Abstract
Objective
The aim of the current study was to evaluate secretory leukocyte protease inhibitor (SLPI) expression in anal biopsies from HIV-positive (HIV+) individuals, and compare that to anal intraepithelial neoplasia (AIN) diagnoses and human papillomavirus (HPV) status.
Design
This is a cross-sectional study of a cohort of 54 HIV+ (31 males and 23 females) from an AIDS clinic in Rio de Janeiro, Brazil.
Methods
The study material consisted of anorectal tissue biopsies obtained from HIV+ subjects, which were used to construct tissue microarray paraffin blocks for immunohistochemical analysis of SLPI expression. Biopsies were evaluated by an expert pathologist and classified as low-grade anal intraepithelial neoplasia (AIN1), high-grade anal intraepithelial neoplasia (AIN2/3), or normal squamous epithelium. Additionally, DNA from the biopsies was extracted and analyzed for the presence of low- or high-risk HPV DNA.
Results
Histologically normal squamous epithelium from the anorectal region showed strong positive SLPI staining in 17/20 (85%) samples. In comparison, 9/17 (53%) dysplastic squamous epithelial samples from AIN1 patients showed strong SLPI staining, and only 5/17 (29%) samples from AIN2-3 patients exhibited strong SPLI staining, which both were significantly fewer than those from normal tissue (p=0.005). Furthermore, there was a significantly higher proportion of samples in which oncogenic high-risk HPV genotypes were detected in low SLPI expressing tissues than that in tissues with high SLPI expression (p=0.040).
Conclusion
Taken together these results suggest that low SLPI expression is associated with high-risk HPV infections in the development of AIN.
Keywords: Secretory leukocyte protease inhibitor, SLPI, Anal intraepithelial neoplasia, Human immunodeficiency virus, human papillomavirus, HPV
INTRODUCTION
Secretory leukocyte protease inhibitor (SLPI) is an 11.7 kDa serine protease inhibitor, which is produced by different epithelial cell types and in salivary glands, and hence is found in high concentrations in secretions from the cervix, nasal cavity, upper respiratory tract, prostate, and in the saliva.1-3 Moreover, there is evidence that the protease activity of SLPI likely plays a role in the protection of the mucosa against proteolysis.2,4 Since many viruses require protease activity for infectivity, it is not surprising that SLPI has been demonstrated to inhibit viral infections, including those by HIV-1 and influenza A.5-11 Interestingly, however, SLPI protection against HIV-1 infection has been shown to be independent of its anti-protease activity,12-14 which suggests that SLPI may block the interaction between HIV and a cell surface receptor. Particularly, SLPI blocked HIV-1 entry into macrophages through an interaction with annexin A2,15 which exists at the cell surface with S100A10 as a heterotetrameric receptor (A2t).16 Furthermore, SLPI has been shown to block human papillomavirus (HPV) entry via annexin A2 in both epithelial and Langerhans cells in vitro.17,18 Conversely, reductions in the amount of SLPI secreted by epithelial cells was shown to promote HPV16 infection through annexin A2 in vitro.19
Interestingly, several studies have reported a direct correlation between the presence of HPV and low expression levels of SLPI in vivo in head and neck squamous cell carcinoma (HNSCC) patients.20-23 In particular, it was shown that SLPI expression at the gene and protein level was significantly lower in metastatic versus non-metastatic HNSCC tissues,20 and that high levels of SLPI were correlated with protection against HPV infection.21 Additionally, high-risk HPV-positive (HPV+) HNSCC tumors were shown to express higher levels of the HPV receptor annexin A2 than those of HPV-negative tumors, suggesting that in HNSCC, higher levels of SLPI may be protective against HPV infection through annexin A2.23 Importantly, these studies demonstrate that there is a correlation between SLPI expression and HPV infection in HNSCC in vivo, though it is unknown if such a correlation exists in other HPV-associated lesions and or/cancers.
Anal intraepithelial neoplasia (AIN) is a precursor to anal cancer and is caused by persistent high-risk HPV infections.24 Though the incidence of AIN and anal cancer are very low in the general population, both are relatively common in individuals infected with HIV-1.25 It is well established that HPV can be found in the dysplastic squamous epithelium of AIN lesions, whereas HIV-1 is more often found in the underlying stromal cells including stromal macrophages.26-29 However, to date, there has been no prior research regarding SLPI distribution patterns or expression levels in the anal epithelium of AIN lesions. Additionally, while previous studies investigating SLPI have employed enzyme linked immunosorbent assays (ELISA) or western blot analyses to analyze SLPI levels,29-34 immunohistochemical analyses provide more information with respect to the cells of origin of SLPI, and its modulation in tissues during viral infection. Given that the expression of SLPI is associated with susceptibility to HPV infection in HNSCC, and that HPV-induced AIN is more common in HIV+ individuals, the purpose of the present study was to perform an immunohistochemical analysis to determine SLPI distribution patterns and expression levels in anal biopsies from HIV+ individuals, and compare the resultant data to AIN diagnoses and HPV status.
METHODS
Study population and Tissue samples
The study material consisted of anorectal tissue samples obtained from HIV+ subjects at the DST/AIDS Clinical Laboratory (LapClin-AIDS-INI) of the Evandro Chagas National Institute of Infectious Diseases (INI), Oswaldo Cruz Foundation (FIOCRUZ), Rio de Janeiro, Brazil. Eight patients underwent additional follow up biopsies at 1 and 5 months from baseline. All patients were followed up in order to monitor the progression of the lesion. Additionally, clinical parameters including CD4 counts and HIV RNA status were assessed at the time of tissue biopsy. The anorectal specimens were used to construct two tissue microarray (TMA) paraffin blocks. The biopsies were used only if sufficient tissue was present to submit as part of the TMAs. Biopsies were evaluated by an expert pathologist and classified as low-grade anal intraepithelial neoplasia (AIN1), high-grade anal intraepithelial neoplasia (AIN2/3), or normal squamous epithelium. In total, 54 tissue samples fit the criteria for inclusion, which included those from 31 males and 23 females. Additionally, laboratory and clinical variables including age at the time of the biopsy, gender, and smoking status were obtained from the cohort studies databank of LapClin-AIDS-INI. Written informed consent was obtained from all participants in strict compliance with the ethical guidelines involving human subjects in Brazil as required in the Resolution n.466/2012 of the National Health Council. The study was approved by the INI FIOCRUZ Institutional Review Board (IRB).
Immunohistochemical analysis
The preparation of the TMA blocks was performed as previously described.35 Following the construction of the array blocks, 4 μm sections were cut with a microtome and placed on silane-coated slides for immunohistochemical analysis following published procedures.26-29 Briefly, the 4 μm paraffin-embedded sections were dehydrated, incubated in 3% hydrogen peroxide for 10 min, and incubated in trypsin for 20 min. The sections were blocked with 10% goat serum at room temperature for 20 min and treated with a goat anti-human SLPI antibody (1:100; Minneapolis, MN, USA) at for 1h. After rinsing, the sections were treated with biotin-conjugated antibodies from the MACH 4 Kit (Biocare Medical, Concord, CA, USA) for 20 min, and streptavidin immune complexes were identified with a diaminobenzidine (DAB) substrate immunochemistry (Biocare) and hematoxylin stain. Sections were mounted, dehydrated, and sealed with a coverslip. For the negative control, sections were treated identically except that the primary antibody was replaced with goat IgG. All steps were performed at room temperature. Semiquantitative analysis of SLPI stained cells was performed by one expert pathologist (GJN). SLPI staining was scored as 0, 1+, 2+, or 3+ based on the percent of positive target cells (normal squamous epithelia in the controls and dysplastic squamous cells in the AIN lesions) in each specimen as follows: 0 = 0%; 1-19% = 1+; 20-49% = 2+; and ≥50% = 3+, respectively. Histology slides of normal cervical, brain, and placental tissues obtained from a tissue block were stained for SLPI to validate the antibody.
HPV genotyping
Tissue biopsies were stored in liquid nitrogen before embedding in Tissue-Tek medium (Sakura Finetek, Torrance, CA, USA). Using a cryostat, three 5 μm thick sections were cut for DNA testing. DNA extraction was performed using the Illustra Tissue Genomic Prep Mini Spin Kit (GE Healthcare, Buckinghamshire, United Kingdom) according to the manufacturer's protocol. The purified DNA was quantified by spectrophotometry using the NanoDrop spectrophotometer (ND 1000, Thermo Fisher Scientific, Wilmington, MA, USA). All samples were tested for the amplification of a 110 base pair fragment of the β-globin gene using primers PC03 (ACACAACTGTGTTCACTAGC) and PC04 (CAACTTCATCCACGTTCACC). Samples negative for β-globin were excluded from the HPV DNA analysis. HPV DNA (100 ng) isolated from HeLa cells was used a positive control, and the addition of no DNA served as the negative control. DNA samples with concentrations below 3 ng/μL were subjected to thermostable amplification of total DNA with Phi29 DNA polymerase using the GenomePhi V2 DNA Amplification Kit (GE Healthcare). For detection of HPV DNA, genomic material was subjected to polymerase chain reaction (PCR) for amplification of conserved regions of the L1 gene for low- and high-risk HPV genotypes using primer pairs flanking these regions. Oligonucleotides (dNTPs) used for all reactions were acquired from Invitrogen (Sao Paulo, Brazil). Samples negative for HPV in the first reaction were then subjected to nested PCR using the GP5+ and GP6+ primers to amplify HPV DNA fragments of approximately 155 base pairs as previously reported.36 The PCR products were purified using the NucleoSpin Gel and PCR Clean-up Kit (Macherey Nagel, Düren, Germany). Sequencing was carried out using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and analyzed with the ABI Prism 3730 Genetic Analyzer (Applied Biosystems). The obtained sequences were further analyzed using MEGA software version 6.0 (available at http://www.megasoftware.net/, and BLAST software (available http://blast.ncbi.nlm.nih.gov/Blast.cgi). We assumed that sequences with double peaks in the chromatogram, i.e. more than one base at the same position, were indicative of multiple infections. In these cases, multiple infections were analyzed using the High+Low PapillomaStrip Kit (Operon, Zaragoza, Spain), which identifies 18 low-risk and 19 high-risk HPV types.
Statistical analysis
Analyses were performed based on SLPI expression, HPV status, histopathological diagnosis, and clinical parameters. Fisher's exact and Pearson's chi-square tests were performed to assess differences between groups using SPSS 20 software (SPSS Inc., Chicago, IL, USA). All tests were two-sided, and p<0.05 was considered statistically significant.
RESULTS
Patient characteristics
Clinical and laboratory data provided by the cohort studies databank at LapClin-AIDS-INI identified individuals who were seropositive for HIV-1, and who had concurrent anal biopsies. It was from this data bank that we obtained the tissues used in this study. Of the 54 study participants, 31 (57.4%) were male, 23 (42.6%) were female, with ages ranging from 24.7 to 66.8 years old, and all were seropositive for HIV-1. The histopathological diagnoses of the tissue samples were as follows: 17 cases of AIN1, 17 cases of AIN2/3, and 20 histologically normal squamous epithelial samples that were used as controls. The average age was 42.5±9.1 years for individuals with normal squamous epithelium; 43.1±6.7 years for AIN1; and 44.2±11.6 years for AIN2/3 with no statistical differences in age among the groups (p=0.963 by Kruskal-Wallis test). The age, sex, HPV type detected, CD4 counts, HIV RNA status, and current smoking status of the study population are summarized in Table 1. As seen in the table, the vast majority of the study population was HPV+ (only 1 negative, and 1 unknown), though the detection of low-risk, high-risk, or a combination of both low- and high-risk HPV genotypes was variable.
Table 1.
Patient characteristics
| Characteristic | N (%) | |
|---|---|---|
| Number of patients | 54 (100%) | |
|
| ||
| Age | < 40 years | 19 (35.2) |
| 40-49 years | 23 (42.6) | |
| > 50 years | 12 (22.2) | |
|
| ||
| Sex | Male | 31 (57.4) |
| Female | 23 (42.6) | |
|
| ||
| HPV type | Negative | 1 (1.9) |
| Low riska | 12 (22.2) | |
| High riskb | 36 (66.7) | |
| Low & High riskc | 4 (7.4) | |
| Unknown | 1 (1.9) | |
|
| ||
| Current CD4 | < 50 | 1 (1.9) |
| 50-99 | 1 (1.9) | |
| 100-199 | 2 (3.7) | |
| 200-349 | 12 (22.2) | |
| 350-499 | 17 (31.5) | |
| ≥ 500 | 21 (38.9) | |
|
| ||
| HIV RNA viral load |
Undetectable | 35 (64.8) |
| Detectable | 19 (35.2) | |
|
| ||
| Current Smoking |
No | 39 (72.2) |
| Yes | 14 (25.9) | |
| Unknown | 1 (1.9) | |
Low risk HPV types detected: 6, 11, 40, 42, 43, and 54
High risk HPV types detected: 16, 18, 31, 33, 35, 51, 58, and 59
Both low and high risk HPV types were detected in these specimens
HPV DNA genotyping
HPV DNA was detected in 96.3% (52/54) of the total anal biopsies analyzed. By sequencing, we identified eight high-risk oncogenic HPV types (16, 18, 31, 33, 35, 51, 58, and 59); six low-risk HPV types (6, 11, 40, 42, 43, 54); and three HPV types classified as probable high-risk (53, 66, and 69). The most common type found in the anal biopsies was high-risk HPV16, which was detected in 20.4% (11/54) of the samples; followed by low-risk HPV6, which was detected in 16.7% (9/54). Multiple HPV infections were found in 4 specimens (7.4%).
SLPI expression in normal anal squamous epithelium and AIN tissues
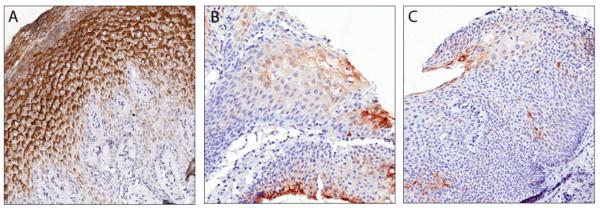
Prior to immunohistochemical analysis of normal anal squamous epithelium and AIN tissues, the SLPI antibody was validated by staining normal cervical transformation zone, which exhibited an intense SLPI signal in the epithelia, whereas brain, and kidney tissues each showed no signal in agreement with previous reports (data not shown).2 We next evaluated the distribution of SLPI in the histologically normal anorectal epithelium. Each of these tissues showed histologically normal squamous epithelium and many showed adjacent normal columnar epithelia of the rectum. In these normal tissues, SLPI tended to be more highly expressed in the more differentiated squamous cells whereas there was weaker SLPI staining towards the basal zone (Figure 1A). Overall, 17/20 (85.0%) of these samples had high SLPI expression scores defined as either 2+ (4/20) or 3+ (13/20) (Table 2 and Figure 2A). We next evaluated the expression and distribution patterns of SLPI in the AIN biopsies. AIN1 lesions were found to have generally weak SLPI staining in the middle and upper thirds of the lesion (Figure 1B). Specifically, only 9/17 (52.9%) of the AIN1 tissues received high SLPI expression (2+/3+) scores (Table 2 and Figure 2A). In the AIN2/3 lesions, no (7/17) or weak (5/17) SLPI staining was observed in the majority of the tissues (Figure 1C), whereas only 5/17 (29.4%) of the samples received high SLPI expression scores (Table 2 and Figure 2A). Moreover, the number of samples receiving high SLPI expression scores from AIN tissues was significantly lower compared to that from histologically normal anorectal tissues (p<0.005).
Figure 1. Immunohistochemical staining of SLPI in anal biopsies.
A. Histologically normal squamous epithelium that lines the anus shows strong SLPI staining (brown) in the more differentiated squamous cells in the middle and superficial layers, and less SLPI expression in the basal and stromal layers (40× magnification). B. Epithelium from an AIN1 lesion shows weaker SLPI staining in dysplastic squamous cells (40× magnification). C. Epithelium from an AIN3 lesion shows a further reduction in SLPI staining in dysplastic squamous cells (20× magnification).
Table 2.
SLPI expression scores across different characteristics
| SLPI signal | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | |||||||
| 0 | 1+ | 2+ | 3+ | Total | p valuea | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Histopathology | Normal | - | 3 (15.0) | 4 (20.0) | 13 (65.0) | 20 (100.0) | 0.003* |
| AIN1 | 4 (23.5) | 4 (23.5) | 6 (35.3) | 3 (17.6) | 17 (100.0) | ||
| AIN2/3 | 7 (41.2) | 5 (29.4) | 2 (11.8) | 3 (17.6) | 17 (100.0) | ||
| Total | 11 (20.4) | 12 (22.2) | 12 (22.2) | 19 (35.2) | 54 (100.0) | ||
|
| |||||||
| HPV Typeb | Low risk | - | 2 (16.7) | 4 (33.3) | 6 (50.0) | 12 (100.0) | 0.177 |
| High risk | 10 (25.0) | 10 (25.0) | 8 (20.0) | 12 (30.0) | 40 (100.0) | ||
| Total | 10 (19.2) | 12 (23.1) | 12 (23.1) | 18 (34.6) | 52 (100.0) | ||
|
| |||||||
| Current CD4 | < 200 | 1 (25.0) | 2 (50.0) | 1 (25.0) | - | 4 (100.0) | 0.590 |
| 200-350 | 2 (16.7) | 2 (16.7) | 4 (33.3) | 4 (33.3) | 12 (100.0) | ||
| > 350 | 8 (21.1) | 8 (21.1) | 7 (18.4) | 15 (39.5) | 38 (100.0) | ||
| Total | 11 (20.4) | 12 (22.2) | 12 (22.2) | 19 (35.2) | 54 (100.0) | ||
|
| |||||||
| HIV RNA viral load |
Undetectable | 5 (14.3) | 9 (25.7) | 8 (22.9) | 13 (37.1) | 35 (100.0) | 0.526 |
| Detectable | 6 (31.6) | 3 (15.8) | 4 (21.1) | 6 (31.6) | 19 (100.0) | ||
| Total | 11 (20.4) | 12 (22.2) | 12 (22.2) | 19 (35.2) | 54 (100.0) | ||
|
| |||||||
| Current smoking statusc |
Non-smokers | 9 (23.1) | 8 (20.5) | 8 (20.5) | 14 (35.6) | 39 (100.0) | 0.767 |
| Smokers | 2 (14.3) | 4 (28.6) | 4 (28.6) | 4 (28.6) | 14 (100.0) | ||
| Unknown | - | - | - | 1 (100.0) | 1 (100.0) | ||
| Total | 11 (20.4) | 12 (22.2) | 12 (22.2) | 19 (35.2) | 54 (100.0) | ||
Fisher's exact test
HPV-negative (N = 1, 1.9%) and missing data (N = 1, 1.9%) samples were excluded from analysis
Missing data (N = 1, 1.9%) sample was excluded from analysis
p<0.05
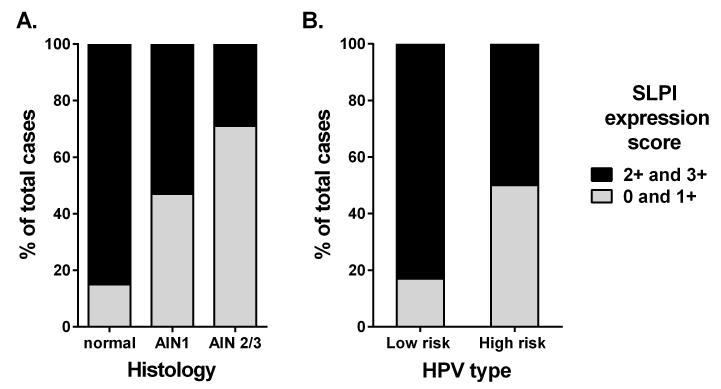
Figure 2. Proportion of SLPI expression scores according to anal histology and HPV type.
A. The proportion of high SLPI expression scores (2+ and 3+) and low SLPI expression scores (0 and 1+) for normal anal squamous epithelium, AIN1, and AIN2/3. The proportions were found to be statistically different by chi-squared test (p=0.005). B. The proportion of high SLPI expression scores (2+ and 3+) and low SLPI expression scores (0 and 1+) for samples with low-risk and high-risk HPV infections. Samples where both low- and high-risk HPV types were detected were counted as high-risk. The proportions were found to be statistically different by chi-squared test (p=0.040).
SLPI expression across HPV status and other clinical parameters
Next, we evaluated the distribution of SLPI expression scores in regards to HPV status as well as other well-established immune biomarkers of HIV infection, in particular CD4 counts and HIV viral load. We found that there was a significantly greater proportion of high SLPI expression (10/12) in tissues where low-risk HPV types were detected compared to tissues where high-risk HPV types were detected (20/40) (p<0.040) (Figure 2B). Hence there was an inverse correlation between high-risk HPV infection and the level of SLPI expression, though this association was less apparent when viewed across individualized SLPI expression scores, which was likely due to the small sample size (Table 2). While the majority of samples were found to be HPV+, only one sample was found to be HPV negative and was histologically normal as expected. Additionally, one AIN2/3 sample was found to be of unknown HPV type, which may have been due to the presence of a high-risk HPV genotype that was not covered in the primers used herein. No associations with SLPI signal were evident between other clinical parameters that could have impacted SLPI protein expression, such as smoking, CD4 counts or HIV RNA status (Table 2). Interestingly, while smoking is known to play a role in the development of anogenital cancers,37 and we previously reported a correlation between SLPI expression and smoking status,21 no such correlation was observed herein (p=0.767), which may have been due to too few of smokers (N=14) in the current study population.
AIN diagnoses across HPV type
The distribution of AIN diagnoses across HPV types was assessed, and the Fisher’s exact test revealed that there was no statistical correlation between AIN status and HPV type (p=1.000). For example, there was a similar distribution of high-risk HPV+ samples among normal (14/36), AIN1 (10/36), and AIN2/3 (12/36) (Table 3).
Table 3.
Anal histopathology status across HPV type
| HPV type | Histopathology | ||||
|---|---|---|---|---|---|
| Normal | AIN1 | AIN2/3 | Total | p valuea | |
| N (%) | N (%) | N (%) | N (%) | ||
| Low risk | 4 (33.3) | 4 (33.3) | 4 (33.3) | 12 (100.0) | 1.000b |
| High risk | 15 (37.5) | 12 (30.0) | 13 (32.5) | 40 (100.0) | |
| Total | 19 (36.5) | 16 (30.8) | 17 (32.7) | 52 (100.0) | |
Fisher's exact test
HPV-negative (N = 1, 1.9%) and missing data (N = 1, 1.9%) samples were excluded from analysis
DISCUSSION
There are several sites in the human body where different types of epithelial tissue meet at distinct transitions. These sites are typically referred to as transformation zones, such as when the squamous epithelium distal to mucosal sites merge with the glandular/columnar epithelium typical of mucosal layers.38 The two most studied transformation zones are those of the cervix and anorectal junction, and each of these are classic sites of transmission for HIV-1 and HPV.26-28 The target cells for HIV-1 in the transformation zone are mucosal Langerhans cells, which can transmit the virus to underlying macrophages or disseminate the virus directly to T cells.26,27 In comparison, the target cells for HPV are the squamous metaplastic cells, which reside only in the “active” area of the transformation zone where over 95% of cervical cancers occur.28 Thus, many investigators have examined the levels of SLPI in cervical secretions given its role in viral infections,29,31,39 while less is known about SLPI expression in anal epithelium. Data regarding the influence of HIV-1 infection on SLPI concentrations in bodily fluids is conflicting. For example, several studies have concluded that SLPI levels are decreased in cervical secretions and saliva of HIV-1 infected individuals.29-33 However, other studies have indicated that HIV-1 infection may increase salivary SLPI.40,41 For these reasons, in the current study we chose to investigate SLPI levels in an HIV+ cohort to reduce potential confounding. Moreover, this cohort is far more afflicted by precancerous anal lesions and anal cancer, which was the focus of this study.
One of the observations from the current study was that epithelial cells in the anorectal junction were the primary source of SLPI expression. Moreover, the squamous epithelium was observed to produce abundant SLPI levels in all layers except the basal layer. Interestingly, SLPI is a ligand for annexin A2,15 which we have shown is a receptor for HPV,17,42 and annexin A2 expression is restricted to the basal and suprabasal layers of stratified epithelium,43 matching the tropism of HPV infection. This may suggest that even small overall reductions of SLPI expression in the epithelial layer may dramatically affect the amount of SLPI bound to annexin A2 at the basal cell surface, thus promoting HPV infection through its putative receptor. This notion is further supported by our data showing the significantly reduced levels of SLPI observed in tissues infected by high-risk HPV types and in high-grade AIN lesions. Additionally, glandular epithelium expressed much less SLPI than squamous epithelium in the anorectal junction, and minimal SLPI was observed in the stroma. As epithelial cells can secrete directly into the mucosal lumen, it can be inferred that inhibition by SLPI in the anorectal junction occurs primarily in the epithelial layers and subsequently in the lumen to which they secrete.
As mentioned, our cooperating group previously reported that SLPI expression levels were inversely correlated with HPV+ HNSCC.20,21 Specifically, it was demonstrated that HNSCC patients with high-risk HPV infections showed little to no SLPI expression, whereas HNSCC patients without HPV infections had high SLPI expression.21 However, in the current study, such a direct comparison could not be performed as HPV was detected in all but one patient. This is likely because highly sensitive PCR methods can detect subclinical HPV infection as well as clinical HPV infection that has resulted in cytological changes in the epithelium. This particular study population is known to be a high-exposure high-risk group,44-46 therefore the high prevalence of HPV infection was not surprising. As such, we evaluated the potential relationship between the proportions of samples with different SLPI expression levels and either low- or high-risk HPV infection. Through this analysis, we found that there was an inverse correlation between high-risk HPV infection and the level of SLPI expression, which is in agreement with our previous results.20,21 Interestingly, we also previously found that smoking increased SLPI expression, and as such, smokers were less likely to develop HPV+ HNSCC.21 However, no such correlation was observed herein, which may have been due to the small numbers of current smokers in the study cohort, or potentially because smoking has less of an influence on SLPI levels in the anorectal region compared to the head and neck region.
A question that remains is whether reduced SLPI expression results in HPV infection, as in those with low expression levels have increased susceptibility leading to AIN development, or whether HPV infection itself actively down-regulates SLPI. Since participants of this study had both HPV and HIV-1 infections, it is difficult to assess if reduced SLPI expression in AIN represents an effect of viral infection. When comparing AIN grade to SLPI expression, it was clear that in less differentiated squamous cells, there was generally weaker SLPI staining, which may suggest that the de-differentiation of squamous cells during AIN progression leads to reduced SLPI. However, future studies with larger sample sizes will allow for multivariate analyses to address whether certain factors influence both AIN and SLPI levels, since this was a limitation of the current study. In any case, a scenario where SLPI expression is reduced likely increases the potential for HPV to infect more cells, and hence promotes AIN development. Though the complete ramifications of reduced SLPI expression in AIN awaits further study, we speculate that it may permit more effective HPV co-infection due to the increased availability of the putative annexin A2 receptor in the basal epithelium of anorectal lesions.
Acknowledgements
The authors would like to thank the Beckman Center for Immune Monitoring Core Facility (supported through NCI grant 5P30 CA014089) for excellent technical assistance. Support for the Kast laboratory from The Netherlands American Foundation, Ella Selders, Christine Ofiesh, Yvonne Bogdanovich and Sammie’s Circle is gratefully acknowledged.
Source of Funding: This study was supported by National Institutes of Health grant R01 CA074397 to WM Kast, who also holds the Walter A. Richter Cancer Research Chair. AFN was a Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq-CSF) scholar and received support from PAPES VII/CNPq- FIOCRUZ, Brazil and LIPMED - IOC-FIOCRUZ, Brazil. BG received support from Proep and FAPERJ-Cientista do Nosso Estado, Brazil. AWW received support from the ARCS Foundation John and Edith Leonis Award and a SC-CTSI (NIH/NCRR/NCATS) Grant #TL1TR000132.
Footnotes
Conferences: Parts of this research were presented at the 29th Annual International Papillomavirus Conference & Clinical Workshops, Seattle, WA, August 20-25, 2014.
Conflicts of Interest: All authors declare no conflicts of interest.
REFERENCES
- 1.Abe T, Kobayashi N, Yoshimura K, et al. Expression of the secretory leukoprotease inhibitor gene in epithelial cells. J Clin Invest. 1991 Jun;87(6):2207–2215. doi: 10.1172/JCI115255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franken C, Meijer CJ, Dijkman JH. Tissue distribution of antileukoprotease and lysozyme in humans. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1989 Apr;37(4):493–498. doi: 10.1177/37.4.2926127. [DOI] [PubMed] [Google Scholar]
- 3.Kramps JA, Franken C, Dijkman JH. ELISA for quantitative measurement of low-molecular-weight bronchial protease inhibitor in human sputum. Am Rev Respir Dis. 1984 Jun;129(6):959–963. doi: 10.1164/arrd.1984.129.6.959. [DOI] [PubMed] [Google Scholar]
- 4.Hiemstra PS. Novel roles of protease inhibitors in infection and inflammation. Biochemical Society Transactions. 2002 Apr;30(2):116–120. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 5.Drannik AG, Henrick BM, Rosenthal KL. War and peace between WAP and HIV: role of SLPI, trappin-2, elafin and ps20 in susceptibility to HIV infection. Biochemical Society Transactions. 2011 Oct;39(5):1427–1432. doi: 10.1042/BST0391427. [DOI] [PubMed] [Google Scholar]
- 6.Kido H, Okumura Y, Yamada H, Le TQ, Yano M. Proteases essential for human influenza virus entry into cells and their inhibitors as potential therapeutic agents. Curr Pharm Des. 2007;13(4):405–414. doi: 10.2174/138161207780162971. [DOI] [PubMed] [Google Scholar]
- 7.Konopka K, Shine N, Pretzer E, Duzgunes N. Secretory leukocyte protease inhibitor (SLPI): oxidation of SLPI does not explain its variable anti-HIV activity. J Dent Res. Dec. 1999;78(12):1773–1776. doi: 10.1177/00220345990780120201. [DOI] [PubMed] [Google Scholar]
- 8.Scott A, Weldon S, Taggart CC. SLPI and elafin: multifunctional antiproteases of the WFDC family. Biochemical Society Transactions. 2011 Oct;39(5):1437–1440. doi: 10.1042/BST0391437. [DOI] [PubMed] [Google Scholar]
- 9.Skott P, Lucht E, Ehnlund M, Bjorling E. Inhibitory function of secretory leukocyte proteinase inhibitor (SLPI) in human saliva is HIV-1 specific and varies with virus tropism. Oral Dis. 2002 May;8(3):160–167. doi: 10.1034/j.1601-0825.2002.01807.x. [DOI] [PubMed] [Google Scholar]
- 10.Tugizov SM, Herrera R, Veluppillai P, et al. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology. 2011 Jan 20;409(2):211–222. doi: 10.1016/j.virol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahl SM, McNeely TB, Janoff EN, et al. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-I. Oral Dis. 1997 May;3(Suppl 1):S64–69. doi: 10.1111/j.1601-0825.1997.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 12.Hiemstra PS, Maassen RJ, Stolk J, Heinzel-Wieland R, Steffens GJ, Dijkman JH. Antibacterial activity of antileukoprotease. Infect Immun. 1996 Nov;64(11):4520–4524. doi: 10.1128/iai.64.11.4520-4524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNeely TB, Shugars DC, Rosendahl M, Tucker C, Eisenberg SP, Wahl SM. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997 Aug 1;90(3):1141–1149. [PubMed] [Google Scholar]
- 14.Bingle CD, Vyakarnam A. Novel innate immune functions of the whey acidic protein family. Trends Immunol. 2008 Sep;29(9):444–453. doi: 10.1016/j.it.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Ma G, Greenwell-Wild T, Lei K, et al. Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV-1 infection. J Exp Med. 2004 Nov 15;200(10):1337–1346. doi: 10.1084/jem.20041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waisman DM. Annexin II tetramer: structure and function. Molecular and Cellular Biochemistry. 1995;149/150:301–322. doi: 10.1007/BF01076592. [DOI] [PubMed] [Google Scholar]
- 17.Woodham AW, Da Silva DM, Skeate JG, et al. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS ONE. 2012;7(8):e43519. doi: 10.1371/journal.pone.0043519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodham AW, Raff AB, Raff LM, et al. Inhibition of langerhans cell maturation by human papillomavirus type 16: a novel role for the annexin A2 heterotetramer in immune suppression. J Immunol. 2014 May 15;192(10):4748–4757. doi: 10.4049/jimmunol.1303190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skeate JG, Porras TB, Woodham AW, et al. Herpes Simplex Virus downregulation of secretory leukocyte protease inhibitor enhances Human Papillomavirus type 16 infection. J Gen Virol. 2015 Nov 10; doi: 10.1099/jgv.0.000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordes C, Hasler R, Werner C, et al. The level of secretory leukocyte protease inhibitor is decreased in metastatic head and neck squamous cell carcinoma. Int J Oncol. 2011 Jul;39(1):185–191. doi: 10.3892/ijo.2011.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann M, Quabius ES, Tribius S, et al. Human papillomavirus infection in head and neck cancer: the role of the secretory leukocyte protease inhibitor. Oncol Rep. 2013 May;29(5):1962–1968. doi: 10.3892/or.2013.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quabius ES, Moller P, Haag J, et al. The role of the antileukoprotease SLPI in smoking-induced human papillomavirus-independent head and neck squamous cell carcinomas. Int J Cancer. 2013 Aug 28; doi: 10.1002/ijc.28462. [DOI] [PubMed] [Google Scholar]
- 23.Quabius ES, Gorogh T, Fischer GS, et al. The antileukoprotease secretory leukocyte protease inhibitor (SLPI) and its role in the prevention of HPV-infections in head and neck squamous cell carcinoma. Cancer Lett. 2015 Feb 1;357(1):339–345. doi: 10.1016/j.canlet.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 24.Stanley MA, Winder DM, Sterling JC, Goon PK. HPV infection, anal intra-epithelial neoplasia (AIN) and anal cancer: current issues. BMC Cancer. 2012;12:398. doi: 10.1186/1471-2407-12-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin F, Bower M. Anal intraepithelial neoplasia in HIV positive people. Sex Transm Infect. 2001 Oct;77(5):327–331. doi: 10.1136/sti.77.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicol AF, Fernandes AT, Grinsztejn B, et al. Distribution of immune cell subsets and cytokine-producing cells in the uterine cervix of human papillomavirus (HPV)-infected women: influence of HIV-1 coinfection. Diagn Mol Pathol. 2005 Mar;14(1):39–47. doi: 10.1097/01.pas.0000143309.81183.6c. [DOI] [PubMed] [Google Scholar]
- 27.Nicol AF, Nuovo GJ, Wang Y, et al. In situ detection of SOCS and cytokine expression in the uterine cervix from HIV/HPV coinfected women. Exp Mol Pathol. 2006 Aug;81(1):42–47. doi: 10.1016/j.yexmp.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Nuovo GJ, Forde A, MacConnell P, Fahrenwald R. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol. 1993 Jul;143(1):40–48. [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell CM, Balkus J, Agnew KJ, et al. Bacterial vaginosis, not HIV, is primarily responsible for increased vaginal concentrations of proinflammatory cytokines. AIDS Res Hum Retroviruses. 2008 May;24(5):667–671. doi: 10.1089/aid.2007.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachdeva N, Oshima K, Cotter A, et al. Analysis of immunological markers associated with pregnancy and HIV-1 infection: relevance in perinatal transmission in HIV-1-infected pregnant women with low plasma viral load. Am J Reprod Immunol. 2008 Sep;60(3):264–273. doi: 10.1111/j.1600-0897.2008.00627.x. [DOI] [PubMed] [Google Scholar]
- 31.Morrison C, Fichorova RN, Mauck C, et al. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr. 2014 Jun 1;66(2):109–117. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 32.Nittayananta W, Kemapunmanus M, Yangngam S, Talungchit S, Sriplung H. Expression of oral secretory leukocyte protease inhibitor in HIV-infected subjects with long-term use of antiretroviral therapy. J Oral Pathol Med. 2013 Mar;42(3):208–215. doi: 10.1111/jop.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moutsopoulos NM, Nares S, Nikitakis N, et al. Tonsil epithelial factors may influence oropharyngeal human immunodeficiency virus transmission. Am J Pathol. 2007 Aug;171(2):571–579. doi: 10.2353/ajpath.2007.061006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierce Campbell CM, Guan W, Sprung R, et al. Quantification of secretory leukocyte protease inhibitor (SLPI) in oral gargle specimens collected using mouthwash. J Immunol Methods. 2013 Dec 31;400-401:117–121. doi: 10.1016/j.jim.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pires AR, Andreiuolo Fda M, de Souza SR. TMA for all: a new method for the construction of tissue microarrays without recipient paraffin block using custom-built needles. Diagn Pathol. 2006;1:14. doi: 10.1186/1746-1596-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000 Jan;38(1):357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daling JR, Sherman KJ, Hislop TG, et al. Cigarette smoking and the risk of anogenital cancer. Am J Epidemiol. 1992 Jan 15;135(2):180–189. doi: 10.1093/oxfordjournals.aje.a116270. [DOI] [PubMed] [Google Scholar]
- 38.McNairn AJ, Guasch G. Epithelial transition zones: merging microenvironments, niches, and cellular transformation. Eur J Dermatol. 2011 May;21(Suppl 2):21–28. doi: 10.1684/ejd.2011.1267. [DOI] [PubMed] [Google Scholar]
- 39.Moriyama A, Shimoya K, Ogata I, et al. Secretory leukocyte protease inhibitor (SLPI) concentrations in cervical mucus of women with normal menstrual cycle. Mol Hum Reprod. 1999 Jul;5(7):656–661. doi: 10.1093/molehr/5.7.656. [DOI] [PubMed] [Google Scholar]
- 40.Pushpanshu K, Sathawane RS, Kaushik R. Estimation and comparison of salivary secretory leukocyte protease inhibitor in human immunodeficiency virus patients and healthy individuals. Indian J Palliat Care. 2014 Jan;20(1):26–30. doi: 10.4103/0973-1075.125551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin AL, Johnson DA, Stephan KT, Yeh CK. Salivary secretory leukocyte protease inhibitor increases in HIV infection. J Oral Pathol Med. 2004 Aug;33(7):410–416. doi: 10.1111/j.1600-0714.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 42.Woodham AW, Taylor JR, Jimenez AI, et al. Small molecule inhibitors of the annexin A2 heterotetramer prevent human papillomavirus type 16 infection. J Antimicrob Chemother. 2015 Feb 23; doi: 10.1093/jac/dkv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dreier R, Schmid KW, Gerke V, Riehemann K. Differential expression of annexins I, II and IV in human tissues: an immunohistochemical study. Histochem Cell Biol. 1998 Aug;110(2):137–148. doi: 10.1007/s004180050275. [DOI] [PubMed] [Google Scholar]
- 44.Stier EA, Sebring MC, Mendez AE, Ba FS, Trimble DD, Chiao EY. Prevalence of anal human papillomavirus infection and anal HPV-related disorders in women: a systematic review. Am J Obstet Gynecol. 2015 Sep;213(3):278–309. doi: 10.1016/j.ajog.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schofield AM, Sadler L, Nelson L, et al. A prospective study of anal cancer screening in HIV positive and negative men who have sex with men; results of Analogy. AIDS. 2016 Feb 1; doi: 10.1097/QAD.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 46.Park LS, Hernandez-Ramirez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS. 2016 Jan;30(2):273–291. doi: 10.1097/QAD.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]