Abstract
Informed collecting, conservation, monitoring and utilization of genetic diversity requires knowledge of the distribution and structure of the variation occurring in a species. Hordeum vulgare subsp. spontaneum (K. Koch) Thell., a primary wild relative of barley, is an important source of genetic diversity for barley improvement and co-occurs with the domesticate within the center of origin. We studied the current distribution of genetic diversity and population structure in H. vulgare subsp. spontaneum in Jordan and investigated whether it is correlated with either spatial or climatic variation inferred from publically available climate layers commonly used in conservation and ecogeographical studies. The genetic structure of 32 populations collected in 2012 was analyzed with 37 SSRs. Three distinct genetic clusters were identified. Populations were characterized by admixture and high allelic richness, and genetic diversity was concentrated in the northern part of the study area. Genetic structure, spatial location and climate were not correlated. This may point out a limitation in using large scale climatic data layers to predict genetic diversity, especially as it is applied to regional genetic resources collections in H. vulgare subsp. spontaneum.
Introduction
Crop wild relatives (CWR) are vital for food security because they provide novel alleles for crop improvement and adaptation [1–3]. Their diversity is threatened by global and climate change [4,5], and more knowledge about the geographical distribution of their genetic variation, and the processes that shape it, is required to more effectively collect, conserve, monitor and use this variation. Genetic data are still lacking for many CWR. Ecogeographical information, which combines environmental and spatial data, is increasingly used as a proxy for genetic diversity to improve collecting, conservation, monitoring and use of CWR [6–11]. This approach assumes that ecogeographical diversity among collecting sites is correlated with genetic diversity because the distribution of genetic variation in wild plant species is affected by environment (via natural selection) and geographical separation (via isolation by distance). It follows that conserving populations sampled from the widest possible range of ecogeographical conditions is expected to maximize the genetic diversity conserved [12].
Ecogeographical data has been used to identify areas and populations for in situ conservation [7,10,13], to assemble core collections [14] and to identify germplasm potentially useful for crop improvement [15,16]. Habitat suitability modelling, also known as species distribution modelling or niche modelling, has been used to identify gaps in existing collections and to prioritize areas for collecting [8,17–19]. Habitat suitability modelling predicts the potential geographical distribution of a species using the known distribution and environmental data, which often come in the form of climatic, edaphic, geophysical and/or land use variables.
Maxted et al. [19] have cautioned that the expected correlation between genetic and ecogeographical diversity may not hold for all species and habitats. CWR are often found in ruderal areas and agricultural landscapes where natural, adaptive responses to climate might be altered through anthropogenic influences [20–23]. Of these, the breakdown of isolation by distance due to elevated gene flow may be particularly important.
Barley is the fourth most important cereal crop worldwide in terms of production, yield and area harvested, and is one of the crops in which CWR use in breeding programs is particularly prominent [24]. Hordeum vulgare subsp. spontaneum (K. Koch) Thell. (hereafter Spontaneum) is the progenitor of barley and represents an important genetic resource in barley breeding for traits such as powdery mildew, leaf scald or leaf rust resistance [25–31], yield [32], drought and temperature tolerance [33,34] and agronomic traits such as malting quality [35,36]. Recently, a multi-parental nested association mapping population, using 24 Spontaneum donor accessions to induce genetic variation, was set up and tested to study regulation of flowering time in barley [37]. The Fertile Crescent has been considered the primary center of origin and domestication of barley [38,39]. Other studies suggest additional domestication events in areas east of the Fertile Crescent [40], Tibet [41], Ethiopia and the Western Mediterranean [42,43].
Efforts have been made since the 1970s to characterize wild barley germplasm across its distribution range using morphological characters, isozymes and molecular markers [44–55]. The highest genetic variation lies within the Fertile Crescent, and there specifically in Jordan and Israel [55,56]. A few studies have compared diversity in Spontaneum between Jordan and neighboring countries. Baek et al. [57] found that the number of alleles as well as the percentage of country specific alleles is significantly higher in Jordan than in Israel. Analysis of SNP diversity indicated Jordan and southern Syria as a likely site of domestication [54].
Past studies have investigated the correlation between genetic diversity and environment in Spontaneum. They have documented an association between genetic diversity, at single loci, and geography, acrosstemperature or rainfall gradients [44,49,52,58–61]. Genetic differentiation has also been shown to occur, in sympatry, between opposing slopes in the Evolution Canyon in Israel. This has been attributed to adaptation to different microclimates [62,63]. In Jordan, Jaradat [64] characterized kernel protein content and genetic diversity at four esterase loci in 12 wild populations. Ribosomal DNA (rDNA) polymorphism was used to study accessions from 27 collecting sites [65]. The distribution of alleles was found to be correlated with ecogeographical factors such as rainfall, temperature, and geographical location. Baek et al. [57] used 18 SSRs to study genetic diversity in accessions from 16 collecting sites and reported associations between ecogeographical variables and allele frequencies at individual loci. Hübner et al. [66] studied Spontaneum in Israel and attempted to correlate genetic population structure—as opposed to polymorphism or allele frequencies at individual loci—with climate variables. No studies of the correlation between environment and population structure of Spontaneum in Jordan have yet been published.
In this study, we sampled Spontaneum populations across their range in Jordan and analyzed this collection with a set of 37 SSRs. Our aim was to describe the patterns of genetic diversity and population structure of Jordanian Spontaneum and to determine the degree to which the genetic structure estimated with our markers is correlated with spatial and climatic variables derived from global data sources commonly used in conservation and ecogeographical studies [18,67–69].
Material and Methods
Plant material and germination
Single spikes of 12–15 individuals were collected from each of 42 Spontaneum populations during a barley collecting mission carried out in 2012, which covered the entire distribution of Spontaneum in Jordan. The collecting had been formalized in a letter of agreement between the Jordanian National Center for Agricultural Research and Extension (NCARE) and Bioversity International, which encompassed the permit to collect Spontaneum from all visited sites. The collecting was carried out with the continuous participation of NCARE staff and no rare or threatened species was collected. Seeds from each spike were germinated to produce leaf tissue for DNA extraction. Up to eight seeds per spike were rolled into germination paper and placed in an incubator at 25°C for germination. 50–100 mg of 3–5 day old leaf tissue was harvested from one germinated seed per spike. 32 populations (Table 1) (where leaf tissue was available from at least 11 individuals) were used for the study. This resulted in a total of 373 genotypes, with 8–13 individuals per population (S1 Table). The spatial distribution of populations is shown in Fig 1.
Table 1. Collecting site description.
| Collecting site number | Latitude | Longitude | Elevation (m) | Number of individuals used in study |
|---|---|---|---|---|
| 1 | 32.70233 | 35.72325 | 94 | 12 |
| 2 | 32.69611 | 35.737528 | 119 | 13 |
| 3 | 32.67656 | 35.804833 | 467 | 11 |
| 4 | 32.59239 | 35.666944 | 87 | 11 |
| 5 | 32.58686 | 35.998194 | 475 | 12 |
| 6 | 32.51183 | 35.645444 | 119 | 12 |
| 7 | 32.47747 | 35.969694 | 608 | 12 |
| 8 | 32.43733 | 35.691806 | 484 | 12 |
| 9 | 32.37594 | 35.7365 | 785 | 12 |
| 10 | 32.33386 | 35.91375 | 957 | 11 |
| 11 | 32.32931 | 36.092667 | 866 | 12 |
| 12 | 32.32144 | 35.750139 | 770 | 12 |
| 13 | 32.27547 | 35.891333 | 564 | 11 |
| 14 | 32.23847 | 35.889472 | 379 | 13 |
| 15 | 32.14508 | 35.856639 | 561 | 12 |
| 16 | 32.13858 | 35.646806 | 141 | 12 |
| 17 | 32.11461 | 35.86625 | 629 | 14 |
| 18 | 32.06989 | 35.715139 | 1044 | 11 |
| 19 | 32.06694 | 35.720583 | 1058 | 12 |
| 20 | 32.04581 | 35.775167 | 911 | 12 |
| 21 | 32.01156 | 35.733778 | 589 | 11 |
| 22 | 31.77919 | 35.798833 | 805 | 9 |
| 23 | 31.70953 | 35.960611 | 709 | 13 |
| 24 | 31.67036 | 35.785889 | 745 | 12 |
| 25 | 31.56639 | 35.791417 | 646 | 11 |
| 26 | 31.54019 | 35.773639 | 677 | 11 |
| 27 | 31.18831 | 35.696583 | 773 | 12 |
| 28 | 31.04872 | 35.708861 | 1222 | 12 |
| 29 | 30.68108 | 35.622222 | 1566 | 12 |
| 30 | 30.65542 | 35.610194 | 1257 | 12 |
| 31 | 30.42178 | 35.512 | 1580 | 8 |
| 32 | 30.39875 | 35.496861 | 1680 | 11 |
Fig 1. Collecting sites in Jordan.
Ecogeographical and climate data of collecting sites
Geographical coordinates, altitude, slope, and aspect of the collecting sites were recorded with a GPS Garmin Emap device (datum: WGS84) and habitat type was recorded. Climate data was obtained from the WorldClim database version 1.4 (http://www.worldclim.org), a global and freely available source for climate data layers generated through interpolation of average monthly climate data from weather stations [70]. Layers for current climate conditions (1950–2000) for the 19 bioclimatic variables (Bioclim; see Table 2) were downloaded. Values for the 19 variables were extracted for each collecting site using DIVA-GIS. Collecting sites included ruderal habitats, barley field margins as well as nature reserves, covered an altitudinal range from 87 to 1680 m, a latitudinal range from 30.39875–32.70233 decimal degrees, a longitudinal range from 35.49686111–36.09266667 decimal degrees, an annual precipitation range from 229–491 mm and a mean annual temperature range from 12.5–21.5°C. Collecting site information is provided in S2 Table.
Table 2. Coding of bioclimatic variables according to WorldClim at http://www.worldclim.org/bioclim.
| Code | Description |
|---|---|
| Bioclim 1 | Annual Mean Temperature |
| Bioclim 2 | Mean Diurnal Range (Mean of monthly (max temp—min temp)) |
| Bioclim 3 | Isothermality (Bioclim2/ Bioclim7) (* 100) |
| Bioclim 4 | Temperature Seasonality (standard deviation *100) |
| Bioclim 5 | Max Temperature of Warmest Month |
| Bioclim 6 | Min Temperature of Coldest Month |
| Bioclim 7 | Temperature Annual Range (Bioclim5- Bioclim6) |
| Bioclim 8 | Mean Temperature of Wettest Quarter |
| Bioclim 9 | Mean Temperature of Driest Quarter |
| Bioclim 10 | Mean Temperature of Warmest Quarter |
| Bioclim 11 | Mean Temperature of Coldest Quarter |
| Bioclim 12 | Annual Precipitation |
| Bioclim 13 | Precipitation of Wettest Month |
| Bioclim 14 | Precipitation of Driest Month |
| Bioclim 15 | Precipitation Seasonality (Coefficient of Variation) |
| Bioclim 16 | Precipitation of Wettest Quarter |
| Bioclim 17 | Precipitation of Driest Quarter |
| Bioclim 18 | Precipitation of Warmest Quarter |
| Bioclim 19 | Precipitation of Coldest Quarter |
DNA extraction and genotyping
DNA was purified from 3–5 day old leaf tissue with the Qiagen DNeasy® 96 Plant Kit. Thirty-seven EST-derived SSR primers were used for genotyping [71–73] (Table 3). Loci were distributed across all 7 barley chromosomes. PCR was carried out in 5-μl reactions consisting of 2–10 ng genomic DNA, 1x Qiagen Multiplex PCR Master Mix, 225 nM of each primer pair. All fragments were amplified using MJ Research (Waltham, Massachusetts) PTC200 thermocyclers and the following PCR profile: an initial denaturing step of 15 min at 95°C followed by 40 cycles with denaturation at 94°C for 60 s, annealing at 60°C for 30 s and extension at 72°C for 15 s. After 40 cycles, a final extension step was performed at 72°C for 10 min. Amplification products were resolved by capillary electrophoresis on the ABI 3130xl Genetic Analyzer. Fragment sizes were calculated using GeneScan 400HD (ROX) internal size standards and scored with GeneMapper software (v. 5.0) (Life Technologies, Thermo Fisher Scientific Inc.).
Table 3. Characteristics of SSR markers.
| Marker ID | Location | Ind no. | Allele no. | PIC | Allelic richness |
|---|---|---|---|---|---|
| GBM1002 | 1H | 367 | 12 | 0.736 | 5.44 |
| GBM1013 | 1H | 373 | 7 | 0.383 | 3.194 |
| GBM1029 | 1H | 373 | 6 | 0.412 | 3.038 |
| GBM1334 | 1H | 372 | 5 | 0.538 | 3.317 |
| GBM1461 | 1H | 373 | 20 | 0.914 | 9.082 |
| GBM1035 | 2H | 373 | 6 | 0.66 | 4.264 |
| GBM1036 | 2H | 372 | 5 | 0.497 | 3.289 |
| GBM1047 | 2H | 364 | 7 | 0.664 | 4.269 |
| GBM1208 | 2H | 367 | 7 | 0.563 | 3.693 |
| GBM1218 | 2H | 369 | 4 | 0.591 | 3.593 |
| GBM1459 | 2H | 373 | 6 | 0.578 | 3.931 |
| GBM1043 | 3H | 372 | 5 | 0.495 | 3.735 |
| GBM1110 | 3H | 373 | 11 | 0.761 | 5.362 |
| GBM1280 | 3H | 372 | 5 | 0.661 | 4.038 |
| GBM1405 | 3H | 372 | 8 | 0.792 | 5.795 |
| GBM1413 | 3H | 372 | 5 | 0.517 | 3.171 |
| GBM1003 | 4H | 372 | 9 | 0.713 | 5.164 |
| GBM1015 | 4H | 373 | 19 | 0.834 | 7.016 |
| GBM1020 | 4H | 371 | 7 | 0.663 | 3.96 |
| GBM1323 | 4H | 371 | 7 | 0.642 | 4.535 |
| GBM1026 | 5H | 372 | 4 | 0.412 | 2.379 |
| GBM1054 | 5H | 368 | 7 | 0.682 | 4.822 |
| GBM1064 | 5H | 373 | 5 | 0.531 | 3.263 |
| GBM1176 | 5H | 373 | 7 | 0.58 | 3.892 |
| GBM1363 | 5H | 373 | 5 | 0.387 | 2.817 |
| GBM1008 | 6H | 366 | 9 | 0.75 | 5 |
| GBM1021 | 6H | 367 | 15 | 0.87 | 7.503 |
| GBM1063 | 6H | 373 | 10 | 0.746 | 5.26 |
| GBM1075 | 6H | 370 | 5 | 0.34 | 2.792 |
| GBM1212 | 6H | 372 | 4 | 0.376 | 2.208 |
| GBM1404 | 6H | 373 | 3 | 0.359 | 2.453 |
| GBM1033 | 7H | 373 | 6 | 0.687 | 4.268 |
| GBM1060 | 7H | 370 | 4 | 0.528 | 3.008 |
| GBM1326 | 7H | 373 | 10 | 0.816 | 5.926 |
| GBM1419 | 7H | 373 | 8 | 0.7 | 4.675 |
| GBM1464 | 7H | 365 | 22 | 0.888 | 8.074 |
| GBM1516 | 7H | 373 | 6 | 0.593 | 4.033 |
| Mean | 371 | 7.9 | 0.6 | 4.4 |
The following values are presented for each marker: chromosome location (Location), number of individuals scored (Ind no.), number of alleles (Allele no.), polymorphism information content (PIC), allelic richness.
Genetic diversity
Summary statistics of the marker data such as number of alleles, sample adjusted allelic richness, and observed heterozygosity were calculated with GDA [74] and FSTAT version 2.93.2 [75]. The number of multi-locus genotypes was determined with GeneticStudio (http://dyerlab.bio.vcu.edu). Polymorphism information content (PIC) per locus was calculated with PICcalc [76].
Population differentiation among sites
FST was used to measure differentiation between populations and was calculated with FSTAT. Inter-individual distances were calculated using a simple matching coefficient with DARwin software version 5.0.158 [77] and used to build a neighbor-joining tree. Because Spontaneum is a highly selfing species, the program InStruct [78] was used to infer population structure. InStruct is an extension of the approach used in STRUCTURE [79] and can specifically account for self-pollination and inbreeding. InStruct was run in mode v = 3 (infer population structure and individual selfing rates) for K = 1–10. For each K, 5 chains were run, with 200,000 MCMC iterations, a burn-in of 100,000 and a thinning interval of 10 steps. Results from independent chains were summarized using CLUMPP [80] and graphical representations of cluster assignments were rendered with DISTRUCT [81]. The ad hoc measure of change in likelihood between successive K values, ΔK [82] was calculated to identify the appropriate number of clusters. As recommended by Gao et al. [78], clustering results were compared with results obtained using STRUCTURE v. 2.3.3 [79] and Structure Harvester [83]. STRUCTURE was run with 5 independent runs for each value of K from 1 to 8, with a burn in period of 105 followed by 105 iterations.
Description of environmental variation in Jordan
We used a procedure developed by Newman and Rissler [84] to delineate distinct environments within the study area. A habitat suitability model was generated for Spontaneum with MaxEnt version 3.3.3k [85]. Occurrence data in Jordan was downloaded from Genesys (https://www.genesys-pgr.org). Occurrences showing a geographical coordinate quality rank > 70 [86] were included. The 19 Bioclim layers for current climate conditions (1950–2000), at a resolution of 2.5 arc-minutes, were used. Ten thousand sites were sampled pseudo-randomly from the study area, in proportion to their suitability, as estimated in the habitat suitability model. The environmental data associated with each site was then extracted from all Bioclim layers. Following normalization of each environmental variable, the data set was subjected to k-means clustering such that each pseudo-randomly selected site was assigned to one of k classes. By coloring each site according to habitat, regions within the study area that had similar mean environmental conditions could be visualized.
Association between genetic diversity, geography and environment
Correlations between allelic richness, InStruct clustering results and environmental data were tested using JMP 5.1 (SAS Institute, Cary, NC, USA). Means were compared using the Tukey-Kramer HSD test. Pearson product-moment and Spearman's Rho rank correlation coefficients were calculated. Isolation by distance (IBD) was estimated using R (http://www.r-project.org/). Geographic distances were calculated as straight-line distances with the GeographicDistanceMatrixGenerator version 1.2.3 [87] and log transformed. Genetic distances were calculated as FST/(1- FST) [88] and as population-wise allelic differences using the FPTEST [89]. Two-tailed Mantel tests were carried out with 105 permutations. To test isolation by environment (IBE), environmental distances between sites were estimated. A principal coordinate analysis (PCO) was performed using data from all Bioclim variables and altitude. Environmental distance was then approximated as the simple Euclidean distance between points on the first principal coordinate axis, which accounted for 49% of the environmental variation across sampling sites. The multivariate measure of environmental distance represented a conservative approach aiming to avoid overfitting, as many of the Bioclim parameters covaried significantly. Two-tailed Mantel tests were carried out to estimate IBE. As environmental and geographical distances were significantly correlated, IBD and IBE were also tested using a partial Mantel test. In addition, correlation of the distance matrix calculated with FPTEST to individual Bioclim variables was examined using appropriate Holm-Bonferroni correction [90] to avoid type I error inherent in multiple comparisons.
Results
Genetic diversity
A total of 291 alleles were identified. Alleles per locus ranged from 3 to 22, with an average of 7.9. The mean number of alleles per locus averaged across sites was 2.8. PIC varied from 0.34 to 0.914 with a mean of 0.62. Allelic richness per locus varied from 2.2–9.1. All populations showed low observed heterozygosity (Ho) ranging between 0–0.025. Spontaneum is a highly self-pollinating species and previous studies on Spontaneum reported similar levels of heterozygosity [66,91]. A total of 370 multi-locus genotypes were identified. Only three populations (5, 15, 18) showed a single multi-locus genotype twice. Allelic richness per population ranged from 1.4 to 3.3 with a mean of 2.63.
Population differentiation among sites
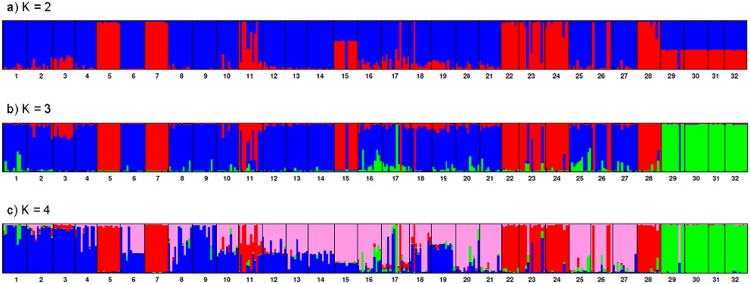
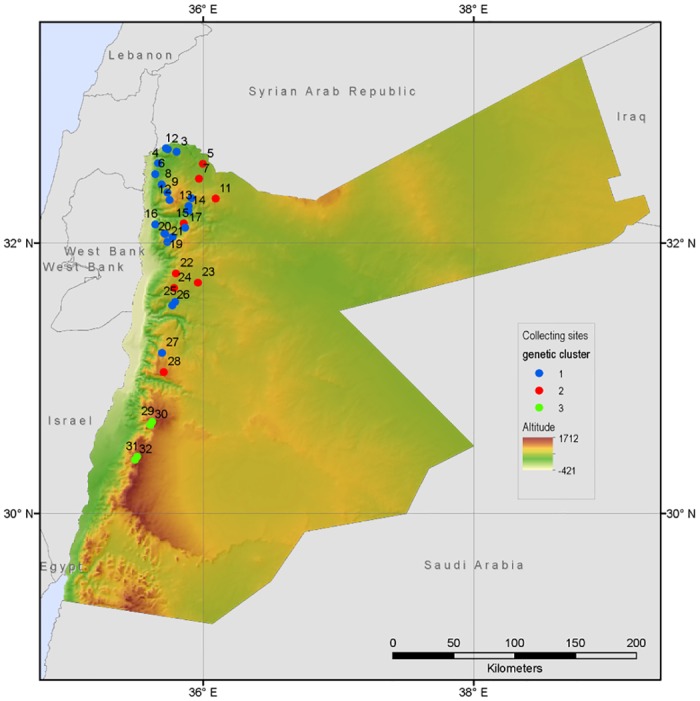
Differentiation among populations measured as FST was 0.33, i.e. 33% of variation was distributed between populations and 67% within populations, similar to previous studies [57,59,92,93]. The ΔK method [82] applied to InStruct and STRUCTURE results suggested subdivision into three clusters. Fig 2 shows the individual assignment coefficients for K = 2 to K = 4. Partitioning into three genetic clusters produced one group of populations predominantly located in the northwestern part of the collecting area and a second cluster which showed a longitudinal extension from the northeast southwards. A small third cluster was geographically separated in the southern part of the collecting area. The geographical distribution of the three clusters is shown in Fig 1. The InStruct assignment was compatible with the neighbor-joining tree based on inter-individual genetic distances (Fig 3). Assignment coefficients (q) varied across the study area. The average assignment coefficient of individuals to cluster 1 was significantly lower (q = 0.902; p = <0.0001) than those of cluster 2 (q = 0.966) and cluster 3 (q = 0.99). The assignment coefficient was inversely correlated with latitude (Pearson coefficient: r = -0.17; p = 0.001; Spearman’s rank coefficient: r = -0.204; p<0.0001) and positively correlated with altitude (Pearson coefficient: r = 0.166; p = 0.0013; Spearman’s rank coefficient: r = 0.235; p<0.0001) indicating that the level of admixture was higher in the north. While there were 10 populations whose respective individuals were all strongly assigned to the same genetic cluster (q ≥ 0.8), the remaining populations contained some individuals either strongly assigned to a different cluster (physical admixture), and/or some genetically admixed individuals (0.49 < q < 0.8) (Table 4). Eight populations were physically admixed, with 1–6 individuals assigned to a different cluster. 18 populations contained 1–9 genetically admixed individuals (four of these populations were also physically admixed). In populations assigned to cluster 3, only the population in site 29 showed physical admixture (one individual assigned to cluster 1), no genetic admixture was identified in any of the four populations of cluster 3. All other physical admixture stems from individuals either assigned to cluster 1 but growing within a site assigned to cluster 2 or vice versa. 88% of the 43 genetically admixed individuals belong to populations assigned to cluster 1, the remaining to cluster 2.
Fig 2. Assignment of individuals to genetic clusters identified by InStruct, for K = 2 to K = 4.
Populations are sorted from left to right by decreasing latitude. Clusters are depicted in the following colours: cluster 1 = blue; cluster 2 = red; cluster 3 = green; cluster 4 = pink.
Fig 3. Neighbor-joining tree showing inter-individual genetic distances.
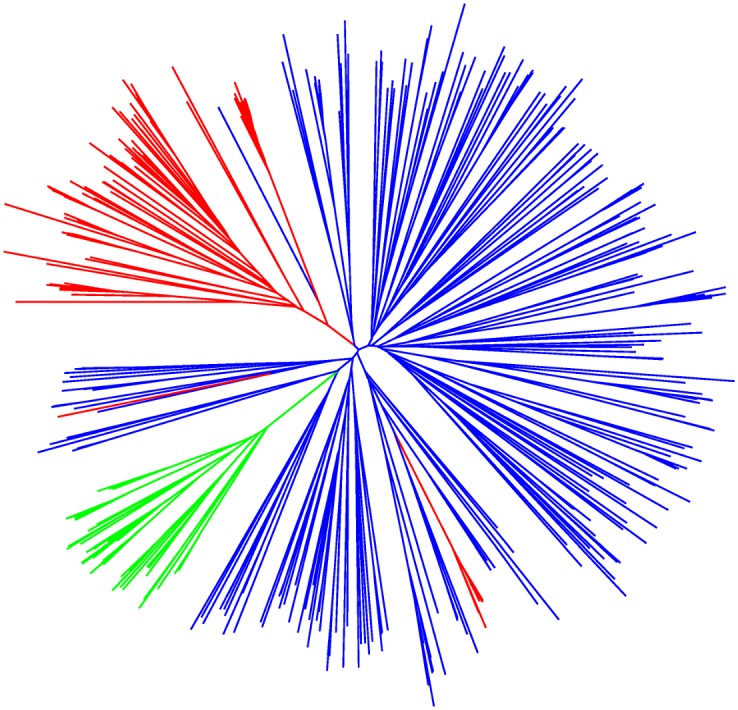
Genetic clusters are depicted in the following colours: cluster 1 = blue; cluster 2 = red; cluster 3 = green.
Table 4. Genetic and physical admixture.
| Physical admixture | Genetic admixture | |||
|---|---|---|---|---|
| Cluster | No. of populations | No. of individuals | No. of populations | No. of individuals |
| 1 | 4 | 11 | 14 | 38 |
| 2 | 3 | 5 | 4 | 5 |
| 3 | 1 | 1 | 0 | 0 |
Association between genetic diversity, geography and environment
K-means clustering was used to delineate different environments that might be inhabited by Spontaneum in Jordan. Regions of the study area with distinct environmental conditions are depicted in Fig 4. They are predominantly arranged as north-south stripes corresponding to the three main topographical regions described by Al-Eisawi [94] (rift valley along the western border; mountain range extending from the north in Irbid to the south in Ras An-Naqab, and the eastern desert). Although the sampling scheme also followed a north-south transect, populations were sampled from the majority of the distinct environments identified (Fig 4). The geographical distribution of the genetic clusters did not match the geographical distribution of these environmental partitions.
Fig 4. Habitat types in Jordan identified through k-means clustering.
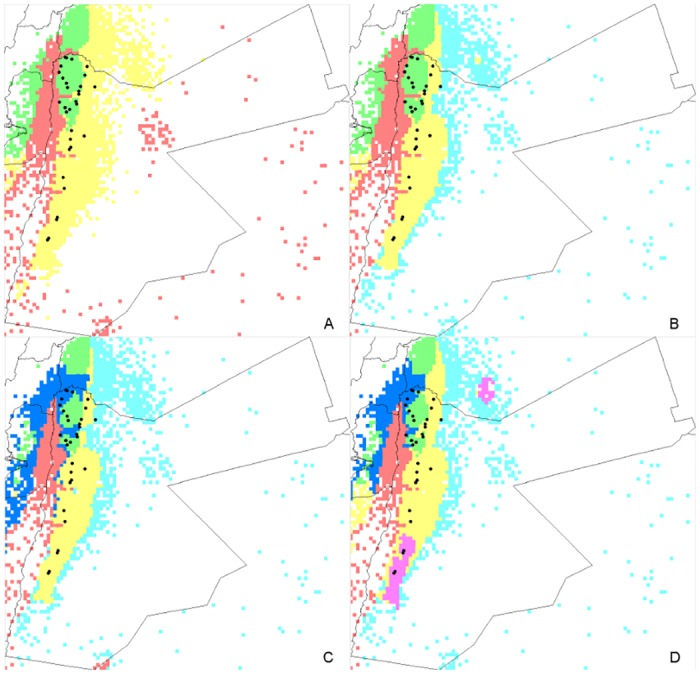
Black dots represent the Spontaneum collecting sites.
When comparing populations collected from nature reserves and those collected from ruderal habitats, roadsides or field margins, no significant differences in genetic diversity measures were found. No physical admixture was detected in populations collected in reserves, while they do show genetic admixture. Plants collected in reserves were significantly smaller (37.0 cm) than those collected from ruderal areas or field margins (72.6 cm; p = 0.0047). Also population size observed in reserves was significantly smaller (p = 0.0112, Tukey-Kramer HSD test; based on observed size of all wild populations sampled during the 2012 barley collecting mission). The average habitat suitability, according to the habitat suitability model, was significantly lower in reserves than in the other sites (p = 0.0289).
Average values of geographical, geophysical and Bioclim variables, allelic richness, and selfing rates are compared among clusters in Table 5. Average longitude and Bioclim 6 were the only variables that were significantly different between all three clusters. Cluster 3 collecting sites were significantly different for several variables including: higher elevation, lower latitude, lower values for temperature-related Bioclim variables 1, 5, 8, 9, 10, 11 (these Bioclim variables are highly correlated, r>0.8) and lower selfing rates. Cluster 1 showed significantly higher allelic richness and higher values for Bioclim 13. No significant differences were found for habitat type, aspect, soil type and Bioclim 2, 3, 7, 15, 16 and 19. Bioclim 14, 17 and 18 were zero at all sites. Allelic richness of loci and of populations was weakly correlated with latitude (loci: Spearman’s rank coefficient r = 0.079, p = 0.0065; Pearson’s coefficient r = 0.147, p<0.0001; populations: Pearson’s coefficient r = 0.36, p = 0.0432).
Table 5. Comparison of average values for geographical, geophysical and Bioclim variables, allelic richness and selfing rate at collecting sites among genetic clusters.
| Variable | Cluster 1 | Cluster 2 | Cluster 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Level | Mean | p | Level | Mean | p | Level | Mean | p | |
| Allelic richness | A | 2.91 | B | 2.25 | <0.0001 | B | 2.0 | <0.0001 | |
| Selfing rate | A | 0.831 | 0.0294 | A | 0.833 | 0.0220 | B | 0.0813 | |
| Altitude (m) | B | 564 | <0.0001 | B | 749 | 0.0005 | A | 1521 | |
| Latitude | A | 32.195 | <0.0001 | A | 31.968 | <0.0001 | B | 30.539 | |
| Longitude | A | 35.76 | 0.0015 (1–3) | B | 35.9 | 0.0038 (2–1) | C | 35.56 | <0.0001 (3–2) |
| Aspect | A | 233.99 | ns | A | 185.07 | ns | A | 199.04 | ns |
| Slope | A | 3.54 | 0.0030 | B | 0.99 | A | 4.81 | 0.0024 | |
| Bioclim 1 | A | 18.03 | <0.0001 | A | 16.67 | 0.0122 | B | 13.78 | |
| Bioclim 2 | A | 11.68 | ns | A | 12.21 | ns | A | 11.60 | ns |
| Bioclim 3 | A | 42.94 | ns | A | 44.10 | ns | A | 42.82 | ns |
| Bioclim 4 | A | 625.73 | 0.0151 | AB | 620.55 | B | 598.47 | ||
| Bioclim 5 | A | 32.07 | 0.0005 | A | 30.94 | 0.0249 | B | 27.96 | |
| Bioclim 6 | A | 4.9 | <0.0001 (1–3) | B | 3.25 | 0.03 (2–1) | C | 0.86 | 0.0332 (3–2) |
| Bioclim 7 | A | 27.17 | ns | A | 27.69 | ns | A | 27.1 | ns |
| Bioclim 8 | A | 10.1 | 0.0002 | A | 8.68 | 0.0287 | B | 6.06 | |
| Bioclim 9 | A | 25.0 | <0.0001 | A | 23.59 | 0.0145 | B | 20.65 | |
| Bioclim 10 | A | 25.04 | <0.0001 | A | 23.63 | 0.0130 | B | 20.65 | |
| Bioclim 11 | A | 10.1 | 0.0002 | A | 8.68 | 0.0287 | B | 6.06 | |
| Bioclim 12 | A | 388.0 | 0.0448 (1–3) | AB | 323.88 | B | 289.75 | ||
| Bioclim 13 | A | 92.1 | B | 73.75 | 0.0314 | B | 67.0 | 0.0238 | |
| Bioclim 15 | A | 113.44 | ns | A | 113.02 | ns | A | 115.12 | ns |
| Bioclim 16 | A | 250.1 | ns | A | 206.88 | ns | A | 187.0 | ns |
| Bioclim 19 | A | 250.1 | ns | A | 206.88 | ns | A | 187.0 | ns |
Bioclim 1- Bioclim 19 = Bioclimatic variables as per definition on http://www.worldclim.org/bioclim (see Table 2); ns = non-significant; levels marked with different letters indicate significant difference among cluster averages (p<0.05) based on the Tukey HSD test. Numbers in brackets after p values indicate the two clusters being compared.
Genetic and geographical distance were significantly correlated (FST based distance: r = 0.3, p = 0.0003; FPTEST based distance: r = 0.2, p = 0.02), suggesting isolation by distance, when analyzed over all 32 populations, while the Mantel tests for isolation by environment were not significant. Environmental and geographic distance were strongly correlated (r = 0.4, p = 0.0001), indicating possible confounding effects. These were accounted for using a partial Mantel test which confirmed significant IBD among all studied populations (r = 0.25, p = 0.004), but did not find significant IBE (S1 Resource). Several climate variables were found to be different in cluster 3 compared to cluster 1 and 2 (Table 5). Cluster 3 was furthermore geographically separated from clusters 1 and 2, which are themselves partly overlapping. The correlation analyses were therefore repeated for populations belonging to clusters 1 and 2 only. No significant IBD or IBE was found between cluster 1 and 2, and neither were environmental and geographic distances significantly correlated. No significant correlations existed between single Bioclim variables and distance matrix calculated with FPTEST (S1 Resource).
Discussion
The present study examined the current geography of genetic structure and its correlation with landscape scale climatic and spatial variation in Spontaneum populations in Jordan. Correlation analyses showed large scale IBD across the study area but did not reveal a correspondence between climate and genetic structure. Analysis of population structure suggested that the 32 Spontaneum populations could be divided into three major, genetically differentiated clusters (Fig 1). Genetic diversity was concentrated in the northern part of the study area, across a range of environments, where populations are characterized by physical and genetic admixture, and high allelic richness. Allelic richness and admixture decrease towards the south; the southernmost populations are not admixed, exhibit low allelic richness and contain physically smaller plants.
Genetic structure is not correlated with climatic variation inferred from global layers
Three genetic clusters were distributed along a longitudinal gradient in the North (clusters 1 and 2), with a distinct cluster (cluster 3) in the South. The study area was characterized by a longitudinal distribution of distinct habitat types as shown in Fig 4, of which the central mountain range was the most variable. At the large scale across the entire study area, where geographical and environmental distances were strongly correlated, significant IBD implied that physical distance was important for genetic differentiation among populations, but environmental variation was found to have no effect. Results were different at a slightly smaller scale, across the central and northern part of the study area, where clusters 1 and 2 spread across an environmentally heterogeneous landscape. Here, geographical and environmental distances were both uncorrelated with genetic distance either measured by FST or by population-wise allelic differences.
Spontaneum prefers disturbed, human-made or influenced habitats [20,22], sympatric with its domesticate [95–98]. These habitats favor anthropogenic movement of material—inclusion and transport with cultivated barley seed lots or hitchhiking on livestock fur or human clothing—which interferes with natural diffusion and selection processes. This may alter the expected distribution of genetic diversity across the landscape and lead to weak or nonexistent correlations between ecogeographical and genetic diversity as found in our study. Natural dispersal and selection processes may not have been the principle force shaping genetic structure in some regions of Jordan.
Spontaneum is a highly self-pollinating species. In self-pollinating species much genetic diversity is distributed among populations rather than within populations, population to population variation is greater than in out-crossing species and the genetic structure is more variable [99]. Given their low gene flow and very localized gene transfer, genetic structure has been found at local scale [63,100,101]. This local variation is unlikely to be detected by globally available layers commonly used to represent landscape scale spatial and climatic variation.
Global climate data such as the Bioclim layers provided by WorldClim climatic data are used in a range of studies and applications [11,19,67–69,85,86,102], and the inherent assumption is that they are robust proxies for genetic data, which is often not available. Our results suggest that there may be some limitations on this assumption. Our study did not find a correlation between climate, as represented by commonly used global, interpolated data layers, and genetic structure for Spontaneum. Thus global climatic data would not be especially useful for predicting existing genetic diversity in Jordan. A ruderal habitat preference and high self-pollination might explain why the general expectation of tight correlation between genetic and ecogeographical diversity does not hold. If collecting and conservation actions are designed without previous knowledge of genetic structure, it will be important to consider species biology and habitat preferences when using ecogeographical diversity to predict genetic diversity.
Sampling and monitoring genetic diversity within Spontaneum populations
All Spontaneum populations sampled here, irrespective of cluster assignment, contained many unique multi-locus genotypes. Only three populations showed a single multi-locus genotype twice, and no multi-locus genotype was repeated among populations. Allelic richness, which is a good metric to assess and monitor genetic diversity [103], increased significantly towards the northern part of the study area. Here, populations were also characterized by admixture. More than half of the populations in clusters 1 and 2 showed considerable genetic admixture as well as physical admixture, a characteristic that was also found by Hübner et al. [66] in Israel. Hübner et al. [93] observed a fairly high rate of gene flow in Spontaneum attributed to sporadic outcrossing events [104] and gene flow through seed dispersal. These mechanisms likely contribute to physical and genetic admixture in Jordan as well.
Due to the reduced level of diversity expected within populations of highly selfing species, germplasm collections are often limited to a few samples per population. The heterogeneity found within populations in this study cautions against such sampling strategies. Modeling studies have shown that collections of highly selfing species need substantially more samples than are commonly recommended to capture existing diversity [105]. The distribution of genetic structure we have described for Spontaneum in Jordan prescribes further collecting and monitoring in the northern part of the country, in particular the area occupied by cluster 1.
Ex situ and in situ conservation of Spontaneum
Natural populations of Spontaneum have been reported to harbour large neutral genetic diversity, and also show considerable diversity in disease resistance and quantitative traits of agronomic importance [45,106–108]. Despite evidence of high genetic, adaptive and quantitative diversity in Jordanian Spontaneum populations, the number of ex situ barley accessions from Jordan in global collections is lower than those from neighboring countries. Although in general the number of Spontaneum accessions in ex situ collections seems relatively high compared with other CWR samples in genebanks, they are derived from a limited number of populations [109]. Maxted and Kell [24] suggest that, although Spontaneum is widespread and locally common [110], individual populations might contain important adaptive traits, thus populations should be actively conserved throughout the geographical range. Vincent et al. [111] identified Jordan as one of the countries where wild Hordeum should be conserved and suggested the establishment of a network of several reserves in the Israel/Jordan region to more effectively conserve the genetic diversity of wild Hordeum. These assessments describe the obvious need to promote in situ conservation of Spontaneum in Jordan and to enlarge ex situ collections. Our description of the distribution of genetic diversity across the Jordanian landscape provides a tool to evaluate the propriety of existing in situ conservation activities and supports the application of proper sampling techniques for future ex situ acquisitions.
Supporting Information
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Gur A, Zamir D. Unused natural variation can lift yield barriers in plant breeding. PLoS Biology. 2004;2(10):e245 10.1371/journal.pbio.0020245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajjar R, Hodgkin T. The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica. 2007;156:1–13. [Google Scholar]
- 3.McCouch S, Baute GJ, Bradeen J, Bramel P, Bretting PK, Buckler E, et al. Feeding the future. Nature. 2013;499:23–24. 10.1038/499023a [DOI] [PubMed] [Google Scholar]
- 4.Jarvis A, Lane A, Hijmans RJ. The effect of climate change on crop wild relatives. Agriculture, Ecosystems & Environment. 2008;126:13–23. [Google Scholar]
- 5.FAO. Report of the Fourteenth Regular Session of the Commission on Genetic Resources for Food and Agriculture. CGRFA 14/13/report. 2013. Available: http://www.fao.org/nr/cgrfa/cgrfa-meetings/cgrfa-comm/fourteenth-reg/en/.
- 6.Parra-Quijano M, Draper D, Torres E, Iriondo JM. Ecogeographical representativeness in crop wild relative ex situ collections In: Maxted N, Ford-Lloyd BV, Kell SP, Iriondo JM, Dulloo E, Turok J, editors. Crop wild relative conservation and use. CAB International, Wallingford, UK; 2008. pp. 249–273. [Google Scholar]
- 7.Parra-Quijano M, Iriondo JM, Frese L, Torres E. Spatial and ecogeographical approaches for selecting genetic reserves in Europe In: Maxted N, Dulloo ME, Ford-Lloyd BV, Frese L, Iriondo JM, Pinheiro de Carvalho MAA, editors. Agrobiodiversity Conservation: securing the diversity of crop wild relatives and landraces. CAB International, Wallingford, UK; 2012. pp. 20–28. [Google Scholar]
- 8.Parra-Quijano M, Iriondo JM, Torres E. Improving representativeness of genebank collections through species distribution models, gap analysis and ecogeographical maps. Biodivers Conserv. 2012;21:79–96. [Google Scholar]
- 9.Steiner JJ. Exploring the relationship of plant genotype and phenotype to ecogeography In: Greene SL, Guarino L, editors. Linking genetic resources and geography: Emerging strategies for conserving and using crop biodiversity. Crop Science Society of America Inc., Madison, WI; 1999. pp. 39–50. [Google Scholar]
- 10.Dulloo ME, Labokas J, Iriondo JM, Maxted N, Lane A, Laguna E, et al. Genetic reserve location and design In: Iriondo JM, Maxted N, Dulloo ME, editors. Conserving plant genetic diversity in protected areas. CAB International; 2008. pp. 23–64. [Google Scholar]
- 11.Parra-Quijano M, Iriondo JM, Torres E. Review. Applications of ecogeography and geographic information systems in conservation and utilization of plant genetic resources. Spanish J Agric Res. 2012;10(2):419–429. [Google Scholar]
- 12.Maxted N, Van Slageren MW, Rihan J. Ecogeographic surveys In: Guarino L, Ramanatha Rao V, Reid R, editors. Collecting plant genetic diversity: Technical guidelines. CAB International, Wallingford, UK; 1995. pp. 255–286. [Google Scholar]
- 13.Maxted N, Magos Brehm J, Kell S. Resource book for preparation of national conservation plans for crop wild relatives and landraces. Food and Agriculture Organization of the United Nations, Italy; 2013. [Google Scholar]
- 14.Parra-Quijano M, Iriondo JM, de la Cruz M, Torres E. Strategies for the development of core collections based on ecogeographical data. Crop Sci. 2011;51:656–666. [Google Scholar]
- 15.Khazaei H, Street K, Bari A, Mackay M, Stoddard FL. The FIGS (focused identification of germplasm strategy) approach identifies traits related to drought adaptation in Vicia faba genetic resources. PLoS ONE 2013;8(5):e63107 10.1371/journal.pone.0063107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thormann I, Parra-Quijano M, Endresen DTF, Rubio-Teso ML, Iriondo MJ, Maxted N. Predictive characterization of crop wild relatives and landraces Technical guidelines version 1. Bioversity International, Rome, Italy; 2014. [Google Scholar]
- 17.Jarvis A, Williams K, Williams D, Guarino L, Caballero PJ, Mottram G. Use of GIS for optimizing a collecting mission for a rare wild pepper (Capsicum flexuosum Sendtn.) in Paraguay. Genet Resour Crop Evol. 2005;52:671–682. [Google Scholar]
- 18.Ramirez-Villegas J, Khoury C, Jarvis A, Debouck DG, Guarino L. A gap analysis methodology for collecting crop genepools: A case study with Phaseolus beans. PLoS ONE. 2010;5(10):e13497 10.1371/journal.pone.0013497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxted N, Dulloo ME, Ford-Lloyd BV, Iriondo MJ, Jarvis A. Gap analysis: a tool for complementary genetic conservation assessment. Divers Distrib. 2008;14:1018–1030. [Google Scholar]
- 20.Ellstrand NC. Dangerous liaisons? When cultivated plants mate with their wild relatives. Johns Hopkins University Press, Baltimore, USA; 2003. [Google Scholar]
- 21.Andersen MS, de Vicente CM. Gene flow between crops and their wild relatives. Johns Hopkins University Press, Baltimore, USA; 2010. [Google Scholar]
- 22.Jain SK. Genetic reserves In: Frankel OH, Hawkes JG, editors. Crop genetic resources for today and tomorrow. Cambridge University Press, UK; 1975. pp. 379–396. [Google Scholar]
- 23.Jarvis S, Fielder H, Hopkins J, Maxted N, Smart S. Distribution of crop wild relatives of conservation priority in the UK landscape. Biol. Cons. 2015;191:444–451. [Google Scholar]
- 24.Maxted N, Kell SP. Establishment of a global network for the in situ conservation of crop wild relatives: status and needs. FAO Commission on Genetic Resources for Food and Agriculture, Rome, Italy; 2009. [Google Scholar]
- 25.Fischbeck G, Schwarzbach E, Sobel F, Wahl I. Mehltauresistenz aus Israelischen Populationen der zweizeiligen Wildgerste (Hordeum spontaneum). Z. Pflanzenzüchtung. 1976;76:163–166. [Google Scholar]
- 26.Ivandic V, Walther U, Graner A. Molecular mapping of a new gene in wild barley conferring complete resistance to leaf rust (Puccinia hordei Otth). Theor Appl Genet. 1998;97:1235–1239. [Google Scholar]
- 27.Backes G, Madsen LH, Jaiser H, Stougaard J, Herz M, Mohler V, et al. Localization of genes for resistance against Blumeria graminis f.sp. hordei and Puccinia graminis in a cross between a barley cultivar and wild barley (Hordeum vulgare subsp. spontaneum) line. Theor Appl Genet. 2003;106:353–362. [DOI] [PubMed] [Google Scholar]
- 28.Dreiseitl A, Bockelman HE. Sources of powdery mildew resistance in a wild barley collection. Genet Resour Crop Evol. 2003;50:345–350. [Google Scholar]
- 29.Genger RK, Williams KJ, Raman H, Read BJ, Wallwork H, Burdon JJ, et al. Leaf scald resistance genes in Hordeum vulgare and Hordeum vulgare ssp. spontaneum: parallels between cultivated and wild barley. Aust J Agric Res. 2003;54(12):1335–1342. [Google Scholar]
- 30.von Korff M, Wang H, Léon J, Pillen K. AB-QTL analysis in spring barley. I. Detection of resistance genes against powdery mildew, leaf rust and scald introgressed from wild barley. Theor Appl Genet. 2005;111(3):583–90. [DOI] [PubMed] [Google Scholar]
- 31.Repkova J, Dreiseitl A, Lizal P, Kyjovska Z, Teturova K, Psotkova R, et al. Identification of resistance genes against powdery mildew in four accessions of Hordeum vulgare ssp. spontaneum. Euphytica. 2006;151:23–30. [Google Scholar]
- 32.von Korff M, Wang H, Leon J, Pillen K. AB-QTL analysis in spring barley. II. Detection of favourable exotic alleles for agronomic traits introgressed from wild barley (Hordeum vulgare ssp. spontaneum). Theor Appl Genet. 2006;112:1221–1231. [DOI] [PubMed] [Google Scholar]
- 33.Chen G, Li C, Shi Y, Nevo E. Wild barley, Hordeum spontaneum, a genetic resource for crop improvement in cold and arid regions. Sciences in cold and arid regions. 2008;1:0115–0124. [Google Scholar]
- 34.Lakew B, Henry RJ, Eglinton J, Baum M, Ceccarelli S, Grando S. SSR analysis of introgression of drought tolerance from the genome of Hordeum spontaneum into cultivated barley (Hordeum vulgare ssp vulgare). Euphytica. 2013;191(2):231–243. [Google Scholar]
- 35.Erkkila MJ, Leah R, Ahokas H, Cameron-Mills V. Allele-dependent barley grain β-Amylase activity. Plant Physiol. 1998;117:679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Korff M, Wang H, Léon J, Pillen K. AB-QTL analysis in spring barley: III. Identification of exotic alleles for the improvement of malting quality in spring barley (H. vulgare ssp. spontaneum). Mol Breed. 2008;21(1):81–93. [Google Scholar]
- 37.Maurer A, Draba V, Jiang Y, Schnaithmann F, Sharma R, Schumann E, et al. Modeling the genetic architecture of flowering time control in barley through nested association mapping. BMC Genomics. 2015;16:290 10.1186/s12864-015-1459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zohary D, Hopf M. Domestication of plants in the Old World, 2nd edition Oxford University Press; 1993. [Google Scholar]
- 39.Badr A, Mueller K, Schaefer-Pregl R, El Rabey H, Effgen S, Ibrahim HH, et al. On the origin and domestication history of barley (Hordeum vulgare). Mol Biol Evol. 2000;17(4):499–510. [DOI] [PubMed] [Google Scholar]
- 40.Morrell PL, Clegg MT. Genetic evidence for a second domestication of barley (Hordeum vulgare) east of the Fertile Crescent. Proc Natl Acad Sci USA. 2007;104:3289–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai F, Nevo E, Wu D, Comadran J, Zhou M, Qiu L, et al. Tibet is one of the centers of domestication of cultivated barley. Proc Natl Acad Sci USA. 2012;109(42):16969–16973. 10.1073/pnas.1215265109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molina-Cano JL, Russell JR, Moralejo MA, Excacena JL, Arias G, Powell W. Chloroplast DNA microsatellite analysis supports a polyphyletic origin for barley. Theor Appl Genet. 2005;110:613–619. [DOI] [PubMed] [Google Scholar]
- 43.Orabi J, Backes G, Wolday A, Yahyaoui A, Jahoor A. The Horn of Africa as a center of barley diversification and a potential domestication site. Theor Appl Genet. 2007;114(6):1117–27. [DOI] [PubMed] [Google Scholar]
- 44.Nevo E, Zohary D, Brown AHD, Haber M. Genetic diversity and environmental associations of wild barley, Hordeum sponateneum, in Israel. Evolution. 1979;33(3):815–833. [DOI] [PubMed] [Google Scholar]
- 45.Nevo E, Beiles A, Gutterman Y, Storch N, Kaplan D. Genetic resources of wild cereals in Israel and vicinity. II. Phenotypic variation within and between populations of wild barley, Hordeum spontaneum. Euphytica. 1984;33(3):737–756. [Google Scholar]
- 46.Baum BR, Nevo E, Johnson DA, Beiles A. Genetic diversity in wild barley (Hordeum spontaneum C. Koch) in the Near East: a molecular analysis using Random Amplified Polymorphic DNA (RAPD) markers. Genet Resour Crop Evol. 1997;44(2):147–157. [Google Scholar]
- 47.Pakniyat H, Powell W, Baird E, Handley LL, Robinson D, Scrimgeour CM, et al. AFLP variation in wild barley (Hordeum spontaneum C. Koch) with reference to salt tolerance and associated ecogeography. Genome 1997;40:332–341. [DOI] [PubMed] [Google Scholar]
- 48.Gupta PK, Sharma PK, Balyan HS, Roy JK, Sharma S, Beharav A, et al. Polymorphism at rDNA loci in barley and its relation with climatic variables. Theor Appl Genet 2002;104:473–481. [DOI] [PubMed] [Google Scholar]
- 49.Liviero L, Maestri E, Gulli M, Nevo E, Marmiroli N. Ecogeographic adaptation and genetic variation in wild barley, application of molecular markers targeted to environmentally regulated genes. Genet Resour Crop Evol. 2002;49:133–144. [Google Scholar]
- 50.Ivandic V, Hackett CA, Nevo E, Keith R, Thomas WTB, Forster BP. Analysis of simple sequence repeats (SSRs) in wild barley from the Fertile Crescent: associations with ecology, geography and flowering time. Plant Mol Biol. 2002;48(5–6):511–527. [DOI] [PubMed] [Google Scholar]
- 51.Ozkan H, Kafkas S, Sertac Ozer M, Brandolini A. Genetic relationships among South-East Turkey wild barley populations and sampling strategies of Hordeum spontaneum. Theor Appl Genet. 2005;112(1):12–20. [DOI] [PubMed] [Google Scholar]
- 52.Cronin JK, Bundock PC, Henry RJ, Nevo E. Adaptive climatic molecular evolution in wild barley at the Isa defense locus. Proc Nat Acad Sci USA. 2007;104:2773–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang A, Yu Z, Ding Y. Genetic diversity analysis of wild close relatives of barley from Tibet and the Middle East by ISSR and SSR markers. Comptes Rendus Biologies. 2009;332:393–403. 10.1016/j.crvi.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 54.Russell J, Dawson IK, Flavell AJ, Steffenson B, Weltzien E, Booth A, et al. Analysis of 1000 single nucleotide polymorphisms in geographically matched samples of landrace and wild barley indicates secondary contact and chromosome-level differences in diversity around domestication genes. New Phytologist. 2011;191(2):564–578. 10.1111/j.1469-8137.2011.03704.x [DOI] [PubMed] [Google Scholar]
- 55.Jakob SS, Roedder D, Engler JO, Shaaf S, Oezkan H, Blattner FR, et al. Evolutionary history of wild barley (Hordeum vulgare subsp. spontaneum) analyzed using multilocus sequence data and paleodistribution modeling. Genome Biol Evol. 2014;6(3):685–702. 10.1093/gbe/evu047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu YB, Horbach C. Genetic diversity in a core subset of wild barley germplasm. Diversity. 2012;4(2):239–257. [Google Scholar]
- 57.Baek HJ, Beharav A, Nevo E. Ecological-genomic diversity of microsatellites in wild barley, Hordeum spontaneum, populations in Jordan. Theor Appl Genet. 2003;106(3):397–410. [DOI] [PubMed] [Google Scholar]
- 58.Lin JZ, Brown AHD, Clegg MT. Heterogeneous geographic patterns of nucleotide sequence diversity between two alcohol dehydrogenase genes in wild barley (Hordeum vulgare subsp. spontaneum). Proc Nat Acad Sci USA. 2001;98:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turpeinen T, Tenhola T, Manninen O, Nevo E, Nissilä E. Microsatellite diversity associated with ecological factors in Hordeum spontaneum populations in Israel. Mol Ecol. 2001;10(6):1577–1591. [DOI] [PubMed] [Google Scholar]
- 60.Chen G, Suprunova T, Krugman T, Fahima T, Nevo E. Ecogeographic and genetic determinants of kernel weight and colour of wild barley (Hordeum spontaneum) populations in Israel. Seed Sci. Res. 2004;14:137–146. [Google Scholar]
- 61.Batchu AK, Zimmermann D, Schulze-Lefert P, Koprek T. Correlation between hordatine accumulation, environmental factors and genetic diversity in wild barley (Hordeum spontaneum C. Koch) accessions from the Near East Fertile Crescent. Genetica. 2006;127(1–3):87–99. [DOI] [PubMed] [Google Scholar]
- 62.Yang Z, Zhang T, Bolshoy A, Beharav A, Nevo E. Adaptive microclimatic structural and expressional dehydrin 1 evolution in wild barley, Hordeum spontaneum, at ‘Evolution Canyon’, Mount Carmel, Israel. Mol Ecol. 2009;18:2063–2075. 10.1111/j.1365-294X.2009.04140.x [DOI] [PubMed] [Google Scholar]
- 63.Nevo E. Evolution of wild barley at “Evolution Canyon”: adaptation, speciation, pre-agricultural collection, and barley improvement. Israel J Plant Sci. 2014;62(1–2):22–32. [Google Scholar]
- 64.Jaradat AA. Genetic diversity of four esterase loci in natural populations of Hordeum spontaneum C. Koch from Jordan. Theor Appl Genet. 1992;84:725–729. 10.1007/BF00224176 [DOI] [PubMed] [Google Scholar]
- 65.Sharma S, Beharav A, Balyan HS, Nevo E, Gupta PK. Ribosomal DNA polymorphism and its association with geographical and climatic variables in 27 wild barley populations from Jordan. Plant Sci. 2004;166:467–477. [Google Scholar]
- 66.Hübner S, Hüffken M, Oren E, Haseneyer G, Stein N, Graner A, et al. Strong correlation of wild barley (Hordeum spontaneum) population structure with temperature and precipitation variation. Mol Ecol. 2009;18:1523–1536. 10.1111/j.1365-294X.2009.04106.x [DOI] [PubMed] [Google Scholar]
- 67.Castañeda-Álvarez NP, de Haan S, Juárez H, Khoury CK, Achicanoy HA, Sosa CC, et al. Ex Situ Conservation Priorities for the Wild Relatives of Potato (Solanum L. Section Petota). PLoS ONE. 2014;10(4):e0122599 10.1371/journal.pone.0122599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khoury CK, Castañeda-Alvarez NP, Achicanoy HA, Sosa CC, Bernau V, Kassa MT, et al. Crop wild relatives of pigeonpea [Cajanus cajan (L.) Millsp.]: Distributions, ex situ conservation status, and potential genetic resources for abiotic stress tolerance. Biol Conserv 2015;184:259–270. [Google Scholar]
- 69.Kantar MB, Sosa CC, Khoury CK, Castañeda-Álvarez NP, Achicanoy HA, Bernau V, et al. Ecogeography and utility to plant breeding of the crop wild relatives of sunflower (Helianthus annuus L.). Frontiers in Plant Sci. 2015;6:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climat. 2005;25(15):1965–1978. [Google Scholar]
- 71.Thiel T, Michalek W, Varshney RK, Graner A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor Appl Genet. 2003;106:411–422. [DOI] [PubMed] [Google Scholar]
- 72.Stein N, Prasad M, Scholz U, Thiel T, Zhang H, Wolf M, et al. A 1000-loci transcript map of the barley genome: new anchoring points for integrative grass genomics. Theor Appl Genet. 2007;114:823–839. [DOI] [PubMed] [Google Scholar]
- 73.Varshney RK, Marcel TC, Ramsay L, Russell J, Roder MS, Stein N, et al. A high density barley microsatellite consensus map with 775 SSR loci. Theor Appl Genet. 2007;114(6):1091–1103. [DOI] [PubMed] [Google Scholar]
- 74.Lewis PO, Zaykin D. Genetic Data Analysis: Computer Program for the Analysis of Allelic Data (version 1.1). 2001; Available: http://hydrodictyon.eeb.uconn.edu/people/plewis/downloads/gda-1.1.win32.zip.
- 75.Goudet J. FSTAT, a program to estimate and test gene diversities and fixation indices (Version 2.9.3). 2001; Available: http://www.unil.ch/popgen/softwares/fstat.htm.
- 76.Nagy S, Poczai P, Cernák I, Gorji AM, Hegedűs G, Taller J. PICcalc: an online program to calculate polymorphic information content for molecular genetic studies. Biochemical Genet. 2012;50(9–10):670–672. [DOI] [PubMed] [Google Scholar]
- 77.Perrier X, Jacquemoud-Collet JP. DARwin software. 2006; Available: http://darwin.cirad.fr/.
- 78.Gao H, Williamson S, Bustamante CD. A Markov chain Monte Carlo approach for joint inference of population structure and inbreeding rates from multilocus genotype data. Genetics. 2007;176(3):1635–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jakobsson M, Rosenberg NA. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007;23(14):1801–1806. [DOI] [PubMed] [Google Scholar]
- 81.Rosenberg NA. Distruct: a program for the graphical display of population structure. Mol Ecol Notes. 2004;4:137–138. [Google Scholar]
- 82.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14(8):2611–2620. [DOI] [PubMed] [Google Scholar]
- 83.Earl DA, von Holdt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour. 2012;4(2):359–361. [Google Scholar]
- 84.Newman CE, Rissler LJ. Phylogeographic analyses of the southern leopard frog: the impact of geography and climate on the distribution of genetic lineages vs. subspecies. Mol Ecol. 2011;20:5295–5312. 10.1111/j.1365-294X.2011.05353.x [DOI] [PubMed] [Google Scholar]
- 85.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modeling. 2006;190:231–259. [Google Scholar]
- 86.Parra-Quijano M, Torres E, Iriondo JM and López F. CAPFITOGEN Tools User Manual Version 1.2 International Treaty on Plant Genetic Resources for Food and Agriculture, FAO, Rome, Italy; 2014. [Google Scholar]
- 87.Ersts PJ. Geographic Distance Matrix Generator (version 1.2.3). American Museum of Natural History, Center for Biodiversity and Conservation. Available: http://biodiversityinformatics.amnh.org/open_source/gdmg.
- 88.Rousset F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics. 1997;145(4):1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fu YB. FPTEST: a SAS routine for testing differences in allelic count. Mol. Ecol. Res. 2010;10(2):389–392. [DOI] [PubMed] [Google Scholar]
- 90.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 91.Morrell PL, Toleno DM, Lundy KE, Clegg MT. Low levels of linkage disequilibrium in wild barley (Hordeum vulgare ssp. spontaneum) despite high rates of self-fertilization. Proc Nat Acad Sci USA. 2005;102(7):2442–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Turpeinen T, Vanhala T, Nevo E, Nissilä E. AFLP genetic polymorphism in wild barley (Hordeum spontaneum) populations in Israel. Theor Appl Genet. 2003;106:1333–1339. [DOI] [PubMed] [Google Scholar]
- 93.Hübner S, Günther T, Flavell A, Fridman E, Graner A, Korol A, et al. Islands and streams: clusters and gene flow in wild barley populations from the Levant. Mol Ecol. 2012;21:1115–1129. 10.1111/j.1365-294X.2011.05434.x [DOI] [PubMed] [Google Scholar]
- 94.Al-Eisawi D. Vegetation of Jordan. UNESCO—Cairo office. Regional office for science and technology for the Arab States; 1996.
- 95.Witcombe JR, Bourgois JJ, Rifaie R. Germplasm collections from Syria & Jordan. Plant Genet Resour Newsletter. 1982;50:2–8. [Google Scholar]
- 96.Jaradat AA. Diversity within and between populations of two sympatrically distributed Hordeum species in Jordan. Theor Appl Genet. 1989;78:653–656. 10.1007/BF00262560 [DOI] [PubMed] [Google Scholar]
- 97.Jaradat AA. Ecotypes and genetic divergence among sympatrically distributed Hordeum vulgare and Hordeum spontaneum from the xeric region of Jordan. Theor Appl Genet. 1989;78:857–862. 10.1007/BF00266671 [DOI] [PubMed] [Google Scholar]
- 98.Nevo E. Origin, evolution, population genetics and resources for breeding of wild barley Hordeum spontaneum in the Fertile Crescent In: Shewry PR, editor. Barley: Genetics, Biochemistry, molecular biology and biotechnology. CAB International, Wallingford, UK: 1992. pp 19–43. [Google Scholar]
- 99.Schoen DJ, Brown AHD. Intraspecific variation in population gene diversity and effective population size correlated with the mating system in plants. Proc Nat Acad Sci USA. 1991;88:4494–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nevo E. Genetic diversity and environmental associations of wild barley, Hordeum spontaneum in Turkey. Genetica. 1986;68:203–213. [DOI] [PubMed] [Google Scholar]
- 101.Volis S, Mendlinger S, Ward D. Adaptive traits of wild barley plants of Mediterranean and desert origin. Oecologia. 2002;133:131–138. [DOI] [PubMed] [Google Scholar]
- 102.Parra-Quijano M., Iriondo J.M., Torres E. Ecogeographical land characterization maps as a tool for assessing plant adaptation and their implications in agrobiodiversity studies. Genet Resour Crop Evol. 2012;59(2):205–217. [Google Scholar]
- 103.Hoban S, Arntzen JA, Bruford MW, Godoy JA, Hoelzel R, Segelbacher G, et al. Comparative evaluation of potential indicators and temporal sampling protocols for monitoring genetic erosion. Evol Appl. 2014;7(9):984–998. 10.1111/eva.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abdel-Ghani AH, Parzies HK, Omary A, Geiger HH. Estimating the outcrossing rate of barley landraces and wild barley populations collected from ecologically different regions of Jordan. Theor Appl Genet. 2004;109:588–595. [DOI] [PubMed] [Google Scholar]
- 105.Hoban S, Strand A. Ex situ seed collections will benefit from considering spatial sampling design and species’ reproductive biology. Biol Conserv. 2015;187:182–191. [Google Scholar]
- 106.Nevo E. Genetic diversity in wild cereals: regional and local studies and their bearing on conservation ex situ and in situ. Genet Resour Crop Evol. 1998;45:355–370. [Google Scholar]
- 107.Al-Saghir MG, Malkawi HI, El-Oqlah A. Morphological Diversity in Hordeum spontaneum C. Koch of Northern Jordan (Ajloun Area). Middle-East J Sci Res. 2009;4(1):24–27. [Google Scholar]
- 108.Shakhatreh Y, Haddad N, Alrababah M, Grando S, Ceccarelli S. Phenotypic diversity in wild barley (Hordeum vulgare L. ssp. spontaneum (C. Koch) Thell.) accessions collected in Jordan. Genet Resour Crop Evol. 2010;57(1):131–146. [Google Scholar]
- 109.van Hintum T, Menting F. Diversity in ex situ genebank collections of barley In: von Bothmer R, van Hintum T, Knüpffer H, Sato K, editors. Diversity in barley (Hordeum vulgare). Elsevier Science B. V., Amsterdam, the Netherlands; 2003. pp. 247–257. [Google Scholar]
- 110.von Bothmer R, Jacobsen N, Baden C, Jørgensen RB, Linde-Laursen I. An ecogeographical study of the genus Hordeum 2nd edition Systematic and Ecogeographic Studies on Crop Genepools 7. International Plant Genetic Resources Institute, Rome, Italy; 1995. [Google Scholar]
- 111.Vincent H, von Bothmer R, Knüpffer H, Amri A, Konopka J, Maxted N. Genetic gap analysis of wild Hordeum taxa. Plant Genet Resour-C. 2013;10(3):242–253. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.