Abstract
Biphenotypic sinonasal sarcoma (BSNS) is a recently recognized low-grade sarcoma that exhibits both neural and myogenic differentiation. This unique dual phenotype stems from recurrent rearrangements in PAX3, a transcription factor that promotes commitment along both lineages. While identification of PAX3 rearrangements by fluorescence in situ hybridization (FISH) can confirm a BSNS diagnosis, this assay is not widely available. This study evaluates whether an expanded immunohistochemical panel can facilitate recognition of BSNS without molecular analysis. Eleven cases of BSNS were identified from the surgical pathology archives of two academic medical centers. In 8 cases, the diagnosis was confirmed by FISH using custom probes for PAX3. In 3 cases FISH failed but histologic and immunophenotypic findings were diagnostic for BSNS. All 11 BSNS (100%) were at least focally positive for S100 as well as calponin and/or smooth muscle actin. In addition, 10 of 11 (91%) expressed nuclear β-catenin, 8 of 10 (80%) expressed factor XIIIa, 4 of 11 (36%) expressed desmin, and 3 of 10 (30%) expressed myogenin. All 11 tumors were negative for SOX10. While no single marker resolves immunohistochemical overlap between BSNS and its histologic mimickers such as nerve sheath tumors, an extended immunohistochemical panel that includes β-catenin and SOX10 helps to support the diagnosis of BSNS without the need for gene rearrangement studies.
Keywords: Sinonasal tract, Biphenotypic sinonasal sarcoma, beta-catenin, immunohistochemistry
Background
Biphenotypic sinonasal sarcoma (BSNS) is a recently described low-grade sarcoma that exhibits both neural and myogenic differentiation[1]. BSNS is characterized by infiltrative, hypercellular fascicles of bland spindle cells on histologic sections and consistent immunohistochemical positivity for both S100 and smooth muscle actin (SMA). This unique dual phenotype stems from recurrent rearrangements in PAX3, a transcription factor that normally promotes neural crest and skeletal muscle differentiation[2,3], and is particularly important in the normal development of nasal structures[4]. The original and predominant translocation identified in BSNS is t(2;4)(q35;q31.1), which results in a PAX3-MAML3 fusion protein and activation of PAX3 response elements[5]. Other molecular alterations have also been recently reported in a subset of BSNS, including PAX3-NCOA1 and PAX3-FOXO1 fusions[6,7]. Still other cases of BSNS have PAX3 rearrangements with yet unidentified partners (PAX3-X).
BSNS can pose diagnostic difficulties for pathologists because of its overlap with a broad range of other cellular spindle cell lesions that express either S100 or SMA. Some of the most significant considerations in the differential diagnosis of BSNS include cellular schwannoma, low-grade malignant peripheral nerve sheath tumor (MPNST), leiomyosarcoma, fibrosarcoma, synovial sarcoma (SS), and glomangiopericytoma (GPC). While identification of PAX3 rearrangements by fluorescence in situ hybridization (FISH) can confirm a diagnosis of BSNS, this assay is not available in most diagnostic surgical pathology laboratories. Moreover, if either neural or myogenic differentiation is established immunohistochemically, a presumptive diagnosis may be made along one lineage before the full breadth of differentiation is ever established. In this study, we aim to expand the immunohistochemical profile of BSNS by evaluating additional key markers, including SOX-10, myogenin, β-catenin, and Factor XIIIa in an effort to facilitate the diagnosis of BSNS without the need for molecular analysis.
Materials and Methods
Case selection
This study was approved by The Johns Hopkins Institutional Review Board (IRB00096402). The surgical pathology archives at The Johns Hopkins Hospital were searched for all sinonasal tumors diagnosed as cellular schwannoma, synovial sarcoma, low-grade malignant peripheral nerve sheath tumor, low-grade fibrosarcoma, or low-grade sarcoma, not otherwise specified. All twelve tumors with available slides were reviewed and six cases (50%) were reclassified as BSNS. The original diagnoses were synovial sarcoma (n=2), cellular schwannoma (n=1), low-grade malignant peripheral nerve sheath tumor (n=1), low-grade fibrosarcoma (n=1), and low-grade sarcoma, not otherwise specified (n=1). Additionally, five cases with established diagnoses of BSNS were identified from the surgical pathology files of The Johns Hopkins Hospital and Memorial Sloan-Kettering Cancer Center. Diagnosis of BSNS and inclusion in this study was based on either identification of PAX3 alterations by fluorescent in situ hybridization (FISH) or diagnostic histologic and immunohistochemical findings for BSNS. Four cases included in this study were also included in a previous molecular characterization of BSNS[7]. One case was previously published as a report of synovial sarcoma prior to the description of BSNS[7]. For all cases, relevant demographic, clinical, and follow-up information was tabulated from the electronic medical record and a national mortality registry.
Immunohistochemistry
Immunohistochemistry was performed on all cases, either at the time of original diagnosis or for the purposes this study. Whole-slide sections of formalin-fixed, paraffin embedded tumor tissue were cut at five-micron thickness, deparaffinized, and subjected to antigen retrieval using 10 mM citrate buffer at 92°C for 30 minutes. Immunohistochemistry was performed on all cases using antibodies for S100 (Ventana Medical Systems, Tucson, AZ), either Smooth Muscle Actin (Ventana) or Calponin (Dako, Carpinteria, CA), SOX10 (BioCare Medical, Concord, CA), Desmin (Dako), and β-catenin (BD Biosciences, San Jose, CA). In addition, for a subset of cases (n=6), PAX3 (Bioss Inc., Wolburn, MA) immunohistochemistry was also performed. Depending on tissue availability, immunohistochemistry was also performed in most cases using antibodies for myogenin (Ventana) and Factor XIIIa (Cell Marque, Rocklin, CA). All immunohistochemical signals were visualized using the Ultra view polymer detection kit (Ventana Medical Systems, Inc. Tucson, AZ) on a Ventana BenchmarkXT autostainer (Ventana). Staining was performed according to manufacturer's instructions in the presence of appropriate controls. “Focal” immunoreactivity was regarded as immunostaining in <10% of tumor cells.
Fluorescence in situ hybridization
Custom FISH probes for PAX3, MAML3, and NCOA1 were constructed using bacterial artificial chromosomes (BACs) as previously described[7]. Four-micron whole-slide sections of formalin-fixed, paraffin-embedded tumor tissue were pretreated and hybridized with BAC probes. These slides were then incubated, washed, and mounted with 4',6-diamidino-2-phenylindole (DAPI) in an antifade solution, also as previously described[7]. In each case, two-hundred consecutive nuclei were evaluated for breakapart signals using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany) with Isis 5 software (Metasystems, Newtom, MA). Only nuclei with a complete set of signals were included in scoring. FISH signals were interpreted as positive in cases where at least 20% of nuclei demonstrated break-apart signals. Cases with less than two-hundred nuclei satisfactory for evaluation were considered to be a FISH failure.
Results
Clinical information is summarized in Table 1. Eight of the 11 patients (73%) were females. Patients ranged in age from 33 to 87 years (median, 44 years) at the time of diagnosis. Tumors uniformly involved the upper sinonasal tract, including 4 lesions in the ethmoid sinus, 3 in the frontal sinus, 3 in the nasal cavity, and 1 involving both the nasal cavity and ethmoid sinus. Two patients underwent orbital exenteration due to direct infiltration of the tumor into orbital structures. Of the 7 patients with follow-up information available, five (71.4%) had no evidence of recurrent disease over intervals ranging from 1 month to 26 years (median follow up 4 years). Two patients (28.6%) experienced local recurrences. One of these patients had two recurrences, the second of which extended into the cranial vault. This patient died eight months after the second recurrence due to persistent intracranial tumor.
Table 1.
Clinical Characteristics of Biphenotypic Sinonasal Sarcoma
| Case | Age | Sex | Location | Recurrence | Follow-Up |
|---|---|---|---|---|---|
| 1 | 39 | F | Frontal Sinus | At 2 and 4 years | Died 8 months after second recurrence |
| 2 | 42 | M | Ethmoid Sinus | None | NED for 26 years |
| 3 | 44 | M | Frontal Sinus | None | NED for 11 years |
| 4 | 74 | F | Ethmoid Sinus | NA | NA |
| 5 | 43 | F | Frontal Sinus | None | NED for 2 years |
| 6 | 48 | F | Nasal Cavity/ Ethmoid Sinus |
NA | NA |
| 7 | 70 | F | Ethmoid Sinus | None | NED for 2 months |
| 8 | 37 | F | Upper Nasal Meatus | None | NED for 3 months |
| 9 | 46 | F | Nasal Septum | At 3 years | NED 1 year after recurrence |
| 10 | 87 | M | Ethmoid Sinus | NA | NA |
| 11 | 33 | F | Nasal Cavity | NA | NA |
F: female, M: male, NA: Not available, NED: No evidence of disease
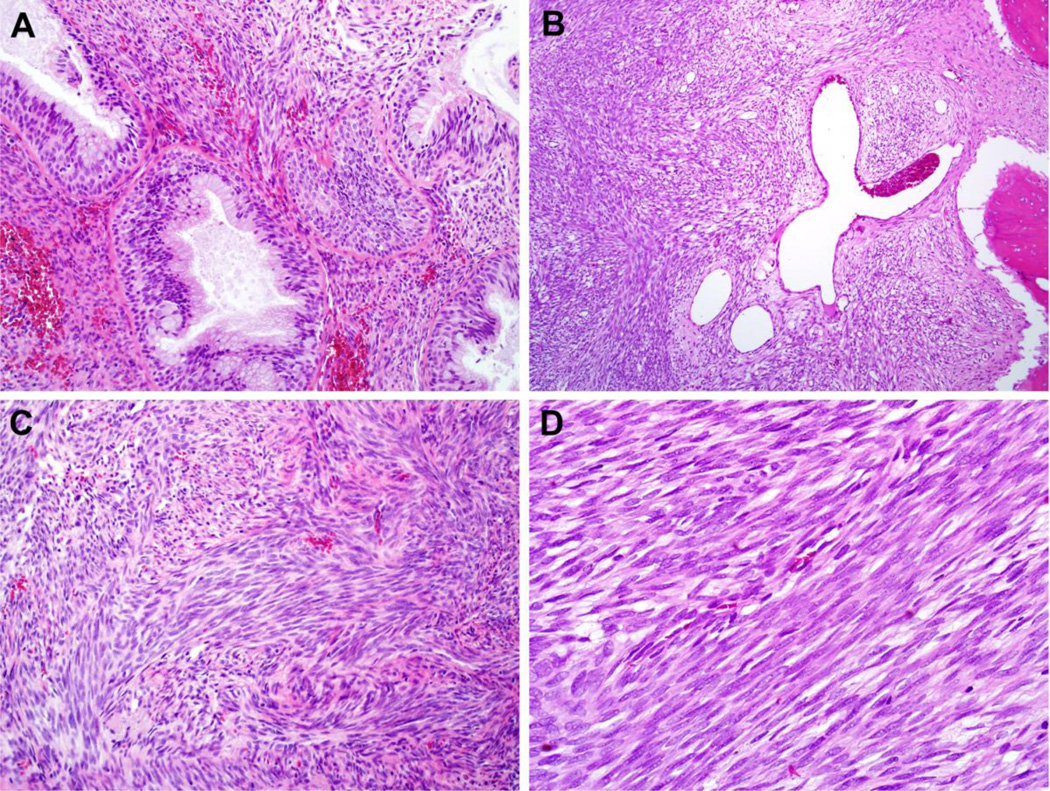
Hematoxylin and eosin (H&E) sections demonstrated poorly-circumscribed tumors with extensive infiltration into surrounding soft tissue and frequent involvement of sinonasal bones. Seven cases had prominent entrapped benign glands lined by proliferations of respiratory epithelium with mucinous metaplasia (Figure 1A). Hemangiopericytoma-like staghorn vessels, often prominent, were seen in nine cases (Figure 1B). The tumors consisted of hypercellular proliferations of monotonous spindle cells arranged in medium to long fascicles with focally prominent herringbone patterns (Figure 1C). Tumor cells had a small amount of eosinophilic cytoplasm with indistinct cytoplasmic borders and scant intracellular collagen depositions. Although it has been described elsewhere in a subset of BSNS[7], overt rhabdomyoblastic differentiation was not a prominent cytologic feature of the tumors in this series. Tumor nuclei were uniform, elongated, and focally wavy with nuclear hypochromasia and finely-granular chromatin (Figure 1D). There was noticeable overlap between adjacent tumor nuclei. Necrosis was absent, and only occasional mitotic figures were identified.
Figure 1.
BSNS frequently demonstrates entrapped benign glands with florid epithelial proliferation (A, X200) as well as prominent staghorn, hemangiopericytoma-like vessels (B, X100). The tumors were composed of dense fascicles of spindle cells with frequent herringbone architecture (C, X200). Tumor cells had a moderate amount of eosinophilic cytoplasm, indistinct cytoplasmic borders, and bland, hypochromatic nuclei with prominent nuclear overlap (D, X400).
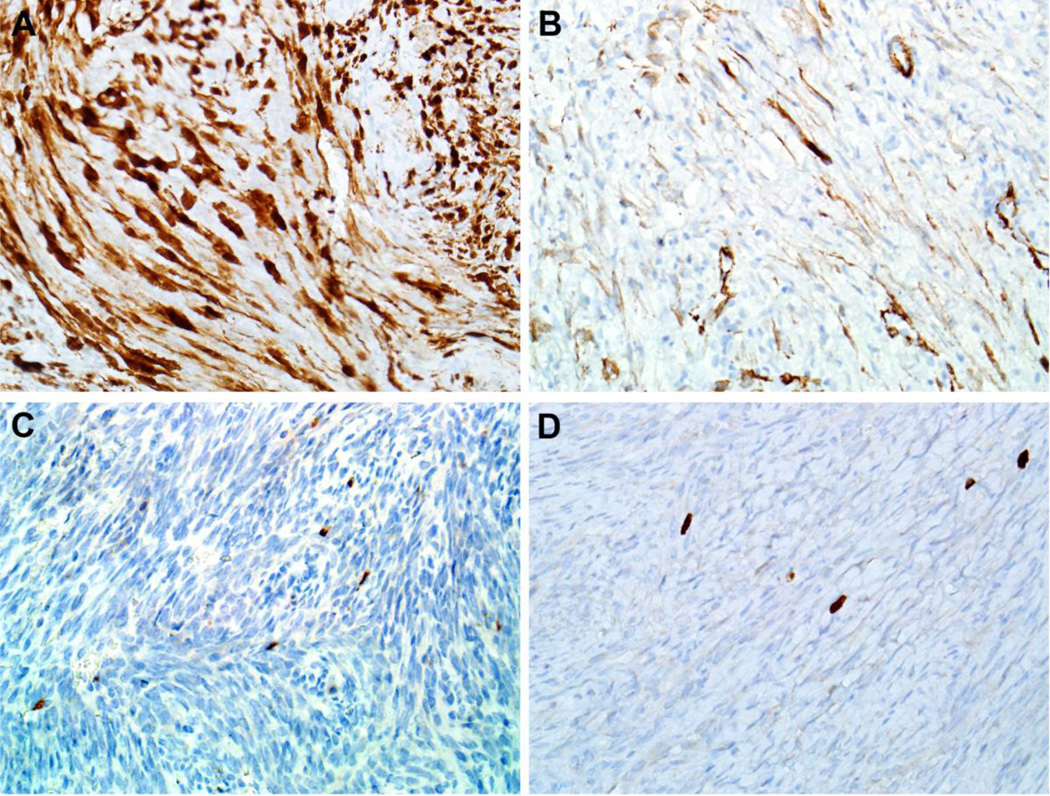
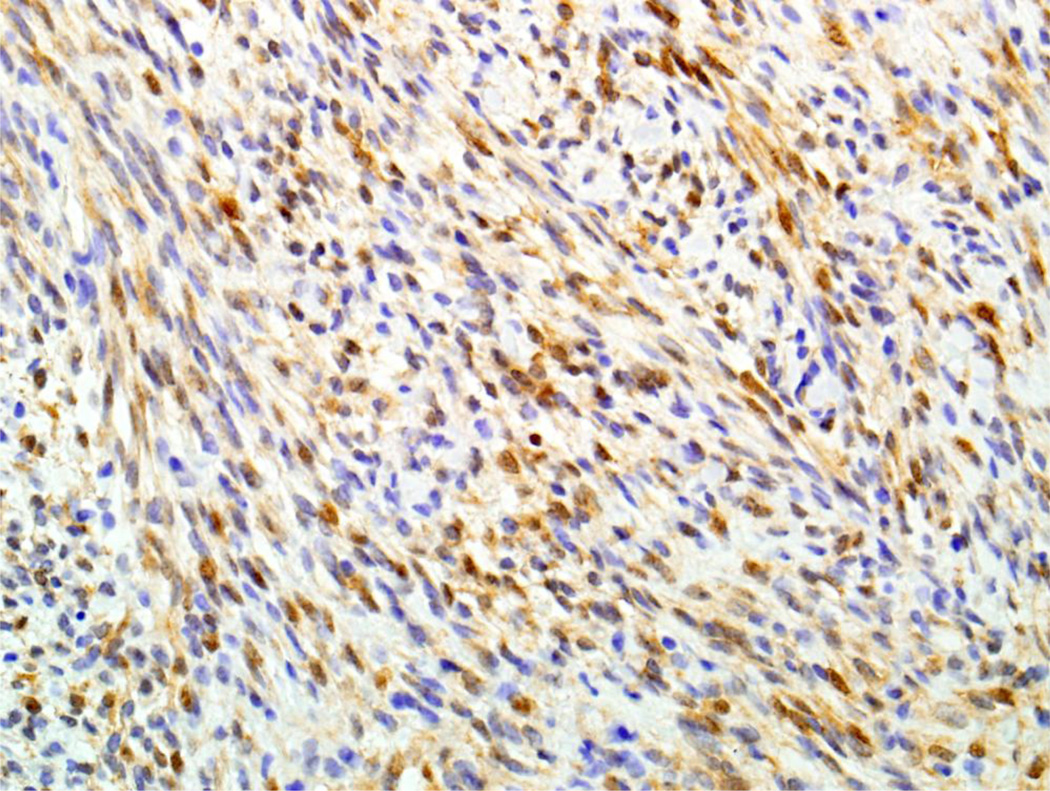
Results of immunohistochemical staining are detailed in Table 2. All 11 cases (100%) demonstrated some degree of both nuclear and cytoplasmic positivity for S100 (Figure 2A). In addition, all 11 cases (100%) also showed variable positivity for at least one smooth muscle marker (SMA or calponin), with only focal staining in 6 cases (Figure 2B). Nuclear β-catenin staining was seen in 10 of 11 (91%) tumors (Figure 3) The β-catenin immunoreactivity was focal in 3 of the positive cases, and in the “non-focal” positives, nuclear staining was seen in 20–70% of tumor cells (mean, 46%) and tended to be weak or moderate in intensity. Additionally, 8 of 10 (80%) tumors tested had focal cytoplasmic positivity for Factor XIIIa, 4 of 11 (36%) showed focal cytoplasmic expression of desmin (Figure 2C), and 3 of 10 (30%) demonstrated focal nuclear staining for myogenin (Figure 2D). None of the 11 tumors showed any expression of SOX10. Immunohistochemistry for PAX3 was noncontributory; in all cases tested, both tumor and normal cells were diffusely positive.
Table 2.
Immunohistochemical Profile of Biphenotypic Sinonasal Sarcoma
| Case | FISH | S100 | SMA or Caponin |
SOX10 | Desmin | Myogenin | β- Catenin |
Factor XIII |
|---|---|---|---|---|---|---|---|---|
| 1 | Failed | F+ | F+ | − | F+ | F+ | F+ | F+ |
| 2 | PAX3- MAML3 |
+ | + | − | F+ | − | + | F+ |
| 3 | PAX3-X | F+ | + | − | − | − | + | − |
| 4 | Tissue unavailable |
+ | F+ | − | − | ND | − | ND |
| 5 | Failed | F+ | F+ | − | − | − | + | F+ |
| 6 | PAX3- MAML3 |
F+ | + | − | − | − | + | − |
| 7 | PAX3- MAML3 |
+ | F+ | − | F+ | F+ | + | F+ |
| 8 | PAX3- NCOA1 |
+ | F+ | − | F+ | F+ | + | F+ |
| 9 | PAX3- MAML3 |
+ | F+ | − | − | − | F+ | F+ |
| 10 | PAX3- MAML3 |
+ | + | − | − | − | + | + |
| 11 | PAX3-X | F+ | + | − | − | − | + | F+ |
ND: Not done, +: Positive, F+: Focally positive (<10% of tumor cells), −: Negative
Figure 2.
All eleven BSNS (100%) demonstrated some degree of S100 positivity (A, X400). Although reactivity was focal in most cases, all BSNS tested were also positive for smooth muscle actin (B, X400). A subset of BSNS demonstrated focal positivity for desmin (C, X400) and myogenin (D, X400).
Figure 3.
Ten of eleven BSNS demonstrated variable degrees of nuclear immunoreactivity for β-catenin.(X400)
FISH results are also summarized in Table 2. PAX3 alterations were identified in all eight cases for which FISH testing could be successfully completed. Five of these cases demonstrated PAX3-MAML3 fusions. One case was positive for a PAX3-NCOA1 fusion. Two other cases demonstrated evidence of PAX3 translocation with an unknown fusion partner (PAX3-X). No FISH results could be obtained in three cases, of which two had insufficient nuclear signals present for scoring and one had insufficient tissue available to attempt testing. As noted above, all three of the cases without FISH showed characteristic histological features of BSNS and immunohistochemical evidence of both neural and myogenic differentiation.
Discussion
BSNS is a recently-described low grade sarcoma that occurs exclusively in the sinonasal tract (both nasal cavity and/or paranasal sinuses, especially ethmoid). It is characterized histologically by a hypercellular proliferation of uniform spindled cells, immunohistochemically by positivity for both S100 and SMA, and molecular genetically by translocations involving the PAX3 gene. BSNS predominantly affects middle-aged (mean, 52 years) women (2:1 ratio). It presents non-specifically as nasal obstruction and facial pressure or pain. BSNS demonstrates slowly progressive growth, with invasion of local structures and frequent recurrences, but no documented metastases. In this series, we present the first documented tumor-related death further supporting the notion that BSNS is best regarded as a low-grade malignant neoplasm (although it is worth noting that this case could not be molecularly confirmed; the FISH for PAX3 failed, possibly due to tissue degradation as the case was over 20 years old).
While identification of PAX3 rearrangements using FISH can confirm the diagnosis of BSNS, this test is not available for most diagnostic pathology laboratories. Moreover, immunohistochemical and morphological overlap between BSNS and other cellular spindle cell neoplasms may lead to an alternate diagnosis being rendered before the possibility of BSNS is even considered. This study evaluated eleven cases of BSNS with an extended immunohistochemical panel including β-catenin, SOX10, myogenin, and Factor XIIIa to facilitate better recognition of BSNS and differentiation from its mimickers without the need for molecular testing.
Nuclear staining for β-catenin has previously been observed in two separate case reports of BSNS[8,9], and positivity in an additional 91% of the cases in this series suggests that it is a consistent finding for BSNS. β-catenin is a terminal component of the Wnt pathway that accumulates in the nucleus upon activation of Wnt signaling. Nuclear expression of β-catenin can be seen on IHC in a specific group of neoplasms that have derangements at various points in the Wnt pathway. Although the exact mechanism for nuclear β-catenin expression in BSNS is not entirely clear, PAX3 is well-established to participate in Wnt signaling in neural crest and myogenic development[10–15]. Indeed, upregulation of Wnt5a, Wnt7b, and Wnt9b family genes has been previously reported in in BSNS[5]. Conceivably, β-catenin nuclear expression in BSNSs could reflect activation of these Wnt family members by PAX3 overexpression. Nuclear accumulation of β-catenin could also reflect targeting of other genetic pathways. One reported case of a BSNS detected an inactivating mutation in CDC73 – a tumor suppressor gene and an important transcriptional regulator of WNT/β-catenin signaling[6]. Whatever the precise mechanism of nuclear accumulation of β-catenin, its presence as a consistent immunohistochemical finding could be a helpful tool in diagnosing BSNS as β-catenin is not typically expressed in peripheral nerve sheath tumors, smooth muscle tumors, fibrosarcomas, and other spindle cell neoplasms that occur in the differential diagnosis of BSNS.
This study also confirms a previous report of uniform negativity for SOX10 in BSNS[16]. SOX10 is a transcription factor that is normally expressed in melanocytes, Schwann cells, oligodendrocytes, myoepithelial cells, and salivary acinar cells as well as neoplasms deriving from these lineages. It has emerged as one of the most sensitive and specific immunohistochemical markers of neural crest differentiation[17]. Despite a seemingly neural phenotype in BSNS, as evidenced by upregulation of several neural development pathways and consistent positivity for S100[5], all BSNS thus far evaluated have been negative for SOX10[7]. This is a relevant finding because most cases of peripheral nerve sheath tumors (essentially 100% of schwannomas and 47–67% of MPNSTs), the tumors most likely to be confused with BSNS, are reported to be SOX-10 positive[17–20].
Immunohistochemistry for PAX3 was also attempted, in an attempt to determine whether the PAX3 rearrangements of BSNS resulted in a detectable alteration of protein expression. In all cases tested, however, both the tumor and normal cells were positive in similar intensity. It is unclear whether these results represent a technical failure or a true lack of diagnostic utility. Notably, published experiences with PAX3 immunohistochemistry are relatively sparse and primarily limited to skin, where the antibody is reported to stain both normal melanocytes and melanoma cells[21,22].
When this and previous studies are taken together, a unique immunoprofile for BSNS emerges (Table 3): it is consistently positive for S100, actin, calponin, β-catenin and factor XIIIa; occasionally positive for desmin, myogenin, cytokeratins and EMA; and never positive for SOX10. While none of these immunohistochemical markers are specific for BSNS by themselves, when used as a panel, BSNS can be separated from its mimickers without the need for molecular diagnostic tools in many cases. For example, while nerve sheath tumors (MPNST and cellular schwannoma) are positive for S100 and can even express muscle markers in the case of malignant Triton tumor, they also express SOX10 in most cases and consistently lack nuclear β-catenin[17–20]. It is notable that previous reports of so-called “low grade” MPNST or “low grade” malignant Triton tumor in the sinonasal tract, in retrospect, almost certainly represent examples of BSNS[23]. Other potential mimickers like fibrosarcoma and leiomyosarcoma are also consistently negative for nuclear β-catenin[24–26]. GPC, also known as sinonasal-type hemangiopericytoma, is a benign neoplasm that demonstrates a perivascular myoid cell phenotype. Like BSNS, GPC is almost always SMA-positive and expresses β-catenin in a nuclear pattern[27,28]. Moreover, GPC is well-established to express Factor XIIIa[27]. The frequent co-positivity for both β-catenin and Factor XIIIa observed in BSNS could therefore support an erroneous diagnosis of GPC. However, GPC almost always lacks S100 expression and is consistently negative for desmin and myogenin[27–29]. Even the patterns of nuclear β-catenin immunoreactivity appear to be different: GPC tends to have very diffuse, strong staining while the staining BSNS is less intense and more focal. The most difficult differential diagnosis from an IHC standpoint is between BSNS and SS, a soft tissue neoplasm defined by a X;18 translocation. Subsets of SS have been reported to express S100 (15–38% of cases)[19,30], SMA (21% of cases)[30], nuclear β-catenin (50–59% of cases)[24,31] and even myogenin (10% of cases)[32]. Conversely, like SS, BSNS is sometimes positive for cytokeratin and EMA[1]. Even TLE1, a relatively sensitive and specific nuclear marker for SS[33], has been reported to have been diffusely positive in a single case of BSNS[6]. Factor XIIIa is only marker that is positive in BSNS in this study that has not been reported as positive in SS- with only a few cases studied[34]. While each of the markers can occasionally be seen in SS, the combined co-expression of S100, actin, β-catenin, and in some cases, desmin and myogenin strongly favor BSNS over SS. It is also important to note that SS is extremely rare in the sinonasal tract, with only ten reported cases overall[35–37], just two of which have molecular confirmation. Moreover, as noted above, at least one case of sinonasal synovial sarcoma (from our institution) [38] was subsequently revealed to be BSNS. Regardless, molecular testing for SS is still available if SS remains a significant diagnostic concern.
Table 3.
Immunohistochemical profiles of biphenotypic sinonasal sarcoma and its histologic mimickers.
| S10 0 |
Acti n |
β- catenin (nuclea r) |
SOX1 0 |
Fact or XIIIa |
Cytokera tin |
Desm in |
Myogen in |
|
|---|---|---|---|---|---|---|---|---|
|
Biphenotypic sinonasal sarcoma |
+ or F+ |
+ or F+ |
+ or F+ | − | F+ | −/F+ | −/F+ | −/F+ |
|
Malignant Peripheral Nerve Sheath Tumor |
F+ | − /F+ |
− | F+/− | −/F+ | −/F+ | −/F+ | |
|
Cellular schwannoma |
+ | − | − | + | − | − | − | − |
| Leiomyosarcoma | − | + | − | − | −/+ | + | − | |
| Fibrosarcoma | − | − | − | − | − | − | − | |
| Glomangiopericytoma | − | + | + | − | + | − | − | − |
| Synovial Sarcoma | −/+ | −/+ | +/− | − | F+ | −/F+ | −/F+ |
+: consistently positive; − : consistently negative; F+: focally positive (<10% of cells); −/F+: usually negative, but sometimes focally positive; F+/−: usually focally positive, but sometimes negative; blank: unknown due to limited data.
In conclusion, BSNS is a novel low-grade sarcoma unique to the sinonasal tract that can pose a difficult differential diagnosis with a wide range of cellular spindle cell neoplasms that express either actin or S100. This study describes an extended immunohistochemical panel that can help facilitate recognition of BSNS and differentiation it from its mimics. The consistent positivity for β-catenin and negativity for SOX10 described here can prove very useful at distinguishing BSNS from low-grade nerve sheath tumors. While none of these stains independently distinguish BSNS from SS, they provide additional points of comparison that, in sum, can help to favor a diagnosis of BSNS. Overall, an expanded panel of immunohistochemical stains that includes β-catenin can help clarify the diagnosis of BSNS without the need for molecular analysis.
Acknowledgments
Funding: This study was funded by the National Institutes of Health/National Institute of Dental and Craniofacial Research Head and Neck SPORE Grant P50 DE019032.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- 1.Lewis JT, Oliveira AM, Nascimento AG, Schembri-Wismayer D, Moore EA, Olsen KD, Garcia JG, Lonzo ML, Lewis JE. Low-Grade Sinonasal Sarcoma with Neural and Myogenic Features: A Clinicopathologic Analysis of 28 Cases. Am J Surg Pathol. 2012;36:517–525. doi: 10.1097/PAS.0b013e3182426886. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham M, Relaix F. The Role of Pax Genes in the Development of Tissues and Organs: Pax3 and Pax7 Regulate Muscle Progenitor Cell Functions. Annu Rev Cell Dev Biol. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- 3.Monsoro-Burq AH. Pax Transcription Factors in Neural Crest Development. Semin Cell Dev Biol. 2015;44:87–96. doi: 10.1016/j.semcdb.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Zalc A, Rattenbach R, Aurade F, Cadot B, Relaix F. Pax3 and Pax7 Play Essential Safeguard Functions against Environmental Stress-Induced Birth Defects. Dev Cell. 2015;33:56–66. doi: 10.1016/j.devcel.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Bledsoe KL, Graham RP, Asmann YW, Viswanatha DS, Lewis JE, Lewis JT, Chou MM, Yaszemski MJ, Jen J, Westendorf JJ, Oliveira AM. Recurrent Pax3-Maml3 Fusion in Biphenotypic Sinonasal Sarcoma. Nat Genet. 2014;46:666–668. doi: 10.1038/ng.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong WJ, Lauria A, Hornick JL, Xiao S, Fletcher JA, Marino-Enriquez A. Alternate Pax3-Foxo1 Oncogenic Fusion in Biphenotypic Sinonasal Sarcoma. Genes Chromosomes Cancer. 2016;55:25–29. doi: 10.1002/gcc.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang SC, Ghossein RA, Bishop JA, Zhang L, Chen TC, Huang HY, Antonescu CR. Novel Pax3-Ncoa1 Fusions in Biphenotypic Sinonasal Sarcoma with Focal Rhabdomyoblastic Differentiation. Am J Surg Pathol. 2016;40:51–59. doi: 10.1097/PAS.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers KA, Han LM, Chiu AG, Aly FZ. Low-Grade Sinonasal Sarcoma with Neural and Myogenic Features--Diagnostic Challenge and Pathogenic Insight. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:e265–e269. doi: 10.1016/j.oooo.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Wong WJ, Lauria A, Hornick JL, Xiao S, Fletcher JA, Marino-Enriquez A. Alternate Pax3-Foxo1 Oncogenic Fusion in Biphenotypic Sinonasal Sarcoma. Genes Chromosomes Cancer. 2015 doi: 10.1002/gcc.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang AG, Papalopulu N, Goulding MD, Kintner C. Expression of Pax-3 in the Lateral Neural Plate Is Dependent on a Wnt-Mediated Signal from Posterior Nonaxial Mesoderm. Dev Biol. 1999;212:366–380. doi: 10.1006/dbio.1999.9319. [DOI] [PubMed] [Google Scholar]
- 11.Brunelli S, Relaix F, Baesso S, Buckingham M, Cossu G. Beta Catenin- Independent Activation of Myod in Presomitic Mesoderm Requires Pkc and Depends on Pax3 Transcriptional Activity. Dev Biol. 2007;304:604–614. doi: 10.1016/j.ydbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Buckingham M, Relaix F. Pax3 and Pax7 as Upstream Regulators of Myogenesis. Semin Cell Dev Biol. 2015;44:115–125. doi: 10.1016/j.semcdb.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 Cooperate to Mediate Fgf8 and Wnt Signals During Xenopus Neural Crest Induction. Dev Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Sasai N, Sasai Y. Neural Crest Determination by Co-Activation of Pax3 and Zic1 Genes in Xenopus Ectoderm. Development. 2005;132:2355–2363. doi: 10.1242/dev.01823. [DOI] [PubMed] [Google Scholar]
- 15.Wiggan O, Hamel PA. Pax3 Regulates Morphogenetic Cell Behavior in Vitro Coincident with Activation of a Pcp/Non-Canonical Wnt-Signaling Cascade. J Cell Sci. 2002;115:531–541. doi: 10.1242/jcs.115.3.531. [DOI] [PubMed] [Google Scholar]
- 16.Huang SC, Ghossein RA, Bishop JA, Zhang L, Chen TC, Huang HY, Antonescu CR. Novel Pax3-Ncoa1 Fusions in Biphenotypic Sinonasal Sarcoma with Focal Rhabdomyoblastic Differentiation. Am J Surg Pathol. 2015 doi: 10.1097/PAS.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miettinen M, McCue PA, Sarlomo-Rikala M, Biernat W, Czapiewski P, Kopczynski J, Thompson LD, Lasota J, Wang Z, Fetsch JF. Sox10--a Marker for Not Only Schwannian and Melanocytic Neoplasms but Also Myoepithelial Cell Tumors of Soft Tissue: A Systematic Analysis of 5134 Tumors. Am J Surg Pathol. 2015;39:826–835. doi: 10.1097/PAS.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang Y, Pekmezci M, Folpe AL, Ersen A, Horvai AE. Diagnostic Utility of Sox10 to Distinguish Malignant Peripheral Nerve Sheath Tumor from Synovial Sarcoma, Including Intraneural Synovial Sarcoma. Mod Pathol. 2014;27:55–61. doi: 10.1038/modpathol.2013.115. [DOI] [PubMed] [Google Scholar]
- 19.Karamchandani JR, Nielsen TO, van de Rijn M, West RB. Sox10 and S100 in the Diagnosis of Soft-Tissue Neoplasms. Appl Immunohistochem Mol Morphol. 2012;20:445–450. doi: 10.1097/PAI.0b013e318244ff4b. [DOI] [PubMed] [Google Scholar]
- 20.Nonaka D, Chiriboga L, Rubin BP. Sox10: A Pan-Schwannian and Melanocytic Marker. Am J Surg Pathol. 2008;32:1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 21.Medic S, Ziman M. Pax3 Expression in Normal Skin Melanocytes and Melanocytic Lesions (Naevi and Melanomas) PloS one. 2010;5:e9977. doi: 10.1371/journal.pone.0009977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plummer RS, Shea CR, Nelson M, Powell SK, Freeman DM, Dan CP, Lang D. Pax3 Expression in Primary Melanomas and Nevi. Mod Pathol. 2008;21:525–530. doi: 10.1038/modpathol.3801019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heffner DK, Gnepp DR. Sinonasal Fibrosarcomas, Malignant Schwannomas, and "Triton" Tumors. A Clinicopathologic Study of 67 Cases. Cancer. 1992;70:1089–1101. doi: 10.1002/1097-0142(19920901)70:5<1089::aid-cncr2820700513>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Ng TL, Gown AM, Barry TS, Cheang MC, Chan AK, Turbin DA, Hsu FD, West RB, Nielsen TO. Nuclear Beta-Catenin in Mesenchymal Tumors. Mod Pathol. 2005;18:68–74. doi: 10.1038/modpathol.3800272. [DOI] [PubMed] [Google Scholar]
- 25.Carlson JW, Fletcher CDM. Immunohistochemistry for Beta-Catenin in the Differential Diagnosis of Spindle Cell Lesions: Analysis of a Series and Review of the Literature. Histopathology. 2007;51:509–514. doi: 10.1111/j.1365-2559.2007.02794.x. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya B, Dilworth HP, Iacobuzio-Donahue C, Ricci F, Weber K, Furlong MA, Fisher C, Montgomery E. Nuclear Beta-Catenin Expression Distinguishes Deep Fibromatosis from Other Benign and Malignant Fibroblastic and Myofibroblastic Lesions. The American journal of surgical pathology. 2005;29:653–659. doi: 10.1097/01.pas.0000157938.95785.da. [DOI] [PubMed] [Google Scholar]
- 27.Thompson LD, Miettinen M, Wenig BM. Sinonasal-Type Hemangiopericytoma: A Clinicopathologic and Immunophenotypic Analysis of 104 Cases Showing Perivascular Myoid Differentiation. Am J Surg Pathol. 2003;27:737–749. doi: 10.1097/00000478-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Tse LL, Chan JK. Sinonasal Haemangiopericytoma-Like Tumour: A Sinonasal Glomus Tumour or a Haemangiopericytoma? Histopathology. 2002;40:510–517. doi: 10.1046/j.1365-2559.2002.01396.x. [DOI] [PubMed] [Google Scholar]
- 29.Terada T, Kato T. Sinonasal-Type Hemangiopericytoma of the Nasal Cavity and Paranasal Sinus. Int J Clin Oncol. 2012;17:169–173. doi: 10.1007/s10147-011-0263-x. [DOI] [PubMed] [Google Scholar]
- 30.Pelmus M, Guillou L, Hostein I, Sierankowski G, Lussan C, Coindre JM. Monophasic Fibrous and Poorly Differentiated Synovial Sarcoma: Immunohistochemical Reassessment of 60 T(X;18)(Syt-Ssx)-Positive Cases. Am J Surg Pathol. 2002;26:1434–1440. doi: 10.1097/00000478-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Horvai AE, Kramer MJ, O'Donnell R. Beta-Catenin Nuclear Expression Correlates with Cyclin D1 Expression in Primary and Metastatic Synovial Sarcoma - a Tissue Microarray Study. Archives of Pathology & Laboratory Medicine. 2006;130:792–798. doi: 10.5858/2006-130-792-CNECWC. [DOI] [PubMed] [Google Scholar]
- 32.Cessna MH, Zhou H, Perkins SL, Tripp SR, Layfield L, Daines C, Coffin CM. Are Myogenin and Myod1 Expression Specific for Rhabdomyosarcoma? A Study of 150 Cases, with Emphasis on Spindle Cell Mimics. Am J Surg Pathol. 2001;25:1150–1157. doi: 10.1097/00000478-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Foo WC, Cruise MW, Wick MR, Hornick JL. Immunohistochemical Staining for Tle1 Distinguishes Synovial Sarcoma from Histologic Mimics. Am J Clin Pathol. 2011;135:839–844. doi: 10.1309/AJCP45SSNAOPXYXU. [DOI] [PubMed] [Google Scholar]
- 34.Nemes Z. Differentiation Markers in Hemangiopericytoma. Cancer. 1992;69:133–140. doi: 10.1002/1097-0142(19920101)69:1<133::aid-cncr2820690124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Salcedo-Hernandez RA, Lino-Silva LS, Luna-Ortiz K. Maxillary Sinus Sarcomas: Epidemiological and Clinicopathological Experience of 25 Years in a National Reference Cancer Center. Indian J Otolaryngol Head Neck Surg. 2014;66:359–364. doi: 10.1007/s12070-012-0522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramaniam MM, Shuen CS, Petersson F. Poorly Differentiated Synovial Sarcoma of the Sphenoid Sinus: Report of the First Case and Review of Synovial Sarcomas of the Sinonasal Tract. Histopathology. 2012;61:1232–1237. doi: 10.1111/j.1365-2559.2012.04340.x. [DOI] [PubMed] [Google Scholar]
- 37.Wong HT, Ho CY, Nazarina AR, Prepageran N. Synovial Sarcoma of the Ethmoidal Sinus. J Laryngol Otol. 2014;128:1022–1023. doi: 10.1017/S0022215114002151. [DOI] [PubMed] [Google Scholar]
- 38.Gallia GL, Sciubba DM, Hann CL, Raman SP, Westra WH, Tufaro AP, Olivi A. Synovial Sarcoma of the Frontal Sinus. Case Report. J Neurosurg. 2005;103:1077–1080. doi: 10.3171/jns.2005.103.6.1077. [DOI] [PubMed] [Google Scholar]