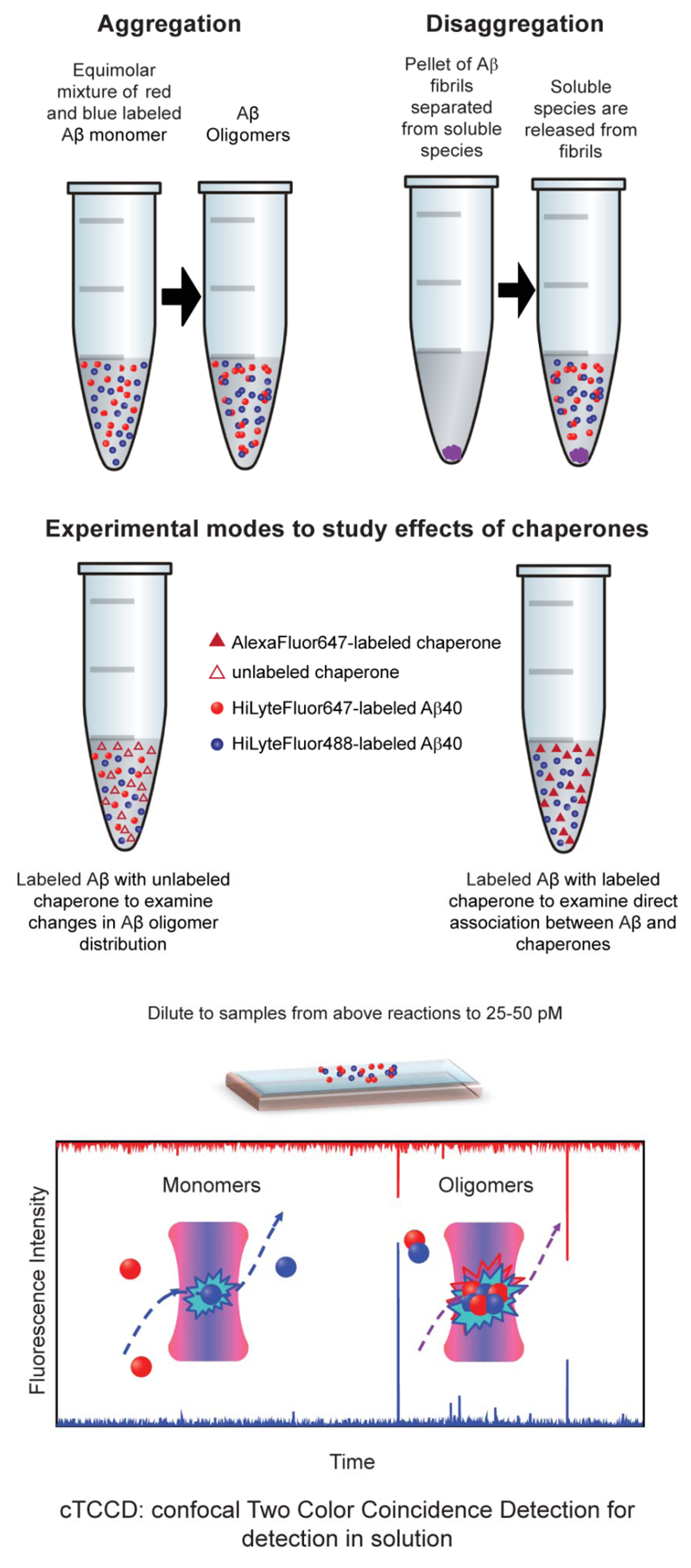
Abstract
The aberrant aggregation of the amyloid-β peptide into β-sheet rich, fibrillar structures proceeds via a heterogeneous ensemble of oligomeric intermediates that have been associated with neurotoxicity in Alzheimer’s disease (AD). Of particular interest in this context are the mechanisms by which molecular chaperones, part of the primary biological defenses against protein misfolding, influence Aβ aggregation. We have used single-molecule fluorescence techniques to compare the interactions between distinct aggregation states (monomers, oligomers, amyloid fibrils) of the AD-associated amyloid-β(1-40) peptide, and two molecular chaperones, both of which are upregulated in the brains of patients with AD and have been found colocalized with Aβ in senile plaques. One of the chaperones, αB-crystallin, is primarily found inside cells while the other, clusterin, is predominantly located in the extracellular environment. We find that both chaperones bind to misfolded oligomeric species and form long-lived complexes thereby preventing both their further growth into fibrils and their dissociation. From these studies, we conclude that these chaperones have a common mechanism of action based on sequestering Aβ oligomers. This conclusion suggests that these chaperones, both of which are ATP-independent, are able to inhibit potentially pathogenic Aβ oligomer-associated processes whether they occur in the extracellular or intracellular environment.
The aggregation of the amyloid-β peptide (Aβ), a fragment of the amyloid precursor protein (APP), is associated with the pathogenesis of Alzheimer’s disease (AD) (1). Although large fibrillar plaques comprised of fibrillar forms of Aβ have conventionally been viewed as a hallmark of AD, recent evidence has implicated oligomeric aggregates of Aβ generated during the process of fibril formation as a primary cause of AD-related neurotoxicity (2, 3). It is therefore vital in the context of understanding the origins of AD and the development of therapeutic strategies, to understand the properties of these oligomeric species and how they interact with the variety of cellular components. Oligomeric aggregates are by nature transient and heterogeneous in both size and structure, rendering them challenging to characterize using bulk techniques. We have chosen to develop a series of single-molecule fluorescence methods, which are capable of resolving such heterogeneity, to examine these oligomers.
The first of these methods used in the present study is confocal two-color coincidence detection (cTCCD), which has the capacity to detect and characterize oligomeric species formed during the aggregation of fluorescently labeled peptides and proteins (4–6). To monitor the aggregation of Aβ peptides with cTCCD, equal amounts of Aβ40 monomers labeled with a HiLyteFluor488-fluorescent tag (Anaspec) and Aβ40 monomers labeled with a HiLyteFluor647 fluorescent tag (Anaspec) are mixed. As the monomers aggregate into oligomeric assemblies, species containing two differently colored fluorophores are formed, and can be readily distinguished from monomers that contain are labeled with only a single fluorophore. When the sample is excited simultaneously with two wavelengths of light, the coincidence of fluorescence signals from the sample in the detection channels with time can be used to distinguish oligomers from monomers and the oligomeric population can be monitored as the aggregation reaction proceeds. Additionally, the size of the oligomeric species can be estimated using the fluorescence intensities of the time-coincident fluorescent bursts. We have already used this method to study the aggregation of several peptide and protein systems including Aβ40 under various aggregation conditions and in the presence or absence of other molecules (4–6). In this study we have also used a second single-molecule technique, total internal reflection microscopy (TIRFM), which allows imaging of the species on a surface to gain insight into their morphology as well as their oligomeric state.
In this work, we have used this single-molecule approach to monitor the size distribution of the oligomers formed during the aggregation and disaggregation of Aβ40 and to examine the interactions of Aβ40 monomers, oligomers and fibrils with molecular chaperones, a key component of the biological defense system against protein misfolding and aggregation in both intracellular and extracellular environments (Figure 1) (7). In a previous study, we examined the interactions between the ATP-independent, predominantly-extracellular chaperone, clusterin, and the Aβ40 peptide, stimulated by a recent discovery of genetic links between clusterin and AD and also because amyloid deposits containing Aβ are largely extracellular (5, 8, 9). In the present work, we extend this previous study to examine the effects of a second chaperone, αB-crystallin, which is also ATP-independent and functions similarly to clusterin. αB-crystallin is of considerable comparative interest as it is found predominantly in the intracellular rather than extracellular space (10). Interestingly, the expression levels of both chaperones are upregulated in the brains of those with AD, and both chaperones have been found colocalized with senile amyloid plaques (11–14). The question of the role of αB-crystallin in AD is also highly relevant in the context of increasing interest in the occurrence and toxicity of intracellular as well as extracellular aggregates of Aβ (15, 16). By combining two single-molecule approaches, cTCCD and TIRFM, we have been able to investigate the mechanisms of action of these two chaperones in vitro and relate these to their roles in a cellular context.
Figure 1. A schematic diagram of aggregation and disaggregation reactions examined by cTCCD.
This figure is adapted from reference (5).
Materials and Methods
Aβ preparation and aggregation assays
HiLyteFluor488 and HiLyteFluor647-labeled Aβ40 peptides were purchased from Anaspec (San Jose, USA). Monomeric starting solutions were prepared and aggregation reactions of all peptides were carried out as described previously (5).
Preparation and labeling of αB-crystallin
Human recombinant αB-crystallin was prepared as described previously (17).The AlexaFluor647 Protein Labeling Kit was purchased from Molecular Probes (Eugene, USA) and αB-crystallin was labeled according to the manufacturer’s guidelines.
cTCCD and TIRFM data acquisition
Data acquisition and analysis for both aggregation and disaggregation studies were performed according to previously described protocols (5).
Statistical Analysis
Two tailed independent t-tests were used for comparison of the values from two measurements. Single-factor ANOVA was used for comparison of multiple values.
Results
In order to derive a mechanistic understanding of the action of the two chaperones, we first examined their effects on the aggregation of the Aβ40 peptide using cTCCD and TIRFM. Data were collected for experiments with αB-crystallin using similar protocols to those used in a previous study with clusterin except where specified in the text (5).
αB-crystallin, like clusterin, inhibits oligomer formation by Aβ40 monomers
We first examined how αB-crystallin affects the aggregation of Aβ40 when added at the start of the reaction—when the peptide is predominantly monomeric. Although studies of these chaperones have shown that they can act at sub-stoichiometric ratios (18), we have conducted most of our studies at a 1:1 (molar) chaperone to Aβ monomer concentration ratio, as such a stoichiometry corresponds approximately to the situation in cerebrospinal fluid or in the cytosol of a number of cell types (19–22).
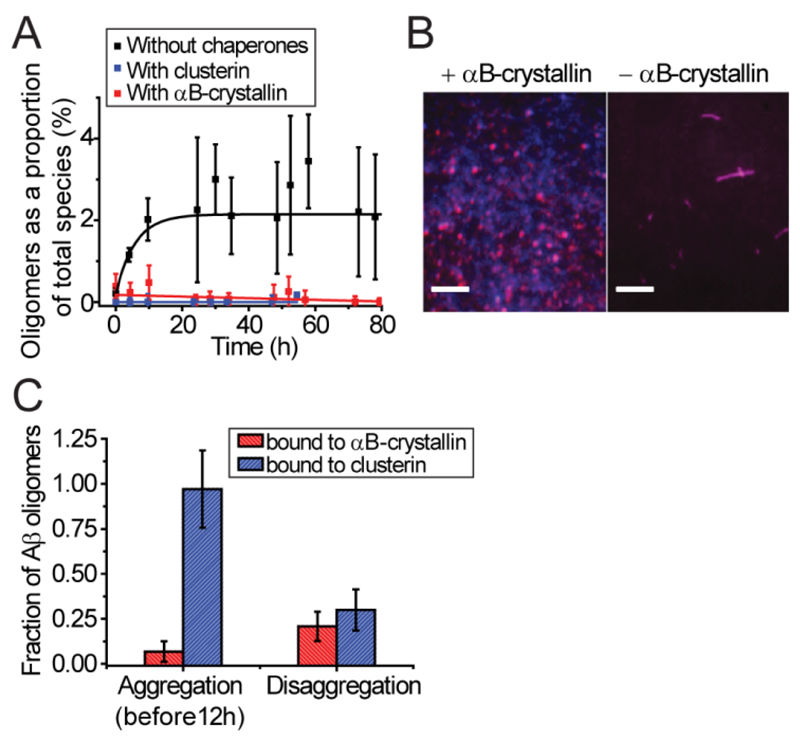
When equimolar amounts of either αB-crystallin or clusterin were added to a monomeric solution of Aβ40, our single-molecule measurements reveal that the formation of oligomers is inhibited in comparison to the situation observed in the absence of chaperones (Figure 2A); this finding is in accord with bulk measurements which report inhibition of fibril formation in the presence of both of these chaperones (23–25). In addition, analysis of the single-molecule data in this study shows no detectible complex formation between Aβ40 monomers and αB-crystallin (see Supporting Figure S1, see t=0), a result again in agreement with previous findings from the studies of Aβ40 with clusterin (5).
Figure 2. αB-crystallin and clusterin affect the distributions of Aβ40 species present when added to the aggregation reaction at different times.
(A) The fraction of oligomers produced over time when monomeric Aβ40 was allowed to aggregate in the absence of chaperones (black) or in the presence of added αB-crystallin or clusterin (red, blue respectively) (600 nM [Aβ40], N=3). (B) Representative TIRFM images showing the approximate morphology of Aβ40 species present after 24 h of aggregation in the absence or presence of αB-crystallin added 3-4 h after initiation of the reaction. Scale bars are 5 µm. (C) The fraction of Aβ40 oligomers bound by either αB-crystallin or clusterin during the aggregation and disaggregation reactions (N=12 or more for all bars). All error bars are SEM. The data for the aggregation reaction in the absence of chaperones and in the presence of clusterin are reproduced from previous work for comparison (5).
We then examined the effects of αB-crystallin on the Aβ40 aggregation reaction when added at an equimolar ratio to a mixture of Aβ40 monomers and oligomers. To accomplish this, we incubated a solution of monomeric fluorescently labeled Aβ40 for 3-4 hrs. This time is close to the midpoint of the reaction process when studied by bulk methods and is a point during the course of the aggregation at which a population of oligomers is readily detectable by cTCCD (5). Imaging using TIRFM of the species present in the reaction mixture after 24 h reveals that in the absence of αB-crystallin, the species present after 24 h are fibrillar in nature while those present after 24 h in the presence of αB-crystallin are predominantly monomeric and oligomeric (Figure 2B). These findings suggest that both chaperones act similarly not only to prevent any oligomeric species present from growing further into fibrillar structures but also, when present initially, to inhibit monomers from forming oligomers.
We then sought to investigate whether the mechanism of inhibition of fibril growth by αB-crystallin involved binding and sequestration of oligomeric species, as found for clusterin in our previous study. Therefore, we tested for any direct interaction between αB-crystallin and Aβ40 by performing complementary cTCCD experiments on labeled Aβ40 in the presence of unlabeled chaperones and experiments on samples where both chaperones and Aβ40 were labeled with different fluorophores. In the first set of experiments, we added AlexaFluor647-labeled αB-crystallin at equimolar ratios to samples taken from an aggregating solution of HiLyteFluor488-labeled Aβ40 at various times after the initiation of the reaction. In the second set of experiments, we used cTCCD to measure the quantity and distribution of oligomers in a mixture of Aβ40 monomers labeled with HiLyteFluor488 and Aβ40 monomers labeled with HiLyteFluor647 but in the absence of chaperones. Examining the results of both experiments enabled a comparison of the fraction of Aβ species in oligomers relative to the fraction of Aβ species associated with chaperones. It was also confirmed in control experiments that the fluorescent labeling of αB-crystallin does not affect its capacity to inhibit the aggregation of Aβ (see Supporting Figure S2).
When these experiments had previously been conducted with clusterin, the proportion of Aβ40 in stable complexes with clusterin matched the proportion of Aβ40 in oligomeric complexes throughout the aggregation reaction (5). In the present study, analogous experiments did not result in evidence for the formation of stable complexes between any of the fluorescently labeled Aβ40 species and αB-crystallin within the first 12 hours of aggregation (Figure 2C, Supporting Figure S1). In order to be detected by cTCCD, a complex has to persist for at least an hour at the picomolar concentrations necessary for the cTCCD measurements. In previous studies we have found that the amyloid species detected by cTCCD are representative (in size) of those present at higher concentrations (5), but the lack of detectable complex formation between αB-crystallin and Aβ40 oligomers by cTCCD does not exclude the presence of complexes of significantly lower stability during the aggregation process. In fact, the observation that both αB-crystallin and clusterin act to inhibit Aβ40 fibril formation even at substoichiometric (to monomeric Aβ) ratios suggests that this mechanism of chaperones is a result of action on the oligomeric species (18) (see Supporting Figure S2).
αB-crystallin binds and sequesters oligomers formed during fibril disaggregation
In the absence of chaperones, Aβ40 fibrils placed in a buffer solution undergo disaggregation and dissociate to yield monomers and a small fraction of oligomeric species (5, 26). In addition, the oligomers formed during the disaggregation process have been shown to be stabilized as a result of binding to clusterin, potentially in a similar manner to the sequestration of oligomers formed during the aggregation reaction. Given the inability to detect complex formation between Aβ40 oligomers and αB-crystallin during the aggregation process, we sought to examine whether αB-crystallin interacts with the oligomers formed from the disaggregation process by adding the chaperone to a solution containing pre-formed fibrils of Aβ40 labeled with HiLyteFluor488. Again fluorescent labeling of the chaperone did not alter its behavior during the fibril disaggregation studies (see Supporting Figure S3).
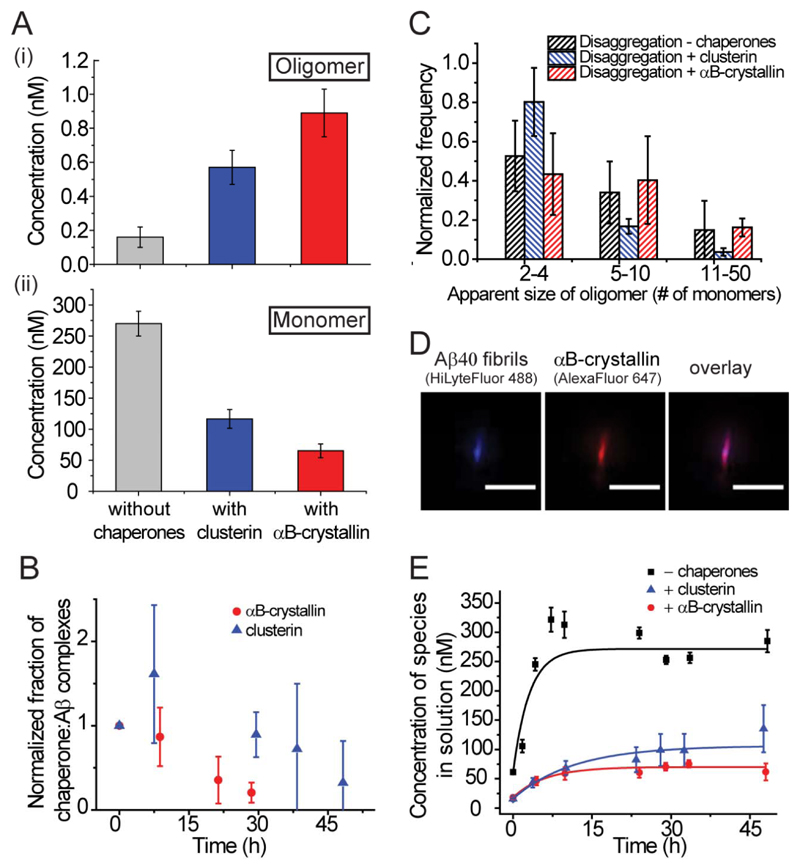
In these experiments, αB-crystallin was found to bind directly to the oligomers formed from the disaggregation of Aβ40 fibrils with sufficient stability to resist dissociation upon dilution to the concentrations requisite for single-molecule experiments (Figure 2C). The results suggest that these complexes contained approximately one αB-crystallin molecule per Aβ monomer (see Supporting Figure S4). The addition of αB-crystallin to the solutions containing fibrils also resulted in a 4-5 fold increase in the population of oligomers observable in the disaggregation products of the fibrils (Figure 3A). This increase in the observable oligomer population can be attributed to the stabilization of the oligomeric species relative to the fibrillar and monomeric states by the binding of αB-crystallin, to a similar degree to that observed with clusterin (5). The stabilization can be quantified by changes to the apparent free energies of formation of these oligomers in the presence of both chaperones (Table 1).
Figure 3. αB-crystallin and clusterin influence the disaggregation of fibrils and bind to oligomers of all sizes.
(A) The oligomer (i) and monomer (ii) concentrations present during disaggregation reactions performed in the presence and absence of chaperones (N = 12, without αB-crystallin, N = 8 with clusterin, N = 4 with αB-crystallin, error bars are SEM). Differences in monomer concentrations between all samples have P-values < 0.02 and differences in oligomer concentrations in the presence of both chaperones when compared to the absence of chaperones have P-values < 0.008). There is, however, no significant difference between oligomer concentration in the presence of clusterin and αB-crystallin (P-value 0.11). (B) The normalized fraction of αB-crystallin–associated Aβ40 oligomers (red) and clusterin-associated Aβ40 oligomers (blue) (N = 3) dissociating over time. These oligomers are formed during disaggregation, incubated with chaperones and the resulting complexes are diluted to nanomolar concentrations to observe dissociation. (C) Distribution of the apparent sizes of oligomers formed in the absence of chaperones and found in complexes with chaperones during the disaggregation reactions (disaggregation without chaperones, N = 10; disaggregation with clusterin, N = 3; disaggregation with αB-crystallin, N = 4). (D) A representative TIRFM image depicting HiLyteFluor-488-labeled Aβ40 fibrils (blue, left) bound with AlexaFluor647-labeled αB-crystallin (red, middle). Scale bar is 5 µm. (E) The variation of the concentration of species (both monomeric and oligomeric) released into solution with time during a disaggregation experiment (N=12, without chaperones, N = 8 with clusterin, N = 4 with αB-crystallin, error bars are SEM, fibrils formed form 8 µM of monomeric Aβ40). The data for the disaggregation reaction performed in the absence of chaperones and in the presence of clusterin are reproduced from previous work for comparison (5).
Table 1. Kinetic and Thermodynamic Data.
| Parameter | Without either chaperone (N) | With αB-crystallin (N) | With clusterin (N) |
|---|---|---|---|
| Rate of monomer and oligomer release from fibril (s-1) | (9.3 ± 3.1)×10-5 (12) | (4.6 ± 1.4)×10-5 (5) | (1.7 ± 0.3)×10-5 (8) |
| Rate of chaperone release from fibrils (s-1) | n/a | (4.4 ± 2.7)×10-5 (3) | (9.8 ± 0.9)×10-7 (3) |
| Rate of oligomer release from fibril (s-1) | n/a | (1.7 ± 1.8)×10-4 (5) | -- |
| Final concentration of monomeric species (nM) | 270 ± 20 (12) | 66 ± 4.5 (5) | 120 ± 20 (8) |
| Final concentration of oligomeric species (nM) | 0.16 ± 0.06 (12) | 0.89 ± 0.14 (5) | 0.42 ± 0.1 (8) |
| Final soluble chaperone concentration (nM) | n/a | 35 ± 34 (3) | 90 ± 14 (3) |
| ΔG° dimer (kJ/mol) | -18.2 ± 0.5 (3) | -25.3 ± 1.0 (5) | -25.8 ± 2.6 (4) |
| ΔG° larger than dimer (kJ/mol) | -38.9 ±2.7 (12) | -43.1 ± 0.5 (5) | -43.9 ± 1.0 (12) |
Rates were derived from fitting a dissociation function to the plot of all soluble species (monomeric and oligomeric) released with time during disaggregation experiments. All thermodynamic values are free energies of formation (ΔG°) for oligomers of different sizes and were determined from apparent size distributions of the various species. Errors in rate values are SD and in thermodynamic values are SEM. All data on Aβ40 in the absence and presence of clusterin are reproduced from a previous study and presented here for comparison (5).
The ability to observe persistent complexes between αB-crystallin and Aβ40 oligomers enabled the investigation of their kinetic stability. The oligomer complexes formed between clusterin and Aβ40 oligomers during the disaggregation reaction were observed to have a half-time for dissociation at nanomolar concentrations of 50 ± 10 h (5). The analogous complexes formed between αB-crystallin and the Aβ40 oligomers released during fibril disaggregation persisted for over 30 h, and the half-time for dissociation was measured to be 17 ± 2 h (Figure 3B). The dissociation rate of αB-crystallin from Aβ40 fibrils is therefore significantly faster than that of clusterin although the distribution of sizes of the Aβ40 species bound to αB-crystallin is remarkably similar to that of the species bound to clusterin and to those released in the absence of either chaperone (Figure 3C).
A variety of studies of aggregation reactions, including single-molecule studies of α-synuclein, a protein whose aggregation is associated with Parkinson’s disease, has revealed that there are time-dependent changes in the structural properties of the oligomeric species (27–29). On the basis of these findings, the apparently greater affinity of αB-crystallin for oligomeric species formed from the disaggregation of fibrillar species relative to those formed during the aggregation reaction could be attributed to structural differences between the oligomers from the disaggregation reaction and those from the aggregation reaction. In particular, it is likely that oligomers from the disaggregation reaction have a greater β-sheet character and a high level of exposed hydrophobicity, a chemical signature for αB-crystallin substrates and well-correlated with toxicity in previous studies of similar oligomers (6, 30–33).
Further analysis of the effects of both of the chaperones on the disaggregation process of Aβ40 fibrils indicates that αB-crystallin binds along the entire fibril surface in a manner similar to that previously observed for clusterin (Figure 3D). We determined a KD of 1.2 ± 0.4 μM for the binding of the αB-crystallin to the fibrils, a value consistent with data from bulk experiments (17). The binding of αB-crystallin to the fibrils also decreases the overall disaggregation rate of the fibril from (8.9 ± 3.3) × 10-5 s-1 to (4.6 ± 1.4) × 10-5 s-1 (Figure 3E, Table 1). We could also define the dissociation rate of the αB-crystallin from the fibrils to be 4.4 ±2.7×10-5, compared to 9.8 ± 0.9 × 10-7 s-1 for clusterin (Table 1). From these data, it seems that αB-crystallin inhibits the disaggregation of Aβ40 fibrils and sequesters any oligomers that are produced during the disaggregation process preventing them from any further dissociation into monomers.
Discussion
In this work we have compared the action of two ATP-independent chaperones, αB-crystallin and clusterin, on a variety of Aβ40 species—monomers, oligomers and fibrils. We find a remarkable number of similarities between the effects of the chaperones, primarily that both molecules inhibit the oligomerization of monomeric peptide molecules, prevent dissociation or further growth of oligomers, and stabilize the oligomers that dissociate from fibrils. The binding of Aβ40 oligomers by these species sequesters them in long-lived complexes and is likely to represent a primary mechanism by which the chaperones prevent oligomer dissociation into monomers and their further growth into fibrils.
The major differences between the actions of αB-crystallin and clusterin on the Aβ40 species involved in the aggregation process are in the magnitude of the effects described above: notably, the αB-crystallin:Aβ40 complexes formed with oligomers from the disaggregation reaction are much more stable than complexes between αB-crystallin and the Aβ40 oligomers formed in the early stages of the aggregation reaction, and therefore are directly observable in the single-molecule experiments. This finding suggests that there is a structural difference between the oligomers formed from the aggregation of monomers and those formed by the disaggregation of fibrils. This can be attributed to their fibrillar origin, as the oligomers that dissociate from the fibrils would possess a more extensive β-sheet structure than those that form from monomers in solution; such a structural difference has been observed for a number of other aggregating proteins in both simulations and experiments (27–29). In contrast to αB-crystallin, clusterin forms stable complexes with oligomers formed throughout the course of the aggregation reaction.
The results also indicate a difference between the two chaperones in their dissociation rate from oligomers formed during the disaggregation of fibrils. In the case of αB-crystallin, these oligomer:chaperone complexes have a half-time of dissociation that is approximately a factor of three shorter than that of the analogous complexes with clusterin and the behavior of the chaperones that interact with the Aβ40 fibrils show similar trends. The dissociation rates of these complexes are all considerably longer than those required for clearance of chaperone-client-protein complexes which suggests that if the chaperones act to sequester oligomers in a cellular context, this interaction will persist for long enough to permit clearance of potentially toxic species (34).
The differences in the dissociation rates of the complexes of the oligomers with the two chaperones determined in vitro can be correlated with the differences in their major cellular locations and the concentration of the two chaperones, in vivo. Clusterin is present at low concentrations (20 to 60 nM) in the cerebrospinal fluid (where Aβ is also present at similar endogenous concentrations (19)) whereas αB-crystallin is present at much higher concentrations within cells; indeed, this protein represents up to 2% of the soluble protein in muscle and other cell types (20–22). Although Aβ aggregation has been conventionally thought to occur in the extracellular space, observations of damaging effects of intracellular Aβ have been reported (15, 16). There is, therefore, a need for defense mechanisms in both the intracellular and extracellular space to abate the toxic effects of Aβ oligomers. An extracellular chaperone present at relatively low concentrations, as is the case of clusterin, would need to bind with high affinity to Aβ oligomers in order to inhibit their interaction with cellular components. In contrast, intracellularly, αB-crystallin is present at much higher concentrations and therefore may not need to bind to these oligomers with such high an affinity to sequester the variety of oligomers present.
In conclusion, the differences between the interactions of αB-crystallin and clusterin with Aβ oligomers are primarily in magnitude rather than in nature. Therefore, it appears that the mechanism of action of the two chaperones is similar despite differences in their primary physiological location (10, 18). As it two chaperones have been found to act in a similar manner to inhibit the aggregation of misfolded globular proteins, it seems that both can act to sequester potentially toxic oligomers, and presumably target them for destruction by the degradative cellular machinery. Just as there may be a generic toxicity of these oligomeric amyloid species towards cellular processes (3), there may be generic protective mechanisms to handle these aberrant oligomeric species. The data presented in this paper therefore suggests the idea that it is when these mechanisms do not function normally, or are overwhelmed, a spectrum of disease states manifest (35, 36).
Supplementary Material
Acknowledgements
We thank Dr. Glyn Devlin for stimulating discussions in the early stages of this work
Funding Source Statement: P.N. is supported by a Marshall Scholarship from the Marshall Aid Commemoration Commission and a Graduate Research Fellowship from the National Science Foundation. S.M. is supported by a Royal Society Dorothy Hodgkin Fellowship. M.R.W. acknowledges the support of the Australian Research Council (DP0773555 and DP0984341). The work of D.K. and C.M.D. is supported by the Wellcome Trust and D.K. by the Augustus Newman Foundation.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
amyloid- β
- Aβ40
amyloid-β(1-40)
- cTCCD
confocal two-color coincidence detection
- TIRFM
total internal reflection microscopy
- CSF
cerebrospinal fluid
References
- 1.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 2.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 3.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo JS, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 4.Orte A, Birkett NR, Clarke RW, Devlin GL, Dobson CM, Klenerman D. Direct characterization of amyloidogenic oligomers by single-molecule fluorescence. Proc Natl Acad Sci U S A. 2008;105:14424–14429. doi: 10.1073/pnas.0803086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narayan P, Orte A, Clarke RW, Bolognesi B, Hook S, Ganzinger KA, Meehan S, Wilson MR, Dobson CM, Klenerman D. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-β1−40 peptide. Nat Struct Mol Biol. 2012;19:79–83. doi: 10.1038/nsmb.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cremades N, Cohen Samuel IA, Deas E, Abramov AndreyY, Chen Allen Y, Orte A, Sandal M, Clarke Richard W, Dunne P, Aprile Francesco A, Bertoncini Carlos W, et al. Direct observation of the interconversion of normal and toxic forms of α-synuclein. Cell. 2012;149:1048–1059. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 8.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert J-C, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 10.Carver JA, Rekas A, Thorn DC, Wilson MR. Small heat-shock proteins and clusterin: intra- and extracellular molecular chaperones with a common mechanism of action and function? IUBMB Life. 2003;55:661–668. doi: 10.1080/15216540310001640498. [DOI] [PubMed] [Google Scholar]
- 11.Shinohara H, Inaguma Y, Goto S, Inagaki T, Kato K. aB crystallin and HSP28 are enhanced in the cerebral cortex of patients with Alzheimer's disease. J Neurol Sci. 1993;119:203–208. doi: 10.1016/0022-510x(93)90135-l. [DOI] [PubMed] [Google Scholar]
- 12.Renkawek K, Voorter C, Bosman G, van Workum F, de Jong W. Expression of αB-crystallin in Alzheimer's disease. Acta Neuropathol (Berl.) 1994;87:155–160. doi: 10.1007/BF00296185. [DOI] [PubMed] [Google Scholar]
- 13.Calero M, Rostagno A, Matsubara E, Zlokovic B, Frangione B, Ghiso J. Apolipoprotein J (clusterin) and Alzheimer's disease. Microsc Res Tech. 2000;50:305–315. doi: 10.1002/1097-0029(20000815)50:4<305::AID-JEMT10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Lidström AM, Bogdanovic N, Hesse C, Volkman I, Davidsson P, Blennow K. Clusterin (Apolipoprotein J) protein levels are increased in hippocampus and in frontal cortex in Alzheimer's disease. Exp Neurol. 1998;154:511–521. doi: 10.1006/exnr.1998.6892. [DOI] [PubMed] [Google Scholar]
- 15.Bayer TA, Wirths O. Intracellular accumulation of amyloid-β - a predictor for synaptic dysfunction and neuron loss in Alzheimer's disease. Frontiers in aging neuroscience. 2010;2:8. doi: 10.3389/fnagi.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedrich RP, Tepper K, Rönicke R, Soom M, Westermann M, Reymann K, Kaether C, Fändrich M. Mechanism of amyloid plaque formation suggests an intracellular basis of Aβ pathogenicity. Proceedings of the National Academy of Sciences. 2010;107:1942–1947. doi: 10.1073/pnas.0904532106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shammas Sarah L, Waudby Christopher A, Wang S, Buell Alexander K, Knowles Tuomas PJ, Ecroyd H, Welland Mark E, Carver John A, Dobson Christopher M, Meehan S. Binding of the molecular chaperone aB-crystallin to Aβ amyloid fibrils inhibits fibril elongation. Biophys J. 2011;101:1681–1689. doi: 10.1016/j.bpj.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannini B, Cascella R, Zampagni M, van Waarde-Verhagen M, Meehan S, Roodveldt C, Campioni S, Boninsegna M, Penco A, Relini A, Kampinga HH, et al. Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers. Proceedings of the National Academy of Sciences. 2012;109:12479–12484. doi: 10.1073/pnas.1117799109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid-β proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MR, Yerbury JJ, Poon S. Extracellular chaperones and amyloids. In: Asea AAA, Brown IR, editors. Heat Shock Proteins and the Brain: Implications for Neurodegenerative Diseases and Neuroprotection. Springer; Netherlands: 2008. pp. 283–315. [Google Scholar]
- 21.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 22.de Jong WW, Caspers G-J, Leunissen JAM. Genealogy of the a-crystallin--small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 23.Kudva YC, Hiddinga HJ, Butler PC, Mueske CS, Eberhardt NL. Small heat shock proteins inhibit in vitro Aβ1-42 amyloidogenesis. FEBS Lett. 1997;416:117–121. doi: 10.1016/s0014-5793(97)01180-0. [DOI] [PubMed] [Google Scholar]
- 24.Kumita JR, Poon S, Caddy GL, Hagan CL, Dumoulin M, Yerbury JJ, Stewart EM, Robinson CV, Wilson MR, Dobson CM. The extracellular chaperone clusterin potently inhibits human lysozyme amyloid formation by interacting with prefibrillar species. J Mol Biol. 2007;369:157–167. doi: 10.1016/j.jmb.2007.02.095. [DOI] [PubMed] [Google Scholar]
- 25.Yerbury JJ, Poon S, Meehan S, Thompson B, Kumita JR, Dobson CM, Wilson MR. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007;21:2312–2322. doi: 10.1096/fj.06-7986com. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez L, Madurga S, Pukala T, Vilaseca M, López-Iglesias C, Robinson CV, Giralt E, Carulla Nl. Aβ40 and Aβ42 amyloid fibrils exhibit distinct molecular recycling properties. J Am Chem Soc. 2011;133:6505–6508. doi: 10.1021/ja1117123. [DOI] [PubMed] [Google Scholar]
- 27.Cremades N, Cohen SIA, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne PD, Aprile FA, Bertoncini CW, et al. Direct observation of the interconversion of normal and pathogenic forms of a-synuclein. Cell. 2012 doi: 10.1016/j.cell.2012.03.037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheon M, Chang I, Mohanty S, Luheshi LM, Dobson CM, Vendruscolo M, Favrin G. Structural reorganisation and potential toxicity of oligomeric species formed during the assembly of amyloid fibrils. PLoS Comput Biol. 2007;3:1727–1738. doi: 10.1371/journal.pcbi.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Culyba EK, Powers ET, Kelly JW. Amyloid-β forms fibrils by nucleated conformational conversion of oligomers. Nat Chem Biol. 2011;7:602–609. doi: 10.1038/nchembio.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treweek TM, Ecroyd H, Williams DM, Meehan S, Carver JA, Walker MJ. Site-directed mutations in the C-terminal extension of human αB-crystallin affect chaperone function and block amyloid fibril formation. PLoS ONE. 2007;2:e1046. doi: 10.1371/journal.pone.0001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lührs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Döbeli H, Schubert D, Riek R. 3D structure of Alzheimer's amyloid-β(1–42) fibrils. Proc Natl Acad Sci U S A. 2005;102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ladiwala ARA, Litt J, Kane RS, Aucoin DS, Smith SO, Ranjan S, Davis J, VanNostrand WE, Tessier PM. Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity. J Biol Chem. 2012 doi: 10.1074/jbc.M111.329763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolognesi B, Kumita JR, Barros TP, Esbjorner EK, Luheshi LM, Crowther DC, Wilson MR, Dobson CM, Favrin G, Yerbury JJ. ANS binding reveals common features of cytotoxic amyloid species. ACS Chem Biol. 2010;5:735–740. doi: 10.1021/cb1001203. [DOI] [PubMed] [Google Scholar]
- 34.Wyatt A, Yerbury J, Berghofer P, Greguric I, Katsifis A, Dobson C, Wilson M. Clusterin facilitates in vivo clearance of extracellular misfolded proteins. Cell Mol Life Sci. 2011:1–13. doi: 10.1007/s00018-011-0684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 36.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.