Abstract
We hypothesized that differences in cardiac baroreflex sensitivity (BRS) would be independently associated with aortic stiffness and augmentation index (AI), clinical biomarkers of cardiovascular disease (CVD) risk, among young sedentary and middle-aged/older sedentary and endurance-trained adults. A total of 36 healthy middle-aged/older (age 55-76 years, n=22 sedentary; n=14 endurance-trained) and 5 young sedentary (age 18-31 years) adults were included in a cross-sectional study. A subset of the middle-aged/older sedentary adults (n=12) completed an 8-week aerobic exercise intervention. Invasive brachial artery blood pressure waveforms were used to compute spontaneous cardiac BRS (via sequence technique) and estimated aortic pulse wave velocity (PWV) and AI (AI, via brachial-aortic transfer function and wave separation analysis). In the cross-sectional study, cardiac BRS was 71% lower in older compared with young sedentary adults (P<0.05), but only 40% lower in older adults who performed habitual endurance exercise (P=0.03). In a regression model that included age, sex, resting heart rate, mean arterial pressure (MAP), body mass index and maximal exercise oxygen uptake, estimated aortic PWV (β±SE = −5.76 ± 2.01, P=0.01) was the strongest predictor of BRS (Model R2=0.59, P<0.001). The 8 week exercise intervention improved BRS by 38% (P=0.04) and this change in BRS was associated with improved aortic PWV (r=−0.65, P=0.044, adjusted for changes in MAP). Age- and endurance exercise-related differences in cardiac BRS are independently associated with corresponding alterations in aortic PWV among healthy adults, consistent with a mechanistic link between variations in the sensitivity of the baroreflex and aortic stiffness with age and exercise.
Keywords: pulse wave velocity, blood pressure, augmentation index, cardiovagal
INTRODUCTION
Cardiac baroreflex sensitivity (BRS), a critical physiological feedback mechanism that regulates short-term (e.g., beat to beat) arterial blood pressure, declines with advancing age in adults who are sedentary1-4 and is linked to cardiac electric instability contributing to an increased risk of lethal ventricular arrhythmias.5, 6 In contrast, the age-related reductions in cardiac BRS are smaller in middle-aged/older adults who perform habitual aerobic exercise 1-3 and BRS improves in previously sedentary middle-aged/older adults following short-term (3-6 months) aerobic exercise interventions.1, 2, 7 Thus, preservation of cardiac BRS may be one mechanism that contributes to reduced cardiovascular disease (CVD) risk and sudden cardiac death among middle-aged/older adults who perform habitual aerobic exercise.
Given that the aortic arch is the anatomical location of one set of arterial stretch baroreceptors it is reasonable to postulate that stiffness of the aortic wall modulates in vivo baroreceptor function. Indeed, higher carotid-femoral pulse wave velocity (PWV), the ‘gold standard’ clinical expression of aortic stiffness, is associated with reduced cardiac BRS in middle-aged8 and older adults with CVD risk factors such as hypertension, smoking, diabetes and dyslipidemia.9 There is strong evidence that age- and exercise- associated differences in cardiac BRS are associated with corresponding changes in common carotid artery compliance2, 3, and improvements in cardiac BRS after a 3 month aerobic exercise intervention are strongly associated with increases in carotid artery compliance in previously sedentary middle-aged/older adults. 2 However, the influence of exercise on the relation between cardiac BRS and carotid-femoral PWV has not been examined as thoroughly as with carotid stiffness. Specifically, there are no data on whether fitness/endurance-exercise-related differences in carotid-femoral PWV are associated with corresponding alterations in cardiac BRS among middle-aged/older adults with low CVD risk factor burden.
Carotid-femoral PWV increases with aging10, 11 particularly in adults who follow a sedentary lifestyle12-15 and is a robust predictor of clinical CVD events (e.g., myocardial infarction, unstable angina, heart failure).16 In contrast, middle-aged/older adults who perform habitual aerobic exercise demonstrate less stiffening of the aorta than their age-matched sedentary peers.13-15, 17 However, whether regular aerobic exercise initiated in previously sedentary middle-aged/older adults reduces stiffness of the aorta remains unresolved with some studies reporting improvement in carotid-femoral PWV 18, 19, whereas other studies have reported no effect particularly if hypertension was present.20-22
Aortic augmentation index (AI), the ratio of the augmented pressure to the pulse pressure at the ascending aorta that is related to arterial stiffness and forward and reflected pressure waves, is also independently associated with elevated CVD risk in some studies.23, 24 Higher AI, resulting in part from greater reflected waves, contributes to greater systolic blood pressure (BP) and pulse pressure (PP) in the central arteries (aorta and carotid arteries), the anatomical sites of the mechanical stretch stimulus for the baroreceptors. The higher systolic BP and PP results in a stronger mechanical stimulus for the baroreceptors which should increase BRS. On the other hand, chronically elevated AI may contribute to stiffening of the central arteries and, thus, reduce BRS. In that AI is lower and BRS greater in middle-aged and older adults who are endurance-exercise trained15, exercise-related differences in BRS would be expected to be inversely associated with AI. However, we are unaware of any other previous study that has assessed this potential relation.
Therefore, we hypothesized that estimated aortic PWV and AI would be independent predictors of alterations in spontaneous cardiac BRS with aging and would explain part of the modulatory influence of habitual aerobic exercise among middle-aged/older adults. To determine this, we calculated cardiac BRS using the sequence technique in a subset of subjects from a previous study where we estimated aortic PWV from invasive brachial artery blood pressure waveforms and validated it against carotid-femoral PWV. 14 First, we examined the effects of older age and habitual aerobic exercise on spontaneous BRS and estimated aortic PWV and AI in healthy young and middle-aged/older adults in a cross-sectional study. We studied middle-aged and older adults without CVD risk factors in order to isolate the effects of aging without the confounding effect of age-related comorbidities. Second, twelve of the sedentary middle-aged/older adults from the cross-sectional study completed a short-term aerobic exercise intervention (i.e., daily moderate/vigorous intensity walking for 8 weeks) to determine whether expected increases in cardiac BRS after the aerobic exercise training are associated with predicted reductions in aortic PWV and AI independent of any training-associated changes in blood pressure and/or heart rate.
METHODS
Subjects
A subset of 41 subjects from our previous report 14 with available invasive brachial blood pressure waveforms were used for the study. See Supplemental Methods for details.
Cross-sectional study
Subjects with available brachial artery pressure waveforms were subdivided into 3 groups based on age and exercise training status including: 5 young sedentary adults (age 18-31 years), 22 middle-aged/older sedentary adults (age 55-71 years) and 14 middle-aged/older endurance-exercise trained adults (age 55-76 years) who had been performing regular vigorous aerobic-endurance exercise (e.g., competitive distance running, cycling, and/or triathlons) >45 min/day, ≥5 days/week for at least the previous 5 years. 25
Exercise intervention and maximal exercise testing
Of the 22 middle-aged/older sedentary adults with invasive blood pressure files available, 12 underwent an 8-week aerobic exercise intervention program as part of a randomized, controlled study as previously described.25 However, there were not enough blood pressure waveforms available from the control group in the original study to achieve proper statistical power, therefore only the exercise intervention group was included. See supplemental Methods for details of the exercise intervention and maximal exercise testing.
Anthropometrics
Anthropometrics were assessed as previously described.25 See Supplemental Methods for more details.
Spontaneous cardiac BRS
Calculation of spontaneous cardiac BRS was performed at the University of Iowa via the sequence technique using the Analyzer component of the HemoLab software package (Harald-Stauss Scientific, Iowa City, IA). See Supplemental Methods for more details.
Estimated aortic PWV and AI
Aortic PWV and AI were derived from an intra- brachial artery blood pressure waveform obtained from a pressure transducer (Cardiocap 5) attached to a 20 gauge catheter as previously described.14 See Supplemental Methods for more details.
Statistical analyses
All analyses were performed using SPSS 22.0, (SPSS, Inc.) and Microsoft Excel. All data are presented as mean ± standard error. Statistical significance was set at an alpha level of P < 0.05. See Supplemental Methods for additional details.
RESULTS
Subject characteristics
Cross-sectional subject characteristics are presented in Table 1. Older sedentary and endurance trained adults had higher body weight, body mass index (BMI), total body fat percentage, waist circumference, waist:hip ratio, and systolic and diastolic blood pressures compared with the young sedentary adults (all P<0.05). Endurance-trained older adults had a significantly lower BMI and total body fat, but age, height, waist circumference, waist:hip ratio and blood pressure were not different than sedentary age-matched peers (all P<0.05). Heart rate in endurance trained older adults was not different than the young sedentary adults, but endurance-trained older adults had a significantly lower resting heart rate compared to older sedentary adults consistent with an aerobic exercise training adaptation (P<0.05). The groups differed significantly in percentage of males vs. females χ2(df=2, n=41) =10.88, P=0.004. Specifically, the percentage of males/females was different between the older trained and older sedentary (P<0.01) and young sedentary (P<0.01), but not between young sedentary and older sedentary (P=0.30).
Table 1.
Subject characteristics of subjects in cross-sectional study
| Young Sedentary (n=5) | Older Sedentary (n=22) | Older Endurance Trained (n=14) | |
|---|---|---|---|
| Age (years) | 22 ± 2 | 62 ± 1* | 61 ± 2* |
| Male, no. (%) | 1 (20) | 8 (36) | 11 (79)*† |
| Weight (kg) | 61 ± 2 | 72 ±0.2* | 71 ± 2.6* |
| Height (cm) | 174 ± 4 | 168 ± 2 | 173 ± 2 |
| Body mass index (kg/m2) | 20.7 ± 0.5 | 25.6 ± 0.5* | 23.5 ± 0.6*† |
| Total body fat (%) | 28 ± 4 | 35 ± 2* | 20 ± 1*† |
| Waist circumference (cm) | 70 ±1 | 83 ± 2* | 80 ± 2* |
| Hip circumference (cm) | 97 ± 2 | 101 ± 1* | 96 ± 1† |
| Waist/Hip ratio | 0.73 ± 0.01 | 0.82 ± 0.02* | 0.84 ± 0.02* |
| Systolic blood pressure (mmHg) | 102 ± 10 | 121 ± 3* | 121 ± 4* |
| Diastolic blood pressure (mmHg) | 57 ± 2 | 72 ± 2* | 75 ± 3* |
| Resting heart rate (beats/min) | 53 ± 2 | 66 ± 2* | 54 ± 2† |
| VO2 max (ml/kg/min) | 38.7 ± 2.1 | 26.2 ± 0.9* | 45.9 ± 1.4*† |
Data are mean ± SE.
P<0.05 vs Young Sedentary.
P<0.05 vs Older.
VO2max, maximal exercise oxygen consumption; LDL, low-density lipoprotein; HDL, high density lipoprotein.
Spontaneous cardiac BRS
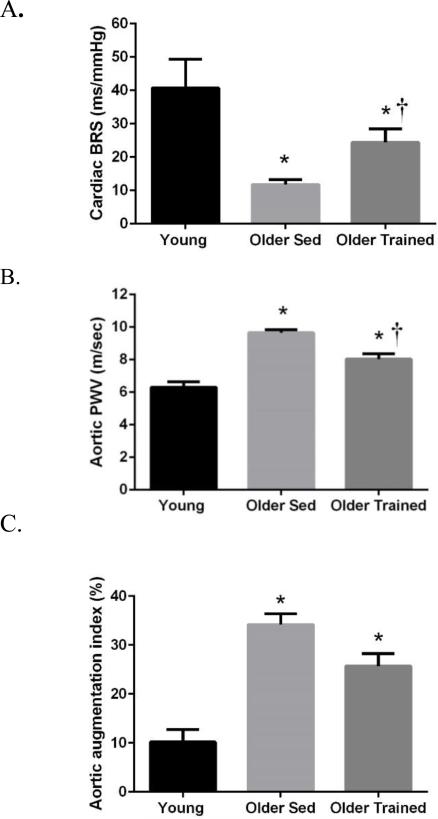
Spontaneous cardiac BRS was 71% lower in older compared with young sedentary adults (11.7 ± 1.4 vs. 40.7 ± 8.6 ms/mmHg, P<0.05), confirming the age-related decline in spontaneous BRS (Figure 1A). Endurance-trained older adults demonstrated a 109% higher BRS compared to their sedentary age-matched peers (24.4 ± 4.0 vs. 11.7 ± 1.4 ms/mmHg, P=0.01) consistent with a beneficial effect of habitual endurance exercise on BRS. Furthermore, there was a 40% lower BRS in endurance-trained older compared with younger adults (24.4 ± 4.0 vs. 40.7 ± 8.6 ms/mmHg, P=0.03), confirming that the age-related reduction in BRS is smaller in older adults who perform habitual endurance-exercise. These group differences remained significant even after covarying for sex.
Figure 1.
A) Spontaneous cardiac baroreflex sensitivity (BRS) adjusted for sex, B) aortic pulse wave velocity (PWV) adjusted for sex, mean arterial pressure and heart rate, and C) aortic AI adjusted for sex, heart rate and height, in young sedentary, older sedentary and older endurance-trained adults. *P<0.05 vs. Young. †P<0.05 vs Older Sedentary.
Estimated aortic PWV and AI
Estimated aortic PWV data are presented in Figure 1B that illustrates the age- and exercise- related differences in aortic PWV. Consistent with our previous study14, aortic PWV was ~52% higher in older sedentary adults (9.7 ± 0.2 vs. 6.1 ± 0.4 m/sec, P<0.01), confirming the increased stiffening of the aorta with aging. Endurance-trained older adults had ~18% lower aortic PWV values compared with sedentary older adults (8.0 ± 0.3 vs. 9.7 ± 0.2 m/sec, P<0.01), supporting the concept that chronic endurance exercise attenuates the age-related increase in aortic stiffness. However, habitual aerobic exercise did not completely abolish the age-related increase in aortic stiffness in older humans, because aortic PWV in the endurance-trained older adults remained elevated compared to the young sedentary adults (8.0 ± 0.3 vs. 6.1 ± 0.4 m/sec, P<0.0001). Importantly, these between-group differences remained significant after adjusting for sex, mean arterial pressure, BMI and heart rate (P<0.01).
As expected, older sedentary adults exhibited greater aortic AI compared with young sedentary adults (33 ± 2 vs. 10 ± 4%, P<0.01), and this difference remained significant after adjusting for sex, heart rate, and height. AI appeared to be attenuated in exercise-trained older adults compared with their sedentary counterparts (26 ± 2 vs. 33 ± 2%, P=0.02) (Figure 1C), however, after adjusting for sex, heart rate and height this difference was abolished (P=0.18) suggesting that habitual aerobic exercise did not have obvious beneficial effects on AI in our cohort of older adults. Not surprisingly, the endurance-trained adults displayed greater aortic AI compared to young sedentary adults (26 ± 2 vs. 10 ± 4%, P<0.01) and this difference remained significant after adjusting for sex, heart rate, and height. These data are consistent with the idea that habitual aerobic exercise does not completely prevent the age-related increase in aortic AI.
Relations between cardiac BRS and estimated aortic PWV and AI
In the entire group, cardiac BRS was inversely associated with aortic PWV (r=−0.68, P<0.01), and this association remained significant after adjusting for age and mean arterial pressure (partial r=−0.55, p<0.01) and subsequently for heart rate (r=−0.45, P<0.01). Among middle-aged/older adults only (trained and sedentary), BRS remained inversely associated with aortic PWV (r=−0.58, P<0.01), even after adjusting for age and mean arterial pressure (r=−0.57, P<0.01) and subsequently for heart rate (r=−0.49, P<0.01).
Hierarchical regression modeling was then performed to determine whether aortic PWV was associated with cardiac BRS after controlling for key covariates such as age, sex, resting heart rate, mean arterial pressure, BMI and VO2max. In the first step of the model (Model 1), age in years significantly predicted BRS (P=0.023) so that for each year increase in age the predicted BRS value decreased by −0.44 ms/mm/Hg (Table 2). Then, aortic PWV was added to the regression model (Model 2) which improved the overall R2 b~10% (Pchange=0.01) and aortic PWV (P=0.011) and mean arterial pressure (P=0.041) remained significantly associated with BRS (Model R2=0.59, P<0.001) (Table 2). Thus, for each m/sec increase in aortic PWV the BRS decreased by −5.76 ms/mm/Hg (P=0.0011) (Table 2). To rule out a possible issue of multicollinearity, we ran multicollinearity diagnostics for all independent variables in the multiple regression model and found that the collinearity tolerance statistics were all greater than 0.20 suggesting that our model is likely not confounded by multicollinearity. Furthermore, because body weight enters into the calculation of aortic PWV (used to calculate reflecting distance) and calculation of relative VO2max (normalized for body weight), we also calculated the model without entering BMI as an independent variable. The correlation coefficient of the model remained almost unchanged and aortic PWV became the only significant correlate of BRS with MAP dropping out of the model.
Table 2.
Hierarchical regression model on cardiac baroreflex sensitivity (BRS) among young sedentary, middle-aged/older sedentary and middle-aged/older endurance-trained healthy adults
| Predictor | Coefficients for Individual Predictors | Model Coefficients (p-value) | ||||
|---|---|---|---|---|---|---|
| B (SE) | β | Partial r | p-value | Model R2 | ΔR2 from Previous | |
| 0.495 (0.001) | 0.495 (0.001) | |||||
| Age (yrs) | −0.44 (0.19) | −0.38 | −0.39 | 0.023 | ||
| Resting HR (bpm) | −0.11 (0.23) | −0.08 | −0.08 | 0.640 | ||
| MAP (mm/Hg) | −0.34 (0.21) | −0.27 | −0.27 | 0.117 | ||
| BMI (kg/m2) | −0.05 (0.92) | −0.01 | −0.01 | 0.960 | ||
| VO2max (ml/kg/min) | 0.31 (0.31) | 0.21 | 0.18 | 0.323 | ||
| Sex (Female) | −4.96 (5.24) | −0.16 | −0.17 | 0.351 | ||
| 0.593 (<0.001) | 0.097 (0.01) | |||||
| Age (yrs) | −0.16 (0.20) | −0.14 | −0.10 | 0.415 | ||
| Resting HR (bpm) | −0.07 (0.21) | −0.05 | −0.04 | 0.741 | ||
| MAP (mm/Hg) | −0.42 (0.20) | −0.33 | −0.25 | 0.041 | ||
| BMI (kg/m2) | 1.16 (0.95) | 0.22 | 0.14 | 0.231 | ||
| VO2max (ml/kg/min) | 0.09 (0.30) | 0.60 | 0.03 | 0.771 | ||
| Sex (Female) | −2.98 (4.84) | −0.10 | −0.07 | 0.54 | ||
| Aortic PWV (m/sec) | −5.76 (2.011) | −0.57 | −0.31 | 0.011 | ||
Note. “B” = Unstandardized Coefficient, “SE” = Standard Error, “β” = Standardized Coefficient. MAP, mean arterial pressure; BMI, body mass index; VO2max, maximal exercise oxygen consumption.
Lastly, in the entire group BRS was inversely related to AI (r=−0.39, P<0.05), but not in middle-aged/older sedentary and endurance trained subjects only (r=−0.18, P=0.28). In a hierarchial regression model applied to determine whether aortic AI was associated with BRS, after controlling for age, sex, height, resting heart rate, mean arterial pressure, BMI and VO2max, AI was not significantly associated with BRS (P=0.56) (data not shown).
Exercise intervention subject characteristics
Subject characteristics for the 12 subjects who underwent the exercise intervention of 8 weeks of moderate-intensity endurance exercise are presented in Table 3. There was a 12.8% reduction in resting heart rate (P=0.02) and a 6.5% increase in VO2 max (P<0.01) after compared with before the 8 weeks of aerobic exercise, consistent with a physiological training effect. In contrast, there was no change in weight, BMI, body fat percentage, waist circumference, hip circumference, and waist: hip ratio after the 8 weeks of aerobic exercise training. Similarly, systolic (P=0.10) and diastolic blood pressures (P=0.19) did not significantly change following 8 weeks aerobic exercise consistent with previous studies of this duration. The volume of exercise for the subjects was previously reported. 25
Table 3.
Subject characteristics of older sedentary adults before vs. after 8 weeks of daily aerobic exercise (n=12)
| Baseline | 8 Weeks Exercise | |
|---|---|---|
| Age (years) | 63 ± 1 | - |
| Male/Female | 4/8 | - |
| Weight (kg) | 70.9 ± 3.4 | 70.7 ± 3.5 |
| Body mass index (kg/m2) | 25.6 ± 0.8 | 25.5 ± 0.9 |
| Total body fat (%) | 34.7 ± 2.5 | 34.5 ± 2.8 |
| Waist circumference (cm) | 83.4 ± 3.0 | 84.5 ± 3.6 |
| Hip circumference (cm) | 100.5 ± 1.7 | 103.1 ± 1.9 |
| Waist/Hip ratio | 0.83 ± 0.03 | 0.82 ± 0.03 |
| Systolic blood pressure (mmHg) | 120 ± 5 | 114 ± 4 |
| Diastolic blood pressure (mmHg) | 73 ± 3 | 70 ± 2 |
| Resting heart rate (beats/min) | 68 ± 4 | 59 ± 2* |
| Maximal exercise VO2 (ml/kg/min) | 27.0 ± 1.3 | 28.7 ± 1.5* |
Data are mean ± SE. VO2 max, maximal exercise oxygen consumption. LDL, low-density lipoprotein cholesterol; HDL, high density lipoprotein cholesterol.
P<0.05 vs. Baseline.
Cardiac BRS, estimated aortic PWV and aortic AI before and after 8 weeks of exercise training
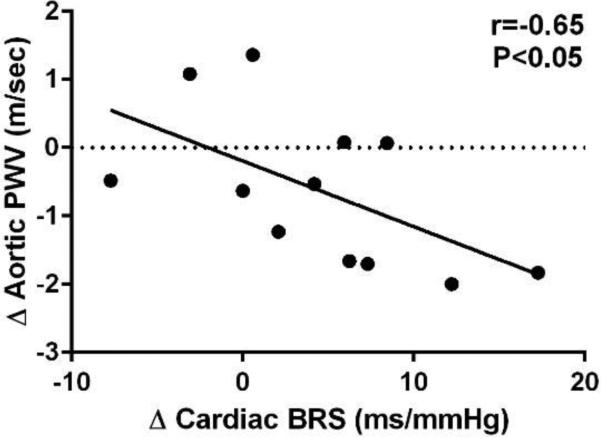
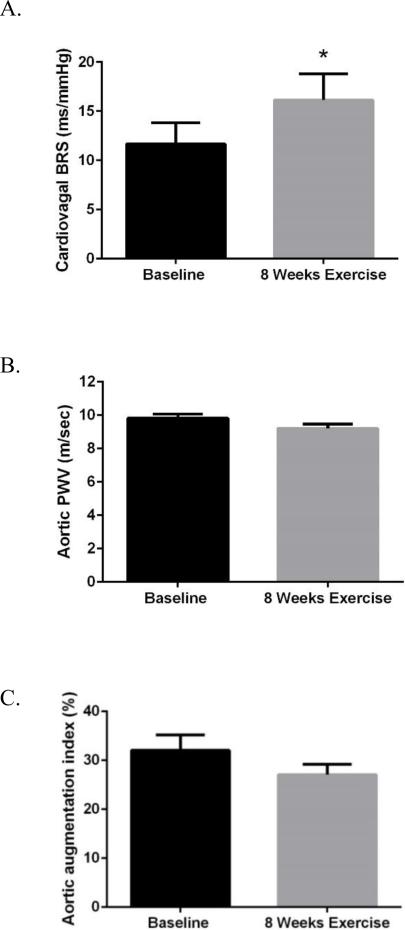
Spontaneous cardiac BRS increased by 38% (11.7 ± 2.2 vs 16.1 ± 2.7 ms/mmHg, P=0.04) after the 8 weeks of aerobic exercise (Figure 3A). There was a trend for a decrease in aortic PWV (9.8 ± 0.2 vs. 9.2 ± 0.3 m/sec, P=0.08) before vs. after 8 weeks of aerobic exercise (Figure 3B). However, after adjusting for exercise training-induce change in mean arterial pressure this trend was lost (P=0.22), suggesting that reduction in mean arterial pressure contributed to the reduction in aortic PWV(even though mean arterial pressure was not significantly different before and after training in the whole group of subjects, P=0.11). Furthermore, there was no significant differences in aortic AI (32.1 ± 3.1 vs. 27.1 ± 2.1%, P=0.13) before vs. after the 8 week exercise intervention (Figure 3C).
Figure 3.
Bivariate correlation between the change in spontaneous cardiac baroreflex sensitivity (BRS) with the change in aortic pulse wave velocity (PWV) adjusted for the change in mean arterial pressure following 8 weeks of aerobic exercise among middle-aged/older adults (n=12) .
Relations between the change in cardiac BRS with change in estimated aortic PWV and AI after exercise intervention
The increase in cardiac BRS after 8 weeks of aerobic exercise was associated with the reduction in aortic PWV (r=−0.59, P=0.048), and this relation was maintained after adjusting for the change in mean arterial pressure and change in resting heart rate (r=−0.65, P=0.044). These results suggest that the improvement in BRS related to the exercise training-induced small change in aortic stiffness (Figure 3). In contrast, there was no correlation between the change in BRS and change in aortic AI (r=−0.22, P=0.50) after 8 weeks of aerobic exercise.
DISCUSSION
The primary new finding from the present study is that age- and endurance exercise-related differences in aortic stiffness, as measured by estimated aortic PWV, are associated with corresponding differences in spontaneous cardiac BRS among healthy adults. Importantly, this relation remained significant in multiple regression modeling adjusting for key covariates know to affect PWV and BRS including age, sex, resting heart rate, mean arterial pressure, BMI and VO2max. Specifically, we found that in a model that included the above covariates, aortic PWV was an independent predictor of cardiac BRS among healthy adults. Furthermore, improvements in BRS after 8 weeks of aerobic exercise training in previously sedentary older adults were correlated with corresponding modest reductions in aortic PWV and that correlation remained significant after adjusting for resting heart rate and mean arterial pressure. Taken together, our cross-sectional and longitudinal data demonstrate that the age- and endurance exercise-mediated effects on spontaneous BRS may be mechanistically linked to corresponding effects in aortic stiffness among healthy adults.
Our data are consistent with previous studies using the sequence technique to assess cardiac BRS in humans 26, 27, as well as studies using phase IV of the vagal maneuver 1, 2 or the modified Oxford technique 3, 28, that all report that cardiac BRS is reduced with sedentary aging in humans. Consistent with results of Monahan et al.1, 3, spontaneous BRS was ~71% lower in older compared with younger sedentary adults in our study and the age-related reduction in BRS was attenuated in endurance-trained older adults in that BRS was reduced only 40% compared with the young adults. In addition, several studies reported an independent association between cardiac BRS and large elastic artery stiffness in healthy adults, but these studies used ultrasound and tonometry based measurements of local carotid artery compliance and stiffness. 2, 3 While the studies by Monahan et al. found strong associations between BRS and carotid artery compliance 1, 3, a key anatomical location of one set of arterial baroreceptors, the novel aspect of our study is the finding of a significant inverse association between BRS and aortic stiffness among young and older healthy adults. Importantly, this association remained significant even after adjusting for important covariates such as the age, sex, heart rate, mean blood pressure and VO2max suggesting a mechanistic link between the BRS and aortic stiffness. Our data extend previous findings of associations between cardiac BRS and carotid artery wall stiffness in middle-aged/older adults varying in fitness3, 12 to the stiffness of the aortic wall, the other location of the arterial baroreceptors. Importantly, our data in healthy older adults are also consistent with previous studies that reported inverse associations between carotid femoral PWV and cardiac BRS in adults with clinical disease such as hypertension8, 29, end-stage kidney disease30, and older adults with multiple risk factors (smoking, diabetes)9. Furthermore, our results also are in line with Tarumi et al. (2015),31 who reported a significant correlation between carotid femoral PWV and cardiac BRS (r=−0.34, P<0.05) in older adults, one-half of whom had mild cognitive impairment. However, Tarumi et al. (2015) and did not adjust for potential confounders in multiple regression models as in our study. Thus, the novelty of our study is that it extends the findings of Tarumi et al. because aortic PWV remained independently associated with cardiac BRS even after adjusting for differences in age, sex, BMI, BP, heart rate and fitness (V02max).
Importantly, we 14 and others 12, 13, 15 have reported that the age-associated increase in aortic PWV in sedentary older adults is significantly attenuated by performance of habitual aerobic exercise. The mechanism by which this adaptation could alter cardiac BRS is not known but could be related to either the mechanical or neural components of the integrated baroreflex response. Our data suggest that the age- and endurance exercise-related differences in BRS could be at least in part the result of a more compliant aorta in the young and the older endurance trained adults whereby greater mechanical stretch of a less stiff aortic wall during an acute elevation in blood pressure would allow more afferent signal to the brainstem inhibiting sympathetic efferent outflow. However, the relative contribution of the mechanical transduction of increased pressure into stretch of the baroreceptors in the wall of the aorta vs. carotid arteries to potential differences in these afferent signals to the brain and to BRS cannot be determined from the results of our study. In addition, although both the neural and mechanical components of the baroreflex appear to contribute to the age-related decline in the integrated cardiac BRS, Hunt et al.32 demonstrated that the neural component contributes more to the preserved cardiac BRS than the mechanical component in older endurance-trained compared with sedentary adults. As such, these data suggest that the preserved mechanical component of baroreceptor gain mediated by less aortic stiffness with habitual endurance training in older adults in our study may only partially explain the preserved cardiac BRS in our cohort. Rather, alterations in one or more elements of the neural component such as afferent neural output from baroreceptors, central integration, efferent autonomic outflow, and/or responsiveness of the SA node, may have contributed to the enhanced cardiac BRS in our endurance-trained older adults. It also could explain the significant improvement in cardiac BRS despite only modest improvements in aortic PWV after the 8 week exercise intervention. Nonetheless, the relative contribution of the mechanical vs. neural component of cardiac BRS in our cohort remains unknown.
Increased AI can lead to elevated central blood pressure and left ventricular afterload augmenting myocardial oxygen demand increasing the risk of myocardial ischemia in older adults. Consistent with previous studies13, 15, we found that the age-related increase in estimated aortic AI in sedentary adults was attenuated in older adults who were endurance exercise-trained, but this difference was lost after adjusting for sex, heart rate and height indicating that habitual endurance exercise did not obviously prevent the age-associated increase in AI in our cohort. Moreover, aortic AI did not remain significantly associated with BRS in multiple regression analysis. Aortic AI represents a complex interaction between the forward and reflected aortic pressure waves that increases until middle-age and then plateaus in older age10, 11 even as carotid-femoral PWV increases exponentially. Thus, it is well established that AI is influenced by non-arterial stiffness components such as systolic ejection timing, sex, heart rate and height. Thus, although we hypothesized that AI would be associated with cardiac BRS AI did not remain a significant predictor of BRS in multiple regression models that included sex, heart rate and height the primary non-stiffness-related factors that can modulate AI. However, the lack of an association between AI and BRS may be related to two opposing effects of an increase in AI. First, a greater AI will increase central pulse pressure and, thus, enhance the mechanical stimulus for the baroreceptors, leading to an increase in BRS. Conversely, a chronically elevated AI may cause accelerate arterial stiffening of the anatomical sites of the baroreceptors and, thus, reducing BRS. Thus, these opposing effects of an increased AI may explained in part the lack of an association between AI and BRS.
Of the 22 older sedentary adults, 12 participated in 8 weeks of a moderate to vigorous intensity (70-75% heart rate max) aerobic exercise program consisting of daily walking 6 days/week for ~45 minutes per day. Consistent with most 2, 3, 7 but not all 33 studies, we observed a 38% increase in spontaneous BRS following 8 weeks of aerobic exercise training. The divergent results between our and the previous studies may be explained in part by differences in methods used to assess BRS and/or the sex-related differences in response to training, i.e., the negative study was in all postmenopausal women.33 In addition, we found a trend for a decrease in estimated aortic PWV among the older adults after the 8 week exercise intervention but that was lost after adjusting for mean arterial pressure. Previous studies on whether aerobic exercise training initiated in older sedentary adults can improve aortic PWV are conflicting2, 12, 18, 19, 22, 34 likely because of differences in age of the subjects, volume of exercise, duration of the intervention and presence or not of CVD risk factors and medications in these studies. In our cohort, it is possible that 8 weeks was not enough time to favorably alter aortic stiffness, that we were not powered enough to detect a difference, or both. Indeed, post-hoc power calculations revealed that we had only 42% power to detect at the alpha 0.05 level the observed 0.6 m/sec difference in aortic PWV after vs. before exercise in our cohort and we would have needed a sample size of 28 to achieve 80% power. Despite this, the increase in BRS after compared to before exercise was significantly associated with the corresponding changes in aortic PWV that remained significant after adjusting for changes in resting heart rate and blood pressure. Taken together, these data provide support that favorable alterations in cardiac BRS as a result of regular aerobic exercise may be mechanistically linked to even small changes in aortic stiffness even when considering changes in blood pressure among older healthy adults.
We recognize several limitations in our study. First, our study included a small sample of young sedentary adults. However, we were still able to detect the expected age-related differences in cardiac BRS, aortic PWV and AI. Thus, adding more young subjects would not likely change the results of our study because we show the expected age-related difference in the primary outcomes. Second, our measure of aortic PWV was estimated, however, our previous study demonstrated reasonable correlation with direct measures of carotid-femoral PWV.14 Third, our intervention study did not include a non-exercise time control group, however the improvement in cardiac BRS after the 8 weeks of exercise were consistent with previous studies and supported our cross-sectional study findings. Fourth, spontaneous cardiac BRS using the sequence technique assesses spontaneous cardiac BRS from beat-to-beat oscillations in blood pressure during resting conditions around a single operating point on the baroreflex function curve. Therefore, it does not provide information on arterial BRS during acute physiological perturbations in blood pressure. However, spontaneous cardiac BRS estimated by the sequence technique does correlate well with dynamic changes in BRS assessed by the Modified Oxford technique.35 We also did not directly assess sympathetic BRS by measuring muscle sympathetic nerve activity so it is unknown if aortic stiffness modulates the sympathetic arm of the arterial baroreflex, although one study reported such an association between sympathetic BRS and MRI-based aortic stiffness in older adults.36 Finally, breathing frequency is known to affect the cardiac BRS. However, subjects were resting supine in quiescent state for at least 15-20 min prior to onset of collecting invasive blood pressure data and habitual aerobic exercise training would not be expected to result in significant adaptations in breathing rate or tidal volume.
In conclusion, our data support the idea that age- and endurance- exercise associated differences in spontaneous cardiac BRS are associated with corresponding alterations in aortic stiffness among healthy adults. Importantly, this relation remained significant after adjusting for multiple key covariates whereby aortic stiffness remained an independent predictor of cardiac BRS in multiple regression modeling. Furthermore, improvements in BRS after 8 weeks of aerobic exercise in previously sedentary older adults was associated with small reductions in estimated aortic PWV even after adjusting for changes in heart rate and blood pressure. Thus, these data support the idea that differences in aortic stiffness with aging and habitual endurance exercise are mechanistically linked to changes in sensitivity of the baroreceptor reflex in humans, although direct cause and effect cannot be discerned from our study. Future studies using a longer duration and randomized, controlled exercise training and pharmacological interventions are needed to directly test whether favorably modulating aortic stiffness is associated with improvements in both the cardiac BRS in aged adults.
Supplementary Material
Figure 2.
A) Spontaneous cardiac baroreflex sensitivity (BRS), B) aortic pulse wave velocity (PWV), and C) aortic AI in 12 middle-aged/older sedentary adults before and after 8 weeks of aerobic exercise training. *P<0.05 vs. Baseline.
Summary Table.
What is known about this topic
Effects of age and exercise training on cardiac baroreflex sensitivity (BRS) are associated with corresponding effects on common carotid artery compliance
Improvements in cardiac BRS after a 3 month aerobic exercise intervention are strongly associated with increases in carotid artery compliance in previously sedentary middle-aged/older adults.
There is little known about the independent role of aortic stiffness, as measured by the gold standard aortic pulse wave velocity (PWV), on cardiac BRS among healthy middle-aged older adults who differ by fitness status
What this study adds
Effects of age and endurance exercise training on cardiac BRS are associated with corresponding effects on aortic stiffness among healthy adults
Aortic stiffness was an independent predictor of cardiac BRS in a multiple regression model including age, sex, mean arterial pressure, BMI, heart rate and fitness (i.e., VO2max)
Improvements in cardiac BRS after 8 weeks of aerobic exercise training in previously sedentary middle-aged/older adults was associated with reductions in aortic PWV even after adjusting for exercise-mediated changes in heart rate and blood pressure.
Acknowledgments
Study was supported by National Institutes of Health awards AG-000279, AG-043722, HL-014388, AG-013038, UL1 RR-024979, American Heart Association award SDG143400012.
Footnotes
Conflicts of interest: None
References
- 1.Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol. 2000;529(Pt 1):263–71. doi: 10.1111/j.1469-7793.2000.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol. 2001;281:H284–9. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 3.Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation. 2001;104:1627–32. doi: 10.1161/hc3901.096670. [DOI] [PubMed] [Google Scholar]
- 4.Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;29:424–31. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- 5.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–80. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- 6.La Rovere MT, Bigger JT, Jr., Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 7.Deley G, Picard G, Taylor JA. Arterial baroreflex control of cardiac vagal outflow in older individuals can be enhanced by aerobic exercise training. Hypertension. 2009;53:826–32. doi: 10.1161/HYPERTENSIONAHA.109.130039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michas F, Manios E, Stamatelopoulos K, Koroboki E, Toumanidis S, Panerai RB, Zakopoulos N. Baroreceptor reflex sensitivity is associated with arterial stiffness in a population of normotensive and hypertensive patients. Blood Press Monit. 2012;17:155–9. doi: 10.1097/MBP.0b013e32835681fb. [DOI] [PubMed] [Google Scholar]
- 9.Mattace-Raso FU, van den Meiracker AH, Bos WJ, van der Cammen TJ, Westerhof BE, Elias-Smale S, Reneman RS, Hoeks AP, Hofman A, Witteman JC. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam Study. J Hypertens. 2007;25:1421–6. doi: 10.1097/HJH.0b013e32811d6a07. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the framingham heart study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46:1753–60. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H, Dinenno F, Monahan K, Clevenger C, DeSouza C, Seals D. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–32. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- 14.Pierce GL, Casey DP, Fiedorowicz JG, Seals DR, Curry TB, Barnes JN, Wilson DR, Stauss HM. Aortic pulse wave velocity and reflecting distance estimation from peripheral waveforms in humans: detection of age- and exercise training-related differences. Am J Physiol Heart Circ Physiol. 2013;305:H135–42. doi: 10.1152/ajpheart.00916.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–62. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–46. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gates PE, Tanaka H, Graves J, Seals DR. Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur Heart J. 2003;24:2213–20. doi: 10.1016/j.ehj.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. 2009;32:1531–5. doi: 10.2337/dc09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel T, Lepretre PM, Brechat PH, Lonsdorfer-Wolf E, Kaltenbach G, Lonsdorfer J, Benetos A. Effect of a short-term intermittent exercise-training programme on the pulse wave velocity and arterial pressure: a prospective study among 71 healthy older subjects. Int J Clin Pract. 2013;67:420–6. doi: 10.1111/ijcp.12021. [DOI] [PubMed] [Google Scholar]
- 20.Oudegeest-Sander MH, Olde Rikkert MG, Smits P, Thijssen DH, van Dijk AP, Levine BD, Hopman MT. The effect of an advanced glycation end-product crosslink breaker and exercise training on vascular function in older individuals: a randomized factorial design trial. Exp Gerontol. 2013;48:1509–17. doi: 10.1016/j.exger.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrier KE, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension. 2001;38:222–6. doi: 10.1161/01.hyp.38.2.222. [DOI] [PubMed] [Google Scholar]
- 22.Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–13. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- 23.Weber T, Auer J, O'Rourke M F, Kvas E, Lassnig E, Lamm G, Stark N, Rammer M, Eber B. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J. 2005;26:2657–63. doi: 10.1093/eurheartj/ehi504. [DOI] [PubMed] [Google Scholar]
- 24.Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, Perez G, Mendez AJ. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45:980–5. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 25.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 2011;120:13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davy KP, DeSouza CA, Jones PP, Seals DR. Elevated heart rate variability in physically active young and older adult women. Clin Sci (Lond) 1998;94:579–84. doi: 10.1042/cs0940579. [DOI] [PubMed] [Google Scholar]
- 27.Davy KP, Miniclier NL, Taylor JA, Stevenson ET, Seals DR. Elevated heart rate variability in physically active postmenopausal women: a cardioprotective effect? Am J Physiol. 1996;271:H455–60. doi: 10.1152/ajpheart.1996.271.2.H455. [DOI] [PubMed] [Google Scholar]
- 28.Monahan KD, Eskurza I, Seals DR. Ascorbic acid increases cardiovagal baroreflex sensitivity in healthy older men. Am J Physiol Heart Circ Physiol. 2004;286:H2113–7. doi: 10.1152/ajpheart.01054.2003. [DOI] [PubMed] [Google Scholar]
- 29.Tomiyama H, Matsumoto C, Kimura K, Odaira M, Shiina K, Yamashina A. Pathophysiological contribution of vascular function to baroreflex regulation in hypertension. Circ J. 2014;78:1414–9. doi: 10.1253/circj.cj-14-0064. [DOI] [PubMed] [Google Scholar]
- 30.Gupta A, Jain G, Kaur M, Jaryal AK, Deepak KK, Bhowmik D, Agarwal SK. Association of impaired baroreflex sensitivity and increased arterial stiffness in peritoneal dialysis patients. Clin Exp Nephrol. 2015 Sep 5; doi: 10.1007/s10157-015-1158-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Tarumi T, de Jong DL, Zhu DC, Tseng BY, Liu J, Hill C, Riley J, Womack KB, Kerwin DR, Lu H, Munro Cullum C, Zhang R. Central artery stiffness, baroreflex sensitivity, and brain white matter neuronal fiber integrity in older adults. Neuroimage. 2015;110:162–70. doi: 10.1016/j.neuroimage.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt BE, Farquhar WB, Taylor JA. Does reduced vascular stiffening fully explain preserved cardiovagal baroreflex function in older, physically active men? Circulation. 2001;103:2424–7. doi: 10.1161/01.cir.103.20.2424. [DOI] [PubMed] [Google Scholar]
- 33.Davy KP, Willis WL, Seals DR. Influence of exercise training on heart rate variability in post-menopausal women with elevated arterial blood pressure. Clin Physiol. 1997;17:31–40. doi: 10.1046/j.1365-2281.1997.01010.x. [DOI] [PubMed] [Google Scholar]
- 34.Matsubara T, Miyaki A, Akazawa N, Choi Y, Ra SG, Tanahashi K, Kumagai H, Oikawa S, Maeda S. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am J Physiol Heart Circ Physiol. 2014;306:H348–55. doi: 10.1152/ajpheart.00429.2013. [DOI] [PubMed] [Google Scholar]
- 35.Parlow J, Viale JP, Annat G, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans. Comparison with drug-induced responses. Hypertension. 1995;25:1058–68. doi: 10.1161/01.hyp.25.5.1058. [DOI] [PubMed] [Google Scholar]
- 36.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59:98–104. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.