Abstract
Carcinogenesis is accompanied by increased protein and activity levels of extracellular cell-surface proteases that are capable of modifying the tumor micro-environment by directly cleaving the extracellular matrix, as well as activating growth factors and proinflammatory mediators involved in proliferation and invasion of cancer cells, and recruitment of inflammatory cells. These complex processes ultimately potentiate neoplastic progression leading to local tumor cell invasion, entry into the vasculature, and metastasis to distal sites. Several members of the type II transmembrane serine protease (TTSP) family have been shown to play critical roles in cancer progression. In this review the knowledge collected over the past two decades about the molecular mechanisms underlying the pro-cancerous properties of selected TTSPs will be summarized. Furthermore, we will discuss how these insights may facilitate the translation into clinical settings in the future by specifically targeting TTSPs as part of novel cancer treatment regimens.
Keywords: cell surface proteolysis, protease inhibitors, type II transmembrane serine proteases
Introduction
The first reviews on the type II transmembrane serine protease (TTSP) family were published at the turn of the millennium describing an emerging class of cell surface proteolytic enzymes (Hooper et al., 2001; Netzel-Arnett et al., 2003). At that time the knowledge about this family of proteases mainly comprised basic biochemical characteristics as well as expression data. Early on, it was evident that many TTSPs were differentially expressed in cancer. In the years following, the knowledge about the physiological and disease-related functions of TTSPs has rapidly accumulated. The generation of genetic loss-of-function and gain-of-function animal models and the characterization of human mutations have greatly enhanced our understanding of TTSP functions in a wide variety of organ systems under normal conditions and during disease initiation and progression, including cancer (Bugge et al., 2007, 2009; Szabo and Bugge, 2008; List, 2009; Antalis et al., 2010) (Table 1). Thus, it has been demonstrated that TTSPs play key roles in diverse processes including epithelial differentiation, homeostasis, iron metabolism, hearing, and blood pressure regulation (Szabo and Bugge, 2011). Furthermore, considerable headway has been made towards characterizing the regulation by cognate inhibitors, and identification of physiologically relevant pro-teolytic substrates (Table 1). Early studies demonstrated that dysregulated expression of TTSPs is associated with several cancers. In more recent studies, promotional or causal roles of TTSPs have been demonstrated in vivo using genetic engineering or administration of protease inhibitors to manipulate protease levels and activity. As evidence continues to accumulate about their functional roles in cancer development and progression, TTSPs represent exciting future therapeutic targets. This review will focus on four TTSPs (matriptase, hepsin, TMPRSS2 and TMPRSS4) that to date have been most extensively studied in cancer. We will evaluate studies describing their roles in solid tumor progression, and their potential for targeted therapy.
Table 1.
Table depicting the associated cancers, known in vivo substrates, and mutant phenotypes for the reviewed TTSP family members.
| Name | Associated cancer type | In vivo substrates (tissue type) | Mutant phenotypes -physiological function |
|---|---|---|---|
| Matriptase | Mouse loss-of-function models | ||
| TMPRSS2 | N. R. |
|
|
| TMPRSS4 | N. R. | ||
| Hepsin |
|
References:
N.R., Not reported.
Matriptase
This protease is one of the most extensively studied TTSPs with more than 300 published articles characterizing expression profiles, gene-regulation, structural biology, regulation by endogenous inhibitors, identification of critical substrates, determination of physiological and pathophysiological functions, and development of synthetic inhibitors and imaging tools. Approximately one third of the studies describe proposed roles of matriptase in cancer by employing cell culture models, in vivo studies using tumor grafting, or genetically engineered mouse models. Matriptase is upregulated in breast, cervical, colorectal prostate, endometrial, esophageal squamous cell carcinoma, gastric, head and neck, and pancreatic carcinoma; and in tumors of the lung, liver, and kidney among others (Cheng et al., 2006; Bugge et al., 2007; List, 2009; Webb et al., 2011). Increased matriptase expression correlates with advanced clinicopathological stages in many of these cancers, and de novo expression is found in ovarian and cervical carcinoma where expression levels also correlate with histopathological grade (Tanimoto et al., 2001, 2005; Lee et al., 2005). Matriptase activity is mainly regulated by the transmembrane serine protease inhibitors, hepatocyte growth factor activator inhibitor-1 and -2 (HAI-1 and HAI-2) in vivo (Szabo et al., 2007, 2008, 2009a,b). In expression studies, an imbalance between matriptase, HAI-1 and HAI-2 exists in several cancer types including ovarian and colorectal cancer where the matriptase/ HAI-1 ratio is increased and in prostate and endometrial carcinoma where matriptase/HAI-2 ratios are increased (Oberst et al., 2002; Saleem et al., 2006; Vogel et al., 2006; Bergum and List, 2010; Nakamura et al., 2011). A causal effect of matriptase expression was demonstrated in mice with transgenic expression of matriptase in the epidermis. These transgenic mice develop spontaneous squamous cell carcinoma, dermal inflammation and increased susceptibility to carcinogen-induced tumorigenesis (List et al., 2005). A follow-up study identified the preform of hepatocyte growth factor (pro-HGF) as a critical proteolytic target for matriptase in epidermal carcinogenesis using mouse genetic analysis (Szabo et al., 2011). When matriptase transgenic mice are crossed to mice with conditional epidermal deletion of the membrane receptor for pro-HGF/ HGF, c-Met, the matriptase-mediated malignancy is prevented, demonstrating that the oncogenic potential of matriptase in mouse epidermis is dependent on the HGF/ c-Met signaling pathway (Szabo et al., 2011) (Figure 1). Importantly, from a matriptase inhibitor perspective, the oncogenic properties of matriptase are completely abolished in double transgenic mice that also express transgenic epidermal HAI-1, which demonstrates that restoring the matriptase/HAI-1 balance in the epidermis rescues the skin from undergoing carcinogenesis (List et al., 2005). In this study, transgenic HAI-1 was expressed constitutively before the onset of transgenic matriptase-mediated tumor formation, thus representing a preventative study. Recently, it was shown that inducible matriptase inhibition initiated after the establishment of carcinomas is also efficient for cancer intervention (Sales et al., 2015). Induction of transgenic HAI-2 impaired malignant progression and caused regression of established individual tumors. Tumor regression correlated with reduced accumulation of tumor-associated inflammatory cells, likely caused by diminished expression of pro-tumorigenic inflammatory cytokines (Sales et al., 2015). These data suggest that matriptase may be a therapeutic target for both squamous cell carcinoma chemoprevention and for the treatment of established tumors.
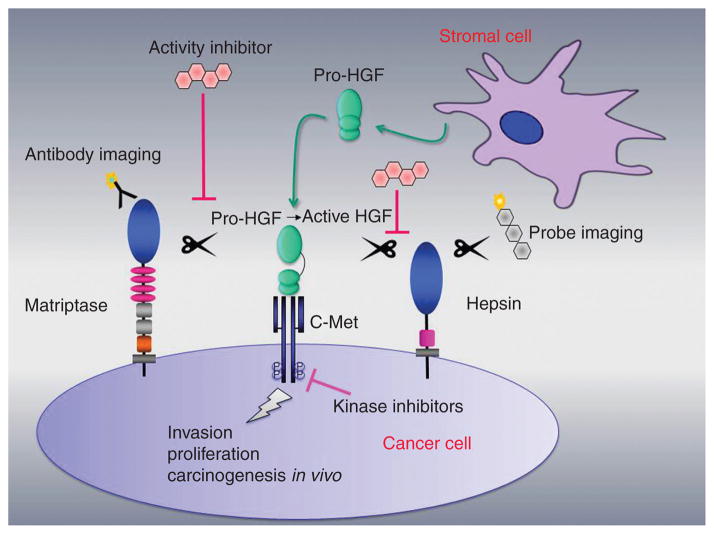
Figure 1. Simple schematic representation showing two TTSPs, matriptase and hepsin, that are localized on the cell surface of cancer cells.
Proteolytic cleavage of stromal cell-secreted pro-HGF to signaling competent HGF elicits the HGF/c-Met pro-oncogenic signaling pathway in cancer. Matriptase activates pro-HGF in squamous cell carcinomas (Szabo et al., 2011) and in breast cancer in vivo (Zoratti et al., 2015). Hepsin has been reported to activate pro-HGF in immortalized mammary epithelial cells (Tervonen et al., 2016) and in prostate cancer cells (Owen et al., 2010). TMPRSS2 (not shown) has been proposed to activate pro-HGF in prostate cancer (Lucas et al., 2014). The development of inhibitors of matriptase, hepsin or TMPRSS2 may represent alternatives to existing receptor tyrosine kinase inhibitors to impair invasion, proliferation and metastasis. Diagnostic imaging of protease activity is also being explored using, e.g. specific antibodies and activity-based probes.
Other studies that have assessed the consequence of matriptase/inhibitor imbalance include orthotopic xenografting of prostate cancer cells which showed that HAI-2 overexpression or matriptase silencing in N2 cells significantly decreased tumorigenicity and metastatic capability in mice (Tsai et al., 2014). In a pancreatic cancer model using orthotopic transplantation it was demonstrated that transgenic expression of HAI-1 in S2-CP8 cells, which display low levels of endogenous HAI-1, significantly decreased the development of distant metastasis in mice (Ye et al., 2014). The role of matriptase in vivo has also been studied in detail in breast cancer. In an oncogene-induced mouse mammary carcinoma model, hypomorphic matriptase mice with reduced levels of matriptase displayed a significant delay in tumor formation and blunted tumor growth (Zoratti et al., 2015). The reduced tumor growth was associated with a profound decrease in cancer cell proliferation. Mechanistic studies demonstrated that the proliferation deficiency was caused by the impairment of carcinoma cells, in cell lines and in vivo, to initiate the activation of the c-Met signaling pathway in response to fibroblast-secreted pro-hepatocyte growth factor (pro-HGF) (Figure 1). In primary mammary carcinoma cells and human breast cancer cell lines, addition of HAI-1 and HAI-2 inhibited pro-HGF mediated c-Met signaling and cell proliferation (Zoratti et al., 2015). Importantly, inhibition of matriptase catalytic activity using a selective small-molecule inhibitor efficiently abrogates the activation of c-Met, Gab1 and AKT, in response to pro-HGF, which functionally leads to attenuated cancer cell proliferation and invasion. The selective inhibitor of matriptase, IN-1 used in the study contains a ketobenzothiazole serine trap and was designed based on the auto-catalytic domain (RQAR) of matriptase (Colombo et al., 2012). It still remains to be tested whether IN-1 is suitable as an anti-tumor drug in vivo. Other inhibitors have been developed including MCoTI-II (based on cyclic microproteins of the squash Momordica cochinchinensis trypsin-inhibitor family) which inhibits the proteolytic activation of pro-HGF by matriptase but not by hepsin, in both purified and cell-based system (Gray et al., 2014).
In prostate cancer, several matriptase inhibitors have been tested in xenograft models. In an ectopic subcutaneous model, a small molecule inhibitor of matriptase, CVS-3983, significantly suppressed the growth of human prostate cancer cell lines in nude mice (Galkin et al., 2004). The authors propose that the effect was mainly cause by the abrogation of invasion since tumors remained localized to the site of injection in treated mice and failed to invade the subscapular area as compared to tumors in vehicle treated mice. Similarly, using matriptase inhibitors based on bis-basic secondary amides of sulfonylated 3-amidinophenylalanine in an orthotopic xenograft mouse model of prostate cancer resulted in reduced tumor growth, and tumor dissemination (Steinmetzer et al., 2006). Together, these findings demonstrate that matriptase is critically involved in cancer progression and lay the groundwork for future studies developing and testing small-molecule matriptase inhibitors and their potential as novel targeted therapeutic drugs in cancer.
Hepsin
Hepsin/TMPRSS1 was the first serine protease characterized to contain a transmembrane domain, and was named based on its original identification in hepatocytes (Leytus et al., 1988). Hepsin is also expressed in kidney, pancreas, stomach, prostate and thyroid (Tsuji et al., 1991a,b). Studies of knockout mouse models of hepsin have demonstrated that this protease plays an important role in cochlear development, is involved in regulating levels of the thyroid secreted hormone, thyroxine, and is responsible for pro-HGF activation in the liver (Guipponi et al., 2007; Hanifa et al., 2010; Hsu et al., 2012).
Many cancers display increased expression levels of hepsin including cancer of the prostate, breast (Xing et al., 2011), ovary (Tanimoto et al., 1997), kidney (Betsunoh et al., 2007), and endometrium (Matsuo et al., 2008). Several studies have revealed that hepsin is critically involved in prostate cancer progression. Klezovitch and colleagues used transgenic mice to show that the overexpression of hepsin, under the control of the probasin (PB) promotor, leads to a disorganized basement membrane and promotes prostate cancer metastasis to the liver, bone and lungs when crossed to the LPB-Tag prostate cancer model (Klezovitch et al., 2004).
The Wnt/β-Catenin signaling pathway has been shown to play an important role in prostate cancer progression. Dysregulation of this pathway by prostate-specific deletion of the adenomatous polyposis coli (Apc) gene results in high-grade prostatic intraepithelial neoplasia (PIN) lesions with rare occurrences of microinvasive characteristics in mice (Bruxvoort et al., 2007; Valkenburg et al., 2014). Crossing PB-Hepsin mice to the prostate-specific Apc-deletion model (APCPBKO) to generate PB-Hepsin/APCPBKO mice results in progression from high-grade PIN lesions to large invasive adenocarcinomas (Valkenburg et al., 2015). Prostate tumors from PB-Hepsin/APCPBKO mice are hyperproliferative, and contained significant numbers of apoptotic cells (Valkenburg et al., 2015).
A separate transgenic mouse model of prostate cancer has been developed by the overexpression of the human c-Myc proto-oncogene in the prostate epithelium. These transgenic mice develop PIN lesions that progress to invasive adenocarcinomas at approximately 6 months of age and show common molecular features with human prostate tumors (Ellwood-Yen et al., 2003). When PB-hepsin mice are crossed into this model of prostate cancer, bitransgenic mice develop adenocarcinomas earlier than PB-Myc mice and histological analysis detected higher grade adenocarcinoma indicating that hepsin cooperates with c-Myc to promote tumor progression in this model (Nandana et al., 2010).
PB-Hepsin/LPB-Tag bitransgenic mice were subsequently used to assess whether targeted inhibition of hepsin attenuates prostate cancer progression in vivo. Tang et al. identified a small molecule inhibitor of hepsin, hepIn-13, and treated PB-hepsin/LPB-Tag mice with oral doses of the inhibitor 13-weeks after mice had initially developed low-grade prostate tumors. Tumor metastasis was observed in the non-treated control group whereas HepIn-13 blocked metastasis to bone, liver, and lung in a dose-dependent manner (Tang et al., 2014).
In an orthotopic xenograft model of prostate cancer, LnCaP cells were stably transfected to overexpress hepsin (LnCaP-34) and injected into the anterior lobe of the prostate. Increased final tumor weights were observed in mice injected with LnCaP-34 cells compared to LnCaP cells expressing endogenous levels of hepsin (LnCaP-17 cells) (Li et al., 2009). Additionally, metastatic lesions to the periaortic lymph nodes were observed in mice injected with LnCaP-34 cells and not found in mice injected with LnCaP-17 cells (Li et al., 2009). To determine whether inhibition of hepsin decreases the tumor growth in vivo, mice injected with LnCaP-34 cells were treated with a PEGylated form of Kunitz domain-1, a potent hepsin active site inhibitor derived from HAI-1. Treatment of established orthotopic LnCaP-34 xenografts tumors with PEGylated Kunitz domain-1 significantly decreased contralateral prostate invasion and lymph node metastasis (Li et al. 2009).
In addition to prostate cancer, hepsin has been shown to have increased expression in breast cancer (Xing et al., 2011). To determine whether hepsin promotes tumor progression in breast cancer, Tervonen et al. performed grafting studies using primary mouse mammary epithelial cells from transgenic mice harboring a Whey acidic protein (Wap) promoter-controlled c-Myc transgene. The primary cells, that also expressed doxycyclin-inducible hepsin, were transplanted into cleared fat pads of syngeneic wild-type virgin hosts. Chronic doxycyclin-induced expression of hepsin resulted in decreased tumor latency in these mice, thus indicating a promotional role for hepsin in breast cancer progression (Tervonen et al., 2016). Interestingly, hepsin acutely downregulated its cognate inhibitor, HAI-1, in human MCF10A immortalized mammary epithelial cells, thereby further increasing the hepsin/HAI-1 ratio. Furthermore, hepsin induced cellular changes in MCF10A cells commonly associated with invasive phenotypes and endowed cells with a strong capacity to proteolytically activate pro-HGF, leading to activation of the c-Met receptor (Figure 1).
Hepsin is also frequently overexpressed in ovarian cancer, where 80% of ovarian carcinomas express hepsin mRNA whereas normal ovaries do not express hepsin mRNA (Tanimoto et al., 1997). In a subcutaneous transplantation study designed to determine whether hepsin expression promotes tumor growth, the parental SKOV3 human ovarian carcinoma, SKOV3 cells expressing WT-hepsin, or SKOV3 cells expressing a catalytically dead (CD) mutant form of hepsin were subcutaneously implanted into nude mice. SKOV3 cells expressing WT-hepsin displayed increased tumor volume compared to both the CD-hepsin and parental control cells, indicating that over-expression of active hepsin in ovarian cancer promotes ovarian tumor growth (Miao et al., 2008)
TMPRSS2 and TMPRSS4
TMPRSS2 and TMPRSS4 are two other members of the TMPRSS/Hepsin subfamily of TTSPs and remain relatively uncharacterized. Like many other TTSPs, expression of TMPRSS2 is localized to several types of epithelial tissues, including in the colon, small intestine, lung, kidney, pancreas, and most notably in the prostate (Jacquinet et al., 2001), where expression is highest. Both the physiological function and substrates of TMPRSS2 have yet to be identified; therefore much of what is known about TMPRSS2 originates from its association with cancer. TMPRSS2 has long been associated with prostate cancer following the identification of the oncogenic gene fusion product with erythroblast transformation specific (ETS) transcription factors, such as ETS-related gene (ERG) (Clark and Cooper, 2009; Shah and Small, 2010). Despite this, the role of the native TMPRSS2 protein and its proteolytic activity in cancer is vastly understudied. In normal prostate epithelia, TMPRSS2 expression is localized to the cell membrane, however, mislocalization to the cytoplasm in prostate cancer has been observed (Lucas et al., 2008). In addition, both primary and metastatic prostate tumors from patients display increases in TMPRSS2 levels, with increasing expression correlating with elevated Gleason score, suggesting that TMPRSS2 may play a pro-oncogenic and pro-metastatic role (Lucas et al., 2008). Knockdown studies in human LNCaP prostate cancer cells demonstrated that reduction of TMPRSS2 expression in cancer cells decreased cellular invasion, tumor size, and incidence of metastases following xenografting in mice (Ko et al., 2015). Interestingly, loss of TMPRSS2 expression does not impact cellular proliferation of LnCaP cells in culture (Ko et al., 2015). However, a role of the tumor micro-environment is not taken into consideration in mono-culture, therefore, a role for TMPRSS2 for cancer cell proliferation cannot conclusively be excluded. It is worth noting that in a similar study, shRNA-mediated silencing of TMPRSS2 in LNCaP-derived, bone metastatic castration-resistant (LNCaP C4-2B) cells led to a significant reduction in cell proliferation and cell invasion compared with scrambled shRNA controls (Lucas et al., 2014).
In a study employing mice with a targeted deletion of the Tmprss2 gene, it was demonstrated that the protease regulates cancer cell invasion and metastasis to distant organs in the TRansgenic Adenocarcinoma Mouse Prostate (TRAMP) model of prostate carcinogenesis (Lucas et al., 2014). TRAMP tumors in Tmprss2−/− mice were significantly larger than those in control mice, however, the incidence of metastasis to distant solid organs was substantially lower in the TRAMP tumors arising in the Tmprss2−/− background. It was demonstrated that TMPRSS2 activated signaling incompetent pro-HGF to active HGF in vitro and it is hypothesized that TMPRSS2-activated HGF consistently promotes invasion and metastasis, but differentially enhances or suppresses proliferation. Importantly, a TMPRSS2 chemical inhibitor (bromhexine hydrochloride) suppressed distant metastasis to lung and liver sites in TRAMP mice (Lucas et al., 2014).
These links to prostate malignancy makes TMPRSS2 a promising target for drug therapies. Of notable interest is that no phenotype affecting normal development or physiological function has been observed in TMPRSS2 deficient mice. This suggests that targeted ablation of TMPRSS2 in cancer may have minimal side effects (Kim et al., 2006). Upstream, TMPRSS2 expression in prostate has been shown to be driven by androgen receptor signaling; one notable consequence of this is the pro-oncogenic TMPRSS2-ERG fusion protein. This link between androgen receptor signaling and TMPRSS2 has further pushed interest in androgen receptor inhibitors as possible targets for inhibiting prostate cancer growth and metastasis. The pro-oncogenic potential of TMPRSS2 has, as mentioned above, been linked to its activity, specifically pertaining to its role in activating the HGF/cMET pathway (Lucas et al., 2014). More recently, work has uncovered matriptase as a possible substrate for TMPRSS2. A recent study found that levels of active matriptase are increased in prostate cancer without increases in matriptase transcript levels (Ko et al., 2015). Furthermore, orthotopic grafts of LNCaP cells over-expressing TMPRSS2 exhibited increased levels of active matriptase, as well as increased metastases, when compared to grafts expressing a control vector or catalytically dead TMPRSS2 (Ko et al., 2015). Proteolytic homeostasis is important for maintaining normal tissue function, and increases in TMPRSS2 expression may shift the balance between matriptase activity and cognate inhibitors. Additionally, beyond the HGF pathway, TMPRSS2 has been shown to activate protease-activated receptor 2 (PAR-2) in LNCaP cells (Wilson et al., 2005), inciting another pathway which may be involved in promoting metastasis (Wilson et al., 2004). In addition to the TMPRSS2 inhibitor bromhexine hydrochloride mentioned above, other synthetic inhibitors based on substrate analogs have been developed and demonstrated to inhibit TMPRSS2 activity, as measured by cleavage of influenza hemagglutinin (HA) (Meyer et al., 2013). TMPRSS2 in airway epithelia is important for influenza infection, and inhibition of TMPRSS2 with small molecule antagonists can prevent influenza infection (Garten et al., 2015). However, these small molecule inhibitors also have strong affinities for other proteases, such as matriptase, making the precise therapeutic mechanism by which these molecules function unclear.
The physiological role of TMPRSS4 is currently not known, however, a mutation in this gene has been associated with autosomal recessive cerebral atrophy (ARCA) (Lahiry et al., 2013). TMPRSS4 has been demonstrated to be upregulated in pancreatic, colorectal, thyroid, lung, and several other cancers (Wallrapp et al., 2000; Kebebew et al., 2005; Kim et al., 2010; Larzabal et al., 2011). Because of this broad expression profile in cancer, TMPRSS4 has been a focal point of anti-cancer research in recent years. TMPRSS4 has been shown to promote proliferative processes in lung and thyroid cancer cells, while shRNA targeting of TMPRSS4 transcripts causes reductions in proliferation (Guan et al., 2015; Hamamoto et al., 2015; de Aberasturi et al., 2016). Work using cultured lung cancer cells demonstrated that TMPRSS4 promotes a mesenchymal and invasive phenotype, suggesting a role for epithelial to mesenchymal transition (EMT) (de Aberasturi et al., 2016). Interestingly, increases in markers for cancer stem cells (CSCs) such as aldehyde dehydrogenase (ALDH) and octamer-binding transcription factor 4 (OCT-4) are strongly positively correlated with TMPRSS4 expression (de Aberasturi et al., 2016). Several reports have suggested that TMPRSS4 associates with poor prognosis and survival in a variety of different cancers (Cheng et al., 2013a,b; Wu et al., 2014; de Aberasturi and Calvo, 2015; Wang et al., 2015), which may be a result of an increase in the CSCs population, although the factors leading to TMPRSS4 upregulation are still not identified. One recent finding suggests that increased TMPRSS4 in cancer may result from gene-silencing of tissue factor pathway inhibitor 2 (TFPI-2), a consequence of improper methylation (Hamamoto et al., 2015). However, the mechanism which connects TFPI-2 and TMPRSS4 expression remains unknown.
While the physiological substrates for TMPRSS4 are not yet fully elucidated, targeting the activity of this protease may have benefits in the treatment of several types of cancer. With several studies reporting reduced invasive and proliferative potential in lung and thyroid cells following silencing of TMRSS4 expression (Larzabal et al., 2011; Guan et al., 2015) inhibitors of TMPRSS4 may provide an edge in cancer treatment. Also, overexpression of TMPRSS4 in cell culture causes cancer cells to be more resistant to several chemotherapeutics (de Aberasturi et al., 2016). A few small molecule inhibitors derived from 2-hydroxydairylamide have been developed with inhibitory effects on the proteolytic activity of TMPRSS4, with the consequence of impacting cancer cell invasiveness in colorectal SW480 cancer cells (Kang et al., 2013). Given that TMPRSS4 impacts signaling pathways such as cyclic AMP response element-binding protein (CREB)-cyclin D1 (Guan et al., 2015), inhibition of TMPRSS4 could impact many downstream processes important for a variety pro-oncogenic processes.
In conclusion, several TTSPs are candidate targets for therapy and combined basic and translational research is needed to further move the findings from cell and animal models into the clinic. Meanwhile, several groups continue to develop new TTSP inhibitors, including inhibitors of matriptase and hepsin, which may provide an alternative non-kinase strategy to the existing c-Met inhibitors that are already in clinical use, to block cell pro-oncogenic signaling in cancer (Figure 1) (Han et al., 2014).
Imaging TTSPs in cancer
Because many TTSPs are highly upregulated in cancer, they represent potential molecular imaging targets to be used, not only for basic research purposes, but also for diagnostic imaging of cancer in the clinic. Early detection of primary tumors is critical for successful cancer management and therapy. Molecular imaging differs from traditional diagnostic imaging techniques such as computed tomography (CT), and magnetic resonance imaging (MRI), by measurement of biologic processes at the cellular and molecular levels including proteolytic activity, that are involved in the disease progression.
The use of activity-based probes in mouse models has confirmed that proteolytic activity is a viable marker for cancer imaging in vivo for e.g. cysteine proteases (Sanman and Bogyo, 2014). Antibodies provide an alternate approach for targeting proteases and have the advantage of being very potent and specific (Figure 1). Antibodies that exclusively bind to the active form of matriptase with picomolar affinity were generated and validated in cell culture before evaluating their in vivo targeting capability (Darragh et al., 2010). Fluorescently labeled anti-matriptase IgG was administered to mice bearing xenografted tumors that were positive or negative for matriptase. Antibodies localized to matriptase expressing tumors, permitting visualization of matriptase activity, whereas tumors without matriptase expression were not visible (Darragh et al., 2010). In a follow-up study by the same group, it was demonstrated that the anti-matriptase IgG was capable of detecting matriptase in formalin-fixed paraffin-embedded tissue samples. Importantly, the significance of active matriptase at the protein level in colon cancer was further documented using immunofluorescence showing that active matriptase was undetectable in healthy colon whereas it was detected in adenocarcinomas of every stage (LeBeau et al., 2013). Furthermore, the preclinical utility of the antibody for the detection and quantitation of active matriptase in vivo was investigated using the nuclear imaging modality SPECT. The matriptase probe showed uptake in both xenografted cell lines and patient derived xenografts (PDX) using near infrared fluorescence imaging (NIR) optical imaging (LeBeau et al., 2013).
Using a different approach Napp et al. performed optical imaging in combination with a fluorescently labeled antibody directed against matriptase, and an activatable probe to determine expression and activity matriptase in orthotopic pancreatic (AsPC-1) xenografts in vivo (Napp et al., 2010). By applying NIR imaging in combination with a Cy5.5 labeled matriptase-specific antibody (recognizing both active and inactive matriptase), it was demonstrated that matriptase is expressed in vivo in primary tumors as well as in distant metastases. To study matriptase activity in vivo, an activatable probe, substrate S*DY-681, consisting of an eight amino acid peptide with a matriptase-preferred cleavage site and two fluorscent dyes quenching each other in the native state was utilized. Upon proteolytic cleavage, the fluorescence dyes separate, resulting in amplification of fluorescence. Matriptase activity was detected in tumors and this activity could be inhibited with synthetic active-site matriptase inhibitors (Napp et al., 2010).
Kelly and coworkers used phage display to isolate hepsin binding peptides with nanomolar affinity in monomeric form and high specificity. The identified peptides were able to detect human prostate cancer on tissue microarrays and in cell based assays. Imaging agents were synthesized by conjugating multiple peptides to fluorescent nanoparticles to further improve avidity through multivalency and pharmacokinetics. When hepsin targeted nanoparticles were injected into mouse xenograft models, they bound specifically to hepsin-expressing LNCaP xenografts compared to PC3 xenografts that did not express hepsin (Kelly et al., 2008).
The development of sensitive and selective imaging agents and inhibitors for TTSPs will provide a platform technology that can be used in diagnosis, treatment and evaluation of treatment efficacy.
Acknowledgments
This work was supported by a NIH Ruth L. Kirschstein National Research Service Award T32-CA009531 (A.S.M.), NCI NIH RCA60565A (K.L.) and NCI NIH 3R01CA160565-04S grant (K.L., F.A.V.), and NIGMS/NIH grant R25 GM 058905-15 (F.A.V.).
Contributor Information
Andrew S. Murray, Department of Pharmacology, Wayne State University School of Medicine and Barbara Ann Karmanos Cancer Institute, 540 East Canfield, Detroit, MI 48201, USA; and Department of Oncology, Wayne State University School of Medicine and Barbara Ann Karmanos Cancer Institute, Detroit, MI 48201, USA; and Cancer Biology Graduate Program, Wayne State University School of Medicine and Barbara Ann Karmanos Cancer Institute, Detroit, MI, USA
Fausto A. Varela, Department of Pharmacology, Wayne State University School of Medicine and Barbara Ann Karmanos Cancer Institute, 540 East Canfield, Detroit, MI 48201, USA; and Pharmacology Graduate Program, Wayne State University School of Medicine and Barbara Ann Karmanos Cancer Institute, Detroit, MI, USA
Karin List, Email: klist@med.wayne.edu, Department of Pharmacology, Wayne State University School of Medicine and Barbara Ann Karmanos Cancer Institute, 540 East Canfield, Detroit, MI 48201, USA; and Department of Oncology, Wayne State University School of Medicine and Barbara Ann Karmanos Cancer Institute, Detroit, MI 48201, USA.
References
- Alef T, Torres S, Hausser I, Metze D, Türsen U, Lestringant GG, Hennies HC. Ichthyosis, follicular atrophoderma, and hypotrichosis caused by mutations in ST14 is associated with impaired profilaggrin processing. J Invest Dermatol. 2008;129:862–869. doi: 10.1038/jid.2008.311. [DOI] [PubMed] [Google Scholar]
- Antalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochem J. 2010;428:325–346. doi: 10.1042/BJ20100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrahami L, Maas S, Pasmanik-Chor M, Rainshtein L, Magal N, Smitt J, van Marle J, Shohat M, Basel-Vanagaite L. Autosomal recessive ichthyosis with hypotrichosis syndrome: further delineation of the phenotype. Clin Genet. 2008;74:47–53. doi: 10.1111/j.1399-0004.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- Baba T, Kawaguchi M, Fukushima T, Sato Y, Orikawa H, Yorita K, Tanaka H, Lin CY, Sakoda S, Kataoka H. Loss of membrane-bound serine protease inhibitor HAI-1 induces oral squamous cell carcinoma cells’ invasiveness. J Pathol. 2012;228:181–192. doi: 10.1002/path.3993. [DOI] [PubMed] [Google Scholar]
- Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Ben Amitai D, Lurie R, Pasmanik-Chor M, Indelman M, Zvulunov A, Saban S, et al. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet. 2007;80:467–477. doi: 10.1086/512487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergum C, List K. Loss of the matriptase inhibitor HAI-2 during prostate cancer progression. Prostate. 2010;70:1422–1428. doi: 10.1002/pros.21177. [DOI] [PubMed] [Google Scholar]
- Betsunoh H, Mukai S, Akiyama Y, Fukushima T, Minamiguchi N, Hasui Y, Osada Y, Kataoka H. Clinical relevance of hepsin and hepatocyte growth factor activator inhibitor type 2 expression in renal cell carcinoma. Cancer Sci. 2007;98:491–498. doi: 10.1111/j.1349-7006.2007.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocheva G, Rattenholl A, Kempkes C, Goerge T, Lin CY, D’Andrea MR, Ständer S, Steinhoff M. Role of matriptase and proteinase-activated receptor-2 in nonmelanoma skin cancer. J Invest Dermatol. 2009;129:1816–1823. doi: 10.1038/jid.2008.449. [DOI] [PubMed] [Google Scholar]
- Bruxvoort KJ, Charbonneau HM, Giambernardi TA, Goolsby JC, Qian CN, Zylstra CR, Robinson DR, Roy-Burman P, Shaw AK, Buckner-Berghuis BD, et al. Inactivation of Apc in the mouse prostate causes prostate carcinoma. Cancer Res. 2007;67:2490–2496. doi: 10.1158/0008-5472.CAN-06-3028. [DOI] [PubMed] [Google Scholar]
- Bugge TH, List K, Szabo R. Matriptase-dependent cell surface proteolysis in epithelial development and pathogenesis. Front Biosci. 2007;12:5060–5070. doi: 10.2741/2448. [DOI] [PubMed] [Google Scholar]
- Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, Lin CY, List K, Szabo R, Fasano A, Bugge TH, Antalis TM. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci USA. 2010;107:4200–4205. doi: 10.1073/pnas.0903923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MF, Tzao C, Tsai WC, Lee WH, Chen A, Chiang H, Sheu LF, Jin JS. Expression of EMMPRIN and matriptase in esophageal squamous cell carcinoma: correlation with clinicopathological parameters. Dis Esophagus. 2006;19:482–486. doi: 10.1111/j.1442-2050.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- Cheng D, Kong H, Li Y. TMPRSS4 as a poor prognostic factor for triple-negative breast cancer. Int J Mol Sci. 2013a;14:14659–14668. doi: 10.3390/ijms140714659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Liang B, Li Y. High TMPRSS4 expression is a predictor of poor prognosis in cervical squamous cell carcinoma. Cancer Epidemiol. 2013b;37:993–997. doi: 10.1016/j.canep.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Cheng MF, Huang MS, Lin CS, Lin LH, Lee HS, Jiang JC, Hsia KT. Expression of matriptase correlates with tumour progression and clinical prognosis in oral squamous cell carcinoma. Histopathology. 2014;65:24–34. doi: 10.1111/his.12361. [DOI] [PubMed] [Google Scholar]
- Chou FP, Chen YW, Zhao XF, Xu-Monette ZY, Young KH, Gartenhaus RB, Wang JK, Kataoka H, Zuo AH, Barndt RJ, et al. Imbalanced matriptase pericellular proteolysis contributes to the pathogenesis of malignant B-cell lymphomas. Am J Pathol. 2013;183:1306–1317. doi: 10.1016/j.ajpath.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JP, Cooper CS. ETS gene fusions in prostate cancer. Nat Rev Urol. 2009;6:429–439. doi: 10.1038/nrurol.2009.127. [DOI] [PubMed] [Google Scholar]
- Colombo E, Désilets A, Duchêne D, Chagnon F, Najmanovich R, Leduc R, Marsault E. Design and synthesis of potent, selective inhibitors of matriptase. ACS Med Chem Lett. 2012;3:530–534. doi: 10.1021/ml3000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darragh MR, Schneider EL, Lou J, Phojanakong PJ, Farady CJ, Marks JD, Hann BC, Craik CS. Tumor detection by imaging proteolytic activity. Cancer Res. 2010;70:1505–1512. doi: 10.1158/0008-5472.CAN-09-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aberasturi AL, Calvo A. TMPRSS4: an emerging potential therapeutic target in cancer. Br J Cancer. 2015;112:4–8. doi: 10.1038/bjc.2014.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aberasturi AL, Redrado M, Villalba M, Larabal L, Pajares MJ, Garcia J, Evans SR, Garcia-Ros D, Bodegas ME, Lopez L, et al. TMPRSS4 induces cancer stem cell-like properties in lung cancer cells and correlates with ALDH expression in NSCLC patients. Cancer Lett. 2016;370:165–176. doi: 10.1016/j.canlet.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Förbs D, Thiel S, Stella MC, Stürzebecher A, Schweinitz A, Steinmetzer T, Stürzebecher J, Uhland K. In vitro inhibition of matriptase prevents invasive growth of cell lines of prostate and colon carcinoma. Int J Oncol. 2005;27:1061–1070. [PubMed] [Google Scholar]
- Galkin AV, Mullen L, Fox WD, Brown J, Duncan D, Moreno O, Madison EL, Agus DB. CVS-3983, a selective matriptase inhibitor, suppresses the growth of androgen independent prostate tumor xenografts. Prostate. 2004;61:228–235. doi: 10.1002/pros.20094. [DOI] [PubMed] [Google Scholar]
- Gao L, Liu M, Dong N, Jiang Y, Lin CY, Huang M, Wu D, Wu Q. Matriptase is highly upregulated in chronic lymphocytic leukemia and promotes cancer cell invasion. Leukemia. 2012;27:1191–1194. doi: 10.1038/leu.2012.289. [DOI] [PubMed] [Google Scholar]
- Garten W, Braden C, Arendt A, Peitsch C, Baron J, Lu Y, Pawletko K, Hardes K, Steinmetzer T, Böttcher-Friebertshäuser E. Influenza virus activating host proteases: Identification, localization and inhibitors as potential therapeutics. Eur J Cell Biol. 2015;94:375–383. doi: 10.1016/j.ejcb.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Gray K, Elghadban S, Thongyoo P, Owen KA, Szabo R, Bugge TH, Tate EW, Leatherbarrow RJ, Ellis V. Potent and specific inhibition of the biological activity of the type II transmembrane serine protease matriptase by the cyclic microprotein MCoTI-II. Thromb Haemost. 2014;112:402–411. doi: 10.1160/TH13-11-0895. [DOI] [PubMed] [Google Scholar]
- Guan H, Liang W, Liu J, Wei G, Li H, Xiu L, Xiao H, Li Y. Transmembrane protease serine 4 promotes thyroid cancer proliferation via CREB phosphorylation. Thyroid. 2015;25:85–94. doi: 10.1089/thy.2014.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guipponi M, Tan J, Cannon PZ, Donley L, Crewther P, Clarke M, Wu Q, Shepherd RK, Scott HS. Mice deficient for the type II transmembrane serine protease, TMPRSS1/hepsin, exhibit profound hearing loss. Am J Pathol. 2007;171:608–616. doi: 10.2353/ajpath.2007.070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto J, Soejima K, Naoki K, Yasuda H, Hayashi Y, Yoda S, Nakayama S, Satomi R, Terai H, Ikemura S, et al. Methylation-induced downregulation of TFPI-2 causes TMPRSS4 overexpression and contributes to oncogenesis in a subset of non-small-cell lung carcinoma. Cancer Sci. 2015;106:34–42. doi: 10.1111/cas.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Harris PK, Jones DE, Chugani R, Kim T, Agarwal M, Shen W, Wildman SA, Janetka JW. Inhibitors of HGFA, matriptase, and hepsin serine proteases: a nonkinase strategy to block cell signaling in cancer. ACS Med Chem Lett. 2014;5:1219–1224. doi: 10.1021/ml500254r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanifa S, Scott HS, Crewther P, Guipponi M, Tan J. Thyroxine treatments do not correct inner ear defects in tmprss1 mutant mice. Neuroreport. 2010;21:897–901. doi: 10.1097/WNR.0b013e32833dbd2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Huang HP, Yu IS, Su KY, Lin SR, Lin WC, Wu HL, Shi GY, Tao MH, Kao CH, et al. Serine protease hepsin regulates hepatocyte size and hemodynamic retention of tumor cells by hepatocyte growth factor signaling in mice. Hepatology. 2012;56:1913–1923. doi: 10.1002/hep.25773. [DOI] [PubMed] [Google Scholar]
- Hoang CD, D’Cunha J, Kratzke MG, Casmey CE, Frizelle SP, Maddaus MA, Kratzke RA. Gene expression profiling identifies matriptase overexpression in malignant mesothelioma. Chest. 2004;125:1843–1852. doi: 10.1378/chest.125.5.1843. [DOI] [PubMed] [Google Scholar]
- Hooper JD, Clements JA, Quigley JP, Antalis TM. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem. 2001;276:857–860. doi: 10.1074/jbc.R000020200. [DOI] [PubMed] [Google Scholar]
- Huang A, Zhou H, Zhao H, Quan Y, Feng B, Zheng M. TMPRSS4 correlates with colorectal cancer pathological stage and regulates cell proliferation and self-renewal ability. Cancer Biol Ther. 2013;15:297–304. doi: 10.4161/cbt.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquinet E, Rao NV, Rao GV, Zhengming W, Albertine KH, Hoidal JR. Cloning and characterization of the cDNA and gene for human epitheliasin. Eur J Biochem. 2001;268:2687–2699. doi: 10.1046/j.1432-1327.2001.02165.x. [DOI] [PubMed] [Google Scholar]
- Jin JS, Chen A, Hsieh DS, Yao CW, Cheng MF, Lin YF. Expression of serine protease matriptase in renal cell carcinoma: correlation of tissue microarray immunohistochemical expression analysis results with clinicopathological parameters. Int J Surg Pathol. 2006;14:65–72. doi: 10.1177/106689690601400111. [DOI] [PubMed] [Google Scholar]
- Jung H, Lee KP, Park SJ, Park JH, Jang YS, Choi SY, Jung JG, Jo K, Park DY, Yoon JH, et al. TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition. Oncogene. 2007;27:2635–2647. doi: 10.1038/sj.onc.1210914. [DOI] [PubMed] [Google Scholar]
- Kang JY, Dolled-Filhart M, Ocal IT, Singh B, Lin CY, Dickson RB, Rimm DL, Camp RL. Tissue microarray analysis of hepatocyte growth factor/Met pathway components reveals a role for Met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node-negative breast cancer. Cancer Res. 2003;63:1101–1105. [PubMed] [Google Scholar]
- Kang S, Min HJ, Kang MS, Jung MG, Kim S. Discovery of novel 2-hydroxydiarylamide derivatives as TMPRSS4 inhibitors. Bioorg Med Chem Lett. 2013;23:1748–1751. doi: 10.1016/j.bmcl.2013.01.055. [DOI] [PubMed] [Google Scholar]
- Kebebew E, Peng M, Reiff E, Duh QY, Clark OH, McMillan A. ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Ann Surg. 2005;242:363–361. doi: 10.1097/01.sla.0000179623.87329.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KA, Setlur SR, Ross R, Anbazhagan R, Waterman P, Rubin MA, Weissleder R. Detection of early prostate cancer using a hepsin-targeted imaging agent. Cancer Res. 2008;68:2286–2291. doi: 10.1158/0008-5472.CAN-07-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppner A, Andreasen D, Mérillat AM, Bapst J, Ansermet C, Wang Q, Maillard M, Malsure S, Nobile A, Hummler E. Epithelial sodium channel-mediated sodium transport is not dependent on the membrane-bound serine protease CAP2/Tmprss4. PloS One. 2015;10:e0135224. doi: 10.1371/journal.pone.0135224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Heinlein C, Hackman RC, Nelson PS. Phenotypic analysis of mice lacking the TMPRSS2-encoded protease. Mol Cell Biol. 2006;26:965–975. doi: 10.1128/MCB.26.3.965-975.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kang HY, Nam EH, Choi MS, Zhao XF, Hong CS, Lee JW, Lee JH, Park YK. TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin α5 and its signaling pathways. Carcinogenesis. 2010;31:597–606. doi: 10.1093/carcin/bgq024. [DOI] [PubMed] [Google Scholar]
- Klezovitch O, Chevillet J, Mirosevich J, Roberts RL, Matusik RJ, Vasioukhin V. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell. 2004;6:185–195. doi: 10.1016/j.ccr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Ko CJ, Huang CC, Lin HY, Juan CP, Lan SW, Shyu HY, Wu SR, Hsiao PW, Huang HP, Shun CT, et al. Androgen-induced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth, and metastasis. Cancer Res. 2015;75:2949–2960. doi: 10.1158/0008-5472.CAN-14-3297. [DOI] [PubMed] [Google Scholar]
- Kosa P, Szabo R, Molinolo AA, Bugge TH. Suppression of Tumorigenicity-14, encoding matriptase, is a critical suppressor of colitis and colitis-associated colon carcinogenesis. Oncogene. 2011;31:3679–3695. doi: 10.1038/onc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiry P, Racacho L, Wang J, Robinson JF, Gloor GB, Rupar CA, Siu VM, Bulman DE, Hegele RA. A mutation in the serine protease TMPRSS4 in a novel pediatric neurodegenerative disorder. Orphanet J Rare Dis. 2013;8:126. doi: 10.1186/1750-1172-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larzabal L, Nguewa PA, Pio R, Blanco D, Sanchez B, Roriguez MJ, Pajares MJ, Catena R, Montuenga LM, Calvo A. Overexpression of TMPRSS4 in non-small cell lung cancer is associated with poor prognosis in patients with squamous histology. Br J Cancer. 2011;105:1608–1614. doi: 10.1038/bjc.2011.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larzabal L, de Aberasturi AL, Redrado M, Rueda P, Rodriguez MJ, Bodegas ME, Montuenga LM, Calvo A. TMPRSS4 regulates levels of integrin α5 in NSCLC through miR-205 activity to promote metastasis. Br J Cancer. 2014;110:764–774. doi: 10.1038/bjc.2013.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau AM, Lee M, Murphy ST, Hann BC, Warren RS, Delos Santos R, Kurhanewicz J, Hanash SM, VanBrocklin HF, Craik CS. Imaging a functional tumorigenic biomarker in the transformed epithelium. Proc Natl Acad Sci USA. 2013;110:93–98. doi: 10.1073/pnas.1218694110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Yong Song S, Choi JJ, Lee SJ, Kim BG, Park CS, Lee JH, Lin CY, Dickson RB, Bae DS. Increased expression of matriptase is associated with histopathologic grades of cervical neoplasia. Hum Pathol. 2005;36:626–633. doi: 10.1016/j.humpath.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Leytus SP, Loeb KR, Hagen FS, Kurachi K, Davie EW. A novel trypsin-like serine protease (hepsin) with a putative transmembrane domain expressed by human liver and hepatoma cells. Biochemistry. 1988;27:1067–1074. doi: 10.1021/bi00403a032. [DOI] [PubMed] [Google Scholar]
- Li W, Wang BE, Moran P, Lipari T, Ganesan R, Corpuz R, Ludlam MJ, Gogineni A, Koeppen H, Bunting S, et al. Pegylated kunitz domain inhibitor suppresses hepsin-mediated invasive tumor growth and metastasis. Cancer Res. 2009;69:8395–8402. doi: 10.1158/0008-5472.CAN-09-1995. [DOI] [PubMed] [Google Scholar]
- Li T, Zeng ZC, Wang L, Qiu SJ, Zhou JW, Zh XT, Yu HH, Tang ZY. Radiation enhances long-term metastasis potential of residual hepatocellular carcinoma in nude mice through TMPRSS4-induced epithelial-mesenchymal transition. Cancer Gene Ther. 2011;18:617–626. doi: 10.1038/cgt.2011.29. [DOI] [PubMed] [Google Scholar]
- Liang B, Wu M, Bu Y, Zhao A, Xie F. Prognostic value of TMPRSS4 expression in patients with breast cancer. Med Oncol. 2013;30:497. doi: 10.1007/s12032-013-0497-8. [DOI] [PubMed] [Google Scholar]
- List K. Matriptase: a culprit in cancer? Future Oncol. 2009;5:97–104. doi: 10.2217/14796694.5.1.97. [DOI] [PubMed] [Google Scholar]
- List K, Haudenschild CC, Szabo R, Chen W, Wahl SM, Swaim W, Engelholm LH, Behrendt N, Bugge TH. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene. 2002;21:3765–3779. doi: 10.1038/sj.onc.1205502. [DOI] [PubMed] [Google Scholar]
- List K, Szabo R, Wertz PW, Segre J, Haudenschild CC, Kim SY, Bugge TH. Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/ MT-SP1. J Cell Biol. 2003;163:901–910. doi: 10.1083/jcb.200304161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, Burke B, Nielsen BS, Gutkind JS, Bugge TH. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 2005;19:1934–1935. doi: 10.1101/gad.1300705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List K, Currie B, Scharschmidt TC, Szabo R, Shireman J, Molinolo A, Cravatt BF, Segre J, Bugge TH. Autosomal ichthyosis with hypotrichosis syndrome displays low matriptase proteolytic activity and is phenocopied in ST14 hypomorphic mice. J Biol Chem. 2007;282:36714–36723. doi: 10.1074/jbc.M705521200. [DOI] [PubMed] [Google Scholar]
- List K, Kosa P, Szabo R, Bey AL, Wang CB, Molinolo A, Bugge TH. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am J Pathol. 2009;175:1453–1463. doi: 10.2353/ajpath.2009.090240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JM, True L, Hawley S, Matsumura M, Morrissey C, Vessella R, Nelson PS. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J Pathol. 2008;215:118–125. doi: 10.1002/path.2330. [DOI] [PubMed] [Google Scholar]
- Lucas JM, Heinlein C, Kim T, Hernandez SA, Malik MS, True LD, Morrissey C, Corey E, Montgomery B, Mostaghel E, et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Nakamura K, Takamoto N, Kodama J, Hongo A, Abrzua F, Nasu Y, Kumon H, Hiramatsu Y. Expression of the serine protease hepsin and clinical outcome of human endometrial cancer. Anticancer Res. 2008;28:159–164. [PubMed] [Google Scholar]
- Meyer D, Sielaff F, Hammami M, Bottcher-Friebertshauser E, Garten W, Steinmetzer T. Identification of the first synthetic inhibitors of the type II transmembrane serine protease TMPRSS2 suitable for inhibition of influenza virus activation. Biochem J. 2013;452:331–343. doi: 10.1042/BJ20130101. [DOI] [PubMed] [Google Scholar]
- Miao J, Mu D, Ergel B, Singavarapu R, Duan Z, Powers S, Oliva E, Orsulic S. Hepsin colocalizes with desmosomes and induces progression of ovarian cancer in a mouse model. Int J Cancer. 2008;123:2041–2047. doi: 10.1002/ijc.23726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Hongo A, Kodama J, Abarzua F, Nasu Y, Kumon H, Hiramatsu Y. Expression of matriptase and clinical outcome of human endometrial cancer. Anticancer Res. 2009;29:1685–1690. [PubMed] [Google Scholar]
- Nakamura K, Hongo A, Kodama J, Hiramatsu Y. The role of hepatocyte growth factor activator inhibitor (HAI)-1 and HAI-2 in endometrial cancer. Int J Cancer. 2011;128:2613–2624. doi: 10.1002/ijc.25606. [DOI] [PubMed] [Google Scholar]
- Nandana S, Ellwood-Yen K, Sawyers C, Wills M, Weidow B, Case T, Vasioukhin V, Matusik R. Hepsin cooperates with MYC in the progression of adenocarcinoma in a prostate cancer mouse model. Prostate. 2010;70:591–600. doi: 10.1002/pros.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napp J, Dullin C, Müller F, Uhland K, Petri JB, van de Locht A, Steinmetzer T, Alves F. Time-domain in vivo near infrared fluorescence imaging for evaluation of matriptase as a potential target for the development of novel, inhibitor-based tumor therapies. Int J Cancer. 2010;127:1958–1974. doi: 10.1002/ijc.25405. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, Antalis TM. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–258. doi: 10.1023/a:1023003616848. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett S, Currie BM, Szabo R, Lin CY, Chen LM, Chai KX, Antalis TM, Bugge TH, List K. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem. 2006;281:32941–32945. doi: 10.1074/jbc.C600208200. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett S, Buzza MS, Shea-Donohue T, Désilets A, Leduc R, Fasano A, Bugge TH, Antalis TM. Matriptase protects against experimental colitis and promotes intestinal barrier recovery. Inflamm Bowel Dis. 2012;18:1303–1314. doi: 10.1002/ibd.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst MD, Johnson MD, Dickson RB, Lin CY, Singh B, Stewart M, Williams A, al-Nafussi A, Smyth JF, Gabra H, et al. Expression of the serine protease matriptase and its inhibitor HAI-1 in epithelial ovarian cancer: correlation with clinical outcome and tumor clinicopathological parameters. Clin Cancer Res. 2002;8:1101–1107. [PubMed] [Google Scholar]
- Owen KA, Qiu D, Alves J, Schumacher AM, Kilpatrick LM, Li J, Harris JL, Ellis V. Pericellular activation of hepatocyte growth factor by the transmembrane serine proteases matriptase and hepsin, but not by the membrane-associated protease uPA. Biochem J. 2010;426:219–228. doi: 10.1042/BJ20091448. [DOI] [PubMed] [Google Scholar]
- Riddick AC, Shukla CJ, Pennington CJ, Bass R, Nuttall RK, Hogan A, Sethia KK, Ellis V, Collins AT, Maitland NJ, et al. Identification of degradome components associated with prostate cancer progression by expression analysis of human prostatic tissues. Br J Cancer. 2005;92:2171–2180. doi: 10.1038/sj.bjc.6602630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M, Adhami VM, Zhong W, Longley BJ, Lin CY, Dickson RB, Reagan-Shaw S, Jarrard DF, Mukhtar H. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev. 2006;15:217–227. doi: 10.1158/1055-9965.EPI-05-0737. [DOI] [PubMed] [Google Scholar]
- Sales KU, Friis S, Konkel JE, Godiksen S, Hatakeyama M, Hansen KK, Rogatto SR, Szabo R, Vogel LK, Chen W, et al. Non-hematopoietic PAR-2 is essential for matriptase-driven pre-malignant progression and potentiation of ras-mediated squamous cell carcinogenesis. Oncogene. 2014;34:346–356. doi: 10.1038/onc.2013.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales KU, Friis S, Abusleme L, Moutsopoulos NM, Bugge TH. Matriptase promotes inflammatory cell accumulation and progression of established epidermal tumors. Oncogene. 2015;34:4664–4672. doi: 10.1038/onc.2014.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AJ, Parr C, Davies G, Martin TA, Lane J, Mason MD, Jiang WG. Genetic reduction of matriptase-1 expression is associated with a reduction in the aggressive phenotype of prostate cancer cells in vitro and in vivo. J Exp Ther Oncol. 2006;6:39–48. [PubMed] [Google Scholar]
- Sanman LE, Bogyo M. Activity-based profiling of proteases. Annu Rev Biochem. 2014;83:249–273. doi: 10.1146/annurev-biochem-060713-035352. [DOI] [PubMed] [Google Scholar]
- Shah S, Small E. Emerging biological observations in prostate cancer. Expert Rev Anticancer Ther. 2010;10:89–101. doi: 10.1586/era.09.161. [DOI] [PubMed] [Google Scholar]
- Srikantan V, Valladares M, Rhim JS, Moul JW, Srivastava S. HEPSIN inhibits cell growth/invasion in prostate cancer cells. Cancer Res. 2002;62:6812–6816. [PubMed] [Google Scholar]
- Steinmetzer T, Schweinitz A, Stürzebecher A, Dönnecke D, Uhland K, Schuster O, Steinmetzer P, Müller F, Friedrich R, Than ME, et al. Secondary amides of sulfonylated 3-amidinophenylalanine. New potent and selective inhibitors of matriptase. J Med Chem. 2006;49:4116–4126. doi: 10.1021/jm051272l. [DOI] [PubMed] [Google Scholar]
- Szabo R, Bugge TH. Type II transmembrane serine proteases in development and disease. Int J Biochem Cell Biol. 2008;40:1297–1316. doi: 10.1016/j.biocel.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Szabo R, Bugge TH. Membrane-anchored serine proteases in vertebrate cell and developmental biology. Annu Rev Cell Dev Biol. 2011;27:213–235. doi: 10.1146/annurev-cellbio-092910-154247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26:1546–1556. doi: 10.1038/sj.onc.1209966. [DOI] [PubMed] [Google Scholar]
- Szabo R, Hobson JP, List K, Molinolo A, Lin CY, Bugge TH. Potent inhibition and global co-localization implicate the transmembrane Kunitz-type serine protease inhibitor hepatocyte growth factor activator inhibitor-2 in the regulation of epithelial matriptase activity. J Biol Chem. 2008;283:29495–29504. doi: 10.1074/jbc.M801970200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R, Hobson JP, Christoph K, Kosa P, List K, Bugge TH. Regulation of cell surface protease matriptase by HAI2 is essential for placental development, neural tube closure and embryonic survival in mice. Development. 2009a;136:2653–2663. doi: 10.1242/dev.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R, Kosa P, List K, Bugge TH. Loss of matriptase suppression underlies spint1 mutation-associated ichthyosis and postnatal lethality. Am J Pathol. 2009b;174:2015–2022. doi: 10.2353/ajpath.2009.090053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R, Rasmussen AL, Moyer AB, Kosa P, Schafer JM, Molinolo AA, Gutkind JS, Bugge TH. c-Met-induced epithelial carcinogenesis is initiated by the serine protease matriptase. Oncogene. 2011;30:2003–2016. doi: 10.1038/onc.2010.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Mahajan SS, Nguyen LT, Béliveau F, Leduc R, Simon JA, Vasioukhin V. Targeted inhibition of cell-surface serine protease Hepsin blocks prostate cancer bone metastasis. Oncotarget. 2014;5:1352–1362. doi: 10.18632/oncotarget.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto H, Yan Y, Clarke J, Korourian S, Shigemasa K, Parmley TH, Parham GP, O’Brien TJ. Hepsin, a cell surface serine protease identified in hepatoma cells, is overexpressed in ovarian cancer. Cancer Res. 1997;57:2884–2887. [PubMed] [Google Scholar]
- Tanimoto H, Underwood LJ, Wang Y, Shigemasa K, Parmley TH, O’Brien TJ. Ovarian tumor cells express a transmembrane serine protease: a potential candidate for early diagnosis and therapeutic intervention. Tumour Biol. 2001;22:104–114. doi: 10.1159/000050604. [DOI] [PubMed] [Google Scholar]
- Tanimoto H, Shigemasa K, Tian X, Gu L, Beard JB, Sawasaki T, O’Brien TJ. Transmembrane serine protease TADG-15 (ST14/Matriptase/MT-SP1): expression and prognostic value in ovarian cancer. Br J Cancer. 2005;92:278–283. doi: 10.1038/sj.bjc.6602320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervonen TA, Belitškin D, Pant SM, Englund JI, Marques E, Ala-Hongisto H, Nevalaita L, Sihto H, Heikkilä P, Leidenius M, et al. Deregulated hepsin protease activity confers oncogenicity by concomitantly augmenting HGF/ MET signalling and disrupting epithelial cohesion. Oncogene. 2016;92:278–283. doi: 10.1038/onc.2015.248. [DOI] [PubMed] [Google Scholar]
- Tripathi M, Potdar AA, Yamashita H, Weidow B, Cummings PT, Kirchhofer D, Quaranta V. Laminin-332 cleavage by matriptase alters motility parameters of prostate cancer cells. Prostate. 2011;71:184–196. doi: 10.1002/pros.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WC, Chao YC, Lee WH, Chen A, Sheu LF, Jin JS. Increasing EMMPRIN and matriptase expression in hepatocellular carcinoma: tissue microarray analysis of immunohistochemical scores with clinicopathological parameters. Histopathology. 2006;49:388–395. doi: 10.1111/j.1365-2559.2006.02516.x. [DOI] [PubMed] [Google Scholar]
- Tsai CH, Teng CH, Tu YT, Cheng TS, Wu SR, Ko CJ, Shyu HY, Lan SW, Huang HP, Tzeng SF, et al. HAI-2 suppresses the invasive growth and metastasis of prostate cancer through regulation of matriptase. Oncogene. 2014;33:4643–4652. doi: 10.1038/onc.2013.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A, Torres-Rosado A, Arai T, Le Beau MM, Lemons RS, Chou SH, Kurachi K. Hepsin, a cell membrane-associated protease. Characterization, tissue distribution, and gene localization. J Biol Chem. 1991a;266:16948–16953. [PubMed] [Google Scholar]
- Tsuji A, Torres-Rosado A, Arai T, Chou SH, Kurachi K. Characterization of hepsin, a membrane bound protease. Biomed Biochim Acta. 1991b;50:791–793. [PubMed] [Google Scholar]
- Uhland K, Siphos B, Arkona C, Schuster M, Petri B, Steinmetzer P, Mueller F, Schweinitz A, Steinmetzer T, Van De Locht A. Use of IHC and newly designed matriptase inhibitors to elucidate the role of matriptase in pancreatic ductal adenocarcinoma. Int J Oncol. 2009;35:347–357. [PubMed] [Google Scholar]
- Valkenburg KC, Yu X, De marzo AM, Spiering TJ, Matusik RJ, Williams BO. Activation of Wnt/β-catenin signaling in a subpopulation of murine prostate luminal epithelial cells induces high grade prostate intraepithelial neoplasia. Prostate. 2014;74:1506–1520. doi: 10.1002/pros.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkenburg KC, Hostetter G, Williams BO. Concurrent Hepsin overexpression and denomatous polyposis coli deletion causes invasive prostate carcinoma in mice. Prostate. 2015;75:1579–1585. doi: 10.1002/pros.23032. [DOI] [PubMed] [Google Scholar]
- Vogel LK, Saebo M, Skjelbred CF, Abell K, Pedersen ED, Vogel U, Kure EH. The ratio of Matriptase/HAI-1 mRNA is higher in colorectal cancer adenomas and carcinomas than corresponding tissue from control individuals. BMC Cancer. 2006;6:176. doi: 10.1186/1471-2407-6-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallrapp C, Uehara H, Izumi K. A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res. 2000;60:2602–2606. [PubMed] [Google Scholar]
- Wang CH, Guo ZY, Chen ZT, Zhi XT, Li DK, Dong ZR, Chen ZQ, Hu Sy, Li T. TMPRSS4 facilitates epithelial-mesenchymal transition of hepatocellular carcinoma and is a predictive marker for poor prognosis of patients after curative resection. Sci Rep. 2015;5:12366. doi: 10.1038/srep12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SL, Sanders AJ, Mason MD, Jiang WG. Type II transmembrane serine protease (TTSP) deregulation in cancer. Front Biosci. 2011;16:539–552. doi: 10.2741/3704. [DOI] [PubMed] [Google Scholar]
- Welman A, Sproul D, Mullen P, Muir M, Kinnaird AR, Harrison DJ, Faratian D, Brunton VG, Frame MC. Diversity of matriptase expression level and function in breast cancer. PloS One. 2012;7:e34182. doi: 10.1371/journal.pone.0034182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Gallagher S, Warpeha K, Hawthorne SJ. Amplification of MMP-2 and MMP-9 production by prostate cancer cell lines via activation of protease-activated receptors. Prostate. 2004;60:168–174. doi: 10.1002/pros.20047. [DOI] [PubMed] [Google Scholar]
- Wilson S, Greer B, Hooper J, Zijlstra A, Walker B, Quigley J, Hawthorne S. The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells. Biochem J. 2005;388:967–972. doi: 10.1042/BJ20041066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SR, Cheng TS, Chen WC, Shyu HY, Ko CJ, Huang HP, Teng CH, Lin CH, Johnson MD, Lin CY, et al. Matriptase is involved in ErbB-2-induced prostate cancer cell invasion. Am J Pathol. 2010;177:3145–3158. doi: 10.2353/ajpath.2010.100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XY, Zhang L, Zhang KM, Zhang MH, Ruan TY, Liu CY, Xu JY. Clinical implication of TMPRSS4 expression in human gallbladder cancer. Tumour Biol. 2014;35:5481–5486. doi: 10.1007/s13277-014-1716-4. [DOI] [PubMed] [Google Scholar]
- Xing P, Li JG, Jin F, Zhao TT, Liu Q, Dong HT, Wei XL. Clinical and biological significance of hepsin overexpression in breast cancer. J Investig Med. 2011;59:803–810. doi: 10.2310/JIM.0b013e31821451a1. [DOI] [PubMed] [Google Scholar]
- Xuan JA, Schneider D, Toy P, Lin R, Newton A, Zhu Y, Finster S, Vogel D, Mintzer B, Dinter H, et al. Antibodies neutralizing hepsin protease activity do not impact cell growth but inhibit invasion of prostate and ovarian tumor cells in culture. Cancer Res. 2006;66:3611–3619. doi: 10.1158/0008-5472.CAN-05-2983. [DOI] [PubMed] [Google Scholar]
- Ye J, Kawaguchi M, Haruyama Y, Kanemaru A, Fukushima T, Yamamoto K, Lin CY, Kataoka H. Loss of hepatocyte growth factor activator inhibitor type 1 participates in metastatic spreading of human pancreatic cancer cells in a mouse orthotopic transplantation model. Cancer Sci. 2014;105:44–51. doi: 10.1111/cas.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Kosa P, Liu X, Swaim WD, Lai Z, Cabrera-Perez J, Di Pasquale G, Ambudkar IS, Bugge TH, Chiorini JA. Matriptase deletion initiates a Sjögren’s syndrome-like disease in mice. PLoS One. 2014;9:e82852. doi: 10.1371/journal.pone.0082852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Cao J, Zhang X. Expression of serine protease SNC19/matriptase and its inhibitor hepatocyte growth factor activator inhibitor type 1 in normal and malignant tissues of gastrointestinal tract. World J Gastroenterol. 2005;11:6202–6207. doi: 10.3748/wjg.v11.i39.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti GL, Tanabe LM, Varela FA, Murray AS, Bergum C, Colombo É, Lang JE, Molinolo AA, Leduc R, Marsault E, et al. Targeting matriptase in breast cancer abrogates tumour progression via impairment of stromal-epithelial growth factor signalling. Nat Commun. 2015;6:6776. doi: 10.1038/ncomms7776. [DOI] [PMC free article] [PubMed] [Google Scholar]