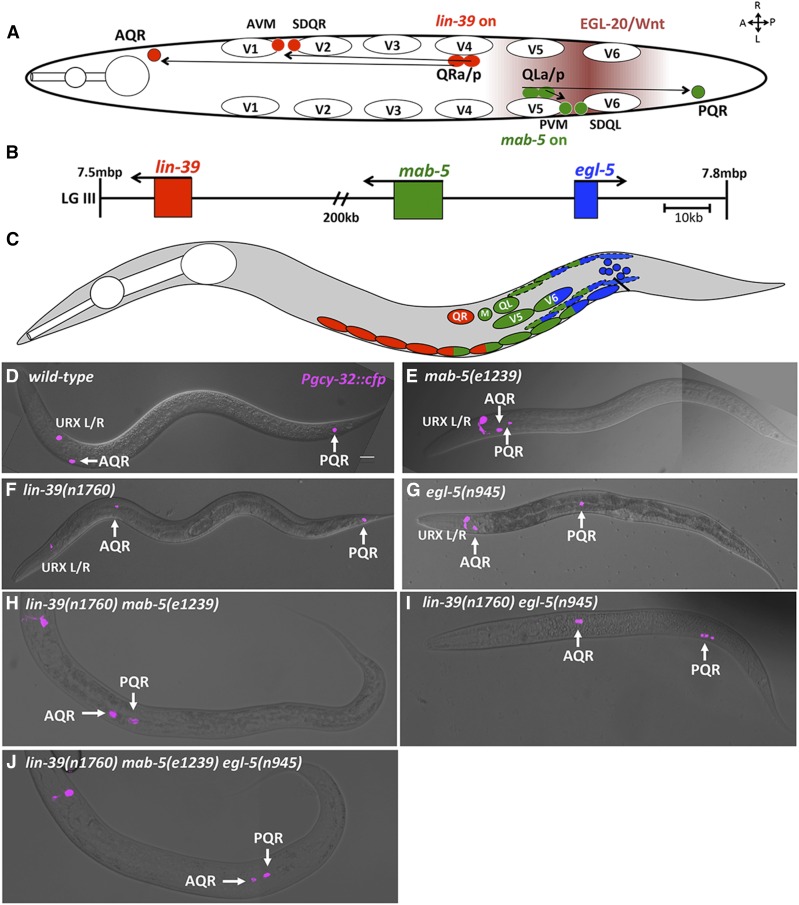
The bilateral neuroblasts QR and QL, born in the posterior between the vulva and anus, give rise to the AQR and PQR neurons, respectively (Sulston and Horvitz 1977) (Figure 1A). In wild-type animals, AQR migrates anteriorly to a region near the anterior deirid, and PQR migrates posteriorly to a position posterior to the anus in the phasmid ganglion (White et al. 1986; Chapman et al. 2008) (Figure 1A). The Hox transcription factors lin-39, mab-5, and egl-5 are expressed in specific regions ranging from anterior to posterior and resemble a Hox cluster on chromosome III (Kenyon 1986; Chisholm 1991; Clark et al. 1993; Salser et al. 1993; Wang et al. 1993; Van Auken et al. 2000) (Figure 1, B and C). MAB-5 is required in QL to direct QL descendant migrations including PQR (Salser and Kenyon 1992) (Figure 1, A and E). LIN-39 has been shown to cell-autonomously promote anterior migration of QR descendants, and lin-39 is normally inhibited by MAB-5 in QL.a/p to allow for posterior migration (Figure 1, A and F) (Harris et al. 1996; Wang et al. 2013).
Figure 1.
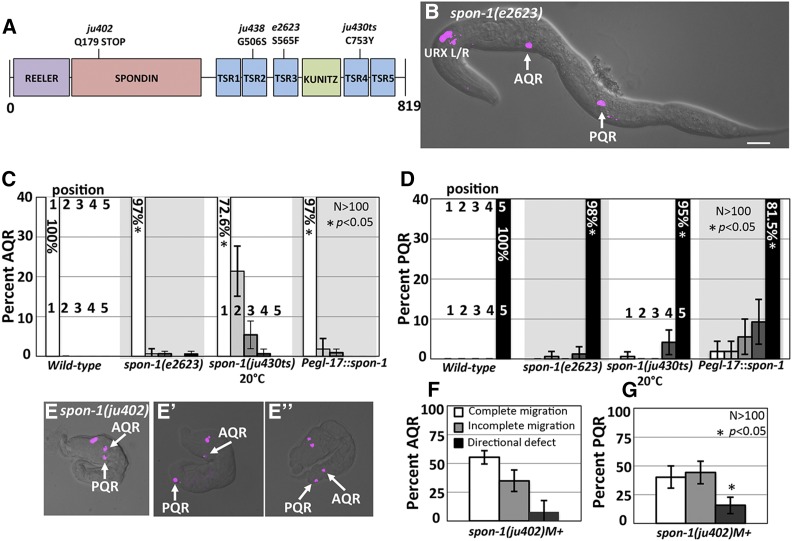
C. elegans Hox genes lin-39, mab-5, and egl-5 affect Q descendant migrations. (A) Diagram of a dorsal view of wild-type Q descendant migration. EGL-20/Wnt (maroon shading) induces MAB-5 in QL and descendants, which directs posterior migration. QR and descendants do not respond to EGL-20/Wnt, and express lin-39, driving anterior migration. (B) Position on LGIII (7.5–7.8 Mbp) of the three C. elegans Hox genes that effect postembryonic development. (C) representation of cells that express lin-39 (red) , mab-5 (green), and egl-5 (blue) during the L1 larval stage. Dashed ovals represent BWMs, solid ovals the P cells, and blue circular cells near the anus represent the rectal epithelium where egl-5 is expressed. (D–J) Positions of Q descendants AQR and PQR in L4/young adult animals. lqIs58[Pgcy-32::cfp] micrographs were merged with DIC micrographs in wild-type and mutants. In all micrographs unless otherwise noted, dorsal is up, anterior is left. Bar, 10 μm.
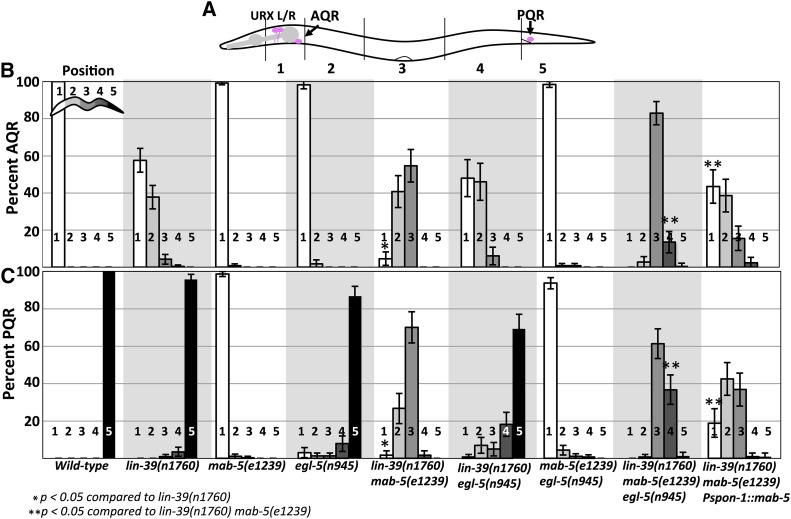
We scored AQR and PQR position using Pgcy-32::cfp along five positions in the animal as previously described (see Materials and Methods and Figure 2A) (Chapman et al. 2008). As expected, lin-39 mutants showed shortened anterior migration of the QR descendant AQR (Figure 1F and Figure 2B). lin-39 displayed minor (4%), but significant defects in PQR migration (Figure 2C). mab-5 mutants affected only PQR migration, with 100% of PQR misdirected, mostly residing in the normal anterior position of AQR (Figure 1E and Figure 2, B and C). egl-5 mutants were reported to have weak QL defects (Desai and Horvitz 1989; Chisholm 1991). We found that egl-5 affected both AQR and PQR migration: 2% of AQR failed to migrate fully and 16% of PQR failed to migrate, or migrated anteriorly (Figure 1G and Figure 2, B and C).
Figure 2.
AQR and PQR migration defects in lin-39, mab-5, and egl-5. (A) Schematic of an animal with scoring zones indicated (see Materials and Methods). The wild-type positions of AQR (position 1) and PQR (position 5) are indicated. (B and C) Percent of AQR and PQR residing at each position in adult animals as visualized by lqIs58[Pgcy-32::cfp]. Error bars represent 2× standard error of the proportion. Fisher’s exact test was used to determine significance of difference. Genotypes with the Pspon-1::mab-5 transgene represent combined results from two independently derived arrays with similar effects.
Previous studies found that lin-39 and mab-5 act in parallel in QR descendant migration (Clark et al. 1993; Wang et al. 1993). In a lin-39mab-5 double mutant, AQR migration defects were significantly stronger compared to lin-39 alone, and misdirected PQRs failed in their anterior migration (Figure 1H and Figure 2, B and C). These data suggest that MAB-5 acts in parallel with LIN-39 in anterior migration.
mab-5egl-5 double mutants showed no significant difference in AQR migration from egl-5 alone (Figure 2B). Most PQR were directed anteriorly in the mab-5egl-5 double, but some failed in their anterior migration, consistent with the weak anterior AQR migration defects in egl-5 single mutants. lin-39egl-5 double mutants had AQR defects similar to an additive effect of lin-39 and egl-5 single mutants (Figure 1I and Figure 2, B and C). PQR defects in lin-39egl-5 animals were significantly increased compared to an additive effect of both single mutants, suggesting they act in parallel (Figure 2, B and C). Similarly, the lin-39mab-5egl-5 triple mutant showed significantly more severe AQR and PQR migration defects compared to any double Hox mutant (Figure 1J and Figure 2, B and C). Most AQR and PQR remained near their birth positions with minimal anterior or posterior migration. These results suggest that lin-39, mab-5, and egl-5 are required in parallel for both anterior and posterior migration of QL and QR descendants AQR and PQR, and in their absence, very little migration occurs.
mab-5 and egl-5 can act in BWM to control AQR and PQR migration
These data suggest that the Hox genes LIN-39, MAB-5, and EGL-5 act in parallel pathways to promote both anterior and posterior migration of QR and QL descendants AQR and PQR. mab-5 is not expressed in QR descendants (Salser et al. 1993; Harris et al. 1996; Salser and Kenyon 1996), and lin-39 autonomously drives anterior QR descendant migration and is repressed in QL by mab-5 (Harris et al. 1996; Wang et al. 2013). However, both are expressed in other posterior cells, including P cells, and MAB-5 in posterior BWMs and seam cells (Salser et al. 1993; Wang et al. 1993; Clandinin et al. 1997; Maloof et al. 1999; Yang et al. 2005; Wagmaister et al. 2006a,b) (Figure 1C). Thus, the effects of mab-5 on AQR migration and lin-39 on PQR migration might be due to their roles in cells other than Q descendants. However lin-39 is expressed briefly in QL lineage, which could be important for initial migration (Wang et al. 1993).
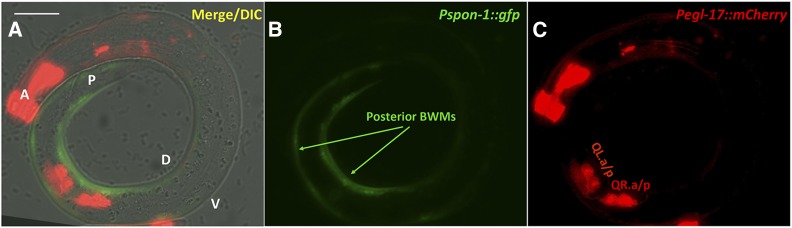
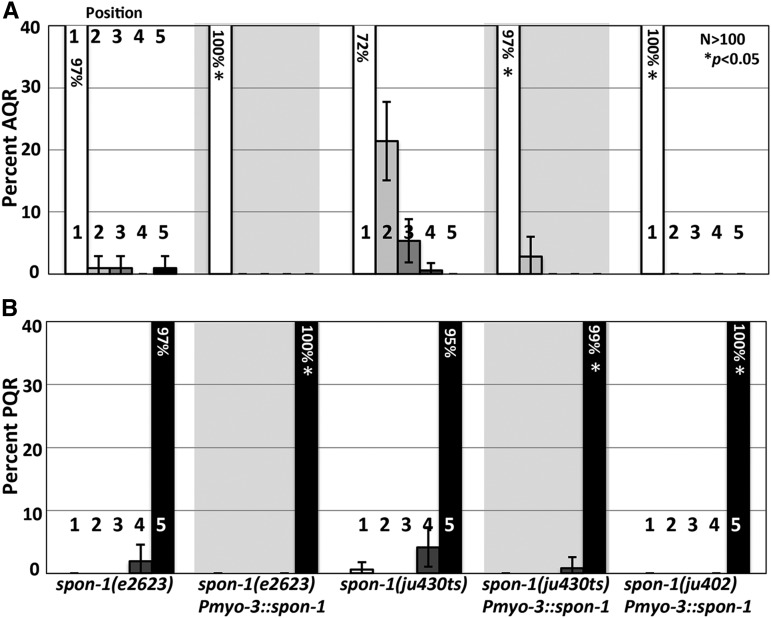
MAB-5 is expressed in posterior BWMs (Salser et al. 1993) (Figure 1C). We drove expression of MAB-5 specifically in BWMs using the promoter of the spon-1 gene (Woo et al. 2008). At the time of Q descendant migration, Pspon-1::gfp was expressed most strongly in posterior BWMs in the region of the Q cells, but not in the Q cells themselves (Figure 3). Pspon-1::mab-5 significantly rescued the AQR and PQR defects seen in the lin-39mab-5 double mutant [e.g., 4–44% of AQR in position 1, and 2–19% of PQR in position 1 (P < 0.05)] (Figure 2, B and C). These results show that MAB-5 can have a role in AQR and PQR migration through activity in BWMs. Importantly, direction of PQR migration was not rescued by Pspon-1::mab-5 (e.g., PQRs still migrated anteriorly). Direction of migration is an established cell-autonomous role of mab-5 and not expected to be rescued by muscle-specific Pspon-1::mab-5. This also shows that Pspon-1::mab-5 did not lead to expression of mab-5 in the Q descendants, as transgenic expression of mab-5 in Q descendants leads to posterior migration of AQR and PQR (Josephson et al. 2016). These data argue that mab-5 has a nonautonomous role in anterior AQR and PQR migration.
Figure 3.
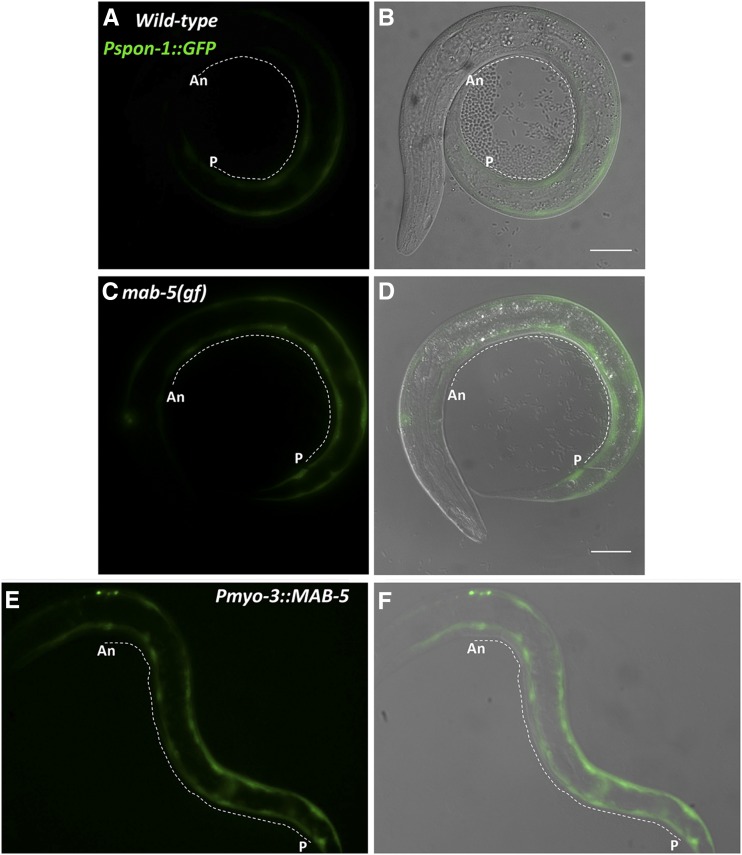
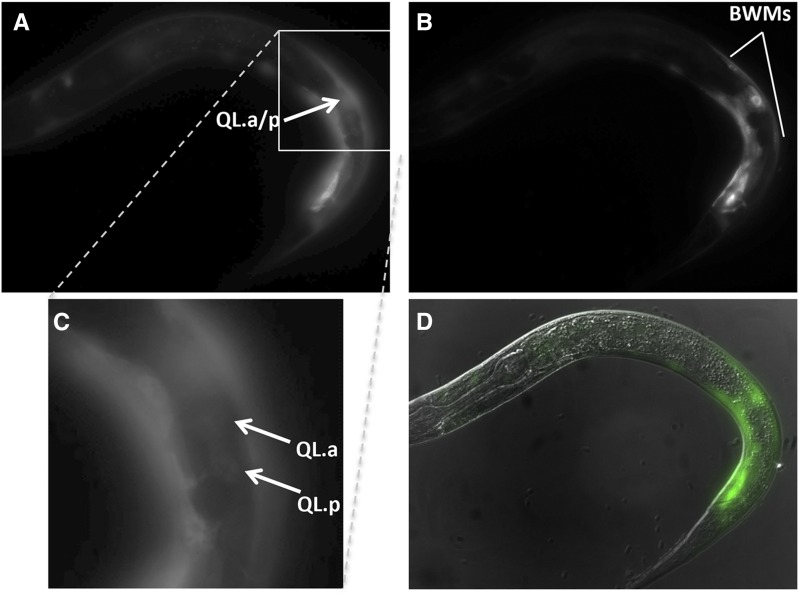
Pspon-1::gfp expression. Micrographs of an L1 larva at 4–4.5 hr posthatching are shown. (A) Pspon-1::gfp expression in posterior BWMs. (B) Qx.a/p visualized using rdvIs1[Pegl-17::mCherry]. (C) Merged Pspon-1::gfp, Pegl-17::mCherry, and DIC images. The animal is coiled such that the anterior (A) is near the posterior (P). Dorsal (D) and ventral (V) are indicated. Bar, 10 μm.
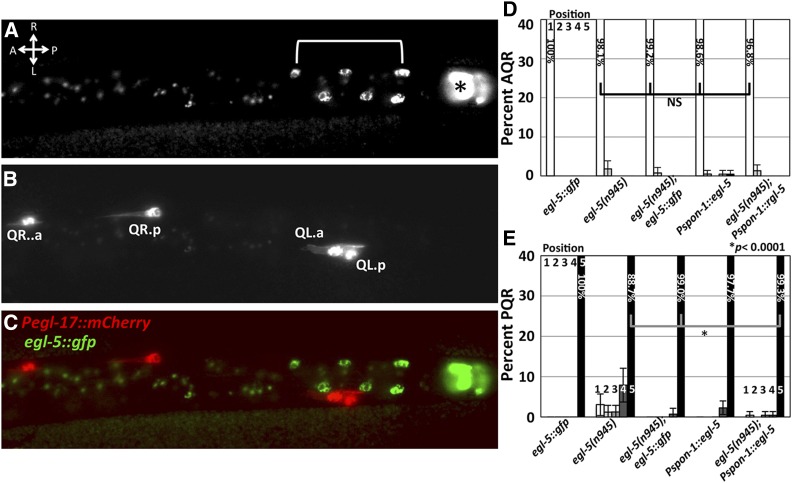
egl-5 is expressed in posterior cells, but not in the Q cells (Ferreira et al. 1999). We examined expression of the full-length egl-5::gfp(wgIs54) transgene from the modENCODE project (Niu et al. 2011). This transgene rescued PQR migration defects of egl-5(n945) (Figure 4), but showed no detectable expression in the Q cells. egl-5::gfp was expressed in other posterior cells, consistent with previously described expression (Figure 4) (Ferreira et al. 1999). Similar to above, we tested the nonautonomous role of EGL-5 by expressing it from the spon-1 promoter. Pspon-1::egl-5::cfp weakly effected PQR migration on its own and rescued the stronger PQR migration defects of egl-5 (Figure 4) but did not rescue the coiler phenotype of egl-5 (data not shown). These data are consistent with a nonautonomous role of EGL-5 in Q descendant migration, likely in posterior BWM.
Figure 4.
Full-length egl-5::gfp expression. Fluorescent micrographs of posterior region of an L1 larva at 5–5.5 hr posthatching, dorsal view. Anterior is left. Bar, 5 μm. (A) egl-5::gfp (wgIs54). The asterisk indicates rectal epithelial expression of egl-5::gfp, and brackets indicate nuclei of posterior BWMs or P cells. The punctate fluorescence anterior to the bracketed nuclei is background autofluorescence of the gut. (B) Pegl-17::mCherry. (C) Merged image. (D and E) egl-5::gfp and Pspon-1::egl-5 transgenic rescue of egl-5(n945) as described for Figure 2. Combined results of two independent Pspon-1::egl-5 arrays that showed similar effects are shown.
SPON-1 is required for proper Q descendant migration
Potential MAB-5 transcriptional targets in Q descendant migration were identified previously by whole-animal RNA-seq on wild-type and mab-5 mutants coupled with functional suppression of mab-5(e1751) gof (Tamayo et al. 2013). spon-1 transcripts were over-represented in mab-5(gof), and reduction of spon-1 function partially suppressed posterior AQR migration in mab-5(gof) (Tamayo et al. 2013). These results indicate that spon-1 expression is regulated by MAB-5 and that SPON-1 is required for posterior AQR migration in mab-5(gof). spon-1 encodes a molecule similar to vertebrate F-spondin, a secreted basement membrane molecule, and is required for proper muscle cell attachment and neural development (Woo et al. 2008) (Figure 5A).
Figure 5.
AQR and PQR migration defects in spon-1 mutants. (A) Diagram of the predicted 819-residue SPON-1 molecule with Reeler, Spondin, Thrombospondin (TSR), and Kunitz serine protease inhibitor (KUNITZ) domains shown. The positions of mutations are indicated. (B) A spon-1(e2623) young adult animal with defects in AQR and PQR migration (merged cfp and DIC micrographs). Bar, 20 μm. (C and D) AQR and PQR migration defects in spon-1 as described in Figure 2. Error bars represent 2× standard error of the proportion. (E–E′′) spon-1(ju402)M+ arrested L1 animals with Pgcy-32::cfp. (E) PQR reversal in migration direction. (E′) Complete AQR and PQR migration. (E′′) AQR directional defect. (F and G) Percent of AQR (F), and PQR (G) that show defects in spon-1(ju402)M+-arrested L1 animals.
spon-1 null mutation results in embryonic lethality due to muscle cell detachment (Woo et al. 2008). We first used the viable hypomorphic alleles e2623 and ju430ts and the embryonic lethal putative null ju402 to analyze AQR and PQR migration (Woo et al. 2008) (Figure 5A). Both hypomorphic mutants displayed AQR and PQR migration defects (Figure 5, B–D). spon-1(ju402) animals with wild-type maternal contribution (M+) displayed the paralyzed, arrested at twofold-stage-of-elongation characteristic of BWM defects. Despite elongation arrest at the twofold stage, many embryos still hatched and displayed AQR and PQR migration (Figure 5E). Arrested ju402 L1 larvae showed AQR and PQR migration defects (45 and 60%, respectively), with directional migration defects observed for both (Figure 5, E–G). Combined with the effects in weaker hypomorphic spon-1 mutants, these results show that SPON-1 function is required for the ability to migrate, as well as direction of migration in the A/P axis.
SPON-1 can act in BWMs to control Q descendant migration
spon-1(+) expression driven in all BWMs using the myo-3 promoter rescued AQR and PQR defects of hypomorphic e2623 and ju430 mutants (Figure 6). It also rescued the lethality and AQR and PQR defects of the null ju402 mutant (Figure 6). This suggests that SPON-1 can function in BWMs to control AQR and PQR migration. The myo-3 promoter does not show the posterior BWM expression bias observed with the spon-1 promoter (Figure 3), yet Pmyo-3::spon-1 efficiently rescued directional AQR and PQR defects in spon-1(ju402). This suggests that spon-1 might play a permissive rather than instructive role in migration, but an instructive role cannot be excluded.
Figure 6.
Muscle-derived SPON-1 rescues AQR and PQR migration. (A and B) Quantification of AQR (A) and PQR (B) positions as in Figure 2. Pmyo-3::spon-1 represents the transgene expressing SPON-1 in BWM cells. Asterisk indicates significant (n > 100, P < 0.05 Fisher’s exact test) difference from corresponding spon-1 mutant (except for ju402, which is lethal). Data for transgenic arrays are the combined results from two independently derived arrays with similar effects.
In a wild-type background, expression of SPON-1 in BWMs by Pmyo-3::spon-1 caused weak but significant defects (Table 1), suggesting that ectopic SPON-1 expression might perturb cell migration. While Pspon-1::gfp expression is not normally observed in the Q lineages (Figure 3), expression of spon-1 in the Q neuroblasts using the Q cell-specific Pegl-17 promoter (Branda and Stern 2000; Cordes et al. 2006) caused defects in AQR (3%) and PQR (19%) migration in a wild-type background (Figure 1 and Table 1). We used this transgenic construct to test which parts of the SPON-1 molecule can perturb AQR and PQR migration. Vertebrate F-spondin is cleaved, forming multiple fragments (Zisman et al. 2007). The fragment containing thrombospondin repeats (TSR)1–4 binds to a lipoprotein receptor-related protein (LRP), which repels axons, while the TSR5–6 fragment and the Reeler fragment both serve as attractants to developing axons. Weak and variable defects were observed with both fragments (Table 1), suggesting that both the TSR repeats and the Reeler/Spondin domain might participate in perturbing AQR and PQR migration. The C. elegans LRP molecule LRP-1 was not required for the effects of full-length spon-1 expression, as lrp-1(ku156) had no affect on AQR/PQR migration and did not modify the Pegl-17::spon-1 phenotype (data not shown).
Table 1. mab-5 and spon-1 ectopic expression.
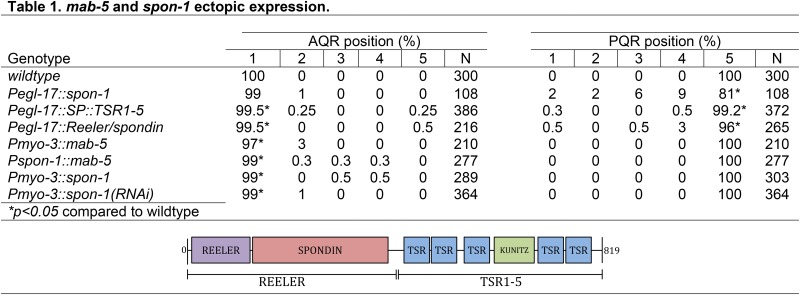 |
Animals were scored with Pgcy-32::cfp marking AQR and PQR. All genotypes with transgenes except Pmyo-3::mab-5 and wild-type were scored as combined results of two or more independently-derived extrachromosomal arrays with similar effects. Pmyo-3::mab-5 is an integrated line. “Reeler” constructs contain the first 430 amino acids of SPON-1, while “SP::TSR-5” constructs contain the endogenous signal peptide (29 residues) followed by residues 431–819.
MAB-5 promotes spon-1 expression in BWMs
spon-1 transcripts were over-represented in the transcriptome of mab-5 gain-of-function animals, indicating that MAB-5 stimulates spon-1 expression (Tamayo et al. 2013). Previous reports indicated that Pspon-1::gfp was expressed in BWM cells (Woo et al. 2008). We analyzed Pspon-1::gfp expression at the time when the Q descendants are beginning their migrations in early L1 larvae 4–4.5 hr posthatching. Expression was observed in posterior BWM cells (Figure 3), but not in the Q cells as determined by the Q cell-specific marker rdvIs1 (Pegl-17::mCherry) (Branda and Stern 2000; Ou et al. 2010) (Figure 3). Pspon-1::gfp expression was in BWM cells adjacent to the Q neuroblasts, with expression extending posteriorly to the tail, but only a short distance anteriorly (Figure 3).
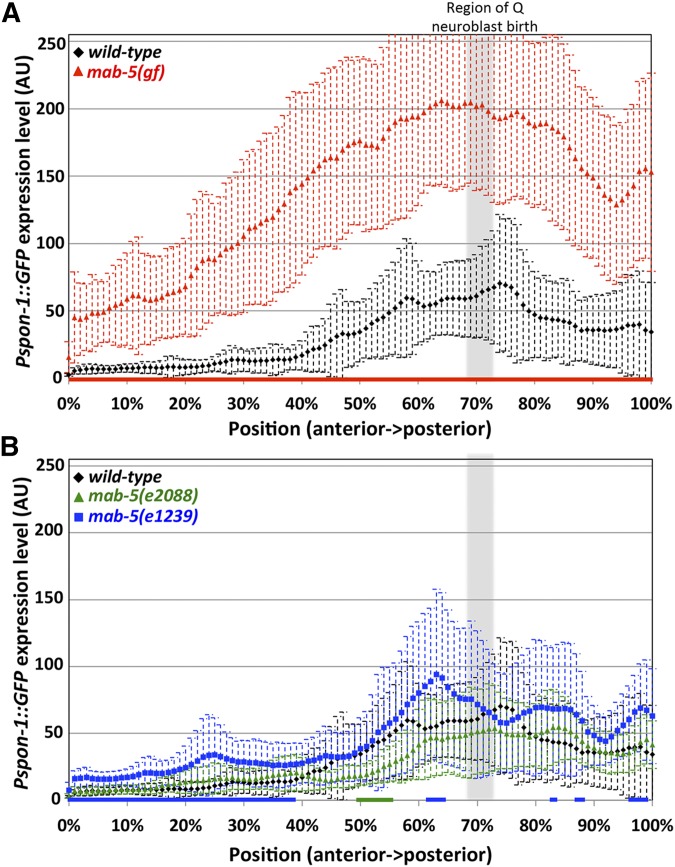
The gain-of-function mab-5(e1751) allele causes ectopic expression of mab-5 in several tissues, including the QR lineage (Salser and Kenyon 1992; Salser et al. 1993). We found an increase in expression of the Pspon-1::gfp transgene in mab-5(gof) (Figure 7). While no expression was observed outside of BWMs, the extent of Pspon-1::gfp expression was increased anteriorly, with robust expression frequently present in anterior BWMs, sometimes reaching into the head (Figure 7, C and D). We quantified pixel intensity along line scans through the BWMs from anterior to posterior to quantify GFP intensity along the A/P axis (Figure 7 and Figure 8) (see Materials and Methods). Wild-type animals had little detectable expression along the first anterior 20%, after which GFP intensity rose steadily with the region of highest GFP intensity also correlated to the location of Q birth (Figure 3 and Figure 8A). mab-5(gof) had a significant increase in Pspon-1::gfp expression along the entire animal but still maintained the highest intensity around the Q cell birthplace (Figure 7, C and D and Figure 8A). These results indicate that mab-5(gof) increased Pspon-1::gfp in BWMs. This result is consistent with the RNA-seq results showing spon-1 over-representation in mab-5(e1751)gof animals (Tamayo et al. 2013). A whole-organism RNA-seq strategy was used in this study, so the sum of expression in all cells of the animal, including BWMs, was assayed. mab-5 is expressed in QL and descendants as well as in other cells in the posterior, including posterior BWMs (Salser et al. 1993). We confirmed posterior BWM expression of the full-length mab-5::gfp transgene muIs16 in early L1 animals at the time when Q descendants begin migration (Hunter et al. 1999) (Figure 9).
Figure 7.
Pspon-1::gfp expression in mab-5 gain of function. Fluorescent micrographs of Pspon-1::gfp-expressing L1 larvae 4–4.5 hr posthatching. Fluorescent Pspon-1::gfp (A, C, and E) and merged DIC (B, D, and F) micrographs. Dashed lines indicate regions of BWM used in line scans for the analysis in Figure 6 (An, anterior near the posterior pharyngeal bulb; P, posterior near the anus) (see Materials and Methods). Bar, 10 μm.
Figure 8.
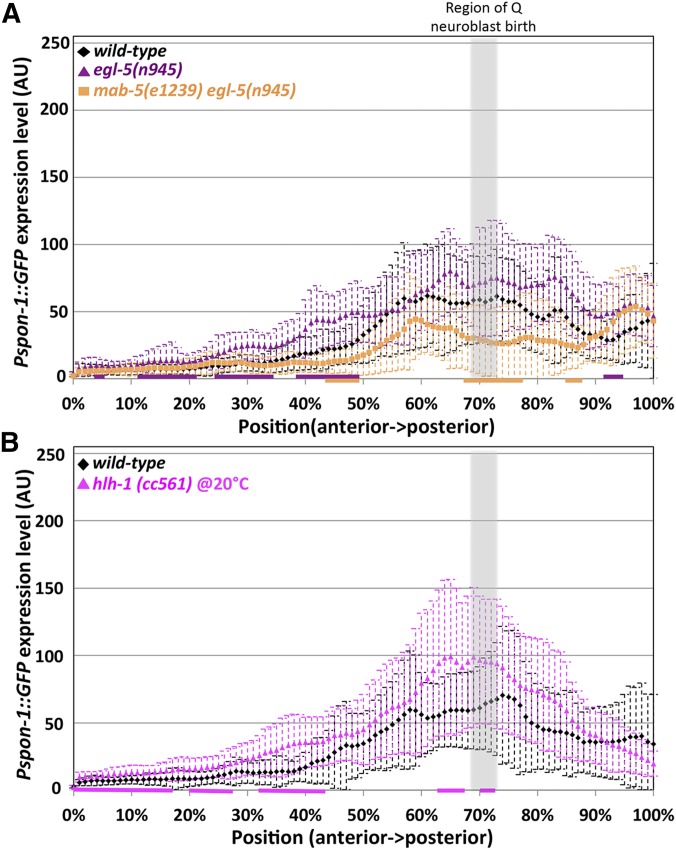
Quantification of Pspon-1::gfp intensity in BWMs. Graphs represent intensities of Pspon-1::gfp expression determined by line scans through BWM quadrants in L1 animals 4–4.5 hr posthatching (see Materials and Methods). The y-axis represents the intensity of GFP in arbitrary units (AU). The x-axis corresponds to position on the animal (0% is the posterior pharyngeal bulb, 100% is at the anus). The approximate region of Q neuroblast birth is shaded gray. Dashed error bars represent one standard deviation. Colored bars along the x-axis correspond to regions of significant difference compared to wild-type (Q < 0.05, multiple comparison corrected Student’s t-test, n = 40 BWM quadrants) (two per animal, dorsal and ventral combined). (A) mab-5(e1751) gain of function. (B) mab-5 putative null mutants e2088 and e1239.
Figure 9.
Expression of mab-5::gfp (muIs16) in wild-type animals. Fluorescent micrographs of muIs16 animals 4–4.5 hr posthatching with left (A and C) and right (B) side of animal in focus. C is an enlargement of section in A to show faint expression in QLa/p. (D) Merge of A and B and DIC. Bars, 5 μm.
We drove mab-5 expression in all BWMs using the myo-3 promoter. Pmyo-3::mab-5 animals were grossly misshapen and could not be reliably quantified with line scans. However, in early L1 larvae, Pmyo-3::mab-5 caused uniform expression of Pspon-1::gfp in all BWM cells from head to tail (Figure 7, E and F). These results show that in the BWMs, MAB-5 can activate Pspon-1::gfp expression. Together with the previous RNA-seq results (Tamayo et al. 2013), these data suggest that MAB-5 drives endogenous spon-1 expression in posterior BWMs adjacent to the Q cells.
MAB-5 is not required for spon-1 expression in BWMs
Consistent with the previous RNA-seq study that showed no effect on spon-1 transcript levels in mab-5(lof), two lof alleles, mab-5(e1239) and mab-5(e2088), had generally the same Pspon-1::gfp expression pattern as wild-type (Figure 8B) (Tamayo et al. 2013). Thus, while MAB-5 was sufficient to drive spon-1 expression in BWMs, it was not required for spon-1 expression.
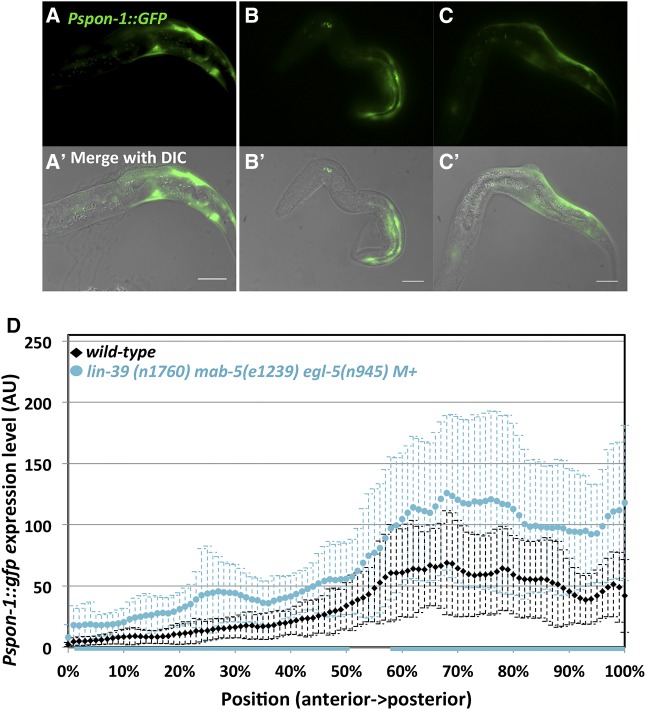
Because spon-1 expression persisted in mab-5(lof) animals, we speculated that other Hox genes egl-5 and lin-39 might act in parallel with mab-5 to activate spon-1 expression, especially given the parallel roles in AQR and PQR migration noted here (Figure 2). We tested egl-5 single mutants, mab-5egl-5 double mutants, and a triple hox lin-39mab-5egl-5 mutant on Pspon-1::gfp expression. None had any striking differences from wild-type animals (Figure 10A and Figure 11). However, despite triple Hox mutants’ grossly misshapen bodies, they maintained a slightly increased expression pattern (Figure 11). An hlh-1 binding site predicted by chromatin immunoprecipitation sequencing (ChIP-seq) is upstream of spon-1 (Lei et al. 2010; Niu et al. 2011). hlh-1 is the C. elegans myoD homolog and is required for BWM formation (Chen et al. 1992, 1994). We tested the hypomorphic hlh-1(cc561) (Harfe et al. 1998) allele on Pspon-1::gfp expression and found no significant difference from wild-type (Figure 10B). Taken together, this suggests other factors might cooperate with MAB-5 to promote spon-1 expression.
Figure 10.
egl-5 and hlh-1 are not required for body wall expression of Pspon-1::gfp. Graphs represent intensities of Pspon-1::gfp expression determined by line scans through BWM quadrants in L1 animals 4–4.5 hr posthatching (see Materials and Methods). The y-axis represents the intensity of GFP in arbitrary units (AU). The x-axis corresponds to position on the animal (0% is the posterior pharyngeal bulb, 100% is at the anus). The approximate region of Q neuroblast birth is shaded gray. Dashed error bars represent one standard deviation. Colored bars along the x-axis correspond to regions of significant difference compared to wild type (Q < 0.05, multiple comparison corrected Student’s t-test, n = 40 BWM quadrants) (two per animal, dorsal and ventral combined). (A) Wild-type, egl-5, and mab-5 egl-5 double mutant. (B) Wild-type and hlh-1(cc561) hypomorphic allele.
Figure 11.
Pspon-1::gfp in lin-39 mab-5 egl-5 triple mutant. Fluorescent micrographs (A–C) and merged DIC micrographs (A′, B′, and C′) of three lin-39(n1760) mab-5(e1239) egl-5(n945) M+ animals with Pspon-1::gfp. Animals are 4–4.5 hr posthatching. Bar, 10 μM. Anterior is to the left, and dorsal is up. (D) Graphs representing intensity of Pspon-1:gfp expression in lin-39 mab-5 egl-5 M+ as in Figure 8.
In double mutants of the spon-1(e2623) hypomorphic allele and mab-5(null) alleles e2088 and e1239, AQR migration defects generally resembled spon-1 alone (Table 2). Doubles with hypomorphic mab-5 alleles mu114 and bx54 displayed significantly more AQR migration failure (Table 2). Misdirected PQR anterior migration also failed in double mutants, with hypomorphic mab-5 alleles having the stronger effect (Table 2). The lack of strong genetic interaction between mab-5 and spon-1 loss-of-function mutations is consistent with our finding that mab-5 is not required for spon-1 expression. While we do not understand the nature of the genetic interactions with the mab-5 hypomorphs, the results suggest that residual mab-5 activity in spon-1(e2623) antagonizes anterior AQR and PQR migration.
Table 2. mab-5; spon-1 double mutant analysis.
| AQR position (%) | PQR position (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | 1 | 2 | 3 | 4 | 5 | N | 1 | 2 | 3 | 4 | 5 | N |
| spon-1(e2623) | 98 | 1 | 1 | 0 | 0 | 158 | 0 | 1 | 0 | 1 | 98 | 159 |
| mab-5(e2088) | 100 | 0 | 0 | 0 | 0 | 249 | 96 | 2 | 1 | 0 | 1 | 249 |
| mab-5(e2088); spon-1(e2623) | 100 | 0 | 0 | 0 | 0 | 146 | 87 | 9 | 2 | 2 | 0 | 249 |
| mab-5(e1239) | 99 | 1 | 0 | 0 | 0 | 283 | 99 | 1 | 0 | 0 | 0 | 283 |
| mab-5(e1239); spon-1(e2623) | 97 | 2 | 0 | 0 | 1 | 316 | 92 | 3 | 3 | 1 | 1 | 316 |
| mab-5(bx54) | 100 | 0 | 0 | 0 | 0 | 161 | 86 | 11 | 2 | 1 | 0 | 161 |
| mab-5(bx54); spon-1(e2623) | 88* | 7 | 5 | 1 | 0 | 176 | 80 | 13 | 5 | 1 | 2 | 176 |
| mab-5(mu114) | 100 | 0 | 0 | 0 | 0 | 202 | 83 | 11 | 4 | 0 | 1 | 202 |
| mab-5(mu114); spon-1(e2623) | 89* | 9 | 0 | 0 | 0 | 226 | 72 | 20 | 7 | 0 | 0 | 225 |
P < 0.05 compared to corresponding additive effect (not tested for PQR).
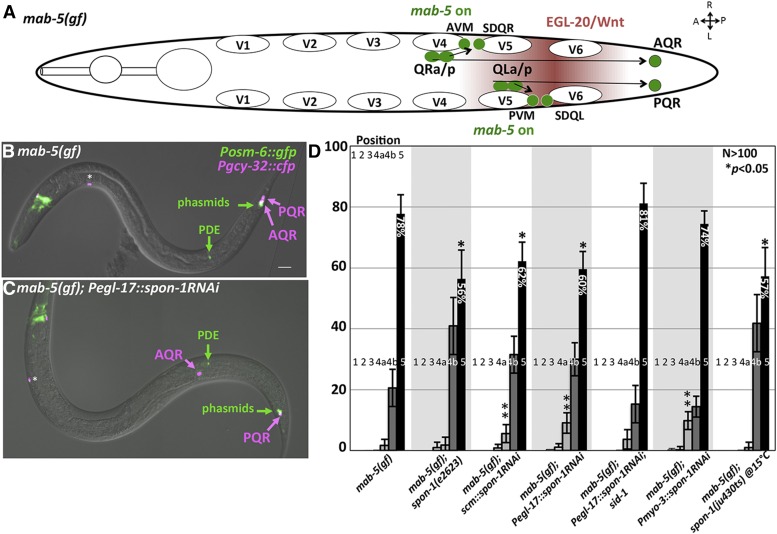
SPON-1 suppresses mab-5 gain of function
The gain-of-function mab-5(e1751) allele causes posterior migration of both AQR and PQR (Figure 12, A and B) (Chapman et al. 2008; Tamayo et al. 2013). Previously, SPON-1 was shown to be required for the full effect of mab-5(gof), as spon-1 knock-down with feeding RNAi partially suppressed posterior AQR migration in mab-5(gof) (Tamayo et al. 2013). The spon-1e2623 and ju430ts mutations also significantly suppressed posterior AQR migration to a similar extent in mab-5 gain of function (Figure 12D): mab-5(e1751) displayed 78% of AQR neurons migrating posteriorly to the anus to the normal position of PQR, whereas mab-5(e1751); spon-1(e2623) displayed 56%, and mab-5(e1751); spon-1(ju430ts) at 15° had 57% of PQR posterior to the anus (P < 0.05) (Figure 12D).
Figure 12.
Suppression of mab-5(gof) by spon-1. (A) Diagram of Q descendant migration in mab-5(e1751) gain-of-function mutants as described in Figure 1A. mab-5 is ectopically expressed in QR lineages, causing its descendants including AQR to migrate posteriorly. (B and C) Merged DIC and fluorescent micrographs of mab-5(gof) animals. Posm-6::gfp marks the PDE neuron, which serves as a landmark for Q birth position. The asterisk indicates an unidentified cell body present in mab-5(gof), but not wild type, that expresses Pgcy-32::cfp. (D) Quantification of AQR position in mab-5(e1751)gof mutants alone and in double mutant combination (see Figure 2). Asterisks indicate a significant (P < 0.05, Fisher’s exact test) reduction in the percentage of AQR residing in position 5 compared to mab-5(gof). Double asterisk refers to genotypes with a significant increase in anterior migration (position 4a or more anterior) compared to mab-5(gof). The locations 4a and 4b refer to location within position 4, with 4a anterior to PDE, and 4b posterior to PDE. Error bars represent 2× standard error of the proportion. Pscm and Pegl-17 spon-1 RNAi genotypes represent a combination of two independently derived transgenic lines with similar effects, and the Pmyo-3 line represents combined results of three independent transgenic lines with similar effects.
We used transgenic RNAi of spon-1 driven from the myo-3, egl-17, and scm promoters to knock down spon-1 (Esposito et al. 2007; Sundararajan and Lundquist 2012). Alone, Pmyo-3:: spon-1(RNAi) weakly affected AQR (1% defective) migration (Table 1). Because SPON-1 synthesis occurs in BWM and is required for embryogenesis, viable Pmyo-3::spon-1(RNAi) transgenes likely cause weak disruption of BWM-derived SPON-1 (i.e., too weak to cause lethality). However, each RNAi construct suppressed mab-5(gof) (Figure 12, C and D). mab-5(e1751) rarely displayed AQR that were positioned anterior to the PDE neurons, the place of Q cell birth. In mab-5(e1751); Pegl-17::spon-1(RNAi) animals, AQR neurons were observed anterior to the PDE neuron (Figure 12C), illustrating suppression of mab-5(e1751)gof. In C. elegans, RNAi can spread from one cell to another via the double-stranded RNA channel SID-1, which has been used as a tool for cell-specific RNAi (Winston et al. 2002; Calixto et al. 2010). Suppression caused by the Q cell-specific Pegl-17::spon-1(RNAi) transgene was abolished by the sid-1 mutation (Figure 12D). This suggests that suppression was due to RNAi spreading from the Q cells (likely to BWM), and that restriction of RNAi to the Q cells did not perturb spon-1 function. In sum, these data indicate that SPON-1, likely from the BWMs, is partially required for posterior AQR migration observed in mab-5(gof).
Discussion
Two main themes emerge from this work. First, our data indicate that the Hox factors MAB-5, EGL-5, and possibly LIN-39 have nonautonomous parallel roles in Q descendant migrations. For MAB-5, this role is in contrast to the well-described autonomous function in QL. The nonautonomous roles of LIN-39 and MAB-5 are not apparent in single mutants due to redundancy, although lin-39 mutants show some PQR migration defects. Second, this work shows that the secreted basement membrane molecule SPON-1, similar to vertebrate F-spondin, might be a transcriptional target of MAB-5 in the BWM cells that nonautonomously influences Q cell migrations.
A nonautonomous role of MAB-5 in Q descendant migration
Here we report a previously undescribed role of the Hox gene mab-5 in the migration of the QR and QL descendants AQR and PQR. Previous studies showed that MAB-5 autonomously regulates posterior migration of QL descendants (Salser and Kenyon 1992) and that LIN-39 is autonomously required for anterior migration of QR descendants (Harris et al. 1996; Wang et al. 2013). We found that lin-39mab-5 double mutants displayed enhanced AQR anterior migration defects compared to lin-39, suggesting that mab-5 and lin-39 act in parallel pathways for anterior AQR migration. Furthermore, lin-39 mutants alone displayed posterior PQR migration defects. MAB-5 expression is not observed in the QR/AQR lineage (Salser et al. 1993), and when expressed in this lineage, drives posterior migration. Additionally, lin-39 is transiently expressed in QL/PQR lineage but is inhibited by MAB-5 expression in this lineage when QL descendant migration occurs (Wang et al. 2013). These data suggest that LIN-39 and MAB-5 might have roles outside of the Q cells to regulate anterior AQR and posterior PQR migration. lin-39 and mab-5 are expressed in other posterior cells, including mab-5 in posterior and midbody BWM (Salser et al. 1993; Clandinin et al. 1997; Maloof et al. 1999; Yang et al. 2005; Wagmaister et al. 2006a,b). Expression of mab-5 in posterior BWMs rescued AQR migration defects in the lin-39mab-5 double mutant to resemble lin-39 alone. It also rescued anterior migration defects of PQR in the lin-39mab-5 double. Of note, BWM expression of mab-5 did not rescue the directional defects of PQR in the lin-39mab-5 double mutant, as all PQR still migrated anteriorly. Posterior migration of PQR is a cell-autonomous role of mab-5, and BWM expression of mab-5 would not be expected to rescue directional defects. Together, these data point to a nonautonomous role of MAB-5 in anterior AQR and PQR migration. The posterior PQR defects of lin-39 could be due to a nonautonomous role or could be due to transient lin-39 expression in the QL lineage (which is known to occur in mab-5 mutants). This nonautonomous role for MAB-5 on cell migration is in contrast to much of the work done with Hox genes that primarily has focused on cell-autonomous roles of these genes, but there is precedence for Hox genes in vertebrates noncell autonomously controlling axon guidance (Gavalas et al. 1997).
The lin-39 mab-5 egl-5 triple mutant shows little or no Q descendant migration
egl-5 mutants displayed PQR migration defects and weak AQR migration defects, consistent with previous reports of Q lineage defects in egl-5 (Chisholm 1991). Functional full-length egl-5::gfp expression was not observed in the Q lineages, and expressing EGL-5 specifically in posterior BWMs rescued egl-5 defects. This suggests that egl-5 might also nonautonomously regulate PQR migration, although expression in the Q lineages cannot be excluded as a possibility. The posterior PQR migration defects might be stronger in egl-5 mutants, because the QL descendants migrate posteriorly through the region that expresses egl-5.
egl-5 did not enhance mab-5 or lin-39 defects, but did enhance AQR defects in the lin-39mab-5egl-5 triple, which showed minimal migration of AQR and PQR away from the Q cell birthplace. This suggests that these three Hox genes act together to promote migration of the Q descendants, and in their absence, little or no anterior or posterior migration away from the Q cell birthplace occurs. In light of evidence presented here of a nonautonomous role of MAB-5, the nearly complete lack of AQR and PQR migration in the lin-39egl-5mab-5 triple is likely due to failure of both autonomous and nonautonomous roles of these molecules in AQR and PQR migration.
SPON-1/F-spondin controls Q descendant migration
Previously, RNA-seq identified spon-1/F-spondin as being positively regulated by MAB-5 in Q descendant migration (Tamayo et al. 2013). We found that mutations in spon-1 caused AQR and PQR incomplete migration and directional defects. spon-1 mutants partially suppressed mab-5(gof), consistent with previous results using RNAi against spon-1 (Tamayo et al. 2013). Restriction of spon-1 RNAi to the Q lineages was insufficient for suppression, whereas RNAi in surrounding tissues and/or BWM resulted in suppression. This result suggests that spon-1 acts nonautonomously in suppression of mab-5(gof). This is consistent with Pspon-1::gfp expression, which we observed in posterior BWMs adjacent to the Q cells but not in the Q cells themselves.
spon-1 was expressed in posterior BWMs adjacent to the Q neuroblasts, and spon-1 mutants displayed directional AQR and PQR migration defects. These results suggest that SPON-1 might provide directional guidance information for Q descendant migration. However, expression of SPON-1 from all BWM cells, from anterior to posterior, efficiently rescued AQR and PQR defects, a result not expected of a cue providing directional information. Therefore, SPON-1 might generally promote the ability of cells to migrate. The localized expression of spon-1 adjacent to the Q cells in early L1 might represent a need for high levels of SPON-1 or newly synthesized SPON-1 to generally stimulate cell migration at that time. While Pmyo-3::spon-1 does not provide localized expression, it might provide high levels of expression throughout larval development, when it is needed for cell migration. However, this does not explain the directional migration defects in spon-1 mutants. Pmyo-3::spon-1 expression caused weak AQR and PQR defects alone, consistent with a potential role in directed guidance. However, the preponderance of evidence suggests a permissive role of SPON-1 in cell migration, which differs from F-spondin in vertebrates, where it acts as a repellent to migrating neural crest cells (Debby-Brafman et al. 1999). However, there are also cases where F-spondin serves as an attractant and permissive signal to developing axons (Burstyn-Cohen et al. 1998, 1999; Zisman et al. 2007).
Increased levels of MAB-5 stimulates Pspon-1::gfp expression in BWM
We have shown that a Pspon-1::gfp transcriptional reporter was expressed at higher levels and more broadly in mab-5(gof), consistent with previous RNA-seq showing that endogenous spon-1 transcripts are over-represented in mab-5(gof) animals (Tamayo et al. 2013). Expression of mab-5 in all BWMs resulted in robust Pspon-1::gfp expression in all BWMs, even those in the anterior. These experiments indicate that increased MAB-5 activity in the BWMs drives Pspon-1:gfp expression. A ChIP-seq study using MAB-5 (Niu et al. 2011) did not identify the spon-1 locus as a potential MAB-5 target. Thus, MAB-5 might indirectly regulate spon-1 expression in BWM. However, this ChIP-seq study was done with L3 larvae, long after Q migration, so any transient interaction at the spon-1 promoter in L1 would have been missed. Because we only see increased Pspon-1::gfp levels in animals with overexpression of mab-5, it is possible that the increase in Pspon-1::gfp is due to aberrant binding to the spon-1 promoter or other enhancers that may not occur in wild-type animals.
Complete loss of SPON-1 function causes embryonic lethality, and mab-5 mutants are not lethal, suggesting that MAB-5 is not the only factor that regulates spon-1 expression. Indeed, mab-5(lof) mutants did not affect Pspon-1:gfp, consistent with RNA-seq showing no effect of mab-5(lof) on spon-1 transcript accumulation (Tamayo et al. 2013). The lin-39mab-5egl-5 triple mutant also was able to express Pspon-1::gfp readily and may have increased spon-1 expression. This indicates that neither LIN-39 nor EGL-5 cooperate with MAB-5 in Pspon-1::gfp expression. The spon-1 locus contains a predicted hlh-1 binding region (Niu et al. 2011), but hlh-1 also did not influence spon-1 expression. While MAB-5 is sufficient to drive spon-1 expression, other factors might be required redundantly with mab-5 in normal spon-1 expression. Furthermore, LIN-39 and EGL-5 might be required for the expression of factors that act in parallel to factors regulated by mab-5 (e.g., spon-1). This redundancy could be in the same cell or in distinct cells, each expressing factors that influence AQR and PQR migration.
In addition to the well-characterized cell-autonomous function of LIN-39 and MAB-5 in Q migration, we find possible roles outside of the Q lineage to promote migration, roles that have been masked by redundancy of function of these molecules. We show evidence for a novel nonautonomous role of the Hox gene mab-5 in Q migrations. lin-39, mab-5, and egl-5 have distinct expression patterns, yet appear to have overlapping functions in promoting Q lineage migrations. We speculate that LIN-39, MAB-5, and EGL-5 pattern the posterior region of the animal for use as a substrate for Q migrations (a nonautonomous role), and that LIN-39 and MAB-5 control the response of the Q descendants to that posterior pattern (an autonomous role). Our data indicate that secreted basement membrane molecule SPON-1/F-spondin might be a target of MAB-5 in BWM and is important for Q descendant migration. In the absence of lin-39, mab-5, and egl-5, very little Q descendant migration away from the Q cell birthplace occurs, suggesting multiple and parallel pathways are regulated by these Hox factors in Q migrations.
Acknowledgments
We thank Eric Struckhoff for technical assistance; Andrew Chisholm and the Caenorhabditis Genetics Center, funded by National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40-OD010440) for strains; and the Lundquist and Ackley labs for helpful discussions. A.M.M. was a University of Kansas Undergraduate Research Award recipient and M.P.J. was supported by the Madison and Lila Self Graduate Fellowship Program at the University of Kansas. This work was funded by NIH grants R01-NS040945 and R21-NS070417 to E.A.L. and the Kansas Infrastructure Network of Biomedical Research Excellence (NIH grant P20-GM103418).
Footnotes
Communicating editor: M. V. Sundaram
Literature Cited
- Arenkiel B. R., Tvrdik P., Gaufo G. O., Capecchi M. R., 2004. Hoxb1 functions in both motoneurons and in tissues of the periphery to establish and maintain the proper neuronal circuitry. Genes Dev. 18: 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda C. S., Stern M. J., 2000. Mechanisms controlling sex myoblast migration in Caenorhabditis elegans hermaphrodites. Dev. Biol. 226: 137–151. [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn-Cohen T., Frumkin A., Xu Y. T., Scherer S. S., Klar A., 1998. Accumulation of F-spondin in injured peripheral nerve promotes the outgrowth of sensory axons. J. Neurosci. 18: 8875–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn-Cohen T., Tzarfaty V., Frumkin A., Feinstein Y., Stoeckli E., et al. , 1999. F-Spondin is required for accurate pathfinding of commissural axons at the floor plate. Neuron 23: 233–246. [DOI] [PubMed] [Google Scholar]
- Calixto A., Chelur D., Topalidou I., Chen X., Chalfie M., 2010. Enhanced neuronal RNAi in C. elegans using SID-1. Nat. Methods 7: 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Thomson J. N., Sulston J. E., 1983. Induction of neuronal branching in Caenorhabditis elegans. Science 221: 61–63. [DOI] [PubMed] [Google Scholar]
- Chapman J. O., Li H., Lundquist E. A., 2008. The MIG-15 NIK kinase acts cell-autonomously in neuroblast polarization and migration in C. elegans. Dev. Biol. 324: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Krause M., Draper B., Weintraub H., Fire A., 1992. Body-wall muscle formation in Caenorhabditis elegans embryos that lack the MyoD homolog hlh-1. Science 256: 240–243. [DOI] [PubMed] [Google Scholar]
- Chen L., Krause M., Sepanski M., Fire A., 1994. The Caenorhabditis elegans MYOD homologue HLH-1 is essential for proper muscle function and complete morphogenesis. Development 120: 1631–1641. [DOI] [PubMed] [Google Scholar]
- Chisholm A., 1991. Control of cell fate in the tail region of C. elegans by the gene egl-5. Development 111: 921–932. [DOI] [PubMed] [Google Scholar]
- Clandinin T. R., Katz W. S., Sternberg P. W., 1997. Caenorhabditis elegans HOM-C genes regulate the response of vulval precursor cells to inductive signal. Dev. Biol. 182: 150–161. [DOI] [PubMed] [Google Scholar]
- Clark S. G., Chisholm A. D., Horvitz H. R., 1993. Control of cell fates in the central body region of C. elegans by the homeobox gene lin-39. Cell 74: 43–55. [DOI] [PubMed] [Google Scholar]
- Cordes S., Frank C. A., Garriga G., 2006. The C. elegans MELK ortholog PIG-1 regulates cell size asymmetry and daughter cell fate in asymmetric neuroblast divisions. Development 133: 2747–2756. [DOI] [PubMed] [Google Scholar]
- Cowing D. W., Kenyon C., 1992. Expression of the homeotic gene mab-5 during Caenorhabditis elegans embryogenesis. Development 116: 481–490. [DOI] [PubMed] [Google Scholar]
- Debby-Brafman A., Burstyn-Cohen T., Klar A., Kalcheim C., 1999. F-Spondin, expressed in somite regions avoided by neural crest cells, mediates inhibition of distinct somite domains to neural crest migration. Neuron 22: 475–488. [DOI] [PubMed] [Google Scholar]
- Desai C., Horvitz H. R., 1989. Caenorhabditis elegans mutants defective in the functioning of the motor neurons responsible for egg laying. Genetics 121: 703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer J. O., Demarco R. S., Lundquist E. A., 2010. Distinct roles of Rac GTPases and the UNC-73/Trio and PIX-1 Rac GTP exchange factors in neuroblast protrusion and migration in C. elegans. Small GTPases 1: 44–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G., Di Schiavi E., Bergamasco C., Bazzicalupo P., 2007. Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene 395: 170–176. [DOI] [PubMed] [Google Scholar]
- Ferreira H. B., Zhang Y., Zhao C., Emmons S. W., 1999. Patterning of Caenorhabditis elegans posterior structures by the Abdominal-B homolog, egl-5. Dev. Biol. 207: 215–228. [DOI] [PubMed] [Google Scholar]
- Gavalas A., Davenne M., Lumsden A., Chambon P., Rijli F. M., 1997. Role of Hoxa-2 in axon pathfinding and rostral hindbrain patterning. Development 124: 3693–3702. [DOI] [PubMed] [Google Scholar]
- Hafez D. M., Huang J. Y., Richardson J. C., Masliah E., Peterson D. A., et al. , 2012. F-spondin gene transfer improves memory performance and reduces amyloid-beta levels in mice. Neuroscience 223: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe B. D., Branda C. S., Krause M., Stern M. J., Fire A., 1998. MyoD and the specification of muscle and non-muscle fates during postembryonic development of the C. elegans mesoderm. Development 125: 2479–2488. [DOI] [PubMed] [Google Scholar]
- Harris J., Honigberg L., Robinson N., Kenyon C., 1996. Neuronal cell migration in C. elegans: regulation of Hox gene expression and cell position. Development 122: 3117–3131. [DOI] [PubMed] [Google Scholar]
- Higashijima S., Nose A., Eguchi G., Hotta Y., Okamoto H., 1997. Mindin/F-spondin family: novel ECM proteins expressed in the zebrafish embryonic axis. Dev. Biol. 192: 211–227. [DOI] [PubMed] [Google Scholar]
- Ho A., Sudhof T. C., 2004. Binding of F-spondin to amyloid-beta precursor protein: a candidate amyloid-beta precursor protein ligand that modulates amyloid-beta precursor protein cleavage. Proc. Natl. Acad. Sci. USA 101: 2548–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe H. S., Wessner D., Beffert U., Becker A. G., Matsuoka Y., et al. , 2005. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol. Cell. Biol. 25: 9259–9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg L., Kenyon C., 2000. Establishment of left/right asymmetry in neuroblast migration by UNC-40/DCC, UNC-73/Trio and DPY-19 proteins in C. elegans. Development 127: 4655–4668. [DOI] [PubMed] [Google Scholar]
- Hu H., Xin N., Liu J., Liu M., Wang Z., et al. , 2016. Characterization of F-spondin in Japanese flounder (Paralichthys olivaceus) and its role in the nervous system development of teleosts. Gene 575: 623–631. [DOI] [PubMed] [Google Scholar]
- Hunter C. P., Harris J. M., Maloof J. N., Kenyon C., 1999. Hox gene expression in a single Caenorhabditis elegans cell is regulated by a caudal homolog and intercellular signals that inhibit wnt signaling. Development 126: 805–814. [DOI] [PubMed] [Google Scholar]
- Ji N., Middelkoop T. C., Mentink R. A., Betist M. C., Tonegawa S., et al. , 2013. Feedback control of gene expression variability in the Caenorhabditis elegans Wnt pathway. Cell 155: 869–880. [DOI] [PubMed] [Google Scholar]
- Josephson M. P., Chai Y., Ou G., Lundquist E. A., 2016. EGL-20/Wnt and MAB-5/Hox act sequentially to inhibit anterior migration of neuroblasts in C. elegans. PLoS One 11: e0148658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalis A. K., Kissiov D. U., Kolenbrander E. S., Palchick Z., Raghavan S., et al. , 2014. Patterning of sexually dimorphic neurogenesis in the caenorhabditis elegans ventral cord by Hox and TALE homeodomain transcription factors. Dev. Dyn. 243: 159–171. [DOI] [PubMed] [Google Scholar]
- Kenyon C., 1986. A gene involved in the development of the posterior body region of C. elegans. Cell 46: 477–487. [DOI] [PubMed] [Google Scholar]
- Klar A., Baldassare M., Jessell T. M., 1992. F-spondin: a gene expressed at high levels in the floor plate encodes a secreted protein that promotes neural cell adhesion and neurite extension. Cell 69: 95–110. [DOI] [PubMed] [Google Scholar]
- Korswagen H. C., Herman M. A., Clevers H. C., 2000. Distinct beta-catenins mediate adhesion and signalling functions in C. elegans. Nature 406: 527–532. [DOI] [PubMed] [Google Scholar]
- Lei H., Fukushige T., Niu W., Sarov M., Reinke V., et al. , 2010. A widespread distribution of genomic CeMyoD binding sites revealed and cross validated by ChIP-Chip and ChIP-Seq techniques. PLoS One 5: e15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kulkarni R. P., Hill R. J., Chamberlin H. M., 2009. HOM-C genes, Wnt signaling and axial patterning in the C. elegans posterior ventral epidermis. Dev. Biol. 332: 156–165. [DOI] [PubMed] [Google Scholar]
- Maloof J. N., Whangbo J., Harris J. M., Jongeward G. D., Kenyon C., 1999. A Wnt signaling pathway controls hox gene expression and neuroblast migration in C. elegans. Development 126: 37–49. [DOI] [PubMed] [Google Scholar]
- Mello C., Fire A., 1995. DNA transformation. Methods Cell Biol. 48: 451–482. [PubMed] [Google Scholar]
- Mentink R. A., Middelkoop T. C., Rella L., Ji N., Tang C. Y., et al. , 2014. Cell intrinsic modulation of Wnt signaling controls neuroblast migration in C. elegans. Dev. Cell 31: 188–201. [DOI] [PubMed] [Google Scholar]
- Middelkoop T. C., Korswagen H. C., 2014. Development and migration of the C. elegans Q neuroblasts and their descendants (October 15, 2014). WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.173.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W., Lu Z. J., Zhong M., Sarov M., Murray J. I., et al. , 2011. Diverse transcription factor binding features revealed by genome-wide ChIP-seq in C. elegans. Genome Res. 21: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou G., Stuurman N., D’Ambrosio M., Vale R. D., 2010. Polarized myosin produces unequal-size daughters during asymmetric cell division. Science 330: 677–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser S. J., Kenyon C., 1992. Activation of a C. elegans Antennapedia homologue in migrating cells controls their direction of migration. Nature 355: 255–258. [DOI] [PubMed] [Google Scholar]
- Salser S. J., Kenyon C., 1996. A C. elegans Hox gene switches on, off, on and off again to regulate proliferation, differentiation and morphogenesis. Development 122: 1651–1661. [DOI] [PubMed] [Google Scholar]
- Salser S. J., Loer C. M., Kenyon C., 1993. Multiple HOM-C gene interactions specify cell fates in the nematode central nervous system. Genes Dev. 7: 1714–1724. [DOI] [PubMed] [Google Scholar]
- Shen Z., Zhang X., Chai Y., Zhu Z., Yi P., et al. , 2014. Conditional knockouts generated by engineered CRISPR-Cas9 endonuclease reveal the roles of coronin in C. elegans neural development. Dev. Cell 30: 625–636. [DOI] [PubMed] [Google Scholar]
- Studer M., Lumsden A., Ariza-McNaughton L., Bradley A., Krumlauf R., 1996. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature 384: 630–634. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R., 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156. [DOI] [PubMed] [Google Scholar]
- Sundararajan L., Lundquist E. A., 2012. Transmembrane proteins UNC-40/DCC, PTP-3/LAR, and MIG-21 control anterior-posterior neuroblast migration with left-right functional asymmetry in Caenorhabditis elegans. Genetics 192: 1373–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan, L., M. L. Norris, and E. A. Lundquist, 2015 SDN-1/Syndecan acts in parallel to the transmembrane molecule MIG-13 to promote anterior neuroblast migration. G3 (Bethesda) 5: 1567–1574. [DOI] [PMC free article] [PubMed]
- Tamayo J. V., Gujar M., Macdonald S. J., Lundquist E. A., 2013. Functional transcriptomic analysis of the role of MAB-5/Hox in Q neuroblast migration in Caenorhabditis elegans. BMC Genomics 14: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzarfaty-Majar V., Lopez-Alemany R., Feinstein Y., Gombau L., Goldshmidt O., et al. , 2001. Plasmin-mediated release of the guidance molecule F-spondin from the extracellular matrix. J. Biol. Chem. 276: 28233–28241. [DOI] [PubMed] [Google Scholar]
- Van Auken K., Weaver D. C., Edgar L. G., Wood W. B., 2000. Caenorhabditis elegans embryonic axial patterning requires two recently discovered posterior-group Hox genes. Proc. Natl. Acad. Sci. USA 97: 4499–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagmaister J. A., Gleason J. E., Eisenmann D. M., 2006a Transcriptional upregulation of the C. elegans Hox gene lin-39 during vulval cell fate specification. Mech. Dev. 123: 135–150. [DOI] [PubMed] [Google Scholar]
- Wagmaister J. A., Miley G. R., Morris C. A., Gleason J. E., Miller L. M., et al. , 2006b Identification of cis-regulatory elements from the C. elegans Hox gene lin-39 required for embryonic expression and for regulation by the transcription factors LIN-1, LIN-31 and LIN-39. Dev. Biol. 297: 550–565. [DOI] [PubMed] [Google Scholar]
- Wang B. B., Muller-Immergluck M. M., Austin J., Robinson N. T., Chisholm A., et al. , 1993. A homeotic gene cluster patterns the anteroposterior body axis of C. elegans. Cell 74: 29–42. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhou F., Lv S., Yi P., Zhu Z., et al. , 2013. Transmembrane protein MIG-13 links the Wnt signaling and Hox genes to the cell polarity in neuronal migration. Proc. Natl. Acad. Sci. USA 110: 11175–11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whangbo J., Kenyon C., 1999. A Wnt signaling system that specifies two patterns of cell migration in C. elegans. Mol. Cell 4: 851–858. [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S., 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. 314: 1–340. [DOI] [PubMed] [Google Scholar]
- Winston W. M., Molodowitch C., Hunter C. P., 2002. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295: 2456–2459. [DOI] [PubMed] [Google Scholar]
- Woo W. M., Berry E. C., Hudson M. L., Swale R. E., Goncharov A., et al. , 2008. The C. elegans F-spondin family protein SPON-1 maintains cell adhesion in neural and non-neural tissues. Development 135: 2747–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Sym M., Kenyon C., 2005. The roles of two C. elegans HOX co-factor orthologs in cell migration and vulva development. Development 132: 1413–1428. [DOI] [PubMed] [Google Scholar]
- Zisman S., Marom K., Avraham O., Rinsky-Halivni L., Gai U., et al. , 2007. Proteolysis and membrane capture of F-spondin generates combinatorial guidance cues from a single molecule. J. Cell Biol. 178: 1237–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.