Abstract
Traumatic injuries to PNS and CNS axons are not uncommon. Restoration of lost behaviors following severance of mammalian peripheral nerve axons (PNAs) relies on regeneration by slow outgrowths and is typically poor or nonexistent if after ablation or injuries close to the soma. Behavioral recovery after severing spinal tract axons (STAs) is poor because STAs do not naturally regenerate. Current techniques to enhance PNA and/or STA regeneration have had limited success and do not prevent the onset of Wallerian degeneration of severed distal segments. This review describes the use of a recently-developed polyethylene glycol (PEG)-fusion technology combining concepts in biochemical engineering, cell biology and clinical microsurgery. Within minutes after micro-suturing carefully-trimmed cut ends and applying a well-specified sequence of solutions, PEG-fused axons exhibit morphological continuity (assessed by intra-axonal dye diffusion) and electrophysiological continuity (assessed by conduction of action potentials) across the lesion site. Wallerian degeneration of PEG-fused PNAs is greatly reduced as measured by counts of sensory and/or motor axons, and maintenance of axonal diameters and neuromuscular synapses. After PEG-fusion repair, cut- or crush-severed or ablated PNAs or crush-severed STAs rapidly (within days to weeks), more completely, and permanently restore PNA- or STA-mediated behaviors compared to non-treated or conventionally-treated animals. PEG-fusion success is enhanced or decreased by applying anti-oxidants or oxidants, trimming cut ends or stretching axons, exposure to Ca2+-free or - containing solutions, respectively. PEG-fusion technology employs surgical techniques and chemicals already used by clinicians and has the potential to produce a paradigm-shift in the treatment of traumatic injuries to PNAs and STAs.
Keywords: axotomy, axonal regeneration, polyethylene glycol, Wallerian degeneration, nerve repair
The curious nature of mammalian axonal regeneration and axonal repair by PEG-fusion
As discussed and documented in this review, in contrast to most invertebrates, axonal regeneration in mammals is usually curiously slow and poor after severing peripheral nerve axons (PNAs) and curiously non-existent after severing spinal tract axons (STAs). The regenerative abilities and mechanisms of invertebrate axonal regeneration inspired the development and application of PEG-fusion technologies to rapidly and effectively restore behaviors lost after mammalian axonal severance. The details of the PEG-fusion protocols were guided by studies of (1) cellular/molecular mechanisms by which all metazoan eukaryotic cells studied to date naturally or artificially repair traumatic plasmalemmal damage, especially with respect to effects of Ca2+, polyethylene glycol (PEG), and anti-oxidants such as methylene blue (MB) and (2) best-practice clinical surgical procedures developed for PNAs, especially with respect to roles of micro-sutures, alignment of cut axonal ends, nerve tension/stretch, autografts and allografts. The rapid, dramatic, and long-lasting restoration of lost behaviors by PEG-fusion technologies by successfully-PEG-fused allografts after ablation of sciatic nerves in the rat thigh is especially curious in that the allograft is not rejected and that most (perhaps all) repaired axons no longer have their original connection specificities.
Clinical significance of traumatic injuries to axons in the PNS and CNS
Because neuronal cell bodies are of modest size and often have very long axons, most traumatic injuries to the CNS or PNS involve cut or crush damage to axons (axotomy). Axotomies of large bundles containing many PNAs or CNS STAs are not un-common injuries that produce significant motor and sensory impairments (Birch et al., 1998; Bozkurt et al., 2008: Campbell, 2008; Burch 2011). For example, 100,000 patients annually undergo surgery in the US and EU to repair PNA injuries with behavioral deficits that often have a devastating impact on a patient’s quality of life (Allan, 2000).
Complex extremity injuries are an especially important issue for combat military personnel. Modern body armor has evolved to effectively protect the chest and abdomen from many threats, and these advances, coupled with the widespread use and distribution of pre-hospital tourniquets, have not only led to dramatic increases in the number of wounded warriors with survivable injuries (Holcomb et al., 2006: Kragh et al., 2009; Fox and Kreishman, 2010; Fox et al., 2011; Isaacson et al., 2010), but also to a dramatic increase in personnel with mutilated extremities. For example, PNA extremity injuries were over 50% of combat wounds sustained in Iraq and Afghanistan, often resulting in extremity amputations that are very debilitating (Stansbury et al., 2007; Cross et al., 2011).
One of the key determinants for extremity amputation is damage to a segment of a large peripheral nerve, especially with concomitant bone and vascular injury (Green and Wolf, 2011). Moreover, the long-term functional prognosis of a salvaged, but denervated, hand is dismal in civilian or military patients (Wolfe et al., 2010). Such advances and improvements in treating traumatic military or civilian injuries have shifted the dogma of saving “life over limb” to a new goal of saving both “life and limb” (Burch, 2011; Campbell, 2008). This shift in goals will require new techniques to enhance repair of severed PNAs.
The annual incidence of spinal cord injury (SCI) in the USA is approximately 40 cases per 1 million people or 12,500 new cases per year. Currently, the US has 240,000 to 337,000 citizens who have some form of SCI, with a lifetime cost of $1.5 – $4.5 million each for care alone; lost wages and productivity increase this burden many fold (National Spinal Cord Injury Statistical Center, 2015). New techniques have long been needed to enhance the naturally-poor regeneration of severed STAs associated with very poor clinical outcomes.
Current approaches to improve regeneration of severed mammalian PNAs and STAs
Rapid and effective repair of traumatically-injured PNAs and STAs to restore lost behavioral functions has been a largely-unattained goal of clinicians and neuroscientists (Birch et al., 1998; Bittner et al., 2000; Campbell, 2008; Kwon et al., 2010; Wolfe et al., 2010). For PNAs and STAs to successfully regenerate after severance, the damaged plasmalemma at the proximal axonal end must rapidly seal off to prevent loss of cytoplasm and influx of substances toxic to the cell (Bittner and Fishman, 2000).
If axolemmal damage is successfully repaired, surviving mammalian PNAs naturally regenerate by slow (~1mm/d) outgrowths from their severed proximal ends (Wolfe et al., 2010). Severed distal segments also seal off (Bittner and Fishman, 2000), but usually begin to degenerate according to various morphological criteria within 1 – 3d (Wallerian degeneration: Waller, 1850; Schlaepfer and Bunge, 1973; Bozkurt et al., 2007, 2008) and are unable to generate action potentials within 3d (Tsao, 1999).
Injuries more proximal to the cell body have for over a century been associated with increased occurrence of cell death (Ramon y Cajal, 1928; Lucas et al., 1985, 1990), probably due to supra-threshold increases in Ca2+ levels in the cell body (Yoo et al., 2003, 2004; Nguyen et al., 2005). Perikaryial death eliminates any chance of axonal regeneration for that neuron.
Current clinical techniques to repair PNA traumatic injuries include micro-sutures, synthetic or autologous conduits and autologous nerve autografts (Campbell, 2008; Wolfe et al., 2010; Riley et al., 2015). However, these methods have had limited success varying with mechanism of injury, timing of repair, injury distance from the CNS, length of damaged nerve and gap length, motor vs. sensory vs. mixed nerve, the surgeon’s technical ability and technique, postoperative rehabilitation, patient coping, etc. (Williams and Dellon, 2008; Farber et al., 2013; Menorca et al., 2013). Typically, (1) sharp clean nerve cuts have better recoveries than severe crushes, tractions or ablations; (2) PNA injuries closer to their distal sensory and/or motor targets have better recoveries than more proximal injuries; and, (3) shorter (<3mm) gaps between cleanly cut ends have better results than longer (>3mm) gap lengths (Allan, 2000; Burdick, 2006; Burch, 2011; Chim et al., 2013). To avoid stretch/tension damage to PNAs, longer gaps are typically best repaired by conduits or (especially) autografts (Allan, 2000; Bozkurt et al., 2008; Wolfe et al., 2010).
Current techniques for PNA repair make no attempt to limit cell body death or Wallerian degeneration (Burch, 2011; Menorca et al., 2013; Ghergherehchi et al., 2015). Recovery is therefore immediately limited by a decreased pool of surviving neurons available to make appropriate connections. Current repair techniques also depend upon proximal nerve regrowth at 1mm/day (Allan, 2000; Bittner et al., 2000; Campbell, 2008; Wolfe et al., 2010). Hence, potentially-appropriate re-innervation of distal motor or sensory targets takes months in rats and years in humans. Since muscles lose capacity for re-innervation over time, such potential re-innervation and behavioral recovery after proximal PNA injuries has a much-reduced chance to occur (MacKinnon and Dellon, 1998; Burch, 2011; Fox and Kreishman, 2010; Fox et al., 2011). Even the best current techniques to re-appose cut ends by epineural micro-sutures or tubular conduits do not re-establish axonal continuity, produce rapid functional recovery, or prevent Wallerian degeneration (Allan, 2000; Bertelli and Ghizoni, 2009, 2011). Furthermore, behavioral recovery is much worse or non-existent if the cut ends cannot be directly re-apposed after loss of a segment of a PNS nerve in humans (Wolfe et al., 2010) and rats (Bittner et al., 2000, 2012; Yan et al., 2013; Ghergerehchi et al., 2015). Consequently, regenerating PNAs often do not remake functional connections, and behavioral recovery is rarely fully restored even for more-distally cut nerves, and is especially poor following more-proximal cut, crush, traction or ablation injuries (Campbell, 2008; Bertelli et al., 2009, 2011; Taras et al., 2011; Farber et al., 2013; Menorca et al., 2013).
Behavioral recovery after STA severance is very poor. Like PNAs, proximal segments of STAs seal off, but STAs do not grow out more than about a millimeter and do not successfully regenerate naturally (Ramon y Cajal, 1928; Kakulas, 1999; Kwon et al., 2010), in part because CNS myelin contains inhibitory molecules that block axonal growth. These inhibitors include myelin associated glycoprotein, oligodendrocyte myelin glycoprotein and other Nogo proteins (Rowland et al., 2008). CNS axonal regeneration is also prevented by glial scars that have outgrowth-inhibiting molecules such as chondroitin sulfate proteoglycans (Fitch and Silver, 2008). As reported for PNAs, severed distal segments of STAs show morphological signs of Wallerian degeneration within three days (Ramon y Cajal, 1928; Kakulas, 1999; Kwon et al., 2010).
Current strategies to increase behavioral recovery after SCI are focused on enhancing outgrowth from proximal ends of severed STAs. These strategies include donor transplants of peripheral nerve sheaths (David and Aguayo, 1981), embryonic tissues (Giovanni et al., 1997; Miya et al., 1997), growth promoting factors (Chen et al. 2010; Kwon et al., 2010), induced pluripotent stem cells (Lu et al., 2014), transplantation of Schwann cells (Williams and Bunge, 2012), and/or antibodies to oligodendrocytic inhibitors of axonal outgrowth (Schnell and Schwab, 1990; Schwab, 2002; Freund et al., 2009). These outgrowth improvement strategies have had small but significant effects to enhance return of lost behaviors in animal model systems. Relearning or retraining the use of surviving axons may also provide significant benefits when spinal severance is not complete in animal models and in the clinic (Bittner et al., 2000, 2015; Kwon et al., 2010). PEG (Kwon et al., 2009, 2010, Spaeth et al., 2012b), MB (Zhang et al., 2006; Rojas et al., 2009; Britt et al., 2010; Spaeth et al., 2012b) and melatonin (MEL) (Stavisky et al., 2005; Ragdona et al., 2009; Plano, 2010, Spaeth et al., 2010, 2012a) administered in low systemic concentrations have also been reported to have neuroprotective effects following SCI or other injuries to CNS neurons. However, all of these approaches have had limited success to enhance restoration of lost behavioral functions and none of these approaches prevent Wallerian degeneration in rats, other animal models, or in clinical settings.
The nature of axonal degeneration and regeneration of mammalian PNAs and STAs is curiously problematic compared to most invertebrate axons
In contrast to the problematic slow and poor axonal regeneration for severed mammalian axons that undergo rapid Wallerian degeneration, in many invertebrates severed axons do not undergo Wallerian degeneration for months to years and lost behavioral functions are rapidly restored within days (Bittner, 1991; Bittner et al., 2000). Both proximal and distal cut ends separate by 1–3 mm and both rapidly seal off (Krause et al., 1994: Bittner and Fishman, 2000), enabling both axonal halves to survive the trauma of transection. The transfer of proteins and other trophic substances from adjacent glia that usually do not form myelin enable distal segments of PNS and CNS invertebrate axons to survive for many months (Viancour et al., 1981; Bittner, 1991; Sheller et al., 1995, 1998; Tanner et al., 1995a,b). As first reported by Hoy et al. (1967) for severed crayfish motor axons, proximal cut ends of severed invertebrate axons in arthropods, annelids, mollusks, and roundworms grow out at 1–3mm/day to selectively and rapidly (within days) contact and produce action potentials in their distal segments that survive for months to years (Birse and Bittner, 1978; Bittner and Brown, 1981; Bouton and Bittner, 1981; DeReimer et al., 1983; Bittner et al., 2000; Neumann et al., 2011).
Action potentials in a proximal segment of an invertebrate axon induce action potentials in the surviving distal segment because the proximal segment fuses with it (DeReimer et al., 1983, Neumann et al., 2011), forms gap junctions with it (Muller and Carbonetto, 1979), forms chemical synapses with it (Fernandez and Fernandez, 1974; Nordlander and Singer, 1976), or activates it by ephaptic extracellular current spread (Bouton and Bittner, 1981; Bittner, 1991; Bittner et al., 2000). No matter what the exact mechanism of distal segment “activation”, the two severed halves function as one continuous axon leading to rapid (1–7d) and complete return of behaviors lost upon severance.
If the distal segment does not fuse with the proximal segment to become one continuous axon, the proximal segment continues to grow out at 1–2 mm/day. Guided by the surviving distal segment that it continues to activate, the outgrowing proximal segment eventually re-innervates its original post-synaptic targets, at which time the surviving distal segment often rapidly degenerates (Bouton and Bittner, 1981; see Fig. 7 in Bittner et al., 2000 for a diagram). The long-term survival of severed distal segments and rapid and complete behavioral recovery for most invertebrate PNS axons and some CNS axons (Bittner, 1991; Bittner et al., 2000) are in sharp contrast to the rapid axonal degeneration and poor behavioral recovery following severance of mammalian PNAs and STAs.
Polyethylene glycol (PEG) and hypotonic calcium-free salines can artificially induce rapid and permanent repair of invertebrate and vertebrate giant axons in vitro
Data from invertebrate axons that successfully regenerated by activation of surviving distal segments stimulated us to search for ways to induce rapid, near-complete, and permanent repair of severed vertebrate PNAs and STAs (Bittner et al., 1986; Krause and Bittner, 1990; Bittner, 1991; Lore et al., 1999). About 40 years ago, due to its hydrophilic properties, the membrane fusogen PEG was first used to create hybridomas to immortalize cells (Pontecorvo, 1975; Lee and Lentz, 1997; Lentz, 2007). Learning that PEG was employed to make multinucleate cells by fusing the membranes of radically different cell types, we hypothesized that we should be able to “PEG-fuse” the membranes of the two axonal halves of the same cell to make it one continuous axon. Indeed, PEG did rejoin the severed proximal and distal segments of unmyelinated crayfish medial giant axons (MGAs) in isotonic physiological salines containing Ca2+ in vitro with a success rate of about 3% as measured by conduction of action potentials across the site of PEG-fusion and EM evidence of cytoplasmic continuity produced within minutes of applying PEG to the lesion site (Fig. 1A; Bittner et al., 1986).
Figure 1. Morphological (A,B,D) and electrical (C) measures showing that a combination of PEG solutions and PEG hydrogels can rapidly and completely repair (fuse, join together) cut axonal ends in vitro (A,C,D) and in vivo (B).
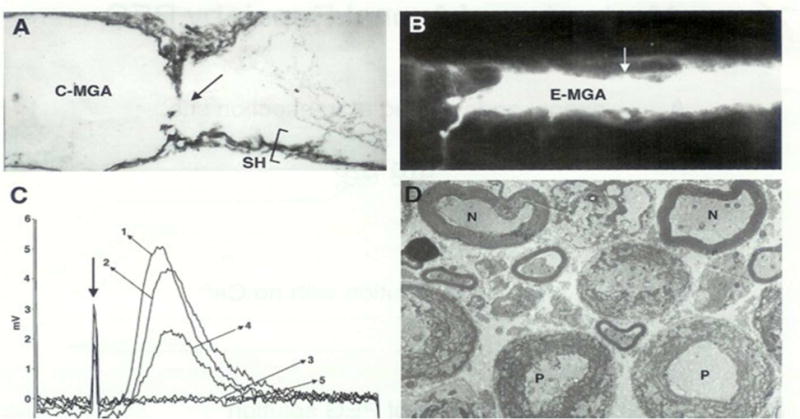
From Bittner at al., 2000 with permission.
A. Electron micrograph of a sagittal (longitudinal) section through a crayfish medial giant axon (C-MGA) PEG-fused in vitro that conducted action potentials (APs) intra-cellularly recorded through the fusion site (arrow) beginning within 10 minutes after PEG-fusion and continuing for 6 hours prior to fixation. Sh = cytoplasmic glial sheath of this un-myelinated axon.
B. Photomicrograph of a Lucifer dye-filled earthworm MGA (E-MGA) cut and PEG-fused in vivo 20 days prior to sampling. A PEG-based hydrogel was then applied to give mechanical strength in vivo. The E-MGA was completely repaired in vivo as evidenced by: (1) Its ability to conduct APs through the fusion site for 20 days; (2) These APs elicited all the behaviors evoked by intact E-MGAs in un-operated earthworms; (3) When this axon was then filled with fluorescein dye at 20 days post PEG-fusion, the dye filled the entire E-MGA which had same morphology as that shown by non-severed, control MGAs. Arrow = site of PEG-fusion of cut ends and site where the PEG-based hydrogel was applied.
C. Compound action potentials (CAPs) stimulated in one end chamber and recorded in the other end chamber of a three-chambered sucrose gap recording device. CAPs were first recorded from intact control bundles of rat spinal axons (trace labeled 1) prior to replacing the physiological saline in the central chamber with hypotonic Ca2+-free saline (trace labeled 2) at ~25° C. The spinal axons were then cut in the central chamber to completely eliminate the CAP (trace labeled 3). The cut ends were PEG-fused, and the central chamber was again perfused with calcium containing physiological saline. Within 15 minutes, CAPs were again recorded from PEG fused spinal axons (traces labeled 4). CAPs continued to be recorded for over 60 minutes before the experiment was terminated by again cutting the spinal axons in the central camber to eliminate the CAP, i.e., to demonstrate that the CAP was not an artifact (trace labeled 5).
D. Electron micrograph of cross sections of rat spinal axons at the site of PEG-induced fusion. APs conducted through the PEG-fusion site for 2 hours before fixation. N = PEG-fused axons of near normal morphology. P = PEG-fused axons of pathological morphology (disrupted myelin sheath and many membranous structures in the axoplasm).
We (Krause and Bittner, 1990) greatly improved this successful PEG-fusion rate by first exposing severed axonal ends to hypotonic Ca2+ - free solutions containing EGTA (to open cut ends and not allow Ca2+ to enter) before applying 500 mM 2–5 kDa PEG in distilled water (to fuse cut ends) followed by rinsing with isotonic Ca2+-containing salines (to restore the axon to physiological conditions). Variants of this prototype PEG-fusion technique were initially based on several inappropriate assumptions about the role of Ca2+, vesicles, and membrane lipids in plasmalemmal repair and survival (see below). Nevertheless, this initial technique succeeded in restoring electrical and morphological integrity to cut axons in 80–100% of all trials in vitro in various invertebrate model systems such as unmyelinated crayfish MGAs and myelinated earthworm MGAs (Krause et al., 1991; Lore et al., 1999). [About the same time, closely apposed ends of severed earthworm MGAs were also artificially fused in vitro by laser beams (Yogev et al. 1991) and electric fields (Todorov et al., 1992).] This initial PEG-fusion technology also worked to reconnect myelinated or unmyelinated sciatic PNAs or spinal STAs from rats, rabbits, and guinea pigs ex vivo (Fig. 1C,D; Lore et al., 1999, Shi and Borgens, 1999; Shi et al., 1999) or in vivo (Borgens et al., 2002). Completely cut-severed earthworm MGAs could be permanently PEG-fused in vivo using a PEG-based hydrogel to increase mechanical strength at the lesion site (Fig. 1B from Lore et al., 1999). However the hydrogel was toxic to mammalian axons. The PEG-fusion site of mammalian cut-severed PNAs lacked mechanical strength and the axons pulled apart in vivo once the animals started to move about (Lore et al., 1999). As discussed and illustrated (Figs. 1–12) in greater detail in subsequent sections, we have recently discovered that cut-severed mammalian nerves can be successfully PEG-fused in vivo by micro-sutures to increase mechanical strength at the site of PEG-fusion and by using various inhibitors or enhancers of plasmalemmal sealing in a well-specified sequence (Britt et al., 2010; Bittner et al., 2012).
Figure 12. PEG-fusion significantly improves BBB scores following SCI.
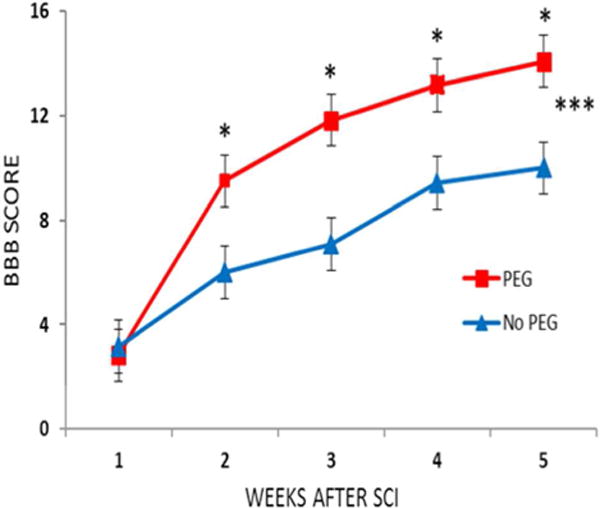
From Bittner et al., 2015 with permission.
A MASCIS rod-drop device (set at 10 gm, 12.25 cm) was used to produce a mild contusive SCI at T9–10 in twelve male Lewis rats randomly assigned to receive PEG-fusion solutions that included MB and PEG or the same series of solutions without PEG. * BBB scores at same postoperative time that differ by p < 0.05 (two-tailed t test). *** The two curves differ by p< 0.001 according to a two-way ANOVA with Bonferroni post-test.
Nerve axons, and other eukaryotic cells, repair (seal) plasmalemmal damage by vesicles
For decades, to survive traumatic injuries, rapid (within minutes) sealing of plasmalemmal damage was assumed to be necessary for various cell types, including neurons (Kelley, 1985). Without rapid plasmalemmal sealing, Ca2+ influx through plasmalemmal lesions would activate enzymes and lysosomes that would rapidly lead to degeneration of cell bodies or cytoplasmic extensions such as axons (Schlaepfer and Bunge, 1973; Bittner and Fishman, 2000). Prior to 1994, biologists assumed after minor damage plasmalemmal lipids would rapidly (within microseconds) spread to seal the hole. After complete axotomy or dendritic transection, plasmalemmal leaflets were assumed to completely and rapidly collapse and fuse to seal the cut ends (Kelley, 1985; Lucas et al., 1985, 1990; Yawo and Kuno, 1985). In contrast, in the course of our initial experiments to induce PEG-fusion, we (Krause et al., 1994) showed that small holes in, or complete transections of, several invertebrate giant axons were sealed by an accumulation of membrane-bound vesicular structures (Fig. 2). In 1994, Steinhardt et al. also showed that plasmalemmal holes in sea urchin eggs and mammalian epithelial cells were sealed by similar accumulation of vesicles.
Figure 2. Model for Ca2+-dependent, vesicle-mediated sealing in which vesicles form a plug at a partly constricted cut end and sites of minor plasmalemmal damage in all eukaryotic cells.
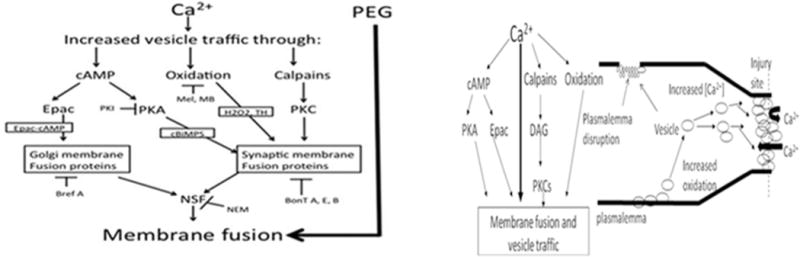
Modified and used with permission from Spaeth et al., 2010, 2012a,b,c.
PKA, PKC: phosphokinase A or C, Epac: exchange protein activated by cAMP, DAG: diacyl-glycerol. PEG bypasses all endogenous sealing pathways and induces sealing of small holes or complete transection within a fraction of a second by collapse and fusion of plasmalemmal leaflets to produce “PEG-Sealing”.
Two decades of research has subsequently revealed that all eukaryotic cells (including neurons) in over 20 preparations studied to date seal plasmalemmal damage by Ca2+-dependent production of vesicles that form a plug, often at a partially-constricted cut end (Krause et al., 1994; Steinhardt et al., 1994; Bi et al., 1995; Eddleman et al., 1997, 1998; Bittner and Fishman, 2000; Detrait et al., 2000a,b; Yoo et al., 2003, 2004; Nguyen et al., 2005; McNeil, 2009; Spaeth et al., 2010, 2012a,b,c; Zuzek et al., 2013; Jimenez et al., 2014). Vesicles from nearby intact membrane (Eddleman et al., 1997, 1998, 2000), lysosomes (Reddy et al., 2001) and/or myelin delaminations (Ballinger et al., 1997) migrate, accumulate, and pack tightly at the damage site to seal small holes within seconds to minutes and to seal complete axonal transections within 5 to 20 minutes (Fig. 2). The Ca2+-induced vesicles interact with each other and with undamaged membrane to reduce the influx of extracellular Ca2+ and other ions until a seal is formed that restores ion influx/efflux to that of intact membranes (Krause et al., 1994; Bittner and Fishman, 2000; Eddleman et al., 2000; Fishman and Bittner, 2003). Complete plasmalemmal repair takes about 24 hours, at which time vesicles are no longer observed at the original lesion site and a continuous axolemma is restored (Lichstein et al., 2000).
In summary, Ca2+ influx has a dual role in death or survival of neurons and other cells after plasmalemmal damage: (1) Ca2+ influx can lead to cell death by activating biochemical pathways responsible for apoptosis, but also (2) Ca2+ influx activates proteins, vesicle accumulation and fusion, and biochemical pathways that enable neurons and other cells to seal membrane damage, stop Ca2+ influx, and thereby survive (Bittner and Fishman, 2000; Fishman and Bittner, 2003; Yoo et al., 2003, 2004; Nguyen et al., 2005).
Artificially increasing and decreasing plasmalemmal sealing
Since these initial studies, we and others have described in some detail 4–5 parallel pathways for endogenous plasmalemmal sealing having various proteins (Fig. 2), many activated by Ca2+, that have isomers involved in vesicle trafficking or fusion at synapses or the Golgi apparatus (McNeil, 2019; Spaeth et al., 2010, 2012a,b,c; Zuzek et al., 2013; Jimenez et al., 2014). Examples of such proteins include isoforms of SNARES, SNAPS, VAMPS, NSF, synaptobrevin, syntaxin, synaptotagmin, calpains, TRIM proteins, phosphokinase A and C (PKA and PKC). The original evolutionary origins of these proteins are almost-certainly the isoforms responsible for plasmalemmal sealing; these proteins were subsequently co-opted in evolution for use in Golgi trafficking and synaptic transmission (Eddleman et al., 1997; Bittner and Fishman, 2000; Fishman and Bittner, 2003; Sudof and Rothman, 2009; Spaeth et al., 2010).
More than 20 substances have been identified (Fig. 2) such as Ca2+, cAMP, PKA, EPAC, synaptobrevin, syntaxin, H2O2, and brilliant blue (FCF) that promote vesicle formation and membrane repair and MB, MEL, phosphokinase inhibitor (PKI). Various substances have also been identified that impede membrane repair such as (η, θ) pseudosubstrate fragments (PSFs) (Spaeth et al., 2010, 2012a,b,c; Zuzek et al., 2013). Especially relevant to developing a bio-engineered series of PEG-fusion solutions described below were data showing that membrane sealing was decreased by anti-oxidants such MB, MEL, and PKI and enhanced by oxidants such as H2O2 or Thimerosal (Figs. 2,3).
Figure 3. Sealing probability (%) vs. post-calcium addition (PC) time of B104 cells with neurites transected >50μm or <50μm from the soma.
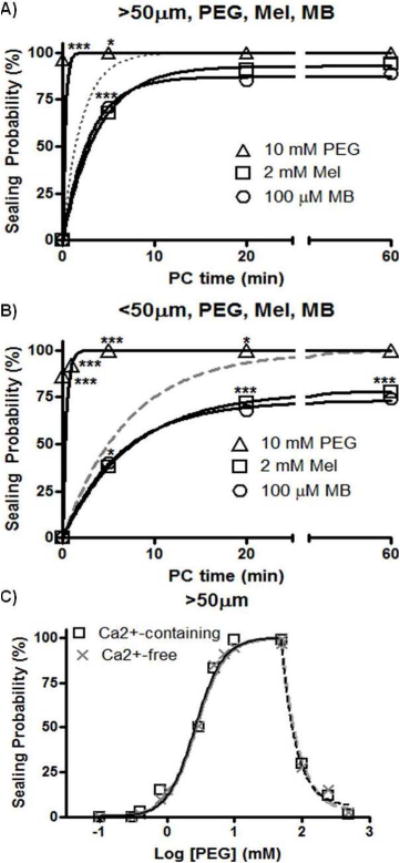
From Spaeth et al., 2012b with permission.
A,B. Neurites transected in Ca2+-free saline containing 2mM MEL (open squares) or 100μM MB (open circles) before adding Ca2+ or 10mM 2kDaPEG (open triangles) for 1min after transection and then adding Ca2+. Solid lines: exponential fit to data. Asterisks: significant differences compared to control sealing (dotted, dashed lines); * = p<0.05, ** <0.01, *** <0.001. B104 cell neurites were transected in Ca2+-free saline containing MEL or MB and then bathed in Ca2+-containing saline (without MEL or MB). Sealing probabilities at each sampling time are compared for different treatments by a Cochran-Mantel-Haenszel χ2 test (CMH); sealing rates for different treatments are compared using Fisher’s Z test (FZT). At various times after Ca2+ addition (post-Ca2+ addition time: PC time), Texas Red dextran dye was added and sealing assessed by dye exclusion.
C. Sealing probability 1min after adding 2kDa PEG vs. log PEG concentration (mM) for B104 cells transected >50μM from the soma and maintained in Ca2+-containing saline (open squares) or Ca2+-free saline (gray X’s). Solid lines: sigmoidal fit to data; Dashed lines: exponential decay fit to data.
In contrast to natural sealing of a damaged plasmalemma, PEG rapidly (<<1sec) and directly “artificially” seals (“PEG-seals”) plasmalemmal damage in vitro and does not utilize any previously reported pathways for membrane fusion in eukaryotic cells (Spaeth et al., 2010, 2012a,b,c) (Figs. 2,3). These data are consistent with hypotheses (Lee and Lentz, 1997; Lore et al., 1999; Lentz, 2007; Spaeth, 2012b) that PEG directly induces membrane fusion by removing waters of hydration at closely apposed membranes, thereby allowing membrane lipids in plasmalemmal leaflets to collapse, fuse and very rapidly (within microseconds) seal cut ends or to spread and seal smaller plasmalemmal holes.
Axonal PEG-fusion in vivo using bioengineered solutions and micro-sutures
We subsequently (Britt et al., 2010; Bittner et al., 2012, 2015; Sexton et al., 2012; Rodriguez-Feo et al., 2013; Ghergherehchi et al., 2015; Riley et al., 2015) used our data on substances that promote or reduce membrane sealing (repair) and concepts on mechanisms and pathways of plasmalemmal sealing to develop and improve a well-defined sequence of bioengineered solutions in combination with micro-sutures to enhance PEG-fusion success (Fig. 4). In a typical preparation, we first confirm in anesthetized rats under sterile surgical conditions that extracellularly-recorded action potentials (compound action potentials, CAPS) taken from a bundle of uncut (intact) nerve axons conduct across the site of any intended lesion (Figs. 1C, 4A, 5A). Using micro-dissection scissors, we then completely cut-sever a bundle of nerve fibers whose cut ends typically separate by 1–3mm. The axons and their sheaths are then carefully trimmed so that the cut ends of host and/or donor nerve axons form smooth flat planes that can be closely apposed with minimal gaps or axonal protrusions (Fig. 4B,C). Cut-severed nerves with carefully-trimmed ends are closely re-apposed with 9-0 or 10-0 micro-sutures to eliminate the 1–3mm gap, but leaving enough space between sutures to allow for diffusion of sterile solutions of PEG, antioxidants, etc., to the lesion site (Fig. 4D–H). [This step is omitted for crush-severance by using a fine-tipped Dumont #5 forceps to crush-sever a 1–2 mm long segment of all axons in a sciatic nerve.] An anti-oxidant(s), usually MB, dissolved in Ca2+-free hypotonic saline is applied to the injury site for 1–2 min (Fig. 4D). Complete single crush- or cut-severance (or two such cuts to remove an ablated segment) is confirmed by visual observation (cut ends separate by several mm) and by again stimulating the axons and demonstrating that severed axons do not conduct CAPS across the lesion site(s) (Figs. 1C, 4E, 5A). Next, PEG in sterile, Ca2+-free, double distilled water (ddH2O) is applied to the lesion site for 1–2min (Fig. 4F). For negative control nerves, PEG is not added to the ddH2O. For another negative control, PEG is sometimes added to ddH20 and applied for 1–2min to the lesion site of nerves that are not micro-sutured, i.e., cut axonal ends that are not closely apposed before adding PEG do not PEG-fuse, but rather they “PEG-seal”, i.e., the cut axonal ends collapse and seal (Spaeth et al., 2012b). Finally, PEG-fused axons are bathed with isotonic Ca2+-containing physiological saline to initiate sealing of any remaining axolemmal disruptions (Fig. 4G). The sciatic nerve is stimulated after all treatments to determine if CAPs again conduct through the lesion site (Figs. 1C, 4H, 5A). Electrophysiological recordings of CAPs from each nerve across a lesion site in vivo are sometimes followed by intra-axonal dye diffusion assessments ex vivo (Figs. 1B, 10A) of axoplasmic and axolemmal morphological integrity (Lore et al., 1999; Britt et al., 2010; Bittner et al., 2012; Ghergherehchi et al., 2015; Riley et al., 2015).
Figure 4. A–G. Bioengineered sequence of solutions and micro-sutures to produce “PEG-sealing” of cut axonal ends.
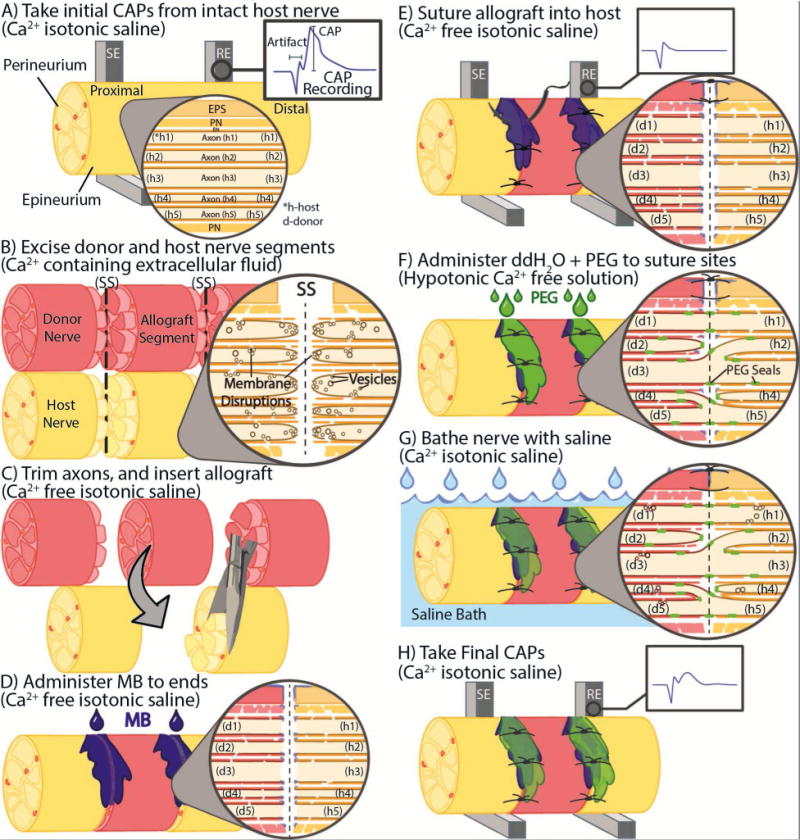
Schematic diagrams showing CAP recording (A), nerve excision (B) and allograft insertion (C) from axons that constitute the sciatic nerve. Higher magnification views in A–G shows five hypothetical axons and their relation to the endoneural (EN), perineural (PN) and epineural (EPS) sheaths. SE: stimulating electrode, RE; recording electrode; SS: severance site. Proximal (central) oriented to the left in A–G. Note that cut axonal ends in the host (h) and donor (d) partially collapse and vesicles begin to seal off the cut ends. The trauma of cutting also damages the axolemma adjacent to the severance site (B).
D–F. Sequence and location of (D) methylene blue (MB) administration, (E) sutures and CAP recording, and F) application of PEG in double distilled water (ddH2O) (C) and their effects on axonal morphology. Note that MB and Ca2+-free isotonic saline unseal axons and prevents vesicle formation (D,E) and that hypotonic solutions open cut axonal ends (F). PEG produces incomplete membrane fusion of apposed axonal ends, possibly connecting more than one axon of unknown specificity on the proximal side of a cut site to one or more axons on the other side of an allograft. For example, motor axons may fuse with motor axons of different specificity or may fuse with sensory axons. Micro-sutures (E) are placed though the epineural sheath to bring axonal ends together, i.e., in close apposition.
G–H. Sequence and location of removal of PEG by isotonic Ca2+-containing saline (G) and recording of CAPs (H). Note that isotonic Ca2+-containing saline causes the formation of vesicles to form that seal off remaining holes in the axolemma at cut axonal ends and adjacent damaged regions (H).
Figure 5. Electrophysiological evidence of PEG-fusion in allografts with trimmed cut ends.
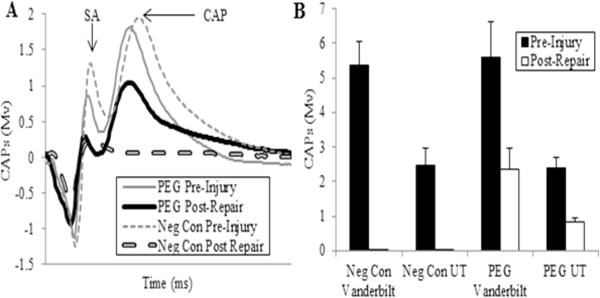
From Riley et al., 2015 with permission.
A. Representative CAP (mV) recordings initially from a negative control (dashed lines) and a PEG-fused (solid lines) allograft pre-injury (thinner lines) and then within 5 min after ablation of a 1 cm segment, insertion of a 1 cm donor segment without (negative control: thicker dashed line) or with PEG-fusion (thicker solid line) of both micro sutured trimmed. SA = arrow points to peak of stimulus artifact. CAP: arrow points to peak amplitude of a CAP.
B. CAPs (mV, mean ± SE) recorded pre injury and immediately post-repair for 4 groups: Negative controls recorded at Vanderbilt University (n=12) and University of Texas at Austin (UT) (n=6), PEG-fused at Vanderbilt University (n=13) and University of Texas at Austin (n=6).
Figure 10. Intra-axonal dye diffusion and axon morphology in allografts.
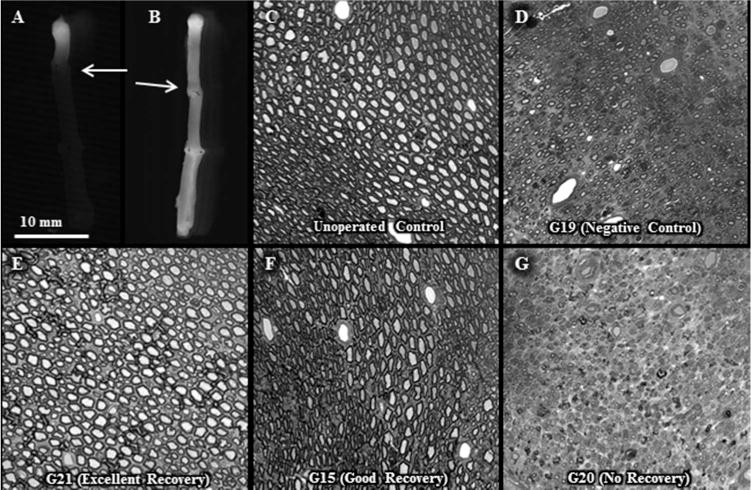
From Riley et al., 2015) with permission.
A, B. No intra axonal dye diffusion of Texas Red at 1d postoperatively in a negative control allograft (A) but observed for a PEG-fused allograft (B). Arrow: site of proximal cut end of host sciatic nerve micro-sutured to proximal end of donor sciatic allograft.
C–G. Increased myelinated axon viability for PEG-fused allografts compared to negative controls or unsuccessful PEG-fusion at 6w postoperatively. Toluidine-blue, plastic embedded sections of sciatic nerves viewed at 20× for an un-operated control (C) and the mid-allograft region at 6wk postoperatively for a negative control (D) and PEG-fused sciatic nerves showing excellent (E), good (F) or no (G) behavioral recovery as assessed by SFI scores (see Fig. 11 and Riley et al., 2015). Note that successful PEG-fusion is correlated with survival of increased numbers of larger diameter myelinated axons within the allograft segment.
As previously defined (Ghergherehchi et al., 2015), unless stated explicitly as a modification, the term PEG-fusion always denotes the following sequence of bio-engineered solutions: “(1) exposure of cut- or crush-severed axonal ends to hypotonic Ca2+- free saline, (2) application of 1% MB in ddH2O for 1–2min, (3) application of PEG in ddH2O for 1–2 min, and (4) extensive rinsing with Ca2+-containing isotonic saline.”
Variables that could affect the ability of PEG-fusion to restore lost functions
From data on plasmalemmal repair described above, we hypothesized that axons with 1–3mm long crush-severances in hypotonic Ca2+-free saline and maintained in Ca2+-free saline would swell/expand so that open axonal ends relatively free of vesicles could come into close apposition and be PEG-fusable; MB or other antioxidants would further reduce vesicle formation. Connective tissue sheaths of crush-severed PNAs remain intact and provide mechanical strength. If crushed or placed in Ca2+-containing salines, axonal ends would fill with vesicles as would the damaged axons between the cut ends and be more difficult (perhaps impossible) to repair by PEG-fusion. Crushes of longer length would have yet-greater inability to PEG-fuse. In the absence of PEG-fusion, the glial sheaths provide mechanical guidance for axons naturally regenerating by outgrowths from surviving proximal ends (Bozkurt et al., 2007; Isaacs, 2010; Burch, 2011). Longer lengths of crushed axons also have poorer natural regeneration.
Similarly, we hypothesize that axons with single cut-severances made in hypotonic Ca2+-free saline and maintained in Ca2+-free saline (plus MB) could be brought into close apposition with micro-sutures and be PEG-fusable. The micro-sutured connective tissue of the epineurium would provide mechanical strength to prevent PEG-fused axons from tearing apart due to nerve stretch/tension during limb movements. If cut or placed in Ca2+-containing salines, the axonal ends would fill with vesicles that could be expelled by placing them in hypotonic salines (with MB) before micro-suturing. Trimming cut ends increases PEG-fusion success by creating a better axonal apposition but decreases success by increasing the amount of stretch tension needed for close apposition. If the gap between cut ends is greater than about 3mm, the surgical literature suggests many nerves are irreversibly damaged and behavioral recovery is very poor or non-existent (Campbell. 2008; Green and Wolfe, 2011). Gaps of > 3mm produced by crush- or cut severance are preferentially repaired by microsutured interposition autograft thereby producing a tension free nerve repair, but also producing a sensory or motor deficit (Birch et al., 1998;Isaacs, 2010;Menorca et al., 2013). Allografts would eliminate such deficits and stretch tension, but might be quickly rejected.
PEG-fusion rapidly rejoins axonal ends crush- severed in Ca2+-free, but not (unless recut) Ca2+-containing salines, as assessed by CAP conduction and intra-axonal dye diffusion
PEG-fusion can rapidly (within minutes) rejoin axonal ends of severed axons as assessed by CAP conduction and intracellular dye diffusion through the severance site (Figs. 1B, C, 4E, 5A, 7A; Lore et al., 1999; Britt et al., 2010; Bittner et al., 2012; Riley et al., 2015). [Cut ends on either side of a severance site are non-specifically fused. See Fig. 5 and Bittner et al., 2012 or Riley et al., 2015.] If sciatic axons crush- or cut-severed in Ca2+-free hypotonic saline are PEG-fused in vivo, Texas red fluorescent dye diffuses into open proximal axonal ends and then diffuses intra-axonally across the lesion site within 24 hours at 4°C, thereby demonstrating axonal morphological continuity through the lesion site. Dye does not diffuse past the lesion site if the cut ends are not PEG-fused. Dye does not diffuse into axonal ends bathed in Ca2+-containing saline with or without PEG even if PEG is added to Ca2+- free solutions containing inhibitors of axolemmal sealing (Spaeth et al., 2012b; Ghergherehchi et al., 2015). That is, PEG bypasses all endogenous sealing pathways to seal axonal transections by collapse and fusion of plasmalemmal membranes at cut axonal ends (“PEG-sealing” in Fig. 2.)
Figure 7. CAP or SFI restoration after PEG-Fusion of axons crush severed- or cut-severed in Ca2+-free or Ca2+-containing salines, with or without careful trimming of cut ends).
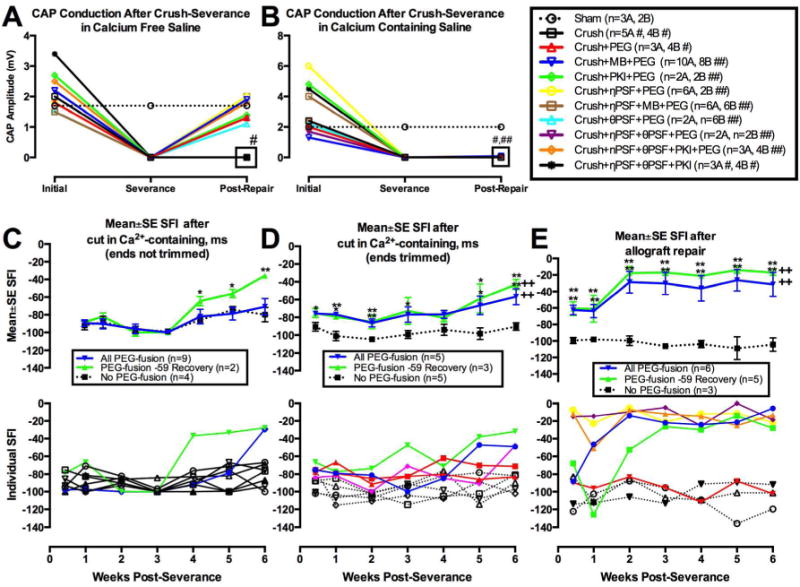
A–D modified from Ghergherehchi et al., 2015 with permission. E modified from Riley et al., 2015 with permission.
A–B. CAPs are restored in vivo within minutes after PEG-fusing sciatic PNAs crushed (A) in Ca2+-free, (B) but not in Ca2+-containing salines having various inhibitors of plasmalemmal sealing as listed in the key to the right of B. Key: the number of animals sampled (n = 4A, 3B) for each protocol plotted in panels A and B (see text).
C–E. Mean SFI ± SE (upper graphs) or individual SFI measures (lower graphs) of behavioral recovery at 3d–6wk postoperatively for sciatic nerves cut-severed (C–D) or ablated (E) in Ca2+-containing salines with or without (negative controls) PEG-fusion.and without (C) or with (D) subsequently carefully trimming cut ends and micro suturing with some axonal stretching (C,D) — or after a carefully-trimmed allograft (E) is inserted and micro-sutured with no stretching of host or donor axons. Key: In upper graphs, “No PEG-fusion” curves show negative control data as black lines and symbols. “PEG-fusion” curves in blue show data for all PEG-fused nerves. “PEG-fusion -59 Recovery” curves in green show data for those PEG-fused sciatic nerves in rats having SFI scores by 6 postoperative weeks of -59 or better. In lower graphs C,D, SFI scores of individual animals with PEG-fused nerves that recovered to SFI scores of -59 or better shown as colored lines, PEG-fused no revovery animals shown as black lines and symbols. E shows individual SFI scores for rats with PEG-fused allografts in colored symbols and lines and negative controls in black symbols and lines. Note that one rat with a PEG-fused allograft did not shopw behavioral recovery (red line and symbols in lower graph of Panel E).
Intra-axonal dye does not diffuse past the lesion site if nerves are crush- or cut-severed in Ca2+-containing saline in vivo, and then subsequently PEG-fused because Ca2+-induced vesicles seal (plug) the open cut ends and impede the ability of PEG to fuse (rejoin) the axolemmas of cut ends of apposed axons (Bittner et al., 2012; Riley et al., 2015; Ghergherehchi et al., 2015). In such cases of vesicle-filled axons, PEG rapidly seals-off the cut ends (PEG-sealing) instead of fusing (joining) them (PEG-fusion). In brief, if axolemmal damage occurs in Ca2+-containing extracellular fluids, (1) Ca2+ influx induces vesicles to form, rapidly migrate and seal the plasmalemmal opening within 5–20 minutes (Krause et al., 1994; Bittner and Fishman, 2000); (2) vesicles at severed ends impede PEG-fusion (Krause and Bittner, 1990; Lore et al., 1999; Spaeth et al., 2012b).
MB and other antioxidants decrease sealing of severed sciatic or B104 cell axons (Spaeth et al., 2010, 2012a,b,c), and increase PEG-fusion success if applied before applying PEG to closely apposed cut axonal ends (Britt et al., 2010). Protein kinase A inhibitor (PKI), protein kinase C isozyme η pseudosubstrate fragment (ηPSF), and protein kinase C isozyme θ pseudosubstrate fragment (θPSF) also decrease the sealing probability of cut axonal ends of B104 cells in vitro (Spaeth et al. 2010, 2012a,b,c; Zuzek et al. 2012) and enhance PEG-fusion as measured by intra-axonal dye diffusion described above or CAP conduction described below.
Figure 7A shows that CAPs were always detected after a sham operation in Ca2+-free saline (Sham; n=3 sciatic nerves). CAP conduction across a crush-severance made in vivo in Ca2+-free saline was restored within minutes in sciatic nerves that PEG-fused in hypotonic saline without adding any antioxidant substances (Crush+PEG, n=3) or if MB was added (Crush+MB+PEG, n=10). CAP conduction was always restored across the crush-severance site if other substances that inhibit axonal sealing were added before adding PEG such as (1) PKI (Crush+PKI +PEG, n=2); (2) nPSF (Crush+nPSF+PEG, n=6); (3) nPSF and MB, (Crush+nPSF+MB+PEG, n=6); (4) θPSF (Crush+θPSF+PEG; n=2); (5) nPSF and θPSF (crush+nPSF+θPSF+PEG; n=2): and, (6) nPSF, θPSF and PKI (Crush+nPSF+θPSF+PKI+PEG, n=3). CAPS were not restored if all PNAs in the sciatic nerve were crush-severed in vivo, but not subsequently PEG-fused in Ca2+-free saline for two experimental protocols (Crush; n=5 and Crush+nPSF+θPSF+PKI; n=3). CAP conduction was not restored if PEG-fusion was not attempted after crush-severance, even if a set of sealing inhibitors were added (Crush; Crush+nPSF+θPSF+PKI).
For examples of attempted PEG-fusion in Ca2+-containing extracellular fluids, Figure 7B shows CAP conduction through the crush-severance site in vivo for the same experimental protocols shown in Figure 2A, with the exception that the crush-severance was made in Ca2+-containing saline. Except for the sham protocol (Sham; n=2), CAP conduction across the crush-severance site was not restored for any PEG protocol (all labeled with ## in the key), including PEG protocols that combined a set of inhibitors of plasmalemmal sealing that would be expected to enhance PEG-fusion success (e.g., Crush+nPSF+θPSF+PKI+PEG; n=4). Similar to Figure 7A, protocols in which crush-severance is not followed by PEG-fusion do not restore CAP through-conduction (Crush; Crush+nPSF+θPSF+PKI). Both protocols are labeled with # in the key to the right of Figure 7B. If a crush-severance was made and maintained for 30 minutes in Ca2+-containing saline, CAP conduction could be restored, if the crush site was excised in Ca2+-free saline and then PEG-fused (Fig. 2C of Ghergherehchi et al., 2015).
PEG-fusion can rapidly rejoin axonal ends cut- severed in Ca2+-free and Ca2+-containing salines, as assessed by CAP conduction and intra-axonal dye diffusion
After cut-severance in Ca2+-free saline, CAP conduction was rapidly restored if sciatic nerves were PEG-fused by our standard technology (Britt et al., 2010; Bittner et al.,. 2012; Ghergherehchi et al., 2015). If sciatic nerves were cut-severed and then immediately PEG-fused in Ca2+-containing saline, CAP conduction was not restored. However, if sciatic nerves were cut-severed and maintained in Ca2+-containing saline for 30 minutes, but then repaired by rinsing the ends in hypotonic Ca2+-free saline before microsuturing and PEG-fused by our standard technique, CAP conduction was restored, i.e., cut-severed ends were joined so that some PNAs were morphologically intact through the lesion site.
Behavioral assessments are the best measures of PEG-fusion success
Although intra-axonal dye diffusion and CAP-conduction through a PEG-fused lesion site provide good evidence that PEG-fusion has rapidly restored membrane and cytoplasmic continuity to some axons of unknown proximal and distal specificity across the lesion, behavioral assessments of functional recovery are by far the most important measure to assess the success of PEG-fusion or any other current method to repair severed PNAs or STAs (Britt et al., 2010; Bittner et al., 2012, 2015; Ghergherehchi et al., 2015; Riley et al., 2015). [CAP amplitudes, counts of axon number, size, etc., distal to a lesion site are useful indicators, but not definitive measures, of regeneration success, e.g., counts of axonal outgrowths that make inappropriate connections that do not restore lost behaviors are not an indicator of successful regeneration.] In our studies (Figs. 6,7), behavioral assessments of PEG-fusion are performed by experienced testers blind to treatment conditions. Rats are handled daily for seven days and baseline behavior scores for tests such as the Sciatic Functional Index (SFI), Foot Fault (FF), or Basso, Beattie, Bresnahan (BBB) are obtained 1–2 days prior to surgery for PNA or STA assessments. After surgery, rats are typically evaluated for behavioral recovery at 1–3 days and then at weekly intervals for 6 to 18 postoperative weeks. The SFI uses footprints to measure gait quality to assess how well sciatic PNAs activate more distal muscle groups subsequently (de Medinaceli et al., 1982; Britt et al., 2010; Wood et al., 2011; Bittner et al., 2012; Ghergherehchi et al., 2015; Riley et al., 2015). Compared to the SFI, FF tests indicate how well sciatic PNAs activate more proximal muscle groups subsequently (Britt et al., 2010; Sexton et al., 2012; Rodriguez-Feo et al., 2013). The BBB is an open field test to assess STA and other spinal functions (Basso et al., 1995).
Figure 6. Mean SFI scores for Cut or Crush treatment groups vs. post-operative week.
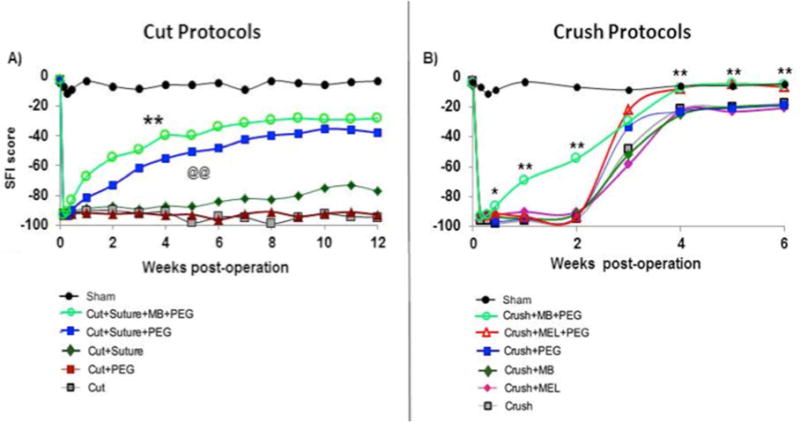
From Bittner et al., 2012 with permission. See Bittner et al., 2012 for tabulated means+/−SEM and detailed statistical comparisons.
A, B. **above cut protocol curves in A identify post-operative data points that significantly (p < 0.01) differ from the Cut (no PEG, no micro-suture) curve. For Crush Protocols (B), asterisks (*) identify individual post-operative data points for Crush+MB+PEG treatment group that differ significantly from data points for the Crush group at the same post-operative time. *= p<0.05, ** = p<0.01. Each group has at least 20 animals, over 300 total animals in the study. @@ indicates significant difference of p<0.01 of MEL group vs. negative control group curves.
For PNA severance, we also record CAPs from sciatic nerves before and after any injury and/or subsequent treatment (Britt et al., 2010; Bittner et al., 2012; Sexton et al., 2012; Rodriguez-Feo et al., 2013; Ghergherehchi et al., 2015; Riley et al., 2015) to correlate our measure of electro-physiological continuity with our measures of behavioral recovery for each rat (Figs. 5, 7A,B, 11E,F). We correlate intracellular dye diffusion with CAP conduction across the lesion site and with behavioral recovery (Britt et al., 2010; Bittner et al., 2012; Ghergherehchi et al., 2015; Riley et al., 2015).
Figure 11.
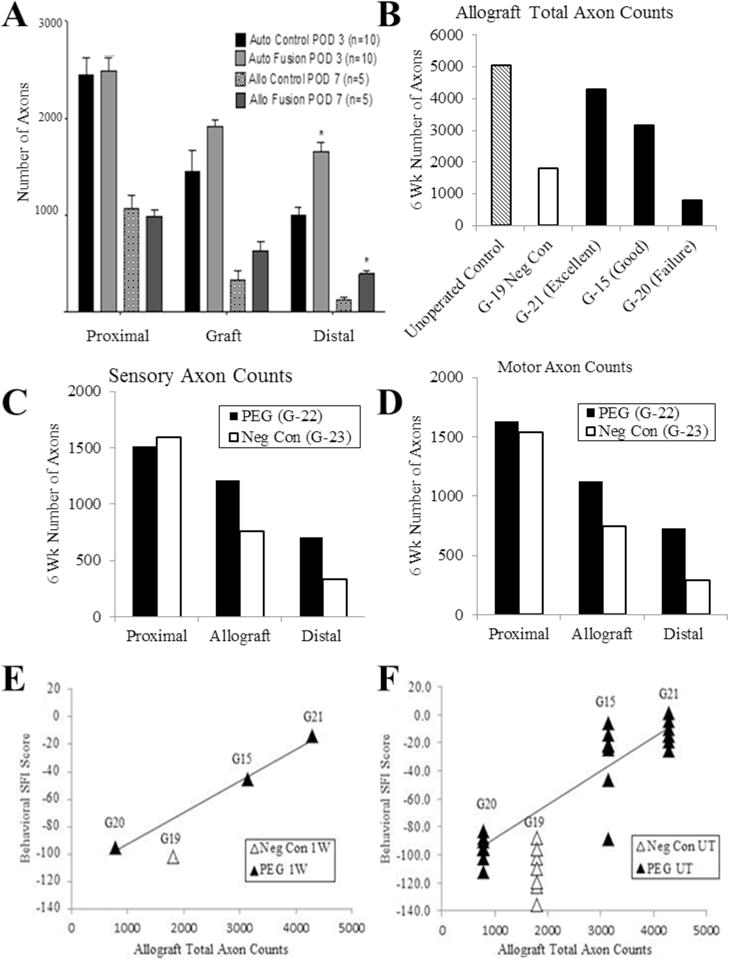
Counts of sensory, motor, or total number of axons in sciatic nerves repaired by PEG-fused autografts or allografts. A. From Sexton et al., 2013 with permission. B–F. From Riley et al., 2015 with permission. A. Total number of viable motor and sensory axons in cross sections of sciatic nerves for negative control (no PEG-fusion) vs. PEG-fused sciatic PNAs in autografts and allografts. Sensory PNAs were identified by immunohistochemical staining for carbonic anhydrase II (CA II) antibodies and motor PNAs by staining for choline-O-acetyl- transferase antibodies (Co-Ach). These data show no statistically significant difference for PEG-treated vs. controls in the proximal sciatic nerve but always a significantly greater number of PNAs in PEG-treated rats within the grafts or distal to the grafts. That is, PEG-fusion retards or reduces Wallerian degeneration. From Sexton et al., 2014.
B. Total viable myelinated axon counts (mean, n=4) from a representative cross-section of sciatic nerves from the allograft region of unoperated control, negative control, and three PEG-fused allografts showing excellent, good or no SFI behavioral recovery 6wk postoperatively.
C, D. Sensory (C) or motor (D) viable myelinated axon counts (mean ± SE, n=6) at 6wk post-operatively from a representative cross section from each of proximal, allograft, and distal regions of PEG-fused and negative control animals.
E. SFI scores at 1w for each animal as listed vs. the number of surviving myelinated axons in allografts at 6wk postoperatively. R2 = 0.9946, p < 0.05. G19 = negative control animal not PEG-fused.
F. SFI scores at 1–6wk individually plotted for each animal as listed vs. the number of surviving myelinated axons in allografts at 6wk postoperatively.
PEG-fusion repair of cut-severed or ablated sciatic nerves is not successful for every rat, perhaps due to variation in surgical skill and other factors as noted in clinical settings (Allan, 2000; Campbell, 2008; Green and Wolfe, 2011). Hence, we often plot SFI data from animals having PEG-fused sciatic nerves in one or more ways as shown in different panels of Figure 7C–E: (1) SFI data from all rats in a group receiving the same protocol (All PEG-Fusion in Fig 7); (2) SFI data from rats in the same group that showed significant (p< 0.003, see below) behavioral recovery as measured by SFI scores better than -59 by six postoperative weeks (PEG-fusion -59 Recovery); and, (3) SFI data from those rats that did not show significant behavioral recovery to an SFI score of -59 by six postoperative weeks (PEG-fusion No -59 Recovery).
Our rationale for subdividing “All PEG-fused animals” is as follows: Mean SFI ± SE scores of negative control rats was −89 ± 10 (n=21) at 6 post-operative weeks and the probability of any rat having a score of -59 was p < 0.003. [No negative control rat had an SFI score better than -66 at 6 post-operative weeks.] Some rats with micro-sutured, cut-severed, PEG-fused sciatic nerves exhibited an SFI score of -59 or better at 6 post-operative weeks in several studies (Bittner et al., 2012; Ghergherehchi et al., 2015) as did some rats having PEG-fused allografts (Riley et al., 2015). That is, for each rat shown in Figure 7C–E, PEG-fusion can fail or succeed to repair a cut-severed sciatic nerve with success defined as an SFI score of -59 or better. This criterion is a very conservative measure of behavioral recovery.
Behavioral recovery after crush- or cut-severance of sciatic PNAs in Ca2+-free salines
In a large study of over 300 animals with at least 20 in each crush- or cut-severed group (Bittner et al., 2012), behavioral recovery was greatest after cut-or crush-severance in Ca2+-free salines when an anti-oxidant (MEL or MB) was applied to reduce vesicle formation in open axonal ends, before applying PEG (Fig. 6A,B); MB was more effective than MEL (Fig. 6A,B). Cut-severed sciatic nerves PEG-fused using our standard technology (using MB) showed significant (p<0.01) enhancement of behavioral recovery at all post-operatives times (Fig. 6A) and recovered on average to near-normal SFI scores of −20. Negative control cut-severed sciatic PNAs showed little, if any, behavioral recovery without micro-sutures and about 20% recovery with micro-sutures (Fig. 6A). PEG applied without micro-sutures to appose the cut ends did not increase PEG-fusion repair over negative controls without micro-sutures which showed no recovery for up to 12 postoperative weeks (Fig. 6A). That is, if two cut ends are not closely apposed (touching), PEG induces cut ends to collapse and seal within a fraction of a second (“PEG-sealing”: Spaeth et al., 2012b; Figs. 2,3) rather than to rejoin the two cut ends (“PEG-fusion”: Lore et al., 1999; Britt et al., 2010; Bittner et al., 2012; Sexton et al., 2012; Rodriguez-Feo et al., 2013; Ghergherehchi et al., 2015; Riley et al., 2015; Fig. 4F). Animals shown in Figure 6 were followed for an additional 6 weeks with no further recovery or change in plateau values for any group.
Figure 6B shows that negative control crush-severed sciatic nerves recovered to near-normal mean SFI scores of −20 and PEG-fused nerves to about 0 mean SFI scores, i.e., equivalent to SFI scores of uninjured animals. PEG-fused crush-severed nerves recovered significantly more rapidly at 3d–2wks than negative control animals (p<0.05 or p<0.01, asterisks in Fig 6B). This enhanced behavioral recovery after PEG-fusion was not observed in a much smaller sample size by Ghergherehchi et al. (2015).
This recent study by Ghergherehchi et al., (2015) has also shown that trimming of cut ends greatly enhances behavioral recovery for PEG-fusion of sciatic nerves cut-severed in Ca2+-free saline. For example, without trimming cut ends, mean ± SE SFI scores of all PEG-fused animals (n=8) were not significantly different from such measures for negative control animals (n=4). Some (3/8) of these animals with PEG-fused sciatic nerves showed significant recovery at five (p<0.05) and six (p<0.01) wks postoperatively, compared to the SFIs of the negative control group. After cut-severance in Ca2+-free saline with trimmed ends, the mean ± SE SFI scores of all PEG-fused animals (n=5) were significantly (p<0.01) different from the negative control animals (n=5) and all 5 PEG-fused animals had individual SFI scores of -59 or better by 6 wks postoperatively. The “All PEG-fusion” curve showed significantly (p<0.01) enhanced behavioral recovery compared to the negative control curve according to an ANOVA.
Behavioral recovery after crush-severance in Ca2+-containing salines
Our recent data (Ghergherehchi et al., 2015) confirm that crush-severance lesions of less than 3mm in length made in Ca2+-containing salines usually behaviorally recover to SFI scores of 0 to −20 in the absence of PEG-fusion repair (negative control rats), as previously reported (Hare et al., 1992; Wood et al., 2011). Our PEG-fusion technology did not significantly (p>0.05) increase SFI recovery for crush-severance lesions made in Ca2+-containing salines, unless the crushed ends were cut and microsutured (see Fig. 3 of Ghergherehchi et al., 2015).
Behavioral recovery after cut-severance in Ca2+-containing salines
Behavioral recovery was significantly greater than negative controls after 6 postoperative weeks for sciatic nerves cut-severed made in Ca2+-containing salines if cut ends were not trimmed (Fig. 7C). Figure 7C also shows that “PEG-Fusion -59 Recovery” animals (2/9) exhibited significant behavioral recovery at weeks 4 and 5 (p<0.05), as well as week 6 (p<0.01), compared to the SFIs of the negative control group. There were no significant differences (p>0.05, ANOVA) between PEG-fusion curves and the negative control curve.
Behavioral recovery was further enhanced if cuts were made in Ca2+-containing salines and cut ends were trimmed (Fig. 7D). That is, Figure 7C shows that after cut-severance in Ca2+-containing saline with trimmed ends, the mean ± SE SFI scores of all five PEG-fused animals was significantly better (p = 0.05 to 0.01) than the mean SFI scores of five negative control animals at 72hrs, 2, 3, 5and 6wks post-operatively. The curves for the two groups of rats with successful PEG-fused sciatic nerves in Figure 7D (All PEG-Fusion, PEG-Fusion -59 Recovery, PEG-Fusion No -59 Recovery) were significantly (p<0.01, ANOVA) better than the negative control curve. Note that the negative control curves in Figure 7 are for sciatic nerves repaired with micro-sutures, typically considered the best repair technique currently in clinical use (Isaacs, 2010).
PEG-fused autografts increase behavioral recovery
Sciatic PNAs with 0.5–1mm ablated segments can be repaired by reinserting the ablated segment as a micro-sutured autograft after carefully trimming all cut ends and PEG-fusing PNAs at both cut ends of the autograft (Sexton et al., 2012). Carefully trimming all cut ends of autografts in which the ablated sciatic segment is carefully re-aligned and re-inserted into the original gap increases the gap length between cut ends to be micro-sutured. This procedure stretches all cut ends during micro-suturing. In such cases, PEG-fusion produces some behavioral recovery, but not to the extent possible with allograft repair (see next section; Ghergherehchi et al., 2015; Riley et al., 2015). Using other sources for autografts (e.g., sural sensory nerves) should eliminate nerve stretching that decreases PEG-fusion success in animal models (Fig. 7) and also successful behavioral recovery in current clinical practice (Campbell, 2008; Fox and Kreishman, 2010; Wolfe et al., 2010). Without PEG-fusion, interposition autografts to replace ablated segments have slightly better outcomes than no treatment, but have much poorer behavioral and morphological outcomes compared to PEG-fused autografts, or especially PEG-fused allografts (see below and Figs. 8–11). Without PEG-fusion, behavioral recovery may be poor in part because mostly-irreversible muscle atrophy may develop before re-innervation by axonal outgrowth at 1–2 mm/day (Allan, 2000; MacKinnon et al., 2001; Campbell, 2008; Williams and Delon, 2008; Fox and Kreishman, 2010; Fox et al., 2011; Birch, 2011) and in part because much Wallerian degeneration occurs in the absence of PEG-fusion (Riley et al., 2015).
Figure 8. Axonal Diameters and G ratios.
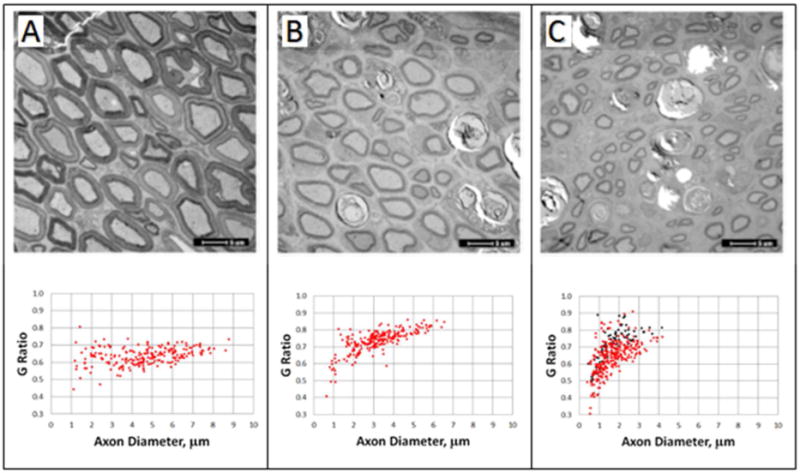
From Ghergherehchi et al., 2015 with permission.
Cross-section EM images of un-operated (A) and single cut rat sciatic nerves with PEG-fusion repair (B) and without PEG-fusion repair (C) 6 weeks postoperatively. Scatterplots of G ratio versus axon diameter, for un-operated (A), PEG-fused (B), and non-PEG fused (C) sciatic nerves 6 weeks post-severance. Red data points: axons measured inside the sciatic epineural sheath; black data points: axons measured outside the sciatic epineural sheath.
PEG-fused allografts dramatically increase SFI recovery and distal segment survival
The best behavioral recoveries (Fig. 7E) and morphological survival of severed distal axonal segments cut in in Ca2+-containing salines (Figs. 8–12) obtained to date have occurred using PEG-fused allografts (Riley et al., 2015). Sciatic PNAs with 0.5–1 mm ablated segments can be repaired with no nerve stretch, sometimes with dramatically rapid and near-complete behavioral recover within a week, by PEG-fusing a sciatic nerve allograft from a donor rat whose length is slightly longer than the ablated host segment after all cut ends are carefully trimmed. Compared to negative controls, many more severed distal axonal segments survive in PEG-fused allografts (Figs. 9–11).
Figure 9.
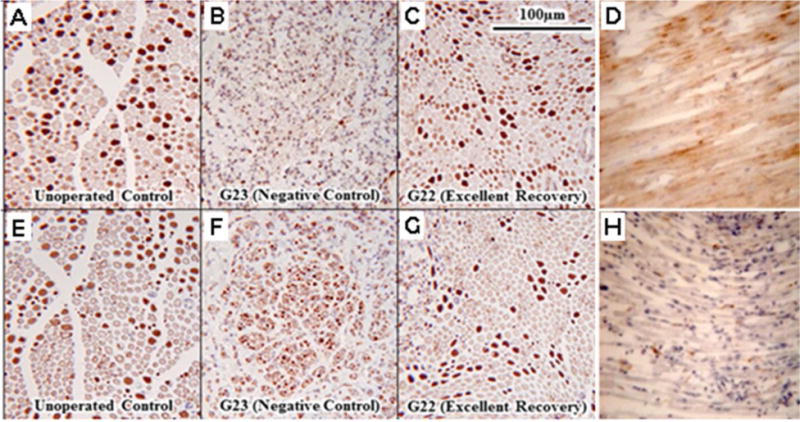
Sciatic axons stained to identify sensory, motor or degenerating axons. A–C, E–G. From Riley et al., 2015 with permission.
Sensory (A–C) or motor (E–G) myelinated axons in paraffin-embedded sections of sciatic nerves viewed at 20× stained for viability by CA II (A–C) or Co-Ach (E–G), respectively, for an unoperated control (A,D) and distal to the allograft at 6wk postoperatively for a negative control (B,F) and PEG-fused sciatic nerve (C,G) showing excellent behavioral recovery. Note that the negative control sciatic nerve (B,F) has very few deeply stained sensory or motor axons whereas the PEG-fused sciatic nerve (C,G) more closely resembles the un-operated control (A,E), i.e., further evidence that PEG-fusion retards or prevents Wallerian degeneration after myelinated axons are severed from their cell body. D,H. Longitudinal sections distal to a complete transection site stained with a marker (NPY1) for Wallerian degenerating PNAs (brown color) for a negative control (D) and PEG-fused rat sciatic axon (H).
Taken together, recently reported data (Bittner et al., 2012; Ghergherehchi et al., 2015, Riley et al., 2015) some of which is shown in Figures 6–7, demonstrate that (1) Trimming cut ends after an axotomy significantly improves PEG-fusion success; (2) All other variables being equal, severing sciatic PNAs in a Ca2+-free environment produces significantly better results than severing in a Ca2+-containing environment; and, (3) Eliminating any tension on, or stretch of, sciatic axons during PEG-fusion enhances behavioral recovery. The dramatic behavioral recovery produced by PEG-fused allografts is likely due to little or no nerve stretching during surgery, even though both ends of the allograft need be PEG-fused. The stretching of nerve axons during micro-suturing the cut ends to close a 1–3mm gap following a single cut-severance almost-certainly results in more nerve ischemia or tearing of tissues held together by sutures (suture failure: Clark et al., 1992).
Morphological evidence that severed distal PNAs survive for many weeks if PEG-fused
Morphological data assessing for successful PEG-fusion of severed PNAs are usually collected from sciatic nerves, tested for CAP conduction across the lesion site, of several behaviorally-tested animals typically sacrificed at each of 1,7,14,30 and 60 days (Figs. 8–11; Sexton et al., 2012; Rodriguez-Feo et al., 2013; Ghergherehchi et al., 2015; Riley et al., 2015). EM tissues were stained en bloc (Figs. 8A–F, 10; Ghergherehchi et al., 2015; Riley et al., 2015). For immunohistochemical stains (Fig. 9), nerves from transcardially perfused were stained using commercial antibodies specifically directed against sensory and motor axons. Viable myelinated axons within and outside the sciatic epineural sheath that had an intact, non-collapsed myelin sheath and no dense-staining axoplasm were counted.
Successful PEG-fusion as measured by SFI scores of rats having cut (Fig. 6,7), autografted, or allografted (Fig. 7) sciatic PNAs produces axonal continuity through the lesion site(s) as measured by axonal conduction (Fig.5, 6A,B) or intracellular dye diffusion through all PEG-fused lesion sites (Fig. 10A, B). Successful PEG-fusion prevents much Wallerian degeneration as measured by light and electron microscopy of plastic embedded cross sections (Figs. 8,10), stains for Wallerian degeneration (Fig. 9D,H), or stains (Fig. 9A–C,E–G) and counts of motor, sensory, or total axons (Fig. 11) in all portions of sciatic cross sections (Sexton et al., 2012; Rodriguez-Feo et al., 2013; Ghergherehchi et al., 2015; Riley et al., 2015). Neuromuscular junctions (NMJs) in animals with PEG-fused allografts are easily discernable for 1–6w postoperatively, and synaptic boutons, secondary folds and terminal Schwann cells appear near-normal. NMJs are intact though they exhibit some redundant basal lamina around the terminal Schwann cells, but not in the synaptic cleft.
Figure 8 shows EM cross sections (upper portion of panels) and G ratios (axon diameter/axon+myelin diameter) of sciatic axons taken inside or outside the epineural sheath (lower portion of each panel) of (A) intact, or just distal to the lesion site of (A) singly cut, or (B) carefully trimmed and PEG-fused or (C) negative control (cut/carefully trimmed, micro-sutured) (Ghergherehchi et al., 2015). These EM sections and measurements for intact axons (Fig. 8A) had a rather wide range of PNA diameters (1–9μm; 4.62 μm mean ± 1.71SD, n=219) and a rather narrow range of G ratios (0.43- 0.81; 0.63 mean ± 0.05SD). Successfully PEG-fused sciatic nerves (Fig. 8B) showed a similar pattern for PNAs at 6 post-operative weeks (axon diameter: 3.25 ± 1.23μm; G ratio: 0.73 ± 0.07, n = 238). In contrast, many small diameter PNAs were found outside the epineural sheath in negative controls (Fig. 8C: black filled circles). Furthermore, those PNAs inside the epineural sheaths of negative controls (Fig. 8F) had smaller diameters (1.75 μm ± 0.75; G ratio: 0.71 ± 0.09, n= 288) compared to PNAs inside the sheath in intact controls (Fig. 8A) or PEG-fused nerves (Fig. 8B). A Student’s t-test with an ANOVA followed by post-hoc analysis showed that each group’s diameters differed significantly (p<0.001) from all other groups.
A few small diameter PNAs were found >0.5cm more distal to the cut in successfully PEG-fused animals. Small diameter nerve axons are a characteristic of PNAs regenerating by outgrowths (Ghergherehchi et al., 2015). These data on PNA diameters following simple cuts with and without PEG-fusion were very similar to preliminary data for allografts with and without successful PEG-fusion.
Successful PEG-fusion of cut-severed sciatic nerves, but not negative control nerves, was associated with intact and well preserved NMJs in soleus muscles, as found for successfully PEG-fused allografts. All these morphological data from simple cuts and allografts are consistent with a hypothesis that PEG-fusion prevents or greatly retards Wallerian degeneration of severed distal PNA segments, and does not prevent regeneration by outgrowth of those axons that were not successfully PEG-fused.
As illustrated for autografts and allografts at 3–7 postoperative days (Fig. 11A), the number of viable axons distal to a cut-lesion site decreases with increasing peripheral distance, but the number of viable axons at any point is always greater for PEG-fused nerves compared to their paired negative controls. This result holds for sensory, motor or total axons for cuts and allografts at 6 postoperative weeks (Fig.11B–D) and other sampling times (Sexton et al., 2012; Riley et al., 2015). An increased number of viable axons distal to a PEG-fusion site also correlates with a better SFI score (Fig. 11E, F). All these data are consistent with a hypothesis that PEG-fusion connects proximal and distal PNA segments thereby retarding or preventing Wallerian degeneration and the more successful PEG-fusions connect a greater number of proximal and distal segments (Ghergherehchi et al., 2015).
PEG-fused sciatic PNAs exhibit survival in all regions of allograft cross sections
PNAs appear intact and morphologically indistinguishable from unoperated axons for both peripheral and central-core regions of successfully-PEG-fused sciatic nerve allografts from animals sacrificed at 6 post-operative weeks with excellent SFI recovery to intact control levels (SFI = +5 to −10). Under higher magnification, there are few structures associated with degenerating axons such as macrophages or macrophage-like cells or highly osmophilic membrane whorls (Riley et al., 2015). Such data suggest that PNAs and other non-neuronal tissue are not being rejected after successful PEG-fusion. Macrophages and osmophilic membrane whorls are frequent, prominent, and evenly distributed in unsuccessfully-PEG-fused nerves. These data also suggest that PEG and other solutions required for PEG-fusion are diffusing to all cross-sectional regions of a 2–3mm diameter rat sciatic nerve (Ghergherehchi et al., 2015).
Mechanisms of PEG-fusion repair after cutting PNS axons
The simplest possible (and perhaps most-intuitive) explanation for any PEG-fusion success in crushes, cuts, autografts, or allografts would be to assume that PEG-fusion reconnects appropriate proximal and distal portions of transected sensory and motor axons. However, for successful PEG-fusion repair of simple transections of mixed sciatic nerves in rats, much less for allografts, there is no readily-conceivable way that we are selectively reconnecting distal and proximal portions of severed motor or sensory axons, much less individually-identifiable axons, especially following ablations and insertions of autografts and donor allografts (Bittner et al., 2012; Riley et al., 2015). In fact for donor allografts, there are no axons with all appropriate connections to PEG-fuse. The ability of our PEG-fusion technology to rapidly restore lost behavioral functions is most curious since at present we do not have basic-science answers as to whether (1) distal portions of sensory or motor axons are re-specified by the PEG-fused proximal portion; (2) distal portions of sensory or motor axons re-specify spinal connections made by the PEG-fused proximal segment; (3) collateral outgrowths from surviving distal and motor axons rather quickly remake a set of new peripheral connections; (4) sensory or motor synapses in central pattern generators in spinal cords or higher CNS brain centers are highly plastic and can quickly alter the interpretation of sensory inputs and use of motor peripheral connections. Furthermore (5), it is very curious that successfully PEG-fused allografts are not obviously rejected (as assessed by histological observations or SFI tests), even when those allografts are taken from different animals of the same or different outbred strains (Riley et al., 2015).
Data can almost certainly be obtained on the existence and extent of the mechanisms underlying PEG-fusion success by tracer and immunological techniques. For example, pre- labeling specific sensory or motor axons in the sciatic nerve, and then re-labeling them after transection with or without PEG-fusion with alternative tracers could allow us to identify potential reinstatements in connectivity (e.g., co-labeling within the same axons) or alterations in axonal identity and the impact of PEG-fusion on such alterations. Similarly, pre-labeling of sensory or motor axons in donor animals, and then re-labeling them after allografting could help elucidate the basis and mechanisms of enhanced recovery seen after allograft repairs. For example, should proximal motor axons become fused to distal sensory axons, does collateral sprouting occur from the motor axons to allow for re-innervation of the target musculature? We could use autografts to exchange portions of purely motor branches with portions of purely sensory branches to assess efficacy of such grafts and plasticity within them. Centrally, the specific locations of the projections from the sensory axons from the separate branches of the sciatic as they project into the dorsal horn, and the differing lumbar levels and dendritic architectures of the motoneurons innervating the muscles of the lower leg and foot could provide a powerful way to examine the central consequences of nerve repair. Following local and discrete tracer injections of cutaneous or muscle targets, preservation of, or potential alterations in their central representations could be assessed after transection with or without PEG-fusion. For example, should proximal sensory axons from a particular branch of the sciatic nerve become fused with distal sensory axons belonging to a different nerve branch, does the central representation now reflect such reassignment? Similarly, should proximal motor axons from a given motor population become fused with distal motor axons innervating a different target muscle, do the re-specified motoneurons adopt a dendritic architecture appropriate to the new target?
To examine the absence vs. presence of immunological rejection for PEG-fused vs. negative control (non-PEG-fused) allografts, one could determine if PEG-fusion allows a more rapid infiltration of recipient GFP-labeled Schwann cells (Whitlock et al., 2010) or if rescuing the graft axons with PEG-fusion facilitates donor Schwann cell survival. Similarly, the cellular composition and quality of wound healing could be evaluated by selected immunohistochemical markers, i.e., to examine the proliferative index (Ki67), apoptotic index (TUNEL), macrophage influx (pan macrophage marker CD68, and subset M1, M2 antibodies), ingrowth of dermal capillaries (Factor VIII), as well as study the degree of new collagen deposition as viewed from Trichrome sections to determine changes in the local wound environment (Nanney et al., 1998). Furthermore, a quantitative analysis of T cell subsets in the animals could be performed to determine the CD4+/CD68+ T cell ratio to determine PEG’s effect on helper/inducer and cytotoxic/suppressor T cell presence after nerve allograft placement (Whitlock et al., 2010; Yan et al., 2013).
Retardation of Wallerian degeneration and PEG-fusion of chronically cut PNAs
Wallerian degeneration of mammalian PNS axons can be retarded for ≥ 6 days by cooling PNS axons in a body part to 23°C, for ≥10 days at 13°C (Sea et al., 1995) or for ≥ 5 days by daily injections of 10mg/kg cyclosporine A (Sunio and Bittner, 1997). Retarding Wallerian degeneration in vivo extends the time window for PEG-fusion repair so that chronically severed rat sciatic axons can be maintained viable for several days and successfully PEG-fused (Lore et al., 1999; Marzullo et al., 2002). Sciatic nerves can be cut in Ca2+-containing saline, sealed, and then re-cut in Ca2+-free saline to unseal and then PEG-fused (Lore et al., 1999; Marzullo et al., 2002; Bittner et al., 2012). Doubly-cut (allograft) segments of mammalian PNS axons maintained chronically in aerated, sterile, Ca2+-free saline at 6°C–9°C for at least 3 days in vitro can be cut again and PEG-fused as allografts (Riley et al, 2015).
PEG-fusion technology can also be used to repair damage to STAs
Spinal compression/contusion injuries that often sever many STAs are responsible for much of the observed functional (behavioral) deficits observed subsequent to SCI. Hence, our PEG-fusion technology might conceivably be applied to repairing STAs in SCI cut or crush injuries. However, following a cut injury, severed STAs and other tissues on either side of the cut typically retract and separate by several millimeters (Bittner et al., 2015). In SCIs, there is no tightly adhering connective tissue comparable to the PNA epineurium or perineurium through which micro-sutures might be put to bring the STA cut ends in close apposition. However, many SCIs result from crush, rather than cut, traumas in which the severed ends of STAs are connected by damaged glial sheaths and damaged axolemmal membranes. Some of these crushed STAs might possibly be repaired by our PEG-fusion technology as are crushed PNAs (Britt et al., 2010; Bittner et al., 2012; Ghergherehchi et al., 2015).
We examined the possibility that PEG-fusion might restore behavioral functions after crush lesions, using a rat model of acute contusive SCI produced by a MASCIS device (Bittner et al., 2015; Fig. 12). In this study, a series of solutions including or not including PEG was applied to the surface of the spinal cord immediately after injury. In the first week after injury, the mean BBB behavior score of the control-vehicle treated group (3.17±1.25 SEM, n=6) was not significantly different from the PEG treated group (2.83±0.91 SEM, n=6). However at 2 to 5 weeks after injury, the mean scores of the PEG treated group were significantly (p<0.05) 3–4 points higher (more recovery) than those of the control group. For the 5 week period, the PEG- fused experimental group scored significantly (p<0.001) better than the control group, according to a two-way ANOVA with Bonferroni post-test. While this first attempt using PEG-fusion to restore behaviors lost to STA severance did not produce recoveries as rapid or as dramatic as that produced by PEG-fusion of cut, ablated or severed PNAs, it does suggest that our PEG-fusion technology may be useful to enhance behavioral recovery after SCI. Behavioral recovery might be further enhanced if we can devise ways to more closely appose more crush- or cut-severed STAs before applying PEG.
Why evolve a curiously problematic nature of axonal regeneration in mammals?
The cellular/system-level mechanisms outlined in 1–5 above for successful PEG-fusion may be unknown, but data can almost certainly be obtained for their identification. It is more difficult to obtain data on why mammals have evolved such a curiously problematic mechanism of axonal regeneration compared to many invertebrate axons and some axons in lower vertebrates (Bittner 1991; Bittner et al., 2000). Mammalian mechanisms of (1) rapid Wallerian degeneration of distal segments together with (2) slow and relatively non-directed outgrowths from surviving proximal segments have a low probability to restore lost behaviors after months to years– compared to much more successful invertebrate mechanisms of (1) long-term survival of severed distal segments for months to years together with (2) slow and directed outgrowths from surviving proximal segments that activate surviving distal segments with a high probability to completely restore lost behaviors within days. We can speculate that rapid degeneration of severed distal segments evolved in mammals (and birds) because of their higher body temperatures that provide better conditions for rapid growth of bacteria. Distal segments are actively phagocytosed and autolysed (Schlaepfer and Bunge, 1973; Raabe et al., 1996a,b; Tsao et al., 1999). [Ectothermic vertebrates have some axons that exhibit long-term survival of distal segments for about 60 days before undergoing phagocytosis and autolysis (Zottoli et al., 1987; Blundon et al., 1990; Bittner, 1991; Bittner et al., 2000).] Furthermore, many more vertebrate axons are myelinated compared to invertebrates, and myelin reduces glial-axon transfer of proteins and other trophic substances (Sheller et al., 1995, 1996; Tanner et al., 1995a,b). CNS axons in vertebrates and invertebrates often exhibit much less ability to successfully regenerate compared to PNS axons, especially for CNS axons that are not as exposed to incidental trauma. In all phyla, cells that are exposed to trauma tend to evolve mechanisms for their regeneration compared to more-protected cells, i.e., evolutionary strategies tend to be “evolve mechanisms (bone, chiton, shells) to protect and don’t regenerate or repair” vs. “don’t protect and evolve mechanisms to regenerate or repair” (Hulsebosch and Bittner 1980, 1981). Regeneration of mammalian CNS axons may also be actively inhibited to allow for specific outgrowth in memory and learning— or perhaps relearning after injury.
Possible clinical and basic-science significance of PEG-fusion technologies
Our PEG-fusion protocols could quickly translate to important clinical procedures that dramatically and permanently restore, within minutes-to-days, much behavior lost by cut- or crush-axonal severance, a result not obtained by any other chemical or surgical treatment published to date. Translation of our protocols into clinical procedures is probable since cut- or crush-severed PNS or CNS axons in other mammals including rat, rabbit, and guinea pig can also be PEG-fused (Lore et al., 1999; Shi et al., 1999; Shi and Borgens, 1999; Borgens et al., 2002; Bittner et al., 2012). Furthermore, all drugs in our PEG-fusion protocols are benign, non- toxic in the concentrations used, readily available, and are already FDA-approved for human use (Bittner et al., 2012; Riley et al., 2015). Success with allograft repair of PNS and/or CNS nerve gaps might well lead to establishment of donors and tissue banks for PNS (sciatic, ulnar, etc.) and CNS (spinal) nerves similar to those established for corneas, hearts, kidneys, etc.
The applicability of PEG-fusion protocols beyond treating acute PNS and CNS nerve injuries is indicated by our previously published data showing that (1) Cut-severed segments of distal axons in rat tail nerves can be maintained morphologically and functionally intact for 5–10 days by cooling this body part in vivo (Sea et al., 1995) or by cyclosporin A injections (Sunio and Bittner, 1997); (2) Distal axonal segments of cut-severed rat spinal nerves maintained intact for days by cooling in vitro can be PEG-fused (Marzullo et al., 2002); and (3) Previously cut- or crush-severed axons that have sealed in Ca2+-containing saline can be recut, apposed and successfully sealed (Lore et al., 1999; Bittner et al., 2012; Ghergherehchi et al., 2015). Such data imply that PNA or STA injuries might be repairable by PEG-fusion technologies within 3 days post-trauma if no measures are taken and up to 10 days if the animal or spinal cord is cooled or treated with cyclosporine A.
The use of retrograde transported tracers and motor vs. sensory labeling (Cammer and Tansey, 1987; Byers et al., 2012; Sexton et al., 2012; Rodriguez-Feo et al. 2013; Liu et al., 2014; Riley et al., 2015) of PNAs by immunohistochemistry may provide important new basic-science insights into axonal specification and synaptic plasticities (Nguyen et al., 2005).
Our use of anti-oxidants (especially MB), oxidants or PEG might also have basic-science neuroprotective effects and clinical implications to enhance survival of severed axons (Stavisky et al., 2005; Rojas et al., 2009; Kwon et al., 2010; Spaeth et al., 2010, 2012a,b,c), whether or not they have been PEG-fused, by increasing the number of PEG-fused axons and/or by improving survival of severed axons not PEG-fused. In the latter case, rescued axons might subsequently successfully regenerate by conventional outgrowths at ~1mm/day (Lore et al., 1999; Bittner et al., 2000). These slowly regenerating outgrowths combined with retraining of PEG-fused axons might account for the slow increase in SFI and/or FF behavioral recovery after the second post-operative week (Britt et al., 2010; Bittner et al., 2012; Ghergherehchi et al., 2015 Riley et al., 2015).
Our PEG-fusion procedures could produce a paradigm shift in the clinical treatment of traumatic injuries to peripheral nerves for which the gold-standard for simple cuts has been micro-suturing the severed ends (Isaacs, 2010; Wolfe et al., 2010). The ability to PEG-fuse severed axons in autografts (or possibly eventually allografts) to rapidly restore behavioral deficits might dramatically alter functional outcomes for patients with mutilated extremity injuries and potentially even change the types of injuries for which salvage is attempted after extensive injury or oncologic resection. For example, the long-term function of a salvaged, but denervated, hand is especially dismal (Campbell, 2008; Wolfe et al., 2010) and limb amputation often occurs if a segment of a major nerve is lost (Brininger, 2008, Burch, 2011; Taras, 2011).
Our data suggest that our PEG-fusion technology may also improve spinal lesion repair in which the primary deficit is severance of spinal axons in dorsal, ventral and/or lateral columns of white matter (Kakulas, 1999; Kwon et al., 2010). The possible use of allografts after SCI may be of critical basic-science and clinical importance since severed spinal rostral and caudal axonal segments separate by several millimeters or more, and cannot be re-apposed by microsutures through connective tissue sheaths such as the epineurium and endoneurium that are found in PNS nerve trunks, but do not exist for CNS axons.
Significance.
Severed PNS axons often do not successfully regenerate, especially if lesioned proximally or if >3mm gaps are created by crushing, ablation, or cut-end retraction; severed CNS spinal axons do not naturally regenerate. PNS and CNS axons distal to the severance site undergo rapid Wallerian degeneration. Successful PEG-fusion of severed or ablated PNS or CNS spinal axons prevents or retards their Wallerian degeneration, restores axonal continuity through the lesion site within minutes and restores lost behavioral functions within days to weeks. PEG-fusion techniques may produce a paradigm-shift in the treatment of traumatic injuries to PNS and CNS axons.
Acknowledgments
Supported by a DOD grant OR120216 to WPT and an NIH grant R01 NS081063 to GDB and TS and a Lone Star Paralysis Grant to GDB and JDP. We especially thank Andrew Poon for creating the schematic diagrams shown in Figure 5.
Footnotes
Conflict of Interest
No author has a conflict of interest.
Role of Authors
All authors participated in the writing of this paper and collaborated with GDB in obtaining and publishing data and concepts described in one or more portions of this paper.
LITERATURE CITED
- Allan CH. Functional results of primary nerve repair. Hand Clin. 2000;16:67–72. [PubMed] [Google Scholar]
- Ballinger ML, Blanchette AR, Krause TL, Smyers ME, Fishman HM, Bittner GD. Delaminating myelin membranes help seal the cut ends of severed earthworm giant axons. J Neurobiol. 1997;33:945–960. doi: 10.1002/(sici)1097-4695(199712)33:7<945::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bertelli JA, Ghizoni MF. Results of c5 root grafting to the musculocutaneous nerve using pedicled, vascularized ulnar nerve grafts. J Hand Surg Am. 2009;34:1821–1826. doi: 10.1016/j.jhsa.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Bertelli JA, Ghizoni MF. Very distal sensory nerve transfers in high median nerve lesions. J Hand Surg Am. 2011;36:387–393. doi: 10.1016/j.jhsa.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Bi G, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995;131:147–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch R, Bonney G, Wynn-Parry CB. Surgical Disorders of the Peripheral Nerves. New York: Churchill Livingstone; 1998. Clinical aspects of nerve injury; pp. 145–190. [Google Scholar]
- Birse SC, Bittner GD. Regeneration of earthworm giant axons following transection or ablation. J Neurophysio. 1981;45:724–742. doi: 10.1152/jn.1981.45.4.724. [DOI] [PubMed] [Google Scholar]
- Bittner GD. Long-term survival of anucleate axons and its implications for nerve regeneration. Trends Neurosci. 1991;14:188–193. doi: 10.1016/0166-2236(91)90104-3. [DOI] [PubMed] [Google Scholar]
- Bittner GD, Ballinger ML, Raymond MA. Reconnection of severed nerve axons with polyethylene glycol. Brain Res. 1986;367:351–365. doi: 10.1016/0006-8993(86)91617-3. [DOI] [PubMed] [Google Scholar]
- Bittner GD, Brown MA. Long term survival of enucleated segments of glial cytoplasm in the leech Macrobdella decora. Brain Res. 1981;218:357–364. doi: 10.1016/0006-8993(81)91314-7. [DOI] [PubMed] [Google Scholar]
- Bittner GD, Fishman HM. Axonal sealing following injury. In: Ingoglia N, Murray M, editors. Nerve regeneration. New York: Marcel Dekker; 2000. pp. 337–369. [Google Scholar]
- Bittner GD, Keating CP, Kane JR, Britt JM, Spaeth CS, Fan JD, Zuzek A, Wilcott RW, Thayer WP, Winograd JM, Gonzalez-Lima F, Schallert T. Rapid, effective, and long-lasting behavioral recovery produced by microsutures, methylene blue, and polyethylene glycol after completely cutting rat sciatic nerves. J Neurosci Res. 2012;90:967–980. doi: 10.1002/jnr.23023. [DOI] [PubMed] [Google Scholar]
- Bittner GD, Rokkappanavar KK, Peduzzi JD. Application and implications of PEG-fusion as a novel technology to repair injured spinal cords. Neural Regener Res In Press. 2015 doi: 10.4103/1673-5374.162772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner GD, Schallert T, Peduzzi JD. Degeneration, trophic interactions and repair of severed axons: A reconsideration of some common assumptions. The Neuroscientist. 2000;6:88–109. [Google Scholar]
- Blundon JA, Sheller RA, Moehlenbruck JW, Bittner GD. Effect of temperature on long- term survival of anucleate giant axons in crayfish and goldfish. J Comp Neurol. 1990;297:377–391. doi: 10.1002/cne.902970305. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Shi R, Bohnert D. Behavioral recovery from spinal cord injury following delayed application of polyethylene glycol. J Exp Biol. 2002;205:1–12. doi: 10.1242/jeb.205.1.1. [DOI] [PubMed] [Google Scholar]
- Bouton MS, Bittner GD. Regeneration of motor axons in crayfish limbs: distal stump activation followed by synaptic reformation. Cell Tissue Res. 1981;219:379–392. doi: 10.1007/BF00210156. [DOI] [PubMed] [Google Scholar]
- Bozkurt A, Brook GA, Moellers S, Lassner F, Sellhaus B, Weis J, Woeltje M, Tank J, Beckmann C, Fuchs P, Damink LO, Schügner F, Heschel I, Pallua N. In vitro assessment of axonal growth using dorsal root ganglia explants in a novel three-dimensional collagen matrix. Tissue Eng. 2007;13:2971–2979. doi: 10.1089/ten.2007.0116. [DOI] [PubMed] [Google Scholar]
- Bozkurt A, Deumens R, Scheffel J, O’Dey DM, Weis J, Joosten EA, Führmann T, Brook GA, Pallua N. CatWalk gait analysis in assessment of functional recovery after sciatic nerve injury. J Neurosci Methods. 2008;173:91–98. doi: 10.1016/j.jneumeth.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Brininger TL, Antczak A, Breland HL. Upper extremity injuries in the U.S. military during peacetime years and wartime years. J Hand Ther. 2008;21:115–122. doi: 10.1197/j.jht.2007.10.010. quiz 123. [DOI] [PubMed] [Google Scholar]
- Britt JM, Kane JR, Spaeth CS, Zuzek A, Robinson GL, Gbanaglo MY, Estler CJ, Boydston EA, Schallert T, Bittner GD. Polyethylene glycol rapidly restores axonal integrity and improves the rate of motor behavior recovery after sciatic nerve crush injury. J Neurophysiol. 2010;104:695–703. doi: 10.1152/jn.01051.2009. [DOI] [PubMed] [Google Scholar]
- Burch R. Nerve repair. In: Wolfe S, Pederson W, Hotchkiss R, Kozin S, editors. Green’s Operative Hand Surgery. New York: Churchill Livingstone; 2011. pp. 1035–107. [Google Scholar]
- Burdick JA, Ward M, Liang E, Young MJ, Langer R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials. 2006;27:452–459. doi: 10.1016/j.biomaterials.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Burkhardt GE, Gifford SM, Propper B, Spencer JR, Williams K, Jones L, Sumner N, Cowart J, Rasmussen TE. The impact of ischemic intervals on neuromuscular recovery in a porcine (Sus scrofa) survival model of extremity vascular injury. J Vasc Surg. 2011;53:165–173. doi: 10.1016/j.jvs.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Byers JS, Huguenard AL, Kuruppu D, Liu NK, Xu XM, Sengelaub DR. Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J Comp Neurol. 2012;520:2683–2696. doi: 10.1002/cne.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammer W, Tansey FA. Immunocytochemical localization of carbonic anhydrase in myelinated fibers in peripheral nerves of rat and mouse. J Histochem Cytochem. 1987;35:865–870. doi: 10.1177/35.8.3110266. [DOI] [PubMed] [Google Scholar]
- Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Chim H, Miller E, Gliniak C, Cohen ML, Guyuron B. The role of different methods of nerve ablation in prevention of neuroma. Plast Reconstr Surg. 2013;131:1004–1012. doi: 10.1097/PRS.0b013e3182879ec2. [DOI] [PubMed] [Google Scholar]
- Cross JD, Ficke JR, Hsu JR, Masini BD, Wenke JC. Battlefield orthopaedic injuries cause the majority of long-term disabilities. J Am Acad Orthop Surg. 2011;19(Suppl 1):S1–7. doi: 10.5435/00124635-201102001-00002. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “ridges” after central nervous system injury in adult rats. Science. 1981;241:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- DeRiemer SA, Elliott EJ, Macagno ER, Muller KJ. Morphological evidence that regenerating axons can fuse with severed axon segments. Brain Res. 1983;272:157–161. doi: 10.1016/0006-8993(83)90373-6. [DOI] [PubMed] [Google Scholar]
- Detrait E, Eddleman CS, Yoo S, Fukuda M, Nguyen MP, Bittner GD, Fishman HM. Axolemmal repair requires proteins that mediate synaptic vesicle fusion. J Neurobiol. 2000a;44:382–391. doi: 10.1002/1097-4695(20000915)44:4<382::aid-neu2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Detrait ER, Yoo S, Eddleman CS, Fukuda M, Bittner GD, Fishman HM. Plasmalemmal repair of severed neurites of PC12 cells requires Ca(2+) and synaptotagmin. J Neurosci Res. 2000b;62:566–573. doi: 10.1002/1097-4547(20001115)62:4<566::AID-JNR11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Doherty JG, Burns AS, O’Ferrall DM, Ditunno JF., Jr Prevalence of upper motor neuron vs. lower motor neuron lesions in complete lower thoracic and lumbar spinal cord injuries. J Spinal Cord Med. 2002;25:289–292. doi: 10.1080/10790268.2002.11753630. [DOI] [PubMed] [Google Scholar]
- Eddleman CS, Ballinger ML, Smyers ME, Godell CM, Fishman HM, Bittner GD. Repair of plasmalemmal lesions by vesicles. Proc Natl Acad Sci USA. 1997;94:4745–4750. doi: 10.1073/pnas.94.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleman CS, Ballinger ML, Smyers ME, Fishman HM, Bittner GD. Endocytotic formation of vesicles and other membranous structures induced by Ca2+ and axoplasmic injury. J Neurosci. 1998;18:4029–4041. doi: 10.1523/JNEUROSCI.18-11-04029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleman CS, Bittner GD, Fishman HM. Barrier permeability at cut axonal ends progressively decreases until an ionic seal is formed. Biophys J. 2000;79:1883–1890. doi: 10.1016/S0006-3495(00)76438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber SJ, Glaus SW, Moore AM, Hunter DA, Mackinnon SE, Johnson PJ. Supercharge nerve transfer to enhance motor recovery: a laboratory study. J Hand Surg Am. 2013;38:466–77. doi: 10.1016/j.jhsa.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J, Fernandez MS. Morphological evidence for an experimentally induced synaptic field. Nature. 1974;251:428–430. doi: 10.1038/251428a0. [DOI] [PubMed] [Google Scholar]
- Fishman HM, Bittner GD. Vesicle-mediated restoration of a plasmalemmal barrier in severed axons. News Physiol Sci. 2003;18:115–118. doi: 10.1152/nips.01429.2002. [DOI] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Patel B, Clouse WD. Update on wartime vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23:13–25. doi: 10.1177/1531003511400625. [DOI] [PubMed] [Google Scholar]
- Fox LC, Kreishman MP. High-energy trauma and damage control in the lower limb. Semin Plast Surg. 2010;24:5–10. doi: 10.1055/s-0030-1253241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller ER. Anit-Nogo- A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates – re-examination and extension of behavioral data. Eur J Neurosci. 2009;29(5):983–996. doi: 10.1111/j.1460-9568.2009.06642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghergherehchi CL, Bittner GD, Hastings RL, Mikesh M, Riley DC, Trevino RC, Schallert T, Thayer WP, Bhupanapadu Sunkesula SR, Ha TN, Munoz N, Pyarali M, Bansal A, Poon AD, Mazal AT. Effects of extracellular calcium and surgical techniques on restoration of axonal continuity by PEG-fusion following complete cut- or crush-severance of rat sciatic nerves. J Neurosci Res. 2015 doi: 10.1002/jnr.23704. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanini MA, Reier PJ, Eskin TA, Wirth E, Anderson DK. Characteristics of human fetal spinal cord grafts in the adult rat spinal cord: influences of lesion and grafting conditions. Exp Neurol. 1997;148:523–543. doi: 10.1006/exnr.1997.6703. [DOI] [PubMed] [Google Scholar]
- Green DP, Wolfe SW. Green’s Operative Hand Surgery. 6th. Philadelphia: Elsevier/Churchill Livingstone; 2011. [Google Scholar]
- Hare GM, Evans PJ, Mackinnon SE, Best TJ, Bain JR, Szalai JP, Hunter DA. Walking track analysis: a long-term assessment of peripheral nerve recovery. Plast Reconstr Surg. 1992;89:251–258. [PubMed] [Google Scholar]
- Holcomb JB, Stansbury LG, Champion HR, Wade C, Bellamy RF. Understanding combat casualty care statistics. J Trauma. 2006;60:397–401. doi: 10.1097/01.ta.0000203581.75241.f1. [DOI] [PubMed] [Google Scholar]
- Hoy RR, Bittner GD, Kennedy D. Regeneration in crustacean motoneurons: evidence for axonal fusion. Science. 1967;156:251–252. doi: 10.1126/science.156.3772.251. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Bittner GD. Evolution of abilities to regenerate CNS neurons. Am Naturalist. 1980;115:276–284. [Google Scholar]
- Hulsebosch CE, Bittner GD. Regeneration of axons and nerve cell bodies in the CNS of annelids. J Comp Neurol. 1981;198:77–88. doi: 10.1002/cne.901980108. [DOI] [PubMed] [Google Scholar]
- Isaacs J. Treatment of peripheral nerve injuries: current concepts. J Hand Surgery. 2010;35A:491–497. doi: 10.1016/j.jhsa.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Isaacson BM, Weeks SR, Pasquina PF, Webster JB, Beck JP, Bloebaum RD. The road to recovery and rehabilitation for injured service members with limb loss: a focus on Iraq and Afghanistan. US Army Med Dep J. 2010;2010:31–6. [PubMed] [Google Scholar]
- Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F. ESCRT machinery is required for plasma membrane repair. Science. 2014;343:1247136. doi: 10.1126/science.1247136. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. A review of the neuropathology of human spinal cord injury with emphasis on special features. J Spinal Cord Med. 1999;22:119–124. doi: 10.1080/10790268.1999.11719557. [DOI] [PubMed] [Google Scholar]
- Kelley JP. Reactions of neurons to injury. In: Kandel ER, Schwartz JH, editors. Principals of Neural Science. Amsterdam: Elsevier; 1985. pp. 187–195. [Google Scholar]
- Kragh JF, Jr, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, Holcomb JB. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann Surg. 2009;249:1–7. doi: 10.1097/SLA.0b013e31818842ba. [DOI] [PubMed] [Google Scholar]
- Krause TL, Bittner GD. Rapid morphological fusion of severed myelinated axons by polyethylene glycol. Proc Natl Acad Sci USA. 1990;87:1471–1475. doi: 10.1073/pnas.87.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause TL, Fishman HM, Ballinger ML, Bittner GD. Extent and mechanism of sealing in transected giant axons of squid and earthworms. J Neurosci. 1994;14:6638–6651. doi: 10.1523/JNEUROSCI.14-11-06638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause TL, Marquis RM, Lyckman AW, Ballinger ML, Bittner GD. Rapid artificial restoration of electrical continuity across a crush lesion of a giant axon. Brain Res. 1991;561:350–353. doi: 10.1016/0006-8993(91)91615-8. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Roy J, Lee JH, Okon E, Zhang H, Marx JC, Kindy MS. Magnesium chloride in a polyethylene glycol formulation as a neuroprotective therapy for acute spinal cord injury: preclinical refinement and optimization. J Neurotrauma. 2009;26:1379–1393. doi: 10.1089/neu.2009.0884. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Sekhon LH, Fehlings MG. Emerging repair, regeneration, and translational research advances for spinal cord injury. Spine. 2010;35(21 Suppl):S263–70. doi: 10.1097/BRS.0b013e3181f3286d. [DOI] [PubMed] [Google Scholar]
- Lee J, Lentz BR. Evolution of lipidic structures during model membrane fusion and the relation of this process to cell membrane fusion. Biochemistry. 1997;36:6251–6259. doi: 10.1021/bi970404c. [DOI] [PubMed] [Google Scholar]
- Lentz BR. PEG as a tool to gain insight into membrane fusion. Eur Biophys J. 2007;36:315–326. doi: 10.1007/s00249-006-0097-z. [DOI] [PubMed] [Google Scholar]
- Lichstein JW, Ballinger ML, Blanchette AR, Fishman HM, Bittner GD. Structural changes at cut ends of earthworm giant axons in the interval between dye barrier formation and neuritic outgrowth. J Comp Neurol. 2000;416:143–157. doi: 10.1002/(sici)1096-9861(20000110)416:2<143::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Liu NK, Byers JS, Lam T, Lu QB, Sengelaub DR, Xu XM. Inhibition of cPLA2 has neuroprotective effects on motoneuron and muscle atrophy following spinal cord injury. J Neurotrauma. 2014 doi: 10.1089/neu.2014.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore AB, Hubbell JA, Bobb DS, Ballinger ML, Loftin KL, Smith JW, Smyers ME, Garcia HD, Bittner GD. Rapid induction of functional and morphological continuity between severed ends of mammalian or earthworm myelinated axons. J Neurosci. 1999;19:2442–2454. doi: 10.1523/JNEUROSCI.19-07-02442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, Goldstein LSB, Tuszynski MH. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83(4):789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JH, Emery DG, Higgins ML, Gross GW. Neuronal survival and dynamics of ultrastructural damage after dendrotomy in low calcium. J Neurotrauma. 1990;7:169–192. doi: 10.1089/neu.1990.7.169. [DOI] [PubMed] [Google Scholar]
- Lucas JH, Gross GW, Emery DG, Gardner CR. Neuronal survival or death after dendrite transection close to the perikaryon: correlation with electrophysiologic, morphologic, and ultrastructural changes. Cent Nerv Syst Trauma. 1985;2:231–55. doi: 10.1089/cns.1985.2.231. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–1429. doi: 10.1097/00006534-200105000-00016. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE, Dellon AL. Surgery of the Peripheral Nerves. New York: Thieme; 1988. [Google Scholar]
- Marzullo TC, Britt JM, Stavisky RC, Bittner GD. Cooling enhances in vitro survival and fusion-repair of severed axons taken from the peripheral and central nervous systems of rats. Neurosci Lett. 2002;327:9–12. doi: 10.1016/s0304-3940(02)00378-6. [DOI] [PubMed] [Google Scholar]
- McNeil P. Membrane repair redux: redox of MG53. Nat Cell Biol. 2009;11:7–9. doi: 10.1038/ncb0109-7. [DOI] [PubMed] [Google Scholar]
- Menorca RM, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29:317–30. doi: 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya D, Giszter S, Mori F, Adipudi V, Tessler A, Murray M. Fetal transplants alter the development of function after spinal cord transection in newborn rats. J Neurosci. 1997;17:4856–4872. doi: 10.1523/JNEUROSCI.17-12-04856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller KH, Carbonetto S. The morphological and physiological properties of a regenerating synapse in the CNS of the leech. J Comp Neurol. 1979;185:485–516. doi: 10.1002/cne.901850305. [DOI] [PubMed] [Google Scholar]
- Nanney LB, Skeel A, Luan J, Polis S, Richmond A, Wang MH, Leonard EJ. Proteolytic cleavage and activation of pro-macrophage-stimulating protein and upregulation of its receptor in tissue injury. J Invest Dermatol. 1998;111:573–81. doi: 10.1046/j.1523-1747.1998.00332.x. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center (NSCISC) Facts and Figures at a Glance. Source: https://www.nscisc.uab.edu/
- Neumann B, Nguyen KC, Hall DH, Ben-Yakar A, Hilliard MA. Axonal regeneration proceeds through specific axonal fusion in transected C. elegans neurons. Dev Dyn. 2011 doi: 10.1002/dvdy.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MP, Bittner GD, Fishman HM. Critical interval of somal calcium transient after neurite transection determines B104 cell survival. J Neurosci Res. 2005;81:805–816. doi: 10.1002/jnr.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlander RH, Singer M. Electron microscopy of severed motor fibers in the crayfish. Z Zellforsch. 1972;1256:157–181. doi: 10.1007/BF00307214. [DOI] [PubMed] [Google Scholar]
- Plano S, Piedrafita E, Miana-Mena FJ, Fuentes-Broto L, Martínez-Ballarín E, López-Pingarrón L, Sáenz MA, García JJ. Melatonin and structurally-related compounds protect synaptosomal membranes from free radical damage. Int J Mol Sci. 2010;11:312–328. doi: 10.3390/ijms11010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontecorvo G. Production of mammalian somatic cell hybrids by means of polyethylene glycol treatment. Somatic Cell Genet. 1975;1:397–400. doi: 10.1007/BF01538671. [DOI] [PubMed] [Google Scholar]
- Raabe TD, Bittner GD. Phosphorylation of neurofilament proteins in isolated goldfish Mauthner axoplasm. J Neurochem. 1996a;66:1214–1221. doi: 10.1046/j.1471-4159.1996.66031214.x. [DOI] [PubMed] [Google Scholar]
- Raabe TD, Nguyen T, Archer C, Bittner GD. Mechanisms for the maintenance and eventual degradation of neurofilament proteins in the distal segments of severed goldfish mauthner axons. J Neurosci. 1996b;16:1605–13. doi: 10.1523/JNEUROSCI.16-05-01605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radogna F, Nuccitelli S, Mengoni F, Ghibelli L. Neuroprotection by melatonin on astrocytoma cell death. Ann NY Acad Sci. 2009;1171:509–513. doi: 10.1111/j.1749-6632.2009.04900.x. [DOI] [PubMed] [Google Scholar]
- Ramon Y, Cajal S. Translated May RM. Oxford University Press; London: 1928. Degeneration and regeneration of the nervous system. [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)- regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Riley DC, Bittner GD, Mikesh M, Cardwell NL, Pollins AC, Ghergherehchi CL, Bhupanapadu Sunkesula SR, Ha TN, Hall BT, Poon AD, Pyarali M, Boyer RB, Mazal AT, Munoz N, Trevino RC, Schallert T, Thayer WP. Polyethylene glycol-fused allografts produce rapid behavioral recovery after ablation of sciatic nerve segments. J Neurosci Res. 2015;93:572–583. doi: 10.1002/jnr.23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Feo CL, Sexton KW, Boyer RB, Pollins AC, Cardwell NL, Nanney LB, Shack RB, Mikesh MA, McGill CH, Driscoll CW, Bittner GD, Thayer WP. Blocking the P2X7 receptor improves outcomes after axonal fusion. J Surg Res. 2013;184:705–713. doi: 10.1016/j.jss.2013.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, John JM, Lee J, Gonzalez-Lima F. Methylene blue provides behavioral and metabolic neuroprotection against optic neuropathy. Neurotox Res. 2009;15:260–273. doi: 10.1007/s12640-009-9027-z. [DOI] [PubMed] [Google Scholar]
- Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- Schlaepfer WW, Bunge RP. Effects of calcium ion concentration on the degeneration of amputated axons in tissue culture. J Cell Biol. 1973;59:456–470. doi: 10.1083/jcb.59.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- Sea T, Ballinger ML, Bittner GD. Cooling of peripheral myelinated axons retards Wallerian degeneration. Exp Neurol. 1995;133:85–95. doi: 10.1006/exnr.1995.1010. [DOI] [PubMed] [Google Scholar]
- Sexton KW, Pollins AC, Cardwell NL, Del Corral GA, Bittner GD, Shack RB, Nanney LB, Thayer WP. Hydrophilic polymers enhance early functional outcomes after nerve autografting. J Surg Res. 2012;177:392–400. doi: 10.1016/j.jss.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheller RA, Smyers ME, Grossfeld RM, Ballinger ML, Bittner GD. Heat shock proteins in crayfish medial giant axons: High constitutive levels and transfer of inducible isoforms from glia. J Comp Neurol. 1998;396:1–11. doi: 10.1002/(sici)1096-9861(19980622)396:1<1::aid-cne1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Sheller RA, Tytell M, Smyers M, Bittner GD. Glia-to-axon communication: enrichment of glial proteins transferred to the squid giant axon. J Neurosci Res. 1995;41:324–334. doi: 10.1002/jnr.490410305. [DOI] [PubMed] [Google Scholar]
- Shi R, Borgens RB. Acute repair of crushed guinea pig spinal cord by polyeyhylene glycol. J Neurophysiol. 1999;81:2406–2414. doi: 10.1152/jn.1999.81.5.2406. [DOI] [PubMed] [Google Scholar]
- Shi R, Borgens RB, Blight AR. Functional reconnection of severed mammalian spinal cord axons with polyethylene glycol. J Neurotrauma. 1999;16:727–738. doi: 10.1089/neu.1999.16.727. [DOI] [PubMed] [Google Scholar]
- Spaeth CS, Boydston EA, Figard LR, Zuzek A, Bittner GD. A model for sealing plasmalemmal damage in neurons and other eukaryotic cells. J Neurosci. 2010;30:15790–15800. doi: 10.1523/JNEUROSCI.4155-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth CS, Fan JD, Spaeth EB, Robison T, Wilcott RW, Bittner GD. Neurite transection produces cytosolic oxidation, which enhances plasmalemmal repair. J Neurosci Res. 2012a;90:945–954. doi: 10.1002/jnr.22823. [DOI] [PubMed] [Google Scholar]
- Spaeth CS, Robison T, Fan JD, Bittner GD. Cellular mechanisms of plasmalemmal sealing and axonal repair by polyethylene glycol and methylene blue. J Neurosci Res. 2012b;90:955–966. doi: 10.1002/jnr.23022. [DOI] [PubMed] [Google Scholar]
- Spaeth CS, Spaeth EB, Wilcott RW, Fan JD, Robison T, Bittner GD. Pathways for plasmalemmal repair mediated by PKA, Epac, and cytosolic oxidation in rat B104 cells in vitro and rat sciatic axons ex vivo. Dev Neurobiol. 2012c;72:1399–1414. doi: 10.1002/dneu.20998. [DOI] [PubMed] [Google Scholar]
- Stansbury LG, Branstetter JG, Lalliss SJ. Amputation in military trauma surgery. J Trauma. 2007;63:940–944. doi: 10.1097/TA.0b013e31814934d8. [DOI] [PubMed] [Google Scholar]
- Stavisky RC, Britt JM, Zuzek A, Truong E, Bittner GD. Melatonin enhances the in vitro and in vivo repair of severed rat sciatic axons. Neurosci Lett. 2005;376:98–101. doi: 10.1016/j.neulet.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263:390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunio A, Bittner GD. Cyclosporin A retards the Wallerian degeneration of peripheral mammalian axons. Exp Neurol. 1997;146:46–56. doi: 10.1006/exnr.1997.6484. [DOI] [PubMed] [Google Scholar]
- Tanner SL, Storm EE, Bittner GD. Maintenance and degradation of proteins in intact and severed axons: implications for the mechanisms of long-term survival of anucleate crayfish axons. J Neurosci. 1995a;15:540–548. doi: 10.1523/JNEUROSCI.15-01-00540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner SL, Storm EE, Bittner GD. Protein transport in intact and severed (anucleate) crayfish medial giant axons. J Neurochem. 1995b;64:1491–1501. doi: 10.1046/j.1471-4159.1995.64041491.x. [DOI] [PubMed] [Google Scholar]
- Taras JS, Jacoby SM, Lincoski CJ. Reconstruction of digital nerves with collagen conduits. J Hand Surg Am. 2011;36:1441–1446. doi: 10.1016/j.jhsa.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Todorov AT, Yogev D, Qi P, Fendler JH, Rodziewicz GS. Electric-field-induced reconnection of severed axons. Brain Res. 1992;582:329–334. doi: 10.1016/0006-8993(92)90151-x. [DOI] [PubMed] [Google Scholar]
- Tsao JW, George EB, Griffin JW. Temperature modulation reveals three distinct stages of Wallerian degeneration. J Neurosci. 1999;19:4718–4726. doi: 10.1523/JNEUROSCI.19-12-04718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viancour TA, Bittner GD, Ballinger ML. Selective transfer of Lucifer yellow CH from axoplasm to adaxonal glia. Nature. 1981;293:65–67. doi: 10.1038/293065a0. [DOI] [PubMed] [Google Scholar]
- Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos Trans R Soc London. 1850;140:423–439. [PMC free article] [PubMed] [Google Scholar]
- Whitlock EL, Myckatyn TM, Tong AY, Yee A, Yan Y, Magill CK, Johnson PJ, Mackinnon SE. Dynamic quantification of host Schwann cell migration into peripheral nerve allografts. Exp Neurol. 2010;225:310–319. doi: 10.1016/j.expneurol.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RR, Bunge MB. Schwann cell transplantation: a repair strategy for spinal cord injury? Prog Brain Res. 2012;201:295–312. doi: 10.1016/B978-0-444-59544-7.00014-7. [DOI] [PubMed] [Google Scholar]
- Williams EH, Dellon AL. Upper extremity nerve repair tips and techniques: a master skills publication. ASSH. 2008:75–88. [Google Scholar]
- Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH. Green’s Operative Hand Surgery. 6th. New York: Churchill Livingstone; 2010. [Google Scholar]
- Wood MW, Kemp SWP, Weber C, Borschel GH, Gordon T. Outcome measures of peripheral nerve regeneration. Annals of Anat. 2011;193:321–333. doi: 10.1016/j.aanat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Yan Y, MacEwan MR, Hunter DA, Farber S, Newton P, Tung TH, Mackinnon SE, Johnson PJ. Nerve regeneration in rat limb allografts: evaluation of acute rejection rescue. Plast Reconstr Surg. 2013;131:499e–511e. doi: 10.1097/PRS.0b013e31828275b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H, Kuno M. Calcium dependence of membrane sealing at the cut end of the cockroach giant axon. J Neurosci. 1985;5:1626–1632. doi: 10.1523/JNEUROSCI.05-06-01626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev D, Todorov AT, Qi P, Fendler JH, Rodziewicz GS. Laser-induced reconnection of severed axons. Biochem Biophys Res Commun. 1991;180:874–80. doi: 10.1016/s0006-291x(05)81146-5. [DOI] [PubMed] [Google Scholar]
- Yoo S, Bottenstein JE, Bittner GD, Fishman HM. Survival of mammalian B104 cells following neurite transection at different locations depends on somal Ca2+ concentration. J Neurobiol. 2004;60:137–153. doi: 10.1002/neu.20005. [DOI] [PubMed] [Google Scholar]
- Yoo S, Nguyen MP, Fukuda M, Bittner GD, Fishman HM. Plasmalemmal sealing of transected mammalian neurites is a gradual process mediated by Ca(2+)-regulated proteins. J Neurosci Res. 2003;74:541–551. doi: 10.1002/jnr.10771. [DOI] [PubMed] [Google Scholar]
- Zhang X, Rojas JC, Gonzalez-Lima F. Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox Res. 2006;9:47–57. doi: 10.1007/BF03033307. [DOI] [PubMed] [Google Scholar]
- Zottoli SJ, Marek LE, Agostini MA, Strittmatter SL. Morphological and physiological survival of goldfish Mauthner axons isolated from their somata by spinal cord crush. J Comp Neurol. 1987;255:272–82. doi: 10.1002/cne.902550210. [DOI] [PubMed] [Google Scholar]
- Zuzek A, Fan JD, Spaeth CS, Bittner GD. Sealing of transected neurites of rat B104 cells requires a diacylglycerol PKC-dependent pathway and a PKA-dependent pathway. Cell Molec Neurosci. 2013;33:31–46. doi: 10.1007/s10571-012-9868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


