Abstract
Objective
To assess whether adding liothyronine (LT3) to levothyroxine (LT4) monotherapy normalizes serum TSH and T4 concentrations in children with congenital hypothyroidism and central resistance to thyroid hormone.
Study design
We retrospectively studied 12 patients with congenital hypothyroidism and central resistance to thyroid hormone (6 treated with LT3+LT4 combined therapy and 6 treated with LT4 monotherapy). In patients receiving combined therapy, we compared serum concentrations of TSH, T4, and T3 before and after addition of LT3. We used repeated measures analysis to compare thyroid function in participants receiving combined therapy versus monotherapy, while accounting for age and intrasubject correlation.
Results
In patients receiving combined therapy, addition of LT3 was associated with normalization of mean TSH (9.2 vs. 4.5 mIU/L, p=0.002), a lower proportion of TSH values above 10 mIU/L (35% vs. 8%, p=0.03), and a decrease in mean serum T4 by 23 ± 9% (p<0.001). Compared with patients receiving LT4 monotherapy, patients receiving combined therapy had lower mean TSH (8.5 ± 0.9 vs. 4.3 ± 0.4, p<0.001), lower odds of TSH elevation above 10 mIU/L (OR 0.20, 95% CI 0.10–0.41, p<0.001), and lower odds of T4 elevation (OR 0.21, 95% CI 0.04–1.09, p=0.06). LT3 treatment did not increase serum T3 levels significantly.
Conclusion
Addition of LT3 to LT4 monotherapy facilitates normalization of both serum TSH and T4 in patients with congenital hypothyroidism and central resistance to thyroid hormone. Larger prospective studies are needed to confirm these findings and to determine the effect of combined therapy on neurodevelopmental outcomes.
Keywords: thyroid hormone resistance, combined therapy
Primary congenital hypothyroidism affects up to 1:2000 infants and leads to poor neurodevelopmental outcomes in the absence of early and adequate thyroid hormone replacement. Current guidelines recommend treatment with levothyroxine (LT4) to restore and maintain normal serum levels of thyrotropin (TSH) and thyroxine (T4).1, 2 However, in up to 43% of infants and 10% of older children with congenital hypothyroidism, TSH elevation fails to normalize despite appropriate LT4 treatment.3–5 This failure of normal thyroid hormone negative feedback on pituitary TSH secretion is a form of central resistance to thyroid hormone, although the mechanisms underlying this phenomenon in congenital hypothyroidism are poorly understood. Treatment of these patients requires the clinician to choose between maintaining normal serum T4 levels while permitting modest TSH elevation, or normalizing TSH at the expense of elevated serum T4. However, studies of children with congenital hypothyroidism have demonstrated that neurocognitive impairment is associated with both undertreatment (reflected by low T4 associated with elevated TSH)6–10 and overtreatment (reflected by elevated T4).11–14 Thus, neither approach to treatment of infants who have central resistance to thyroid hormone (high T4 and high TSH) is optimal based on current data, and existing consensus recommendations offer no evidence-based guidance.1, 2
Given the potential risks associated with elevation of either TSH or T4, some have suggested adding liothyronine (LT3) to LT4 monotherapy in patients with congenital hypothyroidism and central resistance to thyroid hormone. Prior case series of such patients have shown that combined therapy with LT3 and LT4 may help normalize TSH while keeping T3 and T4 in the normal range.15, 16 However, these studies were limited by several factors, most notably their failure to control for the natural history of central resistance to thyroid hormone to resolve over time in patients with congenital hypothyroidism.3
Therefore, we sought to assess the efficacy of adding LT3 treatment to LT4 monotherapy in patients with congenital hypothyroidism and central resistance to thyroid hormone by comparing thyroid function and auxologic variables in individual patients before and during LT3+LT4 combined therapy; and in patients treated with LT3+LT4 combined therapy versus patients treated with LT4 monotherapy. We hypothesized that combined therapy would normalize both serum TSH and T4 in these patients, whereas LT4 monotherapy would not.
METHODS
We searched the electronic medical record (EMR) by ICD9 code and medication prescriptions to identify all patients with congenital hypothyroidism seen at Boston Children’s Hospital from 1999–2014 who had been treated with LT3 for central resistance to thyroid hormone (n=8). All patients were treated initially with LT4 monotherapy, with LT3 added later at the discretion of the treating clinician because of failure to normalize serum TSH in the face of high-normal or elevated serum T4. These patients are referred to as the combined therapy group.
To account for the tendency of central resistance to thyroid hormone in congenital hypothyroidism to resolve spontaneously over time, we also analyzed a group of patients with congenital hypothyroidism and central resistance to thyroid hormone who were managed exclusively with LT4 monotherapy and never received LT3. These patients were identified in the EMR by ICD9 diagnostic code for congenital hypothyroidism and the finding of concurrent supranormal TSH and supranormal T4 on at least two occasions. Manual record review of identified patients was conducted independently by two authors, and patients were included if they met clinical criteria for central resistance to thyroid hormone based on a pattern of failure to suppress TSH in face of elevated T4 levels. Discrepancies in assignment were resolved by discussion after review by a third author (RSB). These patients are referred to as the monotherapy group (n=6).
For all patients, data were abstracted from the medical record including demographic information, birth history, results of laboratory and thyroid imaging studies, heart rate, auxologic variables, and doses of LT4 and LT3. We excluded from analysis any patient with documented nonadherence to therapy at any point (n=2 in the combined therapy group). Patients received various formulations of LT4 and LT3 tablets at the discretion of the treating provider. Due to the retrospective nature of this study, the timing of thyroid function measurements relative to ingested doses of LT4 and LT3 could not be determined. The study was approved by the Boston Children’s Hospital Institutional Review Board.
Statistical analyses
Within the combined therapy group, we used paired t-tests to compare measurements of thyroid function, heart rate, and auxologic variables in each patient before versus after initiating combined therapy. This analysis included data obtained up to two years before and two years after initiation of LT3, except for the a priori exclusion of data obtained prior to one month of age (to avoid inclusion of abnormal thyroid function measurements shortly after diagnosis). Because free T4 was measured in some patients and total T4 in others, we could not calculate mean serum T4 levels across the cohort. Therefore, we analyzed only within-patient changes in free T4 or total T4 before versus after initiating combined therapy, and in our analysis the term “T4” denotes free T4 or total T4, as measured in each patient. Mean values of serum TSH, T4, and T3 over the study period were calculated for each patient using the area-under-the-curve (AUC) to account for variation among patients in timing of measurements and duration of follow-up. To measure accumulated exposure to states of abnormal TSH, T4, and T3 elevation, we also calculated (1) the AUC of TSH that exceeded 5 and 10 mIU/L thresholds and (2) the proportion of T4 and T3 measurements that exceeded the age-specific normal range.
We compared baseline characteristics of patients in the combined therapy versus monotherapy groups using Fisher Exact Test for categorical variables and the Mann-Whitney U-test for continuous variables owing to their skewed distribution. We fit linear and logistic repeated measures regression models to compare the time course of thyroid function in patients receiving combined therapy versus monotherapy. We adjusted each model for age as a time-varying covariate. Further adjustment for sex and gestational age had negligible impact. Data on other baseline covariates (e.g., birth weight, TSH and free T4 at diagnosis) were missing for too many patients in this small sample to be included without introducing selection bias. From variables of the fitted model we constructed pairwise contrasts for mean TSH, odds of TSH exceeding 5 or 10 mIU/L, and odds of supranormal T4 between the following patient groups: (1) combined therapy group pre-LT3 vs. monotherapy group; (2) combined therapy group pre-LT3 vs. combined therapy group on LT3; and (3) combined therapy group on LT3 vs. monotherapy group. The principle of closed testing allowed us to make all three pairwise comparisons as well as the overall comparison (3 groups) with a critical p-value of 0.05 for statistical significance while preserving a family wise Type I error rate of 5%.17 We obtained standard errors for the contrasts by the generalized estimating equation method. Models comparing thyroid function in the combined therapy and monotherapy groups were fit using all available thyroid function measurements for each patient. A sensitivity analysis limited to data obtained within two years before or after initiation of LT3 in the combined therapy group did not significantly alter the results. We used SPSS version 21.0 (IBM Corp, 2012) and SAS version 9.4 (SAS Institute, Cary, NC) for statistical computations.
RESULTS
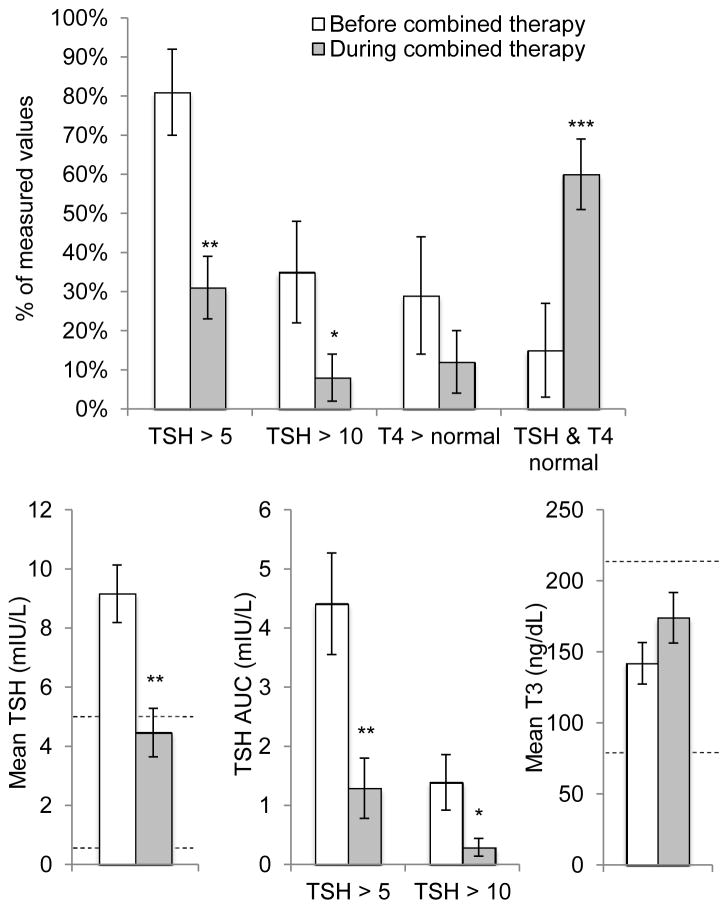
Baseline characteristics of subjects in the combined therapy and monotherapy groups are shown in Table I. To assess the effect of LT3 treatment on biochemical control of hypothyroidism, we compared serum TSH, T4, and T3 concentrations before and during LT3 treatment in each patient in the combined therapy group (Figure 1, Table II). Combined therapy was associated with a significant decrease in mean TSH from supranormal to the normal range (9.2 ± 1.0 vs. 4.5 ± 0.8 mIU/L, p=0.002). While on combined therapy, a smaller percentage of measured TSH values were elevated above 5 mIU/L or above 10 mIU/L compared with prior to LT3 initiation. Combined therapy was also associated with reduced overall exposure to TSH elevation, evident as significant decreases in AUC of TSH above 5 mIU/L and above 10 mIU/L (Figure 1 and Table II).
Table 1.
Baseline characteristics of the study cohort. All patients had central resistance to thyroid hormone and were initially treated with levothyroxine (LT4). Liothyronine (LT3) treatment was later added for patients in the combined therapy group, while the monotherapy group received only LT4.
| Patient | Combined Therapy Group (n=6)
|
Monotherapy Group (n=6)
|
p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Gestational age (wk) | 41 | 41 | 41 | 42 | 40 | 31 | 39 | 41 | 39 | 40 | 41 | 36 | 0.31 |
| Birth weight (kg) | 4.1 | 3.8 | 4.2 | 4.5 | — | 1.0 | 2.1 | 3.6 | 3.7 | 2.8 | 2.7 | 2.5 | 0.13 |
| Sex | M | F | F | M | M | M | F | F | M | F | F | F | 0.24 |
| Age at diagnosis (days) | 14 | 5 | 6 | 9 | 8 | 35 | — | 9 | 4 | — | 3 | 10 | 0.35 |
| TSH at diagnosis (mIU/L) | 460 | 433 | 177 | 100 | 220 | 34 | — | 577 | 734 | — | 272 | 61 | 0.35 |
| Free T4 at diagnosis (ng/dL) | 0.3 | 0.3 | 1.1 | — | 0.3 | 1 | — | — | 0.6 | — | 1.0 | 1.7 | 0.25 |
| Thyroid anatomy | — | NL | EC | EC | AG | — | AG | — | AG | — | AG | NL | 0.34 |
| Age at LT4 initiation (days) | 14 | 5 | 7 | 9 | 8 | 37 | 7 | 10 | 5 | — | 5 | 14 | 0.54 |
| Initial LT4 dose (μg/day) | 50 | 50 | 50 | 37.5 | 50 | 12.5 | 50 | 37.5 | 50 | — | 37.5 | 37.5 | 0.66 |
| Initial LT4 dose (μg/kg/day) | — | 13.9 | 11.9 | — | 11.1 | 6.9 | — | 10.2 | 13.2 | — | 13.2 | — | 0.86 |
Diagnosis is defined by measurement of confirmatory serum thyroid function tests. P-value tests for equal distribution between combined therapy and monotherapy groups by Fisher’s exact test or Mann-Whitney U-test. NL, normal; EC, ectopic; AG, agenesis.
Figure 1.
Thyroid function before and during liothyronine + levothyroxine combined therapy in congenital hypothyroidism patients with central resistance to thyroid hormone initially treated with levothyroxine monotherapy. Data are plotted as mean (SEM). AUC, area under curve. *p<0.05; **p<0.01; ***p<0.001.
Table 2.
Effect of liothyronine (LT3) + levothyroxine (LT4) combined therapy on thyroid function, heart rate, and auxologic characteristics in congenital hypothyroidism patients with central resistance to thyroid hormone initially treated with LT4 monotherapy.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | Median | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Age at LT3 start (yr) | 6.4 | 0.5 | 0.3 | 11.7 | 4.3 | 2.6 | 3.5 | ||||||||
| Initial LT3 dose (μg/day) | 10 | 5 | 2.5 | 5 | 5 | 5 | 5 | ||||||||
| Initial LT3 dose (μg/kg/day) | 0.31 | 0.65 | 0.23 | 0.13 | 0.26 | 0.44 | 0.29 | ||||||||
| ΔLT4 dose at LT3 start (%) | −11 | −20 | 0 | 0 | −12 | −12 | −11.5 | ||||||||
| Mean (SE) | |||||||||||||||
|
| |||||||||||||||
| LT4 | LT4 +LT3 | LT4 | LT4 +LT3 | LT4 | LT4 +LT3 | LT4 | LT4 +LT3 | LT4 | LT4 +LT3 | LT4 | LT4 +LT3 | LT4 | LT4 +LT3 | p | |
|
| |||||||||||||||
| Follow-up (mo) | 24 | 24 | 4 | 5 | 7 | 24 | 24 | 24 | 24 | 12 | 24 | 7 | 18 (4) | 16 (3) | 0.72 |
| TSH and T4 values (n) | 5 | 5 | 6 | 6 | 5 | 7 | 4 | 5 | 7 | 3 | 10 | 4 | 6.2 (0.8) | 5.0 (0.5) | 0.40 |
| TSH, mean (mIU/L) | 10.4 | 7.5 | 12.6 | 3.9 | 8.9 | 4.7 | 6.2 | 2.6 | 6.8 | 2.2 | 10.0 | 5.9 | 9.2 (1.0) | 4.5 (0.8) | 0.002 |
| TSH > 5 mIU/L AUC | 5.5 | 3.2 | 7.6 | 1.5 | 3.9 | 0.84 | 1.8 | 0.07 | 2.7 | 0 | 5.0 | 2.2 | 4.4 (0.9) | 1.3 (0.5) | 0.004 |
| TSH > 10 mIU/L AUC | 2.0 | 0.9 | 3.2 | 0.28 | 0.30 | 0 | 0.07 | 0 | 1.1 | 0 | 1.7 | 0.57 | 1.4 (0.5) | 0.29 (0.47) | 0.04 |
| TSH > 5 mIU/L (%) | 80 | 50 | 100 | 29 | 100 | 38 | 75 | 20 | 29 | 0 | 100 | 50 | 81 (11) | 31 (8) | 0.001 |
| TSH > 10 mIU/mL (%) | 80 | 33 | 50 | 14 | 0 | 0 | 0 | 0 | 29 | 0 | 50 | 0 | 35 (13) | 8 (6) | 0.03 |
|
| |||||||||||||||
| T4 > normal (%) | 0 | 0 | 17 | 50 | 20 | 0 | 100 | 20 | 0 | 0 | 40 | 0 | 29 (15) | 12 (8) | 0.32 |
| ΔT4, mean with LT3 (%) | — | −19 | — | −11 | — | −26 | — | −31 | — | −22 | — | −31 | — | −23 (9) | <0.001 |
| Both TSH and T4 normal (%) | 20 | 60 | 0 | 30 | 0 | 60 | 0 | 60 | 70 | 100 | 0 | 50 | 15 (12) | 60 (9) | <0.001 |
|
| |||||||||||||||
| T3 values (n) | 3 | 5 | 1 | 6 | 2 | 5 | 4 | 5 | 1 | 3 | 5 | 3 | 2.7 (0.6) | 4.5 (0.5) | 0.11 |
| T3, mean (ng/dL) | 196 | 168 | 128 | 178 | 166 | 167 | 103 | 168 | 109 | 115 | 147 | 250 | 142 (15) | 174 (18) | 0.16 |
| T3 > normal (%) | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 67 | 0 (0) | 17 (11) | 0.16 |
|
| |||||||||||||||
| Heart rate, mean (bpm) | — | 72 | 138 | 131 | 122 | 118 | 84 | 88 | 89 | 88 | 122 | 119 | 111 (10) | 102 (9) | 0.27 |
| Weight z-score | 2.2 | 2.2 | 0.7 | 1.0 | 2.3 | 2.0 | −0.2 | −0.3 | 0.9 | 1.0 | −1.7 | −1.3 | 0.7 (0.6) | 0.8 (0.5) | 0.65 |
| Height z-score | 1.4 | 1.3 | 0.3 | 0.5 | 1.6 | 1.1 | 0 | −0.3 | 1.84 | 1.7 | −3.0 | −2.8 | 0.4 (0.7) | 0.3 (0.7) | 0.35 |
| BMI z-score | 2.1 | 2.0 | 0.7 | 1.1 | 1.7 | 1.8 | −0.1 | −0.2 | −0.4 | 0 | −0.8 | 1.1 | 0.5 (0.5) | 1.0 (0.4) | 0.23 |
P-value compares before (LT4) vs. during (LT4+LT3) combined therapy using paired Student’s t-test. AUC, area-under-the-curve; bpm, beats per minute. Upper limit of normal T3 (ng/dL): 2 wk-24 mo, 245; 24 mo-8 yr, 241; 8 yr-21 yr, 210.
Combined therapy was associated with an overall decrease in serum T4 levels, with mean T4 declining by 23 ± 9% (p<0.001) during LT3 treatment. The proportion of supranormal T4 measurements also decreased during combined therapy. Although this decrease was not statistically significant, the lack of significance was attributable to a single patient (#2) with a high proportion of elevated T4 levels for 2 months after starting LT3. In fact, this patient’s serum T4 concentration declined on combined therapy, but a concurrent decrease in the upper limit of normal T4 with increasing age resulted in the T4 levels being categorized as elevated. Overall, the proportion of thyroid function measurements in which both TSH and T4 were normal increased dramatically during combined therapy.
Combined therapy increased mean serum T3 concentration and the proportion of supranormal serum T3 values, but neither effect was statistically significant (Figure 1, Table II). Overall, 17% percent of serum T3 levels measured on combined therapy were elevated. T3 elevation occurred in 3/6 patients, and in each case the elevated T3 levels were <10% above the upper limit of normal and persisted for no more than 2–12 weeks. Heart rate was unaffected by combined therapy, as were z-scores for height, weight, and BMI (Table II).
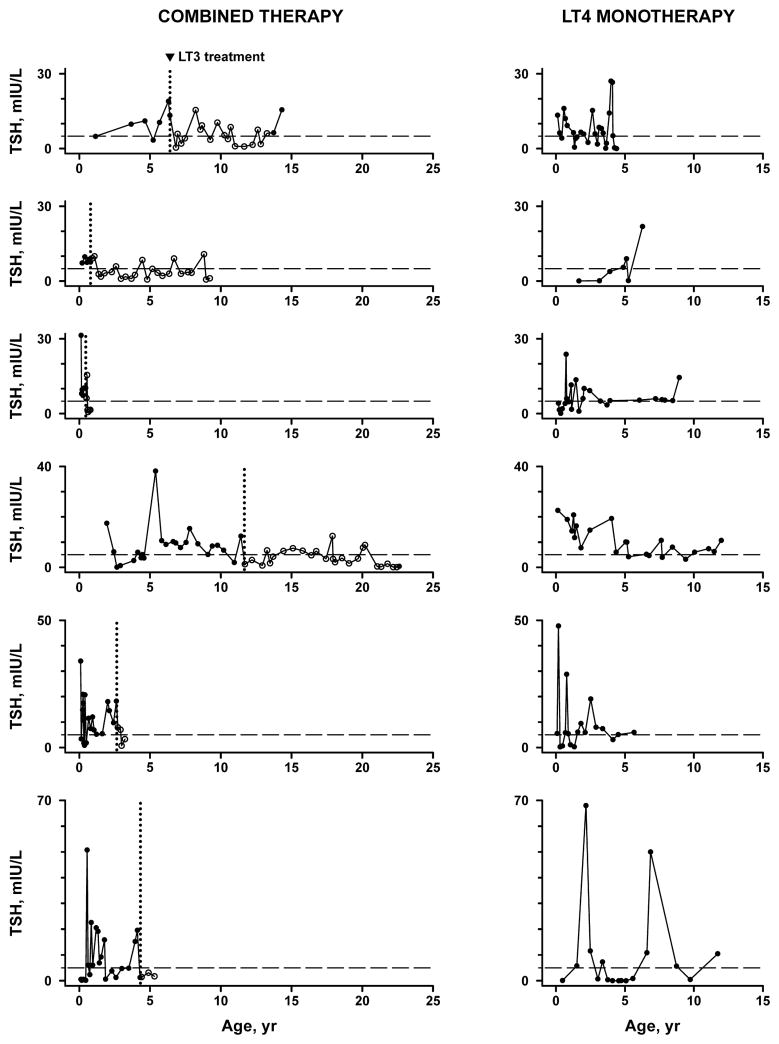
Although combined therapy was associated with normalization of TSH and T4, we wished to ensure that this improvement was not due to the expected resolution of central resistance to thyroid hormone over time (i.e., mean 16 months of follow up in this cohort). Therefore, we compared the patients who received combined therapy to a group of patients with congenital hypothyroidism and central resistance to thyroid hormone treated only with LT4 monotherapy. Six such patients were identified, with baseline characteristics similar to those who received combined therapy (Table I). Repeated measures analysis allowed comparison of TSH and T4 measurements among three categories: combined therapy patients prior to LT3 treatment; combined therapy patients during LT3 treatment; and monotherapy patients (Figure 2; available at www.jpeds.com). Prior to LT3 treatment, patients in the combined therapy group did not differ from those in the monotherapy group in mean TSH or odds of having elevated TSH or T4 (Table III). In the combined therapy group, LT3 treatment was associated with a decrease in mean TSH by 5.1 ± 0.8 mIU/L (p<0.001), as well as significantly reduced odds of TSH elevation above 5 mIU/L and above 10 mIU/L, consistent with the results of the earlier within-patient analysis. Finally, when compared with patients in the monotherapy group, patients on combined therapy had lower mean TSH and lower odds of TSH elevation above 5 mIU/L, TSH elevation above 10 mIU/L, and T4 elevation (Table III).
Figure 2.
Serum TSH levels in patients receiving liothyronine (LT3) + levothyroxine (LT4) combined therapy versus LT4 monotherapy. Open circles (○) represent measurements obtained on combined therapy; filled circles (●) represent those obtained on LT4 monotherapy. Dotted line (---) indicates TSH 5 mIU/L.
Table 3.
Thyroid function in patients with congenital hypothyroidism and central resistance to thyroid hormone receiving liothyronine (LT3) + levothyroxine (LT4) combined therapy versus LT4 monotherapy.
| Patient group | Samples | TSH, mIU/L | TSH >5 mIU/L | TSH >10 mIU/L | T4 > normal |
|---|---|---|---|---|---|
|
Mean ± SEa
|
Percentage of samples
|
||||
| LT4 monotherapy | 116 | 8.5 ± 0.9 | 65 | 28 | 28 |
| Combined therapy, pre-LT3 | 87 | 10.0 ± 0.9 | 72 | 37 | 27 |
| Combined therapy, on LT3 | 80 | 4.3 ± 0.4 | 34 | 6 | 8 |
|
| |||||
|
Difference ± SEb
|
Odds ratio (95% CI)c
|
||||
| Combined therapy, pre-LT3 vs. LT4 monotherapy | — | 1.4 ± 0.9 p=0.12 |
1.44 (0.68–3.04) p=0.34 |
1.52 (0.75–3.08) p=0.24 |
0.95 (0.47–1.88) p=0.87 |
|
| |||||
| Combined therapy, on LT3 vs. Combined therapy, pre-LT3 | — | −5.1 ± 0.8 p<0.001 |
0.17 (0.10–0.31) p<0.001 |
0.13 (0.07–0.25) p<0.001 |
0.22 (0.04–1.11) p=0.07 |
|
| |||||
| Combined therapy, on LT3 vs. LT4 monotherapy | — | −3.6 ± 0.7 p<0.001 |
0.25 (0.14–0.46) p<0.001 |
0.20 (0.10–0.41) p<0.001 |
0.21 (0.04–1.09) p=0.06 |
Unadjusted mean TSH ± standard error.
Difference in mean TSH ± standard error, adjusted for age and within-patient correlation by repeated measures linear regression.
Odds ratio (95% confidence interval) for indicated thyroid hormone status adjusted for age and within-patient correlation by repeated measured logistic regression.
DISCUSSION
In patients with congenital hypothyroidism, maintenance of both TSH and T4 in the normal range is recommended for optimal neurocognitive development. Because this goal is often unachievable in patients with central resistance to thyroid hormone, these patients present the clinical dilemma of whether to permit elevation of TSH or of T4. Evidence from existing studies of congenital hypothyroidism raises concern about both approaches. On one hand, inadequate treatment resulting in low T4 levels with elevated TSH is associated with poor neurocognitive outcomes and reduced IQ.6–9 The risk associated with TSH elevation and normal T4 levels is less clear, but one report has shown an association between repeated TSH elevation and poorer school performance.10 Therefore, maintaining TSH in the normal range is widely recommended, particularly prior to three years of age, when brain development is more rapid.2 On the other hand, overtreatment to supranormal T4 levels is also associated with adverse developmental consequences including altered temperament,11 decreased attention and alertness,12, 13 and decreased IQ.14 Addition of LT3 to LT4 monotherapy is appealing to potentially avoid both over- and undertreatment by simultaneously normalizing both TSH and T4 levels in patients with central resistance to thyroid hormone.
In this study, we found that adding LT3 treatment to standard LT4 monotherapy in such patients simultaneously normalized both TSH and T4. With combined therapy, mean TSH decreased from above normal to the normal range. Combined therapy also substantially decreased the proportion of TSH values above 10 mIU/L, the proportion of T4 values above normal, and mean serum T4 levels. Reductions in the administered dose of LT4 likely played a role in the observed decrease in serum T4, and indeed was part of the rationale for commencing LT3 therapy.
As expected, serum T3 increased with LT3 treatment but generally did not exceed the normal range (Figure 1). Mild T3 elevation occurred briefly in 3/6 patients, but no effect on heart rate or growth was detected. This finding highlights the potential for overtreatment with LT3, which is an important consideration given the overall goal of reducing exposure to elevated thyroid hormone levels. Our retrospective study design did not allow us to assess potential effects of the timing of LT3 administration or potential drawbacks of supplemental LT3 treatment, such as decreased treatment adherence or increased need for laboratory testing and medication adjustments.
Our findings are consistent with two prior case series consisting of a total of 18 patients with congenital hypothyroidism and central resistance to thyroid hormone who showed normalization of TSH and T4 with supplemental LT3 treatment.15, 16 The most significant limitation of both studies was the lack of a comparison group treated with LT4 monotherapy, without which it is difficult to determine whether the observed improvement in TSH and T4 was due to the addition of LT3 or to the normal resolution of central resistance to thyroid hormone. To address this critical issue, we included a monotherapy comparison group in our analysis of LT3 treatment. Although the groups had comparable baseline characteristics, combined therapy was associated with significantly lower mean TSH and a lower proportion of elevated TSH and T4 measurements compared with monotherapy. These results suggest that the observed effect is a result of LT3 treatment and not of resolution of central resistance to thyroid hormone over time. Additional methodological strengths of our study include the use of AUC to analyze changes in TSH, T4, and T3 over an extended period (mean 16–18 months) before and after LT3 treatment, and the use of repeated measures analyses to account for within-patient correlation of values over time.
Our report also extends the findings of prior studies to younger patients and lower doses of LT3. The median age of our cohort at the time of LT3 initiation was 3.5 years, compared with mean ages of 6.2 years and 7.1 years in prior cohorts (one of which excluded patients under 4 years of age).15, 16 Importantly, our study includes three patients started on LT3 before the age of 3 years, demonstrating that LT3 appears effective in normalizing thyroid hormone levels at the age when maintaining euthyroidism is most critical for neurodevelopment. The median initial dose of LT3 in our cohort (0.29 μg/kg/day) is lower than those in prior studies (0.3–0.66 μg/kg/day), in which locally available LT3 formulations may have constrained the investigators to a minimum administered LT3 dose of 6.25 μg,15, 16 whereas doses as low as 2.5 μg are available in many areas of the world. Future studies are needed to determine the optimal dosing of LT3 in this patient population.
The mechanism by which LT3 allows normalization of TSH with lower serum T4 may relate to the fact that circulating T3 accounts for 20–50% of the normal negative feedback regulation of TSH in pituitary thyrotrophs.18 Impaired central feedback of T3 in patients with congenital hypothyroidism might be related to their slightly lower serum T3 levels and T3/T4 ratio,19 which could be due to absence of the 20% of circulating T3 normally secreted by the thyroid and/or to decreased peripheral conversion of T4 to T3. The effect of LT3 treatment might also relate to intrinsic changes in hypothalamic-pituitary-thyroid axis regulation in patients with congenital hypothyroidism and central resistance to thyroid hormone.3, 5 Although the precise mechanisms underlying this phenomenon are unknown, they could involve changes in central thyroid hormone transport, deiodination, or receptors that are more amenable to treatment with LT3 than with LT4.
Our study is limited by its small sample size, although our significant findings suggest no lack of power. Also, although we identified patients in the monotherapy group using objective search criteria as well as independent review of cases and resolution of conflict by a third party, this required clinical judgment, which may have led to bias. However, it is reassuring that the combined therapy and monotherapy groups were similar at baseline.
Even though this retrospective analysis demonstrates that addition of LT3 can normalize thyroid function tests in the population studied—including children under age 3 years, who are at highest risk—it does not address the critical question of whether this intervention improves neurocognitive development, the outcome of ultimate clinical concern. Although patients with elevated T4 and those with elevated TSH and low T4 are clearly at risk for adverse neurocognitive outcomes, the risk to those with elevated TSH and normal T4 remains unclear, and thus the potential benefit of normalizing TSH levels in such patients remains to be determined. In addition, whether normalizing thyroid function tests may benefit older patients who are no longer at risk for abnormal cognitive development is unclear.
In summary, we have demonstrated that addition of LT3 to LT4 monotherapy facilitates the achievement and maintenance of biochemical euthyroidism in children with congenital hypothyroidism and central resistance to thyroid hormone. This is a consequential issue in clinical practice given the management dilemma posed by such patients, who may constitute over one-third of patients with congenital hypothyroidism. Whether combined therapy improves neurodevelopmental outcomes remains to be determined by larger prospective, ideally randomized controlled studies of combined therapy beginning early in life.
Acknowledgments
Funded by the National Institutes of Health (K12 DK094721, K23 ES024803, UL1 TR001102) and Harvard School of Public Health.
Footnotes
The authors declare no conflicts of interest.
Portions of the study were presented as a poster at the meeting of the Pediatric Academic Societies, San Diego, CA, April 25–28, 2015.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rose SR, Brown RS, Foley T, Kaplowitz PB, Kaye CI, Sundararajan S, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290–303. doi: 10.1542/peds.2006-0915. [DOI] [PubMed] [Google Scholar]
- 2.Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm Res Paediatr. 2014;81:80–103. doi: 10.1159/000358198. [DOI] [PubMed] [Google Scholar]
- 3.Fisher DA, Schoen EJ, La Franchi S, Mandel SH, Nelson JC, Carlton EI, et al. The hypothalamic-pituitary-thyroid negative feedback control axis in children with treated congenital hypothyroidism. J Clin Endocrinol Metab. 2000;85:2722–7. doi: 10.1210/jcem.85.8.6718. [DOI] [PubMed] [Google Scholar]
- 4.Kempers MJ, van Trotsenburg AS, van Tijn DA, Bakker E, Wiedijk BM, Endert E, et al. Disturbance of the fetal thyroid hormone state has long-term consequences for treatment of thyroidal and central congenital hypothyroidism. J Clin Endocrinol Metab. 2005;90:4094–100. doi: 10.1210/jc.2005-0197. [DOI] [PubMed] [Google Scholar]
- 5.Bagattini B, Cosmo CD, Montanelli L, Piaggi P, Ciampi M, Agretti P, et al. The different requirement of L-T4 therapy in congenital athyreosis compared with adult-acquired hypothyroidism suggests a persisting thyroid hormone resistance at the hypothalamic-pituitary level. Eur J Endocrinol. 2014;171:615–21. doi: 10.1530/EJE-14-0621. [DOI] [PubMed] [Google Scholar]
- 6.New England Congenital Hypothyroidism Collaborative. Correlation of cognitive test scores and adequacy of treatment in adolescents with congenital hypothyroidism. New England Congenital Hypothyroidism Collaborative. J Pediatr. 1994;124:383–7. doi: 10.1016/s0022-3476(94)70359-0. [DOI] [PubMed] [Google Scholar]
- 7.Heyerdahl S, Kase BF. Significance of elevated serum thyrotropin during treatment of congenital hypothyroidism. Acta Paediatr. 1995;84:634–8. doi: 10.1111/j.1651-2227.1995.tb13716.x. [DOI] [PubMed] [Google Scholar]
- 8.Bongers-Schokking JJ, Koot HM, Wiersma D, Verkerk PH, de Muinck Keizer-Schrama SM. Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. J Pediatr. 2000;136:292–7. doi: 10.1067/mpd.2000.103351. [DOI] [PubMed] [Google Scholar]
- 9.Oerbeck B, Sundet K, Kase BF, Heyerdahl S. Congenital hypothyroidism: influence of disease severity and L-thyroxine treatment on intellectual, motor, and school-associated outcomes in young adults. Pediatrics. 2003;112:923–30. doi: 10.1542/peds.112.4.923. [DOI] [PubMed] [Google Scholar]
- 10.Leger J, Larroque B, Norton J Association Francaise pour le Depistage et la Prevetion des Handicaps de lE. Influence of severity of congenital hypothyroidism and adequacy of treatment on school achievement in young adolescents: a population-based cohort study. Acta Paediatr. 2001;90:1249–56. doi: 10.1080/080352501317130272. [DOI] [PubMed] [Google Scholar]
- 11.Rovet JF, Ehrlich RM, Sorbara DL. Effect of thyroid hormone level on temperament in infants with congenital hypothyroidism detected by screening of neonates. J Pediatr. 1989;114:63–8. doi: 10.1016/s0022-3476(89)80602-x. [DOI] [PubMed] [Google Scholar]
- 12.Rovet J, Alvarez M. Thyroid hormone and attention in congenital hypothyroidism. J Pediatr Endocrinol Metab. 1996;9:63–6. doi: 10.1515/jpem.1996.9.1.63. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez M, Iglesias Fernandez C, Rodriguez Sanchez A, Dulin Lniguez E, Rodriguez Arnao MD. Episodes of overtreatment during the first six months in children with congenital hypothyroidism and their relationships with sustained attention and inhibitory control at school age. Horm Res Paediatr. 2010;74:114–20. doi: 10.1159/000313370. [DOI] [PubMed] [Google Scholar]
- 14.Bongers-Schokking JJ, Resing WC, de Rijke YB, de Ridder MA, de Muinck Keizer-Schrama SM. Cognitive development in congenital hypothyroidism: is overtreatment a greater threat than undertreatment? J Clin Endocrinol Metab. 2013;98:4499–506. doi: 10.1210/jc.2013-2175. [DOI] [PubMed] [Google Scholar]
- 15.Akcay T, Turan S, Guran T, Unluguzel G, Haklar G, Bereket A. T4 plus T3 treatment in children with hypothyroidism and inappropriately elevated thyroid-stimulating hormone despite euthyroidism on T4 treatment. Horm Res Paediatr. 2010;73:108–14. doi: 10.1159/000277627. [DOI] [PubMed] [Google Scholar]
- 16.Strich D, Naugolny L, Gillis D. Persistent hyperthyrotropinemia in congenital hypothyroidism: successful combination treatment with levothyroxine and liothyronine. J Pediatr Endocrinol Metab. 2011;24:347–50. doi: 10.1515/jpem.2011.179. [DOI] [PubMed] [Google Scholar]
- 17.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54:343–9. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 18.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 19.Oto Y, Muroya K, Hanakawa J, Asakura Y, Adachi M. The ratio of serum free triiodothyronine to free thyroxine in children: a retrospective database survey of healthy short individuals and patients with severe thyroid hypoplasia or central hypothyroidism. Thyroid Res. 2015;8:10. doi: 10.1186/s13044-015-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]