Abstract
Data from genetically modified mice suggest that benzodiazepine (BDZ)-site agonists with improved selectivity for α2-subtype GABAA receptors (α2GABAAR) are potentially useful for the treatment of neuropathic pain. Subtype-selective compounds available for preclinical tests in rodents support this concept but have not been approved for human use, hindering proof-of-concept studies in patients. We recently proposed that N-desmethyl clobazam (NDMC), the main metabolite of the licensed BDZ clobazam (CBZ), is responsible for most of the antihyperalgesia observed in mice after CBZ administration. In order to assess a potentially favorable pharmacological profile of NDMC, we analyzed differences in the GABAAR subtype specificity of CBZ, NDMC and diazepam (DZP) in recombinant receptors. DZP and CBZ potentiated sedating α1GABAARs and antihyperalgesic α2GABAARs with similar efficacies, whereas NDMC preferred α2GABAARs over α1GABAARs across a wide concentration range. In vivo, DZP and NDMC reduced neuropathic pain at doses between 3 and 30 mg/kg. At these doses, DZP had strong locomotor sedating effects while NDMC caused no or only weak sedation. Sedative effects of NDMC became apparent when the action of NDMC was restricted to α1GABAARs. However, when GABAAR point-mutated mice were studied that allow the analysis of antihyperalgesia and sedation in isolation, we found that, compared to DZP, NDMC had a significantly improved therapeutic window, consistent with its more favorable α2/α1 in vitro activity ratio. Given that NDMC should share the safety profile of its parent compound CBZ, it should be well-suited for proof-of-concept studies in human volunteers or patients.
Keywords: GABAA receptor subtype, Analgesia, Proof-of-concept, Benzodiazepine, Neuropathic pain, Mouse models
Highlights
-
•
N-desmethyl clobazam is a naturally occurring metabolite of the approved clobazam.
-
•
N-desmethyl clobazam possesses an improved α2/α1 GABAAR selectivity ratio.
-
•
It exerts significant analgesia against neuropathic pain at non-sedative doses.
-
•
N-desmethyl clobazam may be a well-suited for proof-of-concept studies in humans.
1. Introduction
Chronic pain is a medical condition that is often refractory to currently available pharmacotherapy. In particular neuropathic pain is resistant to the majority of conventional analgesics, and drugs that are effective carry a high rate of side-effects. The identification of novel drug targets based on disease mechanisms and the development of new analgesic drugs targeting the biological processes that underlie pain offer the opportunity to improve the current situation.
Many chronic pain states are accompanied by diminished synaptic inhibition at the spinal cord level (Beyer et al., 1985, Roberts et al., 1986, Sivilotti and Woolf, 1994, Zeilhofer et al., 2012). Conversely, pharmacological enhancement of GABAergic inhibition in the spinal cord through locally applied DZP reverses pathologically increased pain sensitivity (hyperalgesia) in rodent models of chronic inflammatory and neuropathic pain (Knabl et al., 2008). The generation of GABAAR point-mutated (“knock-in”) mice which carry a histidine to arginine (H → R) point mutation rendering the mutated GABAARs insensitive to DZP (and many other BDZ site agonists) has allowed attributing different in vivo actions of BDZ to different subtypes GABAARs (Möhler et al., 2002). Analysis of these mice in different pain models indicated that antihyperalgesic actions of BDZ occur mainly through spinal α2GABAARs (Knabl et al., 2008, Paul et al., 2014). Genetically modified (triple GABAA receptor point-mutated) mice, in which only one GABAAR subtype was left BDZ-sensitive, demonstrated that pronounced antihyperalgesia can be achieved with systemic DZP administration without signs of sedation or impaired motor coordination when only α2GABAARs are targeted (Ralvenius et al., 2015). Conversely, many of the typical side effects of classical BDZ such as sedation, addiction, and motor impairment depend on activation of α1GABAARs (Ralvenius et al., 2015, Rudolph et al., 1999, Tan et al., 2010). The observation that desired and unwanted effects of classical BDZ site agonists are mediated by distinct GABAAR subtypes has stimulated the development of new compounds with improved subtype selectivity (Rudolph and Knoflach, 2011). Such compounds were also evaluated in different preclinical pain models (Di Lio et al., 2011, Hofmann et al., 2012, Knabl et al., 2008, Knabl et al., 2009, Munro et al., 2009, Nickolls et al., 2011, Paul et al., 2014, Reichl et al., 2012, reviewed in Zeilhofer et al., 2012) and provided proof-of-concept evidence that the results obtained in genetically modified mice translate into in vivo antihyperalgesic efficacy of novel BDZ site agonists with improved subtype selectivity.
However, in the light of recent concerns raised about the predictive value of animal and, in particular, rodent models in pain research (Tappe-Theodor and Kuner, 2014), it appears important to obtain proof-of-concept data on the translatability of these findings to humans. Classical BDZ site agonists such as clonazepam and CBZ exert weak analgesic effects when given at standard therapeutic doses (Besson et al., 2015, Vuilleumier et al., 2013). Our recent preclinical study (Ralvenius et al., 2015) that compared antihyperalgesic and sedative effects of DZP in genetically modified mice suggests that the doses needed to achieve relevant analgesia can typically not be achieved in human patients because of dose limiting sedation. Compounds devoid of α1GABAAR-mediated sedation should circumvent this problem. However, none of the currently available compounds with improved α2GABAAR selectivity have so far been approved for use in humans. We therefore evaluated alternative possibilities for proof-of-concept studies in humans. In a previous preclinical pharmacokinetic/pharmacodynamic (PK/PD) study on possible antihyperalgesic effects of CBZ we found that antihyperalgesic effects correlated better with blood levels of the main metabolite N-desmethyl clobazam (NDMC) than with blood levels of the parent compound CBZ (Besson et al., 2013). In the present study, we have systematically evaluated the subtype selectivity of NDMC in recombinant GABAARs, its antihyperalgesic effects in a mouse model of neuropathic pain and its propensity towards sedation. We found that NDMC has a better α2/α1-GABAAR selectivity-profile than the parent compound CBZ and better than the canonical BDZ site agonist DZP. This more favorable in vitro profile translated to profound antihyperalgesic activity at doses that caused no or only mild sedation. Because NDMC is a naturally occurring metabolite of CBZ in humans (Grigoleit et al., 1983) and has already been given in-first-in-man clinical studies targeting patients with treatment refractory epilepsy (Haigh et al., 1987), NDMC could constitute a suitable tool compound for proof-of-concept studies exploring its antihyperalgesic potency in chronic pain conditions in humans.
2. Materials and methods
2.1. Drugs
DZP and CBZ were obtained from Lipomed AG, Arlesheim, Switzerland. NDMC was obtained from Imaginechem Co, Ltd, Hangzhou, China. NDMC was tested for purity, which was 99%.
2.2. Mice
Experiments were performed in two strains of wild-type mice (C57BL/6J and 129X1/SvJ), and in homozygous triple and quadruple (H → R) GABAAR point-mutated mice of the 129X1/SvJ background (Ralvenius et al., 2015). Triple and quadruple point-mutated mice were generated by cross-breeding of four strains of single point-mutated mice described previously (Crestani et al., 2002, Löw et al., 2000, Rudolph et al., 1999).
2.3. [3H]flunitrazepam binding to transfected HEK293 cells
HEK293 cells (ATCC) were maintained in DMEM/10% FBS and plated to a density of 800,000 cells onto 10 cm culture dishes 3 h before transfection with plasmids containing the rat subunits α1, β2 and γ2 or α2, β3 and γ2 (8 μg total DNA/culture dish, ratio 1:1:2) using the PEI transfection method. Forty-eight hours after transfection, HEK293 cells were harvested in PBS for [3H]flunitrazepam binding. HEK 293 cells were homogenized in 20 vol 50 mM Tris pH 7.5, protease inhibitor cocktail (complete Mini, Roche Applied Science) and centrifuged at 500 g for 10 min at 4 °C. To obtain the crude membrane fraction, the supernatants were centrifuged for 20 min at 100,000 g (4 °C). The crude membranes were washed 4 times in 5 mM Tris-HCl pH 7.4, 10 mM EDTA by resuspension and centrifugation and stored at −80 °C until used. Crude membranes were then washed once in 50 mM Tris pH 7.5 (containing protease inhibitor cocktail) and aliquots (∼100 μg protein) were incubated with increasing concentrations of DZP, CBZ or NDMC and 1 nM [3H]flunitrazepam (79.8 Ci/mmol, PerkinElmer) in a total volume of 200 μl for 120 min on ice. Subsequently, the samples were filtered onto glass fiber filters using a 12-channel semiautomated cell harvester (Scatron) and washed with ice-cold buffer (50 mM Tris-HCl pH 7.4). Non-specific [3H]flunitrazepam binding was determined using 10 μM flumazenil. The radioactivity retained by the filters was determined by liquid scintillation counting using a Tricarb 2500 liquid scintillation analyzer. Binding data were analyzed using the GraphPad Prism software (version 5.04, GraphPad Software, USA).
2.4. Electrophysiology
The effects of DZP, CBZ and NDMC on currents through recombinant GABAARs were studied in HEK293 cells transiently transfected with rat GABAAR subunits using lipofectamine LTX 46 (Invitrogen). To ensure expression of the γ2 subunit (required for modulation of GABAAR currents by BDZs) in all recorded cells, we transfected cells with a plasmid expressing the γ2 subunit plus eGFP from an IRES, and selected only eGFP-positive cells for recordings (see also Ralvenius et al., 2015). The transfection mixture contained (in μg): 1 αx, 1 βy, 3 γ2/eGFP. Whole-cell patch-clamp recordings were made at room temperature (20–24 °C) at a holding potential of -60 mV 18–36 h after transfection 60. Recording electrodes were filled with solution containing (in mM): 120 CsCl, 10 EGTA, 10 HEPES (pH 7.40), 4 MgCl2, 0.5 GTP and 2 ATP. The external solution contained (in mM): 150 NaCl, 10 KCl, 2.0 CaCl2, 1.0 MgCl2, 10 HEPES (pH 7.40), and 10 glucose. GABA was applied to the recorded cell using a manually controlled pulse (6–10 s) of a low sub-saturating GABA concentration (EC10). EC10 values of GABA were 1 μM, 5 μM, 8 μM, and 1 μM for the four GABAAR combinations (α1β2γ2, α2β3γ2, α3β3γ2 and α5β2γ2), respectively. EC50 values and Hill coefficients (nH) were obtained from fits of normalized concentration-response curves to the Hill equation IGABA = Imax [GABA]nH/([GABA]nH + [EC50]nH). Imax was determined as the average maximal current elicited by saturating concentration of GABA (30 μM–3 mM, depending on the subunit composition). DZP, CBZ and NDMC were dissolved in DMSO (final concentration < 0.1%) and subsequently diluted on the day of the experiment in external solution and were co-applied with GABA without preincubation. Concentration-response curves of the three BDZ were fitted to the Hill equation: E(C) = (Emax·[C]nH)/([C]nH + [EC50]nH).
2.5. Quantitative RT-PCR
Twelve lumbar spinal cords and hippocampi were rapidly removed from euthanized adult wild-type C57BL/6 and 129X1/SvJ mice. mRNA was transcribed into cDNA using the QuantiTect Reverse Transcription Kit (Qiagen no. 205311). Expression of GABAAR subunits was assessed using β-actin as reference gene.
2.6. Animal experiments
All behavioral experiments were performed in 7–10 week old female and male mice by an experimenter blinded to the genotype or treatment of the mice. Care was taken to ensure equal numbers of female and male mice in all groups. Permission for animal experiments was obtained from the Veterinäramt des Kantons Zürich (license numbers 135/2009 and 126/2012). For behavioral experiments, DZP, CBZ and NDMC were suspended in 0.9% saline, 1% Tween80 and applied orally (p.o.) in all experiments.
Neuropathic pain was evoked by applying a chronic constriction injury (CCI; Bennett and Xie, 1988) to the left sciatic nerve proximal to the trifurcation with three loose (5-0 silk) ligatures. Mice which showed signs of paralysis were excluded from subsequent experiments. Effects of DZP and NDMC on thermal and mechanical hyperalgesia were assessed between 7 and 14 days after surgery. Heat hyperalgesia was quantified as the change in the latency of paw withdrawal evoked by exposure of the plantar side of one hindpaw to a defined radiant heat stimulus (Hargreaves test). Mechanical hyperalgesia was assessed with an electronic von Frey apparatus using filament #7 (IITC, Woodland Hills, CA). Three to four measurements were made for each time point and animal for both heat and mechanical hyperalgesia. Percent maximal possible effects (%MPE) were calculated as follows:
%MPE(t) = 100 * (E(t) − Epredrug))/(EpreCCI − Epredrug); E(t), paw withdrawal thresholds or latency at time point t. Epredrug, E after CCI surgery but before DZP application; EpreCCI, E baseline before CCI surgery.
Locomotor activity was measured in an open field arena (20 cm diameter) equipped with four pairs of light beams and photosensors. Drugs were administered immediately before placing the animal into the recording chamber. Locomotor activity was analyzed for the time interval between 72 and 120 min after drug administration.
2.7. NDMC pharmacokinetics
NDMC blood concentrations were evaluated after at various time points after oral administration of 3, 10 and 30 mg kg−1, using the dried blood spot sampling method (Deglon et al., 2011). This technique allows collecting multiple bleeds from the same animal over a large time window. Four μl of whole blood were collected and spotted onto a filter paper card from Whatman (Dassel, Germany) at different time points between 0 and 48 h after NDMC administration. NDMC blood and brain tissue concentration were in addition determined at 2 h after a single oral dose of 1, 3, 10, 30, and 100 mg kg−1. NDMC concentration measurements were performed using a fully validated LC-MS-MS method (Besson et al., 2013). Pharmacokinetic parameters were estimated using a non-compartmental method using WinNonlin® version 5.2 (Pharsight, Mountainview, CA, USA).
3. Results
3.1. GABAAR subtype selectivity
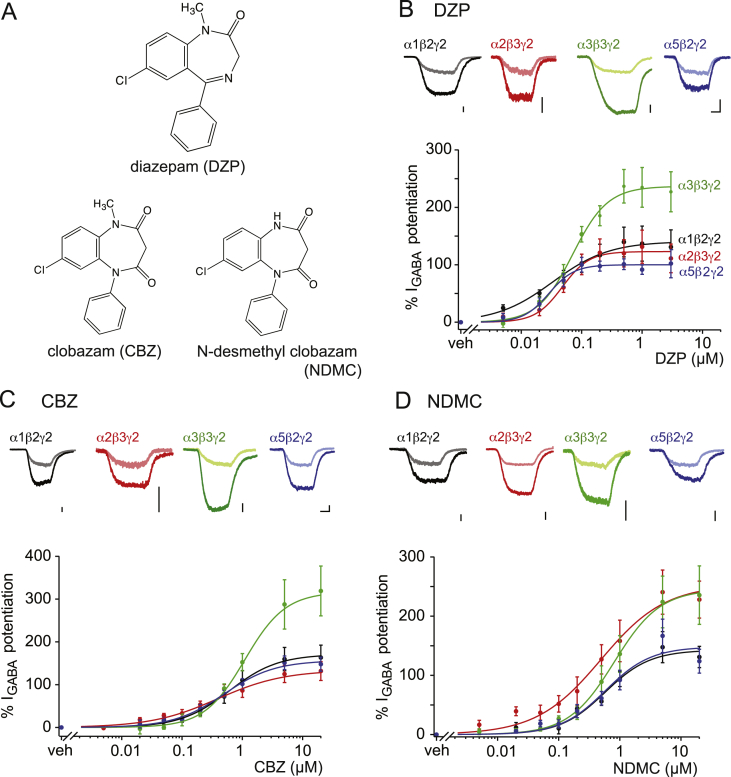
We first analyzed the effect of DZP, CBZ and NDMC as positive allosteric modulators of recombinant GABAARs expressed in HEK293 cells (Fig. 1 and Table 1, for chemical structures see Fig. 1A). In electrophysiological experiments, all three compounds potentiated currents through α1β2γ2, α2β3γ2, α3β3γ2 and α5β2γ2 GABAARs (short α1-, α2-, α3- and α5GABAARs) but did not directly activate GABAAR currents at the concentrations tested (up to 3 μM for DZP, and 20 μM for CBZ and NDMC). Differences were observed with respect to the potency and efficacy of the three compounds at the four GABAAR subtypes. DZP potentiated currents through all four subtypes with EC50 values between 0.029 and 0.071 μM. CBZ and NDMC were less potent with EC50 values between 0.39 and 1.1 μM and between 0.49 and 0.81 μM, respectively (Table 1). Pronounced differences were found when comparing the efficacy of potentiation by the three compounds at the different GABAAR subtypes. Potentiation by DZP was strongest for α3GABAARs (237%), while potentiation of the other three GABAAR subtypes ranged between 100% and 141%. At concentrations < EC50, which are probably more relevant to therapeutic effects of DZP, α1GABAARs were potentiated more strongly than α2-, α3- and α5GABAARs (Fig. 1B). CBZ and DZP had very similar efficacies at the four GABAAR subtypes, but CBZ differentiated less between subtypes at sub-saturating concentrations (Fig. 1C). NDMC potentiated α2 and α3GABAARs to a considerably higher degree (253 and 245%) than α1 and α5 (143 and 148%) (Fig. 1D). Taken together, DZP preferred α1GABAARs at low concentrations and α3GABAARs at high concentrations. CBZ was rather non-specific at low concentrations and preferred α3GABAARs at high concentrations. NDMC showed the strongest potentiation at α2GABAARs over the concentration range tested. We also determined relative affinities of the three compounds to the α1 and α2GABAARs. DZP bound both receptors with more than ten-fold higher affinities than CBZ and NDMC, but differences in affinity between the GABAAR subtypes were generally low (Table 1).
Fig. 1.
Potentiating effects of DZP, CBZ and NDMC on the four BDZ-sensitive GABAAR subtypes. (A) Chemical structure of DZP, CBZ, and NDMC. (B–D) GABA-evoked membrane currents were measured in HEK293 cells transiently transfected with recombinant α1β2γ2, α2β3γ2, α3β3γ2, and α5β2γ2 GABAARs. Top panels, traces show current responses evoked by GABA before and during application of a saturating concentration of DZP, CBZ, or NDMC (1 μM in case of DZP, and 20 μM in case of CBZ and NDMC). Light and dark traces were recorded before and during application of the BDZ, respectively. GABA was applied at EC10 in all experiments (1 μM, 5 μM, 8 μM, and 1 μM, for α1β2γ2, α2β3γ2, α3β3γ2, and α5β2γ2 GABAARs, respectively) for 6–10 s. Scale bars, 2 s and 200 pA. Bottom panels, concentration response curves of DZP, CBZ and NDMC obtained for the four GABAAR subtypes at EC10 of GABA. Data are mean ± SEM. Curves represent fits to the Hill equation with a baseline fixed to 0. n, numbers of cells, 5–7 for all data points.
Table 1.
Binding affinities and electrophysiological properties of DZP, CBZ and NDMC at recombinant GABAARs expressed in HEK203 cells.
| DZP |
CBZ |
NDMC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binding affinity (IC50, μM) | Electrophysiology |
Binding affinity (IC50, μM) | Electrophysiology |
Binding affinity (IC50, μM) | Electrophysiology |
|||||||
| EC50 (μM) | Emax (%) | α2/α1 selectivitya | EC50 (μM) | Emax (%) | α2/α1 selectivitya | EC50 (μM) | Emax (%) | α2/α1 selectivitya | ||||
| α1β2γ2 | 0.019 ± 0.005 | 0.033 ± 0.016 | 141 ± 17 | 0.43 ± 0.13 | 0.61 ± 0.20 | 171 ± 17 | 0.78 ± 0.15 | 0.57 ± 0.18 | 143 ± 13 | |||
| α2β3γ2 | 0.017 ± 0.004 | 0.048 ± 0.012 | 123 ± 11 | 0.88 ± 0.13 | 0.39 ± 0.08 | 0.39 ± 0.19 | 134 ± 16 | 0.78 ± 0.12 | 0.59 ± 0.12 | 0.49 ± 0.24 | 253 ± 32 | 1.77 ± 0.28 |
| α3β3γ2 | ND | 0.071 ± 0.013 | 227 ± 15 | ND | 1.10 ± 0.32 | 317 ± 30 | ND | 0.81 ± 0.30 | 245 ± 29 | |||
| α5β3γ2 | ND | 0.029 ± 0.006 | 100 ± 6.4 | ND | 0.50 ± 0.12 | 157 ± 11 | ND | 0.58 ± 0.18 | 148 ± 15 | |||
ND, not determined.
Data are means ± SD.
Determined as Emax(α2)/Emax(α1).
3.2. Antihyperalgesic actions
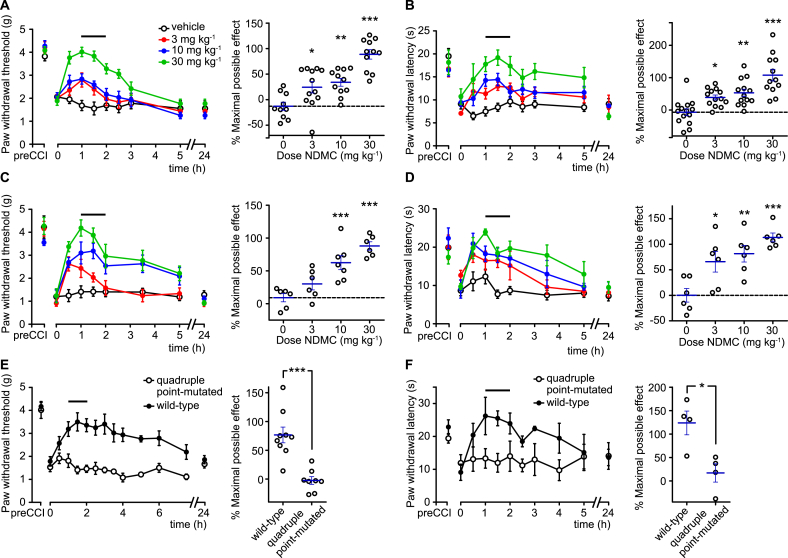
The previous study by Besson et al. (2013) has demonstrated antihyperalgesic effects of systemically applied CBZ in mice. Pharmacokinetic/pharmacodynamic modeling has suggested that most of the antihyperalgesia observed in mice following CBZ administration originated from the CBZ metabolite NDMC. We therefore assessed antihyperalgesic actions of NDMC using the chronic constriction injury (CCI) model of neuropathic pain. Following CCI surgery, all mice developed pronounced mechanical and heat hyperalgesia. Seven to 14 days after surgery, mice were treated systemically (p.o.) with three different doses of NDMC (3, 10, or 30 mg kg−1) or with vehicle. NDMC dose-dependently reduced both heat and mechanical hyperalgesia. No significant differences were observed between results obtained in mice of the 129X1/SvJ background, the genetic background of the GABAAR point-mutated mice used in this study (Fig. 2A,B), and in C57BL/6J mice the mouse strain most frequently used in the preclinical pain studies (Fig. 2C,D). To ensure that NDMC induced antihyperalgesia through the high-affinity BDZ binding site located at the interface between an α subunit and the γ subunit, we made use of quadruple point-mutated mice that carry the H → R mutation in the α1, α2, α3, and α5 subunits, i.e. in all α subunits that can form high affinity BDZ binding sites. In these quadruple point-mutated mice, NDMC (30 mg kg−1) had completely lost its antihyperalgesic effects (Fig. 2E,F).
Fig. 2.
Antihyperalgesic actions of NDMC. Reversal of mechanical hyperalgesia (A,C,E; assessed with von Frey filaments) and thermal hyperalgesia (B,D,F; assessed in the Hargreaves test) by NDMC 7–14 days after CCI surgery in wild-type C57BL/6J (A,B), wild-type 129X1/SvJ (C,D) and quadruple (H → R) 129X1/SvJ point-mutated mice (E,F). Left panels, paw withdrawal threshold or latencies (mean ± SEM) versus time after drug application. Horizontal lines indicates time interval used for the statistical analyses. Right panels, statistical analysis. Black circles, individual mice; blue lines mean ± SEM. (A–D): ***P < 0.001, **P < 0.01, *P < 0.05 significant versus vehicle-treated mice (ANOVA followed by Dunnett’s post hoc test); (A) F(3,41) = 19.8, n = 10, 12, 11, 11 for vehicle and 3, 10 and 30 mg kg−1 NDMC, respectively; (B) F(3,49) = 15.2, n = 14, 13, 13, 12 for vehicle and 3, 10 and 30 mg kg−1 NDMC; (C) F(3,21) = 16, n = 6, 6, 7 and 6 mice, for vehicle and 3, 10 and 30 mg kg−1 NDMC; (D): F(3,20) = 9.8, n = 6, 6, 6 and 6 mice, for vehicle and 3, 10 and 30 mg kg−1 NDMC; (E) unpaired t-test, n = 9 and 8 for wild-type and quadruple point-mutated mice, respectively; (F) unpaired t-test, n = 4 for both groups of mice. NDMC dose in panels E and F was 30 mg kg−1.
3.3. Sedation
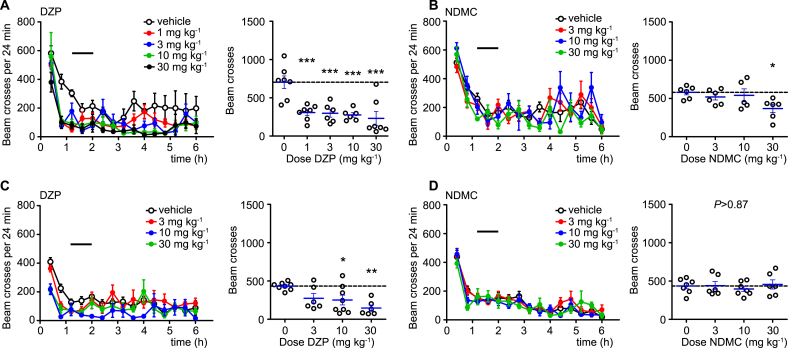
The more favorable α2/α1 selectivity profile of NDMC found in our in vitro experiments should manifest in a reduced propensity to sedation at doses with equipotent antihyperalgesic activity. We therefore compared the effects of DZP and NDMC (3, 10, and 30 mg kg−1 body weight) on locomotor activity in the open field test (Fig. 3). We performed these tests again both in C57BL/6J (Fig. 3A,B) and 129X1/SvJ mice (Fig. 3C,D) and monitored locomotor activity for 6 h after drug application. Statistical analyses were made for the time period between 72 and 120 min, which was the time interval of maximal drug effect in the pain tests described below. In C57BL/6J mice, DZP induced a dose-dependent reduction in locomotor activity, which reached statistical significance already at a dose of 1 mg kg−1 (Fig. 3A). In the same mouse strain, NDMC reduced locomotor activity only slightly and only at the highest dose tested (30 mg kg−1) (Fig. 3B). Reduction in locomotor activity was less pronounced in 129X1/SvJ mice (Fig. 3C,D). In these mice, NDMC did not reduce locomotor activity at any of the doses tested, while reduction in locomotor activity by DZP reached significance only at doses ≥10 mg kg−1.
Fig. 3.
Locomotor sedation by DZP and NDMC. Effects of orally applied DZP (A,C), and NDMC (B,D) in C57BL/6J (A,B) and 129X1/SvJ mice (C,D) on locomotor activity in the open field test. Left panels, number of light beam crosses (mean ± SEM) versus time after drug application. Horizontal lines indicates time interval used for the statistical analyses. Right panels, statistical analyses, performed for the interval 72–120 min. ***P < 0.001; **P < 0.01; *P < 0.05 significant versus vehicle-treated mice (ANOVA followed by Dunnett’s post hoc test. (A) F(4,28) = 9.2, n = 7, 7, 6, 6 and 7 for vehicle and DZP 1, 3, 10 and 30 mg kg−1, respectively. (B) F(3,19) = 3.2, n = 6, 6, 5 and 6 for vehicle and NDMC 3, 10 and 30 mg kg−1. (C) F(3,24) = 5.85, n = 8, 6, 8, 6 for vehicle and DZP 3, 10 and 30 mg kg−1. (D) P > 0.87, F(3,22) = 0.31, n = 7, 6, 7 and 6, for vehicle and NDMC 3, 10 and 30 mg kg−1.
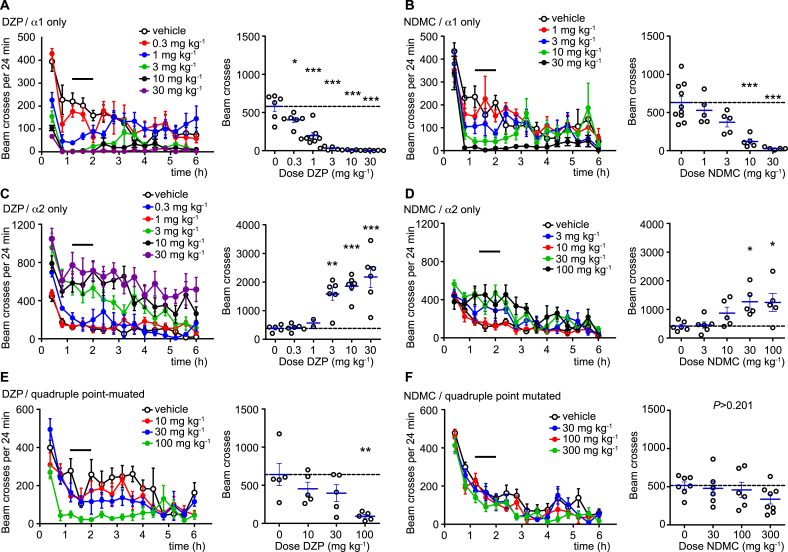
Although reduced sedative effects were expected from the more favorable α2/α1 activity ratio of NDMC, the almost complete lack of sedation was surprising, since NDMC still potentiated α1GABAARs with an efficacy similar to DZP (compare Fig. 1). We therefore tested whether NDMC evoked sedation in triple (H → R) GABAAR point-mutated (129X1/SvJ) mice in which only the α1GABAARs retained a high affinity BDZ binding site (Ralvenius et al., 2015). For brevity, we refer to these mice as HRRR and to mice in which only α2GABAARs retained their high affinity BDZ binding site as RHRR mice. As a pre-requisite of these experiments, we verified that recombinant GABAARs harboring point-mutated α subunits lose their modulation by NDMC (Fig. S1). When locomotor sedating effects of DZP and NDMC were tested in HRRR mice, both compounds exerted pronounced sedative effects. At antihyperalgesic doses (3–10 mg kg−1), locomotor activity was completely suppressed by DZP, while, in case of NDMC, strong (>50%) suppression of locomotor activity became apparent at doses of 10 and 30 mg kg−1 (Fig. 4A,B). Restricting the action of a BDZ site agonist to α1GABAARs thus appears to render mice more susceptible to locomotor sedation. The presence of a motor sedative effect by NDMC in HRRR mice but not in wild-type mice may suggest that α2GABAAR-mediated stimulation of locomotion (Ralvenius et al., 2015) occludes locomotor sedating actions of α1GABAARs. We therefore tested NDMC’s and DZP’s effects on locomotor activity also in RHRR mice. Both compounds indeed increased locomotion in RHRR mice (Fig. 4C,D). It is therefore likely that this locomotor stimulant effect is related to the anxiolytic action of α2GABAAR activation (Löw et al., 2000).
Fig. 4.
Opposing effects of α1 and α2GABAAR activation on locomotor activity. Effects of oral DZP (A,C,E), and NDMC (B,D,F) on locomotor activity in the open field test in mice carrying the H → R point mutation in multiple GABAAR subtypes (α2, α3, and α5, in (A,B) or α1, α3, and α5 in (C,D)) leaving only α1GABAARs (A,B) or α2GABAARs (B,D) BDZ-sensitive. (E,F) Same experiments as (A,B) but performed in quadruple point-mutated mice, in which all four subtypes (α1, α2, α3, and α5) of DZP sensitive GABAARs have been rendered insensitive. Left panels, number of light beam crosses (mean ± SEM) versus time after drug application. Horizontal lines indicates time interval used for the statistical analyses. Right panels, statistical analyses, performed for the interval 72–120 min. ***P < 0.001; **P < 0.01; *P < 0.05 significant versus vehicle-treated mice (ANOVA followed by Dunnett’s post hoc test. (A) F(5,29) = 35.7, n = 6, 6, 8, 5, 5 and 5 for vehicle and 0.3, 1, 3, 10 and 30 mg kg−1, respectively); (B) F(4,25) = 13.2, n = 10, 5, 5, 5 and 5 for vehicle and 1, 3, 10 and 30 mg kg−1. (C) (5,25) = 13.2, n = 5, 6, 2, 6, 6 and 6 for vehicle and 0.3, 1, 3, 10 and 30 mg kg−1. (D) F(4,22) = 4.6 (n = 6, 6, 5, 5 and 5 for vehicle and 3, 10, 30 and 100 mg kg−1. (E) F(3,16) = 4.9, n = 5, 5, 5 and 5 for vehicle and 10, 30 and 100 mg kg−1. (F) F(3,23) = 1.3, n = 7, 6, 6 and 8 for vehicle and 30, 100 and 300 mg kg−1.
In this context, it was interesting to see that wild-type mice of the 129X1/SvJ genetic background were less susceptible to the motor sedating actions of DZP and NDMC than C57BL/6 mice. A previous study reported that GABAAR subunit expression levels vary between mice of different genetic backgrounds and that expression of gabra2, the gene encoding for the GABAARs α2 subunit is unusually low in C57BL/6 mice (Mulligan et al., 2012). We therefore compared its expression levels in spinal cords and hippocampi in the two mouse strains using qRT-PCR. The α2 subunit expression was higher in 129X1/SvJ than in C57BL/6 mice by factors of 3.5 ± 0.03 (unpaired t-test, P < 0.001, n = 12 for both strains) and 4.1 ± 0.04 (P < 0.001, n = 12 for both strains) for spinal cord and hippocampus, respectively. We also found differences in α5 subunit expression, which was higher in C57BL/6 than in 129X1/SvJ mice by factors of 2.8 ± 0.6 (spinal cord, n = 12 for both genotypes) and 2.2 ± 0.4 (hippocampus, P < 0.01, n = 9 and 12 for C57BL/6 and 129X1/SvJ). No significant differences were found between the other subunits contributing to the high affinity BDZ binding sites (α1, α3, and γ2) (Fig. S2).
To address whether a low affinity BDZ binding site could be involved in NDMC-induced sedation, we analyzed also the motor sedative effects in the quadruple point-mutated RRRR mice (Fig. 4E,F). NDMC left locomotor activity unchanged at doses up to 100 mg kg−1 (Fig. 4F), which is 10-fold higher than the dose required for half-maximal antihyperalgesia. By contrast, at high doses (≥30 mg kg−1) of DZP reduced locomotor activity also in these mice (Fig. 5E). This observation is in line with our previous study (Ralvenius et al., 2015). It is likely that this remaining sedation occurs through the low affinity-binding site in the transmembrane domain described by Walters et al. (2000).
Fig. 5.
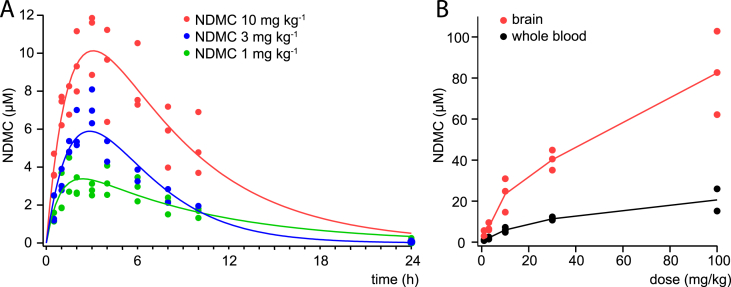
Pharmacokinetics of NDMC in mice. (A) Whole blood NDMC concentrations measured over time after p.o. administration of 3, 10 or 30 mg kg−1 NDMC (green, blue, red lines) to three (C57BL/6) mice per dose. Lines are fits to the Bateman function. (B) Whole blood (black) and brain tissue (red) concentrations determined at 2 h after p.o. administration of NDMC (0.3, 3, 10, 30, and 100 mg kg−1). Data points represent measurements taken from individual (129X1/SvJ) mice. Brain tissue concentrations in ng mg−1 were converted into micromolar concentrations assuming a specific mass of brain tissue of 1 g ml−1.
3.4. Pharmacokinetics of NDMC
In order to relate the results of our behavioral in vivo experiments to the NDMC activity profiles measured in vitro, we determined pharmacokinetic parameters of NDMC in C57BL/6 mice after systemic (p.o.) administration (Fig. 5A and Table 2). Whole blood NDMC concentrations were measured with LC-MS-MS following single oral doses of 3, 10 and 30 mg kg−1 in three mice per group. Maximal blood levels were reached within 2–3 h. Average maximum blood concentrations were 4.1, 6.6, and 11.5 μM and half-life was between 4.6 and 5.6 h (Fig. 5A). We also compared whole blood and brain tissue concentrations reached 2 h after p.o. administration of 1–100 mg kg−1 NDMC in 129X1/SvJ mice (Fig. 5B). Average maximum whole blood concentrations ranged from 1.0 μM (after 1 mg kg−1) to 20.6 μM (after 100 mg kg−1). Brain concentrations determined at the same time point were between 3.4 and 4.0 times higher than whole blood concentrations.
Table 2.
Pharmacokinetics of NDMC in mice after p.o. administration.
| Dose (mg/kg) | Half-life (h) | Cmax (μg/ml) | AUC (h*μg/ml) | Tmax (h) |
|---|---|---|---|---|
| 3 | 5.6 ± 0.6 | 1.19 ± 2.53 | 11.15 ± 1.66 | 2.0 ± 0.9 |
| 10 | 4.6 ± 1.6 | 1.91 ± 1.34 | 14.72 ± 4.38 | 3.0 ± 0.0 |
| 30 | 4.7 ± 0.3 | 3.31 ± 1.01 | 32.28 ± 5.09 | 2.7 ± 0.6 |
Data are means ± SD, n = 3 per group.
3.5. Therapeutic windows of DZP and NDMC
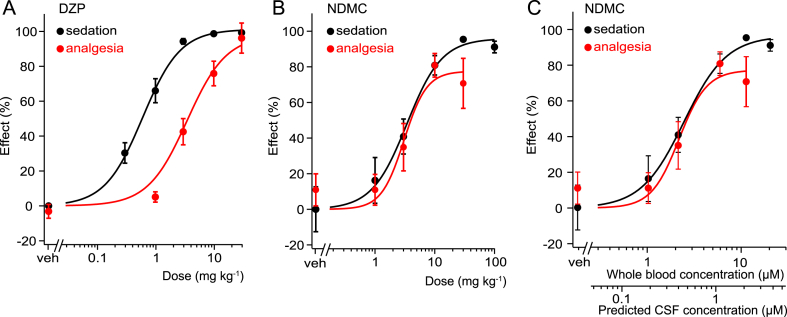
We have previously suggested that one of the major reasons limiting the analgesic efficacy of DZP and other non-selective BDZ in clinical practice is dose-limiting sedation, i.e. sedation already occurs at doses much lower than those necessary for antihyperalgesia (Ralvenius et al., 2015). Classical BDZs hence lack a therapeutic window for antihyperalgesia. The present study suggests that NDMC has a better α2/α1 selectivity profile than DZP, and might thus have a more favorable therapeutic window. We therefore compared the dose dependencies of DZP- and NDMC-induced sedation and antihyperalgesia, assessed as reduced responses to von-Frey filament stimulation (Fig. 6). To avoid confounding effects of anxiolysis in sedation experiments and of sedation on antihyperalgesia, we assessed sedation in triple (H → R) point-mutated mice, in which all GABAARs except α1 had been rendered BDZ-insensitive. Conversely, antihyperalgesia was studied in GABAAR α1(H → R) point-mutated mice to rule out confounding sedation. In case of DZP, dose response curves showed the expected difference in ED50 values with sedation occurring at 6-7-fold lower doses (0.59 ± 0.08 mg kg−1) than antihyperalgesia (3.4 ± 1.3 mg kg−1). By contrast, NDMC exerted antihyperalgesia and sedation with similar ED50 values (3.4 ± 0.8 mg kg−1 and 3.1 ± 0.9 mg kg−1 for sedation and antihyperalgesia, respectively).
Fig. 6.
Therapeutic windows of DZP and NDMC. α1GABAAR-mediated sedation and α2GABAAR-mediated antihyperalgesia by DZP (A) and NDMC (B,C). (A) Sedation in α2/α3/α5(H → R) triple point mutated mice: n = 6, 6, 8, 5 and 5 for vehicle and DZP 0.3, 1, 3, 10 and 30 mg kg−1, respectively; antihyperalgesia in α1(H → R) single point-mutated mice: n = 20, 7, 14, 13 and 6, for vehicle and DZP 1, 3, 10 and 30 mg kg−1, respectively. (B) Sedation in α2/α3/α5 (H → R) triple point-mutated mice: n = 10, 5, 5, 5, 5 and 4 for vehicle and NDMC 1, 3, 10, 30 and 100 mg kg−1, respectively. Antihyperalgesia in α1(H → R) single point-mutated mice: n = 6, 6, 6, 5 and 5 mice, for vehicle and NDMC 1, 3, 10 and 30 mg kg−1, respectively. Data points are mean ± SEM. (C) Same as (B) but antihyperalgesia and sedation plotted versus whole blood concentrations and estimated CSF concentrations (for details of conversion see Discussion).
4. Discussion
The present study suggests that NDMC might be a suitable compound for human proof-of-concept trials assessing a potential antihyperalgesic efficacy of BDZ site agonists with improved subtype selectivity in chronic neuropathic pain patients. This conclusion is based on three observations. (1) In recombinant receptors, NDMC had a more favorable α2- over α1GABAARs activity ratio than its parent compound CBZ and than the classical BDZ agonist DZP. (2) Unlike DZP, NDMC caused either no or only modest sedation at antihyperalgesic doses in two strains of wild-type mice. (3) Even under conditions, which unmasked sedative effects of NDMC (i.e. in the triple point-mutated mice), the therapeutic window of NDMC was significantly better than that of DZP.
In vitro, saturating concentrations of DZP and CBZ potentiated both GABAAR subtypes with similar efficacy, while NDMC clearly favored α2- over α1GABAARs. At low concentrations (<EC50), NDMC preferred α2GABAARs across the entire concentration range tested, while CBZ had about similar effects on α2- and α1GABAARs and DZP favored α1GABAARs. This result is largely consistent with a previous report on the efficacy of CBZ and NDMC at GABAAR subtypes expressed in Xenopus laevis oocytes (Hammer et al., 2015). That study also found an improved α2/α1 selectivity ratio for NDMC of 1.33 ± 0.06 (versus 1.02 ± 0.05 for CBZ) in human GABAARs. The more favorable α2/α1 selectivity ratio very likely underlies the better therapeutic window of NDMC compared to DZP. Our pharmacokinetic analyses revealed that average peak whole blood concentrations after analgesic doses ranged between 4.2 µM (after 3 mg kg−1) and 11.5 μM (after 30 mg kg−1). These concentrations are remarkably similar to blood levels reported for human patients during chronic treatment with antiepileptic doses of NDMC (1000–3000 ng ml−1, equivalent to 3.5–10.5 μM; Haigh et al., 1987). Assuming a cerebrospinal fluid (CSF)/serum concentration ratio of about 0.1 (Laux and Koeppen, 1984) and a serum/whole blood ratio of 1.7 (similar to that of DZP, Jones and Larsson, 2004), the blood levels measured in this study correspond to roughly 0.7–2 μM NDMC in the CSF. Thus, the drug concentrations relevant for the in vivo analgesic effects of NDMC are above the in vitro EC50 values at GABAARs. Accordingly, differences in the in vitro efficacy at concentrations above rather than below EC50 appear to determine the in vivo pharmacological profile of NDMC.
While no or only very weak sedation was observed in wild-type mice after NDMC administration, locomotor sedation became apparent when the action of NDMC was restricted to α1GABAARs, i.e. in α2/α3/α5 (HRRR) triple point-mutated mice. This result was not unexpected because all three compounds tested exhibited similar efficacy at α1GABAARs. Nevertheless, even when analyzed under these conditions, the therapeutic window of NDMC was greatly improved compared to that of DZP.
In the present study, we did not include CBZ in the in vivo experiments, because, in mice, CBZ is very rapidly metabolized into NDMC. Already 15 min after administration of CBZ, blood levels of NDMC exceed those of CBZ (Besson et al., 2013) suggesting that NDMC would make a major contribution to any in vivo effect seen after CBZ administration. In humans, this conversion occurs much more slowly with blood concentrations of CBZ exceeding those of NDMC for more than 24 h after a single oral dose of 20 or 30 mg CBZ (Besson et al., 2015, Pullar et al., 1987). It is hence likely that, in humans, treatment with NDMC – instead of with the parent compound CBZ – would improve the therapeutic margin and result in less sedation at concentrations where antihyperalgesia is expected. On the other hand, after prolonged treatment of humans steady-state NDMC concentrations exceed those of CBZ by a factor of 2.6–3.8 (Tolbert and Bekersky, 2013). This may contribute to the relatively (compared to DZP) low propensity of CBZ to cause sedation during chronic treatment (Miura et al., 2002, Steru et al., 1986). In this context, it might be worth noting that the improved α2/α1 selectivity ratio may not only contribute to a reduced propensity to sedation but may also be relevant for other possible indications such as autism spectrum disorders where activity at non-α1GABAARs improves social interactions (Han et al., 2014, Newman et al., 2015).
Drug discovery and development programs have yielded BDZ site agonists with negligible activity at α1GABAARs such as L-838,417 (McKernan et al., 2000), TPA023 (Atack et al., 2006), and TPA023B (Atack et al., 2011). While the lack of efficacy at α1GABAARs avoids unwanted sedative and other undesired effects, a possible limitation of these compounds is their only partial agonistic activity at α2GABAARs. L-838,417 potentiates recombinant α2β3γ2 GABAARs only by about 40% (McKernan et al., 2000), and potentiation of recombinant α2β3γ2 GABAARs by TPA023 and TPA023B reached only 11% and 38% of that of chlordiazepoxide (Atack et al., 2006, Atack et al., 2011). By contrast, NDMC exhibited even stronger efficacy at α2GABAARs than the full agonist DZP. A correlation of α2GABAAR occupancy and antihyperalgesic effects performed for DZP indicates that significant analgesia (>50% maximum possible effect) is only achieved at about 70% receptor occupancy even when a full agonist is used (Ralvenius et al., 2015). This may indicate limited antihyperalgesic efficacy of subtype selective agonists with only partial agonistic activity at α2GABAARs. Compared to these compounds, NDMC has a less favorable selectivity ratio but much higher activity at α2GABAARs. It remains to be seen whether the α1GABAAR activity of NDMC at antihyperalgesic doses is still a critical limitation.
In summary, our study demonstrates that NDMC possesses not only an improved α2/α1 GABAARs activity ratio in vitro, but also a more favorable in vivo pharmacological profile than classical BDZ. While DZP induced half-maximal sedation at doses much lower than those required for antihyperalgesia, NDMC elicited both actions with similar dose-dependencies. Since NDMC is a naturally occurring metabolite of CBZ (Grigoleit et al., 1983), its safety profile is already known from its parent drug CBZ and it is very unlikely that unexpected adverse effects emerge. NDMC might thus constitute a useful and safe compound for investigator initiated proof-of-concept trials in human volunteers or pain patients.
Author contributions
WTR performed and analyzed all behavior tests, except initial antihyperalgesia tests, which were done by MB and AM. MAA performed and analyzed the electrophysiology experiments. DB performed the binding assays, MB and AM did the pharmacokinetics study, YD checked NDMC purity and did the NDMC analytics. MB, AM, JD, YD, and HUZ analyzed the pharmacokinetic data. HUZ designed experiments, analyzed data and wrote the manuscript. All authors contributed to the manuscript.
Acknowledgements
This study was in part supported by grants of the Swiss National Science Foundation (Special Program on University Medicine (33CM30) to JD and HUZ, an Swiss National Science Foundation project grant (131093) to HUZ and an advanced investigator grant from the European Research Council (DHISP 250128 to HUZ). WTR was supported through a research fellowship from the Stiftung für Medizinische Forschung of the University of Zurich. UR was supported by Award Number R03MH094834 from the National Institute of Mental Health and Award Number R03DA033491 from the National Institute on Drug Abuse. The authors thank Louis Scheurer, Thomas Grampp and Isabelle Kellenberger for excellent technical assistance, and Dennis Kwame Boadum and Carmen Brazerol for animal care.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neuropharm.2016.07.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Atack J., Hallett D.J., Tye S., Wafford K.A., Ryan C., Sanabria-Bohorquez S., Eng W.S., Gibson R.E., Burns H.D., Dawson G.R., Carling R.W., Street L.J., Pike A., De Lepeleire I., van Laere K., Bormans G., de Hoon J.N., van Hecken A., McKernan R.M., Murphy M.G., Hargreaves R.J. Preclinical and clinical pharmacology of TPA023B, a GABAA receptor α2/α3 subtype-selective partial agonis. J. Psychopharmacol. 2011;25:329–344. doi: 10.1177/0269881109354928. [DOI] [PubMed] [Google Scholar]
- Atack J.R., Wafford K.A., Tye S.J., Cook S.M., Sohal B., Pike A., Sur C., Melillo D., Bristow L., Bromidge F., Ragan I., Kerby J., Street L., Carling R., Castro J.L., Whiting P., Dawson G.R., McKernan R.M. TPA023 [7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluor ophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for α2- and α3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J. Pharmacol. Exp. Ther. 2006;316:410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- Bennett G.J., Xie J.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Besson M., Daali Y., Di Lio A., Dayer P., Zeilhofer H.U., Desmeules J. Antihyperalgesic effect of the GABAA ligand clobazam in a neuropathic pain model in mice: a pharmacokinetic-pharmacodynamic study. Basic Clin. Pharmacol. Toxicol. 2013;112:192–197. doi: 10.1111/bcpt.12017. [DOI] [PubMed] [Google Scholar]
- Besson M., Matthey A., Daali Y., Poncet A., Vuillemier P., Curatolo M., Zeilhofer H.U., Desmeules J. GABAergic modulation in central sensitization in humans: a randomized placebo-controlled pharmacokinetic-pharmacodynamic study comparing clobazam with clonazepam in healthy volunteers. Pain. 2015;156:397–404. doi: 10.1097/01.j.pain.0000460331.33385.e8. [DOI] [PubMed] [Google Scholar]
- Beyer C., Roberts L.A., Komisaruk B.R. Hyperalgesia induced by altered glycinergic activity at the spinal cord. Life Sci. 1985;37:875–882. doi: 10.1016/0024-3205(85)90523-5. [DOI] [PubMed] [Google Scholar]
- Crestani F., Keist R., Fritschy J.M., Benke D., Vogt K., Prut L., Bluthmann H., Möhler H., Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deglon J., Thomas A., Daali Y., Lauer E., Samer C., Desmeules J., Dayer P., Mangin P., Staub C. Automated system for on-line desorption of dried blood spots applied to LC/MS/MS pharmacokinetic study of flurbiprofen and its metabolite. J. Pharm. Biomed. Anal. 2011;54:359–367. doi: 10.1016/j.jpba.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Di Lio A., Benke D., Besson M., Desmeules J., Daali Y., Wang Z.J., Edwankar R., Cook J.M., Zeilhofer H.U. HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology. 2011;60:626–632. doi: 10.1016/j.neuropharm.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoleit H.G., Hajdu P., Hundt H.K., Koeppen D., Malerczyk V., Meyer B.H., Muller F.O., Witte P.U. Pharmacokinetic aspects of the interaction between clobazam and cimetidine. Eur. J. Clin. Pharmacol. 1983;25:139–142. doi: 10.1007/BF00544031. [DOI] [PubMed] [Google Scholar]
- Haigh J.R., Pullar T., Gent J.P., Dailley C., Feely M. N-desmethylclobazam: a possible alternative to clobazam in the treatment of refractory epilepsy? Br. J. Clin. Pharmacol. 1987;23:213–218. doi: 10.1111/j.1365-2125.1987.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer H., Ebert B., Jensen H., Jensen A.A. Functional characterization of the 1,5-Benzodiazepine clobazam and its major active metabolite N-Desmethylclobazam at human GABAA receptors expressed in Xenopus laevis oocytes. PLoS ONE. 2015;10:e0120239. doi: 10.1371/journal.pone.0120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Tai C., Jones C.J., Scheuer T., Catterall W.A. Enhancement of inhibitory neurotransmission by GABAA receptors having α2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron. 2014;81:1282–1289. doi: 10.1016/j.neuron.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Kordas K.S., Gravius A., Bolcskei K., Parsons C.G., Dekundy A., Danysz W., Dezsi L., Wittko-Schneider I.M., Saghy K., Gyertyan I., Horvath C. Assessment of the effects of NS11394 and L-838417, α2/3 subunit-selective GABAA receptor-positive allosteric modulators, in tests for pain, anxiety, memory and motor function. Behav. Pharmacol. 2012;23:790–801. doi: 10.1097/FBP.0b013e32835a7c7e. [DOI] [PubMed] [Google Scholar]
- Jones A.W., Larsson H. Distribution of diazepam and nordiazepam between plasma and whole blood and the influence of hematocrit. Ther. Drug Monit. 2004;26:380–385. doi: 10.1097/00007691-200408000-00007. [DOI] [PubMed] [Google Scholar]
- Knabl J., Witschi R., Hösl K., Reinold H., Zeilhofer U.B., Ahmadi S., Brockhaus J., Sergejeva M., Hess A., Brune K., Fritschy J.-M., Rudolph U., Möhler H., Zeilhofer H.U. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Knabl J., Zeilhofer U.B., Crestani F., Rudolph U., Zeilhofer H.U. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain. 2009;141:233–238. doi: 10.1016/j.pain.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Laux G., Koeppen D. Serum and cerebrospinal fluid concentration of clobazam and N-desmethylclobazam. Int. J. Clin. Pharmacol. Ther. Toxicol. 1984;22:355–359. [PubMed] [Google Scholar]
- Löw K., Crestani F., Keist R., Benke D., Brünig I., Benson J.A., Fritschy J.M., Rülicke T., Bluethmann H., Möhler H., Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- McKernan R.M., Rosahl T.W., Reynolds D.S., Sur C., Wafford K.A., Atack J.R., Farrar S., Myers J., Cook G., Ferris P., Garrett L., Bristow L., Marshall G., Macaulay A., Brown N., Howell O., Moore K.W., Carling R.W., Street L.J., Castro J.L., Ragan C.I., Dawson G.R., Whiting P.J. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat. Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Miura Y., Amano S., Torii R., Ihara N. Clobazam shows a different antiepileptic action profile from clonazepam and zonisamide in Ihara epileptic rats. Epilepsy Res. 2002;49:189–202. doi: 10.1016/s0920-1211(02)00032-3. [DOI] [PubMed] [Google Scholar]
- Möhler H., Fritschy J.M., Rudolph U. A new benzodiazepine pharmacology. J. Pharmacol. Exp. Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Mulligan M.K., Wang X., Adler A.L., Mozhui K., Lu L., Williams R.W. Complex control of GABAA receptor subunit mRNA expression: variation, covariation, and genetic regulation. PLoS ONE. 2012;7:e34586. doi: 10.1371/journal.pone.0034586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G., Ahring P.K., Mirza N.R. Developing analgesics by enhancing spinal inhibition after injury: GABAA receptor subtypes as novel targets. Trends Pharmacol. Sci. 2009;30:453–459. doi: 10.1016/j.tips.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Newman E.L., Smith K.S., Takahashi A., Chu A., Hwa L.S., Chen Y., DeBold J.F., Rudolph U., Miczek K.A. α2-containing GABAA receptors: a requirement for midazolam-escalated aggression and social approach in mice. Psychopharmacol. Berl. 2015;232:4359–4369. doi: 10.1007/s00213-015-4069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickolls S., Mace H., Fish R., Edye M., Gurrell R., Ivarsson M., Pitcher T., Tanimoto-Mori S., Richardson D., Sweatman C., Nicholson J., Ward C., Jinks J., Bell C., Young K., Rees H., Moss A., Kinloch R., McMurray G. A comparison of the α2/3/5 selective positive allosteric modulators L-838,417 and TPA023 in preclinical models of inflammatory and neuropathic pain. Adv. Pharmacol. Sci. 2011;2011:608912. doi: 10.1155/2011/608912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J., Yévenes G.E., Benke D., Di Lio A., Ralvenius W.T., Witschi R., Scheurer L., Cook J.M., Rudolph U., Fritschy J.M., Zeilhofer H.U. Antihyperalgesia by α2-GABAA receptors occurs via a genuine spinal action and does not involve supraspinal sites. Neuropsychopharmacology. 2014;39:477–487. doi: 10.1038/npp.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullar T., Haigh J.R., Peaker S., Feely M.P. Pharmacokinetics of N-desmethylclobazam in healthy volunteers and patients with epilepsy. Br. J. Clin. Pharmacol. 1987;24:793–797. doi: 10.1111/j.1365-2125.1987.tb03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralvenius W.T., Benke D., Acuña M.A., Rudolph U., Zeilhofer H.U. Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nature Communications. 2015;6 doi: 10.1038/ncomms7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichl S., Augustin M., Zahn P.K., Pogatzki-Zahn E.M. Peripheral and spinal GABAergic regulation of incisional pain in rats. Pain. 2012;153:129–141. doi: 10.1016/j.pain.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Roberts L.A., Beyer C., Komisaruk B.R. Nociceptive responses to altered GABAergic activity at the spinal cord. Life Sci. 1986;39:1667–1674. doi: 10.1016/0024-3205(86)90164-5. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Crestani F., Benke D., Brünig I., Benson J.A., Fritschy J.M., Martin J.R., Bluethmann H., Möhler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti L., Woolf C.J. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J. Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- Steru L., Chermat R., Millet B., Mico J.A., Simon P. Comparative study in mice of ten 1,4-benzodiazepines and of clobazam: anticonvulsant, anxiolytic, sedative, and myorelaxant effects. Epilepsia. 1986;27(Suppl. 1):S14–S17. doi: 10.1111/j.1528-1157.1986.tb05728.x. [DOI] [PubMed] [Google Scholar]
- Tan K.R., Brown M., Labouebe G., Yvon C., Creton C., Fritschy J.M., Rudolph U., Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe-Theodor A., Kuner R. Studying ongoing and spontaneous pain in rodents–challenges and opportunities. Eur. J. Neurosci. 2014;39:1881–1890. doi: 10.1111/ejn.12643. [DOI] [PubMed] [Google Scholar]
- Tolbert D., Bekersky I. Pharmacokinetics of N-Desmethylclobazam, the active and primary metabolite of clobazam. Neurology. 2013;80 [Google Scholar]
- Vuilleumier P.H., Besson M., Desmeules J., Arendt-Nielsen L., Curatolo M. Evaluation of anti-hyperalgesic and analgesic effects of two benzodiazepines in human experimental pain: a randomized placebo-controlled study. PLoS ONE. 2013;8:e43896. doi: 10.1371/journal.pone.0043896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R.J., Hadley S.H., Morris K.D., Amin J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat. Neurosci. 2000;3:1274–1281. doi: 10.1038/81800. [DOI] [PubMed] [Google Scholar]
- Zeilhofer H.U., Wildner H., Yévenes G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.