Abstract
A growing body of evidence suggests that the amygdala is central to handling the demands of complex social life in primates. In this paper, we synthesize extant anatomical and functional data from rodents, monkeys, and humans to describe the topography of three partially distinct large-scale brain networks anchored in the amygdala that each support unique functions for effectively managing social interactions and maintaining social relationships. These findings provide a powerful componential framework for parsing social behavior into partially distinct neural underpinnings that differ among healthy people and disintegrate or fail to develop in neuropsychiatric populations marked by social impairment, such as autism, antisocial personality disorder, and frontotemporal dementia.
Keywords: Amygdala, Networks, Social life, Social brain, Social network
1. Introduction
The ability to forge and maintain diverse social relationships is critical for primates to survive. Social abilities are particularly crucial for humans. Social relationships are protective in humans, predicting a plethora of positive health outcomes ranging from lower rates of mortality (House, Landis, & Umberson, 1988) to increased survival from heart attacks (Seeman, 1996). On the flipside, loneliness kills (Hawkley & Cacioppo, 2010). Yet humans differ markedly from one another in the size of their social networks (Dunbar & Spoors, 1995; Hill & Dunbar, 2003). Before 2011, comparative studies between non-human primate species linked larger social networks with larger brain regions providing a greater functional capacity for handling the demands of complex social life, including the amygdala (e.g. Barton, 2006; 1988; Barton and Aggleton, 2000). Based on this research, we examined and found that in humans, individual differences in amygdala volume predicted variations in social network size and complexity (Bickart, Wright, Dautoff, Dickerson, & Barrett 2011). Since our initial findings, three papers provided additional support for this link (Kanai, Bahrami, Roylance, & Rees 2011; Sallet et al., 2011; Von Der Heide, Vyas, & Olson 2014), indicating that the amygdala plays a central role in the social life of both human and nonhuman primates. It is clear from these studies and from a wealth of neuroanatomical, neuroimaging and neuropsychology research that the amygdala does not play this role in social life alone. Instead, the amygdala works in conjunction with a broad array of other brain regions that are also important to social cognition, often referred to collectively as the “social brain”. In this review, we synthesize connectional experiments in rodents, monkeys, and humans to develop a neuroanatomical framework wherein the amygdala anchors three partially distinct brain networks that each subserve a distinct domain of social behavior (see Fig. 1). We review the anatomical basis for these networks as well as their putative role in social function. We examine their convergent as well as their discriminant validity for predicting social network size and complexity. Finally, we discuss future directions for research on the amygdala and the social brain with clinical application and multilevel analysis in mind.
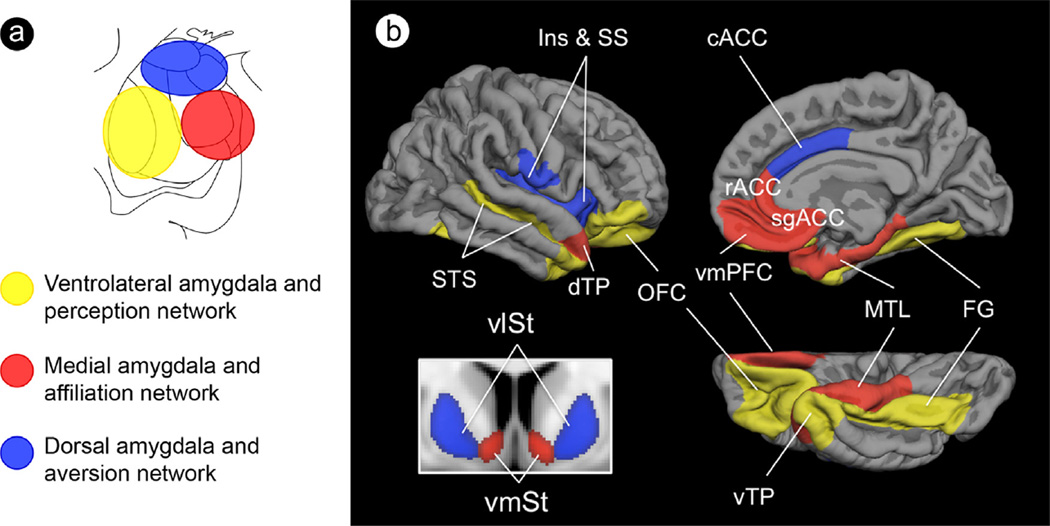
Fig. 1.
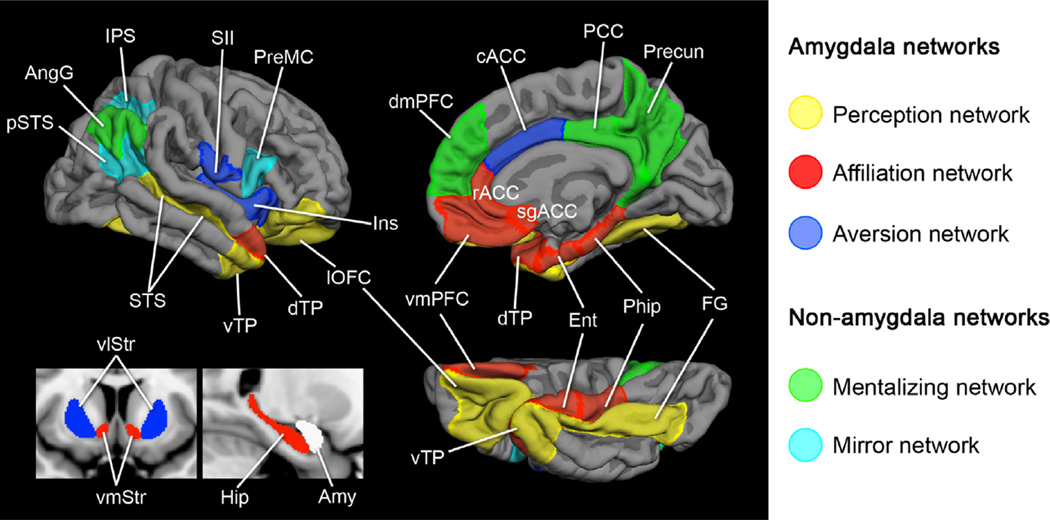
Topographic schematic of amygdala subregions and their affiliated large-scale networks subserving social cognition. A schematic of (a) the amygdala subregions in coronal view that are anchors for (b) three large-scale networks subserving processes important for social cognition. Abbreviations: Ins, insula; SS, somatosensory operculum; STS, superior temporal sulcus; dTP, dorsal temporal pole; OFC, orbitofrontal cortex; cACC, caudal anterior cingulate cortex; rACC, rostral anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; MTL, medial temporal lobe; FG, fusiform gyrus; vTP, ventral temporal pole; vlSt, ventrolateral striatum; vmSt, ventromedial striatum. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.)
2. The amygdala as a hub for large-scale networks in the social brain
Over the last 20 years of rising scientific interest in the neural basis of social cognition, there have been at least 10 review articles summarizing the brain regions that make up the “social brain” (Adolphs, 1999, 2001, 2009; Blakemore & Frith, 2004; Frith, 2007; Frith & Frith, 2007; Lieberman, 2007; Ochsner & Lieberman, 2001; Saxe, 2006) (for a discussion of the social brain, see Box 1). The amygdala and several of its strongly connected targets [particularly the ventromedial prefrontal cortex (vmPFC) and superior temporal sulcus (STS)] are consistently implicated within this broad neural workspace for social cognition (brain regions included in the social brain are tabulated across review articles in the table in Box 1). Tract-tracing work in nonhuman primates (synthesized in the final column of the table in Box 1) reveals that the amygdala shares anatomical connections with almost every other brain region implicated in the social brain, and these connections are especially prominent for less laminated “limbic” cortices and other subcortical structures. Based on the broadly distributed topography of the amygdala’s anatomical connections (Freese & Amaral, 2009), it can be considered a hub within the social brain. Consistent with this view, individuals with amygdala damage have deficits in diverse aspects of social processing (Box 2).
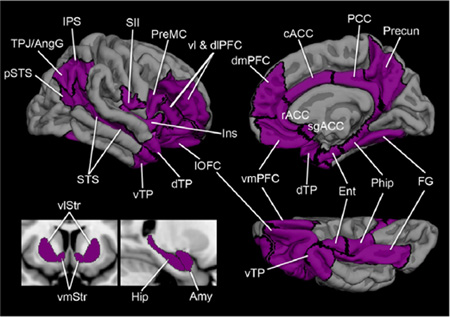
Box 1.
The “the social brain” typically refers to brain regions that consistently show an increase in activation in neuroimaging studies of healthy adults engaging in a variety of social cognitive tasks (e.g., recognizing familiar people, evaluating whether they are trustworthy or not, or making inferences about their thoughts and intentions), or whose damage has been linked to impairments in social cognition (e.g., diminished empathy or warmth towards others, lack of understanding others’ intentions, or disrupted social apprehension). Although there is substantial variability across review papers as to which brain regions play a role in social cognition (Adolphs, 1999, 2001, 2009; Blakemore & Frith, 2004; Brothers, 1990; Frith, 2007; Frith & Frith, 2007; Lieberman, 2007; Ochsner & Lieberman, 2001; Saxe, 2006), there is a relatively high consensus on the importance of the amygdala, as well as connected target regions including the vmPFC, STS, sectors of the orbital and medial surfaces of the prefrontal cortex, divisions of the cingulate cortex, visual association areas of the temporal cortex, the insular cortex, and a region at the junction of the temporal and parietal lobes (see the Table and Figure).
| Brain regions implicated in social cognition |
Percent agreement across 10 review articles |
Strength of connectivity with the amygdala |
|---|---|---|
| Amygdala | 100% | |
| Superior temporal sulcus |
100% | + + + |
| Ventromedial prefrontal cortex |
100% | + + + |
| Caudal anterior cingulate cortex |
80% | + + |
| Rostral anterior cingulate cortex |
80% | + + + |
| Temporoparietal junction/Angular gyrus |
60% | |
| Insula | 60% | + + + |
| Dorsomedial prefrontal cortex |
60% | + |
| Fusiform gyrus | 50% | + + + |
| Subgenual anterior cingulate cortex |
50% | + + + |
| Posterior cingulate cortex |
50% | |
| Temporal pole | 40% | + + + |
| Lateral orbitofrontal cortex |
40% | + + |
| Ventral premotor cortex |
40% | |
| Ventrolateral prefrontal cortex |
30% | + |
| Somatosensory cortex |
30% | + |
| Intraparietal sulcus |
30% | |
| Striatum | 30% | + + |
| Dorsolateral prefrontal cortex |
20% | + |
| Hypothalamus | 20% | + + |
| Brainstem nuclei | 20% | + + |
| Medial temporal lobe |
10% | + + + |
A schematic of brain regions cited as components of the “social brain”.
List of ROIs with abbreviations – cACC, caudal anterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; dTP, dorsomedial temporal pole; Ent, entorhinal cortex; FG, fusiform gyrus; Ins, insula; IPS, intraparietal sulcus; lOFC, lateral orbito-frontal cortex; PHip, parahippocampal cortex; PCC, posterior cingulate cortex; pSTS, posterior superior temporal sulcus; Precun, precuneus; PreMC, premotor cortex; rACC, rostral anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; SII, somatosensory operculum; STS, superior temporal sulcus; TPJ/AngG, temporoparietal junction/angular gyrus; vlSt, ventrolateral striatum; vlTP, ventrolateral temporal pole; vmPFC, ventromedial prefrontal cortex; vmSt, ventromedial striatum.
Gradients in the density of amygdala connectivity appear to mark a broad functional division within the social brain. The amygdala shares the most prominent connections with limbic structures (i.e., less granular cortex and connected subcortical structures), and the density of amygdala connectivity within cortical structures decreases with increasing lamination. This connectional and architectonic gradient suggests a broad division of labor within the social brain. That is, less granular cortex with more prominent amygdala connectivity appears to subserve social behaviors that draw moreso on information from the body (e.g., inferring emotions, as compared to intentions, from the eye region of faces (Stone et al., 2002) or solving moral dilemmas that are personal rather than impersonal (Shamay-Tsoory et al., 2005)). This type of information is typically described as affective in nature (Barrett & Bliss-Moreau, 2009). In contrast, the more granular cortex that is found in other social cognitive networks (e.g., mentalizing and mirror networks) subserves functions that draw less heavily on affective information.
Box 2.
Based on studies in amygdala-damaged patients, it is clear that the amygdala plays a role in perceptual and motivational aspects of social behavior. In line with the view that the amygdala is important for directing attentional resources to relevant social stimuli, amygdala-damaged patients tend to make less eye-contact (Spezio et al., 2007), are insensitive to personal boundaries (Kennedy, Glascher, Tyszka, & Adolphs, 2009), and have difficulty spontaneously orienting to (Adolphs et al., 2005) and interpreting signals from the eye region of others’ faces (Kennedy & Adolphs, 2010; Young et al., 1995). Furthermore, such patients do not exhibit the normal enhancement of fMRI signal in the fusiform gyrus and superior temporal sulcus to facial expressions (Vuilleumier et al., 2004). In fact, the magnitude of reduced fMRI signal in these regions compared to controls correlated with the degree of amygdalar damage. Deficient eye-gaze interpretation has also been attributable to damage in the orbitofrontal cortex (Vecera & Rizzo, 2004) and superior temporal gyrus (Akiyama et al., 2006).
Amygdala-damaged patients also exhibit markedly diminished social apprehension, which is a deficit in motivated interpersonal behavior (Adolphs, Tranel, Damasio, & Damasio, 1995; Bechara, Damasio, Damasio, & Lee, 1999; Tranel & Hyman, 1990). Such patients tend to judge even the most seemingly untrustworthy people as trustworthy and approachable (Adolphs, Tranel, & Damasio, 1998) and cooperate with other people despite apparent violations in trust to which healthy controls respond to in kind (e.g., by rejecting cooperation with the violator) (Koscik & Tranel, 2011). Such deficits in social avoidance judgments and decisions relate to these patients’ real-world impairments where, for example, they exhibit flirtatiousness and inappropriate familiarity with strangers (Adolphs et al., 1995; Bechara et al., 1999; Tranel & Hyman, 1990). As another example, an amygdala-damaged patient who was impaired at detecting cheaters in an experimental task also had difficulty making financial decisions and according to his family, did not realize if someone was taking advantage of him (Stone et al., 2002). He was liked by coworkers, was socially interactive, but sometimes he found social interactions puzzling. The authors of this study further explain that in daily life, such patients are “often vulnerable to scams, bad business deals, and exploitative relationships, a vulnerability that may be affected by deficits in specific aspects of social cognition”.
The amygdala’s connectional organization within the social brain places it in a central position to modulate a variety of brain networks that are important to normal social cognition. For example, the amygdala shares connections with visual association areas in the ventral and lateral temporal cortex implicated in processing social signals from others, such as facial actions (Freese & Amaral, 2005, 2006). The amygdala also connects with prefrontal and striatal areas (McDonald, 1991a) implicated in guiding affiliation with or avoidance of social partners, such as entrusting people based on their approachable appearance or rejecting cooperation with an unfair partner. In our research, we have used the amygdala’s connectional organization as a guide to understand the putative organization of large-scale brain networks within the social brain. By synthesizing connectional experiments in nonhuman animals and functional experiments in human and nonhuman primates, we developed a neuroanatomical framework in which the amygdala anchors three partially distinct corticolimbic networks with dissociable social functions (see Fig. 1): (1) a network supporting perception, performing the sensory processes involved in detecting, decoding and interpreting social signals from others in the context of past experience and current goals, (2) a network supporting affiliation, important for the processes associated with motivating prosocial or affiliative behaviors, such as comforting a loved one in distress and (3) a network supporting aversion, important for the processes enabling avoidant behaviors, such as avoiding an untrustworthy-appearing stranger. In Box 3, we describe how we derived these networks in three samples of healthy adults (Bickart, Hollenbeck, Barrett, & Dickerson, 2012). In the next section, we discuss the existing anatomical and functional research that gives evidence for these three networks.
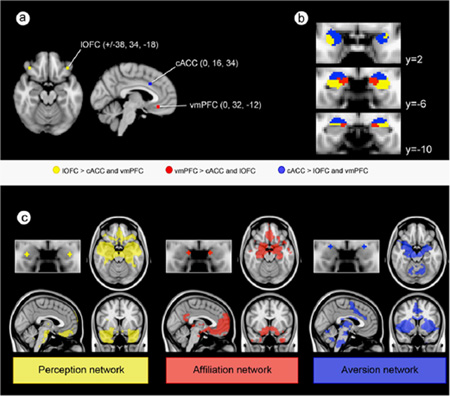
Box 3.
In a recent study (Bickart et al., 2012), we used resting-state functional connectivity analysis (fcMRI) to identify our topographic model of amygdala-based social brain networks. It is a relatively new method for delineating large-scale networks in the living human brain that are composed of regions that tend to function together and share anatomical connections (Deco, Jirsa, & McIntosh, 2011; Fox & Raichle, 2007). In validating fcMRI results against nonhuman animal tract-tracing studies, the topography of putative origins and terminations of large-scale networks is often defined using an iterative seed-target-seed approach (Vincent et al., 2006; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008; Yeo et al., 2011). In our study (Bickart et al., 2012), we used a similar approach to delineate intrinsic networks of the amygdala. After identifying three brain regions of interest (ROIs) outside the amygdala that represent core nodes within each of our amygdala-based networks (lOFC for the perception network, vmPFC for the affiliation network, and cACC, for aversion network), (panel a) (Barbas et al., 2010; Haber & Knutson, 2010; Price & Drevets, 2010), we then identified three voxel clusters within the amygdala (panel b) in a resting-state analysis from a discovery sample of young adults (N=89). Next, we used these connectionally-defined subregions of the amygdala as seeds in another fcMRI analysis of the whole brain to reveal three partially-distinct large-scale networks (panel c) that bear strong resemblance to the known anatomical organization of the amygdala’s connectivity in nonhumans (Fig. 1b). We then replicated these findings in an independent sample (N=31). Since that publication (Bickart et al., 2012), we replicated the networks in three additional independent samples (N=81, 150, and 150) and made further topographic comparisons with other previously established canonical networks. Of eight canonical networks, we found that the ventral attention/salience network (Seeley et al., 2007; Yeo et al., 2011) contains the most tissue in common with the dorsal amygdala network (36% overlap), and the default mode network, especially its medial temporal lobe subsystem (Andrews-Hanna et al., 2010), overlaps most heavily with the medial (60% overlap) and ventrolateral (44% overlap) amygdala networks.
Three connectionally-defined subregions of the amygdala.
(a) A priori seed regions were placed within the ventro-medial prefrontal cortex (vmPFC), caudal anterior cingulate cortex (cACC), and lateral orbitofrontal cortex (lOFC). (b) Each voxel in the amygdala was assigned to the seed region with which it demonstrated the strongest connectivity in the discovery sample (N=89). (c) Seeds are localized within the connectionally-defined amygdala subregions shown in panel b. One sample group mean significance maps for each amygdala seed are displayed in standard views (N=89). The maps are binarized at p<10−5 and overlaid on a T1 MNI152 0.5 mm template brain in radiologic convention to demonstrate the distinct and shared connectivity across maps.
3. An amygdala-based network supporting social perception
3.1. Anatomical tracing evidence
The network supporting perception (Fig. 1 in yellow) is anchored by the ventrolateral sector of the amygdala (including its lateral and basolateral nuclei) and the lateral orbitofrontal cortex (lOFC) and includes connectional targets in sensory association areas of the ventral and lateral temporal cortex (Barbas & De Olmos, 1990; Carmichael & Price, 1995). The ventrolateral amygdala and lOFC receive afferents from sensory association areas of the temporal cortex including the mid to rostral sectors of the temporal gyri, superior temporal sulcus, fusiform gyrus, and ventral temporal pole, as well as the sensory insula that together convey a panoramic view of the external and internal environment (Aggleton, Burton, & Passingham, 1980; Ghashghaei & Barbas, 2002; Hoistad & Barbas, 2008). The ventrolateral amygdala and lOFC send feedback-like glutamatergic projections into sensory association cortices; the amygdala’s projections reach as far back as primary sensory cortices, in a manner capable of modulating perceptual processing of relevant stimuli in accordance with the current affective state and situational context (Barbas, Zikopoulos, & Timbie, 2010; Freese & Amaral, 2006).
3.2. Intrinsic functional connectivity evidence
Recent evidence supports the hypothesis that regions within the network supporting perception share anatomical connections in humans. Using a connectionally-defined ventrolateral subregion of the amygdala (Bickart et al., 2012), we identified an intrinsic connectivity network that includes sensory association areas of the temporal lobe and orbitofrontal cortex (Box 3 panel C). The topography of intrinsic connectivity bears strong resemblance to the underlying anatomical topography of the network in nonhuman animals (Fig. 1). This intrinsic network also contains many of the brain regions that make up an intrinsic network recently referred to as “limbic” (Yeo et al., 2011). These findings suggest that regions within the perception network might function as a unit.
3.3. Functional neuroimaging and electrophysiology evidence
Regions in the network supporting perception are important for detecting and decoding relevant or ambiguous stimuli in the environment. In the social realm, there is perhaps nothing more relevant or ambiguous than the features or actions of conspecifics. Indeed, featural and expressive aspects of faces and bodies including facial identity, facial actions, eye gaze, and lip movement selectively activate neurons within the macaque ventrolateral amygdala, OFC, inferotemporal cortex, superior temporal sulcus, and medial temporal lobe (Baylis, Rolls, & Leonard, 1985; Hasselmo, Rolls, & Baylis, 1989; Haxby Hoffman, & Gobbini, 2002; Leonard, Rolls, Wilson, & Baylis, 1985; Rolls, 1984, 2007; Rolls, Critchley, Browning, & Inoue, 2006). Task-based fMRI studies in humans demonstrate a similar topography of brain responses to socially salient stimuli including facial expressions (Allison, Puce, & McCarthy, 2000; Haxby, Hoffman, & Gobbini, 2000; Morris et al., 1996; Phillips et al., 1997; Winston, O’Doherty, & Dolan, 2003), eye gaze (George, Driver, & Dolan 2001; Kawashima et al. 1999; Richeson, Todd, Trawalter, & Baird, 2008), facial identity (Gobbini & Haxby, 2006; Iidaka et al., 2003; Pourtois et al., 2005; Schwartz et al., 2003; Wright & Liu, 2006), racial or group identity (Cunningham et al., 2004; Freeman, Schiller, Rule, & Ambady, 2010; Hart et al., 2000; Phelps, 2001; Phelps et al., 2000), social hierarchy (Kumaran, Melo, & Duzel, 2012), and trustworthiness (Allison et al., 2000; Bzdok et al., 2011; Cunningham et al., 2004; Engell, Haxby, & Todorov, 2007; George et al., 2001; Gobbini & Haxby, 2006; Hart et al., 2000; Morris et al., 1996; Phelps et al., 2000; Richeson et al., 2008; Said, Baron, & Todorov, 2009; Todorov & Engell, 2008; Todorov et al., 2008; Winston et al., 2002).
3.4. Lesion neuropsychology evidence
In a recent study (Bickart et al., 2013), we reported that the network supporting perception appears to play a necessary role in social perceptual abilities by examining impairments in these abilities in frontotemporal dementia (FTD) patients. Using measures of network-level atrophy derived from structural MRI and a novel clinician-based structured interview and rating scale, the Social Impairment Rating Scale (SIRS), we found that patients with the greatest atrophy in the perception network exhibited a selective lack of awareness or understanding of others’ social and emotional behavior. For example, these patients no longer made as frequent eye-contact, had difficulty following and interpreting body language and gestures, and were relatively insensitive to others’ facial expressions in response to signals such as those indicating a breach in personal boundaries or interruption in conversation. This profile of symptoms is consistent with prior behavioral studies of social perception in FTD patients who demonstrate abnormal eye-contact (Sturm et al., 2010) and deficits in interpreting others’ facial expressions (Keane, Calder, Hodges, & Young 2002; Rosen et al., 2002), eye-gaze (Keane et al., 2002; Kessels et al., 2007; Kipps, Mioshi, & Hodges, 2009a; Kipps, Nestor, Acosta-Cabronero, Arnold, & Hodges, 2009b; Lough et al., 2006; Rankin et al., 2009; Rosen et al., 2002; Snowden et al., 2008; Werner et al., 2007), vocal prosody (Keane et al., 2002; Rankin et al., 2009; Snowden et al., 2008), and body language (Kipps et al., 2009b; Kosmidis, Aretouli, Bozikas, Giannakou, & Ioannidis, 2008; Rankin et al., 2009). In support of our brain-behavior findings, two of these studies traced impairments in the perception of facial expressions (Rosen et al., 2002a) and sarcasm comprehension (Kipps et al., 2009b) back to morphometric changes to regions within the network supporting perception, including the amygdala, lateral orbitofrontal cortex, and temporal pole. Neither examined other key structures within this network, such as the superior temporal sulcus or fusiform gyrus.
4. An amygdala-based network supporting social affiliation
4.1. Anatomical tracing evidence
The network supporting affiliation (Fig. 1, in red) is anchored by nuclei within the medial sector of the macaque amygdala and the vmPFC and includes their connectional mesolimbic targets in the rostral and subgenual anterior cingulate cortices, ventromedial striatum, ventromedial hypothalamus, dorsomedial temporal pole, and medial temporal lobe (An, Bandler, Ongur, & Price, 1998; Carmichael & Price, 1996; Ferry, Ongur, An, & Price, 2000; Fudge, Kunishio, Walsh, Richard, & Haber, 2002; Haber & Calzavara, 2009; Haber & Knutson, 2010; Haber, Kim, Mailly, & Calzavara, 2006; Hsu & Price, 2007; Kondo, Saleem, & Price, 2003, 2005; Kunishio & Haber, 1994; McDonald, 1991b, 1991a; Ongur, An, & Price, 1998; Ongur & Price, 2000; Ongur, Ferry, & Price, 2003; Price, 2007; Price & Drevets, 2010; Saleem, Kondo, & Price 2008). Nodes within this network share convergent connections with nuclei situated in the medial sector of the macaque amygdala including its cortical nuclei, the magnocellular subdivision of the accessory basal nucleus, the medial extent of the parvicellular subdivision of the basolateral nucleus, the amygdalohippocampal transition area, and parts of the medial nucleus (Fudge et al., 2002; McDonald, 1987, 1991b, 1991a).
4.2. Intrinsic functional connectivity evidence
Recent evidence supports the hypothesis that regions within the network supporting affiliation share anatomical connections in humans. Using a connectionally-defined medial subregion of the amygdala (Bickart et al., 2012), we identified an intrinsic connectivity network that includes mesolimbic reward-related areas of the ventromedial prefrontal, anterior cingulate, and medial temporal cortices as well as their connectional targets in the ventro-medial striatum and hypothalamus (Box 3 panel C). The topography of intrinsic connectivity bears strong resemblance to the underlying anatomical topography of the network in nonhuman animals (Fig. 1). The network also contains many of the medial cortical regions of the so-called default mode network (Buckner, Andrews-Hanna, & Schacter, 2008; Greicius, Krasnow, Reiss, & Menon, 2003; Yeo et al., 2011) with additional mesolimbic subcortical regions of the recently-defined limbic network (Yeo et al., 2011). These findings suggest that regions within the network supporting social affiliation might function as a unit.
4.3. Functional neuroimaging and electrophysiology evidence
Regions within this network are involved in goal-directed (instrumental) learning and behavior in rodents, monkeys, and humans (Balleine & O’Doherty, 2010; Knapska, Radwanska, Werka, & Kaczmarek, 2007; Murray, 2007; Waraczynski, 2006), functions that might preferentially subserve learning about and responding to appetitive stimuli (Balleine & O’Doherty, 2010; Knapska et al., 2007; Murray, 2007; Waraczynski, 2006). Within the social realm, regions within the network supporting affiliation are responsive to pictures of loved ones and positive social feedback (e.g., complimentary peer reviews or cooperation from a partner) that elicit prosocial sentiments (e.g., compassion or empathy) and in turn motivate decisions to behave altruistically and cooperate (e.g., donation to charities or repaying trust in kind). For example, in neuroimaging studies when people look at pictures of their own babies or romantic partners the ventral tegmental and striatal areas (Aron et al., 2005; Bartels & Zeki, 2004) as well as the amygdala (Leibenluft, Gobbini, Harrison, & Haxby, 2004) demonstrate increased activity. Stimuli and scenarios that elicit prosocial sentiments like compassion, guilt, pity, gratitude, and pride also activate structures within the social affiliation network including the ventromedial prefrontal cortex, subgenual anterior cingulate cortex, ventral striatum, as well as septal and hypothalamic areas (Moll et al., 2007; Takahashi et al., 2004; Zahn et al., 2009a, 2009b). Similarly, receiving fair treatment from other people in simulated social interactions or positive peer evaluation evokes neural responses in ventromedial prefrontal and mesolimbic structures (Izuma, Saito, & Sadato, 2008) as well as the amygdala (Tabibnia, Satpute, & Lieberman, 2008). These regions also demonstrate increases in activity when people make prosocial decisions such as choosing to treat others fairly or cooperate with them during simulated social interactions (Delgado, Frank, & Phelps, 2005; King-Casas et al., 2005; Li, Xiao, Houser, & Montague, 2009; Rilling et al., 2002, 2004) or when deciding to donate money to charitable causes (Harbaugh, Mayr, & Burghart, 2007; Izuma, Saito, & Sadato, 2009; Moll et al., 2006).
4.4. Lesion neuropsychology evidence
Work with FTD and focal brain lesion patients indicates that the network supporting social affiliation plays a causal role in motivating warm and cooperative behavior towards others. We found that FTD patients with the greatest atrophy in this network exhibited the most severe social and emotional detachment from other people (Bickart et al., 2013). These patients hardly comforted, helped, or showed affection to their friends and loved ones, which in all cases was a substantial departure from their premorbid state. Many became indifferent and unresponsive to the feelings, desires, and needs of other people, often including their closest family members and spouses. Our findings build upon three recent studies in FTD patients that have mapped impairments in prosocial or attachment behavior onto brain regions in the network supporting affiliation. In two of these studies, decreased gray matter in the right ventromedial prefrontal cortex, subgenual anterior cingulate cortex, and the dorsomedial temporal pole correlated with symptoms of diminished empathy (Rankin et al., 2006) and interpersonal warmth (Sollberger et al., 2009), both using voxel-based morphometry (VBM) analyses in large samples of patients with FTD as well as other forms of dementia. The latter study found additional gray matter reductions in the amygdala, anterior hippocampus, entorh-inal cortex, parahippocampus, putamen, caudate, and middle insula mostly in the right hemisphere that correlated with diminished interpersonal warmth (Sollberger et al., 2009). Similarly, in a third study decreases in right ventromedial prefrontal cortex volume correlated with diminished agreeableness in a sample of FTD patients (Rankin et al., 2004). Like FTD patients, vmPFC-damaged patients, who often also have damage to the subgenual and rostral anterior cingulate cortices also demonstrate severely diminished empathy, based on caregiver reports (Hornak et al., 2003; Shamay, Tomer, & Aharon-Peretz 2002; Shamay-Tsoory, Tomer, Berger, & Aharon-Peretz, 2003b; Shamay-Tsoory, Aharon-Peretz, & Perry, 2009). These patients have also exhibited frankly cold behaviors as marked by their untrustworthy and unfair treatment of others in simulated social interactions (Krajbich, Adolphs, Tranel, Denburg, & Camerer, 2009).
5. An amygdala-based network supporting social aversion
5.1. Anatomical tracing evidence
The network supporting social aversion (Fig. 1, in blue) is anchored by nuclei within the rostrodorsal sector of the macaque amygdala and the caudal ACC (cACC) and includes their connectional interoceptive and pain-sensitive targets in the anterior insula and its rostrally adjacent orbitofrontal extension, somato-sensory operculum, ventrolateral striatum, caudolateral hypothalamus, as well as autonomic and dopaminergic nuclei of the brainstem (An et al., 1998; Carmichael & Price, 1996; Ferry et al., 2000; Fudge et al., 2002; Haber & Calzavara, 2009; Haber & Knutson, 2010; Haber et al., 2006; Hsu & Price, 2007; Kondo et al., 2003, 2005; McDonald, 1991b, 1991a; Kunishio & Haber, 1994; Ongur & Price, 2000; Ongur et al., 1998, 2003; Price, 2007; Price & Drevets, 2010; Saleem et al., 2008). Nodes within this network share convergent connections with nuclei situated in the rostrodorsal sector of the macaque amygdala including the central and medial nuclei their cellular extensions into the substantia innominata and the magnocellular subdivision of the basolateral nucleus including its rostral-most sector (Fudge et al., 2002; McDonald, 1987, 1991b, 1991a; Mufson, Mesulam, & Pandya, 1981; Stefanacci & Amaral, 2002).
5.2. Intrinsic functional connectivity evidence
Recent evidence is consistent with the hypothesis that regions within the network supporting social aversion share anatomical connections in humans. Using a connectionally-defined dorsal subregion of the amygdala (Bickart et al., 2012), we identified an intrinsic connectivity network that includes interoceptive pain-related areas of the insula and caudal anterior cingulate cortex as well as their connectional targets in the ventrolateral striatum, hypothalamus, and brainstem (Box 3 panel C). The topography of intrinsic connectivity bears strong resemblance to the underlying anatomical topography of the network (Fig. 1). This intrinsic network also bears strong resemblance to the so-called salience network derived from a seed region in the frontoinsula that is thought to subserve detecting and responding to motivationally relevant stimuli in the environment (Seeley et al., 2007). The strength of intrinsic connectivity within this network is correlated with the intensity of affective experience when viewing aversive images (Touroutoglou, Hollenbeck, Dickerson, & Feldman Barrett, 2012). These findings suggest that regions within the network supporting social aversion functions as a unit.
5.3. Functional neuroimaging and electrophysiology evidence
Regions within the network supporting aversion play a role in habit learning (Pavlovian) and behavior (Balleine & O’Doherty, 2010; Knapska et al., 2007; Murray, 2007; Waraczynski, 2006). These functions preferentially subserve learning about and responding to aversive stimuli (Balleine & O’Doherty, 2010; Hayes & Northoff, 2011; Knapska et al., 2007; Murray, 2007; Waraczynski, 2006). Within the social realm, regions within the network supporting aversion (listed in Fig. 1, in blue) are responsive to untrustworthy-appearing faces and negative social feedback (e.g. disapproval or violations of trust) that elicit sentiments of social aversion (e.g. disgust or contempt) and in turn motivate decisions to not cooperate with an individual or to disengage from a group. For example, stimuli or scenarios that elicit sentiments of social aversion like disgust, contempt, and anger or indignation preferentially activate areas within this network (Buckholtz et al. 2008a; Decety, Jackson, Sommerville, Chaminade, & Meltzoff, 2004; Moll et al., 2005, 2007; Phillips et al., 1998; Rizzolatti et al., 2003; Zahn et al., 2009b). Negative social feedback activates the caudal anterior cingulate cortex and ventral anterior insula (Klucharev, Hytonen, Rijpkema, Smidts, & Fernandez, 2009), and participants who exhibit the greatest increases in caudal anterior cingulate cortex activity change their behavior most in response to the negative social feedback. Similarly, activity in the ventral anterior insula, which is elicited by unfairness (Sanfey, 2007), social exclusion (Eisenberger, Lieberman, & Williams, 2003), and unreciprocated cooperation (Rilling, King-Casas, & Sanfey, 2008) predicts the likelihood that participants will reject cooperation with someone who has treated them unfairly in a previous simulated interaction (Sanfey, Rilling, Aronson, Nystrom, & Cohen 2003). The frontoinsula and neighboring ventral anterior insula display activation when participants decide not to donate to charitable causes (Moll et al., 2006). Regions in this network appear to subserve general avoidance responses in part through their response to pain. Recent neuroimaging studies reveal that—in addition to responding to direct physical pain—areas in the caudal anterior cingulate cortex and anterior thalamus, dorsal and posterior sectors of the insula and its neighboring parietal operculum (including the secondary somatosensory cortex) also respond to second-hand pain and the pain of social rejection (Akitsuki & Decety, 2009; Benuzzi, Lui, Duzzi, Nichelli, & Porro, 2008; Cheng et al., 2007; Cheng, Yang, Lin, Lee, & Decety, 2008; Decety, Jackson, Brunet, & Meltzoff, 2006; Decety & Lamm, 2008; Eisenberger, Jarcho, Lieberman, & Naliboff, 2006; Kross, Egner, Ochsner, Hirsch, & Downey, 2007; Moriguchi et al., 2007).
5.4. Lesion neuropsychology evidence
Supporting a role for this network in motivating apprehensive behavior towards others, we found that FTD patients with the greatest atrophy in this network exhibited the most severe lack of social apprehension (Bickart et al., 2013). These patients became less cautious around strangers and more willing to trust, approach, and strike up conversations with them. They also tended to indiscriminately donate to charities, fall for scams from salesmen, and were more easily taken advantage of by others. Our findings build on previous studies that have traced impairments in social aversion back to morphometric abnormalities in the temporal lobe based on SPECT and PET scans (Snowden et al., 2001; Mendez, Chen, Shapira, Lu, & Miller, 2006). Our finding that patients with atrophy in the aversion network demonstrate diminished social apprehension supports the notion that structures in this network play a necessary role in appropriately judging others as untrustworthy, unfair, or deceptive and in turn making decisions to avoid, punish, or reject them. Furthermore, the finding that patients with the greatest atrophy in this network still tended to seek social rewards or make prosocial decisions to approach others suggests that they had relatively preserved social affiliative behaviors, likely attributable to a relative preservation of affiliative circuitry. In addition, recent fcMRI and neuroanatomical studies in FTD patients have proposed the selective vulnerability of these regions as part of the “salience network” in the pathology of FTD (Seeley, 2008; Seeley et al., 2006, 2008; Zhou et al., 2010).
6. Connectional strength in intrinsic networks subserving social perception and affiliation predict larger, more complex social networks in healthy individuals
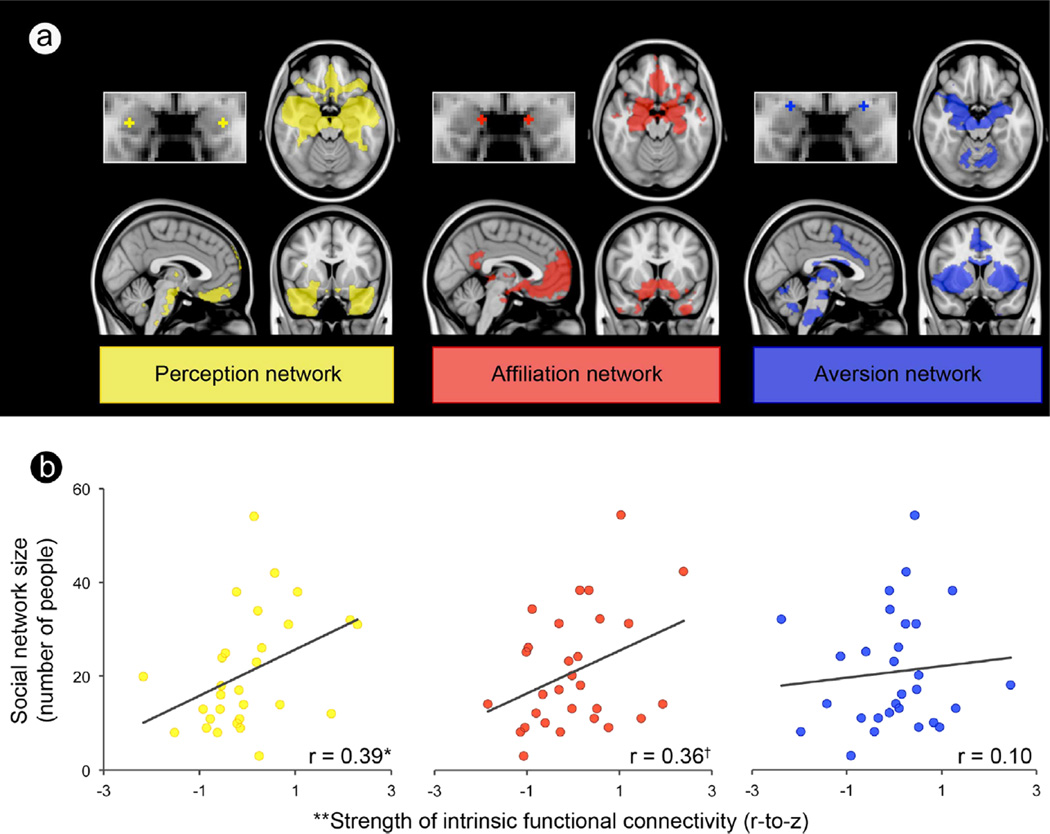
In the context of previous neuroimaging and neuropsychology experiments in humans, our fcMRI findings (Bickart et al., 2012) indicate that the amygdala-based networks supporting perception, affiliation, and aversion are separable both via their topography and functional roles. Importantly, we found stronger intrinsic amygdala connectivity within the networks supporting perception and affiliation, but not in the network supporting aversion, in people who fostered and maintained larger and more complex social networks (Fig. 2). These relations held over and above individual differences in amygdala volume. In fact, individual differences in intrinsic connectivity (along with amygdala volume) predicted 42% of the variance in social network size. Furthermore, people with the largest social networks had stronger connectivity between medial amygdala and vmPFC within the network supporting affiliation, as well as between the ventrolateral amygdale and a number of social brain regions in the temporal cortex including the STS, fusiform gyrus, and hippocampus within the network supporting perception (Fig. 3). These findings indicate that greater functional coherence within amygdala-based networks confers some advantage for handling the demands of complex social life. For example, individuals with stronger connectivity in the network supporting perception might have enhanced ability to detect and decode social signals from others, or as in a recent study (Edelson, Sharot, Dolan, & Dudai, 2011), these individuals may rely more on what others say when recalling events. Those with stronger connectivity in the network supporting affiliation might have an increased tendency or motivation to cooperate with or form bonds with others. Furthermore, the intrinsic connectivity-social network size link was anatomically specific to the two networks. Other networks important for social cognition but not anchored in the amygdala [the mentalizing network (Van Overwalle & Baetens, 2009), the dorsal subsystem of the default mode network (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010), and the mirror network (Van Overwalle & Baetens, 2009)], as seen in Fig. 4], did not explain significant variance in social network size or complexity.
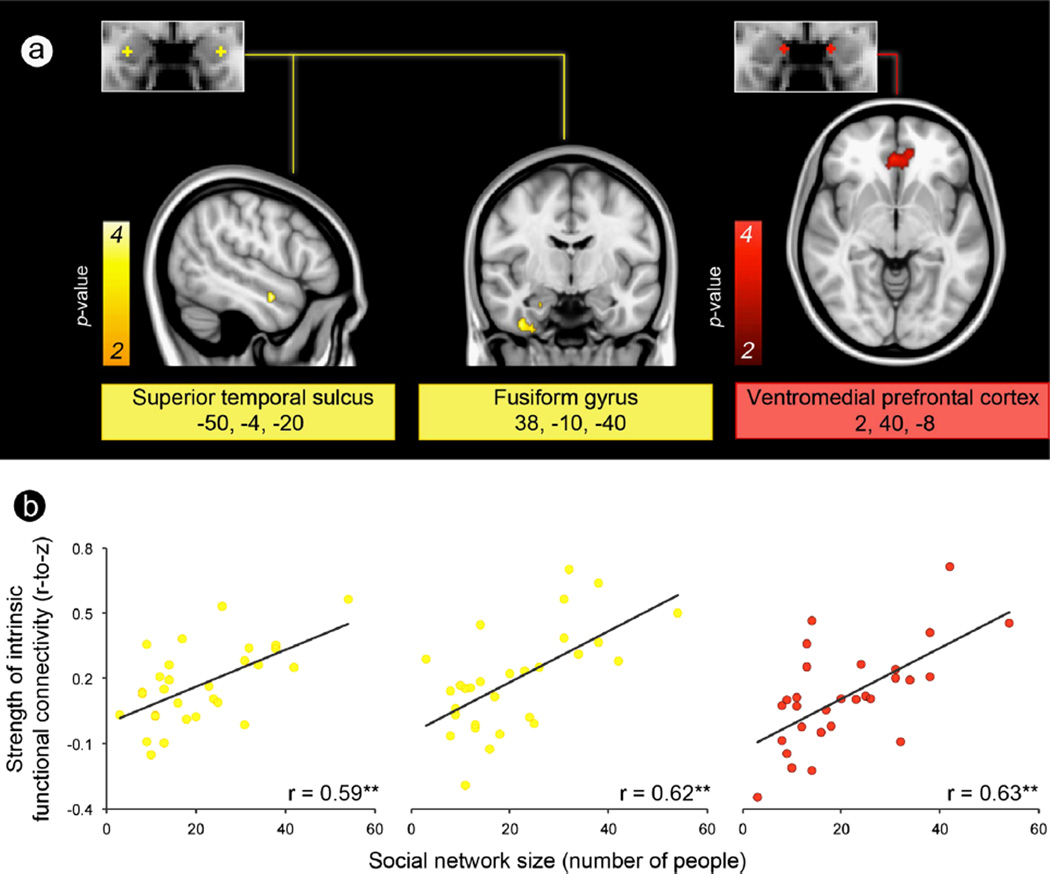
Fig. 2.
A larger social network is predicted by stronger connectivity between amygdala subregions and corticolimbic regions important for perception and affiliative behavior. (a) Each amygdala subregion and its intrinsic connectivity network were independently defined in the discovery sample (shown here) and then used in the test sample to compute the connectivity strength between each amygdala subregion and the rest of the network. (b) Scatter plots show that social network size (y-axis) is predicted by the strength of connectivity between two of the three amygdala subregions and their respective networks (x-axis), over and above amygdala volume, in the test sample. *p<0.05; †p=0.06; **The x-axis displays the residual variance in the strength of the resting-state connectivity measure (Fisher’s r-to-z) after partialling out its shared variance with amygdala volume.
Fig. 3.
Exploratory analyses revealed that the connectivity between amygdala and specific regions within the perception and affiliation networks are the best predictors of social network size. (a) Brain images show location of voxels within the medial and ventrolateral amygdala’s intrinsic connectivity networks (defined in the discovery sample) that correlated with social network size at p <0.01 in the test sample, uncorrected with a cluster size constraint of 10 voxels. Color bars indicate the p-values (10−2–10−4) of correlated voxels, which are overlaid on slices of a T1 MNI152 0.5 mm template brain in radiologic convention. (b) Scatter plots show the effects for peak voxels with the strength of intrinsic connectivity on the y-axis and social network size on the x-axis. **p< 0.001.
Fig. 4.
A schematic of five large-scale brain networks subserving processes important for social behavior. Here, we show three networks that are anchored in the amygdala (amygdala-based networks) and two that are not (control networks). The amygdala is displayed in white indicating that it is the hub of the three amygdala-based networks. List of ROIs with abbreviations–Perception network: lOFC, lateral orbitofrontal cortex; vTP, ventrolateral temporal pole; FG, fusiform gyrus; STS, superior temporal sulcus. Affiliation network: dTP, dorsomedial temporal pole; rACC, rostral anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; Ent, entorhinal cortex; PHip, parahippocampal cortex; vmSt, ventromedial striatum. Aversion network: cACC, caudal anterior cingulate cortex; Ins, insula; SII, somatosensory operculum; vlSt, ventrolateral striatum. Mentalizing network: dmPFC, dorsomedial prefrontal cortex; PCC, posterior cingulate cortex; Precun, precuneus; AngG, angular gyrus (temporoparietal junction). Mirror network: pSTS, posterior superior temporal sulcus; IPS, intraparietal sulcus; PreMC, premotor cortex.
7. Non-amygdala networks within the social brain
Taken together, our fcMRI findings in healthy adults (Bickart et al., 2012), our neuropsychological results in FTD patients (Bickart et al., 2013) along with the existing findings from anatomical tracing, functional, and lesion studies suggest a broad division of labor within the social brain between networks that are anchored in the amygdala and networks that are important for social cognition, but that routinely do not include the amygdala. Regions in the mentalizing network play a common role in inferring others’ thoughts, intentions, and beliefs (Buckner et al., 2008; Saxe, 2006; Van Overwalle, 2011; Van Overwalle & Baetens, 2009), which appears dissociable from the role of brain regions in the network supporting perception that is implicated in understanding others’ feelings (Baron-Cohen et al., 1999; Saxe, 2006; Shamay-Tsoory & Aharon-Peretz, 2007; Shamay-Tsoory, Tomer, Berger, & Aharon-Peretz, 2003a; Shamay-Tsoory, Tomer, Berger, Goldsher, & Aharon-Peretz, 2005; Shaw et al., 2005; Stone, Cosmides, Tooby, Kroll, & Knight, 2002; Zaki & Ochsner, 2012). It is believed that regions within the mirror network support a social cognitive strategy, different from mentalizing, that grounds individuals’ ability to know about others’ goals and intentions by simulating their behaviors (Blakemore & Frith, 2004; Frith, 2007; Frith & Frith, 2007; Saxe, 2006) or their affective experience that is linked to their internal physical state (Zaki, Hennigan, Weber, & Ochsner, 2010). Investigators have previously contrasted the function of this network with that of regions in the network supporting aversion, including the caudal anterior cingulate cortex and insula, that are preferentially involved in simulating others’ feelings, studied largely in the context of representing others’ pain, as opposed to their intentions (Blakemore & Frith, 2004; Frith, 2007; Frith & Frith, 2007; Saxe, 2006).
The amygdala-based and non-amygdala networks within the social brain can be thought of in anatomical terms as being more or less representative of information from the body’s internal milieu. The mentalizing and mirror networks, which are composed mostly of regions with more granular cortex and less amygdala connectivity, appear to support social cognitive functions that draw less on affective input from the body, whereas the amygdala-based networks supporting perception, affiliation, and aversion are composed moreso of regions with less granular “limbic” cortex and appear to support functions that draw more heavily on visceral input. For example, the medial prefrontal cortex exhibits a ventro-dorsal gradient of increasing lamination and decreasing amygdala connectivity (for a discussion, see Barbas, 2007). Perhaps this anatomical gradient reflects a functional gradient in decreasing affective influence on the control of behavior via the medial prefrontal cortex (e.g., (Roy et al., 2012)). Indeed, in the social realm, the ventromedial prefrontal cortex is more consistently involved in motivating decisions to cooperate with or trust others based on moral sentiments (for discussion, see Moll et al., 2005; Schulkin and Moll, 2009) or others’ emotional behavior or appearance (e.g., visually attractive faces: O’Doherty et al., 2003). In contrast, the dorsomedial prefrontal cortex is more consistently involved in thinking about others’ beliefs and intentions based on their behavior (Buckner et al., 2008; Saxe, 2006; Van Overwalle, 2011; Van Overwalle & Baetens, 2009), which may not motivate an adaptive interpersonal response.
A compelling finding from a recent study (Becker et al., 2012) prompts the idea that amygdala-based and non-amygdala networks of the social brain may not only play distinct roles, but they can perform similar functions. In that study, one sister of monozygotic twins, who both have bilateral amygdala lesions, has the ability to maintain a social network and identify caricatured facial poses of fear while the other has neither of these abilities. The authors attributed this difference to a finding on fMRI that showed activation within regions of the mirror network in the twin that has more social function.
8. The amygdala, social cognition, and social connectedness
Evidence from multiple methodologies across species supports a central role for the amygdala in large-scale neural networks that make up the social brain. These findings provide a powerful framework for beginning to parse social cognition into its component processes that have at least partially distinct neural underpinnings. A componential approach allows future research to investigate how separable domains of social cognition and their neurobiological correlates differ among healthy people and disintegrate or fail to develop in neuropsychiatric populations marked by social impairment, such as autism, antisocial personality disorder, and frontotemporal dementia.
Future research on the amygdala’s role in brain networks that support social life will undoubtedly attempt to determine whether, to what degree, and on what timescale connectional differences in amygdalar networks support (or are caused by) social functions. A preliminary finding in macaques suggests that intrinsic connectivity strength between social brain regions (not including the amygdala) might increase as a result of living in larger social groups (Sallet et al., 2011), although more work is needed to bear this out (because the animals were not randomly assigned to living conditions). Longitudinal studies with randomly assigned social group sizes are needed to more carefully track brain changes that might be caused by changes in social network size.
It would also be important to determine the social advantages conferred by greater functional coherence or larger structures within amygdala-based networks that might mediate their link with social network size. This could be accomplished using behavioral analyses that probe multiple social cognitive functions, like the SIRS (Bickart et al., 2013), in combination with social network data, and measures of brain structure and function. The only study of this kind combined measures of cortical volume, mentalizing ability, and social network size (Powell, Lewis, Roberts, Garcia-Finana, & Dunbar 2012). According to that study, mentalizing ability mediates a positive relationship between orbitofrontal cortex volume and social network size.
9. Towards a molecular basis for the amygdala in social life
Elucidating the neuromolecular mechanisms underlying the link between amygdala-based networks and social behavior will likely be required to translate this research to the clinical care of patient populations marked by amygdala dysfunction and associated social disruptions. Studies employing neuropeptide delivery, imaging genetics, and optogenetics provide exciting tools that have already begun mounting evidence for a cellular and molecular understanding of the amygdala-based circuits that make up the social brain.
Members of the nonapeptide family, particularly oxytocin (OXT), are promising targets for treatment of disruptions in social behavior given that the amygdala is one of the core nodes of OXT action in the brain (Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011). In the context of the evidence discussed in this review, recent studies on OXT highlight one potential neuromolecular mechanism by which separate subregions and subnetworks of the amygdala might modulate diverse social functions. For example, OXT-enhanced fMRI signal in the ventrolateral amygdala and functional coupling with the superior colliculus relates to increases in participants’ frequency of fixating their gaze on the eye regions of others’ faces (Gamer, Zurowski, & Buchel, 2010), suggesting a role for OXT in the amygdala network supporting social perception. In response to fearful faces, OXT diminishes activity in the rostral amygdala in the vicinity of a nuclear group that includes the central nucleus (Gamer et al., 2010) and suppresses amygdala coupling with regions of the brainstem (Kirsch et al., 2005), suggesting a role for OXT in the amygdala network supporting social aversion. OXT also potentiates amygdala-dependent socially reinforced learning and emotional empathy (Hurlemann et al., 2010) as well as activity in reward circuitry in response to viewing a female partner’s face (Scheele et al., 2013), suggesting a role for OXT in the network supporting social affiliation. Further support for this latter role, in particular, comes from studies in rodent and avian species. Such studies have exciting potential in this domain given that these species share similarities with humans in the diversity and makeup of group structures, social behaviors, and nonapeptides. According to fMRI work in rodents, OXT may also modulate social behavior by strengthening mother-infant bonds through its action on nuclei in the medial sector of the amygdala and its connectional targets (Febo, Numan, & Ferris, 2005). Increases in nonapeptide activity in a similar circuit across avian species predicts higher degrees of gregariousness and, within species, higher degrees of affiliation with larger groups of birds as well as with birds who are familiar, “friendly”, or mates (Goodson & Wang, 2006; Goodson, Rinaldi, & Kelly, 2009a; Goodson, Schrock, Klatt, Kabelik, & Kingsbury, 2009b; Kelly et al., 2011). Taken together, these studies suggest that anatomical structures of similar size and connectivity can support social groups of different sizes as a function of the underlying neurochemistry. Nevertheless, the interplay between OXT, the amygdala, and social behavior remains quite complex. For example, OXT-related changes in amygdala activity might depend in part on gender (Domes et al., 2010), suggesting there is still much to learn about the conditions that determine the direction of OXT’s effect on the amygdala.
Studies employing imaging genetics have begun to reveal allelic variations that underlie differences in regional brain activation, connectivity, and associated aspects of human sociality. Findings relevant to the amygdala’s role in the social brain have investigated genetic variations encoding such proteins as the oxytocin receptor OXTR (Tost et al., 2010), vasopressin receptor subtype AVPR1A (Meyer-Lindenberg et al., 2009), monoamine oxidase A (Buckholtz & Meyer-Lindenberg, 2008), and the serotonin receptor 5-HTT (Canli & Lesch, 2007). These variations have been found to drive differences in amygdala structure (Good et al., 2003; Meyer-Lindenberg et al., 2006), reactivity (Furmark et al., 2004; Meyer-Lindenberg et al., 2006; Tost et al., 2010), and connectivity (Buckholtz et al., 2008b), which have been linked to differences in aspects of sociality, such as social phobia (Furmark et al., 2004) and various components of social temperament (Buckholtz et al., 2008b; Meyer-Lindenberg et al., 2009; Tost et al., 2010). One study that is particularly relevant to the framework discussed here showed that males with lower expression of monoamine oxidase A demonstrate functional dysregulation within a circuit including the amygdala, rACC, and vmPFC, components of the amygdala network supporting social affiliation, which in turn relates to a tendency toward enhanced reactivity to threatening cues and reduced sensitivity to cues that reinforce prosocial behavior (Buckholtz et al., 2008b).
Optogenetics offers yet another approach for furthering the discovery of neural systems that underlie social behavior, one that enables an unparalleled anatomic precision in delineating the function of specific brain circuits (Yizhar, 2012). For example, one study using optogenetics in rodents revealed a circuit between the basolateral complex of the amygdala and ventral hippocampus that confers bidirectional control over intruder exploration and sociability in general (Felix-Ortiz & Tye, 2014). Future research would benefit from integrating neuropeptide, imaging genetics, and optogenetics with clinical methods for measuring social behavior along with the resting-state fcMRI techniques and the neuroanatomical framework discussed in this review.
Finally, it will be important to understand how the amygdala and its connections support social connectedness in the context of its role in affective reactivity and enhanced anxiety. Structural abnormalities of the amygdala have been related to a range of neurodevelopmental disorders involving disruptions in affect and anxiety and their interactions with social behavior (Schumann, Bauman, & Amaral, 2010). Of course, amygdala function is not specific to social cognition or even to anxiety per se. Many of the structures within amygdala-based networks play a number of roles in other mental processes including a range of emotions such as anger, disgust, happiness, and sadness (Lindquist et al., 2012), as well as other processes involved in learning (Dolan, 2007), object perception (Haxby et al., 2001), attention (Duncan & Barrett, 2007), and memory (Wang et al., 2010). Such findings remind us that an integrated model of how the brain creates the mind, and allows individual minds to function as a social group, requires that we explicitly understand these dependencies. In the social realm, the amygdala can be considered a hub where these processes converge and are used to decode the multimodal, dynamic, and often ambiguous streams of information from other people. In this position, the amygdala and its connections within the social brain appear to be particularly important for discriminating signal from noise, threat from reward, or friend from foe and thereby guiding adaptive interpersonal behavior.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge patients and families who have participated in research, and funding sources including R21-MH097094 and R21-NS084156.
Footnotes
Appendix. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/http://dx.doi.org/10.1016/j.neuropsychologia.2014.08.013.
References
- Adolphs R. Social cognition and the human brain. Trends in Cognitive Sciences. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The social brain: neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. Journal of Neuroscience. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and sub-cortical afferents to the amygdala of the rhesus-monkey (Macaca-mulatta) Brain Research. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Akitsuki Y, Decety J. Social context and perceived agency affects empathy for pain: an event-related fMRI investigation. Neuroimage. 2009;47:722–734. doi: 10.1016/j.neuroimage.2009.04.091. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Kato M, Muramatsu T, Saito F, Nakachi R, Kashima H. A deficit in discriminating gaze direction in a case with right superior temporal gyrus lesion. Neuropsychologia. 2006;44:161–170. doi: 10.1016/j.neuropsychologia.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. Journal of Comparative Neurology. 1998;401:455–479. [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. J Anat. 2007;211:237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. Journal of Comparative Neurology. 1990;300:549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B, Timbie C. Sensory pathways and emotional context for action in primate prefrontal cortex. Biological Psychiatry. 2010;69:1133–1139. doi: 10.1016/j.biopsych.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E. Affect as a Psychological Primitive. Advances in Experimental Social Psychology. 2009;41(41):167–218. doi: 10.1016/S0065-2601(08)00404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Baylis GC, Rolls ET, Leonard CM. Selectivity between faces in the responses of a population of neurons in the cortex in the superior temporal sulcus of the monkey. Brain Research. 1985;342:91–102. doi: 10.1016/0006-8993(85)91356-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Mihov Y, Scheele D, Kendrick KM, Feinstein JS, Matusch A, et al. Fear processing and social networking in the absence of a functional amygdala. Biological Psychiatry. 2012;72:70–77. doi: 10.1016/j.biopsych.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Benuzzi F, Lui F, Duzzi D, Nichelli PF, Porro CA. Does it look painful or disgusting? Ask your parietal and cingulate cortex. Journal of Neuroscience. 2008;28:923–931. doi: 10.1523/JNEUROSCI.4012-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. Journal of Neuroscience. 2012;32:14729–14741. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nature Neuroscience. 2011;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Brickhouse M, Negreira A, Sapolsky D, Barrett LF, Dickerson BC. Atrophy in distinct corticolimbic networks in frontotemporal dementia relates to social impairments measured using the Social Impairment Rating Scale. Journal of Neurology, Neurosurgery, and Psychiatry. 2013;85:438–448. doi: 10.1136/jnnp-2012-304656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Frith U. How does the brain deal with the social world? Neuroreport. 2004;15:119–128. doi: 10.1097/00001756-200401190-00024. [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends in Neuroscience. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Asplund CL, Dux PE, Zald DH, Gore JC, Jones OD, et al. The neural correlates of third-party punishment. Neuron. 2008a;60:930–940. doi: 10.1016/j.neuron.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, et al. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Molecular Psychiatry. 2008b;13:313–324. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Caspers S, Kurth F, Habel U, Zilles K, et al. ALE meta-analysis on facial judgments of trustworthiness and attractiveness. Brain Structure & Function. 2011;215:209–223. doi: 10.1007/s00429-010-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Yang CY, Lin CP, Lee PL, Decety J. The perception of pain in others suppresses somatosensory oscillations: a magnetoencephalography study. Neuroimage. 2008;40:1833–1840. doi: 10.1016/j.neuroimage.2008.01.064. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, Hung D, et al. Expertise modulates the perception of pain in others. Current Biology. 2007;17:1708–1713. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Chris Gatenby J, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychological Science. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. Is the extrastriate body area (EBA) sensitive to the perception of pain in others? Cerebral Cortex. 2008;18:2369–2373. doi: 10.1093/cercor/bhn006. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL, Brunet E, Meltzoff AN. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: an fMRI investigation. Neuroimage. 2004;23:744–751. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, Mcintosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nature Reviews Neuroscience. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Delgado M, Frank R, Phelps E. Perceptions of moral character modulate the neural systems of reward during the trust game. Nature Neuroscience. 2005;8:1611–1618. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2007;362:787–799. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Spoors M. Social networks, support cliques, and kinship. Human Nature: An Interdisciplinary Biosocial Perspective. 1995;6:273–290. doi: 10.1007/BF02734142. [DOI] [PubMed] [Google Scholar]
- Duncan S, Barrett LF. The role of the amygdala in visual awareness. Trends in Cognitive Sciences. 2007;11:190–192. doi: 10.1016/j.tics.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson M, Sharot T, Dolan RJ, Dudai Y. Following the crowd: brain substrates of long-term memory conformity. Science. 2011;333:108–111. doi: 10.1126/science.1203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006;126:132–138. doi: 10.1016/j.pain.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. Journal of Cognitive Neuroscience. 2007;19:1508–1519. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. Journal of Neuroscience. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. Journal of Neuroscience. 2014;34:586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. Journal of Comparative Neurology. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Freeman JB, Schiller D, Rule NO, Ambady N. The neural origins of superficial and individuated judgments about ingroup and outgroup members. Human Brain Mapping. 2010;31:150–159. doi: 10.1002/hbm.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and VI in the macaque monkey. Journal of Comparative Neurology. 2005;486:295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. Synaptic organization of projections from the amygdala to visual cortical areas TE and VI in the macaque monkey. Journal of Comparative Neurology. 2006;496:655–667. doi: 10.1002/cne.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The human amygdala. New York: The Guilford Press; 2009. pp. 3–42. [Google Scholar]
- Frith CD. The social brain? Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2007;362:671–678. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Current Biology. 2007;17:R724–R732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Garpenstrand H, Marteinsdottir I, Langstrom B, Oreland L, et al. Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neuroscience Letters. 2004;362:189–192. doi: 10.1016/j.neulet.2004.02.070. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George N, Driver J, Dolan RJ. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural response to the visual familiarity of faces. Brain Research Bulletin. 2006;71:76–82. doi: 10.1016/j.brainresbull.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Good CD, Lawrence K, Thomas NS, Price CJ, Ashburner J, Friston KJ, et al. Dosage-sensitive X-linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain. 2003;126:2431–2446. doi: 10.1093/brain/awg242. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminals preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Hormones and Behavior. 2009a;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009b;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Research Bulletin. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. Journal of Neuroscience. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, Mclnerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs in group face stimuli. Neuroreport. 2000;11:2351–2355. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Rolls ET, Baylis GC. The role of expression and identity in the face-selective responses of neurons in the temporal visual cortex of the monkey. Behavioural Brain Research. 1989;32:203–218. doi: 10.1016/s0166-4328(89)80054-3. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Annals of Behavioral Medicine. 2010;40:218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Hayes DJ, Northoff G. Identifying a network of brain regions involved in aversion-related processing: a cross-species translational investigation. Frontiers in Integrative Neuroscience. 2011;5:49. doi: 10.3389/fnint.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Dunbar RIM. Social network size in humans. Human Nature: An Interdisciplinary Biosocial Perspective. 2003;14:53–72. doi: 10.1007/s12110-003-1016-y. [DOI] [PubMed] [Google Scholar]
- Hoistad M, Barbas H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage. 2008;40:1016–1033. doi: 10.1016/j.neuroimage.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Price JL. Midline and intralaminar thalamic connections with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology. 2007;504:89–111. doi: 10.1002/cne.21440. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal of Neuroscience. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Terashima S, Yamashita K, Okada T, Sadato N, Yonekura Y. Dissociable neural responses in the hippocampus to the retrieval of facial identity and emotion: an event-related fMRI study. Hippocampus. 2003;13:429–436. doi: 10.1002/hipo.10059. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. Journal of Cognitive Neuroscience. 2009;22:621–631. doi: 10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proceedings of the Royal Society B. 2011;279:1327–1334. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Sugiura M, Kato T, Nakamura A, Hatano K, Ito K, et al. The human amygdala plays an important role in gaze monitoring. A PET study. Brain. 1999;122(Pt 4):779–783. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- Keane J, Calder AJ, Hodges JR, Young AW. Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia. 2002;40:655–665. doi: 10.1016/s0028-3932(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Kingsbury MA, Hoffbuhr K, Schrock SE, Waxman B, Kabelik D, et al. Vasotocin neurons and septal Vla-like receptors potently modulate songbird flocking and responses to novelty. Hormones and Behavior. 2011;60:12–21. doi: 10.1016/j.yhbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. Impaired fixation to eyes following amygdala damage arises from abnormal bottom-up attention. Neuropsychologia. 2010;48:3392–3398. doi: 10.1016/j.neuropsychologia.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Glascher J, Tyszka JM, Adolphs R. Personal space regulation by the human amygdala. Nature Neuroscience. 2009;12:1226–1227. doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels RP, Gerritsen L, Montagne B, Ackl N, Diehl J, Danek A. Recognition of facial expressions of different emotional intensities in patients with frontotemporal lobar degeneration. Behavioural Neurology. 2007;18:31–36. doi: 10.1155/2007/868431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Mioshi E, Hodges JR. Emotion, social functioning and activities of daily living in frontotemporal dementia. Neurocase. 2009a;15:182–189. doi: 10.1080/13554790802632892. [DOI] [PubMed] [Google Scholar]
- Kipps CM, Nestor PJ, Acosta-Cabronero J, Arnold R, Hodges JR. Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain. 2009b;132:592–603. doi: 10.1093/brain/awn314. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucharev V, Hytonen K, Rijpkema M, Smidts A, Fernandez G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiological Reviews. 2007;87:1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology. 2005;493:479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Koscik TR, Tranel D. The human amygdala is necessary for developing and expressing normal interpersonal trust. Neuropsychologia. 2011;49:602–611. doi: 10.1016/j.neuropsychologia.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmidis MH, Aretouli E, Bozikas VP, Giannakou M, Ioannidis P. Studying social cognition in patients with schizophrenia and patients with frontotemporal dementia: theory of mind and the perception of sarcasm. Behavioural Neurology. 2008;19:65–69. doi: 10.1155/2008/157356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. Journal of Neuroscience. 2009;29:2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience. 2007;19:945–956. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Melo HL, Duzel E. The emergence and representation of knowledge about social and nonsocial hierarchies. Neuron. 2012;76:653–666. doi: 10.1016/j.neuron.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishio K, Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. Journal of Comparative Neurology. 1994;350:337–356. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biological Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Rolls ET, Wilson FA, Baylis GC. Neurons in the amygdala of the monkey with responses selective for faces. Behavioural Brain Research. 1985;15:159–176. doi: 10.1016/0166-4328(85)90062-2. [DOI] [PubMed] [Google Scholar]
- Li J, Xiao E, Houser D, Montague PR. Neural responses to sanction threats in two-party economic exchange. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16835–16840. doi: 10.1073/pnas.0908855106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsycholo-gia. 2006;44:950–958. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: a fluorescence retrograde transport study in the rat. Journal of Comparative Neurology. 1987;262:46–58. doi: 10.1002/cne.902620105. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991a;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991b;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Chen AK, Shapira JS, Lu PH, Miller BL. Acquired extroversion associated with bitemporal variant of frontotemporal dementia. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:100–107. doi: 10.1176/jnp.18.1.100. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, et al. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Molecular Psychiatry. 2009;14:968–975. doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, RH A, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souzat R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Moll FT, Ignacio FA, Bramati IE, Caparelli-Daquer EM, et al. The moral affiliations of disgust: a functional MRI study. Cognitive and Behavioral Neurology. 2005;18:68–78. doi: 10.1097/01.wnn.0000152236.46475.a7. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Garrido GJ, Bramati IE, Caparelli-Daquer EMA, Paiva MLMF, et al. The self as a moral agent: link-ling the neural bases of social agency and moral sensitivity. Social Neuroscience. 2007;2:336–352. doi: 10.1080/17470910701392024. [DOI] [PubMed] [Google Scholar]
- Moll J, Schulkin J. Social attachment and aversion in human moral cognition. Neuroscience and Biobehavioral Reviews. 2009;33:456–465. doi: 10.1016/j.neubiorev.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, et al. Empathy and judging other’s pain: an fMRI study of alexithymia. Cerebral Cortex. 2007;17:2223–2234. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Lieberman MD. The emergence of social cognitive neuroscience. American Psychologist. 2001;56:717–734. [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. Journal of Comparative Neurology. 1998;401:480–505. [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Faces and races in the brain. Nature Neuroscience. 2001;4:775–776. doi: 10.1038/90467. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proceedings of the Biological Sciences. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. Portraits or people? Distinct representations of face identity in the human visual cortex. Journal of Cognitive Neuroscience. 2005;17:1043–1057. doi: 10.1162/0898929054475181. [DOI] [PubMed] [Google Scholar]
- Powell J, Lewis PA, Roberts N, Garcia-Finana M, Dunbar RI. Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proceedings of the Biological Sciences. 2012;279:2157–2162. doi: 10.1098/rspb.2011.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsycho-pharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Rosen HJ, Kramer JH, Schauer GF, Weiner MW, Schuff N, et al. Right and left medial orbitofrontal volumes show an opposite relationship to agreeableness in FTD. Dementia and Geriatric Cognitive Disorders. 2004;17:328–332. doi: 10.1159/000077165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Salazar A, Gorno-Tempini ML, Sollberger M, Wilson SM, Pavlic D, et al. Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. Neuroimage. 2009;47:2005–2015. doi: 10.1016/j.neuroimage.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeson JA, Todd AR, Trawalter S, Baird AA. Eye-gaze direction modulates race-related amygdala activity. Group Processes & Intergroup Relations. 2008;11:233–246. [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rilling JK, King-Casas B, Sanfey AG. The neurobiology of social decision-making. Current Opinion in Neurobiology. 2008;18:159–165. doi: 10.1016/j.conb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. Opposing BOLD responses to reciprocated and unreciprocated altruism in putative reward pathways. Neuroreport. 2004;15:2539–2543. doi: 10.1097/00001756-200411150-00022. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Wicker B, Keysers C, Plailly J, Royet JP, Gallese V. Both of us disgusted in My Insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Neurons in the cortex of the temporal lobe and in the amygdala of the monkey with responses selective for faces. Human Neurobiology. 1984;3:209–222. [PubMed] [Google Scholar]
- Rolls ET. The representation of information about faces in the temporal and frontal lobes. Neuropsychologia. 2007;45:124–143. doi: 10.1016/j.neuropsychologia.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Browning AS, Inoue K. Face-selective and auditory neurons in the primate orbitofrontal cortex. Experimental Brain Research. 2006;170:74–87. doi: 10.1007/s00221-005-0191-y. [DOI] [PubMed] [Google Scholar]
- Rosen H, Perry R, Murphy J, Kramer J, Mychack P, Schuff N, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002;125:2286–2295. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said CP, Baron SG, Todorov A. Nonlinear amygdala response to face trustworthiness: contributions of high and low spatial frequency information. Journal of Cognitive Neuroscience. 2009;21:519–528. doi: 10.1162/jocn.2009.21041. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. Journal of Comparative Neurology. 2008;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Andersson JL, O’Reilly JX, Jbabdi S, et al. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- Sanfey AG. Social decision-making: insights from game theory and neuroscience. Science. 2007;318:598–602. doi: 10.1126/science.1142996. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16:235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, et al. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20308–20313. doi: 10.1073/pnas.1314190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2010;49:745–759. doi: 10.1016/j.neuropsychologia.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, et al. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biological Psychiatry. 2003;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Seeley W. Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Current Opinion in Neurology. 2008;21:701–707. doi: 10.1097/WCO.0b013e3283168e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W, Carlin D, Allman J, Macedo M, Bush C, Miller B, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Annals of Neurology. 2006;60:660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of Neurology. 2008;65:249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE. Social ties and health: the benefits of social integration. Annals of Epidemiology. 1996;6:442–451. doi: 10.1016/s1047-2797(96)00095-6. [DOI] [PubMed] [Google Scholar]
- Shamay SG, Tomer R, Aharon-Peretz J. Deficit in understanding sarcasm in patients with prefronal lesion is related to impaired empathic ability. Brain and Cognition. 2002;48:558–563. [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. 2007;45:3054–3067. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Patients with ventromedial prefrontal lesions show impaired affective theory of mind. Brain and Cognition. 2003a;51(229):229. [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2003b;15:324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cognitive and Behavioral Neurology. 2005;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Shaw P, Bramham J, Lawrence EJ, Morris R, Baron-Cohen S, David AS. Differential effects of lesions of the amygdala and prefrontal cortex on recognizing facial expressions of complex emotions. Journal of Cognitive Neuroscience. 2005;17:1410–1419. doi: 10.1162/0898929054985491. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. Journal of Neurology Neurosurgery and Psychiatry. 2001;70:323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Austin NA, Sembi S, Thompson JC, Craufurd D, Neary D. Emotion recognition in Huntington’s disease and fronto temporal dementia. Neuropsychologia. 2008;46:2638–2649. doi: 10.1016/j.neuropsychologia.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Sollberger M, Stanley CM, Wilson SM, Gyurak A, Beckman V, Growdon M, et al. Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia. 2009;47:2812–2827. doi: 10.1016/j.neuropsychologia.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spezio ML, Huang PY, Castelli F, Adolphs R. Amygdala damage impairs eye contact during conversations with real people. Journal of Neuroscience. 2007;27:3994–3997. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. Journal of Comparative Neurology. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Stone VE, Cosmides L, Tooby J, Kroll N, Knight RT. Selective impairment of reasoning about social exchange in a patient with bilateral limbic system damage. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11531–11536. doi: 10.1073/pnas.122352699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, McCarthy ME, Yun I, Madan A, Yuan JW, Holley SR, et al. Mutual gaze in Alzheimer’s disease, frontotemporal and semantic dementia couples. Social Cognitive and Affective Neuroscience. 2010;6:359–367. doi: 10.1093/scan/nsq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Satpute AB, Lieberman MD. The sunny side of fairness: preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry) Psychological Science. 2008;19:339–347. doi: 10.1111/j.1467-9280.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage. 2004;23:967–974. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Todorov A, Engell AD. The role of the amygdala in implicit evaluation of emotionally neutral faces. Social Cognitive and Affective Neuroscience. 2008;3:303–312. doi: 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Baron SG, Oosterhof NN. Evaluating face trustworthiness: a model based approach. Social Cognitive and Affective Neuroscience. 2008;3:119–127. doi: 10.1093/scan/nsn009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Hyman BT. Neuropsychological correlates of bilateral amygdala damage. Archives of Neurology. 1990;47:349–355. doi: 10.1001/archneur.1990.00530030131029. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. A dissociation between social mentalizing and general reasoning. Neuroimage. 2011;54:1589–1599. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vecera SP, Rizzo M. What are you looking at? Impaired ‘social attention’ following frontal-lobe damage. Neuropsychologia. 2004;42:1657–1665. doi: 10.1016/j.neuropsychologia.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Von Der Heide R, Vyas G, Olson IR. The social network-network: size is predicted by brain structure and function in the amygdala and paralimbic regions. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Wang L, Negreira A, LaViolette P, Bakkour A, Sperling RA, Dickerson BC. Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus. 2010;20:345–351. doi: 10.1002/hipo.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waraczynski MA. The central extended amygdala network as a proposed circuit underlying reward valuation. Neuroscience & Biobehavioral Reviews. 2006;30:472–496. doi: 10.1016/j.neubiorev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Werner KH, Roberts NA, Rosen HJ, Dean DL, Kramer JH, Weiner MW, et al. Emotional reactivity and emotion recognition in fronto temporal lobar degeneration. Neurology. 2007;69:148–155. doi: 10.1212/01.wnl.0000265589.32060.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5:277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Wright P, Liu Y. Neutral faces activate the amygdala during identity matching. Neuroimage. 2006;29:628–636. doi: 10.1016/j.neuroimage.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O. Optogenetic insights into social behavior function. Biological Psychiatry. 2012;71:1075–1080. doi: 10.1016/j.biopsych.2011.12.029. [DOI] [PubMed] [Google Scholar]
- Young AW, Aggleton JP, Hellawell DJ, Johnson M, Broks P, Hanley JR. Face processing impairments after amygdalotomy. Brain. 1995;118(Pt 1):15–24. doi: 10.1093/brain/118.1.15. [DOI] [PubMed] [Google Scholar]
- Zahn R, de Oliveira-Souza R, Bramati I, Garrido G, Moll J. Subgenual cingulate activity reflects individual differences in empathic concern. Neuroscience Letters. 2009a;457:107–110. doi: 10.1016/j.neulet.2009.03.090. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, et al. The neural basis of human social values: evidence from functional MRI. Cerebral Cortex. 2009b;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience. 2012;15:675–680. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
- Zaki J, Hennigan K, Weber J, Ochsner KN. Social cognitive conflict resolution: contributions of domain-general and domain-specific neural systems. Journal of Neuroscience. 2010;30:8481–8488. doi: 10.1523/JNEUROSCI.0382-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius M, Gennatas E, Growdon M, Jang J, Rabinovici G, et al. Divergent network connectivity changes in behavioural variant fronto temporal dementia and Alzheimer’s disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.