Abstract
A network meta-analysis of the comparative effectiveness of neurokinin 1 (NK-1) inhibitors in the prophylaxis of highly emetogenic chemotherapy induced nausea and vomiting has been conducted. Eligible studies included randomized trials evaluating aprepitant, fosaprepitant, netupitant (NEPA), casopitant and rolapitant containing regimens in the setting of highly emetogenic chemotherapy. Primary outcomes of interest include complete response (CR) and rate of no significant nausea. After preclusion of ineligible studies, 19 studies were included in the final analysis. The majority of the regimens containing NK-1 inhibitors (including NEPA, aprepitant/palonosetron (palono)/dexamethasone (dexa), casopitant/granisetron (grani) or ondansetron (ondan)/dexa, aprepitant/ondan/dexa) are better than regimens not containing them (palono/dexa, ondan/dexa, grani/dexa) in terms of achieving a CR in the overall phase. Moreover, casopitant/grani or ondan/dexa and aprepitant/grani or ondan/dexa are better than rolapitant/ondan or grani/dexa in terms of CR achievement [odds ratio (OR) 1.62, 95% credible interval (CrI) 1.14–2.23, and OR 1.28, 95% CrI 1.01–1.59, respectively]. Taking into consideration the limitations of cross-trial comparisons, regimens containing neurokinin inhibitors are associated with higher CR rates than regimens not containing them. Moreover, casopitant and aprepitant regimens seem to be more effective than rolapitant regimens.
Keywords: chemotherapy, nausea, neurokinin, vomiting
Introduction
Chemotherapy-induced nausea and vomiting (CINV) are still considered among the major hurdles of administering appropriate anticancer treatment in spite of all major advances in its understanding and its treatment [Aapro et al. 2015; Jordan et al. 2015].
According to the degree of emetogenicity, anticancer drugs can be classified into highly emetogenic, moderately emetogenic, low emetogenic and minimally emetogenic treatment [Roila et al. 2010]; however, some practical considerations face such classification including the very rapid rate of appearance and approval of newer anticancer agents that were not present at the time of the original 2004 Perugia consensus statement [Navari and Aapro, 2016]; another consideration deals with the very broad range of ‘the moderately emetogenic chemotherapy’ category that is from 30% to 90% risk of emesis which means that standardizing one mode of treatment for this group may result in overtreatment for regimens at the lower end of the range or undertreatment for regimens at the upper end of the range. A third issue is related to the fact that this classification was based mainly on the experience with intravenous chemotherapy agents; thus, with the increasing incorporation of oral anticancer agents in the management of patients with cancer, a different emetogenicity classification has to be applied for oral anticancer agents rather than just adopting the older classification [Kottschade et al. 2016].
The process of CINV itself may be classified temporally in a timetable fashion into early phase (between 0 and 24 h), delayed phase (between 24 and 120 h) and overall phase (between 0 and 120 h) [Van Den Brande et al. 2014; Zhou et al. 2015]. Such a temporal classification pattern has proved very beneficial in terms of evaluating and comparing different treatment options for CINV.
Following the rapid establishment of 5HT3 inhibitors as cornerstones of antiemetic treatment, the launching of neurokinin 1 (NK-1) inhibitors in the past decade has further boosted the strength of different antiemetic protocols employed [Hesketh, 2001]. Aprepitant was the first agent approved from this group, followed by fosaprepitant and then several other agents evaluated, including netupitant and rolapitant [Hesketh et al. 2003; Poli-Bigelli et al. 2003].
Objective of the meta-analysis
This network meta-analysis aims to provide a detailed comparative assessment of the efficacy of regimes containing one of the NK-1 inhibitors (aprepitant, fosaprepitant, rolapitant, casopitant, netupitant) in the prevention of CINV from highly emetogenic chemotherapy.
Methodology
Search strategy
A comprehensive search for literature published in English was performed in the following databases: Medline, Cochrane library and Google scholar in order to identify all relevant citations; the date of the last search was 5 September 2015. Meeting abstracts including ASCO (American Society of Clinical Oncology) were also checked. An additional hand search of references of primary original research was conducted for potential ‘cross references’. Citations with the following words in their titles or abstracts were examined: ‘rolapitant’ or ‘aprepitant’ or ‘casopitant’ or ‘fosaprepitant’ or ‘netupitant’, and ‘emesis’ or ‘vomiting’ or ‘nausea’. No protocol has previously been published for this meta-analysis.
Selection criteria
Inclusion criteria:
Clinical studies that evaluate antiemetic regimens based on any of the above agents in the prevention of CINV from highly emetogenic chemotherapy in adults.
Efficacy measures were reported.
Exclusion criteria:
Non-English language records were excluded.
Data extraction
Data were extracted by review authors. All eligible articles underwent initial assessment for relevance. The following data were extracted if available: authors, issuing year, treatment plan, number of patients, complete response (CR) defined as no emesis and no use of rescue drugs, rate of no significant nausea (nausea <25 mm on a visual analogue scale) and rate of no emesis.
Outcome measures
The outcome measures of interest were CR, rate of no significant nausea and rate of no emesis. The three outcome measures were principally evaluated in the overall phase (i.e. from 0 to 120 h after chemotherapy). The cardinal outcome determinants were outlined using descriptive statistics. This meta-analysis follows the guidelines provided by the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses report) [Moher et al. 2009]. Quality of the included studies was assessed through the use of Jadad score (Table 2) [Jadad et al. 1996].
Table 2.
Jadad quality scale of the included studies.
| Study | Randomization | Blinding | An account of all patients | Total score |
|---|---|---|---|---|
| Schnadig et al. [2015] | 1 | 2 | 0 | 3 |
| Rapoport et al. [2015] | 2 | 2 | 1 | 5 |
| Rapoport et al. [2015], HEC1 | 2 | 2 | 1 | 5 |
| Rapoport et al. [2015], HEC2 | 2 | 2 | 1 | 5 |
| Gralla et al. [2014] | 2 | 2 | 1 | 5 |
| Grunberg et al. [2009] | 2 | 2 | 1 | 5 |
| Roila et al. [2009] | 2 | 2 | 1 | 5 |
| Schmitt et al. [2014] | 2 | 2 | 1 | 5 |
| Hu et al. [2014] | 2 | 2 | 1 | 5 |
| Wenzell et al. [2013] | 2 | 0 | 1 | 3 |
| Stiff et al. [2013] | 2 | 2 | 1 | 5 |
| Takahashi et al. [2010] | 2 | 2 | 1 | 5 |
| De Wit et al. [2003] | 2 | 2 | 1 | 5 |
| Schmoll et al. [2006] | 2 | 2 | 1 | 5 |
| Hesketh et al. [2003] | 2 | 2 | 1 | 5 |
| Poli-Bigelli et al. [2003] | 2 | 2 | 1 | 5 |
| Herrington et al. [2008] | 2 | 2 | 1 | 5 |
| Saito et al. [2014] | 2 | 2 | 1 | 5 |
| Ando et al. [2015] | 2 | 0 | 1 | 3 |
Data analysis
The statistical analysis integrated both direct and indirect evidence to get estimates of the relative efficacy of each of the treatments evaluated across the different randomized controlled trials (RCTs). Data were assessed using a Bayesian meta-analyses approach [Lu and Ades, 2004; Caldwell et al. 2005], conducted using WinBUGS 1.4.3 software, and data entry was conducted using NetMETAXL software. Based on the distributions of relative treatment effects, the probability that a certain intervention was more efficacious than another was calculated based on ranking. Both fixed-effects and random-effects models were used. These models estimate odds ratio (ORs) for rates of both CR and rate of no significant nausea and their corresponding 95% credible intervals (CrIs) that capture the uncertainty. Random-effects analyses used vague priors for treatment effects. Sensitivity analyses were performed through reporting results for fixed-effects and random-effects models. A total of 1000 burn in runs and model runs were conducted. Simplified convergence testing was conducted from within the NetMETAXL software.
Ethical approval
This article does not contain any studies with human participants conducted by any of the authors.
Results
Search results
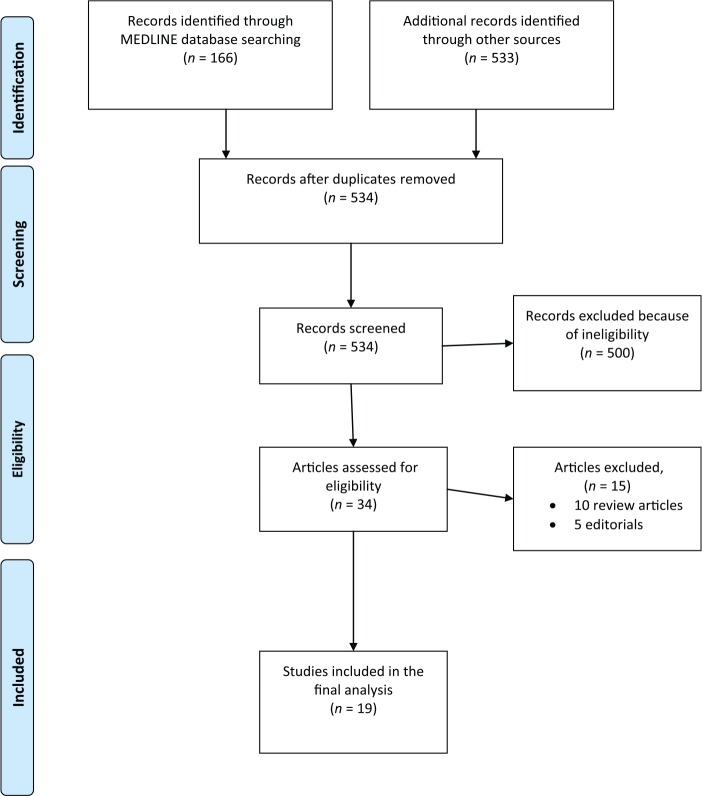
Figure 1 summarizes the PRISMA diagram for the study selection procedure; 699 results were obtained from the searches in Medline (n = 166 studies) and other databases (n = 533). Of these results, 165 were duplicates and 500 did not meet the eligibility criteria and were therefore excluded. Of the 34 possibly eligible studies after the initial screening, a full text search resulted in the removal of 15 studies. Hence, 19 studies were included in the final analysis; 12 phase III studies and 7 randomized phase II studies [Schnadig et al. 2014; De Wit et al. 2003; Hesketh et al. 2003; Poli-Bigelli et al. 2003; Schmoll et al. 2006; Herrington et al. 2008; Grunberg et al. 2009; Roila et al. 2009; Takahashi et al. 2010; Saito et al. 2013; Stiff et al. 2013; Wenzell et al. 2013; Gralla et al. 2014; Hu et al. 2014; Schmitt et al. 2014; Ando et al. 2015; Rapport et al. 2015a; Rapport et al. 2015b] (Table 1). Four studies evaluated rolapitant-based regimens, one study evaluated a netupitant/palonosetron (palono) (NEPA)-based regimen, three studies evaluated aprepitant/palono-based regimens, 10 studies evaluated aprepitant/ondansetron (ondan) or granisetron (grani)-based regimens (one of which is the NEPA randomized study), two studies evaluated casopitant-based regimens and two studies evaluated fosaprepitant-based regimens.
Figure 1.
Flowchart of study selection procedure.
Table 1.
Efficacy outcomes in the included studies.
| Study | Study type | Treatment regimen | CR | Rates of no significant nausea | Rates of no emesis |
|---|---|---|---|---|---|
| Rolapitant studies | |||||
| Schnadig et al. [2015] | Phase II RCT | Arm A: oral rolapitant 200 mg plus granisetron 2 mg and dexamethasone 20 mg (344 patients) Arm B: granisetron and dexamethasone alone (active control) (359 patients) |
Overall: 216 (62.8%) versus 197 (54.9%) | Overall: 216 (62.7%) versus 122 (33.9%). | Overall: 243 (73.0%) versus 215 (60.2%) |
| Rapoport et al. [2015] | Phase II RCT | Arm A: oral rolapitant 180 mg plus ondansetron and dexamethasone (227 patients) Arm B: ondansetron and dexamethasone alone (active control) (227 patients) |
Overall: 143 (62.5%) versus 104 (46.7%) | Overall: 143 (63%) versus 95 (42%) | Overall: 152 (67%) versus 104 (46.7%) |
| Rapoport et al. [2015], HEC1 | Phase III RCT | Arm A: oral rolapitant 180 mg plus granisetron 10 mg/kg and dexamethasone 20 mg on day 1 and dexamethasone (8 mg orally) twice daily on days 2–4 (264 patients) Arm B: granisetron and dexamethasone mg on day 1 and dexamethasone (8 mg orally) twice daily on days 2–4 alone (active control) (262 patients) |
Overall: 185 (70%) versus 148 (56%) | Overall: 189 (72%) versus 165 (63%) | Overall: 199 (75%) versus 155 (59%) |
| Rapoport et al. [2015], HEC2 | Phase III RCT | Arm A: oral rolapitant 180 mg plus granisetron 10 mg/kg and dexamethasone 20 mg on day 1 and dexamethasone (8 mg orally) twice daily on days 2–4 (271 patients) Arm B: granisetron and dexamethasone mg on day 1 and dexamethasone (8 mg orally) twice daily on days 2–4 alone (active control) (273 patients) |
Overall: 183 (68%) versus 165 (60%) | Overall: 197 (71%) versus 175 (64%) | Overall: 192 (71%) versus 165 (64%) |
| NEPA studies | |||||
| Gralla et al. [2014] | Phase III RCT | Arm A: oral NEPA (netupitant 300 mg + palonosetron 0.50 mg) + dexamethasone (309 patients) Arm B: oral aprepitant (125 mg day 1, 80 mg days 2–3) + oral palonosetron 0.50 mg day 1 + dexamethasone (104 patients) |
Overall: 250 (81%) and 79 (76%) | N/R | N/R |
| Casopitant studies | |||||
| Grunberg et al. [2009] | Phase III RCT | All patients received dexamethasone and ondansetron. Patients were randomly assigned to also receive placebo (n = 269), single oral dose of casopitant mesylate (150 mg oral, n = 271), or 3-day intravenous plus oral casopitant mesylate (n = 270) | 175 (66%) patients in the control group versus 228 (86%) in the single-dose oral group versus 214 (80%) in the intravenous group | 184 (69·4%) patients in the control group versus 207 (77·8%) in the single-dose oral group versus 205 (76·2%) in the intravenous group | 179 (67·5%) patients in the control group versus 236 (88·7%) in the single-dose oral group versus 222 (82·5%) in the intravenous group |
| Roila et al. [2009] | Phase III RCT | All patients received dexamethasone and ondansetron plus: Group 1: placebo (n = 84) Group 2: casopitant 50 mg × 3 (n = 82) Group 3: casopitant 100 mg × 3 (n = 81) Group 4: casopitant 150 mg × 3 (n = 81) Group 5: casopitant 150 mg × 1 (n = 83) Group 6: aprepitant (n = 82) |
50 (59.5%) 62 (75.6%) 70 (86.4%) 62 (76.5%) 62 (74.4%) 59 (72%) |
N/R | 64% 78% 89% 78% 78% 79% |
| Aprepitant studies | |||||
| Schmitt et al. [2014] | Phase III RCT | Arm A: aprepitant, granisetron and dexamethasone (n = 181). Arm B: matching placebo, granisetron, and dexamethasone (n = 181) |
104 (58%) versus 74 (41%) | 170 (94%) versus 159 (88%) | 78% versus 65% |
| Hu et al. [2014] | Phase III RCT | Arm A: aprepitant, granisetron and dexamethasone (n = 204) Arm B: matching placebo, granisetron and dexamethasone (n = 207) |
69.6% (142/204) and 57.0% (118/207) | N/R | 70.6% versus 57.0% |
| Wenzell et al. [2013] | Phase II RCT | Arm A: aprepitant, palonosetron and dexamethasone (n = 20) Arm B: aprepitant, ondansetron and dexamethasone (n = 20) |
12 (60%) versus 8 (40%) | N/R | N/R |
| Stiff et al. [2013] | Phase III RCT | Arm A: aprepitant, ondansetron and dexamethasone (n = 90) Arm B: matching placebo, ondansetron and dexamethasone (n = 89) |
73 (81.9%) versus 58 (65.8%) | N/R | 73.3% for aprepitant and 22.5% for placebo |
| Takahashi et al. [2010] | Phase II RCT | Arm A: matching placebo, granisetron and dexamethasone (149 patients) Arm B: aprepitant 40/25 mg (40 mg on day 1 and 25 mg on days 2–5), granisetron and dexamethasone (143 patients) Arm C: aprepitant 125/80 mg (125 mg on day 1 and 80 mg on days 2–5), granisetron and dexamethasone (146 patients) |
50.3% (75/149 subjects), 66.4% (95/143 subjects) and 70.5% (103/146 subjects), respectively | 82 (55%) versus 86 (60%) versus 100 (69%) | 51% versus 74% versus 76% |
| De Wit et al. [2003] | Phase III RCT | Arm A: aprepitant 125 mg before cisplatin and aprepitant 80 mg on days 2–5 (n = 81) Arm B: placebo before cisplatin on days 2–5 (n = 86) All groups received ondansetron 32 mg and dexamethasone 20 mg |
55 (64%) versus 42 (49%) | N/R | N/R |
| Schmoll et al. [2006] | Phase II RCT | Arm A: aprepitant, ondansetron and dexamethasone (n = 243) Arm B: matching placebo, ondansetron and dexamethasone (n = 241) |
175 (72%) versus 147 (61%) | 177 (73.1%) versus 168 (69.7%) | 77% versus 62% |
| Hesketh et al. [2003] | Phase III RCT | Arm A: aprepitant, ondansetron and dexamethasone (n = 260) Arm B: matching placebo, ondansetron and dexamethasone (n = 260) |
187 (72.7%) versus 135 (52.3%) | 190 (73.2%) versus 171(66%) | 77% versus 55% |
| Poli-Bigelli et al. [2003] | Phase III RCT | Arm A: aprepitant, ondansetron and dexamethasone (n = 260) Arm B: matching placebo, ondansetron and dexamethasone (n = 263) |
163 (62.7%) versus 114 (43.3%) | 184 (71%) versus 168 (64%) | 66% versus 44% |
| Herrington et al. [2008] | Phase II RCT | Arm A: aprepitant, palonosetron and dexamethasone (n = 29) Arm A: aprepitant, palonosetron and dexamethasone (n = 30) Arm C: matching placebo, palonosetron and dexamethasone (n = 16) |
16 (55%) versus 19 (63%) versus 7 (43.8%) | N/R | 92% versus 92% versus 50% |
| Fosaprepitant studies | |||||
| Saito et al. [2014] | Phase III RCT | Arm A: fosaprepitant, granisetron and dexamethasone (n = 174) Arm B: matching placebo, granisetron and dexamethasone (n = 173) |
111 (64%) versus 81 (47%) | 90.2% versus 84.9% | 93.6% versus 80.8% |
| Ando et al. [2015] | Phase II RCT | Group A: aprepitant, a 5HT3 receptor antagonist and dexamethasone (48 patients) Group B: fosaprepitant meglumine, a 5HT3 receptor antagonist, and dexamethasone (45 patients) About 40% of patients in each group received palonosetron and 60% received granisetron in addition to the neurokinin inhibitors |
85.4% (41/48) in group A and 82.2% (37/45) in group B | N/R | N/R |
CR, complete response defined as no emesis and no use of rescue medication; NEPA, netupitant/palonosetron; N/R, not reported; RCT, randomized controlled trial; HEC: highly emetogenic chemotherapy.
Population characteristics
A total of 6788 patients were included in the analysis. All patients were enrolled in studies evaluating highly emetogenic chemotherapy and thus have adequate haematological, hepatic and renal functions. The baseline characteristics and the relevant outcomes in each trial are summarized in Table 1.
Quality of included studies
Table 2 summarizes the principal elements of the Jadad quality assessment for each of the included studies, including randomization, blinding and an account of all patients in addition to the overall score.
Results of indirect comparison
The principal clinical outcomes evaluated in the indirect analysis were CR (overall phase) and no significant nausea (overall phase). An OR greater than one indicates improved outcome. A CrI around the point estimate is reported as a measure of uncertainty. A 95% CrI above 1.0 gives a 95% probability of improved outcome. All results were principally reported using a random-effects model with vague priors.
Indirect comparison for the overall phase of CR (from 0 to 120 h after chemotherapy)
These findings suggest that the majority of the regimens containing NK-1 inhibitors [including NEPA, aprepitant/palono/dexamethasone (dexa), casopitant/grani or ondan/dexa, aprepitant/ondan/dexa] are better than regimens not containing them (palono/dexa, ondan/dexa, grani/dexa) in terms of achieving a CR in the overall phase (Figure 2a–c).
Figure 2.
(a) Forest plots of odds ratio (OR) of complete response (CR) associated with different neurokinin-based regimens. (b) League table of different neurokinin-based regimens in terms of CR achievement. (c) Rankogram of different neurokinin-based regimens in terms of CR achievement. CI, credible interval; dexa, dexamethasone; grani, granisetron; ondan, ondansetron; NEPA, netupitant/palonosetron; palono, palonosetron.
Other interdrug indirect comparisons among the neurokinin inhibitors themselves suggested that casopitant/grani or ondan/dexa and aprepitant/grani or ondan/dexa are better than rolapitant/ondan or grani/dexa in terms of CR achievement (OR 1.62, 95% CrI 1.14–2.23, and OR 1.28, 95% CrI 1.01–1.59, respectively).
None of the other interdrug indirect comparisons indicated statistically significant differences between the other neurokinin inhibitors and Table 2b (league table) provided a crude efficacy arrangement for neurokinin inhibitors in terms of ability to achieve CR rates.
Indirect comparison of the overall phase of rate of no significant nausea (from 0 to 120 h after chemotherapy)
None of the interdrug indirect comparisons indicated statistically significant differences between the evaluated regimens and the relevant league table provided a crude efficacy arrangement for neurokinin inhibitors in terms of their ability to achieve a higher rate of no significant nausea (Figure 3a–c).
Figure 3.
(a) Forest plots of odds ratio (OR) of rate of no significant nausea associated with different neurokinin-based regimens. (b) League table of different neurokinin-based regimens in terms of rate of no significant nausea achievement. (c) Rankogram of different neurokinin-based regimens in terms of rate of no significant nausea achievement. CI, credible interval; dexa, dexamethasone; grani, granisetron; ondan, ondansetron; NEPA, netupitant/palonosetron; palono, palonosetron.
Discussion
To my knowledge, this is the most up to date meta-analysis to provide a comparative assessment of the efficacy of neurokinin inhibitor based regimens in the prophylaxis of highly emetogenic CINV. The indirect comparison from this analysis revealed that the majority of the regimens containing NK-1 inhibitors (including NEPA, aprepitant/palono/dexa, casopitant/grani or ondan/dexa, aprepitant/ondan/dexa) are better than regimens not containing them (palono/dexa, ondan/dexa, grani/dexa) in terms of achieving a CR in the overall phase. However, none of the other interdrug indirect comparisons revealed significant differences in terms of rates of no significant nausea.
Gastrointestinal toxicities (including nausea and vomiting) have been recorded for many anticancer therapies and they have always been a major cause of disturbed quality of life as well as lost treatment compliance [Schwartzberg, 2014].
The development of neurokinin inhibitors is considered to be the fruitful consequence of improved understanding of the biology of CINV and the role of different neuronal receptors in its occurrence. The two most important groups of receptors evaluated to date include 5HT3 receptors and NK-1 receptors [Rojas et al. 2014].
The approach to managing CINV has undergone revolutionary changes during the past two decades. Initially, the introduction of 5HT3 inhibitors (e.g. granisetron, dolasetron and ondansetron) in the 1990s changed the landscape of supportive care in patients receiving highly emetogenic chemotherapy. The first decade of the twenty-first century has come with another major breakthrough; that is, the introduction of aprepitant as the first representative of the group of NK-1 inhibitors [Hesketh et al. 2003; Poli-Bigelli et al. 2003; Schmoll et al. 2006]. Since then, a series of other NK-1 inhibitors have been introduced into clinical practice, including fosaprepitant (which is actually a water-soluble prodrug of aprepitant), casopitant (whose approval processes have been halted by the sponsoring company despite interesting phase III results), netupitant (which has been used in combination with palono and summarized as NEPA) and lastly rolapitant [Lasseter et al. 2007; Navari, 2007; Grunberg et al. 2009; Roila et al. 2009].
The above developments have been accompanied by another major achievement with the introduction of a second-generation 5HT3 inhibitor (palono) which has been shown to better older generation 5HT3 inhibitors (grani/ondan) in randomized controlled studies [Aapro et al. 2006; Saito et al. 2009]. The indirect comparison from this analysis has shown that NK-1 inhibitor based regimens not containing palono have a higher ability to achieve CR than the palono/dexa combination.
However, most of the randomized studies proving the superiority of NK-1 inhibitors have used a standard control arm comprising older generation 5HT3 inhibitors (grani or ondan) plus dexa. Thus although the superiority of these regimens against a grani or ondan/dexa combination was clear, the efficacy vis à vis palono/dexa or among the neurokinin inhibitors themselves was not clear for the majority of these antiemetic regimens. This has provided the principal rationale and motive to conduct this analysis in order to study the relative efficacy of these agents which can be investigated further by randomized controlled studies.
What is also interesting about these results is that despite the clear-cut superiority of NK-1 inhibitor regimens over older 5HT3 only regimens in controlling vomiting and achieving CR, the risk of significant nausea is not tackled to the same extent by these agents. This is an interesting area of future research and development in forthcoming antiemetic studies.
Other than the efficacy differences between NK-1 inhibitors discussed above, some other aspects need to be taken into consideration when choosing the appropriate NK-1 inhibitor to be used. For example, pharmacokinetic data have revealed interesting differences among these agents with regard to effect on the cytochrome P450 3A4 metabolizing pathway, with aprepitant and NEPA having a profound effect on this pathway while rolapitant does not [Poma et al. 2013]. This point has to be taken into consideration when prescribing a NK-1 inhibitor to a patient with cancer receiving other drugs potentially metabolized by this pathway. Other points of interest when evaluating these drugs is the minimal cardiac risk imposed by some of the older 5HT3 inhibitors and represented by prolonged QT interval in some of the published studies [George et al. 2010]. This is particularly relevant in older patients and those receiving concomitant medications that may further prolong this interval. This finding may be in favour of using palonosetron-based regimens because of the reduced risk of QT prolongation.
Among the other agents that have shown interesting antiemetic effects has been the atypical antipsychotic olanzapine which is equivalent to aprepitant in reducing emesis but is superior to aprepitant in reducing delayed nausea in a randomized phase III study [Navari et al. 2011].
The principal weakness of this meta-analysis is the presence of some degree of heterogeneity among included studies. Proper sensitivity analyses and meticulous review of all published data have been conducted to overcome this. Moreover, the network meta-analysis has some inherent limitations in performing indirect comparisons mainly due to selection biases.
Conclusion
This meta-analysis has demonstrated that compared with regimens not containing NK-1 inhibitors, the majority of the regimens containing NK-1 inhibitors are better in terms of achieving a CR in the overall phase. Bearing in mind the caveats of a cross-trial comparison, casopitant/grani or ondan/dexa seems to be a better combination than rolapitant/ondan or grani/dexa in terms of CR achievement. Moreover, none of the other inter-drug indirect comparisons revealed significant differences in terms of rates of no significant nausea.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Aapro M., Carides A., Rapoport B., Schmoll H., Zhang L., Warr D. (2015) Aprepitant and fosaprepitant: a 10-year review of efficacy and safety. Oncologist 20: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aapro M., Grunberg S., Manikhas G., Olivares G., Suarez T., Tjulandin S., et al. (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17: 1441–1449. [DOI] [PubMed] [Google Scholar]
- Ando Y., Hayashi T., Ito K., Suzuki E., Mine N., Miyamoto A., et al. (2015) Comparison between 5-day aprepitant and single-dose fosaprepitant meglumine for preventing nausea and vomiting induced by cisplatin-based chemotherapy. Support Care Cancer 24: 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D., Ades A., Higgins J. (2005) Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331: 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit R., Herrstedt J., Rapoport B., Carides A., Carides G., Elmer M., et al. (2003) Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. J Clin Oncol 21: 4105–4111. [DOI] [PubMed] [Google Scholar]
- George E., Hornuss C., Apfel C. (2010) Neurokinin-1 and novel serotonin antagonists for postoperative and postdischarge nausea and vomiting. Curr Opin Anesthesiol 23: 714–721. [DOI] [PubMed] [Google Scholar]
- Gralla R., Bosnjak S., Hontsa A., Balser C., Rizzi G., Rossi G., et al. (2014) A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol 14 March 2014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg S., Rolski J., Strausz J., Aziz Z., Lane S., Russo M., et al. (2009) Efficacy and safety of casopitant mesylate, a neurokinin 1 (NK1)-receptor antagonist, in prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy: a randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 549–558. [DOI] [PubMed] [Google Scholar]
- Herrington J., Jaskiewicz A., Song J. (2008) Randomized, placebo-controlled, pilot study evaluating aprepitant single dose plus palonosetron and dexamethasone for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. Cancer 112: 2080–2087. [DOI] [PubMed] [Google Scholar]
- Hesketh P. (2001) Potential role of the NK1 receptor antagonists in chemotherapy-induced nausea and vomiting. Support Care Cancer 9: 350–354. [DOI] [PubMed] [Google Scholar]
- Hesketh P., Grunberg S., Gralla R., Warr D., Roila F., De Wit R., et al. (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol 21: 4112–4119. [DOI] [PubMed] [Google Scholar]
- Hu Z., Cheng Y., Zhang H., Zhou C., Han B., Zhang Y., et al. (2014) Aprepitant triple therapy for the prevention of chemotherapy-induced nausea and vomiting following high-dose cisplatin in Chinese patients: a randomized, double-blind, placebo-controlled phase III trial. Support Care Cancer 22: 979–987. [DOI] [PubMed] [Google Scholar]
- Jadad A., Moore R., Carroll D., Jenkinson C., Reynolds D., Gavaghan D., et al. (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17: 1–12. [DOI] [PubMed] [Google Scholar]
- Jordan K., Jahn F., Aapro M. (2015) Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Ann Oncol 9 March 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Kottschade L., Novotny P., Lyss A., Mazurczak M., Loprinzi C., Barton D. (2016) Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3 (Alliance). Support Care Cancer 24: 2661–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter K., Gambale J., Jin B., Bergman A., Constanzer M., Dru J., et al. (2007) Tolerability of fosaprepitant and bioequivalency to aprepitant in healthy subjects. J Clin Pharmacol 47: 834–840. [DOI] [PubMed] [Google Scholar]
- Lu G., Ades A. (2004) Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 23: 3105–3124. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151: 264–269. [DOI] [PubMed] [Google Scholar]
- Navari R. (2007) Fosaprepitant (MK-0517): a neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Invest Drugs 16: 1977–1985. [DOI] [PubMed] [Google Scholar]
- Navari R., Aapro M. (2016) Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med 374: 1356–1367. [DOI] [PubMed] [Google Scholar]
- Navari R., Gray S., Kerr A. (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 9: 188–195. [DOI] [PubMed] [Google Scholar]
- Poli-Bigelli S., Rodrigues-Pereira J., Carides A., Julie Ma G., Eldridge K., Hipple A., et al. (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Cancer 97: 3090–3098. [DOI] [PubMed] [Google Scholar]
- Poma A., Christensen J., Pentikis H., Arora S., Hedley H. (2013) Rolapitant and its major metabolite do not affect the pharmacokinetics of midazolam, a sensitive cytochrome P450 3A4 substrate. Support Care Cancer 21: S154. [Google Scholar]
- Rapoport B., Chasen M., Gridelli C., Urban L., Modiano M., Schnadig I., et al. (2015a) Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase III trials. Lancet Oncol 16: 1079–1089. [DOI] [PubMed] [Google Scholar]
- Rapoport B., Chua D., Poma A., Arora S., Wang Y., Fein L. (2015b) Study of rolapitant, a novel, long-acting, NK-1 receptor antagonist, for the prevention of chemotherapy-induced nausea and vomiting (CINV) due to highly emetogenic chemotherapy (HEC). Supportive Care Cancer 23: 3281–3288. [DOI] [PubMed] [Google Scholar]
- Roila F., Herrstedt J., Aapro M., Gralla R., Einhorn L., Ballatori E., et al. (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy-and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21: v232–v243. [DOI] [PubMed] [Google Scholar]
- Roila F., Rolski J., Ramlau R., Dediu M., Russo M., Bandekar R., et al. (2009) Randomized, double-blind, dose-ranging trial of the oral neurokinin-1 receptor antagonist casopitant mesylate for the prevention of cisplatin-induced nausea and vomiting. Ann Oncol. 2009. November;20(11):1867–73. [DOI] [PubMed] [Google Scholar]
- Rojas C., Raje M., Tsukamoto T., Slusher B. (2014) Molecular mechanisms of 5-HT 3 and NK 1 receptor antagonists in prevention of emesis. Eur J Pharmacol 722: 26–37. [DOI] [PubMed] [Google Scholar]
- Saito H., Yoshizawa H., Yoshimori K., Katakami N., Katsumata N., Kawahara M., et al. (2013) Efficacy and safety of single-dose fosaprepitant in the prevention of chemotherapy-induced nausea and vomiting in patients receiving high-dose cisplatin: a multicentre, randomised, double-blind, placebo-controlled phase III trial. Ann Oncol 24: 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Aogi K., Sekine I., Yoshizawa H., Yanagita Y., Sakai H., et al. (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10: 115–124. [DOI] [PubMed] [Google Scholar]
- Schmitt T., Goldschmidt H., Neben K., Freiberger A., Hüsing J., Gronkowski M., et al. (2014) Aprepitant, granisetron, and dexamethasone for prevention of chemotherapy-induced nausea and vomiting after high-dose melphalan in autologous transplantation for multiple myeloma: results of a randomized, placebo-controlled phase III trial. J Clin Oncol 32: 3413–3420. [DOI] [PubMed] [Google Scholar]
- Schmoll H., Aapro M., Poli-Bigelli S., Kim H., Park K., Jordan K., et al. (2006) Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol 17: 1000–1006. [DOI] [PubMed] [Google Scholar]
- Schnadig I, Modiano M, Poma A, Hedley M, Martell R, Schwartzberg L., et al. (2014) Phase 3 trial results for rolapitant, a novel NK-1 receptor antagonist, in the prevention of chemotherapy-induced nausea and vomiting (CINV) in subjects receiving moderately emetogenic chemotherapy (MEC). J Clin Oncol 32(5s Suppl.): abstract 9633. [Google Scholar]
- Schwartzberg L. (2014) Addressing the value of novel therapies in chemotherapy-induced nausea and vomiting. Expert Rev Pharmacoecon Outcomes Res 14: 825–834. [DOI] [PubMed] [Google Scholar]
- Stiff P., Fox-Geiman M., Kiley K., Rychlik K., Parthasarathy M., Fletcher-Gonzalez D., et al. (2013) Prevention of nausea and vomiting associated with stem cell transplant: results of a prospective, randomized trial of aprepitant used with highly emetogenic preparative regimens. Biol Blood Marrow Transplant 19: 49–55. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Hoshi E., Takagi M., Katsumata N., Kawahara M., Eguchi K. (2010) Multicenter, phase II, placebo-controlled, double-blind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Sci 101: 2455–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Brande J., Brouwer A., Peeters M. (2014) Use of antiemetics in the prevention of chemotherapy-induced nausea and vomiting: review and focus on the Belgian situation. Acta Gastroenterol Belg 77: 240–248. [PubMed] [Google Scholar]
- Wenzell C., Berger M., Blazer M., Crawford B., Griffith N., Wesolowski R., et al. (2013) Pilot study on the efficacy of an ondansetron-versus palonosetron-containing antiemetic regimen prior to highly emetogenic chemotherapy. Support Care Cancer 21: 2845–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Popovic M., Pasetka M., Pulenzas N., Ahrari S., Chow E., et al. (2015) Update on the management of chemotherapy-induced nausea and vomiting – focus on palonosetron. Ther Clin Risk Manag 11: 713–729. [DOI] [PMC free article] [PubMed] [Google Scholar]