Abstract
Hodgkin/Reed-Sternberg (HRS) cells in the setting of chronic lymphocytic leukemia (CLL) exist in two forms: type I with isolated HRS cells in a CLL background (Hodgkin-like lesion), and type II with typical classic Hodgkin lymphoma (CHL), a variant of Richter transformation (CHL-RT). The clinical significance of the two morphological patterns is unclear, and their biological features have not been compared. We retrospectively reviewed 77 cases: 26 of type I and 51 of type II CHL-RT; 3 cases progressed from type I to type II. We examined clinical features, EBV status, and clonal relatedness after microdissection. Median age for type I was 62 years vs. 73 years for type II (p=0.01). 27% (type I) vs. 73% (type II) had a history of CLL. HRS cells were positive for EBV in 71% (55/77), similar in type I and II. Clonality analysis was performed in 33 cases (type I and type II combined): HRS cells were clonally related to the underlying CLL in 14 and unrelated in 19. ZAP-70 expression of the CLL cells, but not EBV status or morphological pattern was correlated with clonal relatedness: all 14 clonally related cases were ZAP-70-negative while 74% (14/19) of clonally unrelated cases were ZAP-70-positive. Overall median survival (types I and II) after diagnosis was 44 months. Advanced age was an adverse risk factor for survival, but not histological pattern, type I vs type II. HRS-like cells in a background of CLL carries a similar clinical risk to that of CHL-RT, and may progress to CHL in some cases.
Keywords: Richter transformation, chronic lymphocytic leukemia, Hodgkin lymphoma, Epstein Barr virus, clonality, ZAP-70, Reed Sternberg cell
Introduction
Approximately 2.2% to 8% (average of 5%) patients with chronic lymphocytic leukemia (CLL) transform into high-grade lymphoma during their clinical course, clinically referred to as Richter syndrome (1, 2). Most cases are diffuse large B-cell lymphoma (DLBCL) (1, 3, 4). The classical Hodgkin lymphoma variant of Richter transformation (CHL-RT) is rare (0.4%-0.7%)(1-3), (5-7)
Two different histological patterns with features of CHL in CLL have been described. Type I is defined as HRS cells scattered in a background of CLL cells; while type II has typical CHL morphology showing HRS cells in polymorphous inflammatory background, largely segregated from CLL (8, 9). While type I morphology has many morphologic and phenotypic features in common with CHL, whether it should be considered as true CHL is still controversial(10, 11). Moreover, few data exist on the implications of a type I diagnosis for patient survival, and cases of type I have been omitted from large retrospective reviews on the subject(6). Our extensive experience with type I Hodgkin-like lesions based on our consultation practice gave us the opportunity to investigate the type I pattern in greater detail.
HRS cells in most cases of both types I and II have been reported as EBV-positive(12). A correlation between EBV positivity and history of Fludarabine treatment was noted, identifying the associated immune suppression as a risk factor(5, 6, 13, 14). However, EBV-negative cases are not uncommon(1, 2, 8, 15, 16). It remains challenging to draw a conclusion regarding EBV status and its clinical significance in type I and II processes from the small number of cases published in prior reports.
HRS cells can be clonally related to the underlying CLL or may arise as an independent, clonal process(8, 9, 15, 17-20). The association between a clonal relationship and the morphologic pattern has not been studied. Several studies suggested that clonally related HRS cells might be restricted to EBV-negative cases, whereas EBV-positive HRS cells might originate from an unrelated B-cell clone(8, 11, 18, 20). However, some studies have provided contradictory data arguing against these assumptions(15, 17). What factors determine the clonal relationship between HRS cells and pre-existing CLL are still elusive.
We compared 51 cases of CHL-RT with 26 cases of type I Hodgkin-like lesion submitted to our consultation service. Clinical characteristics, morphology, immunophenotype, EBV status, clonality and overall survival were analyzed.
Materials and methods
Case Selection
The pathology database of the Hematopathology Section, Laboratory of Pathology, National Cancer Institute, was searched for cases of CLL accrued since 1990 and reported as containing HRS cells or evidence of CHL. In total, 91 cases were identified. After initial review, 77 cases with 80 biopsies containing cells with the morphology and immunophenotype of HRS cells were included in this study. Among them, 3 cases had 2 sequential biopsies with the first exhibiting type I morphology and the second type II; these 3 cases were categorized as type I for further analysis. Fourteen cases were excluded due to insufficient information including EBV status, history of CLL, and follow-up. A history of CLL was determined from pathology reports and patient notes provided by referring physicians. The study was approved by the National Cancer Institute Institutional Review Board. Information on survival was obtained using the National Death Registry identified through DOBsearch.com, if not reflected in patient notes.
Immunohistochemical staining and in-situ hybridization
Sections of formalin fixed paraffin-embedded tissue were stained with antibodies against CD30, CD15, Pax5, CD20, EBV LMP1, CD3, CD5, and ZAP-70, using a standard automated technique (Dako or Bench Mark XT). EBV in-situ hybridization was performed using an EBV-encoded digoxigeninlabeled RNA riboprobe, targeting the EBER RNA transcript of EBV infection as previously described(21).
Laser capture microdissection (LCM)
CD30/Pax-5 double stained paraffin-embedded sections were used for laser microdissection, using a PixCell IIe laser capture microscope (Acturus Engineering, Santa Clara, CA). A small 7.5 mm spot size with high power at 100 mW and short duration time of 300 microseconds was used for single cell dissection. Approximately 50 CD30+ HRS cells, 200 Pax5+ CLL cells were dissected and used for direct PCR analysis.
PCR analysis for IGHrearrangement
After microdissection, the thermoplastic membranes containing dissected cells were lifted from LCM cap, mixed with Gene Releaser resin (Bioventures, Murfreesboro, TN), pre-incubated in a Perkin Elmer 480 thermocycler (Applied Biosystems, Foster City, CA), and then used for PCR for IgVH framework III (FR III)/CDRIII gene rearrangement as previously described(21-23). The second semi-nested PCR was performed using 2 μl of 1:100 diluted first PCR product as template with nested JH primer ( 5’ACCAGGGTCCCTTGGCCCCA3’ )(9) and same VH primer of 1st round PCR. The PCR products were then analyzed on 16% polyacrylamide gels and stained with ethidium bromide for visualization(21).
Statistics
Student t-test, Fisher exact test and Log-rank (Mantel-Cox) test were performed using Prism 6 (GraphPad Software Inc., La Jolla, CA). All reported p values are two-sided with a type I error rate of 5% and a p<0.05 set for significance.
Results
Clinical characteristics
Seventy-seven cases with Hodgkin-like (type I) or CHL (type II) features were identified with a male to female ratio of 1.9 (50:27), consistent with a male predominance in CLL(24). The median age at time of diagnosis (type I or type II) was 69 years old (range: 42-86). Fifty-seven per cent (44/77) had a prior history of CLL, with the mean time to Hodgkin or Hodgkin-like lesion from CLL diagnosis of 36 months. The diagnosis of the Hodgkin or Hodgkin-like lesion was made in biopsies from lymph node (58/77, 75%), bone marrow (9/77, 12%), spleen (4/77, 5.2%) and extranodal sites (6/77, 7.8%). The extranodal sites were liver (2), lung (2), pleura (1), and stomach (1).
Type I versus type II patterns by morphology, immunohistochemistry and EBV status
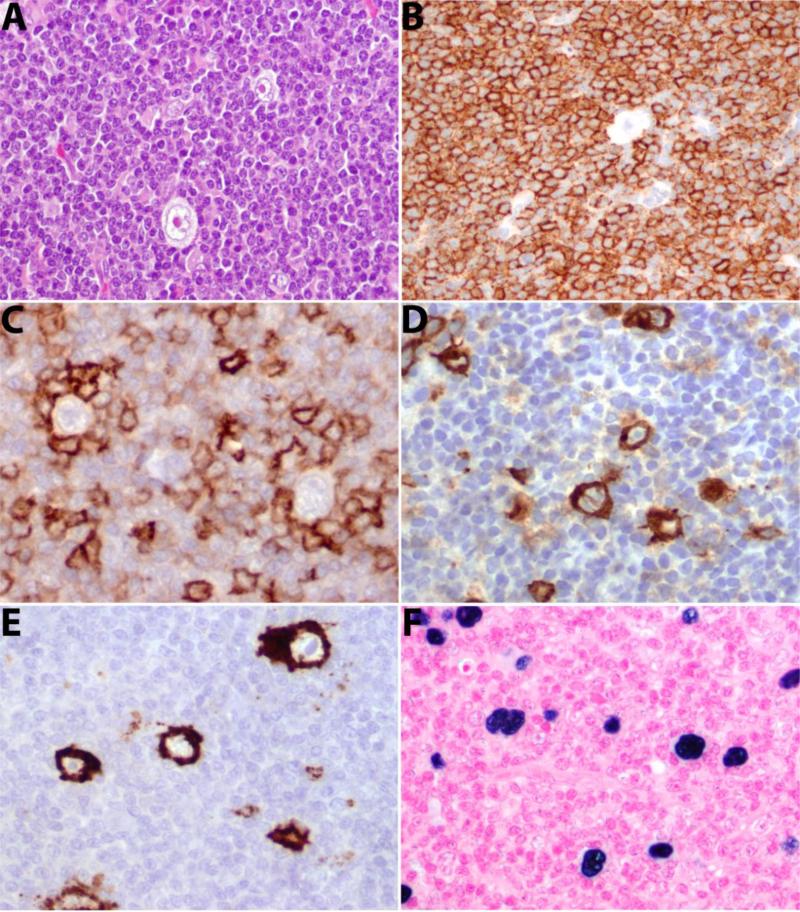
Twenty-six cases were classified as type I with scattered HRS cells in a background of CLL (Figure 1). The cytological features were typical of CLL in most cases, but 2 cases showed slight nuclear irregularity. Four cases showed increased prolymphocytes associated with expanded proliferation centers. Mixed inflammatory cells such as plasma cells and eosinophils were rare. One case had increased eosinophils interspersed with CLL cells; 4 had increased histiocytes with 1 showing focal non-necrotizing granulomas. HRS cells were distributed throughout the tissue sections. We enumerated HRS cells in at least 10 high power fields for each tissue section. The median number of HRS cells was 4 per high power field (range: 1-20). Although the majority of the background lymphocytes were CLL-cells, most individual HRS cells, even in type I, were associated with T-cells (CD3+), often forming rosettes.
Figure 1. Histological features of type I Hodgkin-like lesion.
A. Scattered HRS cells in a background of CLL. B. CD20 is diffusely positive but HRS cells are negative. C. CD5 is weakly positive in background CLL cells and strongly positive in rosetting T-cells. HRS cells are positive for CD30 (D), CD15 (E) and EBV (EBER) (F).
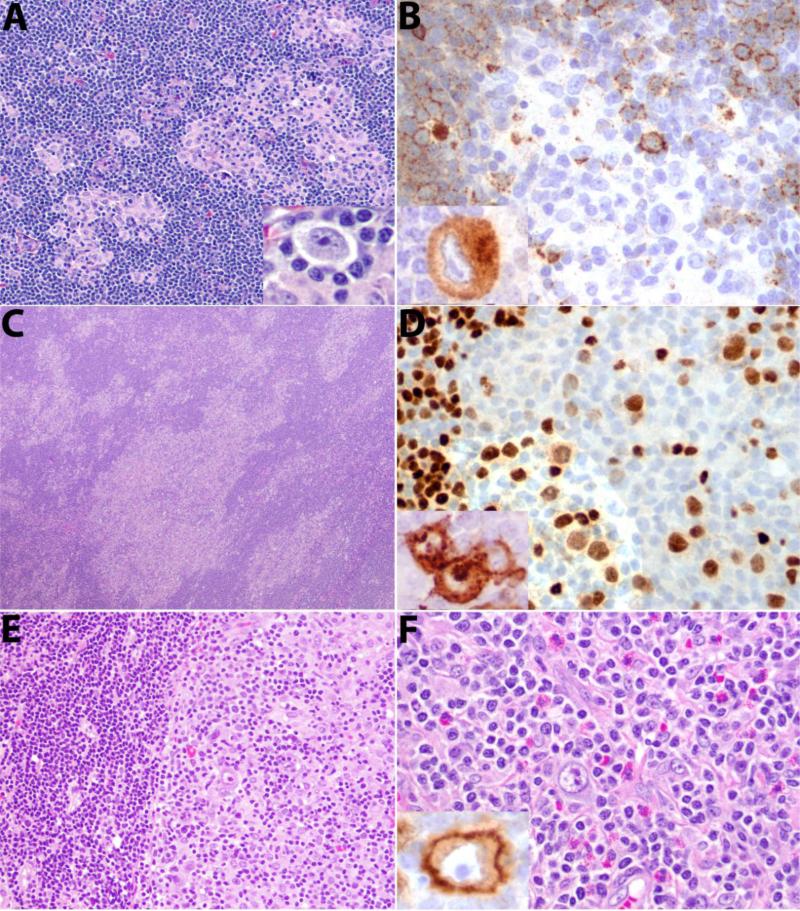
Fifty-one cases were classified as type II, in which HRS cells were present in a polymorphous inflammatory background including reactive lymphocytes, eosinophils, plasma cells and histiocytes. A minority of patients (25%, 13/51) had islands of CHL, with portions of the lymph node containing more a typical CLL (moth-eaten pattern, Figure 2 A-D). In 22 cases (43%) the areas involved by CHL formed a confluent sheet, adjacent to but distinctly segregated from areas of CLL (segregated pattern, Figure 2E-F). Interestingly, in cases with segregated CLL and CHL, the CLL areas usually contained scattered HRS cells, resembling type I. The type II pattern also included synchronous cases with CHL at one site and CLL at another site (separate sites, 31%, 16/51). No clinical differences were observed related to the three histological patterns of type II.
Figure 2. Histological features of type II CHL-RT.
A. Moth-eaten pattern, with foci of CHL in a background of CLL. Insert in A shows HRS cell. B. CD20 is weakly positive in CLL negative in HRS cell and surrounding T-cells. Inset shows CD30 staining of an HRS cell. C. Moth-eaten pattern, more extensive involvement. HRS cells are weakly positive for PAX5 in D, and are positive for CD15 as shown in inset. E. Segregated pattern. A distinct border is seen between CLL and CHL F. Typical histological features of CHL are present with mixed inflammatory background. Inset shows CD30 positivity of HRS cell.
The immunophenotype and EBV status of the HRS cells were similar in both type I and II cases (Table 1). Overall the HRS cells were positive for CD30 (100%) and CD15 (88%). CD20 was variably positive in HRS cells of 49% of cases (52% in type I versus 47% in type II, p=0.44); PAX-5 was positive in all cases examined. Regarding EBV status, 71% (55/77) were positive (50 EBER+, 2 EBER-/LMP1+, 3 LMP1+/EBER not done), and 29% (22/77) negative (17 EBER-/LMP1-, 5 EBER-/LMP1 not done).
Table 1.
Characterization of type I Hodgkin-like lesion and type II CHL-RT
| Type I | Type II | |
|---|---|---|
| Number (N) | 26 | 51 |
| Median age in years (range)* | 62 (42-86) | 73 (46-85) |
| Gender (M:F ratio) | 19:7 (2.7) | 31:20 (1.6) |
| Prior CLL diagnosis# | 27% (7/26) | 73% (37/51) |
| Sites (N)# | LN 25, BM 1 | LN 33, Spleen 4, BM 8, extranodal 6§ |
| CD20+ | 52% (12/23) | 47% (23/49) |
| PAX-5+ | 100% (20/20) | 100% (31/31) |
| CD30+ | 100% (26/26) | 100% (51/51) |
| CD15+ | 78% (18/23) | 92% (46/50) |
| EBV+ | 65% (17/26) | 75% (38/51) |
| ZAP-70+ | 57% (8/14) | 32% (6/19) |
| Clonally related | 29% (4/14) | 53% (10/19) |
p=0.01 by student t-test
p<0.005 by Fisher exact test,
Includes liver (2), lung (2), pleura (1), and stomach (1).
Clinical Correlations with morphology and EBV status
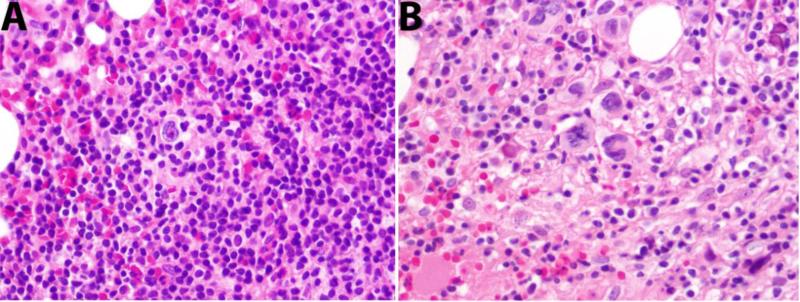
The clinicopathological findings were compared between type I and II. The median age at the time of Hodgkin/Hodgkin-like diagnosis was 62 years in patients with type I as compared to 73 years with type II (p=0.01, Table 1). The male to female ratios were similar. Only 27% of type I cases had a prior diagnosis of CLL in contrast to 73% of type II. Among the patients with a prior CLL diagnosis, the mean time to Hodgkin/Hodgkin-like diagnosis was not different between type I and II (63 months vs. 71 months, p=0.7). All of the 6 cases diagnosed in extranodal sites were type II (Table 1). Three cases had two sequential biopsies from the same or different sites, the first being type I and the second type II (Figure 3 and Supplemental Table 1).
Figure 3. Progression from type I to type II.
A. Type I pattern is present in the bone marrow. B. Two years later CHL diffusely involved the bone marrow (type II) with no histological evidence of CLL.
In order to understand the significance of EBV infection in the Hodgkin/Hodgkin-like process, clinicopathological features were compared between the EBV positive and negative cohorts (Supplemental Table 2). All characteristics examined were similar, including age, gender, time to Hodgkin/Hodgkin-like diagnosis from CLL diagnosis, history of CLL, sites, type I vs II pattern, and expression of ZAP-70.
Clonal relationship between CLL and HRS cells
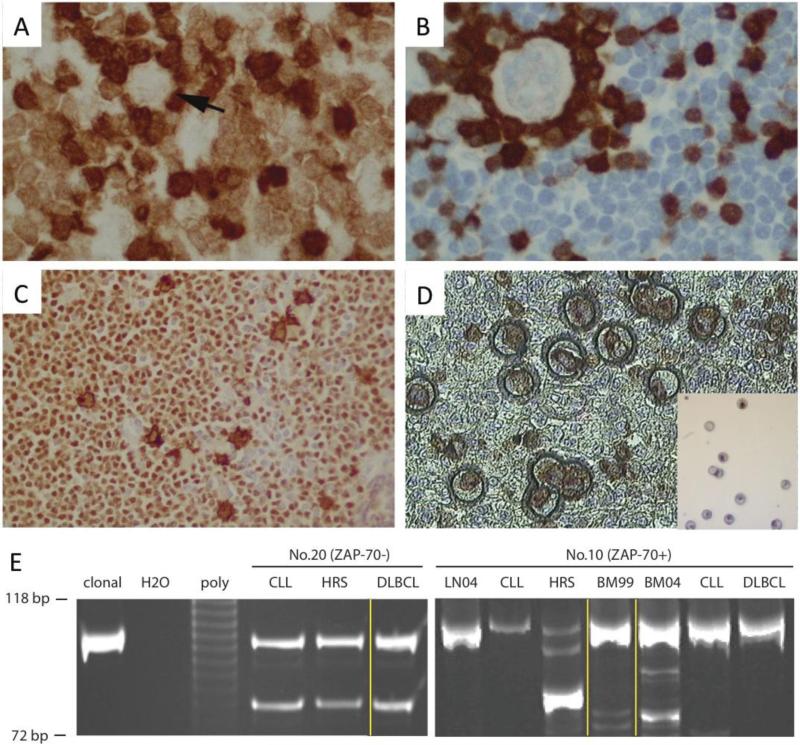
In order to compare clonality, CD30+ HRS cells were microdissected after CD30/Pax5 double staining and directly used for PCR analysis for IGH FRIII/CDRIII rearrangement in 33 cases with adequate materials (Figure 4). The amplified PCR products from HRS cells and CLL cells were compared by gel electrophoresis. To this end, HRS cells in 14 cases were clonally related to the CLL component while 19 cases were unrelated. No clinical differences were observed between clonally related and unrelated cases (Supplemental Table 3).
Figure 4. Microdissection and Clonal Analysis.
A. ZAP-70 positive CLL. Arrow indicates a HRS cell (ZAP-70 negative). T cells are strongly positive for ZAP-70. B. ZAP-70 negative CLL. ZAP-70 positive T cells rosette a HRS cell. C. PAX-5 and CD30 double staining. HRS cells are double positive and background CLL cells are PAX-5 positive but CD30 negative. D. Microdissection. Insert shows HRS cells after microdissection. E Clonal analysis of two concurrent CHL-RT and DLBCL-RT cases. DNA molecular markers with 118 bp and 72 bp shown; clonal, monoclonal positive control; poly, polyclonal control. Microdissected CLL, HRS and DLBCL cells were compared. Yellow lines indicate different sites. Left panel (ZAP-70− case): HRS and CLL cells microdissected from an inguinal lymph node, and DLBCL microdissected from bone marrow. Right panel (ZAP-70+ case): LN04, CLL, HRS from a supraclavicular lymph node in 2004; BM99 from bone marrow in 1999 when CLL was initially diagnosed; BM04, CLL and DLBCL from bone marrow in 2004.
CLL is a heterogeneous entity with two distinct subtypes: IGHV mutated or unmutated(24). Immunostaining for ZAP-70 expression in the CLL cells has been used as a surrogate marker for mutational status(8, 25, 26), and was performed on all cases subjected to clonality assessment. Clonally related and unrelated cases were compared. The underlying CLL was negative for ZAP-70 in all cases with clonally related HRS cells. In contrast, 74% (14/19) of clonally unrelated CLL cases were positive for ZAP-70 (Table 3 and Supplemental Table 3). Examining the data from the perspective of ZAP-70, 14/19 (74%) ZAP-70 negative cases showed a clonal relationship to the HRS cells while 0/14 ZAP-70 positive cases did (Table 3 and Supplemental Table 4). These results indicate that HRS cells arise de novo rather than from the CLL clone in ZAP-70 positive (IGHV unmutated) CLL, while in ZAP-70 negative (IGHV mutated) CLL, HRS cells are predominantly derived from the CLL clone, although clonally unrelated HRS cells were also seen in a minority of these cases.
Table 3.
The difference in clonal relationship between CHL-RT vs DLBCL-RT: Review of the literature and current report
| CHL or Hodgkin-like lesion§ |
DLBCL§§ |
|||
|---|---|---|---|---|
| Clonally related | Clonally unrelated | Clonally related | Clonally unrelated | |
| IGHV unmutated CLL | 0 | 2 | 47 | 6 |
| IGHV mutated CLL | 5 | 0 | 18 | 12 |
| ZAP-70+ CLL | 0 | 14 | 18 | 3 |
| ZAP-70- CLL | 14 | 5 | 10 | 1 |
CHL data: 7 cases from prior reports (IGHV cases) 15, 17, 18, 20 and 33 cases from our study (ZAP-70 cases).
DLBCL data: 83 cases (IGHV cases) are from two prior reports 4, 11. In one of the prior studies, ZAP-70 was also examined4; therefore, the cases (ZAP-70 cases) were combined with 2 cases from our study.
We further examined the correlation of clonal relationship with EBV status. Forty-three per cent (3/7) EBV negative and 42% (11/26) EBV positive cases were clonally related to the associated CLL (Supplemental Table 2). Thus, EBV status did not predict influence the clonal relationship between HRS cells and CLL cells.
Two cases presented with concurrent type II transformation (CHL-RT) and DLBCL-RT. In one ZAP-70 negative case, both CLL and DLBCL involved bone marrow, while the CHL-RT involved an inguinal node. After microdissection, both HRS cells and DLBCL cells shared same clone as the CLL cells (Figure 4E left panel). In a second case the patient was initially diagnosed as CLL, positive for ZAP-70, involving bone marrow, and 5 years later developed CHL-RT in a supraclavicular lymph node and DLBCL-RT in bone marrow. By PCR, the same clone was detected in the original CLL, and the DLBCL, while the clone associated with the HRS cells was distinct (Figure 4E right panel).
Survival analysis
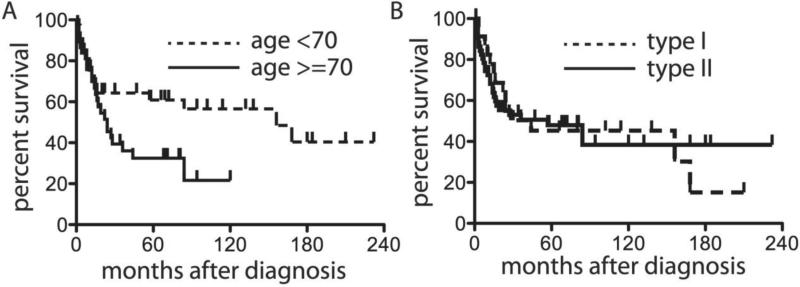
We attempted to analyze what factors affected overall survival following a diagnosis of either type I (Hodgkin-like) or type II (CHL-RT). Forty-one patients had died at the end of this study with a median follow-up of 22 months. The overall median survival after the diagnosis of either type I or type II was 44 months (Supplemental Figure 1A). Advanced age was the strongest predictor for shorter overall survival: patients with age <70 years had a median survival of 156 months after diagnosis compared to 24 months in patients with age ≥70 years (p=0.03, Table 2 and Figure 5A). There was no difference in overall survival between type I and II cases (Figure 5B). Other characteristics including extranodal presentation, negative EBV status and a clonal relationship showed no significant difference in survival (Supplemental Figure 1 and Table 2).
Table 2.
Univariate analysis of survival using Log-rank (Mantel-Cox) test
| Median survival (months) | Hazard ratio (95%CI) | p value | |
|---|---|---|---|
| Age (years) | |||
| <70 (N=40) | 156 | 0.50 (0.26-0.9) | 0.03 |
| ≥70 (N=37) | 24 | ||
| Sex | |||
| Male (N=50) | 57 | 0.91 (0.5-1.7) | 0.78 |
| Female (N =27) | 36 | ||
| Sites | |||
| Nodal (N =71) | 44 | 0.46 (0.1-1.7) | 0.25 |
| Extranodal (N =6) | 14 | ||
| History of CLL | |||
| Yes (N =44) | 57 | 1.1 (0.6-2.1) | 0.70 |
| No (N =33) | 44 | ||
| Morphological types | |||
| Type I (N =26) | 44 | 0.98 (0.5-1.9) | 0.95 |
| Type II (N =51) | 57 | ||
| EBV | |||
| Positive (N =55) | 57 | 0.9 (0.5-1.8) | 0.79 |
| Negative (N =22) | 28 | ||
| ZAP-70 | |||
| Positive (N =14) | 24 | 0.94 (0.4-2.1) | 0.89 |
| Negative (N =19) | 17 | ||
| Clonal relationship | |||
| Unrelated (N =19) | 24 | 0.68 (0.30-1.6) | 0.36 |
| Related (N =14) | 15 |
Figure 5. Kaplan-Meier analysis of survival after type I and type II diagnoses.
Age greater than 70 influences survival (A), but no differences are seen for type I vs. type II (B).
Discussion
The presence of HRS-like cells in a background of CLL was first noted 25 years ago (27), but how this finding relates to CHL as a form of Richter's transformation has been largely unexamined. In this study we compare the clinical and biological features of these two forms of progression in CLL. While it remains uncertain whether the type I pattern (Hodgkin-like lesion) should be considered de facto evidence of CHL, the overall survival was similar for type I and type II from the time of the “Hodgkin” diagnosis, approximately 44 months. In our study for most patients, the diagnosis of a type I Hodgkin-like lesion was the initial finding leading to a diagnosis of CLL; only 27% of patients had a prior diagnosis of CLL as opposed to 73% with a type II pattern. In addition, the median age of type I patients was 11 years younger than type II. These data suggest that the type I pattern is a relatively early event in the evolution of the disease, and one that is not influenced by prior treatment, at least in a subset of patients. In the largest retrospective review of CHL-RT in the literature, Bockorny et al identified 10% of patients presenting with simultaneous CLL and CHL, although those authors did not report cases with type I histology.(6)
It has been questioned whether type I histology should be considered a true form of CHL. We identified three patients in the current study in whom sequential biopsies showed progression from type I to type II. In addition, this progression was reported previously in 2 additional patients (Supplemental Table 1) (12, 27). Additionally, in 22 patients with segregated CLL and CHL in the same biopsy site, the CLL component usually contained scattered HRS-like cells. Thus, the type I pattern appears to have the potential to progress to usual CHL.
The clonal relationship between CLL and HRS cells has been the focus of investigation for many years. Both clonally related and unrelated cases were demonstrated by gel electrophoresis or nucleotide sequencing(8, 9, 11, 16-20). It had been suggested that HRS cells in patients with the type I pattern are clonally related to the underlying CLL, especially when the HRS cells express B cell markers(9, 11). However, Kanzler et al reported that 2 out of 3 cases with the type I pattern were clonally distinct from CLL(17). Our results showed that 29% of type I cases were clonally related as compared to 53% of type II (not statistically different), suggesting that the histological pattern does not predict for a clonal relationship. It must be acknowledged that there are technical challenges in studying Type I cases, since the HRS cells are intimately admixed with the CLL cells and contamination with a small number of CLL cells is difficult to exclude.
Among all other factors we compared, only ZAP-70 positivity strongly correlated with a clonal relationship. ZAP-70 has been used as surrogate marker for IGHV mutational status in CLL, and also has independent clinical and biological significance(28). Most of the ZAP-70 positive CLL are IGHV unmutated, whereas ZAP-70 negative cases are IGHV mutated(8, 25, 26). Both IGHV mutated and unmutated CLL cells are “antigen-experienced” and antigen-selected B-cells, encountering antigen in T-dependent vs. T-independent manner(29). HRS cells in sporadic CHL are believed to be post-germinal center (GC) mutated B-cells harboring mutated IG genes(30). One might conclude that clonally related CHL-RT might be more likely to develop in mutated CLL(25, 31, 32). Several cases reported in the literature fit this assumption: all published 5 IGHV mutated CLL patients had developed clonally related HRS cells(15, 17-19) while 2 IGVH unmutated CLL patients had clonally unrelated CHL-RT(17). The results from our large study further support this scenario. We show that HRS cells from all ZAP-70 positive (IGHV unmutated) CLL cases were clonally unrelated (Table 3), whereas HRS cells from ZAP-70 negative (IGHV mutated) CLL cases often share the same clonal origin with the associated CLL. Infrequently, clonally unrelated HRS cells can occur in ZAP-70 negative (IGHV mutated) CLL as a true secondary neoplasm.
DLBCL-RT shows some differences from CHL-RT in terms of clonal derivation. Two recent studies reporting a total of 83 patients with DLBCL-RT, showed that the majority of cases of DLBCL were clonally related to CLL regardless of IGHV mutational status (Table 3)(4, 11). The results from two interesting cases with concurrent CHL-RT and DLBCL-RT presented in our study further support this observation.
We confirm that the vast majority of cases of both types I and II are associated with EBV infection of the HRS cells, 71% in our series. Data from small case series had suggested that EBV negative HRS cells were often clonally related to CLL cells, whereas EBV positive HRS cells were unrelated(8, 11, 17, 18, 20). We could not confirm that the presence of EBV influenced the clonal relationship with the underlying CLL.
In general, type II transformation, so called CHL-RT has a poor prognosis. Previous studies have shown a wide range in the median survival of CHL-RT patients from 4 to 40 months (2, 6, 7, 33, 34). A history of Fludarabine or other purine analogue treatment was associated with a worse prognosis (2, 6, 33, 34). A comprehensive review of 88 CHL-RT cases reported in the literature demonstrated that median survival without Fludarabine treatment was 25 months in contrast to 8.4 months with Fludarabine(6). The overall median survival of 44 months of combined type I and II diagnoses in the current study is comparable to published data (7).Interestingly, we saw no difference in survival between patients with type I vs. type II histology. However, we confirm that consistent with a recent report, advanced age is a strong predictor for a poor prognosis(7). Additionally, extranodal presentation, negative EBV status and a clonal relationship also showed a trend towards to inferior survival, but did not reach statistical significance (Table 2). Similar findings have been reported in DLBCL-RT(4). Due to the retrospective nature of this study, and lack of detailed treatment information, our survival analysis needs to be interpreted with caution.
We used ZAP-70 as a surrogate marker for IGHV mutational status. Sequencing IGHV is still the gold standard for mutational status; however, fresh/frozen tissue is preferable if Sanger sequencing is utilized. Formalin fixed paraffin embedded (FFPE) tissue has nevertheless been used in very few studies with conflicting results when compared to frozen tissue (35) (36, 37). Given the wide variation in DNA quality obtained from FFPE sections, we elected to use ZAP-70 as a surrogate marker, which could be applied to a larger number of cases. HRS cells in ZAP-70+ (IGHV unmutated) CLL appear to be more often clonally unrelated, whereas clonally related HRS cells were strongly associated with ZAP-70− (IGHV mutated) CLL. However, in the patients analyzed, ZAP-70 status did not appear to influence survival after diagnosis of the Hodgkin-like process.
In summary, our study suggests that type I morphology might be an early stage of transformation that can progress to type II CHL-RT. Our data also indicate that patients with type I and type II had similar overall survival following the diagnosis, indicating the need for therapeutic intervention. However, this study cannot resolve whether the type I pattern should be considered bona fide evidence of CHL, comparable to type II, nor are we in a position to recommend specified treatment regimens for these patients.
Supplementary Material
Acknowledgements
This study was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. Intramural financial support is provided under the following Project: ZIA SC 000550: Lymphoma Disease Discovery and Definition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
References
- 1.Tsimberidou AM, Keating MJ. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer. 2005;103:216–228. doi: 10.1002/cncr.20773. [DOI] [PubMed] [Google Scholar]
- 2.Tsimberidou AM, Keating MJ. Richter's Transformation in Chronic Lymphocytic Leukemia. Semin Oncol. 2006;33:250–256. doi: 10.1053/j.seminoncol.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura N, Abe M. Richter syndrome in B-cell chronic lymphocytic leukemia. Pathol Int. 2003;53:195–203. doi: 10.1046/j.1320-5463.2003.01455.x. [DOI] [PubMed] [Google Scholar]
- 4.Rossi D, Spina V, Deambrogi C, et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood. 2011;117:3391–3401. doi: 10.1182/blood-2010-09-302174. [DOI] [PubMed] [Google Scholar]
- 5.Adiga GU, Abebe L, Wiernik PH. Partially successful treatment of a patient with chronic lymphocytic leukemia and Hodgkin's disease: case report and literature review. Am J Hematol. 2003;72:267–273. doi: 10.1002/ajh.10300. [DOI] [PubMed] [Google Scholar]
- 6.Bockorny B, Codreanu I, Dasanu CA. Hodgkin lymphoma as Richter transformation in chronic lymphocytic leukaemia: a retrospective analysis of world literature. Br J Haematol. 2012;156:50–66. doi: 10.1111/j.1365-2141.2011.08907.x. [DOI] [PubMed] [Google Scholar]
- 7.Parikh SA, Habermann TM, Chaffee KG, et al. Hodgkin transformation of chronic lymphocytic leukemia: Incidence, outcomes, and comparison to de novo Hodgkin lymphoma. Am J Hematol. 2015;90:334–338. doi: 10.1002/ajh.23939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Leval L, Vivario M, De Prijck B, et al. Distinct clonal origin in two cases of Hodgkin's lymphoma variant of Richter's syndrome associated With EBV infection. Am J Surg Pathol. 2004;28:679–686. doi: 10.1097/00000478-200405000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Ohno T, Smir BN, Weisenburger DD, Gascoyne RD, Hinrichs SD, Chan WC. Origin of the Hodgkin/Reed-Sternberg cells in chronic lymphocytic leukemia with “Hodgkin's transformation”. Blood. 1998;91:1757–1761. [PubMed] [Google Scholar]
- 10.Kuppers R, Duhrsen U, Hansmann ML. Pathogenesis, diagnosis, and treatment of composite lymphomas. Lancet Oncol. 2014;15:e435–446. doi: 10.1016/S1470-2045(14)70153-6. [DOI] [PubMed] [Google Scholar]
- 11.Mao Z, Quintanilla-Martinez L, Raffeld M, et al. IgVH mutational status and clonality analysis of Richter's transformation: diffuse large B-cell lymphoma and Hodgkin lymphoma in association with B-cell chronic lymphocytic leukemia (B-CLL) represent 2 different pathways of disease evolution. Am J Surg Pathol. 2007;31:1605–1614. doi: 10.1097/PAS.0b013e31804bdaf8. [DOI] [PubMed] [Google Scholar]
- 12.Momose H, Jaffe ES, Shin SS, Chen YY, Weiss LM. Chronic lymphocytic leukemia/small lymphocytic lymphoma with Reed-Sternberg-like cells and possible transformation to Hodgkin's disease. Mediation by Epstein-Barr virus. Am J Surg Pathol. 1992;16:859–867. doi: 10.1097/00000478-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lazzarino M, Orlandi E, Baldanti F, et al. The immunosuppression and potential for EBV reactivation of fludarabine combined with cyclophosphamide and dexamethasone in patients with lymphoproliferative disorders. Br J Haematol. 1999;107:877–882. doi: 10.1046/j.1365-2141.1999.01765.x. [DOI] [PubMed] [Google Scholar]
- 14.Thornton PD, Bellas C, Santon A, et al. Richter's transformation of chronic lymphocytic leukemia. The possible role of fludarabine and the Epstein-Barr virus in its pathogenesis. Leukemia research. 2005;29:389–395. doi: 10.1016/j.leukres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Fong D, Kaiser A, Spizzo G, Gastl G, Tzankov A. Hodgkin's disease variant of Richter's syndrome in chronic lymphocytic leukaemia patients previously treated with fludarabine. Br J Haematol. 2005;129:199–205. doi: 10.1111/j.1365-2141.2005.05426.x. [DOI] [PubMed] [Google Scholar]
- 16.Rubin D, Hudnall SD, Aisenberg A, Jacobson JO, Harris NL. Richter's transformation of chronic lymphocytic leukemia with Hodgkin's-like cells is associated with Epstein-Barr virus infection. Mod Pathol. 1994;7:91–98. [PubMed] [Google Scholar]
- 17.Kanzler H, Kuppers R, Helmes S, et al. Hodgkin and Reed-Sternberg-like cells in B-cell chronic lymphocytic leukemia represent the outgrowth of single germinal-center B-cell-derived clones: potential precursors of Hodgkin and Reed-Sternberg cells in Hodgkin's disease. Blood. 2000;95:1023–1031. [PubMed] [Google Scholar]
- 18.Kuppers R, Sousa AB, Baur AS, Strickler JG, Rajewsky K, Hansmann ML. Common germinal-center B-cell origin of the malignant cells in two composite lymphomas, involving classical Hodgkin's disease and either follicular lymphoma or B-CLL. Mol Med. 2001;7:285–292. [PMC free article] [PubMed] [Google Scholar]
- 19.Pescarmona E, Pignoloni P, Mauro FR, et al. Hodgkin/Reed-Sternberg cells and Hodgkin's disease in patients with B-cell chronic lymphocytic leukaemia: an immunohistological, molecular and clinical study of four cases suggesting a heterogeneous pathogenetic background. Virchows Arch. 2000;437:129–132. doi: 10.1007/s004280000214. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg A, Maggio E, Rust R, Kooistra K, Diepstra A, Poppema S. Clonal relation in a case of CLL, ALCL, and Hodgkin composite lymphoma. Blood. 2002;100:1425–1429. [PubMed] [Google Scholar]
- 21.Lim MS, Beaty M, Sorbara L, et al. T-cell/histiocyte-rich large B-cell lymphoma: a heterogeneous entity with derivation from germinal center B cells. Am J Surg Pathol. 2002;26:1458–1466. doi: 10.1097/00000478-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Segal GH, Jorgensen T, Scott M, Braylan RC. Optimal primer selection for clonality assessment by polymerase chain reaction analysis: II. Follicular lymphomas. Hum Pathol. 1994;25:1276–1282. doi: 10.1016/0046-8177(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 23.Taddesse-Heath L, Pittaluga S, Sorbara L, Bussey M, Raffeld M, Jaffe ES. Marginal zone B-cell lymphoma in children and young adults. Am J Surg Pathol. 2003;27:522–531. doi: 10.1097/00000478-200304000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 25.Carreras J, Villamor N, Colomo L, et al. Immunohistochemical analysis of ZAP-70 expression in B-cell lymphoid neoplasms. J Pathol. 2005;205:507–513. doi: 10.1002/path.1727. [DOI] [PubMed] [Google Scholar]
- 26.Wiestner A, Rosenwald A, Barry TS, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101:4944–4951. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 27.Williams J, Schned A, Cotelingam JD, Jaffe ES. Chronic lymphocytic leukemia with coexistent Hodgkin's disease: Implications for the origin of the Reed-Sternberg cell. Am J Surg Pathol. 1991;15:33–42. doi: 10.1097/00000478-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Wagner M, Oelsner M, Moore A, et al. Integration of innate into adaptive immune responses in ZAP-70-positive chronic lymphocytic leukemia. Blood. 2016;127:436–448. doi: 10.1182/blood-2015-05-646935. [DOI] [PubMed] [Google Scholar]
- 29.Ghia P, Caligaris-Cappio F. The origin of B-cell chronic lymphocytic leukemia. Semin Oncol. 2006;33:150–156. doi: 10.1053/j.seminoncol.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Kuppers R, Rajewsky K. The origin of Hodgkin and Reed/Sternberg cells in Hodgkin's disease. Annu Rev Immunol. 1998;16:471–493. doi: 10.1146/annurev.immunol.16.1.471. [DOI] [PubMed] [Google Scholar]
- 31.Admirand JH, Rassidakis GZ, Abruzzo LV, Valbuena JR, Jones D, Medeiros LJ. Immunohistochemical detection of ZAP-70 in 341 cases of non-Hodgkin and Hodgkin lymphoma. Mod Pathol. 2004;17:954–961. doi: 10.1038/modpathol.3800145. [DOI] [PubMed] [Google Scholar]
- 32.Brauninger A, Schmitz R, Bechtel D, Renne C, Hansmann ML, Kuppers R. Molecular biology of Hodgkin's and Reed/Sternberg cells in Hodgkin's lymphoma. Int J Cancer. 2006;118:1853–1861. doi: 10.1002/ijc.21716. [DOI] [PubMed] [Google Scholar]
- 33.Jamroziak K, Grzybowska-Izydorczyk O, Jesionek-Kupnicka D, Gora-Tybor J, Robak T. Poor prognosis of Hodgkin variant of Richter transformation in chronic lymphocytic leukemia treated with cladribine. Br J Haematol. 2012;158:286–288. doi: 10.1111/j.1365-2141.2012.09127.x. author reply 289. [DOI] [PubMed] [Google Scholar]
- 34.Tadmor T, Shvidel L, Goldschmidt N, et al. Hodgkin's variant of Richter transformation in chronic lymphocytic leukemia; a retrospective study from the Israeli CLL study group. Anticancer Res. 2014;34:785–790. [PubMed] [Google Scholar]
- 35.Yeung CC, Powers ML, Nguyen TD, et al. Relevance of IgVH gene somatic hypermutation and interphase cytogenetics in lymphomatous presentation of chronic lymphocytic leukemia/small lymphocytic lymphoma. International journal of surgical pathology. 2011;19:563–569. doi: 10.1177/1066896911406918. [DOI] [PubMed] [Google Scholar]
- 36.Walsh SH, Thorselius M, Johnson A, et al. Mutated VH genes and preferential VH3-21 use define new subsets of mantle cell lymphoma. Blood. 2003;101:4047–4054. doi: 10.1182/blood-2002-11-3479. [DOI] [PubMed] [Google Scholar]
- 37.Lai R, Lefresne SV, Franko B, et al. Immunoglobulin VH somatic hypermutation in mantle cell lymphoma: mutated genotype correlates with better clinical outcome. Mod Pathol. 2006;19:1498–1505. doi: 10.1038/modpathol.3800677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.