Abstract
Donor killer immunoglobulin-like receptor (KIR) genotypes associate with relapse protection and survival after allotransplantation for acute myelogenous leukemia. We examined the possibility of a similar effect in a cohort of 614 non-Hodgkin lymphoma (NHL) patients receiving unrelated donor (URD) T-cell replete marrow or peripheral blood grafts. Sixty four percent (n=396) of donor-recipient pairs were 10/10 allele HLA-matched; 26% were 9/10 allele matched. Seventy percent of donors had KIR B/x genotype; the others had KIR A/A genotype. NHL patients receiving 10/10 HLA-matched URD grafts with KIR B/x donors experienced significantly lower relapse at 5 years (26%; CI 21–32% vs. 37%; CI 27–46%, p=0.05) compared with KIR A/A donors, resulting in improved 5 year progression-free survival (PFS) (35%; CI 26–44% vs. 22%; CI 11–35%; p=0.007). In multivariate analysis, use of KIR B/x donors associated with significantly reduced relapse risk (RR 0.63, p=0.02) and improved PFS (RR 0.71, p=0.008). The relapse protection afforded by KIR B/x donors was not observed in HLA-mismatched transplants, and was not specific to any particular KIR-B gene. Selecting 10/10 HLA-matched and KIR B/x donors should benefit patients with NHL receiving URD allogeneic transplantation.
Keywords: non-Hodgkin lymphoma, KIR, allogeneic transplantation, genotype, NK cells
Introduction
Allogeneic hematopoietic cell transplantation (HCT) can cure non-Hodgkin lymphoma (NHL) through the combination of chemotherapy and immune mediated graft-versus-lymphoma (GvL) responses.[1] Long-term survival ranges from 30 to 70%, relapse being the major cause of treatment failure for all NHL histologic subtypes.[2,3] The mechanisms of tumor escape from GvL are poorly understood, but analyses of patients with acute myeloid leukemia (AML) after unrelated donor (URD) HCT reveal the importance of donor killer-cell immunoglobulin-like receptor (KIR) genotype in effective GvL responses.[4–6] NK cells reconstitute promptly after HCT, and express inhibitory KIRs that interact with class I HLA-C1 (ligand for KIR2DL2/3), HLA-C2 (ligand for 2DL1) and HLA-Bw4 epitopes (ligand for 3DL1) to regulate NK cell education and function.[7] NK cells also can express activating KIRs 2DS1, 2DS2, 2DS3 and 2DS5 to co-regulate antitumor effects by binding to HLA-C2 (2DS1) or neo-ligands on tumor cells. Individuals vary in the number of KIR genes contained in their genome. KIR genes are closely linked on chromosome 19q and inherited as haplotype A or B from each parent. The main difference between group A and B haplotypes is that group B contains variable numbers of activating KIR genes, while group A has a fixed gene content of inhibitory but no activating KIR. About 70% of the population has at least one KIR B haplotype. The haplotypes combine to give the A/A and B/x (A/B or B/B) genotypes.[8] The KIR B genotype can be further defined by a KIR B content score determined by the number of centromeric and telomeric motifs containing B-haplotype defining genes (permissible values 0–4). HLA and KIR genes segregate on different chromosomes (6 and 19) and are inherited independently. Although donor selection is guided by HLA matching, we hypothesized that donor KIR interactions with recipient HLA might influence clinical outcomes. Our previous studies showed that KIR B/x donors, and not KIR A/A donors improve leukemia-free survival in AML.[4–6] The impact of KIR polymorphism on relapse and survival of patients with NHL after allo-grafting is unknown. In the current study, we investigated NHL patients receiving allogeneic URD HCT to determine the influence of donor KIR genotype and individual KIR B genes on clinical outcomes.
Patients and Methods
We studied 614 adults (age >18 years) with NHL who underwent T-cell replete URD HCT between 1990 and 2009 facilitated by the National Marrow Donor Program (NMDP). The outcome data were collected at the Center for International Blood and Marrow Transplant Research (CIBMTR). The study protocol was approved by the Institutional Review Board of the NMDP in accordance with the Declaration of Helsinki. Stored donor samples were obtained from the CIBMTR Research Repository and genotyped for KIR.[9] KIR gene content was assessed, allowing each donor to be designated as either KIR A/A or B/x genotype.
Statistical Analysis
Progression-free survival (PFS) and overall survival (OS) were evaluated with Kaplan-Meier estimates.[10] Relapse, non-relapse mortality (NRM) and acute graft-versus-host disease (GVHD) were evaluated using the cumulative incidence function. Clinical variables were tested for the proportional hazard assumption and were adjusted as needed through stratification. Stepwise forward-backward selection was performed to build multivariate Cox proportional hazards models with a threshold of 0.05 for model entry. Donor KIR genotype, the primary variable of interest was forced into the model and adjusted for clinical variables. Other clinical variables analyzed were donor source, GVHD prophylaxis, conditioning regimen, HLA-match, time from diagnosis to transplant, histology group, disease status, in vivo T-cell depletion, age and KPS. To adjust for multiple testing, variable with p<0.01 were considered statistically significant.
Results
Patients, disease and transplant characteristics
The ages of the 614 NHL patients ranged from 19–72 with a median of 50 years. Follicular lymphoma was the most common histology, followed by mantle cell lymphoma, diffuse large B-cell lymphoma and T cell NHL; Burkitt/lymphoblastic lymphomas were excluded (Table 1). Almost all patients were Caucasians and 62% had chemosensitive lymphoma prior to transplant. Most patients were at least 1.5 years from diagnosis to transplant, 41% received myeloablative conditioning regimens and 63% received filgrastim mobilized peripheral blood stem cell (PBSC) grafts. The donor KIR genotype frequencies reflected those of a general Caucasian population; 30% were KIR A/A (n=183) and 70% were KIR B/x (n=431) with KIR-B content scores of 1 (n=243), 2 (n=140) or ≥3 (n=48). We found no correlation between KIR B/x and donor ethnicity (Caucasian vs other 71% vs 68%; p=0.4). Two-thirds of the donor-recipient pairs were 10/10 allele matched at HLA-A, -B, -C, -DRB1 and -DQB1 (n=396); the rest were 1 (n=158) or ≥2 HLA allele (n=60) mismatched. Fully matched recipients were older (52 versus 48 years, p=0.0053), more of them received reduced intensity conditioning (RIC) (62% versus 52%, p=0.011), and received PBSC grafts (69% versus 53%, p=0.0003) vs HLA mismatched transplant recipients. There were no significant differences for other clinical variables (Table 1). We than compared 10/10 HLA matched donor-recipient pairs by donor KIR genotype and found similar patient and graft characteristics in patients with KIR A/A vs KIR B/x donors (Table 1).
Table 1.
Patient, Disease and Transplant Characteristics: HLA mismatched and HLA matched Cohort. HLA-matched donor HCTs are compared by donor KIR genotype.
| Category | HLA Mismatch n=218 |
HLA Match n=396 |
P-value | HLA Match | P-value | |
|---|---|---|---|---|---|---|
|
KIR A/A n=115 |
KIR B/x n=281 |
|||||
|
| ||||||
| Age | 0.0053 | 0.079 | ||||
|
| ||||||
| Median (years) | 48 | 52 | 49 | 52 | ||
|
| ||||||
| Race | 0.0017 | 0.57 | ||||
|
| ||||||
| Caucasian | 194 (89%) | 380 (96%) | 112 (97%) | 268 (95%) | ||
|
| ||||||
| Karnofsky Score | 0.055 | |||||
|
| ||||||
| 90–100 | 131 (60%) | 264 (67%) | 73 (63%) | 191 (68%) | 0.64 | |
|
| ||||||
| ≥80 | 75 (35%) | 101 (25%) | 33 (29%) | 68 (24%) | ||
|
| ||||||
| Missing | 12 (5%) | 31 (8%) | 9 (8%) | 22 (8%) | ||
|
| ||||||
| Lymphoma subset | 0.060 | |||||
|
| ||||||
| Follicular lymphoma | 60 (28%) | 113 (29%) | 36 (30%) | 77 (27%) | 0.91 | |
|
| ||||||
| Diffuse Large B-cell | 36 (17%) | 65 (16%) | 17 (15%) | 48 (17%) | ||
|
| ||||||
| Mantle cell lymphoma | 28 (13%) | 81 (21%) | 22 (20%) | 59 (21%) | ||
|
| ||||||
| T cell lymphoma | 26 (12%) | 60 (15%) | 18 (15%) | 40 (15%) | ||
|
| ||||||
| Other non-Hodgkin lymphoma | 68 (30%) | 77 (19%) | 22 (20%) | 57 (20%) | ||
|
| ||||||
| Chemosensitivity | 0.58 | 0.31 | ||||
|
| ||||||
| Chemosensitive Relapse/PIF | 86 (39%) | 155 (39%) | 40 (35%) | 115 (41%) | ||
|
| ||||||
| Untreated Relapse/PIF | 21 (10%) | 27 (7%) | 9 (8%) | 18 (6%) | ||
|
| ||||||
| Complete Remission# | 47 (22%) | 97 (25%) | 25 (22%) | 72 (26%) | ||
|
| ||||||
| Chemoresistant Relapse/PIF& | 64 (29%) | 117 (29%) | 41 (35%) | 76 (27%) | ||
|
| ||||||
| CMV Serostatus | 0.18 | 0.25 | ||||
|
| ||||||
| Donor −/recipient− | 71 (33%) | 133 (34%) | 37 (32%) | 96 (34%) | ||
|
| ||||||
| Donor + | 78 (36%) | 117 (29%) | 30 (26%) | 87 (31%) | ||
|
| ||||||
| Donor −/recipient+ | 64 (29%) | 141 (36%) | 48 (42%) | 93 (33%) | ||
|
| ||||||
| Unknown | 5 (2%) | 5 (1%) | 0 | 5 (2%) | ||
|
| ||||||
| Conditioning Intensity | 0.011 | 0.77 | ||||
|
| ||||||
| Myeloablative | 104 (48%) | 149 (38%) | 42 (37%) | 107 (38%) | ||
|
| ||||||
| Reduced Intensity | 114 (52%) | 247 (62%) | 73 (63%) | 174 (62%) | ||
|
| ||||||
| GVHD Prophylaxis | 0.63 | 0.15 | ||||
|
| ||||||
| Tacrolimus + MMF | 37 (17%) | 72 (18%) | 27 (23%) | 45 (16%) | ||
|
| ||||||
| Tacrolimus +/− others | 102 (47%) | 199 (50%) | 54 (47%) | 145 (51%) | ||
|
| ||||||
| CSA containing | 76 (35%) | 122 (31%) | 32 (28%) | 90 (32%) | ||
|
| ||||||
| Other prophylaxis | 3 (1%) | 3 (1%) | 2 (2%) | 1 (1%) | ||
|
| ||||||
| In vivo T cell depletion | 0.68 | 0.64 | ||||
|
| ||||||
| No | 149 (89%) | 282 (81%) | 80 (69%) | 202 (72%) | ||
|
| ||||||
| Yes | 69 (11%) | 114 (19%) | 35 (31%) | 79 (28%) | ||
|
| ||||||
| HLA match-A, B, C, DRB1 and DQB11,2 | <0.0001 | |||||
|
| ||||||
| 10/10 allele matched | 0 (0%) | 396 (100%) | 115 (100%) | 281 (100%) | 0.32 | |
|
| ||||||
| 9/10 | 158 (72%) | 0 | ||||
|
| ||||||
| 8/10 | 44 (20%) | 0 | ||||
|
| ||||||
| <8/10 | 16 (7%) | 0 | ||||
|
| ||||||
| Year of Transplant | <0.0001 | 0.32 | ||||
|
| ||||||
| 1990–2000 | 61 (28%) | 55 (14%) | 17 (15%) | 38 (13%) | ||
|
| ||||||
| 2001–2005 | 90 (41%) | 164 (41%) | 41 (35%) | 123 (44%) | ||
|
| ||||||
| 2006–2009 | 67 (31%) | 177 (45%) | 57 (50%) | 120 (43%) | ||
|
| ||||||
| Interval from diagnosis to transplant | 0.40 | 0.49 | ||||
|
| ||||||
| <= 1.5 years | 93 (43%) | 155 (39%) | 42 (37%) | 113 (40%) | ||
|
| ||||||
| > 1.5 years | 125 (57%) | 241 (61%) | 73 (63%) | 168 (60%) | ||
|
| ||||||
| Graft type | 0.0003 | 0.36 | ||||
|
| ||||||
| Bone marrow | 103 (47%) | 124 (31%) | 40 (35%) | 84 (30%) | ||
|
| ||||||
| PBSC | 115 (53%) | 272 (69%) | 75 (65%) | 195 (70%) | ||
|
| ||||||
| Donor KIR genotype$ | 0.69 | |||||
|
| ||||||
| KIR A/A | 68 (31%) | 115 (30%) | 115 (100%) | 0 | ||
|
| ||||||
| KIR B/x | 150 (69%) | 281 (70% | 0 | 281 (100%) | ||
Complete remission 1 (CR1) included MCL (n=19); peripheral T cell (n=18); FL (n=7); DLBCL (n=9), composite lymphoma (n=2).
PIF included FL (n=19); DLBCL (n=18); immunoblastic NHL (n=4); T cell lymphoma and other (n=27); MCL (n=7)
15 KIR genes typed: 2DL1, 2DL2, 2DL3, 2DL4, 2DL5, 2DP1, 2DS1, 2DS2, 2DS3, 2DS4, 2DS5, 3DL1, 3DL2, 3DL3, 3DP1, 3DS1,
Impact of KIR genetics on transplant outcomes
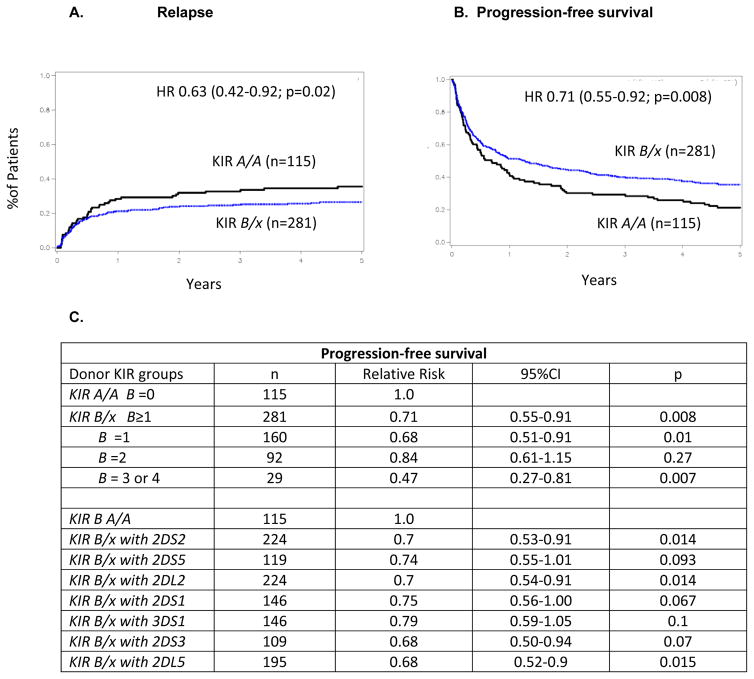
In the 10/10 HLA-matched HCT cohort (n=396), KIR B/x donor grafts resulted in less relapse at 5 years after transplantation (26% [95% CI 21–32%]) compared to KIR A/A donors (37% [27–46%]; p=0.05). This relapse protection translated into improved PFS (KIR B/x 35% [95% CI 26–44] versus (KIR A/A 22% [95% CI 11–35%]; p=0.007) (Figure 1A/B). After adjusting for important clinical variables, KIR B/x donors conferred significant protection against relapse (HR 0.63 [95% CI 0.43–0.92]; p=0.02) and improved PFS (HR 0.71 [95% Cl 0.55–0.91]; p=0.008) compared to KIR A/A donors. In evaluating the protection conferred by individual genes of the KIR B haplotype (KIR2DS2, 2DS5, 2DL2, 2DS1, 3DS1, 2DS3, and 2DL5), we found that each KIR gene was associated with a similar degree of protection against relapse (RRs 0.68–0.79; Figure 1C). Thus individual centromeric and telomeric KIR B genes had similar influences on transplant outcomes and donor with ≥3 KIR B genes conferred the best PFS compared to KIR A/A donors (RR 0.47; 95%CI 0.27–0.81; p=0.007). The protective effect of KIR B/x donors was not observed for our cohort of HLA-mismatched transplants (RR 1.49 [95% CI 0.87–2.55; p = NS). Donor KIR genotypes had no effect on 1 year non-relapse mortality (KIR B/x vs KIR A/A Hazard ratio (HR) 0.8 (95% CI 0.55–1.11); p=0.17), grade II-IV acute GVHD (HR 1.06; 95%CI 0.82–1.38; p=0.67) or chronic GVHD (HR 0.91; 95%CI 0.71–1.15; 0.42). Despite the effect on PFS, HCT recipients had similar OS when transplanted with KIR B/x versus KIR A/A donors (10/10 HLA matched cohort: HR 0.8 (95%CI 0.61–1.06); p=0.12; entire population: HR 0.9 (95%CI 0.74–1.1); p=0.4)). This likely reflects the growing number of immune options available to patients after receipt of an allograft
Figure 1. KIR B/x donors confer relapse protection and superior progression-free survival for NHL patients receiving 10/10 HLA-matched unrelated donor HCT.
Adjusted cumulative incidence curve for relapse (A) and Kaplan Meier curve for progression-free survival (B) are shown for transplants involving donors of different KIR genotype (A/A vs. B/x). (C) The table lists the relative risks [RR] for PFS given by multivariate models that compared KIR A/A to KIR B/x donors based on their B gene content (1, 2, and 3 or 4 KIR B content elements). Also shown are comparisons between different subsets of KIR B/x donors including each of the 7 KIR B genes. Most donors with KIR B/x genotypes have more than one KIR B gene.
Clinical factors affecting transplant outcomes
Main factor associated with improved OS of entire population (n=614) in adjusted multivariate regression was RIC conditioning (HR 0.57 [95% CI 0.42–0.77]; p=0.0008). Shorter OS was associated with chemotherapy-resistant disease (HR 1.6 [95% CI 1.08–2.40]; p=0.02), histology other than follicular lymphoma (HR 1.74–2.06; p=0.0001), and using ≥2 locus HLA-mismatched donors (≤8/10 match HR 1.46 [95%CI 1.06–2.01]; p=0.02; 9/10 match HR 1.09 [95%CI 0.81–1.47; p=0.57). TRM was better with RIC conditioning (HR 0.6; 95% CI 0.5–0.8; p=0.001) and follicular lymphoma histology (HR 0.5; p=0.03) and was not influenced by 9/10 HLA match, GVHD prophylaxis and KIR status (KIR B/x HR 0.97; 95%CI 0.74–1.27; p= 0.81). HCT using donors with ≥2 locus HLA-mismatch had increased TRM (HR 1.5 (95%CI 0.99–2.26; p=0.056). Factors associated with increased relapse were chemotherapy resistance (HR 1.57; p=0.001), in vivo T cell depletion (HR=1.53; p=0.006), histology other that follicular lymphoma (HRs1.66–1.88; p=0.02) and ≥2 locus HLA mismatch (≤8/10 match HR 1.8, p=0.016; 9/10 match HR 1.19; p=0.3). There were no interactions between the in vivo T cell depletion and <10/10 HLA mismatch. Adjusted incidence of grade III-IV acute GVHD was reduced with tacrolimus-other (mostly MTX) GVHD prophylaxis (HR 0.64 [HR 0.38–1.07] compared to tacrolimus-MMF (HR 1.0) and CSA-containing regimen (HR 1.19 [0.71–1.99; overall p=0.012). Use of tacrolimus-other and CSA-based GVHD prophylaxis resulted to similar OS (HR 0.6 [0.46–0.8]) and HR 0.72 [0.53–0.97]). Overall mortality was increased after tacrolimus-MMF combination (HR 1.0; p=0.0014). In adjusted multivariate regression, the 10/10 HLA-matched cohort KIR B/x donors were associated with improved PFS and less relapse. Chemo-resistance, lymphoma histology other than follicular lymphoma and <1.5 years from diagnosis to transplant resulted in inferior PFS (Table 2). Relapse was increased by chemo-resistance, shorter time to HCT and use of in vivo T cell depletion (Table 2).
Table 2.
Multivariate analysis for PFS and relapse for patients undergoing 10/10-matched unrelated donor HCT for NHL.
| Variable | Hazard ratio | 95% confidence intervals | p-value |
|---|---|---|---|
| Progression free survival | |||
| KIR genotype | 0.0075 | ||
| KIR A/A | 1.0 | ||
| KIR B/x | 0.71 | 0.55–0.91 | |
| Disease status | 0.002 | ||
| Chemo sensitive | 1.0 | 0.87–2.83 | |
| Chemo resistant | 1.41 | 0.84–2.35 | |
| Complete Remission | 0.74 | 0.43–1.27 | |
| Lymphoma subset | 0.014 | ||
| Follicular lymphoma | 1.0 | ||
| Diffuse large B cell | 1.68 | 1.14–2.48 | |
| Mantle cell lymphoma | 1.61 | 1.12–2.3 | |
| T cell lymphoma +other | 1.62 | 1.16–2.3 | |
| Interval from Dx to HCT | 0.013 | ||
| ≤ 1.5 year | 1.0 | ||
| >1.5 year | 0.73 | 0.56–0.94 | |
| Relapse rate | |||
| KIR genotype | 0.018 | ||
| KIR A/A | 1.0 | ||
| KIR B/x | 0.63 | 0.43–0.92 | |
| Disease status | 0.039 | ||
| Chemo sensitive | 1.0 | ||
| Chemo resistant | 1.08 | 0.53–2.2 | |
| Complete remission | 0.51 | 0.24–1.09 | |
| T cell depletion in vivo | 0.0037 | ||
| No | 1.0 | ||
| Yes | 1.75 | 1.20–2.55 | |
| Interval from Dx to HCT | |||
| ≤ 1.5 year | 1.0 | 0.0003 | |
| >1.5 year | 0.49 | 0.33–0.72 | |
Discussion
The importance of donor KIR genotypes has been mainly studied in AML where benefit has been reported using haploidentical or matched URD donors. [4–6,11–14]. Here we report a similar benefit conferred by KIR B/x donors after 10/10 HLA-matched URD HCT for mature lymphoid malignancies. Using fully HLA-matched KIR B/x donors lowered relapse by 11% and resulted in significantly better PFS in NHL patients. These effects can potentially be explained by augmented cytolytic function and graft-versus-lymphoma alloreactivity of donor NK cells containing activating KIR. This is consistent with findings in AML by our group and others, as well as new findings in pediatric ALL. [3,6,14–20] While KIR B/x donors conferred significant improvement in PFS and relapse, we noted lack of impact on OS which likely reflects the efficacy of post-transplant therapies and effective immune interventions used in NHL patients who experience post-transplant relapse.[21]
Graft versus tumor effects are delivered by both NK cell and T cell responses against residual malignancy.[15] It is also clear that differential NK cell and T cell susceptibility is governed by HLA class I expression, NK cell receptor repertoires, and NK cell receptor ligands on targets. The balance between donor-derived T and NK cells may regulate the relative anti-tumor response between these cell types. NK cell alloreactivity in a transplant setting was first recognized in AML patients in absence of T cells with HLA-haploidentical donors and grafts prepared with CD34 selection.[16] The impact of genetic polymorphisms of KIR was subsequently reported by several groups showing protective effects of donor KIR B/x genotype in T-cell replete URD HCT in adult AML but not ALL.[3,6,14] The favorable influence of specific activating KIR2DS1 or KIR2DS2 on transplant outcomes in AML was confirmed by several groups in different transplant settings [12,14,17], but data on the benefit of KIR in lymphoid malignancies is scarce. After URD HCT for AML, we showed that donors with increasing number of KIR B defining motifs (≥2) contribute to the protective effect. However, in NHL, we find that a donor KIR gene content score of at least 1 is protective. The protective effect is enhanced ≥3 KIR B defining motifs, and is not limited to any one specific activating KIR gene.
Many reports showed that clinical impact of KIR genetics differ between transplant procedures. In some reports, activating KIR genes have no effect on relapse, yet do result in lower TRM and improved OS using sibling donors.[18,13] The difference between AML and ALL outcomes may be a result of more pronounced HLA-C and HLA-B downregulation on AML and pediatric ALL blasts than adult ALL blasts potentially causing resistance again NK cell mediated cytolysis.[20] Similarly to pediatric ALL, lymphomas variably down regulate HLA class I molecules, particularly HLA-B and C, which engage inhibitory KIR on alloreactive NK cells.[22–24] For example EBV-transformed B-cell lines which completely lack HLA class I are particularly sensitive to NK mediated killing.[25] Indeed, relapse protection, irrespective of disease, perhaps combines cancer mediated downregulation of inhibitory HLA class I alleles with expression of potential neo-ligands for activating KIR on NK cells from KIR B/x donors.[26] This will require detailed study as most of these ligands are not yet established.
The finding is that the benefit of a KIR B/x donor in NHL is limited to the HLA-matched URD HCT setting was unexpected. While it is plausible that altered reconstitution of alloreactive T cells versus NK cells may be dependent on HLA matching, it must be noted that recipients of HLA-mismatched grafts also more frequently received myeloablative conditioning and bone marrow grafts which can alter immune reconstitution, and matched grafts were more often T cell depleted in vivo. It is possible that alloreactive T cells dominant in HLA mismatched URD HCT for NHL mediate GvL, masking a smaller NK cell mediated effect. In contrast, an HLA matched donor with less HLA differences may have less T cell GvL and increase the importance of KIR B/x donor-mediated NK cells in this setting.
Our study confirmed the importance of established favorable prognostic factors for survival such as chemosensitive disease and follicular lymphoma histology. Other notable variables impacting OS in this series were RIC and adverse impact of tacrolimus-MMF GVHD prophylaxis. While RIC conferred a major reduction in TRM leading to improved survival; this contrasts with several prior studies analyzing mixed NHL histologies in which RIC also resulted to higher relapse rates for more aggressive NHL and offset the survival benefit.[27,28] These discrepancies likely reflect the disease and patient heterogeneity of respective cohorts. Recipients of ≤8/10 matched grafts experienced higher relapse rate; however these 60 patients more often had aggressive NHL including T cell and NK cell lymphoma (50% vs 34%) compared to matched donor HCT and given small size of this subset, this data requires caution in inferring conclusions. Another interesting finding is the impact of GVHD regimen on survival. While survival after tacro-other (mostly MTX) and CSA-based combinations was similar, tacrolimus-MMF combination was associated with increased grade 3–4 acute GVHD and inferior survival. Inferior efficacy of tacrolimus-MMF may be explained by inadequate MMF levels; indeed data on MMF dosing and pharmacokinetics with tacrolimus is limited and warrants future investigations.[29,30]
In conclusion, our data suggest that patients with lymphoma benefit with relapse protection after HLA-matched URD HCT when using donors with KIR B/x haplotypes. This effect is broadly seen with the presence of an activating KIR gene and is not limited to a specific KIR. While prospective validation is merited, selecting a KIR B/x donor from amongst available HLA 10/10 allele-matched donors should benefit NHL patients in whom allografting with an unrelated donor is the best treatment option.
Highlights.
Donor KIR genetics influences graft-versus-lymphoma responses.
HLA-matched KIR B/x donors improve progression free survival.
KIR B/x donors benefit non-Hodgkin lymphoma patients undergoing matched unrelated transplantation.
Acknowledgments
Research reported in this publication was supported by the National Center for Advancing Translational Sciences and the National Institutes of Health Award Number UL1TR000114 (VB) and NCI P01 111412 (DJW, TW, SGEM, ET, MH, SRS, LAG, ML, PP, JSM, SC). This work was supported in part by NIH P30 CA77598 utilizing the Masonic Cancer Center, University of Minnesota Oncology Medical Informatics and Services shared resource. The CIBMTR (TW, MH and SRS) is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and others. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Financial Disclosure:
The authors have nothing to disclose.
Conflict of Interests:
The authors report no relevant conflicts of interest.
Authorship Contributions:
V.B., D.J.W, J.S.M and S.C. contributed to the study design, analyzed and interpreted data and wrote the manuscript. S.R.S and M.D.H contributed to the study design, sample procurement, data management and manuscript editing. T.W. performed the biostatistical analyses for the study. E.T and M.L. performed KIR genotyping. S.G.E.M, L.A.G, and P.P. reviewed and edited the manuscript.
References
- 1.Maloney DG. Graft-vs.-lymphoma effect in various histologies of non-Hodgkin’s lymphoma. Leuk Lymphoma. 2003;44(Suppl 3):S99–105. doi: 10.1080/10428190310001623694. [DOI] [PubMed] [Google Scholar]
- 2.Bachanova V, Burns LJ, Wang T, et al. Alternative donors extend transplantation for patients with lymphoma who lack an HLA matched donor. Bone Marrow Transplant. 2015;50(2):197–203. doi: 10.1038/bmt.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Besien K, Carreras J, Bierman PJ, et al. Unrelated donor hematopoietic cell transplantation for non-hodgkin lymphoma: long-term outcomes. Biol Blood Marrow Transplant. 2009;15(5):554–563. doi: 10.1016/j.bbmt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor Killer Cell Ig-like Receptor B Haplotypes, Recipient HLA-C1, and HLA-C Mismatch Enhance the Clinical Benefit of Unrelated Transplantation for Acute Myelogenous Leukemia. J Immunol. 2014;192(10):4592–4600. doi: 10.4049/jimmunol.1302517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parham P. The genetic and evolutionary balances in human NK cell receptor diversity. Semin Immunol. 2008;20(6):311–316. doi: 10.1016/j.smim.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houtchens KA, Nichols RJ, Ladner MB, et al. High-throughput killer cell immunoglobulin-like receptor genotyping by MALDI-TOF mass spectrometry with discovery of novel alleles. Immunogenetics. 2007;59(7):525–537. doi: 10.1007/s00251-007-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Amer Statist. 1958;53(282):457–481. [Google Scholar]
- 11.Giebel S, Nowak I, Wojnar J, et al. Impact of activating killer immunoglobulin-like receptor genotype on outcome of unrelated donor-hematopoietic cell transplantation. Transplant Proc. 2006;38(1):287–291. doi: 10.1016/j.transproceed.2005.11.091. [DOI] [PubMed] [Google Scholar]
- 12.Venstrom JM, Pittari G, Gooley TA, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367(9):805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105(12):4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook MA, Milligan DW, Fegan CD, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004;103(4):1521–1526. doi: 10.1182/blood-2003-02-0438. [DOI] [PubMed] [Google Scholar]
- 15.Gress RE, Miller JS, Battiwalla M, et al. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: Part I. Biology of relapse after transplantation. Biol Blood Marrow Transplant. 2013;19:1537–1545. doi: 10.1016/j.bbmt.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 17.Verheyden S, Schots R, Duquet W, Demanet C. A defined donor activating natural killer cell receptor genotype protects against leukemic relapse after related HLA-identical hematopoietic stem cell transplantation. Leukemia. 2005;19:1446–1451. doi: 10.1038/sj.leu.2403839. [DOI] [PubMed] [Google Scholar]
- 18.Chen DF, Prasad VK, Broadwater G, et al. Differential impact of inhibitory and activating Killer Ig-Like Receptors (KIR) on high-risk patients with myeloid and lymphoid malignancies undergoing reduced intensity transplantation from haploidentical related donors. Bone Marrow Transplant. 2012;47:817–823. doi: 10.1038/bmt.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verheyden S, Ferrone S, Mulder A, et al. Role of the inhibitory KIR ligand HLA-Bw4 and HLA-C expression levels in the recognition of leukemic cells by Natural Killer cells. Cancer Immunol Immunother. 2009;58:855–86520. doi: 10.1007/s00262-008-0601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oevermann L, Michaelis SU, Mezger M, et al. KIR B haplotype donors confer a reduced risk of relapse after haploidentical transplantation in children with acute lymphoblastic leukemia. Blood. 2014 doi: 10.1182/blood-2014-03-565069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wudhikarn K, Brunstein CG, Bachanova V, et al. Relapse of lymphoma after allogeneic hematopoietic cell transplantation: management strategies and outcome. Biol Blood Marrow Transplant. 2011;17(10):1497–504. doi: 10.1016/j.bbmt.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Challa-Malladi M, Lieu YK, Califano O, et al. Combined genetic inactivation of B2-microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B-cell lymphoma. Cancer Cell. 2011;20(6):728–40. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol. 2008;180:6392–6401. doi: 10.4049/jimmunol.180.9.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borgerding A, Hasenkamp J, Engelke M, et al. B-lymphoma cells escape rituximab-triggered elimination by NK cells through increased HLA class I expression. Exp Hematol. 2010;38:213–221. doi: 10.1016/j.exphem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Morvan M, David G, Sebille V, et al. Autologous and allogeneic HLA KIR ligand environments and activating KIR control KIR NK-cell functions. Eur J Immunol. 2008;38(12):3474–3486. doi: 10.1002/eji.200838407. [DOI] [PubMed] [Google Scholar]
- 26.Przewoznik M, Homberg N, Naujoks, et al. Recruitment of natural killer cells in advanced stages of endogenously arising B-cell lymphoma: implications for therapeutic transfer. J Immunother. 2012;35(3):217–22. doi: 10.1097/CJI.0b013e318247440a. [DOI] [PubMed] [Google Scholar]
- 27.Klyuchnikov E, Bacher U, Kröger NM, et al. Reduced-Intensity Allografting as First Transplantation Approach in Relapsed/Refractory Grades One and Two Follicular Lymphoma Provides Improved Outcomes in Long-Term Survivors. Biol Blood Marrow Transplant. 2015;21(12):2091–9. doi: 10.1016/j.bbmt.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 120(20):4256–62. doi: 10.1182/blood-2012-06-436725. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–8. [PubMed] [Google Scholar]
- 30.Jacobson P, Rogosheske J, Barker JN, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther. 2005;78:486–500. doi: 10.1016/j.clpt.2005.08.009. [DOI] [PubMed] [Google Scholar]