Abstract
Background
Attrition along the HIV care continuum slows gains in mitigating the South African HIV epidemic. Understanding population-level gaps in HIV identification, linkage, retention in care and viral suppression is critical to target programming.
Methods
We conducted a population-based household survey, HIV rapid testing, point-of-care CD4 testing, and viral load measurement from dried blood spots (DBS) using multi-stage cluster sampling in two sub-districts of North West Province from January–March, 2014. We used weighting and multiple imputation of missing data to estimate HIV prevalence, undiagnosed infection, linkage and retention in care, medication adherence, and viral suppression.
Results
We sampled 1044 respondents ages 18–49. HIV prevalence was 20.0% (95% CI: 13.7–26.2) for men and 26.7% (95%CI: 22.1–31.4) for women. Among those HIV-positive, 48.4% of men and 75.7% of women were aware of their serostatus; 44.0% of men and 74.8% of women reported ever linking to HIV care; 33.1% of men and 58.4% of women were retained in care; and 21.6% of men and 50.0% of women had DBS viral loads < 5000 copies/mL. Among those already linked to care, 81.7% on ART and 56.0% of those not on ART were retained in care and 51.8% currently retained in care on ART had viral loads < 5000 copies/mL.
Conclusions
Despite expanded treatment in South Africa, attrition along the continuum of HIV care is slowing prevention progress. Improved detection is critically needed, particularly among men. Reported linkage and retention is reasonable for those on ART, however failure to achieve viral suppression is worrisome.
Keywords: South Africa, HIV testing, HIV care continuum, adherence, retention, linkage
Background
South Africa has the largest HIV antiretroviral treatment (ART) program in the world[1], including decentralized ART distribution from primary health clinics and expanded ART eligibility[2, 3]. Despite notable progress, there are an estimated 200,000 AIDS-related deaths each year[4] and only half of those qualifying for ART are currently receiving treatment, which is provided free of charge through the public health system[5]. The ‘cascade’ of attrition along the HIV care continuum from diagnosis of infection through viral suppression[6, 7] in South Africa must be characterized in order to target programming and improve the proportion of people who know their status, engage in care, initiate and maintain treatment, and achieve viral suppression to stem further transmission[8–10].
Currently, there are limited data providing a comprehensive characterization of the HIV care continuum in South Africa. National sero-prevalence data from 2012 indicated that while 65.0% of the population had ever been tested for HIV, only 37.8% of HIV-positive men and 55.0% of HIV-positive women were aware of their HIV status[11]. Available data on linkage to care and retention following HIV diagnosis comes primarily from data on clinical cohorts. Systematic reviews of clinical cohort data across sub-Saharan Africa estimate that 59–66% of people with known HIV status have been assessed for ART eligibility and of those eligible, less than half returned for follow-up care[7, 12]. Patient retention following ART initiation is estimated at 75% and 65% across sub-Saharan Africa after one and three years, respectively[13, 14]. Finally, little is known about population-based viral suppression rates in sub-Saharan Africa. While data from the South African National Health Laboratory Service (NHLS) indicate that approximately 75–80% of ART-initiated patients achieve viral suppression, only 40% of ART patients have viral load data available[15].
Population-based data are scarce but extremely important to understand not only where attrition occurs but also to estimate the complete population burden of disease and the gaps in engagement in clinical care among all people living with HIV (PLWH). We conducted a population-based survey to characterize the HIV care continuum in a rural district of North West Province, South Africa, an area of the country with substantial burden of disease and little available data.
Methods
Study Setting
Data were collected from January–March 2014 in Lekwa-Teemane and Greater Taung sub-districts of North West Province, Republic of South Africa. The area is largely rural, with over 46.0% of the population living in poverty, compared to a national average of 39.9%[16]. The province has the fourth highest HIV prevalence in South Africa, estimated at 20.3% among adults 15–49 years[11] and 29.7% in the antenatal population [17].
Sampling and Recruitment
We employed multi-stage cluster sampling. Twenty-three enumeration areas (EAs) in each sub-district were selected proportionate to size by Statistics South Africa (StatsSA) using 2011 census data. The sampling frame was developed in September 2013 through enumeration of dwelling units (DU) in each EA; fieldworkers recorded the names, ages and sex of DU residents. According to census criteria, residence was defined as sleeping in the DU an average of four or more nights per week. StatsSA then randomly selected 36 inhabited DUs from each EA (1561 DUs in total) for inclusion in the sample and randomly selected one adult (18–49 years) per DU for participation. When more than one DU resident met eligibility criteria (58% of DUs), a second individual was listed as a replacement. Replacement household members were only approached if the first participant was no longer eligible.
Local, trained fieldworkers approached each assigned DU up to five times to locate the selected individual for study participation. Eligibility criteria included being 18–49 years, residing in the home, and able to provide consent (not visibly high, drunk, or with a discernable cognitive impairment). When a participant was located, fieldworkers confirmed eligibility for the study, obtained written informed consent, and conducted a survey by computer-assisted personal interviewing (CAPI) in a private location at the participant’s home in the participant’s language of choice (English, Xhosa, Afrikaans, or Setswana). The survey included questions on demographic characteristics, HIV testing history, health services utilization, and health behaviors.
HIV and CD4 T-cell testing and dried blood spot (DBS) collection
Following the survey, point-of-care HIV rapid antibody testing and pre-and post-test counseling were performed by trained community health workers (CHWs). HIV serostatus was determined by HIV-1/2 antibody rapid testing using the Alere Determine HIV-1/2 rapid test with finger-stick capillary blood (Alere, Bedfordview, South Africa), and, if reactive or indeterminate, confirmed using the First Response HIV 1–2.0 Rapid Whole Blood Test (Premier Medical Corporation Ltd, Daman, India). Participants testing positive or indeterminate were offered point-of-care CD4 testing using the Alere Pima™ CD4 analyzer (Waltham, MA, USA) using 25 μl of capillary blood, following the manufacturer’s instructions. The participant was provided with the test result, counseled on the result’s meaning, and referred for care at the local health care facility if needed. Participants with HIV-positive or indeterminate results were asked to provide finger-prick blood for dried blood spots (DBS) using a Munktell filter card (Ahlstrom Munktell, Helsinki, Finland) for viral load testing. Participants who declined HIV rapid testing in their home were also asked to provide blood for DBS for laboratory HIV diagnosis (serology: ELISA confirmed with Western blot) and viral load testing and offered a study number to call for the results. DBS cards were dried, stored under desiccant at ambient temperature, transported to the testing laboratory within six days of collection, and stored at −70°C. Viral load testing was performed using the COBAS AmpliPrep for sample preparation and COBAS TaqMan HIV-1 2.0 test (Roche Applied Science, Pleasanton CA, USA; lower limit of quantification 400 copies/mL).
Written informed consent was gained for each study procedure; the survey was the only required component. Participants were compensated for their time with an airtime voucher worth approximately $5 USD following completion of all consented procedures. Study procedures were pilot tested in a neighboring sub-district prior to data collection, including a quality assurance program for measurement of viral load using DBS [18]. The protocol was approved by the Committee for Human Research at the University of California, San Francisco; the Human Subjects Division at University of Washington; the Human Sciences Research Council Research Ethics Committee in South Africa; the Policy, Planning, Research, Monitoring and Evaluation Committee for the North West Provincial Department of Health; and the CDC’s Center for Global Health, Human Research Protection.
Measures
Elements of the HIV Care Continuum were defined as follows:
Undiagnosed or newly diagnosed: HIV-positive participants with no reported prior testing or a previous negative test.
-
Linked to care: reported ever seeing a nurse or doctor for HIV care.
Ideal linkage: saw a care provider and completed CD4 testing within three months of diagnosis.
-
Retained in care:
ART adherent: reported taking greater than 90% of their prescribed antiretroviral medication in the past month with no lapse of seven or more days within the past year[20–22].
Viral suppression: viral load of <5000 copies/mL. We used this conservative threshold as there is no definitive cut-point using DBS[23]. We also examined thresholds of <1000 and <3000 copies/mL.
Prior HIV testing behavior was self-reported. Measured CD4 counts among those individuals identified by HIV rapid test as positive were dichotomized at 350 cells/μL, reflecting the 2013 South African ART guidelines which were in effect during the data collection period[24].
Analysis
All analyses were weighted to account for sample design. Weights were created using the inverse probability of selection at each stage (EA, DU and person) and adjusted for non-response to reflect the municipality, age group and sex distributions within the target population [25].
We calculated weighted sample sizes, proportions and 95% confidence intervals (CIs) to describe overall and sex-specific participant demographic characteristics, HIV testing, prevalence and HIV care engagement. Care continuum outcomes were estimated and presented in two ways: 1) among the complete HIV-positive population, in order to provide a comprehensive vision of the care continuum in this population-based sample, and 2) conditionally, including those previously diagnosed with HIV, with each step in the continuum dependent on achieving the previous step, in order to enable comparison with clinical cohort data. To evaluate sex-specific differences in the HIV care continuum, we estimated chi-square statistics using the second-order Rao and Scott correction for bivariate analyses [26], F statistics for age group differences, and generalized linear regression modeling (glm). HIV prevalence, ART eligibility and DBS (viral load) results were adjusted using multiple imputation to account for non-participation or missing data (see Supplemental Digital Content). All analyses were performed using Stata 12 (StataCorp, College Station, TX, USA).
Results
Sample Characteristics
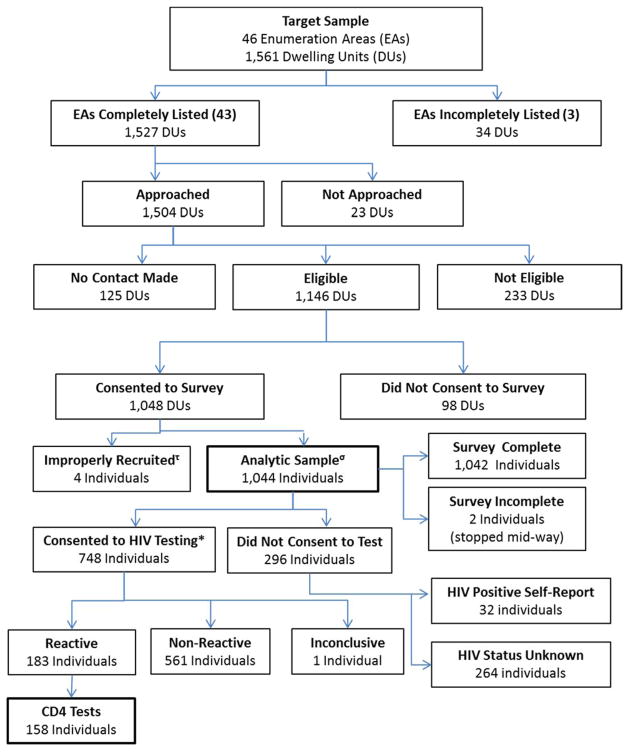
Forty-six EAs were visited for enumeration (Figure 1). Three EAs were excluded as fieldworkers were not granted access to the areas. Of the remaining 1,527 DUs, 98.5% were approached during seven weeks of recruitment. Contact was made at 91.7% of DUs, yielding 1,146 eligible individuals, of whom 1048 (76.0% of contacted DUs; 91.0% of eligible participants) consented to take part in the study. Four individuals were incorrectly recruited, resulting in a total analytic sample of 1,044. The most common reasons for ineligibility were no longer residing in the DU (85.4%) and being unable to provide consent (6.0%). Overall, those who were not located, not eligible, or declined participation were more likely to be male (51.6%) than female (48.4%).
Figure 1.
Participant recruitment, enrollment, and data collection, North West Province, South Africa
*HIV Rapid Test (n=694) or DBS only (n=51); 3 individuals consented but did not test as fieldwork team was unable to locate them when they returned for scheduled testing.
τEnrolled replacement though original selection was eligible and had declined.
σ86.1% (n=899) target individual and 13.9% (n=145) replacement household member.
The final weighted sample reflects the gender and age distribution of the sub-districts (Table 1). Almost all participants were South African citizens or permanent residents (99.4%) and just over half (53.9%) were employed in the past year. Two-thirds had not completed secondary school and about one-quarter of the sample experienced household food insecurity, defined as having gone to bed hungry some to most of the time in the past month.
Table 1.
Participant demographic characteristics, North West Province, South Africa, 2014.
| Participant Characteristics | wgt n*=92,507 | |
|---|---|---|
|
| ||
| wgt %* | 95% CI | |
| Sex | ||
| Male | 47.0 | 41.8–52.2 |
| Female | 53.0 | 47.8–58.2 |
| Age group | ||
| 18–29 Years | 49.0 | 45.2–52.8 |
| 30–39 Years | 28.0 | 25.0–31.3 |
| 40–49 Years | 23.0 | 20.1–26.1 |
| South African citizen/permanent resident | 99.4 | 98.6–99.8 |
| Employed past 12 months | 53.9 | 48.8–59.0 |
| Marital status | ||
| Married/living with partner | 25.8 | 21.5–30.7 |
| Single/In relationship | 70.3 | 65.5–74.8 |
| Single (separated/divorced) | 2.1 | 1.3–3.4 |
| Single (widowed) | 1.7 | 1.1–2.8 |
| Educational attainment | ||
| Primary or less | 21.0 | 17.0–25.5 |
| Some secondary | 43.7 | 38.5–48.9 |
| Completed secondary | 27.6 | 23.4–32.3 |
| College/University or technikon | 7.7 | 4.4–13.4 |
| Food insecurity past month | 25.7 | 20.7–31.3 |
Weights account for sampling, non-response, and age/gender of target population.
HIV Prevalence, CD4, and HIV Testing
Among 745 respondents undergoing either rapid HIV testing or HIV testing using DBS (71.7% of the sample), 183 individuals tested positive. An additional 35 respondents who declined testing reported positive serostatus, resulting in a total of 218 HIV-positive participants. Point-of-care CD4 tests were performed on 158 (94.1%) of participants with an HIV-positive rapid test result. DBS were collected from 157 (93.5%) participants who tested HIV-positive or indeterminate in the field, 51 participants who declined rapid testing, and 1 was collected from an HIV-negative participant in error, resulting in a total of 209 DBS collected.
Overall 20.0% (95% CI: 13.7–26.2) of men and 26.7% (95% CI: 22.1–31.4) of women were HIV positive (Table 2); prevalence was higher among females than males in every age group (Table 2). Males’ prevalence increased with age, peaking at 40–49 years; females’ prevalence peaked at 30–39 years. While 68.9% of men (95% CI: 61.8–75.1) and 89.2% of women (95% CI: 84.2–92.7) reported having tested previously, there were a substantial number of new HIV diagnoses, particularly among men. Among those newly diagnosed, approximately one-third of women and men reported having tested negative in the previous 12 months; one-third of men reported having never tested (Table 2). CD4 cell count varied significantly by sex; CD4 counts were lower for men; this was true among both newly diagnosed men and those who already knew their serostatus (data not shown).
Table 2.
HIV prevalence, testing history, and CD4 results by sex, North West Province, South Africa, 2014.
| Male wgt* n=43,446 |
Female wgt* n=49,061 |
p-value | |||
|---|---|---|---|---|---|
|
| |||||
| wgt* % | 95% CI | wgt* % | 95% CI | ||
| HIV status¥ | 0.07 | ||||
| Positive | 20.0 | 13.7–26.2 | 26.7 | 22.1–31.4 | |
| Negative | 80.0 | 73.8–86.3 | 73.3 | 68.6–77.9 | |
| HIV prevalence by age group | <0.01 | ||||
| Age 18–29 | 9.7 | 3.6–16.0 | 13.8 | 7.8–19.7 | |
| Age 30–39 | 28.4 | 16.0–40.9 | 40.4 | 30.2–50.5 | |
| Age 40–49 | 33.2 | 15.6–50.8 | 35.7 | 26.6–44.9 | |
| HIV tested ever | <0.01 | ||||
| Yes | 68.9 | 61.8–75.1 | 89.2 | 84.2–92.7 | |
| No | 31.2 | 24.9–38.2 | 10.8 | 7.3–15.8 | |
| HIV tested past 12 mosα | <0.01 | ||||
| Yes | 41.8 | 35.3–48.6 | 62.3 | 55.8–68.4 | |
| No | 58.2 | 51.4–64.7 | 37.7 | 31.6–44.2 | |
|
| |||||
|
HIV-Positive Male wgt n*=5,681 |
HIV-Positive Female wgt n*=9,941 |
||||
| Prior knowledge of positive serostatus | |||||
| Yes | 48.4 | 36.5–60.5 | 75.7 | 63.1–85.1 | <0.01 |
| No | 51.6 | 39.5–63.5 | 24.3 | 14.9–36.9 | |
| CD4 category (Pima)β | |||||
| ≤350 cells/μL | 61.9 | 43.8–77.2 | 37.9 | 27.5–49.6 | 0.03 |
| >350 cells/μL | 38.1 | 22.8–56.2 | 62.1 | 50.4–72.6 | |
| Last HIV testedδ | |||||
| < 12 months | 30.3 | 14.7–52.2 | 32.0 | 17.1–51.8 | 0.21 |
| 1–3 yrs ago | 29.6 | 11.6–57.6 | 31.3 | 10.6–63.8 | |
| > 3 yrs ago | 6.6 | 2.0–19.5 | 25.7 | 11.3–48.4 | |
| Never | 33.6 | 15.3–58.6 | 11.0 | 3.3–30.8 | |
Weights account for sampling, non-response, and age/gender of target population
Includes self-report and confirmed;
does not include those who were diagnosed HIV positive prior to 12 months in the denominator;
Among those for whom Pima CD4 results were available;
Among those for whom testing history available.
HIV Continuum of Care
Population-based: among all HIV-positive people
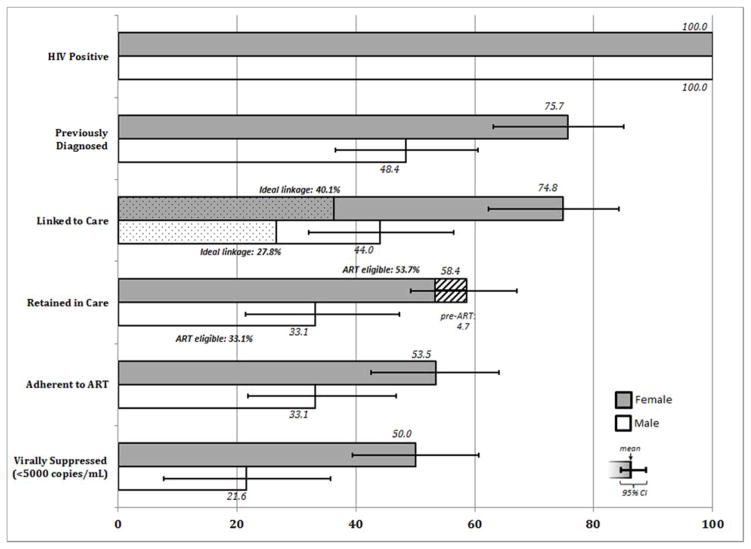
There was major attrition along the HIV continuum of care for the full HIV-positive population, with the most significant drop occurring at the gateway to the care continuum: testing, particularly for men (Figure 2; Table S1). Over half (51.6%, 95% CI: 39.5–63.5) of men and one-quarter (24.3%, 95% CI: 14.9–36.9) of women identified as HIV-positive were previously unaware of their serostatus. The proportion of those who eventually linked to care was similar to the proportion of those previously diagnosed, suggesting that those who are aware of their HIV-positive status will eventually seek care. However, we observed additional attrition associated with retention, treatment and viral suppression. Only 33.1% (95% CI: 21.4–47.3) of HIV-positive men and 58.4% (95% CI: 49.2–67.1) of women were retained in care, of whom the majority had been initiated on ART (Figure 2 shaded area represents the proportion of those retained classified as pre-ART). While 33.1% (95% CI: 21.8–46.8) and 53.5% (95% CI: 42.6–64.1) of HIV-positive men and women reported adherence to medication, only 21.6% (95% CI: 7.6–35.7) and 50.0% (95% CI: 39.4–60.7) of HIV-positive men and women respectively had attained viral suppression, using a conservative threshold of 5000 copies/mL (Figure 2).
Figure 2.
Continuum of HIV Care among all those HIV-positive, North West Province, South Africa
Denominators include all HIV-positive participants and are not conditional upon completing any prior step of the continuum of care.
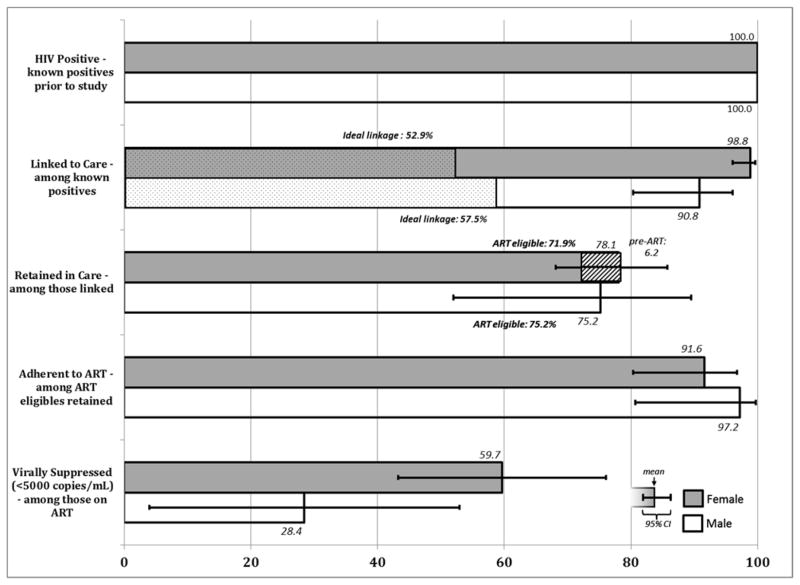
Conditional Cascade: among those achieving each prior step
Next, we examined attrition across the care continuum conditioning on each previous step, among those who already knew their positive HIV status (Figure 3; Table S2). The majority of PLWH, 98.8% of women (95% CI: 96.0–99.6) and 90.8% of men (95% CI: 80.3–96.0) were ever linked to care, though less than 60% linked to care three months of diagnosis (ideal linkage). Among those who ever linked to care, 75.2% (95% CI: 52.0–89.5) of men and 78.1% (95% CI: 68.1–85.7) of women were retained in care; retention being higher among those ART-eligible. The vast majority of men (97.2%, 95% CI: 80.7–99.7) and women (91.6%, 95% CI: 80.3–96.7) retained in care on ART reported adhering to their medication, however, only 28.8% (95% CI 4.7–52.9) of men and 59.7% (95% CI: 43.3–76.1) of women achieved viral load below 5000 copies/mL. We utilized a higher threshold for determining viral suppression with DBS[23] than that which is recommended for plasma. Thus, population viral suppression may be overestimated. Using cut-offs of <1000 and < 3000 copies/mL, respectively, 25.7% (95% 12.5–39.0) and 40.1% (95% CI: 24.4–55.6) of the population retained on ART would be classified as virally suppressed(Table S2). Of note, fewer than 50% of participants who should have had a viral load conducted (all those on ART for at least 6 months), reported past viral load testing.
Figure 3.
Conditional Continuum of HIV Care, North West Province, South Africa
Denominators include all HIV-positive participants that were previously aware of their status and are conditional upon completing each prior step of the continuum of care.
Discussion
These results provide a comprehensive picture of engagement in HIV care from diagnosis to viral suppression in a geographic area with little previous research but extremely high burden of disease. Based on the full HIV-positive population, it is quite clear that the greatest gap in engagement occurs at HIV diagnosis, indicating a critical need for improving case detection, particularly among men. Less than half of HIV-positive men and three-quarters of HIV-positive women were aware of their serostatus prior to our survey. Previous population-based seroprevalence data point to similar rates of reported HIV testing and an even higher proportion of undiagnosed infections [11, 27], particularly among men [27–29]. HIV prevalence in our sample was also similar to that reported for adults in the North West Province in the 2012 national survey (20.3%) [11] and for women in the national antenatal survey (29.7%) [17]. With continued losses along the continuum of care at linkage, retention, and ART adherence, a resulting 50% of women and 21% of men living with HIV in the region are virally suppressed under a best case scenario (using a liberal definition of suppression). This portends continued high rates of transmission in the area unless uptake of testing and treatment initiation improves rapidly and rates of viral suppression are greatly improved.
We also assessed the conditional continuum of care, beginning with those aware of their HIV-positive status, to compare our findings to data from clinical cohorts. We found remarkably similar patterns: among survey participants who were previously aware of their positive serostatus, almost all reported having seen a care provider for HIV, though only 54% reported being assessed for ART eligibility within three months of diagnosis[32, 33]. This estimate, though based on self-report, is consistent with data from South African clinical cohorts, which have found that between 50% and 70% of HIV-positive patients undergo CD4 staging within three months[12, 19, 34, 35]. Our findings regarding retention in care also correspond with estimates from South African clinical cohorts, despite different methods of measurement. Unlike studies that monitor retention within a clinic, we assessed frequency of care without detailed case history tied to a particular clinic. For patients who were known to be eligible for ART, 81.7% reported remaining on ART and receiving care at least every three months in the past year (as clinics provide ART for 1–3 month increments). South African clinical cohorts have documented retention among ART-initiated patients of 75% at one year[19], 81% remaining on treatment for two years[36], and data from the National Health Indicators Report indicate that 82% of initiated patients remained on treatment at three years (among those reported into the national system)[15]. Pre-ART retention, also referred to as stage 2 retention[32], in our study was low: 68% of women and no men were retained in this stage (due to no men being previously diagnosed prior to treatment eligibility, an indication that men do not seek testing until they are already ill). Studies from Cape Town, Johannesburg, and Kwa-Zulu Natal similarly documented that 45–57% of those not ART eligible returned for a subsequent CD4 test in approximate one-year time frames, with men being less likely than women to return for subsequent testing[19, 34, 37].
Our findings demonstrate high reported adherence, but low rates of viral suppression, indicating a potential bias in self-reported adherence data, which could be due to social desirability bias or over-reporting of medication adherence, as well as medication failure. While the effect of social desirability bias would likely over-estimate the proportion of those ‘adherent’ to care, ART adherence is not always over-reported. While pre-exposure prophylaxis (PrEP) clinical trial data among women in Africa indicate extreme over-reporting of medication adherence[38], instances of under-reporting usage of antiretrovirals in large trials have also been documented[39]. A recent population-based HIV survey in South Africa, found high agreement between self-reported ART intake and blood-tested ART exposure (κ = 91.9%). Self-report and blood test discrepancies were demonstrated in both directions, such that just over 7% of those reporting ART intake had negative blood tests and similar numbers reporting no ART intake had positive blood tests for ART[40]. Further analysis of the DBS samples should shed light on drug exposure.
We used a conservative threshold for defining viral suppression due to the sample matrix utilized, as cellular HIV DNA contributes to copy number when using whole blood instead of blood plasma samples[23]. By utilizing 5,000 copies/mL as a threshold we run the risk of overestimating viral suppression. If we lower the threshold to 3,000 copies/mL or even to 1,000 copies, viral suppression among all those on ART is estimated at 40.1% and 25.7% as opposed to 51.8%. Viral suppression in this study is below national targets and estimates from other recent population-based studies[41], but aligns with estimates based on population-level projections from national data[42]. Data from the NHLS suggests viral suppression (defining viral suppression as <400 copies/mL plasma) ranges from 52–75% for North West Province, depending on the district[15]. However, NHLS viral load information is available for less than half of ART-patients; which is consistent with reported rates of viral load testing in our sample. This may indicate that treatment failure is not being properly monitored and could lead to high rates of HIV-1 drug resistance in this rural population. Additionally, food insecurity, which was prevalent in this sample, has been shown to be an independent predictor of incomplete viral suppression after adjustment for ART adherence[43].
Our data has several limitations, as well as strengths. First, linkage, retention, and adherence is based on self-report and not linked to clinic records; participants may not recall dates and/or over-report clinic attendance and adherence. Second, those who are in care are more likely to survive and therefore more likely to be included in our estimates; this survivor bias may lead to inflated estimates of engagement in care. However, our findings on the proportion of those linked to and retained in care are quite similar to findings from clinical cohorts utilizing documented treatment history and accounting for death. Third, our sample cannot account for populations who are highly mobile, unlikely to be included in survey data, and more likely to drop out of care[44]. Both in and out migration in North West Province is extremely common[45]; in our data, approximately 13% of the original sample were no longer at the residence only six months after the area was enumerated. One strength of these data is the potential to capture more inclusive measures of engagement to care, in that our estimates of linkage and retention are not impacted by mobility between clinics or erroneous classification of patients as out of care when they are, in fact, deceased[12]. Further, it should be noted that prevalence may be underestimated in our data, despite adjustments for non-response, as HIV-positive individuals are more likely to refuse testing[30, 31].
Conclusion
Increasing ART coverage can significantly lower the risk of new HIV infections in South Africa[46]. However, treatment expansion will only extend prevention gains if infected individuals are identified early in their HIV disease, enter into care rapidly, remain in care, adhere to ART, and achieve viral suppression. These population-based data provide a comprehensive picture of the HIV care continuum in the North West Province and should be utilized to inform targeted programming. While attrition occurs along all steps of the cascade, the most urgent need is for improved HIV detection, particularly among men. Evidence-based, male-targeted programming, including programs that address male norms that dissuade care seeking and work- and community-based programming outside of the clinic environment, is sorely needed to improve testing uptake, frequency, and early linkage to care. The low proportion of those achieving viral suppression also requires urgent attention. At present population viral load is still too elevated to expect treatment as prevention to achieve its potential impact in this region. Future research to understand the discrepancies between reported adherence and viral suppression is warranted.
Supplementary Material
Acknowledgments
Sources of Support: This project was funded by the Cooperative Agreement 5U2GGH000324-02 from the Centers for Disease Control and Prevention. Additional support for TL was provided through the University of California San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (CFAR), an NIH-funded program (P30 AI027763).
We thank the team at I-TECH South Africa, from data collectors to site supervisors, for study implementation. We thank Elsie Raphela and Charles Koenaite, Lebogang Ntswane, and the combination prevention team for community entry, logistics, and assistance in training the fieldwork teams. We would like to acknowledge our collaborators at StatsSA for sample enumeration and LifeLine Mafikeng for provision of community health workers. We thank the North West Provincial Department of Health (DoH), Dr. Ruth Segomotsi Mompati District DoH, Lekwe Teemane and Greater Taung Sub-district DoH, and the Provincial Research Committee for ongoing support of this project. We thank the participants for their generosity and willingness to be a part of this study. This project was funded by the US Centers for Disease Control and Prevention Cooperative Agreement 5U2GGH000324-02. Additional support for TL was provided through the University of California San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research (CFAR), an NIH-funded program (P30 AI027763).
Footnotes
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the views of CDC or the NIH.
References
- 1.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic. 2010. [Google Scholar]
- 2.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach 2013 revisions. World Health Organization; Geneva: 2013. [PubMed] [Google Scholar]
- 3.Department of Health and Republic of South Africa. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. 2015. Draft: December 2014. [Google Scholar]
- 4.UNAIDS. UNAIDS Country Profiles: South Africa. 2013. cited 2014 December 2014. [Google Scholar]
- 5.Johnson LF. Access to antiretroviral treatment in South Africa, 2004 – 2011. 2012;13:2012. [Google Scholar]
- 6.Cheever LW. Engaging HIV-infected patients in care: their lives depend on it. Clin Infect Dis. 2007;44(11):1500–2. doi: 10.1086/517534. [DOI] [PubMed] [Google Scholar]
- 7.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 10.Padian NS, McCoy SI, Karim SS, Hasen N, Kim J, Bartos M, et al. HIV prevention transformed: the new prevention research agenda. Lancet. 2011;378(9787):269–78. doi: 10.1016/S0140-6736(11)60877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shisana O, RT, Simbayi LC, Zuma K, Jooste S, Zungu N, Labadarios D, Onoya D, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. HSRC Press; Cape Town: 2014. [DOI] [PubMed] [Google Scholar]
- 12.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15(2):17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Department of Health Republic of South Africa and Directorate: Monitoring and Evaluation. Health Indicators Update: Antiretroviral Indicators. 2013. [Google Scholar]
- 16.Bahamondes L, Marchi NM, de Lourdes Cristofoletti M, Nakagava HM, Pellini E, Araujo F, et al. Uniject as a delivery system for the once-a-month injectable contraceptive Cyclofem in Brazil. Contraception. 1996;53(2):115–9. doi: 10.1016/0010-7824(95)00267-7. [DOI] [PubMed] [Google Scholar]
- 17.National Department of Health. The National Antenatal Sentinel HIV and Syphilis Prevalence Survey, South Africa, 2011. Pretoria: 2012. [Google Scholar]
- 18.Prach LM, Puren A, Lippman SA, Carmona S, Stephenson S, Cutler E, et al. Design and Implementation of an External Quality Assessment Program for HIV Viral Load Measurements Using Dried Blood Spots. Journal of Clinical Microbiology. 2015;53(3) doi: 10.1128/JCM.02698-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clouse K, Pettifor AE, Maskew M, Bassett J, Van Rie A, Behets F, et al. Patient Retention From HIV Diagnosis Through One Year on Antiretroviral Therapy at a Primary Health Care Clinic in Johannesburg, South Africa. Journal of acquired immune deficiency syndromes. 2013;62(2):e39–e46. doi: 10.1097/QAI.0b013e318273ac48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke CE, Lee HY, Xing S. Adherence to antiretroviral therapy in managed care members in the United States: a retrospective claims analysis. J Manag Care Pharm. 2014;20(1):86–92. doi: 10.18553/jmcp.2014.20.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haberer JE, Kiwanuka J, Nansera D, Ragland K, Mellins C, Bangsberg DR. Multiple measures reveal antiretroviral adherence successes and challenges in HIV-infected Ugandan children. PLoS One. 2012;7(5):e36737. doi: 10.1371/journal.pone.0036737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vreeman RC, Nyandiko WM, Liu H, Tu W, Scanlon ML, Slaven JE, et al. Measuring adherence to antiretroviral therapy in children and adolescents in western Kenya. J Int AIDS Soc. 2014;17(1):19227. doi: 10.7448/IAS.17.1.19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkin NT. Measurement of HIV-1 viral load for drug resistance surveillance using dried blood spots: literature review and modeling of contribution of DNA and RNA. AIDS Rev. 2014;16(3):160–71. [PubMed] [Google Scholar]
- 24.South African National Department of Health. Antiretroviral Treatment Guidelines, Version 14 March 2013. Pretoria: 2013. [Google Scholar]
- 25.Levy PS, Lemeshow SA. Sampling of Populations: Methods and Applications. 4. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 26.Rao JNK, Scott AJ. On Chi-Squared Tests for Multiway Contingency Tables with Cell Proportions Estimated from Survey Data. 1984;(1):46–60. [Google Scholar]
- 27.Kranzer K, van Schaik N, Karmue U, Middelkoop K, Sebastian E, Lawn SD, et al. High prevalence of self-reported undiagnosed HIV despite high coverage of HIV testing: a cross-sectional population based sero-survey in South Africa. PLOS One. 2011;6(9):e25244. doi: 10.1371/journal.pone.0025244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peacock D, Stemple L, Sawires S, Coates TJ. Men, HIV/AIDS, and human rights. J Acquir Immune Defic Syndr. 2009;51(Suppl 3):S119–25. doi: 10.1097/QAI.0b013e3181aafd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight L, McGrath N, van Rooyen H, Humphries H, van Heerden A, Richter L. Characteristics of sexually experienced HIV testers aged 18 to 32 in rural South Africa: baseline results from a community-based trial, NIMH Project Accept (HPTN 043) BMC Public Health. 2014;14:1164. doi: 10.1186/1471-2458-14-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnighausen T, Tanser F, Malaza A, Herbst K, Newell ML. HIV status and participation in HIV surveillance in the era of antiretroviral treatment: a study of linked population-based and clinical data in rural South Africa. Trop Med Int Health. 2012;17(8):e103–10. doi: 10.1111/j.1365-3156.2012.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Floyd S, Molesworth A, Dube A, Crampin AC, Houben R, Chihana M, et al. Underestimation of HIV prevalence in surveys when some people already know their status, and ways to reduce the bias. AIDS. 2013;27(2):233–42. doi: 10.1097/QAD.0b013e32835848ab. [DOI] [PubMed] [Google Scholar]
- 32.Fox MP, Larson B, Rosen S. Defining retention and attrition in pre-antiretroviral HIV care: proposals based on experience in Africa. Trop Med Int Health. 2012 doi: 10.1111/j.1365-3156.2012.03055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for Improving Entry Into and Retention in Care and Antiretroviral Adherence for Persons With HIV: Evidence-Based Recommendations From an International Association of Physicians in AIDS Care Panel. Ann Intern Med. 2012;156(11):817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kranzer K, Zeinecker J, Ginsberg P, Orrell C, Kalawe NN, Lawn SD, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLOS One. 2010;5(11):e13801. doi: 10.1371/journal.pone.0013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losina E, I, Bassett V, Giddy J, Chetty S, Regan S, Walensky RP, et al. The “ART” of linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5(3):e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox MP, Shearer K, Maskew M, Meyer-Rath G, Clouse K, Sanne I. Attrition through Multiple Stages of Pre-Treatment and ART HIV Care in South Africa. PLoS One. 2014;9(10):e110252. doi: 10.1371/journal.pone.0110252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lessells RJ, Mutevedzi PC, Cooke GS, Newell ML. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. Journal of acquired immune deficiency syndromes. 2011;56(3):e79–86. doi: 10.1097/QAI.0b013e3182075ae2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS One. 2014;9(2):e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marzinke MA, Clarke W, Wang L, Cummings V, Liu TY, Piwowar-Manning E, et al. Nondisclosure of HIV status in a clinical trial setting: antiretroviral drug screening can help distinguish between newly diagnosed and previously diagnosed HIV infection. Clin Infect Dis. 2014;58(1):117–20. doi: 10.1093/cid/cit672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huerga H, Bouhenia M, Farhat JB, Maman D, Etard J-F, Cutsem GV, et al. CROI. Seattle, Washington: 2015. Self-Reported Versus Blood-Tested ART Intake to Estimate ART Coverage in South Africa. [Google Scholar]
- 41.Barnabas RV, van Rooyen H, Tumwesigye E, Murnane PM, Baeten JM, Humphries H, et al. Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. Lancet HIV. 2014;1(2):e68–e76. doi: 10.1016/S2352-3018(14)70024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takuva S, Puren A, Brown A, Delpech V, Macleod W, Pillay Y. CROI. Seattle, Washington: 2015. Disparities in Engagement Within HIV Care in South Africa. [Google Scholar]
- 43.Weiser SD, Palar K, Frongillo EA, Tsai AC, Kumbakumba E, Depee S, et al. Longitudinal assessment of associations between food insecurity, antiretroviral adherence and HIV treatment outcomes in rural Uganda. AIDS. 2014;28(1):115–20. doi: 10.1097/01.aids.0000433238.93986.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor BS, Garduno LS, Reyes EV, Valino R, Rojas R, Donastorg Y, et al. HIV care for geographically mobile populations. Mt Sinai J Med. 2011;78(3):342–51. doi: 10.1002/msj.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Statistics South Africa. Mid-year population estimates, 2014. Pretoria: 2014. [Google Scholar]
- 46.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–71. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.