Abstract
Background
The risk of ventricular arrhythmia with citalopram and escitalopram is controversial. In this study we investigated the association between these two drugs and the risk of ventricular arrhythmia.
Methods
We conducted a population-based retrospective cohort study of older adults (mean age 76 years) from 2002 to 2012 in Ontario, Canada, newly prescribed citalopram (n = 137 701) or escitalopram (n = 38 436), compared to those prescribed referent antidepressants sertraline or paroxetine (n = 96 620). After inverse probability of treatment weighting using a propensity score, the baseline characteristics of the comparison groups were similar. The primary outcome was a hospital encounter with ventricular arrhythmia within 90 days of a new prescription, assessed using hospital diagnostic codes. The secondary outcome was all-cause mortality within 90 days.
Results
Citalopram was associated with a higher risk of a hospital encounter with ventricular arrhythmia compared with referent antidepressants (0.06% vs. 0.04%, relative risk [RR] 1.53, 95% confidence intervals [CI]1.03 to 2.29), and a higher risk of mortality (3.49% vs. 3.12%, RR 1.12, 95% CI 1.06 to 1.18). Escitalopram was not associated with a higher risk of ventricular arrhythmia compared with the referent antidepressants (0.03% vs. 0.04%, RR 0.84, 95% CI 0.42 to 1.68), but was associated with a higher risk of mortality (2.86% vs. 2.63%, RR 1.09, 95% CI 1.01 to 1.18).
Conclusion
Among older adults, initiation of citalopram compared to two referent antidepressants was associated with a small but statistically significant increase in the 90-day risk of a hospital encounter for ventricular arrhythmia.
Introduction
Selective serotonin re-uptake inhibitors (SSRIs; e.g., citalopram, escitalopram, paroxetine and sertraline) are commonly prescribed antidepressants.[1–4] Citalopram and escitalopram have been implicated in ventricular arrhythmias, presumably by lengthening the QT interval of the cardiac cycle.[5–18] The Food and Drug Administration (FDA) and Health Canada caution against the use of citalopram at doses >20 mg/day in patients over 65 years of age).[19–22] The FDA warnings were based on an unpublished trial of 119 patients randomized to placebo or citalopram, demonstrating an increase in the corrected QT interval with citalopram.[19] These safety warnings have been controversial,[23–25] with inconsistent findings in other follow-up studies.[5, 14, 23, 26–29] Many of these studies were limited by the use of QT prolongation rather than ventricular arrhythmia risk,[14, 26, 28] a young population cohort,[5, 23, 27, 28] low statistical power,[26] and not accounting for important confounding factors in the analysis.[29] Escitalopram (the S enantiomer of citalopram) has also been associated with QT interval prolongation and Health Canada warns against the use of >10mg/day of escitalopram for patients 65 years of age or older.[5, 7, 14–15, 28, 30] We conducted this large propensity score-weighted population-based cohort study of older adults to investigate whether initiating citalopram or escitalopram in the outpatient setting is associated with a higher risk of ventricular arrhythmia, compared to initiating sertraline or paroxetine (referent antidepressants).
Methods
Design and Setting
We conducted a population-based retrospective cohort study of older adults from April 1, 2002 to December 31, 2012 in Ontario, Canada, who had received a new outpatient prescription for citalopram, escitalopram, sertraline or paroxetine (the most commonly prescribed SSRIs in Ontario). Ontario has approximately 2 million residents 65 years of age or older, who have full coverage for hospital and physician services, and prescription drugs.[31]
We used datasets held securely in linkable-files without any direct personal identifiers, and analyzed at the Institute for Clinical Evaluative Sciences (ICES). Patient information was anonymized and de-identified prior to analysis. The pre-specified protocol was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre (Toronto, Ontario, Canada). The reporting of this study follows guidelines for observational studies (see S1 Table).[32]
Data Sources
We ascertained patient baseline characteristics, drug use and outcome data using records from eight linked databases. The Ontario Drug Benefit database contains highly accurate records for outpatient prescriptions dispensed to patients aged 65 years or older (error rate less than 1%).[33] The Ontario Registered Persons Database records vital statistics, including date of death. The Canadian Institute for Health Information (CIHI)–Discharge Abstract Database, the CIHI—National Ambulatory Care Reporting System database, and the Ontario Mental Health Reporting System database contain diagnostic and procedural information on all hospitalizations, emergency room and psychiatric facility visits. The ICES Physician Database reports prescriber and specialist referral data. The Ontario Health Insurance Plan database (OHIP) includes health claims for physician services, and the Canadian Organ Replacement Register identifies patients with end-stage kidney disease. We have used these databases previously to research adverse drug events and health outcomes.[34–40] The information obtained was complete, except for neighbourhood income quintile (missing in 0.3% of patients) and prescriber specialty (missing in 12.7% of patients).
We used International Classification of Diseases 9th revision (ICD 9; pre-2002) and 10th revision (ICD 10; post- 2002) codes to assess baseline co-morbidities in the five years prior to the receipt of the relevant prescriptions (S2 Table), in concordance with prior studies [34, 36]. We assessed baseline medications and health care use in the 120 days and 1 year prior to the date of the new SSRI prescription, respectively.
Patients
We established a cohort of older adults in Ontario, Canada, who were dispensed a new outpatient prescription of at least 7 days for citalopram, escitalopram, paroxetine or sertraline between April 2002 and December 2012. The prescription date was the cohort entry date. Patients were separated into three groups based on their prescription: 1) citalopram, 2) escitalopram and 3) referent antidepressants (paroxetine or sertraline). Paroxetine and sertraline were grouped together as they have low cardiac toxicity, and both are prescribed for similar indications as citalopram and escitalopram.[10, 14–15, 27]
We excluded from analyses: patients in their first year of eligibility for prescription drug coverage (age 65) to avoid incomplete medication records; those with antidepressant prescriptions in the 180 days prior to the cohort entry date to ensure new antidepressant use; those discharged from hospital in the two days prior to their cohort entry date to ensure new outpatient prescriptions (patients continuing antidepressants initiated in hospital would have their outpatient prescription dispensed on the same day or the day after hospital discharge); those with a history of ventricular arrhythmia, cardiac arrest or implantable cardiac defibrillator to capture de novo arrhythmic events in follow up; and those with nonstandard daily drug doses to ensure generalizability to usual care and omit data errors. Patients with multiple eligible study drug prescriptions entered the cohort once on the first prescription.
Outcomes
We ascertained all outcomes within 90 days of the cohort entry date, to mimic the duration of follow-up in corresponding clinical trials and to avoid potential crossovers between the groups that could occur with longer follow up.[5, 14] Since, QT prolongation starts within hours of initiating citalopram or escitalopram, we expected drug-related ventricular arrhythmias to occur soon after SSRI initiation.[7, 41–42]
The primary outcome was at least one hospital encounter (emergency room presentation or hospital admission) with ventricular arrhythmia. The secondary outcome was all-cause mortality. Diagnostic codes used to ascertain outcomes are listed in S3 Table (ICD 10 diagnostic codes were used to assess ventricular arrhythmia and this coding system was implemented in Canada in 2002). These codes are entered into the databases by trained personnel based on physician-recorded diagnoses in patients’ medical charts. The ICD 10 codes for ventricular arrhythmia have not been previously validated. However, their sensitivity is expected to be low as ventricular arrhythmias frequently go undetected in routine healthcare (often occuring outside hospital settings, in unmonitored patients, or in the setting of multi-organ medical illness). Previous studies assessing the accuracy of ICD 9 and ICD 10 codes for cardiac arrhythmia (ventricular and supraventricular) show a positive predictive value exceeding 80%.[43–46] We performed an ethics-approved manual review of 202 random charts in our region, looking at hospital encounters (emergency visits or admissions) with the ventricular arrhythmia codes used in this study, and confirmed a positive predictive value of 92% (95% confidence interval [CI] 87 to 95%). All-cause mortality data is accurately coded in our data sources, with a sensitivity of 97.8% and specificity of 100% for the finding of death.[47]
Statistical Analysis
We used inverse probability of treatment weights based on propensity scores to eliminate systematic differences in the baseline characteristics of the compared groups while retaining all individuals in the analysis.[48] The propensity scores provided the probability of receiving a prescription for the exposure drug (citalopram or escitalopram) given a set of measured baseline characteristics. Scores were calculated using multivariable logistic regression models with 48 baseline characteristics (S4 Table)–chosen because of their potential influence on the outcomes or segregation of patients between the compared groups.[49–51] By applying propensity score-based weights to the patients and outcomes, we created weighted groups that were well-balanced on all measured baseline characteristics. We compared baseline characteristics between the groups using standardized differences. This metric describes differences between the group means relative to the pooled standard deviation—a difference greater than 10% is considered meaningful.[48, 52] We calculated relative risks (RR) with 95% confidence intervals (CI) using log-binomial regression models accounting for the weights.
We also evaluated the association for both exposed groups (citalopram and escitalopram) with our outcomes in pre-specified subgroups of patients—defined by the presence or absence of: 1) congestive heart failure, 2) coronary artery disease, 3) chronic kidney disease, and 4) high dose (S5 Table). We hypothesized a higher risk in the presence of these conditions. We identified chronic kidney disease using an algorithm of hospital diagnostic codes validated for older adults in our region.[53] We determined interaction p-values by including interaction terms in the regression models. We interpreted a two-sided p-value of less than 0.05 as statistically significant, and performed all analysis using SAS version 9.3 (SAS Institute, Cary, North Carolina).
Results
Baseline Characteristics
We identified 472 001 patients with prescriptions for the study SSRIs. After applying our selection criteria, we had 137 701 older adults with prescriptions for citalopram, 38 436 for escitalopram and 96 620 for the referent antidepressants (refer to S1 Fig; less than 10% of patients [40 015 patients] were excluded for non-standard daily doses of the SSRI). The mean age was 76 years old (range 66 to 105), and 66% were women. General practitioners wrote 78% of the prescriptions. The distribution of baseline characteristics before and after propensity score weighting are presented in Table 1 for citalopram and Table 2 for escitalopram.
Table 1. Baseline characteristics for the citalopram cohort (pre and post weighting).
| Un-weighted | Weighteda | |||||
|---|---|---|---|---|---|---|
| Citalopram n = 137 701 (%) | Paroxetine or Sertraline n = 96 620 (%) | Standardized Differenceb (%) | Citalopram n = 137 701 (%) | Paroxetine or Sertralinen = 135 746(%) | Standardized Differenceb (%) | |
| DEMOGRAPHICS | ||||||
| Age, years | 76 (7.4) | 75 (7.1) | 19 | 76 (7.4) | 76 (8.7) | 2 |
| Women | 65.5 | 66.8 | 3 | 65.5 | 65.8 | 1 |
| Ruralc | 15.4 | 13.5 | 5 | 15.4 | 15.3 | 0 |
| Long term care | 6.9 | 3.4 | 16 | 6.9 | 6.3 | 2 |
| Income quintiled | ||||||
| One (lowest) | 20.6 | 20.9 | 1 | 20.6 | 20.8 | 0 |
| Two | 21.2 | 21.9 | 2 | 21.2 | 21.8 | 1 |
| Three (medium) | 19.7 | 20.0 | 1 | 19.7 | 20.1 | 1 |
| Four | 19.0 | 18.6 | 1 | 19.0 | 18.5 | 1 |
| Five (highest) | 19.5 | 18.6 | 2 | 19.5 | 18.7 | 2 |
| Year of cohort entrye | ||||||
| 2002–2005 | 41.6 | 60.7 | 39 | 41.6 | 58.1 | 33 |
| 2006–2009 | 39.8 | 25.1 | 32 | 39.8 | 26.7 | 28 |
| 2010–2012 | 18.6 | 14.2 | 12 | 18.6 | 15.2 | 9 |
| COMORBIDITIESf | ||||||
| Charlson Comorbidity Indexg | 0.74 (1.1) | 0.62 (1.0) | 12 | 0.74 (1.1) | 0.73 (1.3) | 1 |
| Dementia | 20.3 | 12.5 | 21 | 20.3 | 19.2 | 2 |
| Schizophrenia/psychotic disorders | 4.0 | 3.4 | 3 | 4.0 | 4.4 | 2 |
| Bipolar disorder | 3.6 | 3.5 | 0 | 3.6 | 3.8 | 1 |
| Unipolar depression/anxiety disorderh | 21.6 | 20.6 | 2 | 21.6 | 21.0 | 1 |
| History of self-harm | 0.2 | 0.1 | 2 | 0.2 | 0.1 | 1 |
| Major haemorrhage | 5.7 | 4.7 | 4 | 5.7 | 5.6 | 0 |
| Haemorrhagic Stroke | 0.6 | 0.4 | 3 | 0.6 | 0.5 | 1 |
| Ischemic Stroke | 4.7 | 3.2 | 7 | 4.7 | 4.5 | 1 |
| Transient Ischemic Attack | 1.3 | 1.1 | 2 | 1.3 | 1.5 | 1 |
| Chronic liver disease | 3.7 | 3.6 | 1 | 3.7 | 3.8 | 0 |
| Chronic Kidney Disease | 6.7 | 5.1 | 7 | 6.7 | 6.5 | 1 |
| Congestive heart failure | 15.8 | 13.7 | 6 | 15.8 | 15.7 | 0 |
| Coronary artery diseasei | 32.4 | 30.6 | 4 | 32.4 | 32.3 | 0 |
| Angina | 24.0 | 23.5 | 1 | 24.0 | 24.0 | 0 |
| Acute myocardial infarction | 4.5 | 3.9 | 3 | 4.5 | 4.5 | 0 |
| Pacemaker | 3.2 | 2.4 | 5 | 3.2 | 3.1 | 0 |
| Atrial fibrillation/flutter | 4.7 | 4.2 | 2 | 4.7 | 4.7 | 0 |
| Peripheral vascular disease | 2.3 | 2.1 | 1 | 2.3 | 2.5 | 1 |
| Chronic lung disease | 30.7 | 30.5 | 0 | 30.7 | 30.8 | 0 |
| Cancerj | 15.7 | 14.1 | 5 | 15.7 | 15.7 | 0 |
| Alcoholism | 2.4 | 2.4 | 0 | 2.4 | 2.5 | 0 |
| Seizure | 1.0 | 0.8 | 2 | 1.0 | 1.0 | 0 |
| Acute kidney injury | 2.4 | 1.6 | 6 | 2.4 | 2.3 | 1 |
| Hospitalization with hyperkalemia | 0.8 | 0.7 | 2 | 0.8 | 0.9 | 1 |
| Venous Thromboembolism | 1.5 | 1.1 | 3 | 1.5 | 1.5 | 0 |
| MEDICATIONSk | ||||||
| Anti-arrhythmics | 2.2 | 2.1 | 1 | 2.2 | 2.3 | 1 |
| Antipsychotics | 7.1 | 5.0 | 9 | 7.1 | 6.9 | 1 |
| Proton pump inhibitors | 31.2 | 27.1 | 9 | 31.2 | 31.0 | 0 |
| Anti-emetic | 2.2 | 1.7 | 3 | 2.2 | 2.0 | 1 |
| Lithium | 0.3 | 0.3 | 1 | 0.3 | 0.3 | 0 |
| Anti-lipemics | 41.6 | 39.4 | 5 | 41.6 | 41.2 | 1 |
| Antihypertensives | 72.1 | 68.8 | 7 | 72.1 | 71.9 | 0 |
| H2RAs | 10.4 | 12.8 | 8 | 10.4 | 10.5 | 0 |
| Pro-kinetics | 4.6 | 4.1 | 3 | 4.6 | 4.5 | 1 |
| Antidiabetics | 16.3 | 15.0 | 3 | 16.3 | 16.0 | 1 |
| Acetylsalicylic acid | 9.6 | 11.5 | 6 | 9.6 | 9.7 | 0 |
| Anticoagulants | 10.4 | 7.7 | 9 | 10.4 | 10.2 | 1 |
| Antiplatelet | 6.5 | 4.7 | 8 | 6.5 | 6.3 | 1 |
| Tri-cyclic antidepressants | 1.4 | 1.6 | 1 | 1.4 | 1.5 | 1 |
| Opioids | 0.0 | 0.1 | 1 | 0.0 | 0.1 | 1 |
| Anti-malarial | 0.7 | 0.7 | 0 | 0.7 | 0.7 | 0 |
| Anti-viral | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 1 |
| Antibiotic | 36.6 | 36.4 | 1 | 36.6 | 36.9 | 1 |
| Antineoplastic | 4.5 | 3.8 | 4 | 4.5 | 4.1 | 2 |
| Benzodiazepine | 39.6 | 42.8 | 5 | 39.6 | 40.3 | 1 |
| NSAIDSl | 21.5 | 24.0 | 6 | 21.5 | 21.7 | 1 |
| Cholinesterase inhibitors | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 0 |
| Anticonvulsants | 3.6 | 3.0 | 4 | 3.6 | 3.4 | 1 |
| DOSEm | ||||||
| High | 6.5 | 12.6 | 21 | 6.5 | 6.8 | 1 |
| PRESCRIBER | ||||||
| General Practitioner | 76.6 | 78.5 | 5 | 76.6 | 76.9 | 1 |
| Psychiatrist | 2.6 | 2.4 | 1 | 2.6 | 2.6 | 0 |
| Internist | 0.8 | 0.7 | 1 | 0.8 | 0.8 | 0 |
| Other | 6.9 | 4.8 | 9 | 6.9 | 6.5 | 2 |
| Missing | 13.1 | 13.5 | 1 | 13.1 | 13.3 | 1 |
| HEALTH CARE USEn | ||||||
| Number of Hospitalizations | ||||||
| 0 | 58.9 | 63.9 | 10 | 58.9 | 59.8 | 2 |
| 1 to 3 | 37.2 | 33.0 | 9 | 37.2 | 36.3 | 2 |
| 4 to 6 | 3.4 | 2.6 | 5 | 3.4 | 3.3 | 1 |
| 7 to 9 | 0.4 | 0.3 | 2 | 0.4 | 0.4 | 0 |
| 10 to 12 | 0.1 | 0.1 | 0 | 0.1 | 0.1 | 0 |
| over 12 | 0.0 | 0.0 | 0 | 0.0 | 0.1 | 1 |
| Number of Emergency room visits | ||||||
| 0 | 52.7 | 59.5 | 14 | 52.7 | 54.3 | 3 |
| 1 to 3 | 39.7 | 34.6 | 11 | 39.7 | 37.9 | 4 |
| Over 3 | 7.6 | 13.0 | 18 | 7.6 | 7.8 | 1 |
| General Practitioner Visits | ||||||
| 0–4 | 13.9 | 15.9 | 6 | 13.9 | 14.5 | 2 |
| 5–9 | 22.4 | 24.2 | 4 | 22.4 | 22.2 | 0 |
| 10–14 | 19.5 | 20.2 | 2 | 19.5 | 19.2 | 1 |
| 15–19 | 12.9 | 13.0 | 0 | 12.9 | 12.9 | 0 |
| 20–24 | 8.6 | 8.2 | 1 | 8.6 | 8.5 | 0 |
| 25–29 | 5.7 | 5.3 | 2 | 5.7 | 5.7 | 0 |
| ≥30 | 17.1 | 13.2 | 11 | 17.1 | 16.9 | 1 |
| At home physician services | 11.0 | 10.2 | 3 | 11.0 | 11.7 | 2 |
| Specialist Consultations | ||||||
| Psychiatrist consults | 7.8 | 6.0 | 7 | 7.8 | 7.6 | 1 |
| Nephrologist consultso | 6.0 | 5.1 | 4 | 6.0 | 6.0 | 0 |
| Cardiologist visits | 42.1 | 37.9 | 9 | 42.1 | 41.7 | 1 |
| Neurologist consults | 11.5 | 9.5 | 7 | 11.5 | 11.3 | 1 |
| Diagnostic tests/Interventions | ||||||
| Electrocardiogram | 87.8 | 86.2 | 5 | 87.8 | 87.6 | 0 |
| Stress test | 35.3 | 34.9 | 1 | 35.3 | 35.0 | 0 |
| Echocardiography | 41.7 | 38.1 | 7 | 41.7 | 41.4 | 1 |
| Cardiac Catheterization | 6.8 | 6.3 | 2 | 6.8 | 6.5 | 1 |
| Holter Monitor | 21.1 | 19.3 | 5 | 21.1 | 21.0 | 0 |
| Coronary angiogram | 7.5 | 6.8 | 3 | 7.5 | 7.0 | 2 |
| Chest X-ray | 78.4 | 76.1 | 5 | 78.4 | 78.2 | 0 |
| Pulmonary function test | 24.8 | 25.0 | 0 | 24.8 | 25.1 | 1 |
| Carotid ultrasound | 19.5 | 17.2 | 6 | 19.5 | 19.3 | 1 |
| Computed Tomography of the Head | 37.0 | 29.7 | 15 | 37.0 | 36.1 | 2 |
| Computed Tomography of other area | 35.7 | 30.2 | 12 | 35.7 | 35.3 | 1 |
| Mammogram | 27.0 | 30.7 | 8 | 27.0 | 27.4 | 1 |
| Bone Mineral Density | 40.1 | 39.6 | 1 | 40.1 | 40.3 | 0 |
Data presented as percent except for age and Charlson Comorbidity Index which are presented as mean (standard deviation).
Abbreviations: Non-steroidal anti-inflammatory drug (NSAID)–excludes acetyl-salicylic acid, Acetylsalicylic acid (ASA), Histamine H2 Receptor antagonist (H2RA), Not applicable (N/A)
a Weighted cohort based on inverse probability of treatment weights, using a propensity score based on 48 baseline characteristics.
b Standardized differences are less sensitive to sample size than traditional hypothesis tests. They provide a measure of the difference between groups divided by the pooled standard deviation; a value greater than 10% is interpreted as a meaningful difference between the groups.
c Defined as a population <10 000 people.
d Income was categorized into fifths of average neighbourhood income on the cohort entry date.
e The year of cohort entry is also referred to as the year of cohort entry date.
f Comorbidities assessed by administrative database codes in the previous 5 years.
g Charlson Comorbidity Index [Charlson ME, Pompei P, Alex KL, Mackenzie CR. A new method for classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987;40(5):373–383. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–1139.] was calculated using 5 years of hospitalization data. “No hospitalizations” received a score of 0.
h The prevalence of depression is low since depression is not usually an in-patient disorder, and thus often not coded in the source databases.
i Coronary artery disease includes receipt of coronary artery bypass graft surgery and percutaneous coronary intervention.
j Major cancers include esophagus, lung, bowel, liver, pancreas, breast, male/female reproductive organs, as well as leukemias and lymphomas.
k Baseline medication use assessed in the previous 120 days.
l Excludes acetylsalicylic acid.
m Refer to S5 Table for definitions of high and low doses.
n Health care use assessed in the one year prior to SSRI prescription.
o Based on the ICES physician database.
Table 2. Baseline characteristics for the escitalopram cohort (pre and post weighting).
| Un-weighted | Weighteda | |||||
|---|---|---|---|---|---|---|
| Escitalopram n = 38 436 (%) | Paroxetine or Sertraline n = 96 620 (%) | Standardized Differenceb (%) | Escitalopram n = 38 436 (%) | Paroxetine or Sertraline n = 113 058 (%) | Standardized Differenceb (%) | |
| DEMOGRAPHICS | ||||||
| Age, years | 76 (7.6) | 75 (7.1) | 9 | 76 (7.6) | 75 (4.5) | 4 |
| Women | 63.0 | 66.8 | 8 | 63.0 | 63.2 | 0 |
| Ruralc | 12.8 | 13.5 | 2 | 12.8 | 12.8 | 0 |
| Long term care | 5.0 | 3.4 | 8 | 5.0 | 4.6 | 2 |
| Income quintiled | ||||||
| One (lowest) | 18.9 | 20.9 | 5 | 18.9 | 20.0 | 3 |
| Two | 20.3 | 21.9 | 4 | 20.3 | 21.4 | 3 |
| Three (medium) | 19.6 | 20.0 | 1 | 19.6 | 20.2 | 2 |
| Four | 20.1 | 18.6 | 4 | 20.1 | 19.0 | 3 |
| Five (highest) | 21.1 | 18.6 | 6 | 21.1 | 19.3 | 4 |
| Year of cohort entrye | ||||||
| 2002–2005 | 0.0 | 60.7 | 176 | 0.0 | 49.0 | 139 |
| 2006–2009 | 20.4 | 25.1 | 11 | 20.4 | 30.5 | 23 |
| 2010–2012 | 79.6 | 14.2 | 173 | 79.6 | 20.6 | 146 |
| COMORBIDITIESf | ||||||
| Charlson Comorbidity Indexg | 0.65 (1.1) | 0.62 (1.0) | 4 | 0.65 (1.1) | 0.64 (0.7) | 2 |
| Dementia | 19.8 | 12.5 | 21 | 19.8 | 18.6 | 4 |
| Schizophrenia/psychotic disorders | 3.9 | 3.4 | 2 | 3.9 | 4.3 | 3 |
| Bipolar disorder | 4.0 | 3.5 | 2 | 4.0 | 4.3 | 2 |
| Unipolar depression/anxiety disorderh | 19.8 | 20.6 | 2 | 19.8 | 20.8 | 3 |
| History of self-harm | 0.2 | 0.1 | 3 | 0.2 | 0.1 | 2 |
| Major haemorrhage | 6.3 | 4.7 | 7 | 6.3 | 6.1 | 1 |
| Haemorrhagic Stroke | 0.4 | 0.4 | 1 | 0.4 | 0.5 | 1 |
| Ischemic Stroke | 2.8 | 3.2 | 2 | 2.8 | 2.7 | 1 |
| Transient Ischemic Attack | 0.8 | 1.1 | 3 | 0.8 | 1.3 | 6 |
| Chronic liver disease | 3.7 | 3.6 | 0 | 3.7 | 4.0 | 2 |
| Chronic Kidney Disease | 7.2 | 5.1 | 9 | 7.2 | 6.9 | 1 |
| Congestive heart failure | 11.9 | 13.7 | 5 | 11.9 | 11.7 | 1 |
| Coronary artery diseasei | 28.3 | 30.6 | 5 | 28.3 | 27.9 | 1 |
| Angina | 18.3 | 23.5 | 13 | 18.3 | 18.3 | 0 |
| Acute myocardial infarction | 3.6 | 3.9 | 1 | 3.6 | 3.6 | 0 |
| Pacemaker | 3.2 | 2.4 | 5 | 3.2 | 3.1 | 1 |
| Atrial fibrillation/flutter | 2.9 | 4.2 | 7 | 2.9 | 3.5 | 4 |
| Peripheral vascular disease | 1.6 | 2.1 | 4 | 1.6 | 2.1 | 5 |
| Chronic lung disease | 28.7 | 30.5 | 4 | 28.7 | 28.6 | 0 |
| Cancerj | 15.3 | 14.1 | 3 | 15.3 | 15.3 | 0 |
| Alcoholism | 2.3 | 2.4 | 0 | 2.3 | 2.4 | 0 |
| Seizure | 0.7 | 0.8 | 2 | 0.7 | 0.8 | 3 |
| Acute kidney injury | 2.8 | 1.6 | 9 | 2.8 | 2.6 | 1 |
| Hospitalization with hyperkalemia | 0.7 | 0.7 | 0 | 0.7 | 0.8 | 2 |
| Venous Thromboembolism | 1.3 | 1.1 | 1 | 1.3 | 1.3 | 1 |
| MEDICATIONSk | ||||||
| Anti-arrhythmics | 1.5 | 2.1 | 5 | 1.5 | 2.1 | 5 |
| Antipsychotics | 7.1 | 5.0 | 9 | 7.1 | 6.9 | 1 |
| Proton pump inhibitors | 35.5 | 27.1 | 18 | 35.5 | 35.5 | 0 |
| Anti-emetic | 2.0 | 1.7 | 2 | 2.0 | 1.8 | 2 |
| Lithium | 0.3 | 0.3 | 0 | 0.3 | 0.4 | 2 |
| Anti-lipemics | 50.6 | 39.4 | 23 | 50.6 | 50.4 | 1 |
| Antihypertensives | 70.7 | 68.8 | 4 | 70.7 | 70.3 | 1 |
| H2RAs | 5.2 | 12.8 | 25 | 5.2 | 5.2 | 0 |
| Pro-kinetics | 4.5 | 4.1 | 2 | 4.5 | 4.5 | 0 |
| Antidiabetics | 17.7 | 15.0 | 7 | 17.7 | 17.5 | 1 |
| Acetylsalicylic acid | 4.4 | 11.5 | 24 | 4.4 | 4.4 | 0 |
| Anticoagulants | 9.4 | 7.7 | 6 | 9.4 | 9.2 | 1 |
| Antiplatelet | 7.5 | 4.7 | 12 | 7.5 | 7.4 | 0 |
| Tri-cyclic antidepressants | 1.1 | 1.6 | 4 | 1.1 | 1.5 | 4 |
| Opioids | 0.0 | 0.1 | 1 | 0.0 | 0.1 | 1 |
| Anti-malarial | 0.7 | 0.7 | 0 | 0.7 | 0.7 | 0 |
| Anti-viral | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 1 |
| Antibiotic | 35.5 | 36.4 | 2 | 35.5 | 36.4 | 3 |
| Antineoplastic | 4.3 | 3.8 | 3 | 4.3 | 4.0 | 2 |
| Benzodiazepine | 35.5 | 42.8 | 13 | 35.5 | 36.0 | 1 |
| NSAIDSl | 17.4 | 24.0 | 16 | 17.4 | 17.5 | 0 |
| Cholinesterase inhibitors | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 0 |
| Anticonvulsants | 4.0 | 3.0 | 6 | 4.0 | 3.4 | 5 |
| DOSEm | ||||||
| High | 9.0 | 12.6 | 11 | 9.0 | 9.4 | 2 |
| PRESCRIBER | ||||||
| General Practitioner | 81.0 | 78.5 | 6 | 81.0 | 81.2 | 1 |
| Psychiatrist | 4.5 | 2.4 | 12 | 4.5 | 4.5 | 0 |
| Internist | 0.5 | 0.7 | 3 | 0.5 | 0.5 | 0 |
| Other | 5.1 | 4.8 | 1 | 5.1 | 4.8 | 1 |
| Missing | 8.9 | 13.5 | 15 | 8.9 | 9.0 | 0 |
| HEALTH CARE USEn | ||||||
| Number of Hospitalizations | ||||||
| 0 | 63.1 | 63.9 | 2 | 63.1 | 62.9 | 0 |
| 1 to 3 | 33.8 | 33.0 | 2 | 33.8 | 34.2 | 1 |
| 4 to 6 | 2.7 | 2.6 | 1 | 2.7 | 2.5 | 1 |
| 7 to 9 | 0.3 | 0.3 | 0 | 0.3 | 0.3 | 0 |
| 10 to 12 | 0.1 | 0.1 | 0 | 0.1 | 0.0 | 0 |
| over 12 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 0 |
| Number of Emergency room visits | ||||||
| 0 | 54.5 | 59.5 | 10 | 54.5 | 56.1 | 3 |
| 1 to 3 | 38.4 | 34.6 | 8 | 38.4 | 36.9 | 3 |
| Over 3 | 7.1 | 13.0 | 20 | 7.1 | 7.0 | 0 |
| General Practitioner Visits | ||||||
| 0–4 | 14.6 | 15.9 | 4 | 14.6 | 14.6 | 0 |
| 5–9 | 25.5 | 24.2 | 3 | 25.5 | 23.8 | 4 |
| 10–14 | 21.5 | 20.2 | 3 | 21.5 | 20.2 | 3 |
| 15–19 | 12.7 | 13.0 | 1 | 12.7 | 13.2 | 1 |
| 20–24 | 7.8 | 8.2 | 1 | 7.8 | 8.5 | 3 |
| 25–29 | 4.7 | 5.3 | 3 | 4.7 | 5.5 | 4 |
| ≥30 | 13.2 | 13.2 | 0 | 13.2 | 14.2 | 3 |
| At home physician services | 7.8 | 10.2 | 8 | 7.8 | 9.9 | 10 |
| Specialist Consultations | ||||||
| Psychiatrist consults | 9.1 | 6.0 | 12 | 9.1 | 8.9 | 1 |
| Nephrologist consultso | 7.1 | 5.1 | 8 | 7.1 | 7.1 | 0 |
| Cardiologist visits | 44.9 | 37.9 | 14 | 44.9 | 44.8 | 0 |
| Neurologist consults | 10.0 | 9.5 | 2 | 10.0 | 10.0 | 0 |
| Diagnostic tests/Interventions | ||||||
| Electrocardiogram | 89.1 | 86.2 | 8 | 89.1 | 88.9 | 1 |
| Stress test | 39.1 | 34.9 | 9 | 39.1 | 37.4 | 5 |
| Echocardiography | 47.7 | 38.1 | 20 | 47.7 | 47.4 | 1 |
| Cardiac Catheterization | 7.1 | 6.3 | 3 | 7.1 | 5.9 | 7 |
| Holter Monitor | 24.2 | 19.3 | 12 | 24.2 | 24.0 | 1 |
| Coronary angiogram | 8.0 | 6.8 | 5 | 8.0 | 6.4 | 8 |
| Chest X-ray | 77.7 | 76.1 | 4 | 77.7 | 77.4 | 1 |
| Pulmonary function test | 26.7 | 25.0 | 4 | 26.7 | 26.6 | 0 |
| Carotid ultrasound | 20.0 | 17.2 | 7 | 20.0 | 19.7 | 1 |
| Computed Tomography of the Head | 37.8 | 29.7 | 17 | 37.8 | 37.1 | 2 |
| Computed Tomography of other area | 41.8 | 30.2 | 25 | 41.8 | 41.4 | 1 |
| Mammogram | 24.4 | 30.7 | 14 | 24.4 | 24.8 | 1 |
| Bone Mineral Density | 41.9 | 39.6 | 5 | 41.9 | 42.3 | 1 |
Data presented as percent except for age and Charlson Comorbidity Index which are presented as mean (standard deviation).
Abbreviations: Non-steroidal anti-inflammatory drug (NSAID)–excludes acetyl-salicylic acid, Acetylsalicylic acid (ASA), Histamine H2 Receptor antagonist (H2RA), Not applicable (N/A)
a Weighted cohort based on inverse probability of treatment weights, using a propensity score based on 48 baseline characteristics.
b Standardized differences are less sensitive to sample size than traditional hypothesis tests. They provide a measure of the difference between groups divided by the pooled standard deviation; a value greater than 10% is interpreted as a meaningful difference between the groups.
c Defined as a population <10 000 people.
d Income was categorized into fifths of average neighbourhood income on the cohort entry date.
e The year of cohort entry is also referred to as the year of cohort entry date.
f Comorbidities assessed by administrative database codes in the previous 5 years.
g Charlson Comorbidity Index [Charlson ME, Pompei P, Alex KL, Mackenzie CR. A new method for classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987;40(5):373–383. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–1139.] was calculated using 5 years of hospitalization data. “No hospitalizations” received a score of 0.
h The prevalence of depression is low since depression is not usually an in-patient disorder, and thus often not coded in the source databases.
i Coronary artery disease includes receipt of coronary artery bypass graft surgery and percutaneous coronary intervention.
j Major cancers include esophagus, lung, bowel, liver, pancreas, breast, male/female reproductive organs, as well as leukemias and lymphomas.
k Baseline medication use assessed in the previous 120 days.
l Excludes acetylsalicylic acid.
m Refer to S5 Table for definitions of high and low doses.
n Health care use assessed in the one year prior to SSRI prescription.
o Based on the ICES physician database.
After weighting, with exception of the date of cohort entry, there was no significant difference between the two sets of comparison groups across all other 77 baseline characteristics measured in this study (see Tables 1 and 2). The year of cohort entry was expected to be different since escitalopram was added to Ontario’s provincial formulary in 2008. Only 6.5% of the citalopram group and 9% of the escitalopram group received prescriptions with high daily doses (defined in S5 Table; refer to S6 Table for yearly percentages).
Outcomes
The primary and secondary outcomes are shown in Table 3 for citalopram and Table 4 for escitalopram. Across the entire cohort, in the 90-day follow up 140 patients (0.05%) had a record of a hospital encounter with ventricular arrhythmia and 8214 (3.01%) died.
Table 3. Relative risks for primary and secondary outcomes of patients prescribed citalopram compared to the referent antidepressants (paroxetine or sertraline).
| Number of events (%) | ||||
|---|---|---|---|---|
| Outcome | Citalopram n = 137 701 | Paroxetine or Sertralineb n = 135 746 | Relative Risk (95% CI) | p-value |
| Ventricular Arrhythmiaa | 87 (0.06%) | 56 (0.04%) | 1.53 (1.03, 2.29) | 0.04 |
| All-Cause Mortality | 4811 (3.49%) | 4238 (3.12%) | 1.12 (1.06, 1.18) | <0.01 |
Patients prescribed paroxetine or sertraline served as the comparator group.
Abbreviations: confidence interval (CI)
a Based on hospital presentation (emergency room or hospitalization)–assessed by hospital diagnostic codes. This underestimated the true event rate because these codes tend to have high specificity but low sensitivity.
b Weighted cohort and results based on inverse probability of treatment weights, based on a propensity score which used 48 baseline characteristics (see Methods section)
Table 4. Relative risks for primary and secondary outcomes of patients prescribed escitalopram compared to the referent antidepressants (paroxetine or sertraline).
| Number of events (%) | ||||
|---|---|---|---|---|
| Outcome | Escitalopram n = 38 436 | Paroxetine or Sertralineb n = 113 058 | Relative Risk (95% CI) | p-value |
| Ventricular Arrhythmiaa | 13 (0.03%) | 15 (0.04%) | 0.84 (0.42, 1.68) | 0.62 |
| All-Cause Mortality | 1100 (2.86%) | 998 (2.63%) | 1.09 (1.01, 1.18) | 0.04 |
Patients prescribed paroxetine or sertraline served as the comparator group.
Abbreviations: confidence interval (CI)
a Based on hospital presentation (emergency room or hospitalization)–assessed by hospital diagnostic codes. This underestimated the true event rate because these codes tend to have high specificity but low sensitivity.
b Weighted cohort and results based on inverse probability of treatment weights, based on a propensity score which used 48 baseline characteristics (see Methods section)
The 90-day risk of ventricular arrhythmia in patients receiving citalopram was higher compared to referent antidepressants (0.06% vs. 0.04%; RR 1.53, 95% CI 1.03 to 2.29, p-value 0.04). Citalopram was also associated with a higher risk of all-cause mortality (3.49% vs. 3.12%, RR 1.12, 95% CI 1.06 to 1.18, p-value <0.01).
For escitalopram, the risk of ventricular arrhythmia compared to the referent antidepressants was not statistically different (0.03% vs. 0.04%; RR 0.84, 95% CI 0.42 to 1.68, p-value 0.62). Escitalopram was associated with a higher risk of all-cause mortality (2.86% vs. 2.63%; RR 1.09, 95% CI 1.01 to 1.18, p-value 0.04).
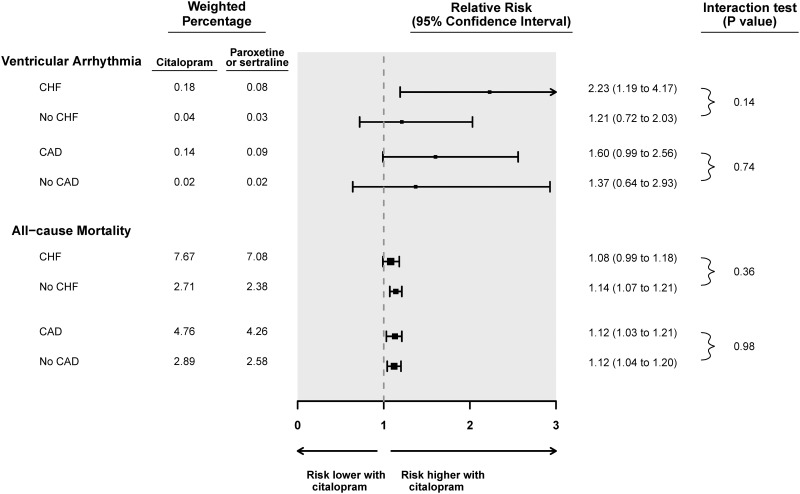
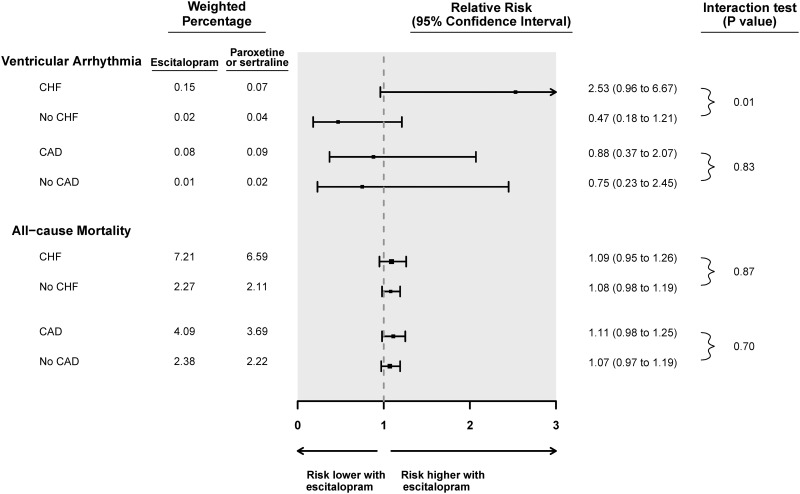
Subgroup Analysis
Subgroup analyses for ventricular arrhythmia and all-cause mortality are shown in Figs 1 and 2 for citalopram and escitalopram, respectively. The presence or absence of congestive heart failure did not significantly alter the association between citalopram and the risk of ventricular arrhythmia or all-cause mortality (p-values for interaction 0.14 and 0.36, respectively). Congestive heart failure did significantly modify the association between escitalopram compared to referent antidepressants and the risk of ventricular arrhythmia (p-value for interaction 0.01). Among those with congestive heart failure on escitalopram, the relative risk of ventricular arrhythmia was 2.53 (95% CI 0.96 to 6.67) whereas the relative risk was 0.47 (95% CI 0.18 to 1.21) for those without congestive heart failure. Congestive heart failure did not modify the association between escitalopram and all-cause mortality (p-value for interaction 0.87).
Fig 1. Subgroup analyses of the association between citalopram prescription and the risk of a hospital encounter with ventricular arrhythmia or all-cause mortality.
Abbreviations: Coronary artery disease (CAD), Congestive heart failure (CHF), Confidence interval (CI).
Fig 2. Subgroup analyses of the association between escitalopram prescription and the risk of a hospital encounter with ventricular arrhythmia or all-cause mortality.
Abbreviations: Coronary artery disease (CAD), Congestive heart failure (CHF), Confidence interval (CI).
Coronary artery disease did not modify the association between SSRI drug and the risk of either ventricular arrhythmia or all-cause mortality (Figs 1 and 2). There were too few patients with chronic kidney disease or on high doses of SSRI to permit subgroup analysis.
Discussion
In this population-based study of older adults newly prescribed SSRIs, we found that compared to paroxetine and sertraline, initiation of citalopram was associated with a small but statistically significant higher 90-day risk of a hospital encounter with ventricular arrhythmia. This increase in arrhythmia risk may have contributed to the observed small higher 90-day risk of death. Initiating citalopram compared to referent SSRIs was associated with a number needed to harm of 5000 (0.02% absolute increase) for the 90-day incidence of a hospital encounter with ventricular arrhythmia—assessed by hospital diagnostic codes. However, because hospital diagnostic codes are insensitive, the risk we observed is likely an underestimate of the true rate. Assuming the codes underestimate the incidence of arrhythmia by a factor of 10, the 90-day absolute risk increase would still be relatively low (1 in 500 patients). There were too few events in the escitalopram group to reliably assess the risk of ventricular arrhythmia. Thus, the higher association of 90-day all-cause mortality, and increased ventricular arrhythmia risk in the subgroup with congestive heart failure should be interpreted cautiously.
The findings of nine other studies (summarized in S7 Table) describing the association between citalopram or escitalopram and QT prolongation, ventricular arrhythmia, a cardiac event or mortality are inconsistent. We used the Downs and Black quality checklist to assess the reporting, external validity, internal validity and statistical power of these nine studies (S8 Table).[54] Based on this checklist, the quality was rated as good for two studies [5, 27], fair for five [14, 23, 26, 28–29] and poor for two (never published).[19,20] Out of the published studies, three focused on QT prolongation.[14, 26, 28] Compared to the other studies, our results on citalopram agree with the findings of Weeke et al (showing increased out-of-hospital cardiac arrest in a case-time-control study of elderly patients on citalopram),[29] but differ from those of two other cohort studies.[23, 27] Zivin et al showed no difference in the 5-year rates of ventricular arrhythmia and mortality in 618 450 US veterans who received a prescription for citalopram, compared to 365 898 patients with prescriptions for sertraline.[23] Leonard et al showed no difference in the 30-day rates of sudden death and ventricular arrhythmia in 294 434 United States Medicaid patients on citalopram compared to 560 822 patients on paroxetine.[27] Both these studies focused on a younger population (>50% were less than 65 years-old).
Escitalopram has previously been associated with a higher risk of the composite outcome of ventricular arrhythmia, cardiac arrest and sudden death in 14 128 pediatric patients, as compared to 32 906 pediatric patients prescribed fluoxetine.[5] We did not find a similar statistical association in our study, recognizing our observed rate of events was quite low.
Our study has several strengths: the use of Ontario’s healthcare databases allows for the assessment of all older residents who received the study antidepressants in routine care; our outcomes were clinically important adverse events; and we used a referent group who were also prescribed SSRIs and robust statistical methodology to balance the groups on 77 baseline characteristics, to reduce confounding.
Our study has limitations. Electrocardiograms were not available in our data sources; instead we relied on diagnostic codes for a hospital encounter with ventricular arrhythmia which have a good positive predictive value but limited sensitivity. However, we do not suspect any systematic difference in diagnostic recording by antidepressant type, suggesting that our relative measures of risk are robust. Also, our results may only generalize to older adults. As with any observational study residual confounding can never be fully eliminated.
Physicians who prescribe citalopram to older patients should be cognizant of the potential risk of ventricular arrhythmia and all-cause mortality. Our results suggest that in the elderly the warnings from regulatory agencies appear warranted. We detected a signal despite over 90% of our citalopram cohort taking a dose of ≤ 20mg/day (as per current recommendations). It is reassuring that the absolute increase in ventricular arrhythmia and mortality risk with citalopram was low. The FDA recommends monitoring of patients taking citalopram with electrocardiography;[19, 20] however, evidence for this approach is lacking.
In outpatient practice, we found a small increase in the 90-day risk of hospital encounter with ventricular arrhythmia in older adults prescribed citalopram compared to those prescribed paroxetine or sertraline. This may have contributed to the observed modestly higher risk of all-cause mortality with citalopram.
Supporting Information
(DOC)
(DOCX)
Abbreviations: CCI—Canadian Classification of Health Interventions (available after 2002), CCP—Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures (before 2002), DSM—IV—Diagnostic and Statistical Manual of Mental Disorders IV coding, ICD 9 —International Classification of Diseases, Ninth Revision, ICD 10 —International Classification of Diseases, Tenth Revision. OHIP—Ontario Health Insurance Plan. * Where applicable a combination of diagnostic and procedure codes were used; a Treatment Code—from the Canadian Organ Replacement Register; b Treatment Organ—from the Canadian Organ Replacement Register: c Excluding cardiac angina: d DSM—IV—coding from Ontario Mental Health Reporting System: e Only includes dialysis visits with nephrologist present.
(DOCX)
a Only ICD 10 Codes were used to identify outcomes due to the timing of our study. These codes had to be associated with a hospital presentation in any position (e.g. most responsible diagnosis, or secondary diagnosis). b Data obtained from the Ontario Registered Persons Database and the Ontario Registrar General Death.
(DOCX)
Abbreviations: Selective serotonin re-uptake inhibitor (SSRI), Local Health Integration Network (LHIN). aLHIN—Local Health Integration Network, health authorities responsible for regional administration of public healthcare services in Ontario; b computed tomography of a body area other than the head; c Refer to eTable 5 for the definitions of high and low doses.
(DOCX)
** These doses are considered to be equivalent amongst the different types of selective serotonin re-uptake inhibitors.
(DOCX)
(DOCX)
Abbreviations: CI = confidence interval. aWe evaluated the quality of individual studies using the Downs and Black quality assessment method, which is a list of 27 criteria to evaluate both randomized and non-randomized trials (eTable 8) [57]. This scale assesses the completeness and clarity of study reporting, external validity, internal validity (e.g. bias and confounding) and power. The tool was modified slightly for use in our review. Specifically, the scoring for question 27 dealing with statistical power was simplified to a choice of awarding either 1 or 0 points depending on whether there was sufficient power to detect a clinically important effect. On the modified scale, we gave all included studies a score from 0 to 28, grouped into the following four quality levels: excellent (26 to 28), good (20 to 25), fair (15 to 19) and poor (less than 14).
(DOCX)
aItem has been modified; specifically, the scoring for this question was simplified to a choice of awarding either 1 or 0 points depending on whether there was sufficient power to detect a clinically important effect.
(DOCX)
Acknowledgments
We thank Gamma Dynacare for use of the outpatient laboratory database and the team at London Health Sciences Centre, St. Joseph’s Health Care, and the Thames Valley Hospitals for providing access to the Cerner laboratory database.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Institute for Clinical Evaluative Sciences (ICES) is a non-profit research corporation funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Parts of the material in the current report are based on data and information compiled and provided by the Canadian Institute of Health Information (CIHI). The research was conducted at the ICES Western facility, which receives financial support from the Academic Medical Organization of Southwestern Ontario, the Schulich School of Medicine and Dentistry at Western University and the Lawson Health Research Institute. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES, the Ontario MOHLTC or CIHI is intended or should be inferred. Dr. Amit Garg was supported by the Dr. Adam Linton Chair in Kidney Health Analytics. Research personnel who worked on this project were supported by the Lilibeth Caberto Kidney Clinical Research Unit.
References
- 1.Newman SC, Schopflocher D. Trends in antidepressant prescriptions among he elderly in Alberta during 1997 to 2004. Can J Psychiatry 2008;53(10):704–7. [DOI] [PubMed] [Google Scholar]
- 2.Mamdani MM, Parikh SV, Austin PC, Upshur RE. Use of antidepressants among elderly subjects: Trends and contributing factors. Am. J Psychiatry 2000;157(3):360–7. [DOI] [PubMed] [Google Scholar]
- 3.Petersen T, Dording C, Neault NB, Kombluh R, Alpert JE, Nierenberg AA, et al. A survey of prescribing practices in the treatment of depression. Prog Neuropsychopharmacol Bio Psychiatry 2002;26(1):177–87. [DOI] [PubMed] [Google Scholar]
- 4.IMS Institute for Health Informatics.The use of medicines in the United States: review of 2011. [Google Scholar]
- 5.Czaja AS, Valuck RJ, Anderson HD. Comparative safety of selective serotonin reuptake inhibitors among pediatric users with respect to adverse cardiac events. Pharmacoepidemiol Drug Saf 2013;22(6):607–15. [DOI] [PubMed] [Google Scholar]
- 6.Patel NH, Golwala H, Stavrakis S, Schechter E. Sertraline-induced ventricular tachycardia. Am J Ther 2013;20(6):e720–2. 10.1097/MJT.0b013e3182192d6e [DOI] [PubMed] [Google Scholar]
- 7.Tseng PT, Lee Y, Lin YE, Lin PY. Low-dose escitalopram for 2 days associated with corrected QT interval prolongation in a middle-aged woman: a case report and literature review. Gen Hosp Psychiatry 2012;34(210):e13–15. [DOI] [PubMed] [Google Scholar]
- 8.Vieweg WV, Hasnain M, Howland RH, Hettema JM, Kogut C, Wood MA, et al. Citalopram, QTc interval prolongation and torsade de pointes. How should we apply the recent FDA ruling? Am J Med 2012;125(9):859–68. 10.1016/j.amjmed.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 9.de Gregorio C, Morabito G, Cerrito M, Dattilo G, Oreto G. Citalopram—induced long WT syndrome and torsades de pointes: role for concomitant therapy and disease. Int J Cardiol 2011;148(2):226–8. 10.1016/j.ijcard.2009.05.060 [DOI] [PubMed] [Google Scholar]
- 10.Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsades de pointes. Dtsch Arztebl Int 2011;108(41):687–93. 10.3238/arztebl.2011.0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyer WF, Blumhardt CL. The safety profile of paroxetine. J Clin Psychiatry 1992;53(Suppl):61–6. [PubMed] [Google Scholar]
- 12.Edwards JG, Goldie A, Papayanni-Papasthatis S. Effect of paroxetine on the electrocardiogram. Psychopharmacology 1989;97(1):96–8. [DOI] [PubMed] [Google Scholar]
- 13.Kuhs H, Rudolf GA. Cardiovascular effects of paroxetine. Psychopharmacology, 1990;102(3):379–82. [DOI] [PubMed] [Google Scholar]
- 14.Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, et al. QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ 2013;346:f288 10.1136/bmj.f288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funk KA, Bostwick JR. A comparison of the risk of QT prolongation among SSRIs. Ann Pharmacother 2013;47(10):1330–41. 10.1177/1060028013501994 [DOI] [PubMed] [Google Scholar]
- 16.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiogram in an independent risk factor for sudden death due to cardiac arrest. Circulation 1991;83(6):1888–94. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Post WS, Dalal D, Blasco-Colmenares E, Tomaselli GF, Guallar E. QT- interval duration and mortality rate: results from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2011;171(19):1727–33. 10.1001/archinternmed.2011.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sala M, Lazzaretti M, De Vidovich G, Caverzasi E, Barale F, d’Allio G, et al. Electrophysiological changes of cardiac function during antidepressant treatment. Ther Adv Cardiovasc Dis 2009;3(1):29–43. 10.1177/1753944708096282 [DOI] [PubMed] [Google Scholar]
- 19.Food US and Administration Drug. FDA drug safety communication: Abnormal heart rhythms associated with high doses of Celexa. 2011. Available: http://www.fda.gov/drugs/drugsafety/ucm269086.htm (accessed 20 April 2014).
- 20.Food US and Administration Drug. FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. 2012. Available: http://www.fda.gov/Drugs/DrugSafety/ucm297391.htm (accessed 20 April 2014).
- 21.Marcum ZA, Vande Griend JP, Linnebur SA. FDA drug safety communications: a narrative review and clinical considertations for older adults. Am J Geriatr Pharmacother 2012;10(4):264–71. 10.1016/j.amjopharm.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canada Health. Celexa (citalopram)–Association with abnormal heart rhythms—For Health Professionals. 2012. Available: http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2012/14672a-eng.php (accessed 21 March 2015).
- 23.Zivin K, Pfeiffer PN, Bohnert AS, Ganoczy D, Blow FC, Nallamothu BK, et al. Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40mg. Am J Psychiatry 2013;170(6):642–50. 10.1176/appi.ajp.2013.12030408 [DOI] [PubMed] [Google Scholar]
- 24.Lam RW. Psychopharmacology for the clinician (antidepressants and QT prolongation). J Psychiatry Neurosci 2013;38(2):E5–6. 10.1503/jpn.120256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howland RH. A critical evaluation of the cardiac toxicity of citalopram: part 2. J psychosoc Nurs Ment Health Serv 2011;49(12):13–16. 10.3928/02793695-20111102-04 [DOI] [PubMed] [Google Scholar]
- 26.van Haelst IM, van Klei WA, Doodeman HJ, Warnier MJ, De Bruin ML, Kalkman CJ, et al. QT interval prolongation in users of selective serotonin reuptake inhibitors in an elderly surgical population: a cross sectional study. J Clin Psychiatry 2014;75(1):15–21. 10.4088/JCP.13m08397 [DOI] [PubMed] [Google Scholar]
- 27.Leonard CE, Bilker WB, Newcomb C, Kimmel SE, Hennessy S. Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf 2011;20(9):903–913. 10.1002/pds.2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchida M, Spencer AE, Biederman J, Castro VM, Kenworthy T, Chan J, et al. A systematic evaluation of the QTc interval and antidepressants in youth: An electronic health record study. J Dev Behav Pediatr. 2015;36(6):434–9. 10.1097/DBP.0000000000000188 [DOI] [PubMed] [Google Scholar]
- 29.Weeke P, Jensen A, Folke F, Gisalson GH, Olesen JB, Andersson C, et al. Antidepressant use and risk of out-of-hospital cardiac arrest: a nationwide case-time-control study. Clin Pharmacol Ther. 2012;92(1):72–9. 10.1038/clpt.2011.368 [DOI] [PubMed] [Google Scholar]
- 30.Canada Health. Antidepressant Cipralex (escitalopram): Updated information regarding dose-related heart risk. 2012. Availbel: http://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2012/13674a-eng.php (accessed 21 March 2015).
- 31.Canada Statistics, Government of Canada Statistics Canada. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo31a-eng.htm (accessed 01 October 2014).
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007;335(7624):806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol 2003;10(2):67–71. [PubMed] [Google Scholar]
- 34.Li DQ, Kim R, McArthur E, Fleet JL, Bailey DG, Juurlink D, et al. Risk of adverse events among older adults following co-prescription of clarithromycin and statins not metabolized by cytochrome P450 3A4. CMAJ 2015;187(3):174–80. 10.1503/cmaj.140950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weir MA, Beyea MM, Gomes T, Juurlink DN, Mamdani M, Blake PG, et al. Orlistat and acute kidney injury: an analysis of 953 patients. Arch Intern Med 2011;171(7):703–4. 10.1001/archinternmed.2011.103 [DOI] [PubMed] [Google Scholar]
- 36.Gandhi S, Fleet JL, Bailey DG, McArthur E, Wald R, Rehman F, et al. Calcium-channel blocker- clarithromycin drug interactions and acute kidney injury. JAMA 2013;310(23):2544–53. 10.1001/jama.2013.282426 [DOI] [PubMed] [Google Scholar]
- 37.Shih AW, Weir MA, Clemens KK, Yao Z, Gomes T, Mamdani MM, et al. Oral bisphosphonate use in the elderly is not associated with acute kidney injury. Kidney Int. Nature Publishing Group 2012;82(8):903–8. [DOI] [PubMed] [Google Scholar]
- 38.Zhao YY, Weir MA, Manno M, Cordy P, Gomes T, Hackamm DG, et al. New fibrate use and acute renal outcomes in elderly adults: a population-based study. Ann Intern Med 2012;156(8):560–9. 10.7326/0003-4819-156-8-201204170-00003 [DOI] [PubMed] [Google Scholar]
- 39.Jain AK, Cuerden MS, McLeod I, Hemmelgam B, Akbari A, Tonelli M, et al. Reporting of the estimated glomerular filtration rate was associated with increased use of angiotensin-converting enzyme inhibitors and angiotensin-II receptor blockers in CKD. Kidney Int 2012;81(12):1248–53. 10.1038/ki.2012.18 [DOI] [PubMed] [Google Scholar]
- 40.Patel AM, Shariff S, Bailey DG, Juurlink DN, Gandhi S, Mamdani M, et al. Statin toxicity from macrolide antibiotic co-prescription: A population -based cohort study. Ann Intern Med 2013;158(12):869–76. 10.7326/0003-4819-158-12-201306180-00004 [DOI] [PubMed] [Google Scholar]
- 41.Isbister GK, Friberg LE, Duffull SB. Application of pharmacokinetic-pharmacodynamic modelling in management of QT abnormalities after citalopram overdose. Intensive Care Med 2006;32(7):1060–5. [DOI] [PubMed] [Google Scholar]
- 42.Friberg LE, Isbister GK, Duffull SB. Pharmacokinetic-pharmacodynamic modelling of QT interval prolongation following citalopram overdoses. Br J Clin Pharmacol 2006;61(2):177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hennessy S, Leonard CE, Freeman CP, Deo R, Newcomb C, Kimmel SE, et al. Validation of diagnostic codes for outpatient originating sudden cardiac death and ventricular arrhythmia in Medicaid and Medicare claims data. Pharmacoepidemiol Drug Saf 2010;19(6):555–62. 10.1002/pds.1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying ventricular arrhythmias using administrative and claims data. Pharmacoepidemiol Drug Saf, 2012;21(Suppl1):148–153. [DOI] [PubMed] [Google Scholar]
- 45.De Bruin ML, van Hemel NM, Leufkens HG, Hoes AW. Hospital discharge diagnosis of ventricular arrhythmias and cardiac arrest were useful for epidemiologic research. J Clin Epidemiol 2005;58(12):1325–9. [DOI] [PubMed] [Google Scholar]
- 46.Quan H, Li B, Saunders LD, Nilsson CI, Alibhai A, Ghali WA, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008;43(4):1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jha P, Deboer D, Sykora K, Naylor CD. Characteristics and mortality outcomes of thrombolysis trial participants and nonparticipants: a population-based comparison. J Am Coll Cardiol 1996;27(6):1335–42. [DOI] [PubMed] [Google Scholar]
- 48.Austen PC. An introduction to propensity score methods for reducing the effect of confounding in observational studies. Multivariate Behav Res 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: A Monte Carlo study. Stat Med 2007;26(4):734–53. [DOI] [PubMed] [Google Scholar]
- 50.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol 2006;163(12):1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joffe MM, Ten Have TR, Feldman HI, Kimmel SE. Model selection, confounder control, and marginal structural models: Review and new applications. Am Stat 2004;58(4):272–79. [Google Scholar]
- 52.Austen P. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Comm Statist Simulation Comput 2009;38(6):1228–34. [Google Scholar]
- 53.Fleet JL, Dixon SN, Shariff SZ, Quinn RR, Nash DM, Harel Z, et al. Detecting chronic kidney disease in population-based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol 2013;14:81 10.1186/1471-2369-14-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Abbreviations: CCI—Canadian Classification of Health Interventions (available after 2002), CCP—Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures (before 2002), DSM—IV—Diagnostic and Statistical Manual of Mental Disorders IV coding, ICD 9 —International Classification of Diseases, Ninth Revision, ICD 10 —International Classification of Diseases, Tenth Revision. OHIP—Ontario Health Insurance Plan. * Where applicable a combination of diagnostic and procedure codes were used; a Treatment Code—from the Canadian Organ Replacement Register; b Treatment Organ—from the Canadian Organ Replacement Register: c Excluding cardiac angina: d DSM—IV—coding from Ontario Mental Health Reporting System: e Only includes dialysis visits with nephrologist present.
(DOCX)
a Only ICD 10 Codes were used to identify outcomes due to the timing of our study. These codes had to be associated with a hospital presentation in any position (e.g. most responsible diagnosis, or secondary diagnosis). b Data obtained from the Ontario Registered Persons Database and the Ontario Registrar General Death.
(DOCX)
Abbreviations: Selective serotonin re-uptake inhibitor (SSRI), Local Health Integration Network (LHIN). aLHIN—Local Health Integration Network, health authorities responsible for regional administration of public healthcare services in Ontario; b computed tomography of a body area other than the head; c Refer to eTable 5 for the definitions of high and low doses.
(DOCX)
** These doses are considered to be equivalent amongst the different types of selective serotonin re-uptake inhibitors.
(DOCX)
(DOCX)
Abbreviations: CI = confidence interval. aWe evaluated the quality of individual studies using the Downs and Black quality assessment method, which is a list of 27 criteria to evaluate both randomized and non-randomized trials (eTable 8) [57]. This scale assesses the completeness and clarity of study reporting, external validity, internal validity (e.g. bias and confounding) and power. The tool was modified slightly for use in our review. Specifically, the scoring for question 27 dealing with statistical power was simplified to a choice of awarding either 1 or 0 points depending on whether there was sufficient power to detect a clinically important effect. On the modified scale, we gave all included studies a score from 0 to 28, grouped into the following four quality levels: excellent (26 to 28), good (20 to 25), fair (15 to 19) and poor (less than 14).
(DOCX)
aItem has been modified; specifically, the scoring for this question was simplified to a choice of awarding either 1 or 0 points depending on whether there was sufficient power to detect a clinically important effect.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.