Abstract
Studies on the treatment patterns and outcomes of elderly patients with metastatic pancreas cancer remain limited. Therefore, an analysis of systemic therapy use, clinical trial participation, and outcomes in elderly patients with metastatic pancreas cancer was performed at our institution. Elderly patients who received systemic therapy had a longer survival compared with those who did not. However, therapeutic clinical trial participation was low and should be encouraged
Background
Pancreas adenocarcinoma has a median age at diagnosis of 71 years. Limited studies have focused on the treatment of elderly patients with pancreas cancer.
Patients and Methods
An analysis of systemic therapy use, clinical trial participation, and overall outcomes of 237 patients with metastatic pancreas adenocarcinoma ≥ 75 years of age evaluated at Memorial Sloan-Kettering Cancer Center between 2005 and 2013 was undertaken.
Results
Median overall survival was 7 months for the entire study population. A total of 197 (83%) patients received systemic therapy, which was significantly associated with longer overall survival (P < .01). No significant difference was detected in survival between age groups 75 to 79, 80 to 84, and ≥ 85 years of age among those who received systemic therapy (P = .49). Seventy-seven (32%) patients participated in a clinical trial of whom 13 (5%) patients were enrolled in a therapeutic trial, including no patients aged ≥ 85 years. Multivariate analysis demonstrated that presence of liver metastases (P < .001), performance status (P < .001), and number of systemic agents (P < .001) were significantly associated with survival.
Conclusion
Receipt of systemic therapy was associated with longer survival in elderly patients ≥ 75 years of age with metastatic pancreas adenocarcinoma. Therapeutic clinical trial participation among these patients was low and future development of prognostic models for appropriate patient selection is warranted.
Keywords: Cancer, Chemotherapy, Elderly, Palliative, Survival
Introduction
With a growing elderly population in the United States, an estimated 70% of all cancer diagnoses will occur in patients older than the age of 65 by the year 2030.1 Pancreatic cancer is a disease that mainly affects the elderly population with a median age of 71 years at diagnosis.2 The incidence of pancreatic cancer has increased in recent years and 53% of patients are initially diagnosed with meta-static disease.3 As a result, prognosis remains poor and pancreatic cancer is now projected to become the second leading cause of cancer-related mortality in the United States by the year 2030.4
Previous studies that specifically focused on elderly metastatic pancreatic cancer patients have been limited. Furthermore, elderly patients remain poorly represented in clinical trials5 and are often not recommended for standard therapy.6,7 Hutchins et al demonstrated that patients 65 years or older were significantly underrep-resented in the Southwest Oncology Group treatment trials, which included trials specifically for pancreas cancer.5 In another study, 11% of physicians explicitly reported that age was a reason for not enrolling elderly patients in clinical trials.8 In terms of standard therapy, Oberstein et al investigated the Surveillance, Epidemiology, and End Results-Medicare database for patients with metastatic pancreas adenocarcinoma diagnosed between 1998 and 2005 and demonstrated that the likelihood of receipt of palliative gemcitabine decreased with advancing age.7 Additionally, Vijayvergia et al also reported differences in patterns of care and outcomes of elderly versus younger patients with metastatic pancreatic cancer. Specifically, the authors showed at their institution that older patients (> 65 years of age) despite having similar performance status and disease characteristics were less likely to receive any chemotherapy and if treated were less likely to receive more than 1 chemotherapy agent.9
The safety and efficacy of gemcitabine-based chemotherapy in elderly patients aged ≥ 70 years with advanced pancreatic cancer was shown to be similar to that of younger patients based on a limited number of retrospective studies.10–12 5-fluorouracil, leucovorin, irinotecan, oxaliplatin (FOLFIRINOX)13 and gemcitabine/nab-paclitaxel14 are newly established combination cytotoxic regimens routinely used in the treatment of metastatic pancreas cancer in addition to single-agent gemcitabine. However, there are many pertinent questions regarding the use of gemcitabine-based therapy and these newer cytotoxic regimens in elderly patients ≥ 75 years of age, an age group that has not been widely studied in pancreas cancer despite that > 40% of patients diagnosed with this disease fall into this age group.2 Furthermore, data on this particular age group with regard to clinical trial participation are sparse and notably, the phase III study that evaluated FOLFIRINOX had an upper limit of age enrollment of 76 years,13 although the phase III trial that evaluated nab-paclitaxel and gemcitabine did include patients older than 75 years of age, which accounted for 10% of the trial enrollment.14
Based on the limited data available on the use of various systemic therapies and clinical trial participation among elderly patients with stage IV pancreas adenocarcinoma, we hypothesized that the frequency of systemic therapy use and clinical trial participation would be low among patients aged ≥ 75 years who underwent treatment for stage IV pancreas adenocarcinoma. Therefore, the primary objective of the current study was to assess the frequency of systemic therapy use, clinical trial participation, and overall survival (OS) in patients ≥ 75 years of age with metastatic pancreas adenocarcinoma who received care at Memorial Sloan Kettering Cancer Center (MSKCC). Additional objectives included characterization of treatment and disease-related morbidity, and modeling to investigate predictors of survival.
Patients and Methods
Patient Selection
After obtaining institutional review board approval for the study, the MSKCC institutional database, comprised of all patients seen at MSKCC, was searched to identify patients aged ≥ 75 years diagnosed with stage IV pancreas adenocarcinoma between January 2005 and December 2013. Inclusion criteria were (1) patients ≥ 75 years of age at diagnosis; (2) diagnosis of stage IV pancreas adenocarcinoma; and (3) patients must have received consultation or evaluation for stage IV pancreas adenocarcinoma at MSKCC. Patients specifically with nonadenocarcinoma histology and stage III disease, were excluded. The cutoff age of 75 was specifically used for this study because of the persistent underrepresentation of this age group among cancer trials.15 As a result, research on cancer therapeutic use among those aged ≥ 75 years has been recommended as a top priority in geriatric oncology among collaborators from the Cancer and Aging Research Group, the National Institute of Aging, and the National Cancer Institute.16 A retrospective chart review was undertaken to abstract data.
Selection of Variables
The following variables were extracted in the chart review: age (divided into groups aged 75–79, 80–84, and ≥ 85 years), sex, other comorbid medical conditions, Eastern Cooperative Oncology Group (ECOG) performance status, primary tumor site, location of metastatic sites, number of medications, laboratory tests (consisting of albumin and carcinoma antigen [CA] 19-9), chemotherapy use (including number of agents given during first-line systemic therapy), and clinical trial participation.
Age was specifically divided into groups 75 to 79, 80 to 84, and ≥ 85 years because previous studies at MSKCC in breast cancer have shown differences in treatment patterns when age groups 75 to 79 versus ≥ 80 years were compared.17 Furthermore, a previous study in pancreas cancer divided age groups into 65 to 69, 70 to 74, 75 to 79, 80 to 84 and ≥ 85 years and demonstrated the magnitude of decreased likelihood to receive gemcitabine treatment increased with each advancing age group.7
The age-adjusted Charlson Comorbidity Index (ACCI) was used to assess the effect of age combined with comorbid medical conditions.18,19 ACCI scores were calculated using the method previously reported and initially developed by Charlson in 1987, which has since been updated and validated in numerous studies including in older patients with cancer.18–20
Other variables selected for investigation in this study were based on previous potential prognostic variables in pancreas cancer identified from previous studies.14,21–23
Statistical Analysis
The primary outcome of the study was median OS. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Associations between different risk factors and OS were assessed using univariate Cox regression models. A multivariable Cox regression model was built based on risk factors found to be significant at the .05 level in the univariate Cox regression models.
Results
A total of 247 patients aged ≥ 75 years with a listed diagnosis of stage IV pancreas adenocarcinoma were identified in the MSKCC database between January 2005 and December 2013. Of these, 237 patients were eligible for inclusion. Most of those who were ineligible for analysis had an initial diagnosis of stage III pancreas cancer and were identified to have developed metastatic disease only after receiving previous systemic therapy (ie, not de novo stage IV disease).
Patient characteristics are detailed in Table 1. Age was divided into 3 age groups with 114 (48%) patients between the ages of 75 and 79, 84 (35%) patients between the ages of 80 and 84, and 39 (17%) patients aged ≥ 85 years. One hundred forty-four (61%) patients in this study had an ECOG performance status of 0 to 1. One hundred twenty-two (52%) patients had a head of pancreas primary tumor and 168 (71%) had evidence of metastatic liver disease. Baseline laboratory values for albumin and CA 19-9 were also recorded and categorized as shown in Table 1. Patient comorbidity was measured using the ACCI. The median ACCI score among patients in this study was 5 (range, 4–14). The most common comorbid medical conditions identified in patients included diabetes mellitus (33%), myocardial infarction (17%), and other malignancies (10%). Additional comorbid medical conditions are listed in Table 2.
Table 1.
Patient Characteristics
| Total Patients (n = 237) | Value |
|---|---|
| Age, n (%) | |
| 75–79 | 114 (48) |
| 80–84 | 84 (35) |
| ≥85 | 39 (17) |
| Sex, n (%) | |
| Male | 104 (44) |
| Female | 133 (56) |
| ECOG Performance Status, n (%) | |
| 0–1 | 144 (61) |
| 2 | 71 (30) |
| ≥3 | 22 (9) |
| Primary Tumor Location, n (%) | |
| Head | 122 (52) |
| Body/tail | 112 (47) |
| Unknown | 3 (1) |
| Metastatic Sites, n (%)a | |
| Liver | 168 (71) |
| Lung | 64 (27) |
| Peritoneum | 72 (30) |
| Node | 119 (50) |
| Bone | 9 (4) |
| Other | 10 (4) |
| Median Comorbidity/Polypharmacy (Range) | |
| Number of comorbid conditions | 1 (0–4) |
| Charlson Comorbidity index | 5 (4–14) |
| Number of medications | 7 (0–18) |
| Serum Albumin (n = 234), n (%)b | |
| ≥4.0 g/dL | 67 (29) |
| 3.5 to <4.0 g/dL | 72 (31) |
| <3.5 g/dL | 95 (40) |
| Serum CA 19-9 (n = 217), n (%)c | |
| Normal (0–40 U/mL) | 37 (17) |
| >ULN to ≤59 times ULN | 110 (51) |
| >59 times ULN | 70 (32) |
Abbreviations: CA = carcinoma antigen; ECOG = Eastern Cooperative Oncology Group; ULN = upper limit of normal.
Multiple patients had multiple sites of metastatic disease and therefore the total is > 237 and the total percentage for the category metastatic sites is > 100%.
Only 234 patients had data on serum albumin level at the time of diagnosis.
Only 217 patients had data on CA 19-9 level at the time of diagnosis.
Table 2.
Comorbidity (n = 237)
| Comorbid Medical Conditions | n | % |
|---|---|---|
| No Comorbid Medical Conditions | 99 | 42 |
| Diabetes Mellitus | 79 | 33 |
| Myocardial Infarction | 40 | 17 |
| Other Malignancy | 23 | 10 |
| Connective Tissue Disease | 12 | 5 |
| Cerebral Vascular Accident | 12 | 5 |
| Peptic Ulcerative Disease | 10 | 4 |
| Peripheral Vascular Disease | 8 | 3 |
| Chronic Obstructive Pulmonary Disease | 6 | 3 |
| Dementia | 5 | 2 |
| Chronic Renal Insufficiency | 4 | 2 |
| Congestive Heart Failure | 2 | 1 |
Thirty-five patients had 2 separate comorbid medical conditions, 11 patients had 3 separate comorbid medical conditions, and 2 patients had 4 separate comorbid medical conditions. As a result the sum total is > 237 and the total percentage is > 100%.
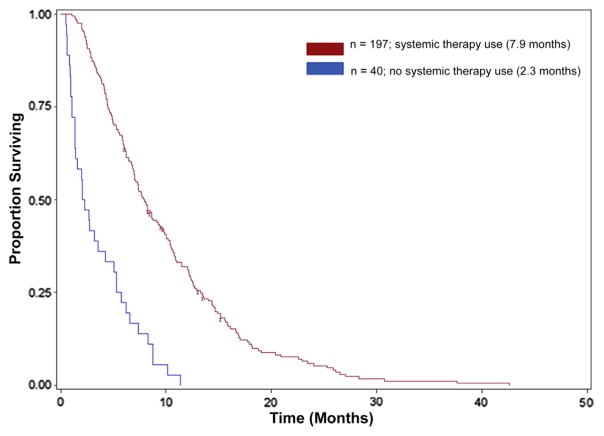
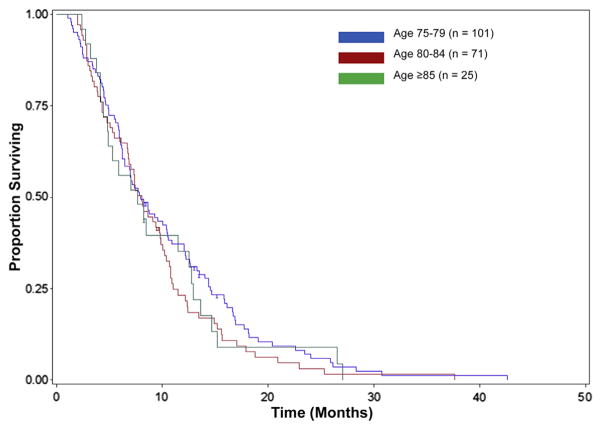
Clinical trial participation and systemic treatment patterns were also determined. Seventy-seven (32%) patients participated in a clinical study (therapeutic, biospecimen, or psychosocial), however, only 13 (5%) patients enrolled specifically in a therapeutic trial and no patients aged ≥ 85 years was enrolled in a therapeutic study (Table 3). A total of 197 (83%) patients received systemic therapy for the treatment of stage IV pancreas adenocarcinoma. The distribution of systemic therapy use and median OS among the 3 age groups (75–79, 80–84, and ≥ 85 years) are also shown in Table 3. Single-agent gemcitabine (55%) was the most prevalent choice of first-line therapy used in our study population, although many patients were also frequently treated with various combination regimens (Table 3). For the entire study population, the median OS was 7 months. Systemic therapy use was associated with longer OS (Figure 1) with a median OS of 7.9 months for patients who received treatment versus 2.3 months for those who did not receive any therapy (P < .01). In addition, no significant difference was detected in survival between the different age groups (Figure 2) among those who received systemic therapy (P = .49).
Table 3.
Clinical Trial Participation, Systemic Therapy Use, and Overall Survival
| Age, Years | Total Cohort | 75–79 | 80–84 | ≥85 |
|---|---|---|---|---|
| N | 237 | 114 | 84 | 39 |
| Clinical Trial Participation | 77 (32%) | 43 (38%) | 29 (34%) | 5 (13%) |
| Participation in a Therapeutic Trial | 13 (5%) | 9 (8%) | 4 (5%) | – |
| Systemic Therapy Use | 197 (83%) | 101 (89%) | 71 (85%) | 25 (64%) |
| Type of First-Line Systemic Therapy | ||||
| Gemcitabine | 108 (55%) | 50 (50%) | 35 (49%) | 23 (92%) |
| Capecitabine | 1 (0.5%) | 1 (1%) | – | – |
| Gemcitabine with erlotinib | 24 (12%) | 13 (13%) | 11 (16%) | – |
| Gemcitabine with oxaliplatin | 19 (10%) | 13 (13%) | 6 (9%) | – |
| Gemcitabine with capecitabine | 12 (6%) | 5 (5%) | 6 (9%) | 1 (4%) |
| Gemcitabine with carboplatin | 1 (0.5%) | – | 1 (1%) | – |
| Gemcitabine with cisplatin | 3 (2%) | 3 (3%) | – | – |
| Gemcitabine with 5-FU | 1 (0.5%) | 1 (1%) | – | – |
| Gemcitabine with nab-paclitaxela | 1 (0.5%) | – | 1 (1%) | – |
| GTX | 1 (0.5%) | 1 (1%) | – | – |
| Gemcitabine with 5-FU and HAI cisplatin | 1 (0.5%) | – | 1 (1%) | – |
| FOLFOX | 5 (3%) | 2 (2%) | 2 (3%) | 1 (4%) |
| FOLFIRINOX | 6 (3%) | 3 (3%) | 3 (4%) | – |
| Clinical trial | 13 (7%) | 9 (9%) | 4 (6%) | – |
| Unknown therapy | 1 (0.5%) | – | 1 (1%) | – |
| Median Overall Survival, Months | 7.0 | 7.1 | 7.3 | 5.9 |
| With systemic therapy | 7.9 | 7.9 | 8.1 | 7.7 |
| No therapy | 2.3 | 1.4 | 2.5 | 4.7 |
Abbreviations: FOLFIRINOX = 5-FU with leucovorin, oxaliplatin, and irinotecan; FOLFOX = 5-FU with leucovorin and oxaliplatin; 5-FU = fluorouracil; GTX = gemcitabine with docetaxel and capecitabine; HAI = hepatic artery infusion.
The current study review was conducted during a time period before Food and Drug Administration approval of gemcitabine/nab-paclitaxel combination therapy.
Figure 1.
Kaplan–Meier Probabilities for Overall Survival for All Patients Treated With Systemic Therapy Versus Patients Not Treated With Systemic Therapy
Figure 2.
Kaplan–Meier Probabilities for Overall Survival for Patients Treated With Systemic Therapy, Based on Age Groups
The association between different patient characteristics and OS are shown in Table 4. Presence of liver metastases (P < .001), ECOG performance status (P < .001), number of agents used in front-line therapy (P < .001), and ACCI score index (P = .01) were all significantly associated with survival in univariate analysis. A multivariate analysis based on the significant univariate findings was then applied, and it revealed that the presence of liver metastases (P < .001) and worse ECOG performance status (P < .001) remained significantly associated with shorter OS and more systemic agents used during front-line therapy (P < .001) remained significantly associated with longer OS.
Table 4.
Univariate and Multivariate Analysis of Factors Associated With Overall Survival
| Factor | Hazard Ratio (95% CI) | P |
|---|---|---|
| Univariate Analysis | ||
| Age (compared with ≥85 group) | ||
| 75–79 | 0.75 (0.52–1.09) | .14 |
| 80–84 | 0.92 (0.63–1.36) | .68 |
| ECOG PS (compared with ≥3) | ||
| 0–1 | 0.18 (0.11–0.29) | <.001 |
| 2 | 0.32 (0.20–0.53) | <.001 |
| Site of primary tumor (compared with tail) | ||
| Head | 0.77 (0.55–1.1) | .15 |
| Body | 0.73 (0.49–1.1) | .11 |
| Site of metastasis (yes or no) | ||
| Liver | 1.72 (1.28–2.31) | <.001 |
| Bone | 1.30 (0.67–2.54) | .44 |
| Lung | 0.92 (0.68–1.23) | .57 |
| Peritoneum | 0.85 (0.64–1.13) | .26 |
| Node | 1.20 (0.93–1.56) | .17 |
| Number of comorbid conditions | 1.13 (0.98–1.31) | .10 |
| Charlson Comorbidity Index | 1.12 (1.02–1.21) | .01 |
| Number of medications | 1.00 (0.97–1.03) | .98 |
| Serum albumin (compared with <3.5 g/dL) | ||
| 3.5 to <4.0 g/dL | 0.84 (0.61–1.15) | .27 |
| ≥4.0 g/dL | 0.74 (0.54–1.02) | .07 |
| Serum CA 19-9 (compared with normal 0–40 U/mL) | ||
| >ULN to ≤59 times ULN | 0.80 (0.55–1.17) | .25 |
| >59 times ULN | 1.15 (0.77–1.73) | .49 |
| Number of systemic therapy agents (compared to none) | ||
| 1 | 0.26 (0.18–0.39) | <.001 |
| 2+ | 0.24 (0.16–0.37) | <.001 |
| Multivariate Analysis | ||
| Presence of liver metastasis | 1.73 (1.28–2.35) | <.001 |
| Charlson Comorbidity Index | 1.07 (0.97–1.17) | .17 |
| ECOG PS (compared with 0–1) | ||
| 2 | 1.76 (1.28–2.42) | <.001 |
| 3+ | 3.55 (2.08–6.06) | <.001 |
| Number of systemic therapy agents (compared with 0) | ||
| 1 | 0.40 (0.26–0.63) | <.001 |
| 2+ | 0.38 (0.24–0.61) | <.001 |
Abbreviations: CA = carcinoma antigen; ECOG PS = Eastern Cooperative Oncology Group performance status; ULN = upper limit of normal.
Treatment- and disease-related morbidity data were noted in the form of any patient hospitalizations during first-line systemic therapy treatment. Although 197 patients received first-line systemic therapy in our study, 170 patients were evaluable for the rate of hospitalizations because 27 patients resumed care with a local oncologist and therefore data regarding hospitalizations for these patients were not captured. Overall, 96 (56%) patients were hospitalized at least once during front-line therapy (Table 5, and see Supplemental Table 1 in the online version). Seventeen patients (10%) required 2 different hospitalizations and 3 patients (2%) had 3 separate hospitalizations. However, of those hospitalized, only 23 (24%) patients were specifically admitted to the hospital as a result of a treatment-related event. Reasons for treatment-related hospital admissions during first-line therapy included: infection (6%), anasarca (4%), fatigue (3%), diarrhea (3%), gemcitabine pneumonitis (2%), dehydration (2%), acute kidney injury (2%), and anemia (1%).
Table 5.
Hospitalizations During First-Line Systemic Therapy
| Patients With Any Grade ≥3 Events Who Required Hospitalization During First-Line Systemic Therapy | n | % |
|---|---|---|
| Total | 96a | 56 |
| Reason for Hospitalization | ||
| Cardiovascular | 6 | 4 |
| Constitutional | 11 | 6 |
| Gastroenterology | 38 | 22 |
| Hematologic | 9 | 5 |
| Infectious disease | 36 | 21 |
| Neurology/psychiatry | 2 | 1 |
| Orthopedics | 5 | 3 |
| Pulmonary | 5 | 3 |
| Renal | 5 | 3 |
Total patient n = 170.
Seventeen patients required 2 different hospitalizations and 3 patients had 3 separate hospitalizations. Therefore, the sum of all separate categories is > 96.
Discussion
As the US population increases in age along with an increasing incidence of pancreas cancer, more patients older than the age of 75 years will be diagnosed with the disease.1,2 This poses a particular challenge to oncologists because the cost-benefit ratio of treatment in terms of prolonging survival must be weighed against potential treatment toxicities and the overall effect on quality of life. Although there is a lack of reference data on elderly patient preferences and physician recommendation patterns to offer and initiate systemic therapy for advanced pancreas adenocarcinoma,24 studies in other gastrointestinal malignancies such as colorectal cancer have indicated that physicians are reluctant to offer elderly patients systemic therapy or enrollment into clinical trials.25 Because of the worse prognosis often associated with advanced pancreas adenocarcinoma, patient motivation to pursue treatment might vary among this older population. As a result, many oncologists could remain hesitant to offer elderly patients many forms of cancer-directed therapies.
Aldoss et al specifically reviewed the role of palliative chemotherapy for metastatic pancreas adenocarcinoma patients aged ≥ 80 years in the Veterans Affairs Cancer Registry.6 Surprisingly, only 13% of the patients received chemotherapy despite chemotherapy treatment resulting in improved median OS. In contrast to the study by Aldoss et al, our study demonstrated that 78% of patients aged ≥ 80 years received systemic therapy at our institution. One potential explanation for the discrepancy could be differences among patient populations between studies; patients typically seen at our cancer center are often self-seeking individuals determined to undergo treatment. Similar to the study by Aldoss et al, systemic treatment was associated with longer OS across all elderly age subgroups, with an average OS difference of 5.6 months between patients who received treatment versus those who did not, although selection of patients for treatment versus no treatment could have been a potential driver for the differences in survival seen. The difference in OS observed is consistent and reasonable for our study population in which 55% of patients received single-agent gemcitabine and only a very limited 4% of patients received newly established combination cytotoxic therapies such as FOLFIRINOX or gemcitabine with nab-paclitaxel as front-line treatment, in part because gemcitabine and nab-paclitaxel was not approved by the Food and Drug Administration during the period of time reviewed for our study. Furthermore, although the percentage of patients who received systemic therapy did decrease slightly with advancing age in our study, this result is similar to that previously reported by Oberstein and colleagues.7
Patients in the current study generally tolerated treatment consistent with results of previous studies in elderly patients who received treatment with gemcitabine.10–12 Although actual rates of significant treatment-related adverse events that required hospitalization were relatively low, overall the number of hospitalizations during front-line treatment was greater than expected and could potentially be explained by the much greater percentage of very elderly patients who received therapy in the current study compared with previous studies and that more patients received various combination systemic treatments rather than just single-agent gemcitabine. To fully discriminate between treatment-related versus underlying disease and associated comorbidities during systemic therapy in this retrospective analysis was difficult. A complete and through review of the medical record was performed in which any hospitalization regardless of length of stay was captured during front-line therapy. Furthermore, a relative comparison of overall hospitalization rates regardless of cause during front-line systemic therapy among elderly patients with metastatic pancreas cancer is difficult because previous studies have not yet reported on this specific parameter.
Clinical trial participation represents another challenging area in which elderly patients with cancer are significantly underrepresented.5 Hoos et al recently reported a rate of 4.6% overall clinical trial participation in pancreas cancer.26 Although the therapeutic clinical trial participation from the current study of patients older than the age of 75 years with stage IV pancreas adenocarcinoma was similar, we found that no patient aged ≥ 85 years was enrolled in a therapeutic study. Because participants of recent large phase III trials in metastatic pancreas adenocarcinoma have typically mean ages in the low 60s,13,14 a major focus going forward should be on enhancing clinical trial participation in the elderly and clinical trial designs that include patients with a less robust performance status. Where therapeutic trial participation is not feasible, consideration of participation in related nontherapeutic studies (eg, biospecimen, psychosocial, and other trial types), should be encouraged.
Establishment of definitive prognostic variables during initial diagnosis could be key in helping oncologists better define which patients should receive single-agent therapy, combination therapy, be enrolled in a clinical trial, or even receive treatment at all. Although age has been described as an independent prognostic factor in past studies of pancreas adenocarcinoma,22,23,27 our study did not confirm this observation because age was not a significant factor when different age groups older than the age of 75 years were compared. Rather, performance status, evidence of liver metastases, and number of therapeutic agents remained significantly prognostic in multivariate analysis. Research into better defining subgroup prognostic factors will be crucial moving forward because it would provide valuable information on patients who might or might not benefit the most from aggressive therapy and possible enrollment in clinical trials.
Furthermore, because the median age at the time of diagnosis is 71 years for pancreas adenocarcinoma,2 oncologists dedicated to treating this disease might also benefit from developing a specialist geriatric interest. Previous studies have demonstrated that the use of geriatric assessments (GAs) can detect health care problems in elderly cancer patients that are often missed during routine oncology care.28 However, several different assessment tools currently exist to evaluate the various domains that make up a comprehensive GA.28 Therefore, future research focused on standardization of the various assessment tools to create an appropriate comprehensive GA specifically for elderly patients with pancreatic cancer is warranted. When established, randomized trials that compare GA-guided therapy versus no GA-guided therapy should be performed in this population.
Limitations of our study include its retrospective design, lack of quality of life assessment during treatment, and, as mentioned, the inability to fully discriminate between treatment-related versus underlying disease and comorbidity-related issues during systemic therapy. The use of varied systemic therapy regimens along with varied dose schedules made dose intensity conclusions difficult in this study. In addition, 27 patients in our study continued to receive first-line therapy under the care of a local oncologist and as a result, data regarding hospitalizations from this group of patients were lost to follow-up. Furthermore, the data for the current study was derived from a single, large, academic cancer center in which the patient population, referral, and treatment patterns are likely to be different compared with other geographic or smaller community-based practices across the United States. Therefore, larger, multi-institutional prospective studies that focus specifically on patients aged ≥ 75 years with metastatic pancreas adenocarcinoma are needed.
Conclusion
Use of systemic therapy is tolerable and might be beneficial in selected elderly patients ≥ 75 years of age with metastatic pancreas adenocarcinoma. However, because the total cost of cancer treatment in the United States has been projected to be as high as $173 billion by the year 2020,29 the overall cost-benefit ratio of treating elderly patients with stage IV pancreas adenocarcinoma with several lines of systemic therapy needs to be determined moving forward. Further research on the identification of key prognostic factors in this important subgroup of patients with metastatic pancreas adenocarcinoma is warranted to permit optimal selection and stratification of patients who can best tolerate and benefit from systemic therapy.
Supplementary Material
Clinical Practice Points.
The pattern of systemic therapy use, clinical trial participation, and outcomes among elderly patients 75 years or older with stage IV pancreas adenocarcinoma is unclear.
This experience at our tertiary academic cancer center reveals that a significant majority of patients ≥ 75 years with metastatic pancreas adenocarcinoma are offered systemic therapy, including many with combination chemotherapy regimens.
Elderly patients ≥ 75 years with stage IV pancreas adenocarcinoma who received systemic therapy had a longer OS than those who did not. Although actual rates of significant systemic treatment-related adverse events that required hospitalization were relatively low among this elderly population, rates of overall hospitalizations during front-line treatment were higher than expected. Establishment of key prognostic factors is warranted to permit optimal selection and stratification of patients who can best tolerate and benefit from systemic therapy.
Therapeutic clinical trial enrollment in patients ≥ 75 years of age with metastatic pancreas cancer remains low and reasons for the lack of participation in this population warrant further investigation to promote future enrollment.
Acknowledgments
This study was funded by the Andrea J. Will Foundation.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
The Supplemental table accompanying this article can be found in the online version at http://dx.doi.org/10.1016/j.clcc.2015.05.005.
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. [Accessed June 1, 2014];SEER Cancer Statistics Review, 1975–2011. Available at: http://seer.cancer.gov/csr/1975_2011.
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 5.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 6.Aldoss IT, Tashi T, Gonsalves W, et al. Role of chemotherapy in the very elderly patients with metastatic pancreatic cancer-A Veterans Affairs Cancer Registry analysis. J Geriatr Oncol. 2011;2:209–14. [Google Scholar]
- 7.Oberstein PE, Hershman DL, Khanna LG, Chabot JA, Insel BJ, Neugut AI. Uptake and patterns of use of gemcitabine for metastatic pancreatic cancer: a population-based study. Cancer Invest. 2013;31:316–22. doi: 10.3109/07357907.2013.789904. [DOI] [PubMed] [Google Scholar]
- 8.Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316) Oncologist. 2012;17:1180–90. doi: 10.1634/theoncologist.2011-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijayvergia N, Dotan E, Devarajan K, Hatahet K, Rahman F, Cohen S. Differences in patterns of care and outcomes of elderly versus younger metastatic pancreatic cancer patients (abstract 9546) J Clin Oncol (Meeting Abstracts) 2013;31(suppl) [Google Scholar]
- 10.Marechal R, Demols A, Gay F, et al. Tolerance and efficacy of gemcitabine and gemcitabine-based regimens in elderly patients with advanced pancreatic cancer. Pancreas. 2008;36:e16–21. doi: 10.1097/MPA.0b013e31815f3920. [DOI] [PubMed] [Google Scholar]
- 11.Locher C, Fabre-Guillevin E, Brunetti F, et al. Fixed-dose gemcitabine in elderly patients with advanced pancreatic cancer: an observational study. Crit Rev Oncol Hematol. 2008;68:178–82. doi: 10.1016/j.critrevonc.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Hentic O, Dreyer C, Rebours V, et al. Gemcitabine in elderly patients with advanced pancreatic cancer. World J Gastroenterol. 2011;17:3497–502. doi: 10.3748/wjg.v17.i30.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 14.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 16.Hurria A, Mohile SG, Dale W. Research priorities in geriatric oncology: addressing the needs of an aging population. J Natl Compr Canc Netw. 2012;10:286–8. doi: 10.6004/jnccn.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurria A, Leung D, Trainor K, Borgen P, Norton L, Hudis C. Factors influencing treatment patterns of breast cancer patients age 75 and older. Crit Rev Oncol Hematol. 2003;46:121–6. doi: 10.1016/s1040-8428(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales K, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 20.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36:453–71. doi: 10.1016/s0959-8049(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 21.Tas F, Aykan F, Alici S, Kaytan E, Aydiner A, Topuz E. Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. Am J Clin Oncol. 2001;24:547–50. doi: 10.1097/00000421-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Park JK, Yoon YB, Kim YT, Ryu JK, Yoon WJ, Lee SH. Survival and prognostic factors of unresectable pancreatic cancer. J Clin Gastroenterol. 2008;42:86–91. doi: 10.1097/01.mcg.0000225657.30803.9d. [DOI] [PubMed] [Google Scholar]
- 23.Stocken DD, Hassan AB, Altman DG, et al. Modeling prognostic factors in advanced pancreatic cancer. Br J Cancer. 2008;99:883–93. doi: 10.1038/sj.bjc.6604568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol. 2003;21:3409–14. doi: 10.1200/JCO.2003.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA. 2010;303:1037–45. doi: 10.1001/jama.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoos WA, James PM, Rahib L, Talley AW, Fleshman JM, Matrisian LM. Pancreatic cancer clinical trials and accrual in the United States. J Clin Oncol. 2013;31:3432–8. doi: 10.1200/JCO.2013.49.4823. [DOI] [PubMed] [Google Scholar]
- 27.Tas F, Sen F, Keskin S, Kilic L, Yildiz I. Prognostic factors in metastatic pancreatic cancer: older patients are associated with reduced overall survival. Mol Clin Oncol. 2013;1:788–92. doi: 10.3892/mco.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wildiers H, Heeren P, Puts M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.