Abstract
Purpose
Despite the overlap between the clinical symptoms/sequelae of polycystic ovarian syndrome (PCOS) and many known reproductive risk factors for breast cancer, the relationship between PCOS and breast cancer remains unclear, possibly because of the complex heterogeneity and challenges in diagnosing PCOS over time. We hypothesized that PCOS, specific PCOS-related symptoms/sequelae, or clusters of PCOS-related symptoms/sequelae, may be differentially associated with pre- vs. postmenopausal breast cancer risk.
Materials and Methods
Cases were 1,508 women newly diagnosed with a first primary in situ or invasive breast, and the 1,556 population-based controls were frequency-matched by age.
Results
History of physician-diagnosed PCOS was reported by 2.2% (n=67), among whom oral contraceptive (OC) use, irregular menstruation, and infertility due to ovulatory dysfunction were common. Using unconditional logistic regression, adjusted odds ratios (95% confidence intervals) for PCOS were increased for premenopausal [2.74 (1.13, 6.63)], but not post-menopausal breast cancer [0.87 (0.44, 1.71)]. We used cluster analysis to investigate whether risk among all women varied by PCOS-related symptoms/sequelae, such as reproductive irregularities, OC use, and components of insulin resistance. In the cluster analysis, odds ratios were elevated among premenopausal women who had a history of OC use and no ovulatory dysfunction [1.39 (1.03, 1.88)], compared to those with fewer number of PCOS-related symptoms/sequelae.
Conclusion
PCOS, and associated PCOS-related symptoms/sequelae including OC use, may play a role in the development of premenopausal breast cancer. Our findings require confirmation in studies with a larger number of premenopausal women with systematically applied diagnostic criteria for PCOS.
Keywords: breast cancer, polycystic ovarian syndrome, premenopausal, oral contraceptives
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine condition in women of reproductive age with an estimated prevalence 2–18% [1,2]. PCOS is a complex, heterogeneous disorder of uncertain etiology characterized by hyperandrogenism, menstrual disturbances, and polycystic ovaries. The clinical manifestation of PCOS includes menstrual dysfunction, sub/infertility, hirsutism, acne, obesity, insulin insensitivity, and the metabolic syndrome [2]. Hyperinsulinemia and, resulting hyperandrogenemia have been investigated as possible causal factors for PCOS [3,4]. Elevated insulin levels contribute to the increased ovarian androgen production and decreased follicular maturation [4]. Because hyperinsulinemia also underpins the pathogenesis of the metabolic syndrome [5], PCOS has been considered the ‘ovarian’ manifestation of the metabolic syndrome [6].
The possible association between PCOS and breast cancer has been investigated because of the large overlap between the clinical manifestations of PCOS and risk factors for breast cancer [7–12]. Late age at menarche, and late age at first birth or nulliparity are typical clinical manifestations of PCOS [2,13], and are established risk factors for breast cancer [14–16]. The inverse relationship between physical activity and breast cancer risk is fairly consistent [17]. At the same time, a few studies have reported a possible inverse association between physical activity and PCOS [18, 19], but the association is not well established. Also, increased levels of androgen and insulin have been reported to be positively associated with the risk of breast cancer [20]. In addition, oral contraceptive (OC) use is one of the clinical sequelae of PCOS because OCs are the most widely applied treatment modality for PCOS to regulate menstrual cycles and decrease levels of free testosterone and hirsutism scores [21]; yet, OC use also has been consistently, albeit modestly, associated with elevated premenopausal breast cancer risk [22]. However, prior studies assessing the possible association between PCOS and breast cancer report conflicting results, including increased risk [23], decreased risk [7] and null results [9, 12, 24]. Inconsistent results may be due to the low prevalence of PCOS, thus making it difficult to conduct adequately powered PCOS-related investigations. Further, the unclear etiology of PCOS along with changes in the diagnostic criteria of PCOS over time and the intra-individual variability of PCOS symptoms, contribute to the challenges in the conceptualization of the study design as well as the statistical analysis when examining the potential PCOS-breast cancer association. However, understanding how the hormonal conditions of PCOS are related to breast cancer development is significant because it may clarify the underlying hormonal etiology of breast cancer, and perhaps PCOS itself.
Our study examines the association between PCOS and breast cancer risk using data from a population-based case-control study. Given the different risk factor profiles [25,26], pathologic, biologic and prognostic features of premenopausal compared to postmenopausal breast cancer [27], we also investigated if PCOS is differentially associated with pre- vs. postmenopausal breast cancer risk. Given the under-diagnosis and the changes in the diagnosis criteria overtime, we, for the first time, used a novel approach of cluster analysis to investigate the association between PCOS-related clinical symptoms/sequelae among all women (regardless of their PCOS diagnosis) and breast cancer risk.
Methods
The study reported here utilizes resources from the Long Island Breast Cancer Study Project (LIBCSP), a population-based study conducted among adult English-speaking female residents of Nassau and Suffolk counties, Long Island, NY. Details of the case-control study methods have been described elsewhere [28]. Institutional Review Board approval was obtained from all participating institutions.
Study Population
Eligible LIBCSP cases were women of all ages and races who were newly diagnosed with a first primary in situ or invasive breast cancer between August 1, 1996, and July 31, 1997. Cases were identified through daily or weekly contact with the 28 hospitals on Long Island and 3 large tertiary care hospitals in New York City.
Eligible controls were women without a personal history of breast cancer who were frequency matched to the expected age distribution of cases by 5-year age group. Controls were identified from among female residents of the same two Long Island counties as the cases using random digit dialing for women under the age of 65 years and Health Care Finance Administration rosters for women aged ≥65 years.
Response rates among eligible cases and controls were 82.1% (N= 1508) and 62.8% (N= 1556), respectively. Participants ranged from 20–98 years of age, one-third were premenopausal, and 93% were white, 5% were black, and 2% were other. The racial distribution did not vary by case-control status, and reflects the racial distribution of the underlying population of Nassau and Suffolk counties at the time of data collection [28]. The distribution of other demographic and breast cancer risk factors among the study participants have been reported previously [28].
Brest cancer incidence in the LIBCSP population has been positively associated with: early age at menarche, late age at first birth and little or no breastfeeding [29]; use of oral contraceptives and hormone replacement [30]; use of alcohol [31], and little or no use of aspirin and other NSAIDs [32]; and, among postmenopausal women only, obesity [33] and physical inactivity [17].
History of PCOS and PCOS-related Clinical Symptoms/Sequelae
Participants completed a 100-minute structured questionnaire conducted by a trained interviewer in the respondent’s home shortly after diagnosis (or date of identification for controls). As part of the case-control questionnaire, interviewers asked participants if a health professional had ever told them that they had polycystic ovarian syndrome (‘yes’, ‘no’ or ‘don’t know’). Interviewers also asked participants: at what age their menstrual periods became regular or if it never became regular; and if their periods became regular naturally, because of birth pills, or in some other way. Other PCOS-related symptoms/sequelae such as acne, obesity (weight and height), OC use, history of infertility, age at menarche and age at first birth were also assessed at the interview, and covariates were defined based on the Rotterdam criteria and previous studies regarding clinical features of PCOS [34, 35]. The interviewers also inquired if the participants had physician-diagnosed diseases that are associated with metabolic syndrome such as hypertension, diabetes mellitus and hypercholesterolemia.
Other Covariate Assessment
The case-control interviewers queried women on demographic characteristics, cigarette smoking and physical activity levels. Details of the methods used to assess smoking [36], and physical activity [17] have been described previously. Among eligible cases, clinical data on the characteristics of their breast cancer diagnosis, including hormone receptor status, were obtained from medical records.
Statistical Analysis
We first examined distributions of demographic and breast cancer risk factors among women with and without PCOS, among all women. Also, because risk factor profiles may vary by pre-and postmenopausal breast cancer [29][30], we examined the prevalence of symptoms/sequelae by menopausal status.
Unconditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations of PCOS and breast cancer risk [37]. All statistical models were implemented in SAS, version 9.2 (SAS Institute Inc., Cary, NC). History of PCOS (yes/no) was evaluated as dichotomous variable; women who were not sure about their PCOS diagnosis were considered not to have PCOS history.
We identified potential confounders through the known epidemiology of breast cancer [15,16], PCOS and analysis of causal diagrams (Fig. 1) [38]. Careful consideration of our causal diagram identified the following as mediators of the association between the study exposure (PCOS) and the study outcome (breast cancer), and were thus excluded from all models: history of infertility; parity/gravidity; body mass index (BMI = weight in kilograms/height in meters squared); physician-diagnosed diabetes mellitus/hypertension/hypercholesterolemia, irregular menstruation; and ever OC use [39]. Covariates considered as potentials confounders included average lifetime physical activity (<0.1 hrs/week, 0.1–3.59 hrs/week, 3.6–10.49 hrs/week, and ≥10.5 hrs/week) and cigarette smoking history (never/ever). Covariates resulting in >10% change in the regression coefficient when added to the model, compared to a model without the covariate, were included as confounders in our final analysis [40]; physical activity, but not smoking, met this criterion. Thus, final multivariable models were adjusted for physical activity, and the frequency matching factor, 5-year age group.
Figure 1.
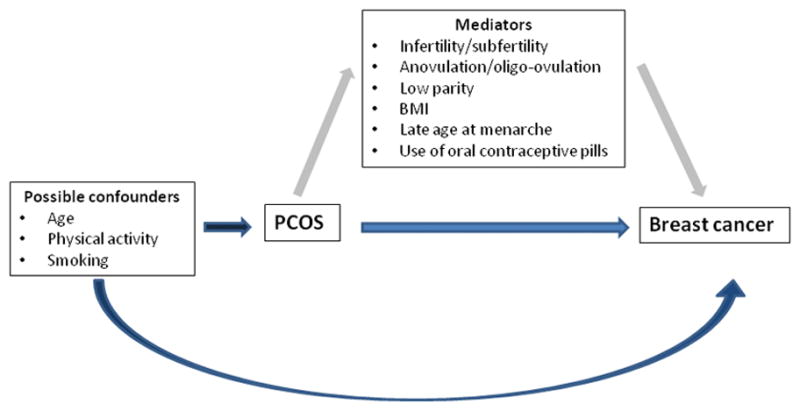
A simplified directed acyclic graph for the association between PCOS and breast cancer.
Menopausal status (premenopausal/postmenopausal) at diagnosis (cases) or at interview (controls) was investigated as a potential effect measure modifier based on our a priori study aims. Departure from the multiplicative null was assessed using the likelihood ratio test [37].
The age range of the LIBCSP study subjects was wide, and thus the criteria used to define a diagnosis of PCOS is likely to have varied over time. Also, many of the symptoms/sequelae of PCOS are likely to be independently associated with breast cancer, and are likely mediators for any association observed between PCOS and breast cancer. Thus, using adjusted logistic regression models, the associations between breast cancer risk and the symptoms/sequelae of PCOS (including acne, increased BMI, OCs use, history of infertility, age at menarche, age at first birth, and history of diabetes mellitus, hypertension, and hypercholesterolemia) were examined among all women, regardless of their PCOS history [41]. Many of the effect estimates for the individual associations between breast cancer risk and each of these PCOS symptoms/sequelae in the LIBCSP study population have already been reported [17, 29, 30, 42], but are reported here to ease interpretation of our cluster analysis (see below).
Using k-means cluster analysis [43], all participants (regardless of their PCOS diagnosis) were grouped according to PCOS symptoms/sequelae. PCOS-related symptoms/sequelae considered in the cluster analysis included: ovulatory dysfunction (fail to initiate regular cycles naturally or history of infertility due to ovulatory dysfunction); metabolic syndrome-related sequelae (history of hypertension, diabetes mellitus, or hypercholesterolemia); late age at menarche; late age at first birth; current or past OC use; and overweight/obesity (current BMI > 25kg/m2). Using model fit and clinical relevance, participants were grouped into six clusters. We then used logistic regression to examine the association between each cluster group (entered as a dichotomous categorical variable, using the cluster with the fewest PCOS-related symptoms as the referent) and breast cancer risk. We also considered these associations stratified by menopausal status.
Results
PCOS characteristics
Among the 3,046 LIBCSP study participants (1,508 cases and 1,556 controls), 67 (2.2%) reported a PCOS diagnosis from a medical professional and 2,951 (96.3%) reported no PCOS history. The 46 women (1.5%) who were not sure about their PCOS diagnosis were considered not to have PCOS history. As shown in Table 1, participants with PCOS history were significantly younger than participants without PCOS history (p=0.008).
Table 1.
The distribution of demographic factors, reproductive and medical history, by PCOS history among all subjects (cases (n=1508) and controls (n = 1556)), LIBCSP 1996–1997.
| Factor | PCOS (n=67) | No PCOS (n=2,997) | Total (n=3,064) |
|---|---|---|---|
| Age | 53.8±12.2 | 58.0±12.8 | 57.8±12.8 |
| BMI (kg/m2) | 27.4±6.2 | 26.5±5.7 | 26.5±5.7 |
| BMI at age 20 (kg/m2) | 21.4±3.9 | 20.9±3.1 | 20.9±3.2 |
| Parity (%) | |||
| 0 | 10 (14.9) | 359 (12.0) | 369 (12.0) |
| 1 | 7 (10.5) | 307 (10.5) | 314 (10.5) |
| ≥2 | 50 (74.6) | 2,331 (77.5) | 2,381 (77.4) |
| Race (%) | |||
| White | 63 (94.0) | 2,777 (92.8) | 2,840 (92.8) |
| Black and other | <5 | 217 (7.2) | 221 (7.2) |
| Age at menarche (years) | 12.4±7.5 | 12.6±1.6 | 12.6±1.6 |
| Age at first birth (years) | 25.1±3.8 | 25.3±4.6 | 25.3±4.6 |
| No. of postmenopausal women (%) | 35 (55.6) | 1,961 (67.4) | 1,996 (65.1) |
| Physical activity (hours/week) | 6.4±10.2 | 7.3±10.0 | 7.3±10.0 |
| Ever use of oral contraceptives (%) | 37 (55.2) | 1335 (44.6) | 1,372 (44.8) |
| Family history of breast cancer (%) | 12 (18.5) | 480 (16.5) | 492 (16.6) |
| Periods never became regular naturally (%) | 16 (24.2) | 299 (10.3) | 315 (10.6) |
| History of infertility due to ovulatory dysfunction (%) | 6 (12.0) | 44 (1.7) | 50 (1.9) |
| Metabolic syndrome-related sequelae (%) | |||
| Hypertension | 21 (33.3) | 999 (34.4) | 1,020 (34.4) |
| Diabetes mellitus | <5 | 236 (8.1) | 239 (8.0) |
| Hypercholesterolemia | 22 (34.9) | 888 (30.5) | 910 (30.6) |
| History of active smoking (%) | 37 (57.8) | 1,616 (55.3) | 1,653 (55.4) |
Participants with PCOS were more likely to have ever used OCs (p=0.05) and to have a history of infertility due to ovulatory dysfunction (p=0.001); and to not to have regular menstrual cycles naturally (p=0.002). In contrast, distributions for parity, age at menarche, age at first birth, BMI, hypertension, diabetes mellitus, hypercholesterolemia, and active smoking were not substantially different from women without PCOS. Among breast cancer cases, the proportion diagnosed with in situ vs. invasive disease was similar for women with and without PCOS. As shown in Fig. 2, metabolic syndrome-related sequelae were the most frequent PCOS-related symptoms/sequelae among all and postmenopausal participants, whereas history of using OCs was the most frequent symptoms/sequelae among premenopausal subjects.
Figure 2.
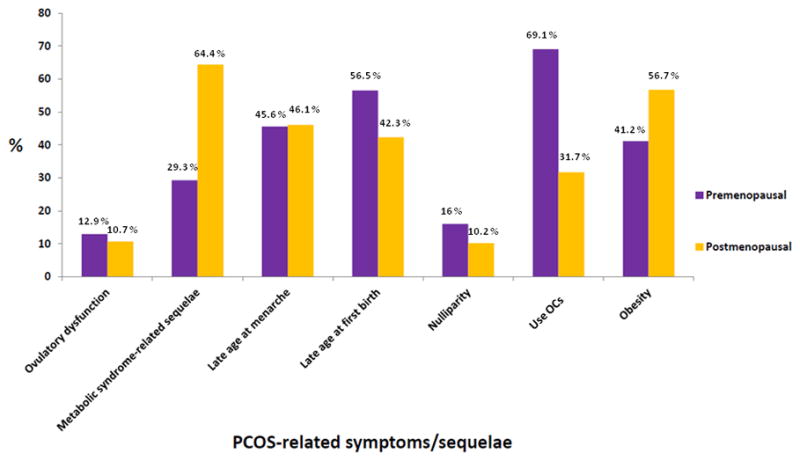
Frequencies (percent) of PCOS-related clinical symptoms/sequelae among all, premenopausal and postmenopausal women, LIBCSP, 1996–1997.
PCOS and breast cancer risk
Among all women, the age-adjusted effect estimate for the association between PCOS and breast cancer incidence was elevated by 43%, but the confidence intervals were wide (age-adjusted OR= 1.43; 95% CI= 0.80, 2.20); the estimate was similar in multivariate models (multivariate adjusted OR= 1.37; 95% CI= 0.81–2.29) (Table 2). However, risk varied significantly by menopausal status (p-value for multiplicative interaction=0.05). In premenopausal women, breast cancer incidence was increased nearly 3-fold among women with PCOS as compared to women without PCOS (multivariate-adjusted OR= 2.74; 95% CI= 1.13–6.63). In contrast, for postmenopausal women, breast cancer incidence was decreased by 33% among women with a history of PCOS (multivariate-adjusted OR= 0.67; 95% CI= 0.33–1.35).
Table 2.
Odds ratios [and 95% confidence intervals] for the association between a history of PCOS and breast cancer incidence, by menopausal status, LIBCSP, 1996–1997.
| History of PCOS | Cases (N=1,508) | Controls (N=1,556) | Age-adjusted OR (95% CI) | Multivariable-adjusted ORa (95% CI) |
|---|---|---|---|---|
| All women | ||||
| No PCOS | 1,503 | 1,448 | Ref | Ref |
| PCOS | 38 | 29 | 1.43 (0.88, 2.34) | 1.37 (0.81, 2.29) |
| Premenopausal women | ||||
| No PCOS | 448 | 489 | Ref | Ref |
| PCOS | 19 | 9 | 2.31 (1.03, 5.17) | 2.74 (1.13, 6.63) |
| Postmenopausal women | ||||
| No PCOS | 973 | 954 | Ref | Ref |
| PCOS | 16 | 19 | 0.87 (0.44, 1.71) | 0.67 (0.33, 1.35) |
Adjusted for age and physical activity (hrs/week)
Individual PCOS-related clinical symptoms/sequelae and breast cancer risk
As shown in Table 3, neither pre- nor postmenopausal breast cancer incidence among all women (regardless of PCOS history) was associated with the history of the initiation of regular menstruation and infertility due to ovulatory dysfunction. As previously reported [17, 29, 30, 42, 44] and as shown in Table 3, breast cancer risk was significantly elevated among all women (regardless of PCOS history) with characteristics that are often associated with PCOS. Women with breast cancer were more likely to be nulliparous, and the association was strongest among postmenopausal women. Among women who had their first birth at later age (≥28 years), the risk of breast cancer was elevated, and ever use of OCs was associated with increased odds of developing premenopausal breast cancer. Obesity and a history of physician-diagnosed diabetes mellitus were associated with increased postmenopausal breast cancer risk, and decreased premenopausal breast cancer risk.
Table 3.
Odds ratios [and 95% confidence intervals] for the associations between PCOS-related sequelae and breast cancer incidence, by menopausal status, LIBCSP, 1996–1997.
Please note: part of these associations have been previously reported by LIBCSP collaborators (Bonnier et al., 1995; Breslow et al., 1980; Clavel-Chapelon et al., 2002; Greenland et al., 1989; Shrier et al., 2008), and are provided here to aid interpretation of results presented in Table 4.
| Symptom/sequelae | All | Premenopausal | Postmenopausal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (%) | Controls (%) | Age-adjusted OR (95% CI) | Cases (%) | Controls (%) | Age-adjusted OR (95% CI) | Cases (%) | Controls (%) | Age-adjusted OR (95% CI) | |
| Ovulatory dysfunction related symptomsa | |||||||||
| Yes | 160 (10.6) | 192 (12.3) | 0.86 (0.68, 1.08) | 59 (12.5) | 67 (13.3) | 0.93 (0.64, 1.36) | 95 (9.4) | 117 (11.8) | 0.80 (0.60, 1.07) |
| No | 1347 (89.4) | 1364 (87.7) | Ref | 412 (87.5) | 436 (86.7) | Ref | 911 (90.6) | 873 (88.2) | Ref |
| Menstrual irregularity | |||||||||
| Yes | 144 (9.6) | 171 (11.0) | 0.87 (0.69, 1.10) | 52 (11.1) | 53 (10.6) | 1.06 (0.70, 1.59) | 86 (8.6) | 111 (11.3) | 0.75 (0.56, 1.01) |
| No | 1357 (90.4) | 1380 (89.0) | Ref | 417 (88.9) | 449 (89.4) | Ref | 916 (91.4) | 875 (88.7) | Ref |
| Infertility caused by ovulatory dysfunction | |||||||||
| Yes | 25 (1.9) | 27 (2.0) | 1.08 (0.63, 1.89) | 13 (3.2) | 17 (3.8) | 0.84 (0.40, 1.76) | 11 (1.3) | 9 (1.1) | 1.08 (0.44, 2.65) |
| No | 1262 (98.1) | 1344 (98.0) | Ref | 388 (96.8) | 427 (96.2) | Ref | 847 (98.7) | 863 (99.0) | Ref |
| Nulliparityc | |||||||||
| Yes | 189 (13) | 165 (11) | 1.27 (1.01, 1.60) | 75 (16) | 79 (16) | 1.12 (0.79, 1.59) | 114 (12) | 86 (9) | 1.37 (1.02, 1.85) |
| No | 1265 (87) | 1308 (89) | Ref | 394 (84) | 415 (84) | Ref | 836 (88) | 891 (91) | Ref |
| Age at menarche (years)c | |||||||||
| <12 | 377 (26) | 406 (28) | Ref | 121 (26) | 146 (29) | Ref | 246 (26) | 260 (27) | Ref |
| 12 | 406 (28) | 381 (26) | 1.15 (0.94, 1.40) | 123 (27) | 133 (27) | 1.12 (0.79, 1.58) | 283 (29) | 248 (26) | 1.17 (0.91, 1.48) |
| 13 | 361 (25) | 338 (23) | 1.14 (0.93, 1.40) | 142 (31) | 119 (24) | 1.43 (1.02, 2.02) | 219 (22) | 219 (23) | 0.99 (0.76, 1.27) |
| 14+ | 299 (21) | 337 (23) | 0.93 (0.75, 1.14) | 79 (17) | 99 (20) | 0.96 (0.66, 1.41) | 220 (23) | 238 (25) | 0.90 (0.70, 1.16) |
| Age at first birth among parous women (years)c | |||||||||
| <22 | 291 (23) | 326 (25) | Ref | 75 (19) | 95 (23) | Ref | 216 (25) | 231 (26) | Ref |
| 22–24.99 | 358 (28) | 395 (30) | 0.99 (0.80, 1.23) | 88 (23) | 95 (23) | 1.15 (0.75, 1.74) | 270 (31) | 300 (34) | 0.83 (0.57, 1.22) |
| 25–27.99 | 268 (21) | 286 (22) | 1.04 (0.82, 1.31) | 85 (22) | 86 (21) | 1.26 (0.82, 1.94) | 183 (48) | 200 (23) | 0.83 (0.56, 1.25) |
| ≥28 | 350 (28) | 299 (23) | 1.33 (1.07, 1.67) | 144 (37) | 143 (34) | 1.32 (0.90, 1.93) | 206 (24) | 156 (18) | 1.18 (0.78, 1.79) |
| Taking OCsc | |||||||||
| Yes | 629 (43) | 659 (45) | 1.08 (0.92, 1.28) | 337 (72) | 330 (67) | 1.33 (1.01, 1.75) | 292 (30) | 329 (34) | 0.96 (0.78, 1.18) |
| No | 834 (57) | 805 (55) | Ref | 131 (28) | 163 (33) | Ref | 681 (70) | 639 (66) | Ref |
| Cardiovascular diseasec | |||||||||
| Yes | 89 (6) | 72 (5) | 1.11 (0.80, 1.54) | 6 (1) | 5 (1) | 1.17 (0.35, 3.87) | 83 (8) | 67 (7) | 1.07 (0.76, 1.51) |
| No | 1394 (94) | 1368 (95) | Ref | 599 (99) | 495 (99) | Ref | 955 (92) | 890 (93) | Ref |
| Metabolic syndrome-related sequelaeb,c | |||||||||
| Yes | 1,162 (80) | 1,192 (81) | 0.89 (0.74, 1.07) | 356 (76) | 372 (75) | 0.78 (0.61, 0.99) | 806 (82) | 820 (84) | 1.09 (0.81, 1.46) |
| No | 294 (20) | 280 (19) | Ref | 111 (24) | 124 (25) | Ref | 177 (18) | 156 (16) | Ref |
| Diabetes | |||||||||
| Yes | 130 (9) | 104 (7) | 1.22 (0.93, 1.60) | 15 (3) | 21 (4) | 0.75 (0.38, 1.47) | 115 (12) | 83 (9) | 1.37 (1.01, 1.84) |
| No | 1314 (91) | 1382 (93) | Ref | 485 (97) | 504 (96) | Ref | 843 (88) | 839 (91) | Ref |
| Hypertension | |||||||||
| Yes | 510 (35) | 487 (33) | 0.97 (0.82, 1.14) | 76 (16) | 76 (15) | 0.98 (0.70, 1.38) | 434 (44) | 411 (42) | 0.98 (0.82, 1.17) |
| No | 947 (65) | 989 (67) | Ref | 399 (84) | 431 (85) | Ref | 552 (56) | 568 (58) | Ref |
| Hypercholesterolemia | |||||||||
| Yes | 442 (30) | 458 (31) | 0.91 (0.78, 1.05) | 73 (16) | 82 (17) | 0.88 (0.63, 1.23) | 369 (37) | 376 (39) | 0.93 (0.79, 1.10) |
| No | 1031 (70) | 1019 (69) | Ref | 383 (84) | 400 (83) | Ref | 628 (63) | 588 (61) | Ref |
| BMI at age 20 (kg/m2) | |||||||||
| <20 | 609 (42.6) | 611 (42.2) | Ref | 209 (45.1) | 202 (40.9) | Ref | 400 (41.4) | 409 (42.9) | Ref |
| 20–24.99 | 722 (50.5) | 708 (48.9) | 1.02 (0.88, 1.19) | 222 (48.0) | 247 (50.0) | 0.87 (0.67, 1.13) | 500 (51.7) | 461 (48.4) | 1.10 (0.92, 1.33) |
| ≥25 | 99 (6.9) | 128 (8.9) | 0.78 (0.59, 1.04) | 32 (6.9) | 45 (9.1) | 0.73 (0.44, 1.19) | 67 (6.9) | 83 (8.7) | 0.81 (0.57, 1.15) |
| BMI at diagnosis (kg/m2) | |||||||||
| <25 | 664 (45.6) | 709 (48.2) | Ref | 281 (60.2) | 278 (55.8) | Ref | 383 (38.7) | 431 (44.3) | Ref |
| 25–29.99 | 459 (31.5) | 431 (29.3) | 1.11 (0.94, 1.32) | 124 (26.6) | 127 (25.5) | 0.96 (0.71, 1.29) | 335 (33.9) | 304 (31.2) | 1.23 (1.00, 1.51) |
| ≥30 | 316 (21.7) | 304 (20.7) | 1.09 (0.90, 1.32) | 61 (13.1) | 86 (18.3) | 0.69 (0.48, 1.00) | 255 (25.8) | 218 (22.4) | 1.34 (1.07, 1.69) |
Having menstrual irregularity or infertility caused by ovulatory dysfunction
History of diabetes or hypertension or hypercholesterolemia or high BMI at age 20 or current BMI.
Previously reported by LIBCSP collaborators.
Clusters of PCOS-related clinical symptoms/sequelae and breast cancer risk
We used cluster analysis to investigate if constellations of various PCOS-related symptoms/sequelae are related to breast cancer risk, which resulted in all women (regardless of their PCOS history) being grouped into six clusters according to PCOS-related symptoms/sequelae (Table 4A). All women in cluster 1 and 2 had used OCs. Women in cluster 1 tended to have higher prevalence of ovulatory dysfunction while those in cluster 3 did not. Cluster 2 was characterized as high prevalence of OC use and low ovulatory dysfunction. Women in cluster 6 had the highest prevalence of metabolic syndrome-related symptoms/sequelae and were more likely to be obese compared to subjects in other clusters. Women in cluster 4 had the lowest prevalence of PCOS-related symptoms/sequelae overall; thus, we selected cluster 4 as the referent category for our cluster analyses.
Table 4A.
Frequency or mean value of PCOS-related clinical symptoms/sequelae within each cluster (identified using cluster analysis) among all cases and controls (n=3064), LIBCSP, 1996–1997.
| Cluster | N | PCOS (%) | BMId (kg/m2) | Nulliparity (%) | Age at menarched | Age at first birthd | Irregular menstrual cycle (%) | Infertility (%) | Diabetes (%) | Hyper- tension (%) | Hyper-cholester-olemia (%) | Ever taking OCs (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1: Women with RSa and OCs | 196 | 10 (5) | 25.3±5.3 | 30 (15) | 12.8±1.8 | 24.7±4.5 | 193 (99) | 54 (28) | 12 (6) | 50 (26) | 56 (29) | 196 (100) |
| 2: Women with OCs without RSb | 1176 | 27 (2) | 26.1±5.7 | 113 (10) | 12.4±1.6 | 25.3±4.8 | <5 | 181 (15) | 68 (6) | 329 (28) | 306 (26) | 1176 (100) |
| 3: Women with RSa without OCs | 122 | 6 (5) | 26.8±5.7 | 14 (12) | 12.8±1.7 | 25.6±5.0 | 122 (100) | 26 (21) | 17(14) | 60 (49) | 54 (44) | 0 |
| 4: Women without RSb/MSc | 936 | 17 (2) | 25.8±5.0 | 130 (14) | 12.7±1.6 | 25.5±4.6 | 6 (0) | 134 (14) | 50 (5) | 0 | 259 (28) | 0 |
| 5: Non-obese women with MSc | 165 | <5 | 26.8±5.3 | 13 (8) | 13.7±1.6 | 24.7±3.8 | 0 | 24 (15) | 17 (10) | 141 (85) | 78 (47) | 0 |
| 6: Obese women with MSc | 6 (1) | 29.3±6.8 | 69 (15) | 12.2±1.4 | 24.9±4.3 | <5 | 72 (15) | 74 (16) | 469 (100) | 182 (39) | 0 |
The menopausal status was unsure or missing in 137 (4%) women.
Reproductive symptoms which includes irregular menstruation and infertility
Symptoms/sequelae related to metabolic syndrome including hypertension, diabetes and hypercholesterolemia
Mean±SD
Breast cancer risk among all women showed little or no variation across clusters of PCOS-related symptoms/sequelae, as compared to cluster 4 (Table 4B). However, premenopausal breast cancer incidence was increased by 39% among women in cluster 2 (age-adjusted OR=1.39; 95% CI =1.03, 1.88), and the confidence intervals excluded the null value. Premenopausal breast cancer incidence was also increased among women in cluster 1 compared to those in cluster 4, however the confidence intervals included the null value (age-adjusted OR=1.35; 95% CI =0.84, 2.17). For postmenopausal breast cancer, the odds ratios tended to decrease for all clusters, however, confidence intervals included the null value.
Table 4B.
Odds ratios [and 95% confidence intervals] of breast cancer incidence within Clusters and stratified by menopausal status, among all case and control women (n=3064), LIBCSP, 1996–1997.
| Cluster | All | Premenopausal | Postmenopausal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (%) | Controls (%) | Age-adjusted OR (95% CI) | Cases (%) | Controls (%) | Age-adjusted OR (95% CI) | Cases (%) | Controls (%) | Age-adjusted OR (95% CI) | |
| 1: Women with RSa and OCs | 93 (47) | 103 (53) | 1.03 (0.75, 1.41) | 47 (50) | 47 (50) | 1.35 (0.84, 2.17) | 40 (44) | 51 (56) | 0.83 (0.53, 1.30) |
| 2: Women with OCs without RSa | 564 (48) | 612 (52) | 1.05 (0.88, 1.26) | 295 (51) | 286 (49) | 1.39 (1.03, 1.88) | 255 (47) | 283 (53) | 0.95 (0.75, 1.20) |
| 3: Women with RSa without OCs | 53 (43) | 69 (57) | 0.73 (0.50, 1.07) | 6 (46) | 7 (54) | 1.16 (0.38, 3.55) | 47 (44) | 60 (56) | 0.70 (0.46, 1.05) |
| 4: Women without RSa/MSb | 462 (49) | 474 (51) | Ref | 107 (43) | 144 (57) | Ref | 345 (52) | 321 (48) | Ref |
| 5: Non-obese women with MSb | 83 (50) | 82 (50) | 0.93 (0.66, 1.30) | <5 | <5 | Non-estimable | 80 (50) | 81 (50) | 0.87 (0.61, 1.23) |
| 6: Obese women with MSb | 253 (54) | 216 (46) | 1.09 (0.87, 1.37) | 14 (44) | 18 (56) | 1.01 (0.48, 2.12) | 239 (55) | 194 (45) | 1.08 (0.85, 1.38) |
Reproductive symptoms which includes irregular menstruation and infertility
Symptoms/sequelae related to metabolic syndrome including hypertension, diabetes and hypercholesterolemia
Discussion
In this population-based study, we observed a pronounced, nearly 3-fold increase in risk for a history of physician-diagnosed PCOS in association with premenopausal, but not postmenopausal, breast cancer. History of irregular menses, infertility, and OC use were more commonly reported by women with PCOS, than those without PCOS. However, in our cluster analysis that considered PCOS-related symptoms/sequelae among all women, OC use was the only symptom/sequelae for which the risk of premenopausal breast cancer was elevated.
Although our results are provocative, they must be interpreted with care. Despite the notable overlap of risk factors between breast cancer and PCOS-related symptoms/sequelae, previous research has reported increased [23], no altered risk [9,12,24], or decreased risk of breast cancer [7], in relation to a history of PCOS. There are few studies showing differences by menopausal status [7,23]. In a population based case-control study evaluating 4,730 women with breast cancer and 4,688 control women aged 20–54 years, Gammon and colleagues reported a 50% decrease in breast cancer risk among women with PCO, which did not vary by menopausal status [7]. In contrast, our finding here showed significantly increased risk of premenopausal breast cancer with PCOS which is consistent with Cowan et al.'s report showing an increased premenopausal breast cancer risk in women with progesterone deficiency [23]. The inconsistency of the results between previous reports and our own may stem from differences in the analytic approach and in the distribution of PCOS symptoms/sequelae across study populations. Previous researchers included adjustments for many variables that are most likely mediators of the PCOS-breast cancer association [7–10,12], and thus should not be included in the statistical models [46]; as shown in Fig. 1, these mediators include history of infertility, parity, BMI, or history of OCs use. Adjusting for mediators is likely to attenuate and reduce the precision of the effect estimate for the PCOS-breast cancer association [45].
The diagnosis of PCOS is made based on its symptoms and the various symptoms may be independently associated with breast cancer risk. Also, because of the wide spectrum of clinical features and the interconnection among those features of PCOS, women with PCOS tend to present with multiple and heterogeneous clinical symptoms or sequelae. Thus, when exploring the association between PCOS and breast cancer, it may be informative to consider the impact of the constellation of various PCOS symptoms and sequelae, individually as well as grouped. Interestingly, in our cluster analysis that included all women, breast cancer risk increase was highest among premenopausal women who had used OCs (cluster 1 and 2). However, there was no notable risk change in women with metabolic syndrome-related symptoms and sequelae (cluster 5 and 6) or in women who had ovulatory dysfunction without OC use (cluster 3). Although our approach cannot definitively differentiate the impact of OC use on breast cancer risk between women with and without PCOS, it is possible that the increased premenopausal breast cancer risk in women with PCOS is not related to PCOS itself or PCOS-related symptoms/sequelae, but is possibly associated, at least in part, with the high prevalence of OCs use in this population.
A possible reason for our observed increase for the PCOS-breast cancer association among premenopausal women only is that PCOS is a disease of premenopausal women given the clinical features of PCOS are not easily discerned among postmenopausal women. So, it may be possible that clinical features and the aberrant hormonal profile of PCOS impact only premenopausal breast cancer, which attenuates with time once exposure to PCOS dissipates. Our cluster analysis among women with and without PCOS, revealed that from among all PCOS-related symptoms/sequelae, the clusters which included women who had ever used OCs were most strongly associated with breast cancer. Given that OCs are used only by premenopausal women, their use by women with PCOS requires closer scientific and clinical examination. To elucidate the exact relationship between OC use and premenopausal breast cancer development in women with PCOS, a mediation analysis would be appropriate, which is not possible in this study due to the case-control design [46].
Our study has several significant strengths. Our novel approach of clustering women according to their history of these factors mitigates the impact of low prevalence and under-diagnosis of PCOS and may be clinically useful and practical. In addition, our cluster approach can be helpful in elucidating the possible association which results from specific clinical symptoms/sequelae of PCOS versus the clinical manifestation of PCOS. However, our clusters were derived using our primarily white population-based study sample and thus may not be applicable to other more racially diverse populations. Also, ours is the first study to exclude all mediators of the PCOS-breast cancer association in the model. Finally, by suggesting the possible role of OCs in women with PCOS, our results help to inform future research focused on the potential breast cancer risk versus any potential benefits associated with OC use among women with PCOS.
There are several limitations in this study. First, despite the large overall sample size, the prevalence of PCOS was low in our study population, and thus we were unable to conduct more detailed statistical analyses, including consideration of: (1) a potential interaction between OC use and PCOS on breast cancer risk; (2) potential heterogeneity of the association with breast cancer subtypes, including subtypes defined by hormone receptor status; and (3) potential differential recall of a history of PCOS due to age at recruitment. Second, the study is based on self-reported history of PCOS, which is subject to errors in recall. However, despite the small number of women who reported a history of PCOS, the overall prevalence of PCOS and the clinical sequelae of PCOS among the LIBCSP study population were consistent with previous reports [2], particularly those based on studies conducted among a population-based sample [1]. Third, our study population is fairly racially homogenous, with more than 90% of participants who self-reported their race as white. Although, we were unable to investigate the role of race on the association between PCOS and breast cancer, the population homogeneity increases internal validity of our study. Fourth, we were unable to consider hirsutism, which is a significant clinical feature of PCOS, as one of PCOS-related symptom/sequelae in our cluster analysis, because assessment of hirsutism was not included in the LIBCSP questionnaire. Finally, the response rate between cases and controls differed. However, the LIBCSP control response rate is comparable to that found in other population-based control studies [28].
In summary, in this population-based study, we found a strong positive association between PCOS and premenopausal breast cancer. We also observed modest increased risks among premenopausal women with select clusters of PCOS-related symptoms and sequelae, which included those who reported OC use. Future investigations, with larger numbers of premenopausal women and systematically applied criteria for defining PCOS, are required to confirm our findings.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (CA/ES 66572, and 5TL1CA133837-05).
Footnotes
Disclosure
The authors report no financial or nonfinancial conflicts of interest.
References
- 1.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 4.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 5.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garruti G, Depalo R, Vita MG, Lorusso F, Giampetruzzi F, Damato AB, et al. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reprod Biomed Online. 2009;19:552–563. doi: 10.1016/j.rbmo.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Gammon MD, Thompson WD. Polycystic ovaries and the risk of breast cancer. Am J Epidemiol. 1991;134:818–824. doi: 10.1093/oxfordjournals.aje.a116156. [DOI] [PubMed] [Google Scholar]
- 8.Anderson KE, Sellers TA, Chen PL, Rich SS, Hong CP, Folsom AR. Association of Stein-Leventhal syndrome with the incidence of postmenopausal breast carcinoma in a large prospective study of women in Iowa. Cancer. 1997;79:494–499. [PubMed] [Google Scholar]
- 9.Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136:99–103. doi: 10.1016/j.ygyno.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Brinton LA, Moghissi KS, Westhoff CL, Lamb EJ, Scoccia B. Cancer risk among infertile women with androgen excess or menstrual disorders (including polycystic ovary syndrome) Fertil Steril. 2010;94:1787–1792. doi: 10.1016/j.fertnstert.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb) 2000;3:101–105. doi: 10.1080/1464727002000198781. [DOI] [PubMed] [Google Scholar]
- 12.Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:748–758. doi: 10.1093/humupd/dmu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenberg N, Glueck C. Medical therapy in women with polycystic ovarian syndrome before and during pregnancy and lactation. Minerva Ginecol. 2008;60:63–75. [PubMed] [Google Scholar]
- 14.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Tamimi R, Hankinson SE, Hazra A, Eliassen AH, Colditz GA. Chapter 18: nongenetic factors in the causation of breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. 5. Lippincott Williams & Wilkins; 2014. pp. 211–267. [Google Scholar]
- 16.Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat. 2014;144:1–10. doi: 10.1007/s10549-014-2852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullough LE, Eng SM, Bradshaw PT, Cleveland RJ, Teitelbaum SL, Neugut AI, et al. Fat or fit: the joint effects of physical activity, weight gain, and body size on breast cancer risk. Cancer. 2012;118:4860–4868. doi: 10.1002/cncr.27433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright CE, Zborowski JV, Talbott EO, McHugh-Pemu K, Youk A. Dietary intake, physical activity, and obesity in women with polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2004;28:1026–1032. doi: 10.1038/sj.ijo.0802661. [DOI] [PubMed] [Google Scholar]
- 19.Lin AW, Lujan ME. Comparison of dietary intake and physical activity between women with and without polycystic ovary syndrome: a review. Adv Nutr. 2014;5:486–496. doi: 10.3945/an.113.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glintborg D, Altinok ML, Mumm H, Hermann AP, Ravn P, Andersen M. Body composition is improved during 12 months treatment with metformin alone or combined with oral contraceptives compared to treatment with oral contraceptives in polycystic ovary syndrome. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2014-1135. jc20141135. [DOI] [PubMed] [Google Scholar]
- 22.Cogliano V, Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F WHO International Agency for Research on Cancer. Carcinogenicity of combined oestrogen–progestagen contraceptives and menopausal treatment. Lancet Oncol. 2005;6:552–553. doi: 10.1016/s1470-2045(05)70273-4. [DOI] [PubMed] [Google Scholar]
- 23.Cowan LD, Gordis L, Tonascia JA, Jones GS. Breast cancer incidence in women with a history of progesterone deficiency. Am J Epidemiol. 1981;114:209–217. doi: 10.1093/oxfordjournals.aje.a113184. [DOI] [PubMed] [Google Scholar]
- 24.Chittenden BG, 1, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009;19:398–405. doi: 10.1016/s1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- 25.Micheli A, Muti P, Secreto G, Krogh V, Meneghini E, Venturelli E, et al. Endogenous sex hormones and subsequent breast cancer in premenopausal women. Int J Cancer. 2004;112:312–318. doi: 10.1002/ijc.20403. [DOI] [PubMed] [Google Scholar]
- 26.Clavel-Chapelon F. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer. 2002;86:723–727. doi: 10.1038/sj.bjc.6600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clavel-Chapelon F, Gerber M. Reproductive factors and breast cancer risk. Do they differ according to age at diagnosis? Breast Cancer Res Treat. 2002;72:107–115. doi: 10.1023/a:1014891216621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gammon MD, Neugut AI, Santella RM, Teitelbaum SL, Britton JA, Terry MB, et al. The Long Island Breast Cancer Study Project: description of a multi-institutional collaboration to identify environmental risk factors for breast cancer. Breast Cancer Res Treat. 2002;74:235–254. doi: 10.1023/a:1016387020854. [DOI] [PubMed] [Google Scholar]
- 29.Shantakumar S, Terry MB, Teitelbaum SL, Britton JA, Millikan RC, Moorman PG, et al. Reproductive factors and breast cancer risk among older women. Breast Cancer Res Treat. 2007;102:365–374. doi: 10.1007/s10549-006-9343-4. [DOI] [PubMed] [Google Scholar]
- 30.Shantakumar S, Terry MB, Paykin A, Teitelbaum SL, Britton JA, Moorman PG, et al. Age and menopausal effects of hormonal birth control and hormone replacement therapy in relation to breast cancer risk. Am J Epidemiol. 2007;165:1187–1198. doi: 10.1093/aje/kwm006. [DOI] [PubMed] [Google Scholar]
- 31.Terry MB, Zhang FF, Kabat G, Britton JA, Teitelbaum SL, Neugut AI, et al. Lifetime alcohol intake and breast cancer risk. Ann Epidemiol. 2006;16:230–240. doi: 10.1016/j.annepidem.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 32.Terry MB, Gammon MD, Zhang FF, Tawfik H, Teitelbaum SL, Britton JA, et al. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004;291:2433–2440. doi: 10.1001/jama.291.20.2433. [DOI] [PubMed] [Google Scholar]
- 33.Eng SM, Gammon MD, Terry MB, Kushi LH, Teitelbaum SL, Britton JA, et al. Body size changes in relation to postmenopausal breast cancer among women on Long Island, New York. Am J Epidemiol. 2005;162:229–237. doi: 10.1093/aje/kwi195. [DOI] [PubMed] [Google Scholar]
- 34.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 35.Jamil AS, Alalaf SK, Al-Tawil NG, Al-Shawaf T. Comparison of clinical and hormonal characteristics among four phenotypes of polycystic ovary syndrome based on the Rotterdam criteria. Arch Gynecol Obstet. 2015 doi: 10.1007/s00404-015-3889-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Shen J, Terry MB, Gammon MD, Gaudet MM, Teitelbaum SL, Eng SM, et al. MGMT genotype modulates the associations between cigarette smoking, dietary antioxidants andbreast cancer risk. Carcinogenesis. 2005;26:2131–2137. doi: 10.1093/carcin/bgi179. [DOI] [PubMed] [Google Scholar]
- 37.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selvin S. Statistical Analysis of Epidemiologic Data. New York: Oxford University Press; 1996. [Google Scholar]
- 39.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breslow NE, Day NE. Statistical methods in cancer research. Volume I - The analysis of case-control studies. IARC Sci Publ. 1980:5–338. [PubMed] [Google Scholar]
- 41.Rothman KJ, Greenland S, Lask TL. Modern Epidemiology. Linppincott, Williams and Wilkins; 2012. [Google Scholar]
- 42.Cleveland RJ, North KE, Stevens J, Teitelbaum SL, Neugut AI, Gammon MD. The association of diabetes with breast cancer incidence and mortality in the Long Island Breast Cancer Study Project. Cancer Causes Control. 2012;23:1193–1203. doi: 10.1007/s10552-012-9989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milligan G. A study of standatdization of variables in cluster analysis. Journal of Classification. 1988;5:181–204. [Google Scholar]
- 44.Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(5):748–758. doi: 10.1093/humupd/dmu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lange T, Rasmussen M, Thygesen LC. Assessing natural direct and indirect effects through multiple pathways. Am J Epidemiol. 2013;179:513–518. doi: 10.1093/aje/kwt270. [DOI] [PubMed] [Google Scholar]


