Summary
Continuous controversy surrounds the predictive value of the degree of vascular invasion (VI) in low-grade encapsulated follicular cell–derived thyroid carcinomas (LGEFCs). Some guidelines advocate conservative therapy in LGEFCs with focal VI. There is therefore a need to assess the survival rates of LGEFC patients with various degrees of VI to better stratify patients for subsequent therapy. Furthermore, the prognostic effect of VI within the different histotypes of LGEFCs is not well known. A total of 276 patients with LGEFCs were subjected to a meticulous histopathologic analysis. They were classified as encapsulated papillary thyroid carcinoma, encapsulated follicular carcinoma (EFC), and encapsulated Hurthle cell carcinoma (EHCC). Of the 276 patients, 24 had extensive VI (EVI) (≥4 foci) and 28 displayed focal (<4 foci) VI. EHCC and EFC showed a much higher rate of EVI than encapsulated papillary thyroid carcinoma. Median follow-up was 6 years. All 14 tumors with adverse behavior harbored distant metastases (DMs), of which 9 had DMs at presentation. All 3 patients without EVI who had aggressive carcinomas harbored DMs at presentation. EVI was an independent predictor of poor recurrence-free survival. Excluding cases with DMs at presentation, only patients with EVI had recurrence, and all relapsed cases were EHCC. EVI is an independent predictor of recurrence-free survival in LGEFCs. EHCC with EVI has a particularly high risk of recurrence. When DMs are not found at presentation, patients with focal VI are at a very low risk of recurrence even if not treated with radioactive iodine.
Keywords: Encapsulated low-grade follicular cell–derived thyroid carcinoma, Vascular invasion, Follicular carcinoma, Hurthle cell carcinoma, Papillary thyroid carcinoma, Prognosis
1. Introduction
Thyroid carcinoma is the cancer with the largest annual increase in the United States [1], accounting for 62980 newly diagnosed cancers per year [2]. Despite the increasing prevalence of thyroid cancers, a vast majority of them, namely, small organ-confined differentiated thyroid carcinomas, are considered low-risk lesions because they follow a highly indolent clinical course and rarely cause death. Several well-recognized organizations, including the American Thyroid Association [3] and the National Comprehensive Cancer Network (NCCN) [4], have published clinical management guidelines advocating for risk stratification using a variety of clinical and pathologic parameters. These societies recommend conservative treatment approaches that do not require completion thyroidectomy or radioactive iodine (RAI) therapy for indolent low-risk thyroid carcinoma.
The extent of vascular invasion (VI) was 1 criterion being adopted by the NCCN for risk assessment [3,4]. According to the NCCN guidelines, minimal VI, defined as a few microscopic foci of VI, in an intrathyroidal well-defined follicular or Hurthle cell carcinoma places a patient into a low-risk group in which RAI administration and completion thyroidectomy are not mandatory. On the other hand, patients with extensive (more than a few foci) VI (EVI) will be classified into a higher risk category, in which completion thyroidectomy and postsurgical RAI therapy are highly recommended [4]. Hence, it is crucial for pathologists to reliably evaluate and report the presence and extent of VI in low-grade thyroid carcinoma to direct risk stratification and subsequent clinical treatment decisions. However, the very definition of VI and the prognostic significance of its extent in thyroid carcinomas have been surrounded by controversies since its first description by Graham [5] in 1924. Although some authors argue that the mere existence of VI, even if just 1 focus, entails a substantial risk of distant metastasis (DM) (35% in 1 study) [6,7], others have shown that tumors with focal VI (defined as less than 4–5 foci) have a significantly better outcome compared with carcinomas with more foci of VI [8–11].
The confusion is compounded in part by a lack of consistency in applying the diagnostic criteria for VI across studies. Mete and Asa [6], for example, did not consider tumor protrusion into vascular space lined by endothelial cells as a diagnostic criterion for VI, whereas other authors did [8–10]. Additional larger-scale studies are therefore needed to clarify the prognostic value of focal and extensive VI in low-grade encapsulated follicular cell–derived thyroid carcinomas (LGEFCs). In this study, we aimed to identify the prognostic impact of extent of VI in patients with various histological types of LGEFCs with the hope that it will help better guide patient stratification and therapy.
2. Material and methods
2.1. Histologic definitions and inclusion criteria
The institutional database was searched for all cases with a diagnosis of thyroid carcinomas operated at Memorial Sloan-Kettering Cancer Center (MSKCC) between 1980 and 2004. All cases from MSKCC with adequate material were examined microscopically under the supervision of a head and neck surgical pathologist with special interest in thyroid neoplasia (R. G.), who was blinded to the patients’ outcome. Cases were included in the study if the tumor was an encapsulated papillary thyroid carcinoma (EPTC), encapsulated follicular carcinoma (EFC), or encapsulated Hurthle cell carcinoma (EHCC). Encapsulated carcinomas with high-grade features (ie, tumor necrosis or mitotic rate of 5 or more mitotic figures per 10 high-power fields [400×; field size, 0.24 mm2]) were excluded. Multicentric tumors defined as containing more than 2 foci of carcinoma were also excluded. The study was approved by the institutional review board of MSKCC.
2.2. Pathology review
Tumor size was measured as the maximum diameter of the resected tumor specimen. Mitotic rate was determined by counting 10 high-power fields (400×) with an Olympus microscope (U-DO model, Center Valley, PA, United States) in the areas of greatest concentration of mitotic figures. Capsular invasion (CI) was defined as complete penetration of the capsule by tumor, and the number of these foci was recorded. The presence of VI was noted only when such foci were present within or beyond the capsule in accordance with criteria outlined by the Armed Forces Institute of Pathology fascicle [12]. Briefly, only when the invasive focus protruded into the lumen of the vessel in a polypoid manner covered by endothelial cells, or when it was attached to the vessel wall or associated with thrombus formation was considered true VI. Areas of VI that were closely adjacent to one another were counted as separate foci. The foci of CI and VI were subdivided into 2 categories: focal (<4 invasive foci) and extensive (≥4 foci). The presence or absence of extrathyroid tumor extension (ETE) into the perithyroid soft tissue stroma as well as the presence of extrathyroid VI was documented. ETE was subdivided into (1) none, (2) focal (presence of 1–2 microscopic foci of ETE measuring ≤1 mm each), and (3) extensive (presence of >2 microscopic foci of ETE [≤1 mm in size each] or any foci >1 mm in size). Microscopic resection margins were categorized as positive (tumor at the inked margin) or negative (no tumor at the inked margin). Finally, the number and metastatic status of the regional lymph nodes were also recorded.
2.3. Clinical review
The patients’ medical records were reviewed for age at diagnosis, sex, type of surgery, and RAI therapy. In view of the fact that many cases from the1980s did not have adequate biochemical data, the patient disease status at recurrence or follow-up was based on a combination of clinical and imaging assessments. These evaluations include history taking, physical examination, RAI scanning, cross-sectional imaging and/or positron emission tomography scanning, or histological examination of the recurrent tumor. Thus, biochemical recurrence was not assessed. Follow-up in each case was conducted by a member of the head and neck disease management team of MSKCC. Status at last follow-up was categorized as no evidence of disease (NED), alive with disease (AWD), and dead of disease (DOD).
2.4. Statistics
All statistical analyses were performed using the SPSS software 22.0 (IBM Corporation, New York, NY). Clinicopathologic characteristics were compared between cases with different status of VI or disease outcome using appropriate statistical tests, that is, log-rank test for survival analysis, χ2 test or Kruskal-Wallis analysis of variance (ANOVA) by ranks for nonparametric variables, and one-way ANOVA for continuous variables. Status of VI was classified into 3 categories: none, focal VI (FVI) defined as 3 foci or less, and extensive VI with 4 or more foci of VI. Similarly, the status of capsular invasion was divided into 3 categories: none, focal (<4 foci), and extensive (≥4 foci). Recurrence-free survival (RFS) was calculated from the date of surgery to the date of recurrence. Prognostic variables that were significant on univariate analyses were subsequently subjected to multivariate analyses using the Cox proportional hazards model. P values less than .05 were considered to be statistically significant.
3. Results
3.1. Patient population and clinicopathologic characteristics of the study cohort according to extent of VI
Of the 276 patients, 224 patients (81.2%) harbored EPTC, 34 (12.3%) EFC, and 16 (6.5%) EHCC. Of the 224 EPTCs, 130 (58%) were follicular variant, 59 (26%) classical variant, 5 (2.5%) tall cell variant, 2 (1%) columnar variant, 1 (0.5%) oncocytic variant, and 27 (12%) microcarcinomas (Table 1).
Table 1.
Clinicopathologic characteristics of the entire study cohort according to extent of VI a
| Row totals |
VI c | Pb | |||
|---|---|---|---|---|---|
| No | Focal | Extensive | |||
| n | 276 | 224 (81.2%) | 28 (10.1%) | 24 (8.7%) | |
| Sex | |||||
| Male | 88 | 64 (72.7%) | 11 (12.5%) | 13 (14.8%) | .026 d |
| Female | 188 | 160 (85.1%) | 17 (9.0%) | 11 (5.9%) | |
| Age | 45.2 ± 0.9 | 47.6 ± 2.5 | 49.0 ± 3.1 | .339 | |
| Pathologic diagnosis | |||||
| Follicular carcinoma | 18 | 8 (44.4%) | 6 (33.3%) | 4 (22.2%) | <.001 d |
| Hurthle cell carcinoma | 34 | 12 (35.3%) | 11 (32.4%) | 11 (32.4%) | |
| Papillary carcinoma | 224 | 204 (91.1%) | 11 (4.9%) | 9 (4.0%) | |
| Variants of PTC | |||||
| Follicular variant | 130 | 112 (86.2%) | 9 (6.9%) | 9 (6.9%) | .008 d |
| Classical variant | 59 | 58 (98.3%) | 1 (1.7%) | 0 | |
| Microcarcinoma | 27 | 26 (96.3%) | 1 (3.7%) | 0 | |
| Tall cell variant | 5 | 5 (100%) | 0 | 0 | |
| Columnar variant | 2 | 2 (100%) | 0 | 0 | |
| Oncocytic variant | 1 | 1 (100%) | 0 | 0 | |
| Size | 2.3 ± 0.1 | 3.2 ± 0.3 | 4.4 ± 0.4 | <.001 d | |
| Mitotic index (/10 HPFs) | |||||
| 0 | 240 | 197 (82.1%) | 25 (10.4%) | 18 (7.5%) | .187 |
| 1–4 | 36 | 27 (75.0%) | 3 (8.3%) | 6 (16.7%) | |
| Capsular invasion c | |||||
| No | 161 | 143 (88.8%) | 8 (5.0%) | 10 (6.2%) | <.001 d |
| Focal | 91 | 69 (75.8%) | 15 (16.5%) | 7 (7.7%) | |
| Extensive | 24 | 12 (50%) | 5 (20.8%) | 7 (29.2%) | |
| Surgical margin status | |||||
| NA | 1 | 0 | 1 (100%) | 0 | .053 |
| Negative | 273 | 222 (81.3%) | 27 (9.9%) | 24 (8.8%) | |
| Positive | 2 | 2 (100%) | 0 | 0 | |
| Extrathyroidal extension | |||||
| None | 269 | 220 (81.8%) | 27 (10.0%) | 22 (8.2%) | .002 d |
| Focal | 4 | 4 (100%) | 0 | 0 | |
| Extensive | 3 | 0 | 1 (33.3%) | 2 (66.7%) | |
| Lymph node status | |||||
| N0 | 82 | 67 (81.7%) | 6 (7.3%) | 9 (11.0%) | .536 |
| N1 | 21 | 19 (90.5%) | 1 (4.8%) | 1 (4.8%) | |
| NA | 173 | 138 (79.8%) | 21 (12.1%) | 14 (8.1%) | |
| Extranodal extension | |||||
| No | 17 | 15 (88.2%) | 1 (5.9%) | 1 (5.9%) | .932 |
| Yes | 1 | 1 (100%) | 0 | 0 | |
| NA | 258 | 208 (80.6%) | 27 (10.5%) | 23 (8.9%) | |
| RAI | |||||
| Yes | 81 | 55 (67.9%) | 11 (13.6%) | 15 (18.5%) | .001 d |
| No | 192 | 167 (87.0%) | 17 (8.9%) | 8 (4.2%) | |
| NA | 3 | 2 (66.7%) | 0 | 1 (33.3%) | |
| Disease status at last FU | |||||
| NED | 263 | 221 (84.0%) | 28 (10.6%) | 14 (5.3%) | <.001 d |
| AWD | 11 | 2 (18.2%) | 0 | 9 (81.8%) | |
| DOD | 2 | 1 (50%) | 0 | 1 (50%) | |
Abbreviations: FU, follow-up; HPF, high-power fields (400×, field size 0.24 mm2); NA, not applicable or not available; NED, no evidence of disease; AWD, alive with disease; DOD, dead of disease; PTC, papillary thyroid carcinoma; VI, vascular invasion; HCC, Hurthle cell carcinoma; RAI, radioactive iodine.
Values expressed are n (% of row total) or mean ± SEM whenever appropriate.
P value was obtained using one-way ANOVA for continuous variables; χ2 test or Kurskal-Wallis ANOVA by ranks for nonparametric variables.
For vascular and capsular invasion, focal invasion was defined as 1 to 3 foci, whereas extensive invasion was defined as 4 or more foci.
The P values are significant.
Twenty-four (8.7%) had EVI (≥4 foci) (Fig. 1), and 28 (10.1%) displayed FVI (<4 foci) (Fig. 2). There was no significant difference among tumors with different VI status in terms of age at presentation, mitotic index, surgical margin status, involvement of cervical lymph node(s), and presence of extranodal extension (Table 1, χ2 test or Kruskal-Wallis ANOVA by ranks, P > .05). In contrast, there was a significant association between EVI and male sex (P = .026), a pathologic diagnosis of EHCC or EFC rather than EPTC (P < .001), large tumor size (P < .001), presence of extensive capsular invasion (P < .001), extensive extrathyroidal extension (P = .002), RAI therapy (P = .001), and adverse outcome at last follow-up (P < .001, Table 1). Among patients with EPTC, encapsulated follicular variant of PTC was associated with a significantly higher incidence of VI compared with all the other variants overall (P = .008) and classical variant of PTC in particular (P = .007, Table 1).
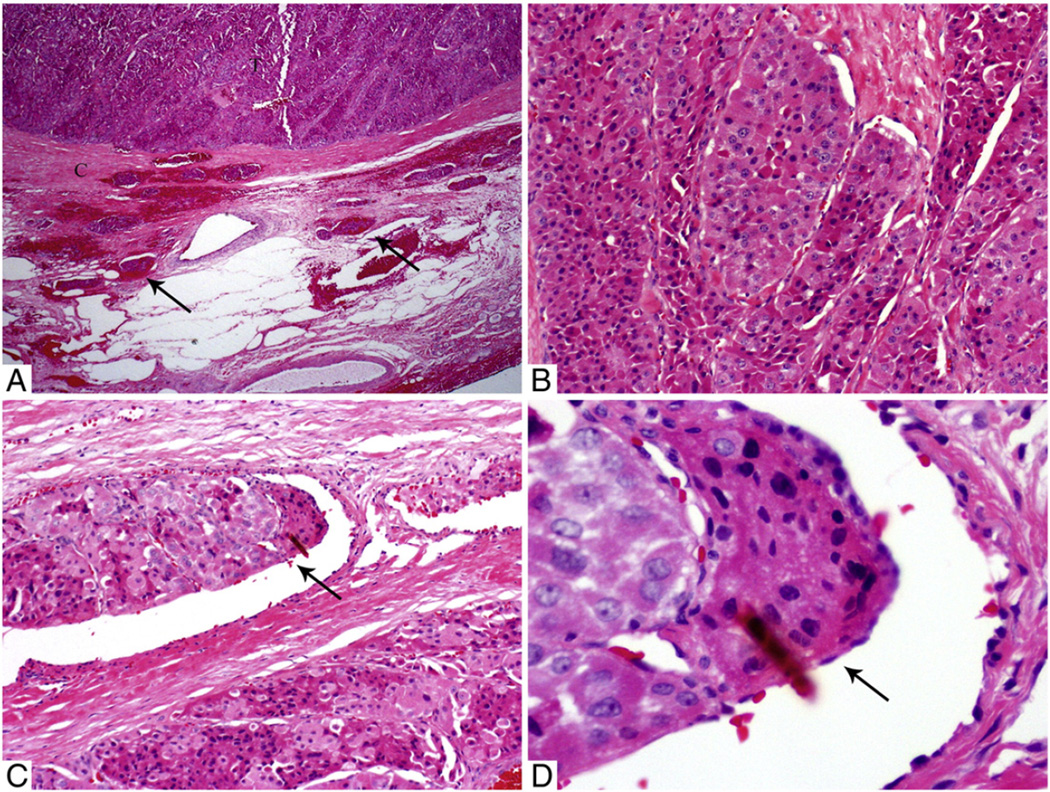
Fig. 1.
Microscopic pictures of a 4-cm EHCC with EVI (7 foci) in a 44-year-old man without distant disease at presentation. The patient developed bone and lung metastasis 3 years after diagnosis. A, Low-power view of the tumor (T) and its capsule (C) with several intra- and extracapsular vessels filled with tumor (arrows). B, Medium-power view of the Hurthle cells growing in nested/trabecular pattern. C, Medium-power view of a tumor thrombus (arrow) hanging in the lumen of an intracapsular vessel. D, High-power view showing the tumor thrombus covered by endothelial cells (arrow).
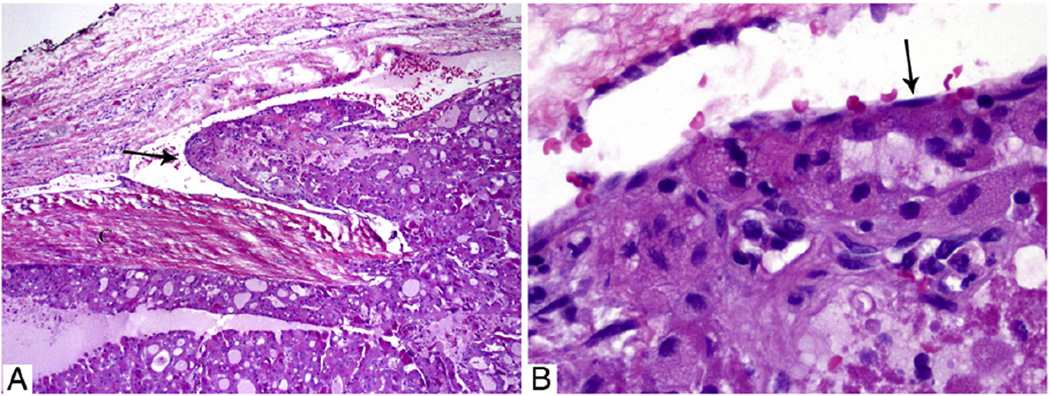
Fig. 2.
Microscopic pictures of a 4.8-cm EHCC with focal VI in a 44-year-old man without distant disease at presentation and not treated by RAI. The patient did not have a recurrence and has no evidence of disease 16 years after diagnosis. A, Medium-power view of the tumor and its capsule with a tumor thrombus hanging (arrow) in the lumen of a vessel located immediately outside the capsule. B, High-power view of the tumor thrombus covered by endothelial cells (arrow).
3.2. Predictors of adverse outcomes in the entire patient population
In our cohort, 13 of 276 patients (4.3%) had adverse outcomes (Table 2). All of the 13 patients harbored DMs, of which 8 had DMs at presentation (Table 3). Median follow-up was 6.0 years (mean ± standard error of mean (SEM), 7.5 ± 0.4 years; up to 28.1 years). In the whole patient cohort, factors associated with distal recurrence included sex (log-rank test, P = .011), older age at presentation (P = .015), large tumor size (P = .048), presence of mitosis (P = .009), EVI (P < .001), HCC diagnosis (P < .001), capsular invasion (P = .026), extrathyroidal extension (P < .016), and RAI therapy (P < .001, Table 2). The Kaplan-Meier plots for RFS stratified by extent of VI and pathology subtype are shown in Figs. 3A and 4A, respectively. On multivariate analysis using Cox proportional hazards model, the presence of CI, EVI, and RAI treatment independently predicted RFS (CI: hazard ratio [HR] = 0.330, P = .048; VI: HR = 46.3, P < .001; and RAI: HR = 0.092, P = .011), whereas sex, age at presentation, presence of mitosis, extrathyroidal extension status, tumor size, and tumor type failed to reach significance (P > .05). Overall, 10 of 24 patients (41.7%) with EVI had poor outcome, with 7 of them harboring EHCC. VI was not identified in 3 patients who had DMs at presentation. All 3 patients harbored encapsulated follicular variant of papillary thyroid carcinoma with capsular invasion (Table 3). Among the 8 patients with DM at presentation, 2 (25%) had a diagnosis of EHCC, whereas the remaining 6 harbored encapsulated follicular variant of PTC.
Table 2.
Clinicopathologic characteristics according to clinical outcome in the whole patient population
| Row totals |
No recurrence (NED) |
Distant recurrence | Pa | ||
|---|---|---|---|---|---|
| AWD | DOD | ||||
| n | 276 | 263 (95.3%) | 11 (4.0%) | 2 (0.7%) | |
| Sex | |||||
| Male | 88 | 80 (90.9%) | 7 (8.0%) | 1 (1.1%) | .01 b |
| Female | 188 | 183 (97.3%) | 4 (2.1%) | 1 (0.5%) | |
| Age | 45.0 ± 0.8 | 59.9 ± 4.0 | 59.0 ± 2.0 | .015 b | |
| Pathologic diagnosis | |||||
| Papillary carcinoma | 224 | 218 (97.3%) | 5 (2.2%) | 1 (0.4%) | <.001 b |
| Follicular carcinoma | 18 | 18 (100%) | 0 | 0 | |
| Hurthle cell carcinoma | 34 | 27 (79.4%) | 6 (17.6%) | 1 (2.9%) | |
| Size | 2.5 ± 0.1 | 4.1 ± 0.5 | 3.6 ± 1.4 | .048 b | |
| Mitotic index (/10 HPFs) | |||||
| 0 | 240 | 231 (96.3%) | 7 (2.9%) | 2 (0.8%) | .009 b |
| 1–4 | 36 | 32 (88.9%) | 4 (11.1%) | 0 | |
| VI | |||||
| No | 224 | 221 (98.7%) | 2 (0.9%) | 1 (0.4%) | <.001 b |
| Focal | 28 | 28 (100%) | 0 | 0 | |
| Extensive | 24 | 14 (58.3%) | 9 (37.5%) | 1 (4.2%) | |
| Capsular invasion | .026 b | ||||
| No | 161 | 157 (97.5%) | 3 (1.9%) | 1 (0.6%) | |
| Focal | 91 | 85 (93.4%) | 6 (6.6%) | 0 | |
| Extensive | 24 | 21 (87.5%) | 2 (8.3%) | 1 (4.2%) | |
| Surgical margin status | .939 | ||||
| NA | 1 | 1 (100%) | 0 | 0 | |
| Negative | 273 | 260 (95.2%) | 11 (4.0%) | 2 (0.7%) | |
| Positive | 2 | 2 (100%) | 0 | 0 | |
| Extrathyroidal extension | .016 b | ||||
| None | 269 | 257 (95.5%) | 10 (3.7%) | 2 (0.7%) | |
| Focal | 4 | 4 (100%) | 0 | 0 | |
| Extensive | 3 | 2 (66.7%) | 1 (33.3%) | 0 | |
| Lymph node status | |||||
| N0 | 82 | 77 (93.9%) | 5 (6.1%) | 0 | .920 |
| N1 | 21 | 21 (100%) | 0 | 0 | |
| NA | 173 | 165 (95.4%) | 6 (3.5%) | 2 (1.2%) | |
| Extranodal extension | .965 | ||||
| NA | 258 | 245 (95.0%) | 11 (4.3%) | 2 (0.8%) | |
| No | 17 | 17 (100%) | 0 | 0 | |
| Yes | 1 | 1 (100%) | 0 | 0 | |
| RAI | <.001 b | ||||
| Yes | 81 | 71 (87.7%) | 9 (11.1%) | 1 (1.2%) | |
| No | 192 | 190 (99.0%) | 1 (0.5%) | 1 (0.5%) | |
| NA | 3 | 2 (66.7%) | 1 (33.3%) | 0 | |
Abbreviations: NED, no evidence of disease; AWD, alive with disease; DOD, died of disease; HCC, Hurthle cell carcinoma; HPF, high-power fields; VI, vascular invasion; NA, not available; RAI, radioactive iodine; RFS, recurrence-free survival.
P value was obtained using log-rank test comparing the RFS between patients with and without recurrence.
The P values are significant.
Table 3.
Clinicopathologic features of patients with adverse outcome
| Pt | Age/sex | Histo | Tumor size (cm) | Capsular invasion (# foci) |
VI (# foci) | Location of DM | Surgery/RAI | Outcome |
|---|---|---|---|---|---|---|---|---|
| Patients without DMs at presentation | ||||||||
| 1 | 50/M | HCC | 2.8 | None (0) | Extensive (23) | Lung | TT/Yes | AWD |
| 2 | 69/M | HCC | 5.0 | Focal (1) | Extensive (4) | Lung, mediastinum | LOB/NA | AWD |
| 3 | 42/F | HCC | 6.0 | Focal (1) | Extensive (7) | Lung, mediastinum, bone | TT/Yes | AWD |
| 4 | 44/M | HCC | 4.0 | Focal (1) | Extensive (7) | Lung, bone | TT/No | AWD |
| 5 | 61/M | HCC | 5.0 | None (0) | Extensive (23) | Bone | TT/No | DOD |
| Patients with DMs at presentation | ||||||||
| 6 | 41/M | HCC | 5.0 | Focal (2) | Extensive (23) | Lung | TT/Yes | AWD |
| 7 | 75/F | HCC | 5.0 | Extensive (9) | Extensive (4) | Lung, mediastinum | LOB/Yes | AWD |
| 8 | 66/M | FVPTC | 5.0 | Extensive (6) | Extensive (8) | Bone | TT/Yes | AWD |
| 9 | 75/M | FVPTC | 4.0 | Focal (1) | None (0) | Bone, soft tissue | TT/Yes | AWD |
| 10 | 62/F | FVPTC | 5.0 | None (0) | Extensive (8) | Lung | TT/Yes | AWD |
| 11 | 64/F | FVPTC | 1.5 | Focal (1) | None (0) | Bone | TT/Yes | AWD |
| 12 | 71/M | FVPTC | 1.6 | None (0) | Extensive (4) | Bone, soft tissue | LOB/Yes | AWD |
| 13 | 57/F | FVPTC | 2.3 | Extensive (4) | None (0) | Bone, kidney | TT/Yes | DOD |
Abbreviations: AWD, alive with disease; DM, distant metastasis; DOD, dead of disease; F, female; FVPTC, follicular variant of papillary thyroid carcinoma; HCC, Hurthle cell carcinoma; Histo, histotypes of the carcinoma; LOB, lobectomy; M, male; Met, metastasis; NA, not available; Pt, patient; RAI, radioactive iodine; TT, total thyroidectomy.
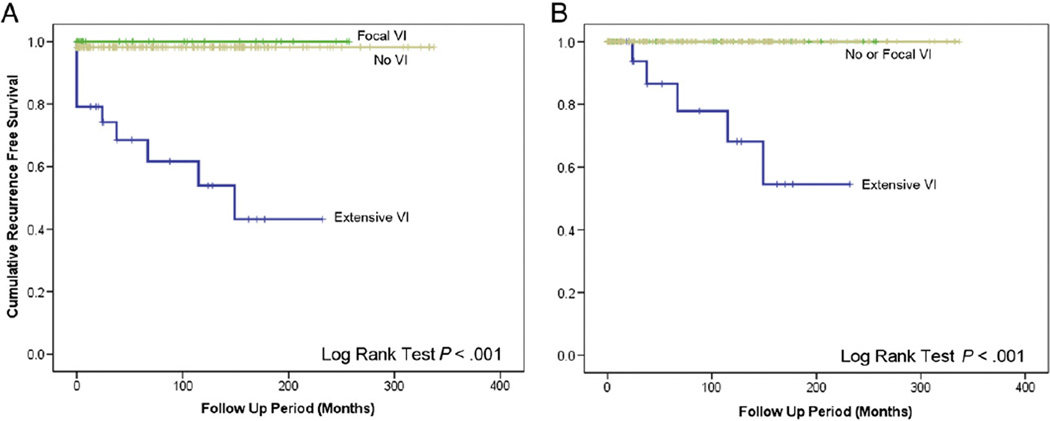
Fig. 3.
RFS according to extent of VI. EVI is correlated with adverse clinical outcome (log-rank test, P < .001). A, All cases. B, Excluding cases with DMs at presentation.
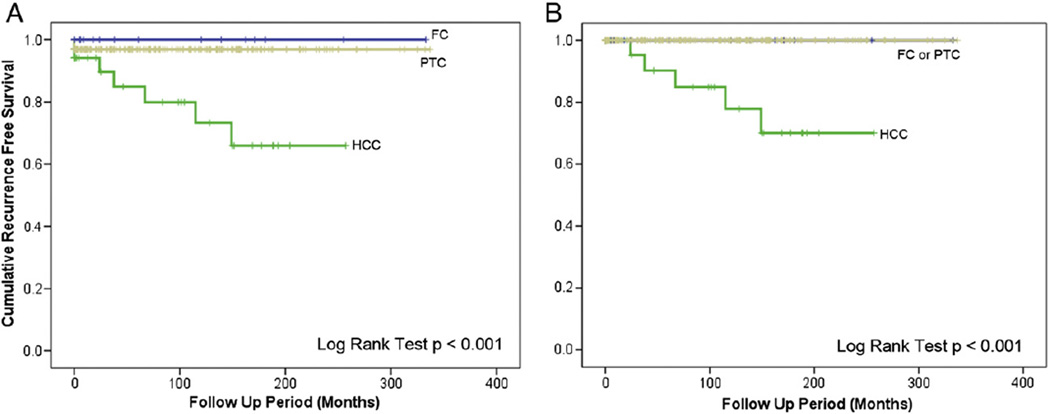
Fig. 4.
RFS according to carcinoma histotype. EHCC is associated with decreased long-term survival compared with EFC and papillary carcinoma (log-rank test, P < .001). A, All cases. B, Excluding cases with DMs at presentation.
3.3. Predictors of adverse outcome in patients without DM at presentation
In patients without DM at presentation, the only factors that were associated with recurrence were male sex (log-rank test, P = .032), EVI (P < .001), and a pathologic diagnosis of EHCC (P < .001, Table 4). Only patients with EVI had recurrence, and all relapsed cases were EHCC. The Kaplan-Meier plots for RFS stratified by extent of VI and pathology subtype are shown in Figs. 3B and 4B, respectively. On multivariate analysis using Cox proportional model, all 3 factors, namely, sex, extent of VI, and pathologic diagnosis, failed to predict clinical outcome independently (P > .05). In patients without DMat presentation, 5 (26%) of 19 patients with EVI had recurrence.
Table 4.
Clinicopathologic characteristics according to clinical outcomes in patients with no evidence of DMs at presentation
| Row total |
No recurrence (NED) |
Distant recurrence | Pa | ||
|---|---|---|---|---|---|
| AWD | DOD | ||||
| n | 267 | 262 (98.1%) | 4 (1.5%) | 1 (0.4%) | |
| Sex | .032 b | ||||
| Male | 83 | 79 (95.2%) | 3 (3.6%) | 1 (1.2%) | |
| Female | 184 | 183 (99.5%) | 1 (0.5%) | 0 | |
| Age | 45.0 ± 0.8 | 51.3 ± 6.2 | 61.0 | .208 | |
| Pathologic diagnosis | <.001b | ||||
| Papillary carcinoma | 217 | 217 (100%) | 0 | 0 | |
| Follicular carcinoma | 18 | 18 (100%) | 0 | 0 | |
| Hurthle cell carcinoma | 32 | 27 (84.4%) | 4 (12.5%) | 1 (3.1%) | |
| Size | 2.5 ± 0.1 | 4.5 ± 0.7 | 5.0 | .111 | |
| Mitotic index (/10 HPFs) | |||||
| 0 | 235 | 231 (98.3%) | 3 (1.3%) | 1 (0.4%) | |
| 1–4 | 32 | 31 (96.9%) | 1 (3.1%) | 0 | |
| VI | <.001 b | ||||
| No | 220 | 220 (100%) | 0 | 0 | |
| Focal | 28 | 28 (100%) | 0 | 0 | |
| Extensive | 19 | 14 (73.7%) | 4 (21.1%) | 1 (5.3%) | |
| Capsular invasion | .449 | ||||
| No | 159 | 157 (98.7%) | 1 (0.6%) | 1 (0.6%) | |
| Focal | 87 | 84 (96.6%) | 3 (3.4%) | 0 | |
| Extensive | 21 | 21 (100%) | 0 | 0 | |
| Surgical margin status | .988 | ||||
| NA | 1 | 1 (100%) | 0 | 0 | |
| Negative | 264 | 259 (98.1%) | 4 (1.5%) | 1 (0.4%) | |
| Positive | 2 | 2 (100%) | 0 | 0 | |
| Extrathyroidal extension | .962 | ||||
| None | 261 | 256 (98.1%) | 4 (1.5%) | 1 (0.4%) | |
| Focal | 4 | 4 (100%) | 0 | 0 | |
| Extensive | 2 | 2 (100%) | 0 | 0 | |
| Lymph node status | .395 | ||||
| N0 | 80 | 77 (96.3%) | 3 (3.8%) | 0 | |
| N1 | 20 | 20 (100%) | 0 | 0 | |
| NA | 167 | 165 (98.8%) | 1 (0.6%) | 1 (0.6%) | |
| Extranodal extension | .857 | ||||
| No | 16 | 16 (100%) | 0 | 0 | |
| Yes | 1 | 1 (100%) | 0 | 0 | |
| NA | 250 | 245 (98.0%) | 4 (1.6%) | 1 (0.4%) | |
| RAI | .433 | ||||
| Yes | 72 | 70 (97.2%) | 2 (2.8%) | 0 | |
| No | 192 | 190 (99.0%) | 1 (0.5%) | 1 (0.5%) | |
| NA | 3 | 2 (66.7%) | 1 (33.3%) | 0 | |
Abbreviations: DM, distant metastases; NED, no evidence of disease; AWD, alive with disease; DOD, died of disease; HCC, Hurthle cell carcinoma; HPF, high-power fields; VI, vascular invasion; NA, not available; RAI, radioactive iodine; RFS, recurrence-free survival.
P value was obtained using log-rank test comparing RFS between patient with and without distant recurrence.
The P values are significant.
3.4. Outcome of patients with focal VI without DM at presentation
Among the 28 patients with focal VI and no DM at presentation, 11 received RAI, whereas the remaining 17 were not treated with RAI. None of these 28 patients had recurrence over a median follow-up period of 5.8 years (up to 21.4 years) despite a mean age of 47.6 years (SEM, 2.5 years) and a mean tumor size of 3.3 cm (SEM, 0.3 cm).
3.5. Tumor sampling
To evaluate the effects of tumor sampling on detection of VI or clinical outcome, we evaluated the number of slides per case, the sections of tumor capsule sampled per tumor, and the sections of tumor capsule per centimeter of tumor among 73 cases within our cohort randomly selected from 1980 to 2004. This subgroup included all 13 cases with recurrence and 60 cases without recurrences. In general, encapsulated lesions were consistently and extensively sampled in our center. For each case, we examined a median of 13 slides (range, 1–47; mean ± SEM, 14 ± 1), 6 tissue sections of tumor capsule (range, 1–21; mean ± SEM, 7 ± 1), and 2.4 sections of tumor capsule per centimeter of tumor (range, 0.6–7.0; mean ± SEM, 2.7 ± 0.2). The tumor sampling did not differ across different time periods (ie, before or after 2000) and was not associated with VI status or clinical outcome (P > .05).
4. Discussion
EVI appears fortunately to be a relatively rare event in LGEFC (8.7% in this study). In accordance with previous studies, we found that EPTC harbors less VI than EFC and EHCC [13,14]. This difference is mainly due to the very low reported rate of VI in EPTC of the classical variant (5%) [14]. The molecular basis for such a difference in angioinvasiveness between EPTC and the other encapsulated thyroid carcinomas is not known at the present time. In these series, patients with EVI had a significantly higher rate of RAI therapy in line with the recommendations emanating from the American Thyroid Association and the NCCN [3,4]. We found a strong correlation between the presence of EVI and extensive CI as well as a large tumor size. These expected results should prompt pathologists to extensively sample the tumor capsule when they encounter large tumors or extensive CI.
More importantly, this study shows that EVI is an independent predictor of recurrence in LGEFC. This is in congruence with the findings of several investigators [8–10,15]. However, this strong relationship between EVI and outcome was not found in all series [5–7]. These discrepant results could be due to different criteria used for the diagnosis of VI. Mete and Asa [6], for example, did not consider the presence of intravascular endothelial-lined tumor as a diagnostic criterion for VI. This is based on the idea that intravascular endothelial-lined tumor is separated from the bloodstream by endothelial cells. However, the endothelization and reorganization of tumor thrombi are well known phenomena seen in certain malignancies. Indeed, in renal cell carcinomas, one can see a large tumor thrombus growing in the inferior vena cava and covered by endothelial cells. A similar picture can be seen in large neck veins including the jugular vein when involved by widely invasive HCC. Other differences between studies could not be explained by variation in the definition of VI. Indeed, Goldstein et al [7] used a definition similar to ours but did not find correlation between extent of VI and poor outcome. These conflicting results could be due to interobserver variability because the identification of VI can be quite subjective. For example, pathologists may have different thresholds in regard to how much a tumor should protrude into the lumen to qualify as VI. We decided to use the definition of VI delineated in the authorative 1992 Armed Forces Institute of Pathology fascicle [12] because it has been adopted in the majority of the published literature.
In our cohort of 276 patients, the largest cohort of LGEFCs published to date, EVI was associated with a recurrence rate of 42% compared with 1% recurrence rate in LGEFCs with only focal or no VI. It is important to note that all 3 patients without EVI who had adverse outcome presented with DM. These 3 individuals had only capsular invasion. This could explain why capsular invasion was found to be an independent predictor of recurrence in the whole patient population. It is our experience that when encapsulated follicular cell–derived thyroid carcinomas with limited focal VI or capsular invasion recur, they present with distant disease. These tumors often have a significant amount of intratumoral fibrosis, raising the possibility of tumor regression. Whatever the mechanism behind this unusual phenomenon, one can reasonably predict the risk of recurrence using extent of VI as long as the patient does not have distant disease at presentation. In our cases lacking DMs at diagnosis, only tumors with EVI recurred. The relapse rate for EVI was 26% in this patient population.
In contrast, all 28 patients with focal VI and no DM at presentation lacked recurrence with a median follow-up of 5.8 years. This subgroup behaved exactly like patients with no VI (Fig. 3B) and included 17 patients that did not receive RAI therapy. Is there a molecular basis for such a difference in behavior between patients with absent/focal and extensive VI? Using expression arrays, our group [16] has previously shown that EHCCs with only capsular and/or focal VI (<4 foci) exhibited a different molecular signature compared with HCCs with extensive/significant VI (ie, >4 foci of VI, extrathyroid VI). Molecular pathways that differentiate HCC with extensive/significant VI from the other Hurthle cell tumors included the PIK3CA-Akt-mTOR and Wnt/beta-catenin pathways. Previous study has shown that nuclear overexpression of beta-catenin promotes VI [17]. Perhaps, beta-catenin plays a central role in regulating the differences in vascular phenotype that is the hallmark of HCC with extensive/significant VI [16]. Whatever the mechanism leading to EVI, the excellent outcome in patients with focal VI including those spared RAI treatment suggests that tumors with focal VI are very indolent. However, in view of the relatively small number of analyzed cases with focal VI, it remains to be confirmed whether or not such patients should receive RAI therapy.
HCC is currently classified as a variant of follicular carcinoma in the WHO 2004 classification [18]. However, recent studies have shown that the copy number profiles of HCC are distinctly different from those of FCs, with copy number gains involving chromosome 4p, 5p, 6p, 7p, 8p 10p, 12p, 16q, and 17p, and copy number loss involving 4q, 6p, 7p, 9q, 12q, and 22 [16]. The mutational profile of HCC is also unique with a 16% frequency of RAS mutations and no PPARG rearrangements, whereas follicular carcinomas have a 45% RAS mutation rate and PPARG rearrangements in 25%–60% of cases [16]. Clinically, HCC follows a more aggressive course compared with other types of differentiated thyroid cancers, generally presenting as tumor of larger size, more advanced clinical stage, and shorter disease-specific survival, indicating that HCC may be a distinct entity with its unique pathologic and clinical signatures [9,19]. Importantly, a minority of HCCs show RAI uptake in metastatic sites (38% in 1 study), in contrast to follicular carcinomas, which are in their vast majority RAI avid [20,21]. We report in this series that EHCCs with EVI had a particularly high risk of recurrence (7/11, 64%) compared with 33% (3/9) in EPTCs with EVI and 0% (0/4) in EFCs with EVI. In patients without DM at presentation, only HCCs with EVI relapsed (Fig. 4B). Based on the above observations, we believe that there is sufficient molecular and clinical evidence to consider reclassifying HCC as a separate entity from follicular carcinomas.
In conclusion, we have found that EVI is an independent prognostic predictor in encapsulated low-grade follicular cell–derived thyroid carcinoma. The extent of VI should be routinely reported, and 4 foci or more of VI should prompt the clinician to consider aggressive therapy, that is, completion thyroidectomy and adjuvant RAI ablation. Among all types of encapsulated low-grade carcinoma studied, HCC with EVI has a particularly high risk of recurrence. This is further evidence that HCCs are biologically and clinically distinct from follicular carcinomas and should not be classified as a variant of the latter. When DMs are not found at presentation, patients with focal VI seem to be at a very low risk of recurrence even if not treated with RAI. However, more studies on LGEFCs with focal VI are needed to confirm our findings and decide if such patients should be treated conservatively.
Footnotes
Disclosure statement: No competing financial interests exist for all contributory authors.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.American Thyroid Association Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 4.Tuttle RM National Comprehensive Cancer Network. Clinical practice guidelines in oncology, thyroid cancer: National Comprehensive Cancer Network. 2014 [Google Scholar]
- 5.Graham A. Malignant epithelial tumors of the thyroid, with special reference to blood vessels. Surg Gynecol Obstet. 1924;39:781–790. [Google Scholar]
- 6.Mete O, Asa SL. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol. 2011;24:1545–1552. doi: 10.1038/modpathol.2011.119. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein NS, Czako P, Neill JS. Metastatic minimally invasive (encapsulated) follicular and Hurthle cell thyroid carcinoma: a study of 34 patients. Mod Pathol. 2000;13:123–130. doi: 10.1038/modpathol.3880023. [DOI] [PubMed] [Google Scholar]
- 8.Collini P, Sampietro G, Pilotti S. Extensive vascular invasion is a marker of risk of relapse in encapsulated non-Hurthle cell follicular carcinoma of the thyroid gland: a clinicopathological study of 18 consecutive cases from a single institution with a 11-year median follow-up. Histopathology. 2004;44:35–39. doi: 10.1111/j.1365-2559.2004.01729.x. [DOI] [PubMed] [Google Scholar]
- 9.Ghossein RA, Hiltzik DH, Carlson DL, et al. Prognostic factors of recurrence in encapsulated Hurthle cell carcinoma of the thyroid gland: a clinicopathologic study of 50 cases. Cancer. 2006;106:1669–1676. doi: 10.1002/cncr.21825. [DOI] [PubMed] [Google Scholar]
- 10.Lang W, Choritz H, Hundeshagen H. Risk factors in follicular thyroid carcinomas. A retrospective follow-up study covering a 14-year period with emphasis on morphological findings. Am J Surg Pathol. 1986;10:246–255. [PubMed] [Google Scholar]
- 11.Brennan MD, Bergstralh EJ, van Heerden JA, McConahey WM. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1991;66:11–22. doi: 10.1016/s0025-6196(12)61170-7. [DOI] [PubMed] [Google Scholar]
- 12.Rosai JCM, Delellis RA. Tumors of the thyroid gland. Washington, DC: Armed Forces Institute of Pathology; 1992. [Google Scholar]
- 13.D’Avanzo A, Treseler P, Ituarte PH, et al. Follicular thyroid carcinoma: histology and prognosis. Cancer. 2004;100:1123–1129. doi: 10.1002/cncr.20081. [DOI] [PubMed] [Google Scholar]
- 14.Rivera M, Tuttle RM, Patel S, Shaha A, Shah JP, Ghossein RA. Encapsulated papillary thyroid carcinoma: a clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern) Thyroid. 2009;19:119–127. doi: 10.1089/thy.2008.0303. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Hirokawa M, Masuoka H, et al. Prognostic factors of minimally invasive follicular thyroid carcinoma: extensive vascular invasion significantly affects patient prognosis. Endocr J. 2013;60:637–642. doi: 10.1507/endocrj.ej12-0419. [DOI] [PubMed] [Google Scholar]
- 16.Ganly I, Ricarte Filho J, Eng S, et al. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J Clin Endocrinol Metab. 2013;98:E962–E972. doi: 10.1210/jc.2012-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H, Masuda N, Shimura T, et al. Nuclear beta-catenin expression at the invasive front and in the vessels predicts liver metastasis in colorectal carcinoma. Anticancer Res. 2008;28:1821–1830. [PubMed] [Google Scholar]
- 18.DeLellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and genetics of tumours of the endocrine organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 19.Goffredo P, Roman SA, Sosa JA. Hurthle cell carcinoma: a population-level analysis of 3311 patients. Cancer. 2013;119:504–511. doi: 10.1002/cncr.27770. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Penabad L, Chiu AC, Hoff AO, et al. Prognostic factors in patients with Hurthle cell neoplasms of the thyroid. Cancer. 2003;97:1186–1194. doi: 10.1002/cncr.11176. [DOI] [PubMed] [Google Scholar]
- 21.Tala HP, Rondeau G, Ghossein RA, et al. Paris, France: International Thyroid Cancer Meeting; 2010. Histologically aggressive types of follicular cell–derived thyroid cancer often have radioactive avid distant metastases: a study of 314 patients with distant metastases at a single institution. Abstract 2679. [Google Scholar]