Abstract
Purpose
To determine the measure of the photopic negative response (PhNR) of the full-field electroretinogram (ERG) that exhibits the optimal level of test-retest repeatability, and examine its repeatability under different conditions using a handheld, nonmydriatic ERG system and self-adhering skin electrodes.
Methods
Multiple ERG recordings (using 200 sweeps each) were performed in both eyes of 20 normal participants at two different sessions to compare its coefficient of repeatability (CoR; where 95% of the test-retest difference is expected to lie) between different PhNR measures and under different testing conditions (within and between examiners, and between sessions).
Results
The ratio between the PhNR trough to b-wave peak and b-wave peak to a-wave trough amplitude (PhNR/B ratio) exhibited the lowest CoR relative to its effective dynamic range (30 ± 4%) when including three recordings. There were no significant changes in the PhNR/B ratio over seven measurements (4 right and 3 left eyes) at either session (P ≥ 0.100), or significant difference in its CoR between different testing conditions (P = 0.314).
Conclusion
The PhNR/B ratio was the measure that minimized variability, and its measurements using a novel handheld ERG system with self-adhering skin electrodes and the protocols described in this study were comparable under different testing conditions and over multiple recordings.
Translational Relevance
The PhNR can be measured for clinical and research purposes using a simple-to-implement technique that is consistent within and between visits, and also between examiners.
Keywords: photopic negative response, ganglion cell function, electroretinogram, glaucoma
Introduction
The photopic negative response (PhNR) of the full-field electroretinogram (ERG) is a slow negative potential following the a- and b-waves that has been reported to originate primarily from the retinal ganglion cells (RGCs) and their axons.1–4 As an objective clinical measure of neuroretinal function, this component has been used primarily to evaluate eyes with glaucoma,5–13 although studies have also investigated its changes in retinal vascular diseases,14–16 conditions causing optic atrophy,17–21 and following vitreoretinal surgery.22 Despite its potential clinical use for these conditions, a major impediment to its widespread and routine application is the difficulty of its implementation in clinical settings and its large magnitude of measurement variability.23,24
However, a handheld, portable, and nonmydriatic full-field ERG system that uses a self-adhering skin electrode array (RETeval; LKC Technologies, Inc., Gaithersburg, MD) that has recently been developed and made commercially available has the potential to overcome some of these barriers. This system provides a way for measuring the PhNR in a clinically efficient manner, due to the ease of electrode placement and recording set-up. The convenience with this device compared with current electrodiagnostic systems improves its potential for widespread implementation in clinical settings. However, it has not been established yet whether PhNR measurements obtained using this technique will have sufficient repeatability for clinical application, especially when seeking to detect slow progressive changes in conditions like glaucoma.
Previous studies have also highlighted how the measure of the PhNR has a significant impact on its repeatability.23,24 The PhNR can be measured in several different ways, either as the negative trough following the b-wave or at a fixed time point.6,20,23,24 It can also be presented either as a raw amplitude or a ratio relative to other components of the ERG response.7,11,18,19,23,24 However, there has been no agreement within the studies to date as to which PhNR measure is characterized by having the lowest magnitude of variability.23,24 In the studies that have compared the repeatability of these various measures,23–25 the extent of repeatability has been presented as a percentage of its mean value in normal subjects. This however is a significant limitation because the mean value does not always represent the effective dynamic range (EDR) of a parameter, and may underestimate the true magnitude of variability relative to the range over which the measure is effective. For instance, the mean value of the PhNR measured from the b-wave peak-to-trough (PT) is substantially larger than that of the measured from baseline-to-trough (BT), despite the EDR being the same. Normalizing the variability of the PhNR measure to the mean value rather than the EDR can therefore artificially reduce its variability. Previous studies have recognized this when seeking to compare different clinical parameters26 or devices,27 and have therefore normalized the magnitude of variability by the EDR of the measure. For the PhNR, it is expected that the EDR will lie between the value of normal participants and the floor amplitude of zero, based on experimental findings previously.1 We have also demonstrated recently that increasing the number of sweeps obtained substantially decreased the level of intrasession variability of the PhNR,28 but it remains to be determined whether this is the case for its intersession variability.
This study therefore sought to determine the test-retest repeatability of the PhNR using the novel handheld ERG system under various testing conditions (including both intra- and intersession) in normal participants, and to determine the optimal measure of the PhNR for minimizing the degree of variability after normalizing its magnitude of repeatability by its EDR. It also sought to determine whether increasing the number of recordings could improve the repeatability of the PhNR.
Methods
All protocols and methods described in this study were approved by the Human Research Ethics Committee of the Royal Victorian Eye and Ear Hospital and were conducted in adherence with the Declaration of Helsinki. Informed consent was obtained from all participants following a thorough explanation of all test procedures.
Participants
In this study, healthy participants without any current or previous history of ocular disease in both eyes were included. Participants were also excluded if they had any systemic disease (e.g., diabetes) or were taking any medication known to affect retinal function (e.g., hydroxychloroquine). They were also excluded if they had any physical or mental impairment that prevented them from participating in this study and/or providing informed consent.
Full-Field Electroretinography
All ERG recordings were performed using the portable, handheld RETeval visual electrodiagnostic device (LKC Technologies), which uses a 60-mm diameter Ganzfeld dome with a soft eyecup for visual stimulation. Responses were recorded using a disposable, self-adhering skin contact electrode array (Sensor Strip; LKC Technologies) that incorporates the active (positive), ground, and reference (negative) electrode in a single strip. Following skin preparation with an isopropyl alcohol wipe, the electrodes were placed on the inferior orbital rim at least 2 mm below the lid margin with the active electrode positioned below the pupil center. Once connected, the device determines if excessive electrode noise (including power line interference) is present, by computing the standard deviation (SD) of the electrical response between 48 and 186 Hz. All tests were performed only when the SD × 2 × √2 of this electrical response did not exceed 55 μV (an internal requirement by the device), and further skin preparation was performed to achieve this. Electrical responses were amplified and digitized to a resolution of 24 bits at a sampling rate of 1.95 kHz.
Visual stimulation was performed using red flashes (peak wavelength, 635 nm) of 4 ms in duration presented against a blue background (of 10 cd/m2, peak wavelength 465 nm; determined to be the optimal stimulus type based on a previous study29) in a similar manner to previous studies.6,23 The device uses a built-in infrared camera that measures the pupil size in real-time and adjusts the flash luminance (cd·s/m2) continuously with each flash to deliver a constant retinal illuminance throughout the measurement using the following formula: photopic flash retinal illuminance (Td·s) = photopic flash luminance (cd·s/m2) × pupil area (mm2). For this study, the stimulus intensity was set at 58 Td·s to provide an equivalent flash luminance of 1.50 cd·s/m2 with a 7-mm diameter pupil used in previous studies. The stimuli were presented at a rate 3.43 Hz to enable reduction of any power line interference through signal averaging. A total of 200 sweeps were obtained for each measurement, each of which took approximately 60 seconds.
Study Design and Testing Procedure
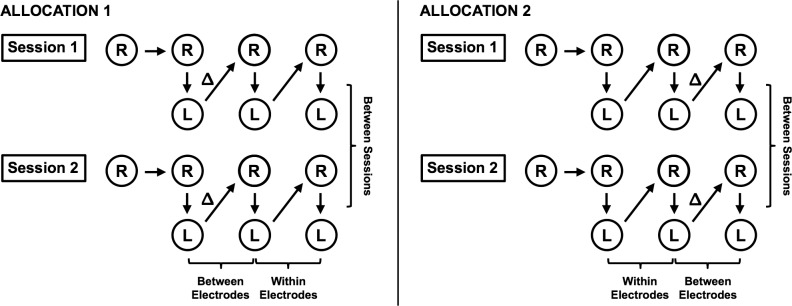
As illustrated in Figure 1, ERG recordings of both eyes were performed at two different sessions spaced, 1 to 3 weeks apart. At each session, a measurement was first performed on the right eye, followed by three measurements of each eye alternating between each (with the right eye always tested first). One investigator of this study (ZW) placed the electrodes at the start of each session and performed all the measurements. To examine the effect of electrode placement and between-examiner variability, the electrodes were replaced with a new set by another study investigator (XH; who was masked to the placement of the electrodes by the other investigator) following at least one or two measurements in both eyes (“Δ” in Fig. 1). Subsequent measurements were performed without any further changes to electrode placement. The time point for the electrode switch was alternated between consecutive participants (to provide a more equivalent comparison between the test-retest repeatability of the measurements within and between examiners), but the order remained the same for each participant on the second session. In this study, the additional measurement performed on the right eye was included to examine whether any systematic change occurs between the first and second measurement. Excluding this first measurement, 600 sweeps in total were collected over the three measurements for each eye at each session.
Figure 1.
Illustration of the testing procedure used for measuring the repeatability of the photopic negative response using the full-field ERG in this study. One measurement of the right eye (R) was first performed, followed by three measurements of both the right and left (L) eyes. The electrodes were replaced following either the first or second measurement (Δ) to allow within electrode and between electrodes repeatability to be examined.
Signal Processing
Analyses of the recorded signals were performed according to the manufacturer's specifications. In short, a zero-phase, 1-Hz high-pass filter was applied to reduce electrode drift and offset, while preserving waveform timing. A 100-Hz low-pass filter was also applied to smooth the traces and remove high-frequency fluctuations. Sweeps with amplitudes exceeding ± 1 mV were considered as outliers and were removed. The final trace was derived by calculating the interquartile mean (excluding the highest and lowest 25%) of the responses from the remaining sweeps at each time sample.30
Outcome Measures
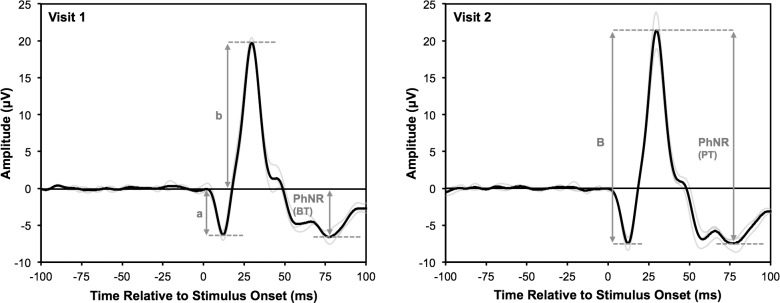
The parameters of the ERG waveform analyzed in this study are shown in Figure 2. The a- and b-waves were considered to be the first negative trough (between 0 and 20 ms post-stimulus onset) and positive peak (between 21 and 55 ms post-stimulus onset), respectively. The amplitude of the b-wave was measured either from the baseline to b-wave peak (b-wave amplitude) or from the a-wave trough to b-wave peak (B-wave amplitude). The PhNR trough was considered to be the most negative trough between 55 and 100 ms post-stimulus onset, to avoid including the trough between the b-wave and i-wave peaks when the PhNR is reduced in eyes with glaucoma. The PhNR was measured either from baseline (BT) or from the b-wave peak (PT), and both of these measures were also expressed as a ratio relative to the b-wave and B-wave amplitude, respectively (BT/b and PT/B ratio, respectively).
Figure 2.
Parameters of the ERG waveform measured in this study, illustrated on the measurements from the right eye (excluding the first measurement) of one participant over the two sessions, representative of the measurements obtained in this study; individual traces are shown (light gray lines), and all three traces were averaged (black line). The a- and b-wave amplitudes (“a” and “b,” respectively) were measured relative to the baseline, and the b-wave peak to a-wave trough was considered the b-wave amplitude (“B”). The PhNR was measured either from the baseline (BT) or to the b-wave peak (PT).
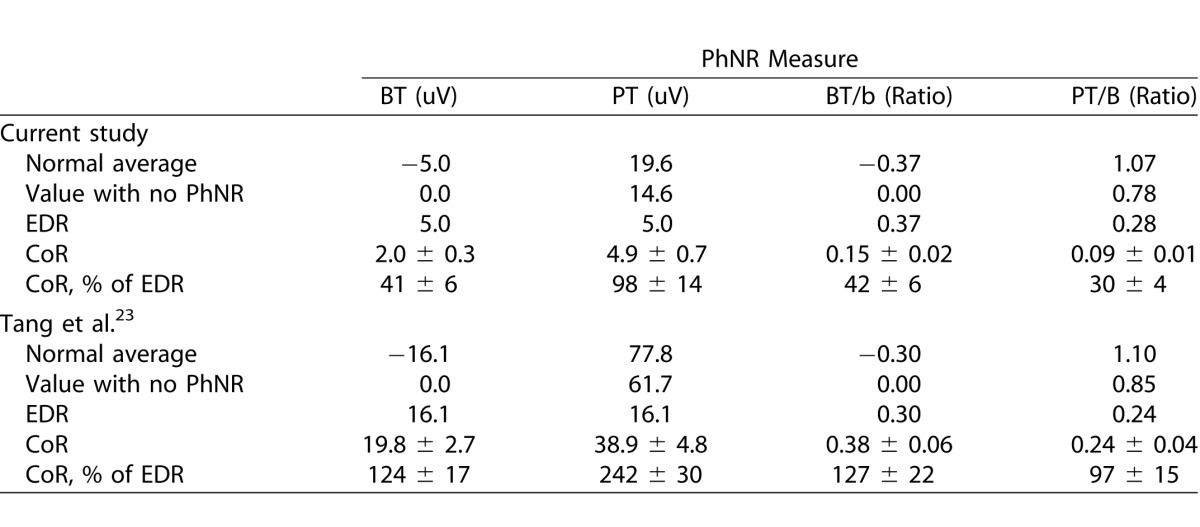
In order to compare the repeatability of the different PhNR measures in this study, we normalized the magnitude of test-retest repeatability by the EDR of the measure. We considered the EDR of a PhNR measure as the difference between (1) the average of the PhNR measure for all the normal participants, and (2) the average of the PhNR measure when the trough was considered to be at its floor value (having an amplitude of zero) for all the normal participants; see Table for the calculations of the EDR for all the PhNR measures in this study.
Table.
Coefficient of Repeatability Relative to the Effective Dynamic Range of Different Outcome Measures for the Photopic Negative Response; Values are Shown Either as a Mean or Mean ± Standard Error of Mean
Statistical Analysis
Generalized estimating equation (GEE) models were used for the analyses in this study due to the hierarchical and repeated-measures nature of the data. An exchangeable correlation structure was used as the type of covariance matrix, which specified homogenous correlations between the two eyes from each participant and repeated measurements within the same and between the two sessions. Robust estimates of the standard errors were used for the parameters examined, and a Wald χ2 test was used to test the significance. The level of significance during pairwise comparisons between multiple conditions, sweeps, and measures were adjusted using a Bonferroni correction. A normal distribution was specified when raw values were analyzed and a Tweedie distribution was specified when the absolute test-retest difference were analyzed.
In this study, the coefficient of repeatability (CoR) of the PhNR/B ratio was obtained from the mean value of the absolute test-retest difference of the PhNR/B ratio from the GEE models, to allow the correlations within the data to be accounted for when estimating this value. The CoR represents a value where 95% of the test-retest difference is expected to lie, and is calculated by multiplying the SD of measurement (which is standard error of measurement ×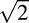 , when two measurements are obtained) by 1.96.31 Based on probability theory, the SD of measurement differences that follow a normal distribution and with a mean of zero can also be calculated by multiplying the absolute test-retest difference by
, when two measurements are obtained) by 1.96.31 Based on probability theory, the SD of measurement differences that follow a normal distribution and with a mean of zero can also be calculated by multiplying the absolute test-retest difference by 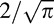 .
.
Results
A total of 20 normal participants were included in this study and were on average 50.5 ± 8.0-years old (range 39- to 66-years old). All participants performed testing on two different sessions that were on average 1.4 ± 0.5 weeks apart (range 1–3 weeks).
Test-Retest Repeatability Using Different Outcome Measures
Prior to examining the systematic change and test-retest repeatability of the PhNR under different conditions, the PhNR measure that provided the optimal level of repeatability was first determined. This was achieved by comparing the CoR of the PhNR measures derived from a final trace that averaged three recordings for each eye at each session (excluding the first measurement of the right eye, consisting of 200 sweeps), and the CoR were calculated as a percentage of the EDR (%CoR). The CoR could be calculated for all the PhNR measures because there were no significant systematic changes in these measures between the two sessions for either eye (P ≥ 0.051), and the test-retest difference was normally distributed for all the measures (Shapiro-Wilk test; P ≥ 0.216).
Among the PhNR measures, the %CoR was the highest for the PT amplitude (98 ± 14%), being significantly higher than all the other measures (P < 0.001). The PT/B ratio exhibited the lowest %CoR (30 ± 4%), although this was not significantly lower compared with the BT amplitude and BT/b ratio on pairwise comparisons (41 ± 6% and 42 ± 6%, respectively; P ≥ 0.425); these findings are outlined in the Table. In addition, the %CoR for the BT/B and PT/b ratio was ±35% and ±42%, respectively, which were higher than the PT/B ratio (data not shown).
The data from a previous study by Tang and colleagues23 (publicly available) was also reanalyzed to compare and examine the intersession test-retest repeatability of the different PhNR measures, and are shown in the Table. Similar to the findings of this study, the %CoR of the PT amplitude was the highest among all the PhNR measures (242 ± 30%), while the PT/B ratio exhibited the lowest %CoR (97 ± 15%). The PT/B ratio was therefore used as the outcome measure for the remaining analyses in this study (and henceforth referred to as the PhNR/B ratio).
Systematic Changes in the Photopic Negative Response at Each Session
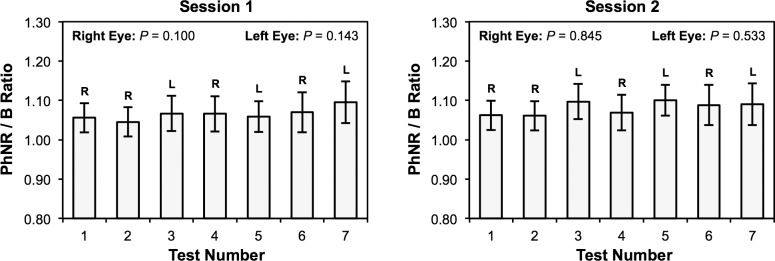
No significant differences were found in the PhNR/B ratio between the four measurements of the right eye (P ≥ 0.100) and three measurements of the left eye over the two sessions (P ≥ 0.143; Fig. 3), suggesting that the PhNR remains unchanged during multiple measurements within each session and that the CoR under different conditions could be determined.
Figure 3.
The mean ratio between the b-wave peak to photopic negative response trough amplitude and b-wave peak to a-wave trough amplitude (PhNR/B ratio) of each eye of all participants over each test during the two separate sessions. No significant differences in the PhNR/B ratio were found between tests for both the right and left eyes at both sessions (P ≥ 0.100). Error bars represent the 95% confidence interval of the mean.
Test-Retest Repeatability Under Different Conditions and Number of Sweeps
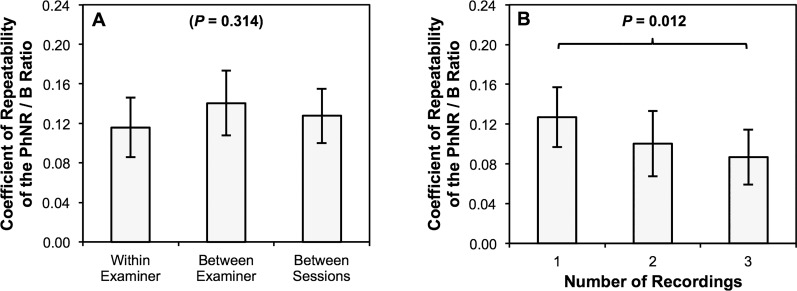
The test-retest repeatability was then compared between three different conditions, with the first measurement from the right eye excluded when examining this. The CoR of the PhNR/B ratio was not significantly different between measurements obtained: (1) using the same electrode placed by one examiner (within examiner), (2) using different electrodes placed by two different examiners (between examiner), and (3) under the same conditions and test sequence between the two sessions (between sessions; P = 0.314; Fig. 4). Pairwise comparisons also revealed that the CoR of the PhNR/B ratio was only significantly lower when comparing the measurements that included 1 and 3 recordings for deriving the final trace (P = 0.012; Fig. 4).
Figure 4.
The coefficient of repeatability of the ratio between the b-wave peak to photopic negative response trough amplitude and b-wave peak to a-wave trough amplitude (PhNR/B ratio) are shown and compared between (A) different conditions, and (B) different number of sweeps included. No significant difference in the magnitude of test-retest repeatability was observed between measurements obtained: with the same electrode placed by one examiner (within examiner), with two different electrodes placed by two different examiners (between examiner) and with the same measurements obtained at different sessions (between sessions). However, magnitude of test-retest repeatability improved significantly with increasing number of sweeps used to derive the final trace, though only when comparing those derived from one and three recordings (P = 0.012). Error bars represent the 95% confidence interval of the mean.
Discussion
This study demonstrated that the ratio between PhNR PT amplitude and the B-wave amplitude (defined as the a-wave trough to b-wave peak; PT/B) was the measure that exhibited the lowest magnitude of test-retest variability (and was henceforth referred to as the PhNR/B ratio as it was the primary outcome measure used in this study). This study also determined that the PhNR/B ratio did not exhibit systematic changes over the seven measurements obtained at each session, and that the magnitude of its repeatability did not vary significantly under different testing conditions (both between sessions or between electrode placement by different examiners) using a handheld, nonmydriatic ERG system with self-adhering skin electrodes. It also demonstrated that improved repeatability could be achieved by increasing the number of recordings obtained.
In this study, we observed that the optimal measure for the PhNR was the PT/B ratio, producing the lowest level of test-retest variability relative to its EDR. Although we had previously reported the PT amplitude as the most repeatable measure,23 reanalysis of the data from that study after normalizing the different measures confirmed that the PT/B ratio also exhibited the lowest level of test-retest variability in that study. Normalization to the EDR has also been performed for both standard automated perimetry (SAP)26 and retinal nerve fiber layer (RNFL) thickness on optical coherence tomography (OCT)27 in eyes with glaucoma, when seeking to compare the number of discriminable steps (or SDs from the normal to floor values based on measurement variability) between different stimulus parameters and devices, respectively. For instance, the global RNFL thickness ranged from having between 8.3 to 10.6 discriminable steps for the three different devices.27 For the PhNR/B ratio in this study, there would be 6.5 discriminable steps from normal to floor values based on its CoR of 30% of the EDR (using a similar method of calculation). Although these findings may suggest that the PhNR may be less useful for monitoring disease progression than the RNFL thickness, its usefulness may lie in its potential prognostic value17 and relevance as a biomarker for neuroretinal function that is reversible,32 although future studies are required to investigate this further.
We also confirmed in this study that there were no systematic changes between the first two PhNR/B ratio measurements within each session. This is consistent with previous findings that there were no significant interocular differences for PhNR measurements within the same session for normal participants (suggesting that there were no significant intrasession changes, if both eyes are assumed to be equal).24 We sought to establish this because a previous study observed an unexplained decline in the pattern electroretinogram (PERG) amplitude between repeated measurements within the same session,33 although such intrasession changes have not been observed with conventional full-field ERG.34 This finding suggests that the first measurement is comparable with all subsequent measurements, and a practice measurement is not required to obtain comparable subsequent responses. We also confirmed that no systematic changes occurred over the seven measurements performed in this study, which is important because clinicians may wish to take multiple measurements in both eyes for participants with noisy recordings in order to obtain a reliable response.
We also observed that there were no significant differences in the repeatability of the PhNR/B ratio within- and between-sessions, and between different electrode placements. We had hypothesized that the intersession variability would be significantly higher than the intrasession variability (as observed in previous studies using other electroretinography techniques34–36) due to differences in electrode placement. We examined this by replacing the electrodes within the same session in a masked fashion by a different examiner. We sought to introduce further interexaminer variability by including a clinician and nonclinician examiner for electrode placements, but yet did not find significant differences in the measurement repeatability. It is possible that these findings are attributed to the self-adhering electrode array used in this study, which appeared to be more stable and easier to set up than the conjunctival fiber and skin gold cup electrodes that we have used previously from our anecdotal observations; further studies are required to determine if this is the case.
Intersession repeatability of the PhNR/B ratio also improved when including an increasing number of sweeps in this study, which is consistent with the findings from our recent study28 and also the notion that the statistical estimate of a mean improves with more samples. We had hypothesized that no further improvements in the measurement repeatability would be observed when deriving a final trace from more than one recording (that consisted of obtaining 200 sweeps) because it would have fallen within the noise levels of the measurement. However, we observed that a final trace that included up to three recordings exhibited a significantly smaller magnitude of variability compared with those derived from just one recording. These findings are useful for both researchers and clinicians when deciding how many sweeps to include when using this testing protocol, depending on the level of measurement precision desired.
The ability to obtain PhNR measurements using a portable, handheld, full-field ERG system that is simple to implement clinically compared with the standard full-field ERG system overcomes previous impediments to its widespread and routine implementation. Interestingly, we also observed that the repeatability of the PhNR BT and PT/B ratio in this study was more than three times better than our previous study, where recordings were obtained using conjunctival electrodes and included only 10 sweeps.23 The repeatability of the PhNR BT measure in this study was also approximately 2 and 1.5 times better than those obtained using skin and conjunctival electrodes, respectively, in another previous study24 that included 40 sweeps (the limits of agreement were 88% and 63% of the mean value of the normal subjects for the skin and conjunctival electrodes, respectively). This improved repeatability is most likely attributed to the larger number of sweeps (through more recordings) used to derive the final trace in this study, and suggests that the protocol used in this study could have a comparable (if not better) level of repeatability than those often used in clinical or research studies.11,17,20–24 However, it is likely that the repeatability of the PhNR may be even better when using corneal bipolar electrodes compared with the self-adhering skin electrodes used in this study, but the use of such electrodes are more invasive and requires experienced examiners. The finding that the PT/B ratio for measuring the PhNR was the most repeatable measure also highlights its use over standard measurements of the PhNR amplitude from the baseline when evaluating eyes with diseases that only affect this component of the full-field ERG, such as glaucoma. However, the finding that the PT/B ratio was the most repeatable measure in normal subjects should not diminish the importance of examining the BT amplitude in diseased eyes, because ocular diseases that affect other components of the full-field ERG response (including the a- and b-waves) will affect the PT/B ratio and thus obscure the ability to detect changes to the PhNR. In addition, improvements in the repeatability of the PhNR (by means of the protocol or measure used) should not be the sole way of judging clinical performance, and future studies are required to examine whether such improvements result in an improved ability to detect and monitor ocular diseases and evaluate treatment efficacy.
Some potential limitations need to be recognized when interpreting the results of this study, including the method by which the EDR was calculated. Although it is assumed that the floor amplitude of the PhNR in response to a brief flash is zero based on animal studies1 and our observations of patients with advanced glaucoma, this has not yet been confirmed by comparing the amplitude of the PhNR with visual sensitivity on SAP.27 In addition, we assumed that the a- and b-waves of the ERG recording would be unchanged when the PhNR response was at its floor, which is an assumption that is required when evaluating any PhNR measure that include these other components (such as the PT/B ratio). However, these assumptions were kept consistent for all methods used to measure the PhNR in this study, and we therefore believe the comparison is valid. The limited sample size and age range of the participants may also prohibit its generalizability for younger or older participants. However, we sought to include participants that were of a similar age to most participants with preperimetric glaucoma, because the PhNR may be most useful for this stage of the disease. Future studies are required to confirm these findings in other age groups and in a larger sample size. In addition, the self-adhering skin electrodes used in this study obtains smaller signal amplitude than corneal or conjunctival electrodes typically used in ERG recordings. For instance, the a-wave trough to b-wave peak amplitude of the self-adhering skin electrodes was 26% of the amplitude for conjunctival electrodes in our previous study,28 smaller compared with skin gold cup electrodes using a full-field red-on-blue stimulation (between 36%28 and 38%24) and when measuring PERG amplitude (30%33). However, these studies have demonstrated that the magnitude of test-retest variability relative to signal amplitude was similar between skin and conjunctival electrodes,24,28,33 and one study showed that the capacity of both electrodes to diagnose glaucoma was similar.33 Nevertheless, future studies are required to establish whether the self-adhering skin electrodes used in this study is comparably effective for detecting and monitoring changes in the PhNR in eyes with glaucoma.
In conclusion, this study showed that the PT/B ratio was the optimal measure of the PhNR and that its CoR fell within ± 30% of its EDR when obtaining measurements using a portable, handheld full-field ERG system that uses self-adhering skin electrodes. These findings highlight the potential clinical use of this technique as an objective measure of neuroretinal function in glaucoma and other ocular conditions.
Acknowledgments
Supported by grants from the Ophthalmic Research Institute of Australia, The Menzies Foundation, the National Health & Medical Research Council (Early Career Fellowship #1104985, ZW), the Miller Foundation, and the Dorothy Adele Edols Charitable Trust. The Centre for Eye Research Australia receives support for operational infrastructure from the Victorian government.
Disclosure: Z. Wu, None; X. Hadoux, None; F. Hui, None; M.G. Sarossy, None; J.G. Crowston, None
References
- 1. Viswanathan S,, Frishman LJ,, Robson JG,, Harwerth RS,, Smith E. The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999; 40: 1124–1136. [PubMed] [Google Scholar]
- 2. Li B,, Barnes G,, Holt W. The decline of the photopic negative response (PhNR) in the rat after optic nerve transection. Doc Ophthalmol. 2005; 111: 23–31. [DOI] [PubMed] [Google Scholar]
- 3. Rangaswamy NV,, Frishman LJ,, Dorotheo EU,, et al. Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci. 2004; 45: 3827–3837. [DOI] [PubMed] [Google Scholar]
- 4. Machida S,, Raz-Prag D,, Fariss RN,, Sieving PA,, Bush RA. Photopic ERG negative response from amacrine cell signaling in RCS rat retinal degeneration. Invest Ophthalmol Vis Sci. 2008; 49: 442–452. [DOI] [PubMed] [Google Scholar]
- 5. Colotto A,, Falsini B,, Salgarello T,, et al. Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci. 2000; 41: 2205–2211. [PubMed] [Google Scholar]
- 6. Viswanathan S,, Frishman LJ,, Robson JG,, Walters JW. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001; 42: 514–522. [PubMed] [Google Scholar]
- 7. Machida S,, Gotoh Y,, Toba Y,, et al. Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2008; 49: 2201–2207. [DOI] [PubMed] [Google Scholar]
- 8. North RV,, Jones AL,, Drasdo N,, Wild JM,, Morgan JE. Electrophysiological evidence of early functional damage in glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2010; 51: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 9. Machida S,, Tamada K,, Oikawa T,, et al. Sensitivity and specificity of photopic negative response of focal electoretinogram to detect glaucomatous eyes. Br J Ophthalmol. 2010; 94: 202–208. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura H,, Hangai M,, Mori S,, Hirose F,, Yoshimura N. Hemispherical focal macular photopic negative response and macular inner retinal thickness in open-angle glaucoma. Am J Ophthalmol. 2011; 151: 494–506, e1. [DOI] [PubMed] [Google Scholar]
- 11. Preiser D,, Lagreze WA,, Bach M,, Poloschek CM. Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci. 2013; 54: 1182–1191. [DOI] [PubMed] [Google Scholar]
- 12. Machida S,, Kaneko M,, Kurosaka D. Regional variations in correlation between photopic negative response of focal electoretinograms and ganglion cell complex in glaucoma. Curr Eye Res. 2014: 1–11. [DOI] [PubMed]
- 13. Kaneko M,, Machida S,, Hoshi Y,, Kurosaka D. Alterations of photopic negative response of multifocal electroretinogram in patients with glaucoma. Curr Eye Res. 2014; 40: 77–86. [DOI] [PubMed] [Google Scholar]
- 14. Machida S,, Gotoh Y,, Tanaka M,, Tazawa Y. Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol. 2004; 137: 938–940. [DOI] [PubMed] [Google Scholar]
- 15. Chen H,, Wu D,, Huang S,, Yan H. The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol. 2006; 113: 53–59. [DOI] [PubMed] [Google Scholar]
- 16. Chen H,, Zhang M,, Huang S,, Wu D. The photopic negative response of flash ERG in nonproliferative diabetic retinopathy. Doc Ophthalmol. 2008; 117: 129–135. [DOI] [PubMed] [Google Scholar]
- 17. Gotoh Y,, Machida S,, Tazawa Y. Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol 2004; 122: 341–346. [DOI] [PubMed] [Google Scholar]
- 18. Miyata K,, Nakamura M,, Kondo M,, et al. Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci. 2007; 48: 820–824. [DOI] [PubMed] [Google Scholar]
- 19. Moon CH,, Hwang SC,, Kim B-T,, Ohn Y-H,, Park TK. Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci. 2011; 52: 8527–8533. [DOI] [PubMed] [Google Scholar]
- 20. Wang J,, Cheng H,, Hu Y-S,, Tang RA,, Frishman LJ. The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci. 2012; 53: 1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abed E,, Piccardi M,, Rizzo D,, et al. Functional loss of the inner retina in childhood optic gliomas detected by photopic negative response. Invest Ophthalmol Vis Sci. 2015; 56: 2469–2474. [DOI] [PubMed] [Google Scholar]
- 22. Ueno S,, Kondo M,, Piao C,, et al. Selective amplitude reduction of the PhNR after macular hole surgery: ganglion cell damage related to ICG-assisted ILM peeling and gas tamponade. Invest Ophthalmol Vis Sci. 2006; 47: 3545–3549. [DOI] [PubMed] [Google Scholar]
- 23. Tang J,, Edwards T,, Crowston JG,, Sarossy M. The test-retest reliability of the photopic negative response (PhNR). Transl Vis Sci Technol. 2014; 3 (6): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mortlock KE,, Binns AM,, Aldebasi YH,, North RV. Inter-subject, inter-ocular and inter-session repeatability of the photopic negative response of the electroretinogram recorded using DTL and skin electrodes. Doc Ophthalmol. 2010; 121: 123–134. [DOI] [PubMed] [Google Scholar]
- 25. Fortune B,, Bui BV,, Cull G,, Wang L,, Cioffi GA. Inter-ocular and inter-session reliability of the electroretinogram photopic negative response (PhNR) in non-human primates. Exp Eye Res. 2004; 78: 83–93. [DOI] [PubMed] [Google Scholar]
- 26. Wall M,, Woodward KR,, Doyle CK,, Zamba G. The effective dynamic ranges of standard automated perimetry sizes iii and v and motion and matrix perimetry. Arch Ophthalmol. 2010; 128: 570–576. [DOI] [PubMed] [Google Scholar]
- 27. Mwanza J-C,, Kim HY,, Budenz DL,, et al. Residual and dynamic range of retinal nerve fiber layer thickness in glaucoma: comparison of three OCT platforms. Invest Ophthalmol Vis Sci 2015; 56: 6344–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Z,, Hadoux X, Fan Gaskin JC, Sarossy MG, Crowston JG. Measuring the photopic negative response of the electroretinogram: viability of skin electrodes and variability across disease severities in glaucoma. Transl Vis Sci Technol. 2016; 5 (2): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rangaswamy NV,, Shirato S,, Kaneko M,, et al. Effects of spectral characteristics of ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci. 2007; 48: 4818–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leonowicz Z,, Karvanen J,, Shishkin SL. Trimmed estimators for robust averaging of event-related potentials. J Neurosci Methods. 2005; 142: 17–26. [DOI] [PubMed] [Google Scholar]
- 31. Bland JM,, Altman DG. Statistics notes: measurement error. BMJ. 1996; 313: 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niyadurupola N,, Luu CD,, Nguyen DQ,, et al. Intraocular pressure lowering is associated with an increase in the photopic negative response (PhNR) amplitude in glaucoma and ocular hypertensive eyes. Invest Ophthalmol Vis Sci. 2013; 54: 1913–1919. [DOI] [PubMed] [Google Scholar]
- 33. Bach M,, Ramharter-Sereinig A. Pattern electroretinogram to detect glaucoma: comparing the PERGLA and the PERG Ratio protocols. Doc Ophthalmol. 2013; 127: 227–238. [DOI] [PubMed] [Google Scholar]
- 34. Hochstein GD,, Molnar FE,, Marmor MF. Intrasession variability of the full-field ERG. Doc Ophthalmol. 2007; 115: 77–83. [DOI] [PubMed] [Google Scholar]
- 35. Gundogan FC,, Sobaci G,, Bayraktar MZ. Intra-sessional and inter-sessional variability of multifocal electroretinogram. Doc Ophthalmol. 2008; 117: 175–183. [DOI] [PubMed] [Google Scholar]
- 36. Porciatti V,, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004; 111: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]