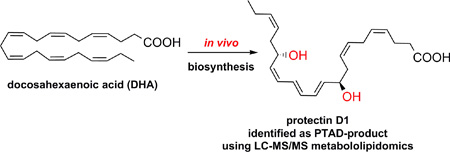
Abstract
The n-3 polyunsaturated fatty acids are substrates for lipoxygenases and cyclooxygenases. During inflammatory processes, these enzymes form several distinct families of oxygenated polyunsaturated fatty acids coined specialized pro-resolving lipid mediators. Structural elucidation of these natural products using LC-MS/MS based metabololipidomics with the pico- to nanogram amounts of biosynthetic material available have been performed. The specialized pro-resolving lipid mediators display stereospecific and potent anti-inflammatory and pro-resolving actions. Most often the different families among these mediators are chemically characterized by two or three chiral, secondary alcohols, separated by either anE,E,Z-triene or an E,Z,E,E-tetraenemoiety. The lipoxygenases also form other oxygenated polyunsaturated natural products, coined double di-oxygenation products, that are constitutional isomers of the protectin and maresin families of specialized pro-resolving lipid mediators. Very often these products exhibit similar chromatographic properties and mass spectrometrical fragment ions as the pro-resolving mediators. In addition, the double di-oxygenation products are sometimes formed in larger amounts than the specialized pro-resolving lipid mediators. Thus, it is not always possible to distinguish between the specialized pro-resolving mediators and their double di-oxygenation isomers in biological systems, using LC/MS-based techniques. Herein, a convenient and easy-to-use protocol to meet this challenge is presented.
Graphical abstract
Introduction
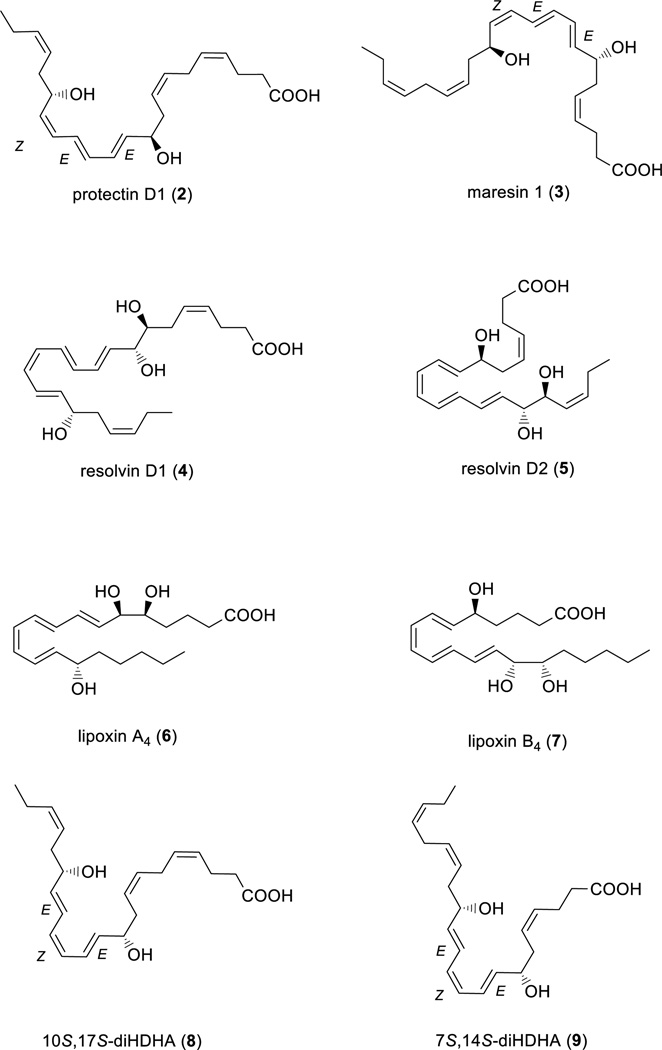
Polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (1, DHA), play a major role in the physiology of living organisms.1 Recent efforts have established that DHA (1) is a substrate in the biosynthesis of several potent anti-inflammatory pro-resolving mediators such as protectin D1 (PD1, 2),2 maresin 1 (MaR1, 3),3 resolvin D1 (RvD1, 4),2a,4 and resolvin D2 (RvD2, 5)2a,4 (see Figure 1 for chemical structures). Other examples of pro-resolving mediators are lipoxin A4 (LXA4, 6)5,6 and lipoxin B4 (LXB4, 7).5,6
Figure 1.
Chemical structures of some SPMs and the two the double di-oxygenation products 10S,17S-diHDHA (8) and 7S,14S-diHDHA (9).
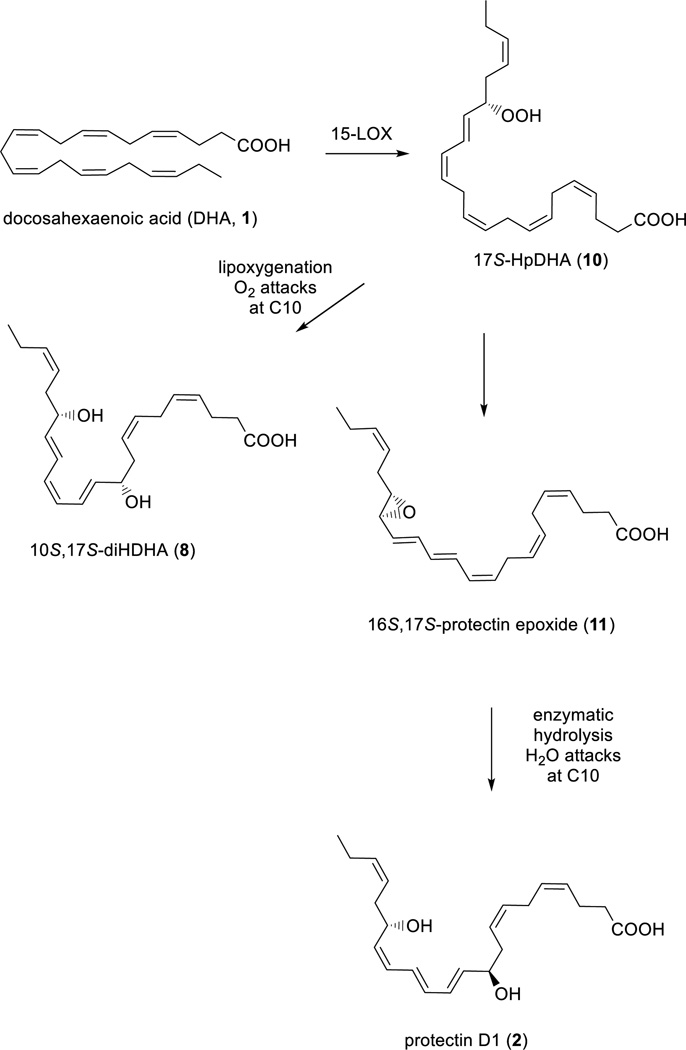
All of these are biosynthesized during the resolution phase of the acute-inflammatory response and have enabled new research areas related to many disease states associated with inflammation.7,8 For example, were cently reported additional evidence for the biosynthesis9 of protectin D1 (2) that confirmed earlier results.10 A human 15-lipoxygenase (15-LOX) type Imediated pathway in combination with enzymatic hydrolysis produces protectin D1 (2) via the two intermediates 17S-hydroperoxide intermediate (10) and16S,17S-protectin epoxide (11) (Scheme 1).9,10 Alternatively, molecular oxygen addition at C10 of 10 yields the double di-oxygenation product 10S,17S-diHDHA (8),2a,10,11 also denoted protectin DX.11
Scheme 1.
Outline of the biosynthetic pathways of protectin D1 (2) and the isomeric double di-oxygenation product 10S,17S-diHDHA (8).
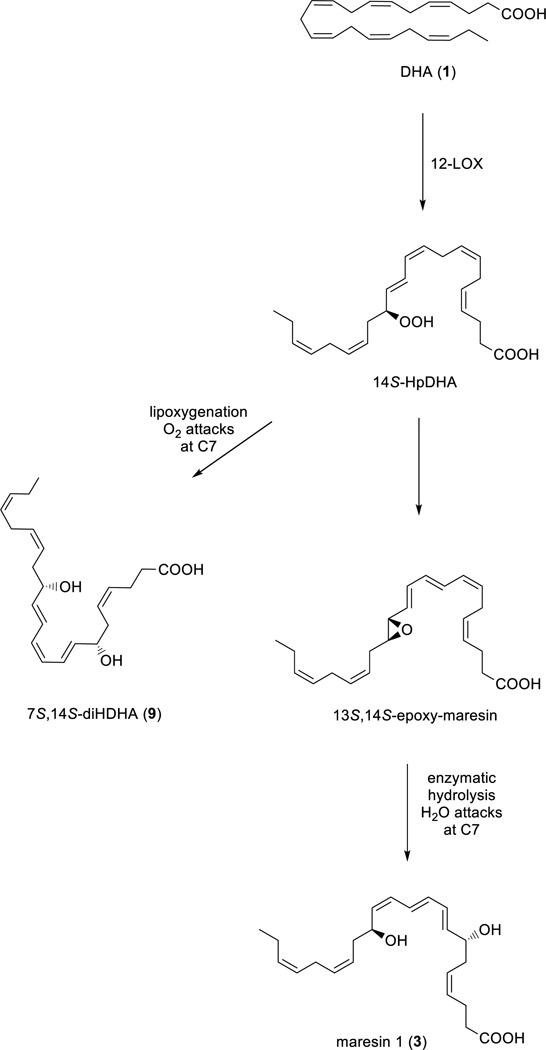
The biosynthesis of maresin 1 (3) and its double di-oxygenation product 7S,14S-diHDHA (9) is carried out by the action of 12-lipoxygenase (12-LOX),12 and shares several steps with the biosynthesis outlined in Scheme 1. Both pathways result in the formation of two chiral, secondary alcohols separated by the six carbon atoms of anE,E,Z-triene moiety, see Scheme 2.
Scheme 2.
Outline of the biosynthetic pathways of maresin (3) and the isomeric double di-oxygenation product 7S,14S-diHDHA (9).
These docosanoid-based families of the different SPMs display high structural complexity due to the presence of several stereogenic centers, both in the form of chiral, secondary alcohols and conjugated E- and Z-double bonds,7 reflecting their biochemical origins, functions and stereo specific bioactions towards different G-coupled receptors (GPCRs).13 Moreover, the individual SPMs often exhibit identical chromatographic properties, resulting in incomplete separation of peaks.2,14 These properties are also found with other lipid mediators, such as the pro-inflammatory mediator leukotriene (LTB4, 12),that displays similar chromatographic behavior to its double lipoxygenation product 5S,12S-diHETE.15 Of the two, it is only LTB4 (12), see Figure 2, that carries potent biological actions.15
Figure 2.
Chemical structures of leukotriene B4 (LTB4,12) and PD1n-3 DPA (13).
Therefore, the unambiguous identification of closely related isomers of the distinct SPM families is of fundamental importance to the elucidation of their chemical structures, but also to the understanding of their biosynthetic origins and function in numerous biological processes. Of particular importance to the structural elucidation of SPMs products biosynthesized during inflammatory processes, are their isomeric double di-oxygenation products.2a,7,11 Two examples are 10S,17S-diHDHA (8)2 and 7S,14S-diHDHA (9),3 see Figure 1 for chemical structures. The double di-oxygenation products 8 and 9 also contain two chiral, secondary alcohols, but the double bond geometry of these two compounds constitute an E,Z,E-triene moiety amid the two alcohols. Of note, these oxygenated lipids display distinct activities and potencies from the SPMs.11,16 Isomerization of the chemically sensitive E,E,Z-triene, found in both protectin D1 (2) and maresin 1 (3), to their corresponding E,E,E-triene diastereomers, is also observed when analyzing SPMs produced in vivo by inflammatory exudates.7 Also, the n-6 PUFA arachidonic acid is biosynthetically converted into anti-inflammatory oxygenated compounds,17 such as the lipoxygenase interaction products termed lipoxin A4 (6) and lipoxin B4 (7), see Figure 1.5,6 Moreover, novel n-3 docosapentaenoic acid derived SPMs have also been reported,14 such as PD1n-3 DPA (13) (Figure 2), which is biosynthesized, via a pathway analogous to that described above for protectin D1 (2).18 PD1n-3 DPA also carries potent pro-resolution and anti-inflammatory actions.18 Hence, the isolation and structural elucidation of novel SPMs with such biologically fascinating actions is of considerable interest in the biomedical research community.19 Towards these ends, advances in LC-MS/MS metabololipidomics techniques have emerged.20,21 In parallel, an approach that may facilitate the exact structural elucidation of structurally challenging SPMs is chemical derivatization, and some derivatization protocols have been reported for the identification and structural elucidation of PUFAs.22 However, harsh derivatization conditions not suitable for the SPMs are employed, and the intensity of the ions obtained from the mass spectra is often low.
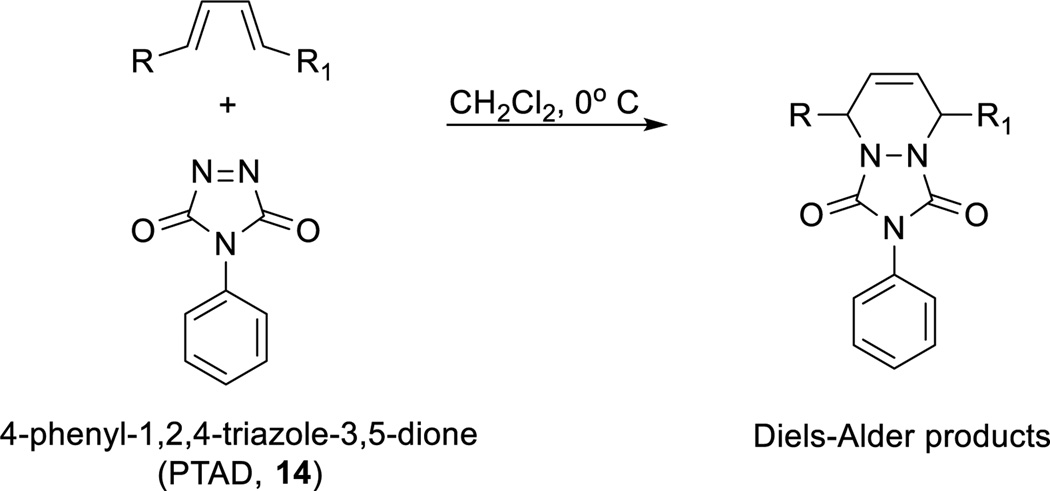
Given the small amounts of material and the chemically sensitive structures of the SPMs, which owes to the presence of two secondary allylic alcohols in conjugation with labile E,E,Z-trienes orE,E,Z,E-tetraenes, caution has to be exercised when deciding on an appropriate derivatization agent. As outlined above, the in vivo biosynthetic pathways of DHA (1) to protectin D1 (2) and maresin (3), yield both product mixtures with E,E,Z- or E,Z,E-trienegeometries of the conjugated double bonds with different bioactions and biological potencies. These geometrical differences caught our interest, since a diene with an E,E-configuration is far more reactive in the Diels-Alder cycloaddition reaction than an E,Z-diene.23 We were also aware of the guiding principles of click-chemistry, outlined by Sharpless and co-workers for cycloaddition reactions,24 that have been applied in the development of synthetic protocols.25,26 For useful applications of the Diels-Alder cycloaddition reaction towards the structural elucidation of SPMs, a highly reactive dienophile must be employed. The 4-substitued-1,2,4-triazole-3,5-diones are examples of such dienophiles.27,28 In particular, the safe, easy-to-handle, commercially available solid, 4-phenyl-1,2,4-triazole-3,5-dione (PTAD, 14), is a premier example of such a highly reactive dieneophile.27
Only dienes that can adopt the s-cis conformation undergo the Diels-Alder reaction (Scheme 3). Any substitution pattern favoring the s-trans conformation, slows down the Diels-Alder cycloaddition, as is the case for cis-1,2-disubstituted dienes.23 Hence, we envisioned that the double di-oxygenation products 8 and 9, due to theirE,Z,E-trienes, would be far less reactive in a Diels-Alder reaction with 14. Given the importance of specific double bond geometries to biological actions and potencies,3,10 our investigations towards development of a protocol for the selective identification of SPMs from their biosynthetic pathway markers are reported.
Scheme 3.
General outline of the Diels-Alder reaction between a 1,4-disubstituted E,E-olefin, present in SPMs, and 14.
Results and Discussion
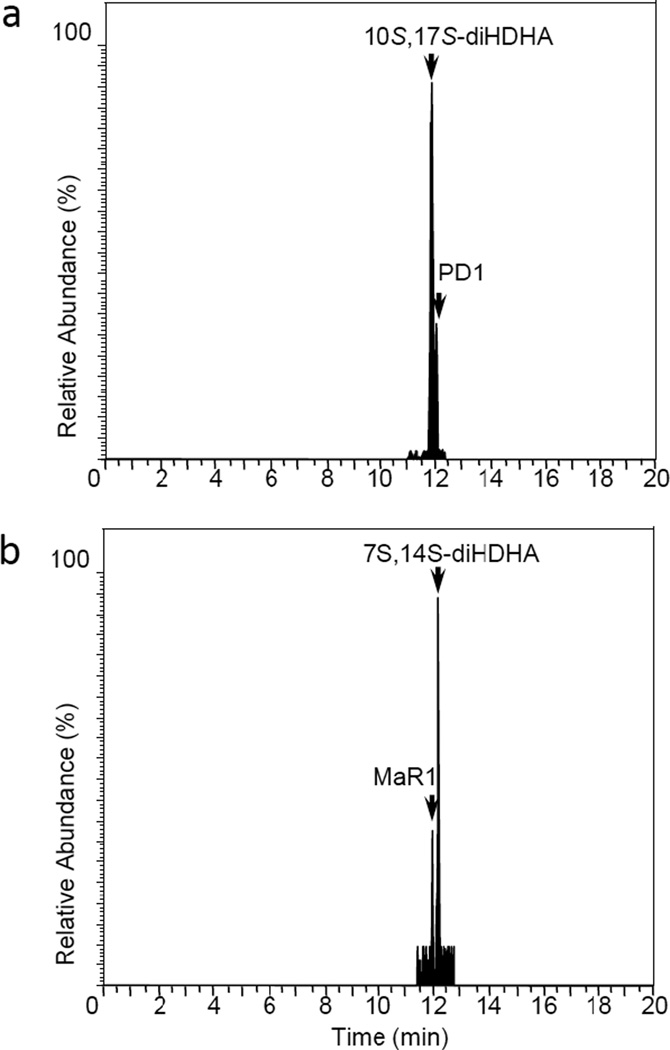
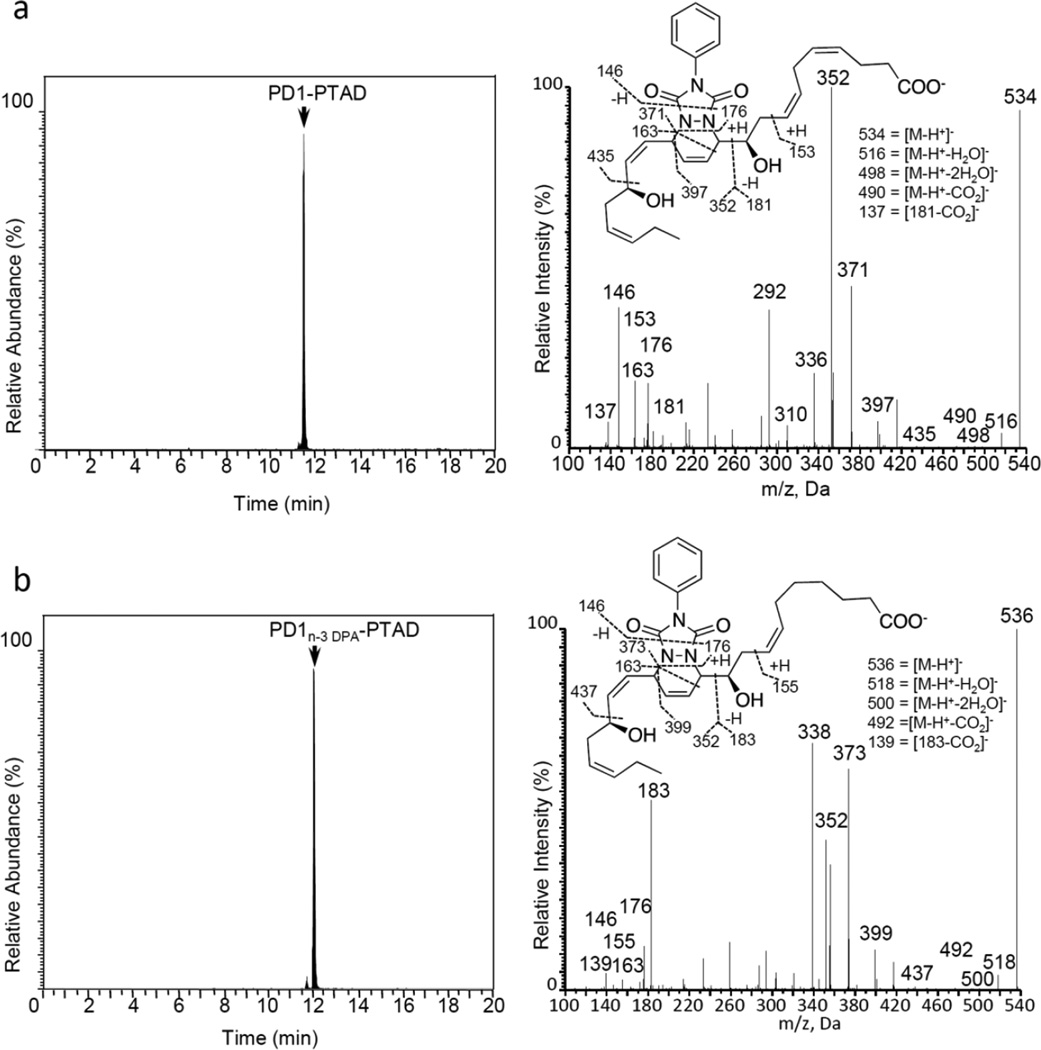
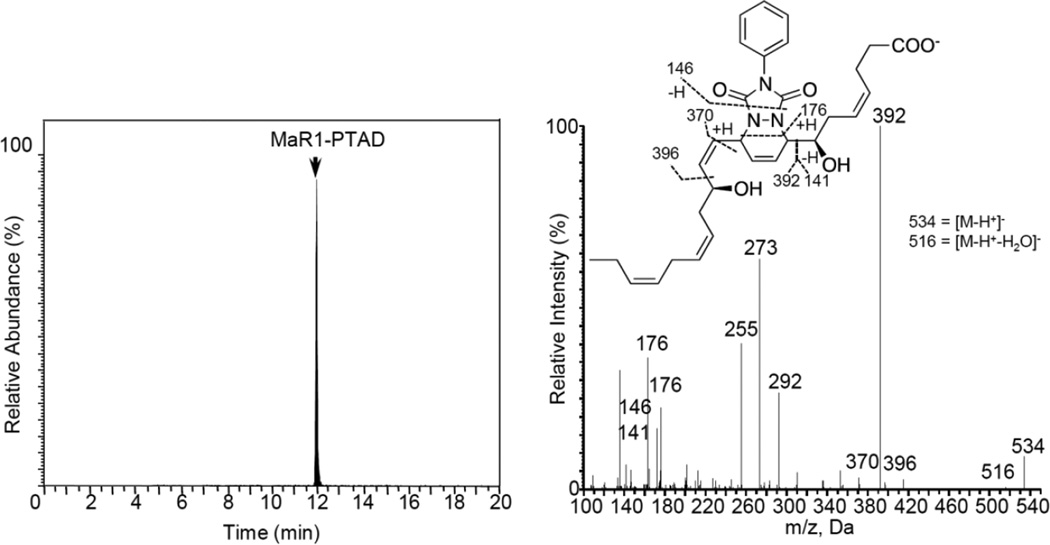
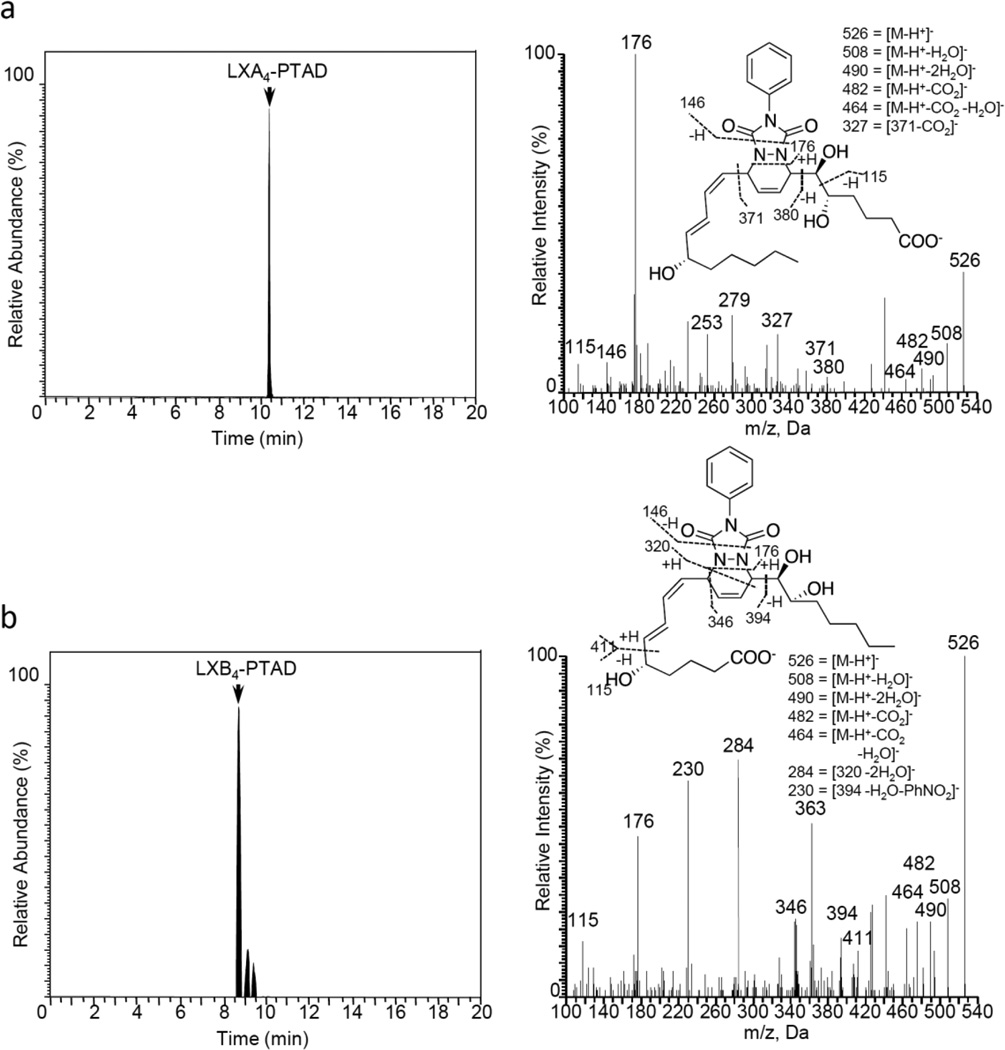
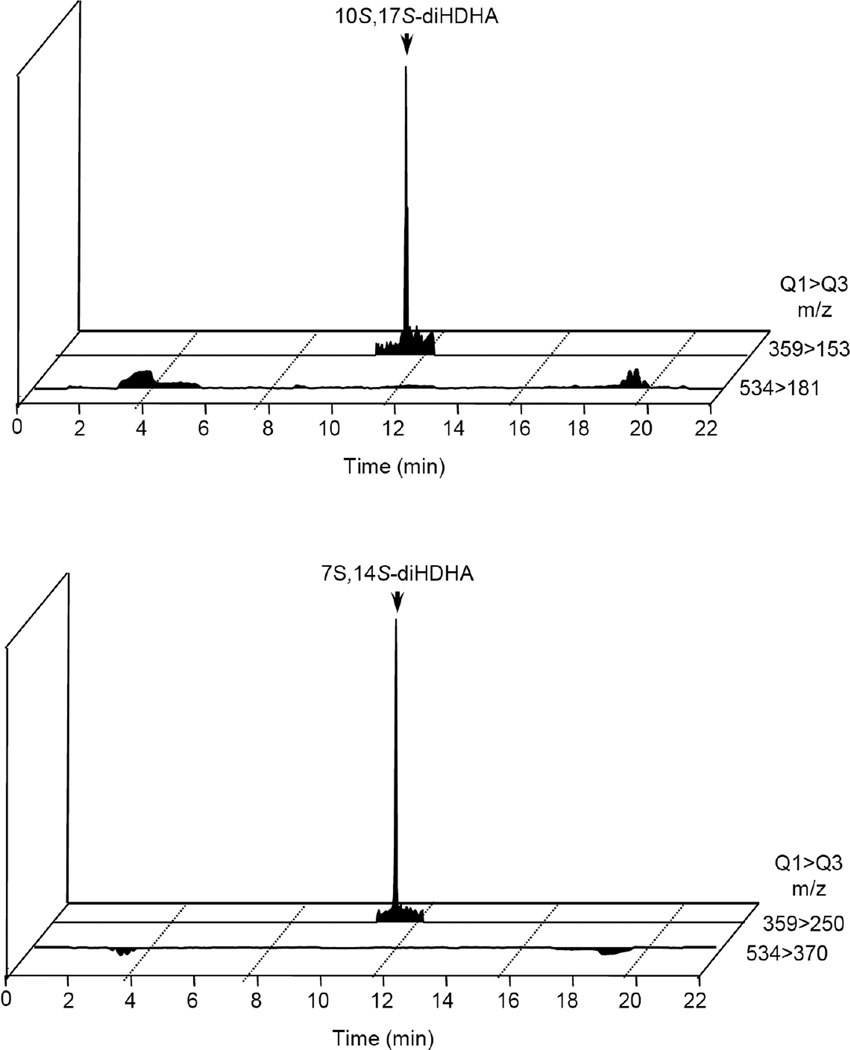
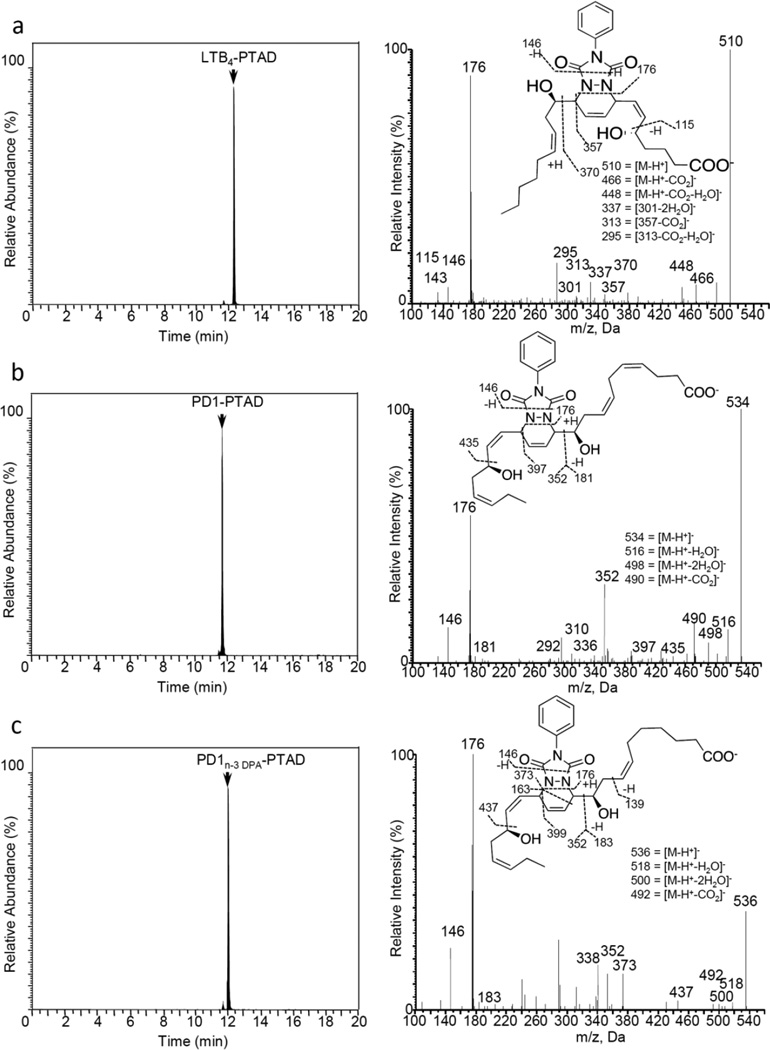
First, E. coli inoculation in mice was performed as reported earlier,29 evoking a self-limited host response in which polymorphonuclear leukocyte (PMN) infiltration reached a maximum at approximately 12 h. After this period, the mice were euthanized and peritoneal exudates collected as reported.29 In Figure 3, the chromatograms of the two DHA-derived SPMs protectin D1 (2) and maresin 1 (3), together with their respective double di-oxygenation isomers 10 and 11, are depicted. To be able to develop a protocol for the selective identification of SPMs we employed validated synthetic protectin D1 (2)30 and its congener PD1n-3 DPA (13)18 that were each separately reacted with 4-phenyl-1,2,4-triazole-3,5-dione (14) and separately profiled using LC-MS/MS-based lipid mediator metabololipidomics (see Experimental section for details).21 The results are depicted in Figure 4. For the two C22-derived SPMs, protectin D1 (2) and PD1n-3 DPA (13), the chromatographic behavior and the respective MS/MS spectra indicated the presence of Diels-Alder products formed between 2 and 14 (Figure 4A), but also between 13 and 14 (Figure 4B). Distinct m/z values for ions assigned to the PTAD-derivatized products of both 2 and 13 were observed at m/z= 534 and 536, respectively. The MS/MS spectra for the Diels-Alder product of 2 (PTAD-PD1) displayed characteristic fragmentation patterns with the following fragments assigned: m/z= 516 [M-H+-H2O]−, m/z= 498 [M-H+-2H2O]− and m/z= 490 [M-H+-CO2]−, see Figure 4A. The PTAD-derivatized products exhibited prominent molecular ion peaks and diagnostic fragment ions due to the double bond position in the SPM 2, as well as the PTAD-moiety. In particular, m/z= 397, m/z= 371 [M-H+-CO2-PhCNO]− and m/z= 352 were diagnostic, see Figure 4A for interpretations. A similar pattern was observed for the PTAD-PD1n-3DPA product at m/z values 518, 500, 492,399, 373 and 352, respectively (Figure 4B). The fragment at m/z= 352 is the same for the PTAD-derivatized products of both 2 and 13, as the C15–C22 fragment is identical for both SPMs. Synthetic material of the isomer of 2, namely maresin 1 (3), reacted just as smoothly with 14, giving rise to m/z= 534 [M-H+]− and m/z= 514 [M-H+-H2O]−, see Figure 5. The loss of the C1–C7 fragment was prominent in the MS-spectra of the MaR1-PTAD product, m/z = 392. The method was conveniently extended to the two DHA-derived SPMs resolvin D1 (RvD1, 4) and resolvin D2 (RvD2, 5), Figure 6. The MS/MS-spectra of the PTAD-derivatives of 4 and 5 also showed diagnostic m/z values. For RvD1 (4), at m/z= 550 [M-H+]− and m/z= 506 [M-H+-CO2]−, and for RvD2 (5) at m/z= 550 [M-H+]−, m/z= 532 [M-H+-H2O]− and m/z = 506 [M-H+-CO2]−. In both cases, the retro-Diels-Alder products were observed as the base peaks at m/z = 176. Moreover, the trihydroxy PTAD-derivatives of LXA4 (6) and LXB4 (7) showed a similar fragmentation patterns for both regioisomeric SPMs. The relevant m/z values were observed at 526 [M-H+]−, 508 [M-H+-H2O]−, 490 [M-H+-2H2O]−, 482 [M-H+-CO2]− and 464 [M-H+-CO2-H2O]− (Figure 7). To our delight, no Diels-Alder reaction took place when reacting 14, in individual experiments, with the two double di-oxygenation products 10S,17S-diHDHA (8) and 7S,14S-diHDHA (9), see Figure 8.These experiments were repeated in the presence of equimolar amounts of protectin D1 (2) with the only observed Diels-Alder products observed being those derived from 2. No PTAD-derivatives of 8 and 9 were detected. Hence, as elective identification of specialized pro-resolving lipid mediators as their PTAD-derivatives, from mixtures with their biosynthetic double di-oxygenation isomers 8 and 9, was achieved. This protocol was further extended to in vivo biosynthetic material. To the mouse exudate extract obtained as described above, an excess of 4-phenyl-1,2,4-triazole-3,5-dione (PTAD, 14) was added. Then, as proof of principle, PTAD-derivatives of both protectin D1 (2) and its congener PD1n-3 DPA (13), were investigated using multiple reaction monitoring (MRM)-mode. The results are depicted in Figure 9. These results and fragmentation patterns are in accord with the discussion outlined above for Figure 4A and Figure 4B, respectively. Moreover, we were also able to detect the Diels-Alder product LTB4-PTAD after reaction of 14 with LTB4 (12), see Figure 9A.31 Of importance, no formation of a Diels-Alder product from the reaction of 14 with the double lipoxygenation product 5S,12S-diHETE, was observed. Overall, the results depicted in Figure 9d emonstrate the utility of this easy-to-use protocol on materials present in picogram levels in biological samples.
Figure 3.
Chromatographic separation of protectin D1and maresin 1 from their double di-oxygenation isomers. Mice were administered E. coli (107 CFU), exudates obtained 12h later and the products extracted and subject to LC-MS/MS metabololipidomics. MRM chromatograms for exudates: (a) Protectin D1 (PD1, 2) and 10S,17S-diHDHA (8) (m/z 359->153). (b) Maresin 1 (MaR1, 3) and 7S,14S-diHDHA (9) (m/z 359->250). Results are representative of n=12 mouse exudates.
Figure 4.
Characterization of the di-hydroxy PTAD-derivatives of docosahexaenoic acid-and n-3 docosapentaenoic acid-derived mediators protectin D1 (PD1, 2) and PD1n-3DPA (13). Products were analyzed using LC-MS/MS metabololipidomics. MRM chromatograms obtained from using (a) m/z 534->181 and (b) m/z 536 ->183. Results are representative of 3 independent preparations.
Figure 5.
Characterization of the di-hydroxy PTAD-derivative of maresin 1 (MaR1, 3). Products were analyzed using LC-MS/MS metabololipidomics. MRM chromatograms obtained using m/z 534->370. Results are representative of 3 independent preparations.
Figure 6.
Characterization of the tri-hydroxy PTAD-derivatives of docosahexaenoic acid-derived mediators resolvin D1 (RvD1, 4) and resolvin D2 (RvD2, 5). Products were analyzed using LC-MS/MS metabololipidomics. MRM chromatograms obtained usingm/z 550->141. Results are representative of 3 independent preparations.
Figure 7.
Characterization of the tri-hydroxy PTAD-derivatives of arachidonic acid derived-mediators lipoxin A4 (LXA4, 6) and lipoxin B4 (LXB4, 7). Products were analyzed using LC-MS/MS metabololipidomics. MRM chromatograms obtained using m/z 526->115. Results are representative of 3 independent preparations.
Figure 8.
4-Phenyl-1,2,4-triazole-3,5-dione (PTAD, 14) reacts selectively with mediators carrying E,E- conjugated double bonds and not their double di-oxygenation isomers. Products were analyzed using LC-MS/MS metabololipidomics. Results are representative of 3 independent preparations.
Figure 9.
Chromatographic separation of PTAD-products, obtained when reacting 14 with SPMs and their double di-oxygenation isomers. Mice were administered E.coli (107 CFU), exudates obtained 12h later and products analyzed using LC-MS/MS metabololipidomics. MRM chromatograms for exudates: (a) Product of leukotriene B4 (LTB4, 12) and PTAD (14) (m/z 510->176). (b) Protectin D1 (PD1, 2) and PTAD (14) (m/z534->181), (c) PD1n-3DPA (13) and PTAD (14) (m/z 536->183). Results are representative of n = 12 mouse exudates.
Conclusions
To summarize, herein we present a straightforward protocol for the selective identification of pro-resolving and anti-inflammatory oxygenated polyunsaturated fatty acids, named specialized pro-resolving lipid mediators. This protocol is based on the guiding principles of click-chemistry, amenable for biological conditions,32 that we have now extended to the Diels-Alder reaction for the selective identification of SPMs. Distinct MS/MS fragmentation patterns were observed for all cycloaddition products obtained upon reaction of the dienophile 4-phenyl-1,2,4-triazole-3,5-dione (14) with different SPMs. In contrast, the non-resolving double di-oxygenation compounds were unreactive towards this reagent. For challenging and difficult-to-separate, co-eluting compounds, biosynthesized simultaneously with the SPMs, this fast, reproducible and easy-to-use protocol facilitates the identification of novel SPMs using LC-MS/MS based metabololipidomic techniques. Of note, the MRM chromatograms depicted in Figures 3 – 7 and 9, contain the entire structures of each individual SPM-derivative, attesting to the chemical stability of these Diels-Alder products. The protocol can easily be extended beyond the analysis of novel SPMs to other natural occurring olefinic compounds and their further metabolites that can be present at very low levels in biological samples. Such efforts have indeed been reported after we initiated our investigations on the SPMs.33
Experimental section
Synthetic materials of resolvin D1 (RvD1, 4), resolvin D2 (RvD2, 5), lipoxin A4 (LXA4, 6), lipoxin B4 (LXB4, 7), 5S,12S-diHETE, 10S,17S-diHDHA (8),7S,14S-diHDHA (9) and leukotriene B4 (12) were obtained from Cayman Chemicals, while protectin D1 (2), maresin 1 (MaR1, 3), and PD1n-3DPA (13) were obtained by total synthesis as in ref.18,30,34 4-Phenyl-1,2,4-triazole-3,5-dione (14), dry MeOH and CH2Cl2 were all purchased from Sigma-Aldrich.
Derivatization of SPM with 4-phenyl-1,2,4-triazole-3,5-dione (14)
A 5% molar excess of a stock solution of 14 (83 mg/mL in CH2Cl2) was added to an EtOH solution containing the SPM, the double di-oxygenation product or the exudate of interest at room temperature. The vial was then sealed and kept on ice for 30 minutes in the dark. The solvent mixture was evaporated under a gentle stream of nitrogen, the reaction mixture re-dissolved in 100 µL of CH2Cl2, and then filtered through a 0.1 cm plug of SiO2–gel in a Pasteur pipette by eluting with 500 µL of hexane:EtOAc (1:1). Solvents were evaporated again and the residue re-dissolved in 100 µL of MeOH. This sample was analyzed by LC-MS/MS using lipid mediator metabololipidomics.21 The same procedure was employed when an inflammatory exudate was analyzed. The derivatization with 14 can be monitored either visually, since the deep red color of the PTAD-reagent 14 disappears when the reaction is complete. Alternatively, the reaction with an individual SPM can be monitored by UV-spectroscopy and the change in the intensity of the λmax-value of the SPM, before and after addition of 4-phenyl-1,2,4-triazole-3,5-dione (14). Observation of the complete disappearance of the λmax-peak assures a full conversion into the Diels-Alder product.
Microbial-initiated inflammation in vivo by E. coli inoculation
Male FVB mice (6 to 8 weeks old) purchased from Charles River Laboratories were fed ad libitum Laboratory Rodent Diet 20–5058 (Lab Diet, Purina Mills). Animal experimental procedures were approved by the Standing Committee on Animals of Harvard Medical School (protocol no. 02570) and complied with institutional and US National Institutes of Health (NIH) guidelines. Mice were given vehicle together with live E. coli (serotype O6:K2:H1; 107 CFU) or saline injections (mock infection) i.p. After 12 h, mice were euthanized and the peritoneal exudate was collected, see reference 29 for details. The local eicosanoid levels and identification PTAD-products were assessed by targeted lipid mediator metabololipidomics as described below.
Lipid Mediator Metabololipidomics.21
The LC-MS/MS system included a Shimadzu LC-20AD HPLC and a Shimadzu SIL-20AC autoinjector (Shimadzu) paired with a QTrap 5500 (ABSciex) coupled to a Poroshell 120 EC column (100 mm × 4.6 mm × 2.7 µm; Agilent) maintained at 50 °C using a column oven (ThermaSphere TS-130; Phenomenex) employed for identification and quantification. For product profiling the following mobile phase was employed: MeOH/H2 O/acetic acid of 55:45:0.1 (vol:vol:vol) with a gradient ramped to 80:20:0.1 (vol:vol:vol) for 8 min, that was held for 3 min and then ramped to 98:2:0.1 (vol:vol:vol) for the next 0.1 min and maintained at 98:2:0.1 (vol:vol:vol) for 4 min, with a flow rate maintained at 0.6 mL/min. The QTrap 5500 was operated in negative ionization mode using scheduled multiple reaction monitoring coupled with information-dependent acquisition (IDA) and an enhanced product ion scan (EPI).
Acknowledgments
The Norwegian Research Council (KOSK-II 197704/V30 and the Leiv Eriksson travel scheme) are gratefully acknowledged for financial support to T. V. H. J. D. was supported by the National Institutes of Health GM Grant PO1GM095467 awarded to C. N. Serhan. J. D. was also supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant number 107613/Z/15/Z).
Contributor Information
Jesmond Dalli, Email: j.dalli@qmul.ac.uk.
Charles N. Serhan, Email: cnserhan@zeus.bwh.harvard.edu.
References
- 1.Calder PC. J. Nutr. 2012:592S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 2.(a) Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. J. Exp. Med. 2002;196:1025. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8491. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ariel A, Pin-Lan L, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. J. Biol. Chem. 2005;280:43079. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 3.(a) Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. J. Exp. Med. 2009;206:15. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. FASEB J. 2012;26:1755. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. J. Biol. Chem. 2003;278:14677. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 5.(a) Serhan CN, Hamberg M, Samuelsson B. Biochem. Biophys. Res. Commun. 1984;118:943. doi: 10.1016/0006-291x(84)91486-4. [DOI] [PubMed] [Google Scholar]; (b) Serhan CN, Hamberg M, Samuelsson B. Proc. Natl. Acad. Sci. U.S.A. 1984;81:5335. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang N, Serhan CN. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:163. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Serhan CN, Petasis NA. Chem. Rev. 2011;111:5922. doi: 10.1021/cr100396c. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabas I, Glass CK. Science. 2013;339:166. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aursnes M, Tungen JE, Collas RA, Vlasakov I, Dalli J, Serhan CN, Hansen TV. J. Nat. Prod. 2015;78:2924. doi: 10.1021/acs.jnatprod.5b00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. J. Immunol. 2006;176:1848. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 11.Chen P, Fenet B, Michaud S, Tomczyk N, Véricel E, Lagarde M, Guichardant M. FEBS Lett. 2009;583:3478. doi: 10.1016/j.febslet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Dalli J, Zhu M, Vlasenko NA, Haegström JZ, Petasis NA, Serhan CN. FASEB J. 2013;27:2573. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN, Chiang N. Curr. Opin. Pharmacol. 2013;13:632. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalli J, Colas RA, Serhan CN. Sci. Rep. 2013;3:1940. doi: 10.1038/srep01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuelsson B. J. Biol. Chem. 2012;287:10070. doi: 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan CN, Friedman G, Yang R, Karamanov S, Belayev LS, Bazan NG, Zhu M, Winkler JW, Petasis NA. Chem. Biol. 2011;18:976. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maderna P, Godson C. Br. J. Pharmacol. 2009;158:947. doi: 10.1111/j.1476-5381.2009.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aursnes M, Tungen JE, Vik A, Collas AR, Cheng C-Y, Dalli J, Serhan CN, Hansen TV. J. Nat. Prod. 2014;77:910. doi: 10.1021/np4009865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN. Nature. 2014;510:92. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalli J, Serhan CN, N C. Blood. 2012;120:e60. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Am. J. Physiol. Cell. Physiol. 2014;307:C39. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spitzer V. Gas Chromatograpy/mass spectrometry analysis of conjugated linoleic acid using different derivatization techniques. In: Yurawecz MP, Mossoba MM, Kramer JKG, Pariza MP, Nelson GJ, editors. Advances in conjugated linoleic acid research. Champaign: AOCS Press; 1999. pp. 110–125. [Google Scholar]
- 23.Sauer J. Angew. Chem. Int. Ed. 1966;5:211. [Google Scholar]
- 24.Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Odlo K, Høidahl EA, Hansen TV. Tetrahedron Lett. 2007;48:2097. [Google Scholar]
- 26.Hansen TV, Wu P, Fokin VV, V V. J. Org. Chem. 2005;70:7761. doi: 10.1021/jo050163b. [DOI] [PubMed] [Google Scholar]
- 27.Fringuelli F, Tatticchi A. Dienes in the Diels-Alder reaction. John Wiley & Sons, Ltd.; 1990. pp. 1–44. [Google Scholar]
- 28.(a) Young DC, Vouros P, Holick MF. J. Chromatogr. 1990;522:295. doi: 10.1016/0021-9673(90)85199-6. [DOI] [PubMed] [Google Scholar]; (b) Sebeidio JL, Juaneda P, Dobson G, Ramilison I, Martin JC, Chardigny JM. Biochem. Biophus. Acta. 1997;1345:5. doi: 10.1016/s0005-2760(97)00015-5. [DOI] [PubMed] [Google Scholar]; (c) Dobson G. J. Am. Oil Chem. Soc. 1998;75:137. [Google Scholar]; (d) Martin JT, Reaney YDL, Wesley GT. J. Am. Oil Chem. Soc. 2001;78:1083. [Google Scholar]; (e) Shah U, Proctor A, Lay JO, Jr, Moon K. J. Am. Oil Chem. Soc. 2012;89:979. [Google Scholar]
- 29.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Nature. 2012;484:524. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aursnes M, Tungen JE, Vik A, Dalli J, Hansen TV. Org. Biomol. Chem. 2014;12:432. doi: 10.1039/c3ob41902a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borgeat P, Samuelsson B. J. Biol. Chem. 1979;254:2643. [PubMed] [Google Scholar]
- 32.McKay CS, Finn MG. Chem. Biol. 2014;21:1075. doi: 10.1016/j.chembiol.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedman CJ, Wiebe DA, Dey S, Plath J, Kemnitz JW, Ziegler TE. J. Chromatogr. B. 2014;953–954:62. doi: 10.1016/j.jchromb.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tungen JE, Aursnes M, Hansen TV. Tetrahedron Lett. 2015;56:1843. [Google Scholar]