Abstract
Non-Hodgkin lymphoma (NHL) constitutes a collection of lymphoproliferative disorders with widely varying biologic, histologic and clinical features. For the B-cell NHLs, great progress has been made due to the addition of monoclonal antibodies and, more recently, other novel agents such as B-cell receptor signaling inhibitors, immunomodulatory agents, and proteasome inhibitors. Autologous hematopoietic cell transplantation (auto-HCT) offers the promise of cure or prolonged remission in some NHL patients. For some patients, however, auto-HCT may never be a viable option, while in others their disease may progress despite auto-HCT. In those settings, allogeneic HCT (allo-HCT) offers the potential for cure. Over the past 10–15 years, considerable progress has been made in the implementation of allo-HCT, such that this approach now is a highly effective therapy for patients up to (and even beyond) age 75. Recent advances in conventional lymphoma therapy, peri-transplant supportive care, patient selection, and donor selection (including the use of alternative hematopoietic cell donors), has allowed broader application of allo-HCT to NHL patients. As a result, an ever-increasing number of NHL patients over age 60–65 years stand to benefit from allo-HCT. In this review, we present data in support of the use of allo-HCT for patients with diffuse large B-cell lymphoma, follicular lymphoma, and mantle cell lymphoma. These histologies account for a large majority of allo-HCT performed for patients over 60 in the U.S. Where possible, we highlight available data in older patients. This body of literature strongly supports the concept that allo-HCT should be offered to fit patients well beyond age 65 and, accordingly, that this treatment should therefore be covered by their insurance carriers.
INTRODUCTION
In many cases of high risk NHL, the only potentially curative option for patients (regardless of age) remains allo-HCT. With recent advances in pre-, peri-, and post- transplant care, allo-HCT can now successfully be applied to a wider group of patients, including those with advanced age. While it is true that advanced age does increase the risk of transplant-related complications and non-relapse mortality, allo-HCT still often represents the only realistic option for cure for many older patients. After careful consideration of risks, benefits, and alternatives to allo-HCT, an increasing number of patients into their 70s are electing to undergo allo-HCT.
We present separate sections in which we briefly review the features of three B-cell NHL subtypes: diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), and mantle cell lymphoma (MCL). These three lymphoma histologies accounted for 73% of allo-HCT performed for patients over age 60 in the U.S. between 2010–2014. [1] In each section, we include a brief overview of the use of non-transplant frontline therapies. We next briefly review outcomes for auto-HCT, and then focus primarily on available data supporting the use of allo-HCT. We then discuss data specifically supporting allo-HCT in elderly lymphoma patients.
DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL)
Overview of DLBCL and non-transplant options for frontline therapy
DLBCL, the most common type of aggressive non-Hodgkin Lymphoma, encompasses several clinical-pathologic entities. While risk factors include HIV infection, solid organ transplantation, and auto-immune disorders, most cases are sporadic and occur predominantly in individuals age >60 years with no obvious predisposing factors. Overall, 60% of those affected will be cured with chemoimmunotherapy. Clinical factors such as the NCCN-International Prognostic Index (NCCN-IPI), however, delineate a wide range of results identifying groups with likelihood of 5-year survival as high as 96% and others as low as 38%. [2,3] A greater understanding of biology and genetics has refined prognostic markers with potential therapeutic implications, such as the putative cell of origin (defined by gene expression profile or immunohistochemistry),[4,5] and translocation or overexpression of the MYC oncogene. [6]
Despite these advances, several disease subsets represent high-risk disease, clinically defined by the NCCN-IPI [2], or biologically identified, for example the “double-hit” and “triple-hit” lymphomas (i.e. those with MYC, and BCL2 and/or BCL6 translocations). Particularly for double and triple hit lymphomas, standard frontline therapies are ineffective for providing long-term remission, and cure is rarely achieved in the relapsed and refractory settings. Selecting the optimal therapy for such patients therefore remains a great challenge. [6,7]
Auto-HCT for DLBCL
High-dose chemotherapy with auto-HCT exploits the steep dose-response curve of some chemotherapy agents, by eradicating disease that could not be eliminated by conventional chemotherapy doses. Two decades ago, one randomized trial conducted in relapsed aggressive lymphomas patients with chemosensitive disease showed a 53% 5-year survival with auto-HCT vs. 32% with continuation of conventional chemotherapy. [8] Even in the rituximab era, some patients whose disease relapses will be long-term survivors after proper second-line therapy and auto-HCT. [9] However, for patients who never achieve CR with frontline therapy, who have a short duration of CR, or relapse with a high IPI, significantly worse results are expected. For example, in the CORAL trial, fewer than 25% of patients who relapsed within one year of diagnosis achieved long-term disease-free survival with auto-HCT. [10]
Investigations designed to improve upon auto-HCT such as modifying the transplant preparative regimen with the addition of radioimmunotherapy to the chemotherapy backbone [11], or use of post-transplant maintenance therapy have not proven beneficial in relapsed/refractory (R/R) DLBCL. [12] Auto-HCT remains the standard of care for chemosensitive R/R DLBCL, but for subjects who experience relapse after auto-HCT, or for whom auto-HCT is not an option because the NHL is not sufficiently chemosensitive, better approaches are needed.
Allogeneic transplantation for DLBCL
Recent studies of allo-HCT involving at least 40 DLBCL patients are shown in Table 1.
Table 1.
Recent studies reporting the outcomes of allogeneic HCT for DLBCL (> 40 patients)
| Author (year) [Reference] |
N | Prior auto- HCT |
Conditioning | Median age (range) |
NRM/TRM (yrs) |
Relapse (yrs) |
OS (yrs) |
|---|---|---|---|---|---|---|---|
| Thomson (2009) [15] |
48 | 69% | RIC (100%) | 46 (23–64) | 32% (4) | 33% (4) | 48% (4) |
| Sirvent (2010) [82] |
68 | 79% | RIC (100%) | 48 (17–66) | 23% (1) | 41% (2) | 49% (2) |
| Lazarus (2010) [16] |
79* | 0% | MA (100%) | 46 (21–59) | 43% (3) | 33% (3) | 26% (3) |
| van Kampen (2011) [21] |
101 | 100% | MA (37%) RIC (63%) |
46 (18–66) | 28% (3) | 30% (3) | 52% (3) |
| Rigacci (2012) [20] |
165 | 100% | MA (30%) RIC (70%) |
43 (16–65) | 19–32% (2) | NR** | 39% (5) |
| Bacher (2012) [17] |
396 | 32% | MA (42%) RIC (58%) |
54 (18–66) | 36–56% (5) | 26–40% (5) | 18–26% (5) |
| Hamadani (2013) [18] |
533*** | 25% | MA (58%) RIC (42%) |
46 (19–66) 53 (20–70) |
53% (3) 42% (3) |
28% (3) 35% (3) |
19% (3) 28% (3) |
| Fenske (2015) [23] |
503 | 100% | MA (25%) RIC (75%) |
52 (19–72) | 31% (5) | 40% (5) | 34% (5) |
Abbreviations: MAC=myeloablative conditioning; NRM=non-relapse mortality; OS=Overall survival; RIC=reduced intensity conditioning. TRM= treatment-related mortality.
Analysis restricted to patients undergoing MA Allo-HCT as first transplant.
Not reported.
Included 85% DLBCL and 15% FL grade 3; all patients had chemo-resistant disease pre-transplant.
Allo-HCT provides the theoretical advantage of a tumor-free graft and the benefit of a graft-versus-lymphoma (GVL) effect. This GVL effect has been well demonstrated by the fact that some patients who experience relapse after auto-HCT will attain cure with allo-HCT. Further, use of donor lymphocyte infusion (DLI), withdrawal of immune suppression, or the combination provides a GVL effect in DLBCL after allo-HCT, leading to cure in some cases. [13–15] Allo-HCT, however, presents challenges including donor availability, the need for prolonged immunosuppression and an increased risk of early treatment-related mortality due to toxicity of conditioning regimen, graft-versus-host disease (GVHD), and infectious complications. Progress has been made in recent years in addressing these challenges.
In the past, allo-HCT utilized myeloablative (MA) conditioning in order to eliminate maximal tumor and permit engraftment by eliminating the host immune system. No prospective trials comparing auto-HCT versus MA allo-HCT for R/R DLBCL have been undertaken. A large retrospective analysis by the Center for International Blood and Marrow Transplant Research (CIBMTR) including 837 auto-HCT and 79 allo-HCT performed between 1995 and 2003 demonstrated higher treatment-related mortality (TRM)/non-relapse mortality (NRM), and higher overall mortality in the allo-HCT group with no decrease in risk of disease progression. [16] Those subjects who underwent allo-HCT, however, were more likely to have high-risk disease features including higher disease stage, received more prior chemotherapy regimens, and had resistant disease.
Despite such limitations, when reviewing the literature in allo-HCT, there is a consistent message that long-term OS in the 20–50% range is possible (see Table 1). The 25–30% NRM rate remains the largest drawback. Implementation of a less intensive preparative regimen (termed reduced-intensity conditioning [RIC]) has been utilized in recent years. Retrospective comparisons between MA and RIC show reduction of NRM, at the expense of some increase in disease relapse, but producing long-term OS rates comparable to MA conditioning. [17,18] Most allo-HCT for DLBCL are now performed using lower intensity regimens, which also permits undertaking this procedure in older patients who otherwise were good transplant candidates but previously would have been excluded from MA transplants due to age. [17] The expanding use of allo-HCT in older patients is evident by the fact that the more recent studies on Table 1 include patients 65–72 years of age. One recent study focusing on the use of allo-HCT in NHL patients in their their 60s and early 70s, found that there was not a major increase in NRM for such patients. [19]
Allogeneic HCT for DLBCL patients following a failed auto-HCT
Approximately 30–40% of DLBCL auto-HCT recipients ultimately will experience relapse or progression of DLBCL and cannot be cured with intensification of chemotherapy. As noted above, because of the GVL effect in DLBCL, such patients are often considered for allo-HCT after a failed prior auto-HCT. [20–23] These studies report TRM/NRM rates of 17–31% but OS rates at 3–5 years of 34–52%. In the most recent and largest study, from the CIBMTR, 503 patients were analyzed. At 3 years, NRM was 30%, with a progression/relapse rate of 38% and OS of 37%, a reasonable result given that the median survival typically seen in this population is in the 3–10 month range. [24,25] In multivariate analysis, advanced age was not a prognostic factor, indicating that chronologic age should not be the key factor used to determine allo-HCT eligibility. A prognostic model was constructed using KPS <80, time from auto-HCT to allo-HCT of < 1 year, and chemo-resistant disease as adverse factors. This model facilitates identification of high-risk patients unlikely to benefit from allo-HCT (3 year OS of 14%) as well as low risk patients who have a good potential to benefit from allo-HCT (3 year OS of 43%). [23]
Summary – DLBCL
These data indicate that medically appropriate DLBCL patients whose disease relapses after auto-HCT have the clearest indication for allo-HCT and potentially can be cured. In this setting, patients preferentially should receive RIC, ideally after debulking conventional therapy. Other subsets of patients who may benefit from allo-HCT are subjects known to have a low chance of cure with auto-HCT, as identified by use of the second-line IPI, failure to attain CR using initial therapy, relapse <12 months after chemoimmunotherapy, or NHL refractory to second and third-line chemotherapy. [10,13,18,26] The recently developed CIBMTR prognostic model described above can guide decision-making. [23] Other appropriate indications include: 1) patients in whom auto-HCT is not feasible, either by failure to obtain an adequate autologous graft for transplant, or coexistence of intrinsic bone marrow disease, particularly myelodysplastic syndrome; 2) DLBCL transformed from an indolent B-cell malignancy relapsing after anthracycline-containing therapy, or a failed auto-HCT; and 3) “double hit” DLBCL.
FOLLICULAR LYMPHOMA (FL)
Overview of FL and non-transplant options for frontline therapy
FL comprises the most common subtype of indolent NHL, accounting for roughly 20% of all cases of NHL. Unlike patients with aggressive NHL, nearly all subjects who complete induction therapy will relapse and require additional lines of therapy. Furthermore, while the OS for many patients is prolonged, with standard therapies FL remains incurable for the vast majority of patients. The follicular lymphoma international prognostic index (FLIPI) incorporates clinically-based features and identifies a subgroup at high risk for early disease-related death. [27] Approximately one quarter of all FL patients fall into this poor-risk category (i.e., ≥3 risk factors), and have an estimated 5-year OS of only 53%.
A proportion of patients may be observed safely without treatment until the development of symptomatic progressive disease, bulky adenopathy, cytopenias, organ obstruction, or malignant fluid collection. [28] Patients who require therapy, but who otherwise have low tumor burden, may be managed with single-agent rituximab. [29] The majority of patients, however, warrant combination chemoimmunotherapy. Currently, bendamustine-rituximab (B-R) may be a preferred front-line approach in high tumor burden FL with improved progression-free survival (PFS) (median not reached vs 41 months, p=0.0072) and decreased toxicity compared to R-CHOP. [30]
There is no standard therapy for R/R FL, and readily available options include anti-CD20 immunotherapies, combination chemotherapy, radioimmunotherapy, the oral PI3 kinase idelalisib, immunomodulatory drugs (lenalidomide), and the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib. [31] More intensive treatments often are considered for high-risk patients, especially those who relapse early after induction therapy, since relapse within 2 years of initial therapy is associated with a 5-year OS of only 50%. [32] In this context, auto-HCT and allo-HCT can provide for long-term survival in R/R FL.
Auto-HCT for FL
Auto-HCT for FL in first remission
The data to support autologous HCT for FL in first remission are limited. Prospective randomized trials comparing chemotherapy with auto-HCT in first complete remission have generally shown a PFS benefit but not OS benefit in favor of up-front auto-HCT. [33–36] Benefit in terms of improved lymphoma-specific survival appears to be offset by a decrease in survival due to secondary malignancies such as myelodysplastic syndrome and acute myeloid leukemia. As a result, auto-HCT is not recommended for consolidation of remission after first-line therapy for low grade FL.
Auto-HCT for R/R FL
While auto-HCT can produce prolonged remissions, it typically is not viewed as curative therapy in R/R FL. Prospective studies are lacking, although one randomized study (N=89) from the pre-rituximab era, closed early due to poor accrual, showed improved 2-year PFS and 4-year OS improved in auto-HCT recipients compared to standard chemotherapy patients [37] A review of two French studies, which included patients who did and did not receive rituximab at the time of relapse, demonstrated that rituximab appeared to have a more prominent impact on event-free survival (EFS) and OS than did receipt of an auto-HCT. [38] At least nine additional single-center retrospective studies and large registry studies of R/R FL have demonstrated PFS in the 30–60% range at 5–10 years, although not all patients in these series were treated with rituximab. [39] Since a large majority of FL patients relapse after auto-HCT, this approach cannot be considered a curative intervention for most FL patients.
Allo-HCT for FL
There are no randomized prospective trials or retrospective studies to support the application of allo-HCT as consolidation for FL in first remission. However, there have been numerous prospective as well as retrospective studies that support the use of allo-HCT in R/R FL. Outcomes for selected studies investigating allo-HCT in R/R FL are presented in Table 2.
Table 2.
Studies evaluating allogeneic HCT in relapsed/refractory FL (> 30 patients).
| Author (year) |
N | Conditioning | Median age (range) |
TRM/NRM (years) |
Relapse (years) |
OS (years) |
Comments |
|---|---|---|---|---|---|---|---|
| Rezvani (2008) [44] | 46 | RIC | 54 (33–66) | 42% (3) | 14% (3) | 52% (3) | - Prospective |
| Hari (2008) [83] | 208 | MAC (58%) RIC (42%) |
44 (27–70) 51 (27–700 |
23% (1) | 8–17% (3) | 62–71% (3) | - Retrospective (CIBMTR) |
| Pinana (2010) [42] | 37 | RIC | 50 (34–62) | 37% (4) | 8% (4) | 57% (4) | - Prospective |
| Thomson (2010) [43] |
82 | RIC | 45 (26–65) | 15% (4) | 26% (4) | 76% (4) | - Prospective |
| Khouri (2012) [84] | 47 | RIC | 53 (33–68) | 15% (8) | 4% (8) | 85% (8) | - Prospective - 45/47 MRD |
| Evens (2013) [45] | 48 | NR* | 50 (27–64) | 24% (3) | 16% (3) | 61% (3) | - Retrospective (NCCN) |
| Robinson (2013) [85] |
149 | RIC | 51 (33–66) | 22% (3) | 20% (5) | 67% (5) | - Retrospective (EBMT) |
| Klyuchnikov (2015) [46] |
268 | RIC | 52 (27–74) | 26% (5) | 20% (5) | 66% (5) | - Retrospective (CIBMTR) - Grade 1 and 2 FL |
| Klyuchnikov (2015) [48] |
70 | RIC | 53 (36–64) | 27% (5) | 20% (5) | 54% (5) | - Retrospective (CIBMTR) - Grade 3 FL |
| Yano (2015) [86] | 46 | RIC | 48 (34–66) | 23% (5) | 15% (5) | 81% (5) | - Retrospective (Japan) |
Abbreviations: MAC=myeloablative conditioning; MRD=matched related donor; NRM=non-relapse mortality; OS=Overall survival; RIC=reduced intensity conditioning. TRM= treatment-related mortality.
Not reported.
Allo-HCT for R/R FL: Prospective Studies
Shea et al [40] reported on a prospective study of 16 patients receiving RIC allo-HCT conditioned with fludarabine/cyclophosphamide and a matched related donor graft. The 3-year EFS was 75% and 3-year OS was 81%; 3 subjects relapsed. A second prospective study of 47 patients conducted at MD Anderson Cancer Center used RIC conditioning (fludarabine, cyclophosphamide, and rituximab conditioning). [41] The median 5 year PFS and OS were 85%; seven patients died, mostly from infectious complications. Another report combined two, prospective multi-center Spanish trials in 37 patients given a fludarabine/melphalan RIC regimen. [42] The relapse incidence was only 8%, but NRM was as high as 71% for patients with active disease prior to allo-HCT. For patients in CR prior to transplant, the 4-year OS was 71%, comparable to other studies. A prospective study from the U.K. enrolled 82 consecutive FL patients treated with fludarabine, melphalan and alemtuzumab and RIC. NRM was 15% and only 26% of patients experienced a relapse at 4 years, and OS was 76% at 4 years. [43] Finally, another prospective multi-center study in 46 FL patients coordinated by the Fred Hutchinson Cancer Research Center [44] noted a high 42% rate of NRM driven, in large part, by use of mismatched unrelated allografts. Relapse rate at 3 years was low at 14%.
Allo-HCT for R/R FL: Retrospective Studies
The NCCN compared outcomes in 48 allo-HCT recipients to patients receiving auto-HCT. [45] The allo-HCT recipients had a 3-year NRM of 26%; in a multivariable analysis, allo-HCT was associated with an increased risk of death compared to auto-HCT (p=0.002). There should be caution in interpretation of these data, however, as the group of FL patients who underwent allogeneic transplantation were a highly select and high-risk group. In contrast, a recent and larger analysis by the CIBMTR indicates that long-term remission is feasible in a significant number of R/R FL patients who receive allo-HCT, and that patients who do not die early in the post-transplant course have prolonged survival compared with patients receiving autologous transplant. Among 268 relapsed patients with grade 1–2 FL who underwent RIC allo-HCT, the 5-year NRM was 26%., the probability of disease progression at 5 years was 20%, and the 5-year PFS was 41%, representing a subset of patients who are likely cured of their disease. The 5-year OS was 66%, and among 24-month survivors, the subsequent risk of death was decreased for patients receiving allogeneic transplantation compared to those receiving autologous transplants (RR 2.09, p=0.04). [46] A recent retrospective study from Memorial Sloan-Kettering compared outcomes for R/R FL patients undergoing auto-HCT versus allo-HCT in the post-rituximab era. In subgroup analysis was performed in patients who had a remission duration of <12 months prior to salvage therapy, 3 year event-free survival was 42% with auto-HCT versus 80% for allo-HCT, suggesting that patients with a very short first remission (less than 1 year) may be better served with an allo-HCT approach. [47]
Allo-HCT for grade 3 FL
A recent report from the CIBMTR examined the outcomes of 61 patients who underwent RIC allo-HCT for grade 3 FL. [48] At 5 years, NRM was 27% and OS 54%, indicating that grade 3 histology should not be an exclusion factor for allo-HCT, and that RIC conditioning can offer long-term survival for patients with grade 3 histology.
Summary – FL
While auto-HCT is not considered a curable intervention for most patients, a subset (~30–35%) may enjoy long-term (> 5 year) remissions with this therapy, justifying its use in 2nd or 3rd remission. Furthermore, given the development of less toxic, novel therapeutic strategies in the modern era, the use of auto-HCT may decline in the coming years. It should be noted that these novel strategies are not currently known to be curative. In contrast, allo-HCT repeatedly has been shown to be a curative therapy for a significant subset of patients with R/R FL. While the rate of early death is increased among FL patients receiving allo-HCT due to increased NRM, the studies cited above demonstrate excellent long-term outcomes in those surviving the early period. With the increasing application of RIC allo-HCT, and continued improvement in supportive care, NRM rates likely will continue to decline for well-selected FL patients who receive expert supportive care. Such patients can expect a reasonable chance of being cured. These observations apply to patients over age 65 years, for whom outcomes with RIC are comparable to, or only minimally less favorable than, those in the 55–64 year age group. [19]
MANTLE CELL LYMPHOMA (MCL)
Overview of MCL and non-transplant options for frontline therapy
MCL comprises ~6% of newly diagnosed NHL. Median age at diagnosis is in the late 60s, with a 4:1 male predominance. Patients often present in advanced disease stage along with extranodal involvement. Modern chemoimmunotherapies alone, or as induction followed by auto-HCT in first remission, undoubtedly have improved patient outcomes; however, disease relapse eventually occurs for nearly all patients. [49]
Although variables such as histology (e.g. blastoid morphology), tumor proliferation index, cytogenetics, gene expression profiling, and microRNA expression profile have prognostic value in MCL, their clinical application as a risk stratification tool remains limited. [45] The MCL International Prognostic Index (MIPI) is a commonly used risk-stratification score based on patient age, performance status, serum LDH, and white blood cell count at diagnosis, with/without Ki-67 proliferation index, that classifies MCL into low, intermediate, and high risk groups with median OS ranging from 29 months to > 5 years. [50] This index also has also been validated to predict outcomes after auto-HCT [51], but has not yet been validated as a tool to direct optimal consolidation therapies in patients responding to frontline therapies.
Using conventional frontline therapy such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), the median remission duration historically was only 12–18 months. [52] Recently, however, incorporation of agents such as bortezomib, bendamustine, and lenalidomide into first-line therapy have improved upon this result, with PFS in the 27–35 month range or longer. [30,53–55] Intensive regimens like R-Hyper-CVAD (rituximab, fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone alternating with methotrexate and cytarabine) without subsequent consolidation led to impressive PFS in some reports, but toxicity concerns have dampened enthusiasm for this approach. [56–58]
Given the relatively short remissions observed in MCL, investigators have tested the concept of maintenance rituximab after conventional frontline therapies; median duration of first remission has improved to the 3–5 year range. As a result, rituximab maintenance is a reasonable option for elderly MCL patients who are not candidates for HCT. [59,60]
Auto-HCT for MCL
Auto-HCT in first remission
In the pre-rituximab era, the poor prognosis seen in relapsed MCL motivated the European MCL Network to conduct a randomized, prospective post-remission trial comparing auto-HCT versus interferon-alpha maintenance. [61] Auto-HCT demonstrated superior PFS; however no OS benefit was seen, potentially due to crossing over of the interferon-alpha-treated patients to subsequently receive auto-HCT off protocol. While similar prospective randomized data in the rituximab era are not available, several large prospective phase 2 trials applying up-front auto-HCT after various intensive induction regimens have yielded median PFS durations in the 5–7 year range. [62–67] Despite the lack of randomized, controlled data demonstrating a survival benefit with auto-HCT as consolidation in first remission, many clinicians have adopted this approach. Certain patients such as those not in CR at the time of up-front autoHCT [68], those with minimal residual disease pre-transplant, [69] or those with high-risk MIPI score [51,64,70] fare considerably less well with upfront auto-HCT consolidation, underscoring the need for novel modalities to consolidate first remission for such patients.
Autologous Transplantation for Relapsed/ Refractory MCL
Initial retrospective studies, including a European Group of Blood and Marrow Transplantation (EBMT) study, [71] suggested that auto-HCT may have limited value in relapsed and refractory patients. More recent studies, however, including a large retrospective study from the CIBMTR, indicated that durable remissions in the 2–4 year range may be observed for select MCL patients whose disease remains chemosensitive. [68,72] Therefore, in relapsed MCL patients with chemosensitive disease, who are not candidates for a potentially curative allogeneic-HCT (due to co-morbidities, donor availability etc.), consolidation with an auto-HCT can be offered, with the understanding that this therapy is not curative. It remains to be seen whether auto-HCT will remain part of the treatment paradigm for R/R MCL in the era of new and novel agents.
Allo-HCT for MCL
Allo-HCT in first remission
Although adoptive immunotherapy in the form of allo-HCT is a potentially curative option for MCL patients, there are no prospective data assessing this approach in first remission. Registry data from the CIBMTR examining the role of allo-HCT versus auto-HCT in first CR or partial remission (PR) showed no benefit in terms of PFS (55% vs. 52% at 5 years) or OS (62% vs. 61% at 5 years); however, early allograft was associated with a significantly higher NRM (25% vs. 3% at 1 year). [68] As a result, use of allo-HCT in chemosensitive MCL patients in first remission generally is not recommended. Early use of allo-HCT can be considered in selected cases for which the chance for success with auto-HCT is poor, such as those with high MIPI score, or those not in CR prior to transplant. It has been hypothesized that the outcome of such patients might be improved by early allografting. This approach would not yet be considered standard care by most clinicians, but warrants prospective investigation.
Allo-HCT for R/R MCL
As shown in Table 3, evidence from several single-center studies and large transplant registries have established that allo-HCT is the only potentially curative modality for R/R MCL, with 35–45% of patients disease-free (and likely cured) 3 years post transplantation [68,73–77] Chemosensitive disease and adequate performance status are predictive of improved allo-HCT outcomes. In recent years, the wider adoption of RIC regimens has extended transplant availability to elderly patients and those with medical comorbidities. The challenging subset of MCL patients with chemotherapy-unresponsive disease, however, have a particularly poor prognosis. A large CIBMTR analysis restricted to such chemorefractory patients showed that approximately one-quarter gain durable disease control with allo-HCT, likely due to a clinically relevant GVL effect. [73] No benefit of MA conditioning was seen even in this high-risk cohort of patients. In otherwise healthy patients with relapsed or refractory MCL and an available sibling or adult unrelated donor, an allo-HCT with curative intent is therefore a reasonable therapeutic option. Advanced age, in medically fit patients without significant comorbidities, should not be considered a contraindication.
Table 3.
Studies evaluating allogeneic HCT in relapsed/refractory MCL (> 30 patients).
| Author (year) |
N | Conditioning | Median age (range) |
TRM/NRM (years) |
Relapse (years) |
OS (years) |
|---|---|---|---|---|---|---|
| Maris (2004) [74] |
33 | RIC | 53 (33–70) | 9% (2) | 16 % (2) | 65% (2) |
| Cook (2010) [75] |
70 | RIC | 52 (25–69) | 18% (3) | 65 % (5) | 37% (5) |
| Le Gouill (2012) [76] |
70 | RIC | 56 (33–67) | 32% (2) | 18 % (2) | 53% (2) |
| Hamadani* (2013) [73] |
202 | MAC (37%) RIC (63%) |
54 (27–69) 59 (42–75) |
38–43% (1) | 32–33% (3) | 25–30% (3) |
| Fenske** (2014) [68] |
88 | RIC | 58 (26–75) | 17% (1) | 38 % (5) | 31% (5) |
| Kruger (2014) [77] |
33 | MAC (21%) RIC (79%) |
59 (33–69) | 24% (5) | 15 % (5) | 73% (5) |
Abbreviations: MAC=myeloablative conditioning; NRM=non-relapse mortality; OS=Overall survival; RIC=reduced intensity conditioning. TRM= treatment-related mortality.
Included only patients with refractory disease.
Excluded patients with refractory disease.
Summary – MCL
Currently, both auto-HCT and allo-HCT play important roles in the management of MCL patients. Auto-HCT is not considered curative therapy for MCL, although intensive induction and consolidation using auto-HCT may provide many patients with remissions that can last 6–8 years or longer. Select MCL patients who did not undergo auto-HCT as part of frontline therapy may also benefit from auto-HCT later in the disease course. Despite the recent emergence of several exciting new agents, for the vast majority of R/R MCL patients, allo-HCT remains the only treatment option with curative potential. With the widespread implementation of RIC, properly selected MCL patients who receive expert supportive care have a reasonable chance of being cured with allogeneic transplantation. This also applies to patients over age 65, for whom outcomes with reduced-intensity conditioning are comparable to those in the 55–64 age group. [19]
ALLOGENEIC TRANSPLANATION IN OLDER LYMPHOMA PATIENTS
Tables 1–3 display many key studies that utilize allo-HCT in DLBCL, FL, and MCL. Several of these reports include patients up to age 70–75 years. One recent registry study by McClune et al specifically analyzed outcomes for patients over age 65 undergoing allo-HCT for lymphoma. [19] In this study, 82 elderly NHL patients underwent allo-HCT. While 100 day NRM was not significantly different for the age ≥65 group, NRM at 1 year was higher for the older age group (34%) versus two younger populations, e.g. age 40–54 years (27%) and age 55–64 years (22%). Relapse rates at 3 years were similar across the 3 age groups (28–33%). Not surprisingly, OS was highest in the age 40–54 patients; however the patients ≥65 years old fared reasonably well with OS of 39% at 3 years. However, it bears emphasizing that, for an individual patient over age 65, the real question should not be whether allo-HCT outcomes are comparable to patients under age 65. Rather, the question should be whether allo-HCT offers the best and reasonable chance for long-term remission or cure for that patient, when considering all treatment options.
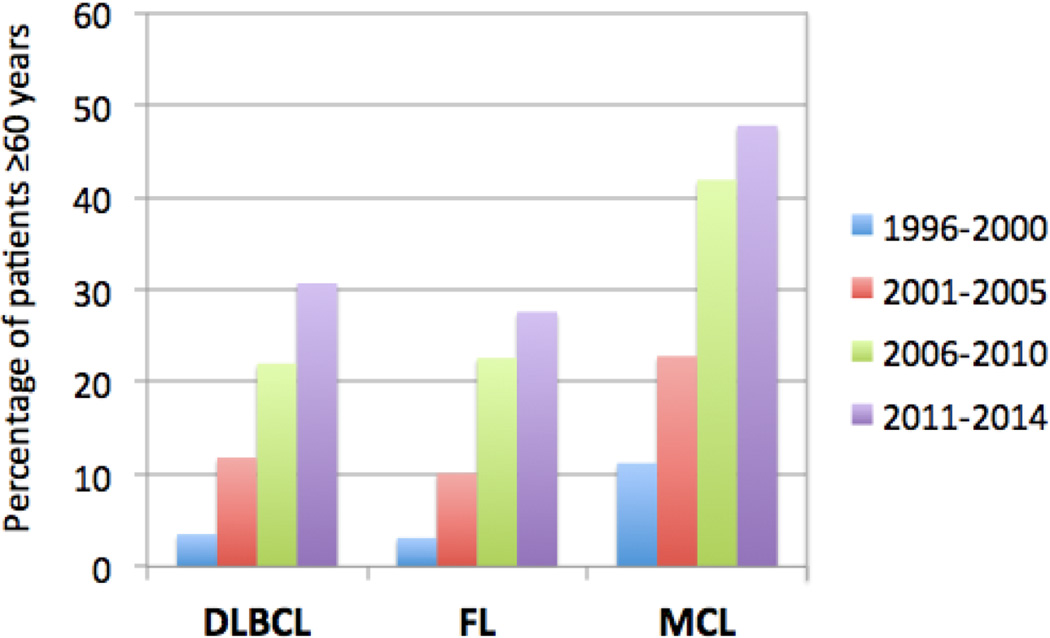
Due in part to the advances in patient selection, donor selection, pre-transplant lymphoma therapy, and peri-transplant supportive care, older patients are increasingly undergoing allo-HCT. As shown in Figure 1, data from the CIBMTR database show a greater use over time of allo-HCT for NHL patients over age 60 years. For example, for the time period of 2006–2010 the percentage of patients undergoing allo-HCT over age 60 for DLBCL, FL, and MCL were 22%, 23%, and 42%, respectively. Those percentages increased during the next time period (2011–2014) to 31%, 28% and 48%, respectively (personal communication, Center for International Blood and Marrow Transplant Research). These figures clearly demonstrate that for DLBCL, FL and MCL, allo-HCT represents an important therapeutic option, even for older patients. In recent years, alternative donor transplantation (umbilical cord blood or haploidentical marrow) has become more widespread, with recent publications showing outcomes that compare favorably to those using matched unrelated donors. [78,79] The haploidentical approach, with post-transplant cyclophosphamide, has successfully been applied to lymphoma patients up to age 75, with 1 year NRM of 10–15% across all age groups from 50–75, and a 3 year survival of approximately 50%. [80] With the growing use and success of alternative donor transplantation, it is expected that an even larger number of older patients will be allo-HCT candidates, since in many cases such patients do not have a matched sibling who is in good enough health to serve as a donor.
Figure 1.
Increase in proportion of NHL patients over age 60 undergoing allo-HCT in the U.S. from 1996–2014.*
*Data provided by the CIBMTR (Center for International Blood and Marrow Transplant Research).
Based on the data presented above, it is therefore increasingly clear that allo-HCT is in fact feasible in older patients. Transplant clinicians will therefore be increasingly challenged to consider allo-HCT for these older patients. On an individual patient level, deciding to proceed with transplant requires a careful consideration of patient issues (co-morbidities, performance status), disease factors (histology and biological features of the lymphoma, clinical aggressiveness, disease burden), and donor factors. These considerations are of particular importance in older patients where the therapeutic window for transplant may be narrow, and the risk for toxicity and disability post-transplant may be higher. With this in mind, it will be very important to prospectively collect quality of life data in elderly NHL patients undergoing allo-HCT, if allo-HCT becomes a covered indication in these patients.
SUMMARY AND CONCLUSIONS
Ideally there would be more prospective randomized clinical trial data to prove superior survival with allo-HCT for FL, DLBCL, and MCL, compared to alternative approaches. To date no such studies have successfully been completed. One such study was attempted in FL but due to poor accrual was not successfully completed. [81] While it is true that a number of exciting targeted, biological, and immunological therapies are emerging in NHL, it is currently unclear how these new therapies might enhance or replace allo-HCT. High quality studies comparing novel agents with allo-HCT, or integrating such agents with allo-HCT will need to be completed to answer such questions. In the meantime, based on the extensive literature currently available and summarized in this review, it is clear that for many NHL patients, allo-HCT is the only currently available therapy which offers cure.
Advances in the HCT field have enabled this therapy to be applied successfully to patients over age 65 years. Access to allo-HCT in the U.S. has historically been quite limited due to a lack of insurance coverage. Despite this, there is now a body of evidence to show that lymphoma patients in the 60s and 70s can in fact tolerate and benefit from allo-HCT. Based on the evidence, we strongly assert that allo-HCT now represents a standard of care for older NHL patients and, as a result, the associated costs should be covered by insurance carriers.
REFERENCES
- 1.Personal communication. Mehdi Hamadani, Center for International Blood and Marrow Transplant Research. 2016 Apr 18; [Google Scholar]
- 2.Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N. Engl. J. Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 5.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 6.Cheah CY, Oki Y, Westin JR, Turturro F. A clinician's guide to double hit lymphomas. Br. J. Haematol. 2015;168:784–795. doi: 10.1111/bjh.13276. [DOI] [PubMed] [Google Scholar]
- 7.Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124:2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 8.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N. Engl. J. Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 9.Hamadani M, Hari PN, Zhang Y, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2014;20:1729–1736. doi: 10.1016/j.bbmt.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vose JM, Carter S, Burns LJ, et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: results from the BMT CTN 0401 trial. J. Clin. Oncol. 2013;31:1662–1668. doi: 10.1200/JCO.2012.45.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gisselbrecht C, Schmitz N, Mounier N, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J. Clin. Oncol. 2012;30:4462–4469. doi: 10.1200/JCO.2012.41.9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klyuchnikov E, Bacher U, Kroll T, et al. Allogeneic hematopoietic cell transplantation for diffuse large B cell lymphoma: who, when and how? Bone Marrow Transplant. 2014;49:1–7. doi: 10.1038/bmt.2013.72. [DOI] [PubMed] [Google Scholar]
- 14.Bishop MR, Dean RM, Steinberg SM, et al. Clinical evidence of a graft-versus-lymphoma effect against relapsed diffuse large B-cell lymphoma after allogeneic hematopoietic stem-cell transplantation. Ann. Oncol. 2008;19:1935–1940. doi: 10.1093/annonc/mdn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson KJ, Morris EC, Bloor A, et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin's lymphoma. J. Clin. Oncol. 2009;27:426–432. doi: 10.1200/JCO.2008.17.3328. [DOI] [PubMed] [Google Scholar]
- 16.Lazarus HM, Zhang MJ, Carreras J, et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol. Blood Marrow Transplant. 2010;16:35–45. doi: 10.1016/j.bbmt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacher U, Klyuchnikov E, Le-Rademacher J, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120:4256–4262. doi: 10.1182/blood-2012-06-436725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamadani M, Saber W, Ahn KW, et al. Impact of pretransplantation conditioning regimens on outcomes of allogeneic transplantation for chemotherapy-unresponsive diffuse large B cell lymphoma and grade III follicular lymphoma. Biol. Blood Marrow Transplant. 2013;19:746–753. doi: 10.1016/j.bbmt.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClune BL, Ahn KW, Wang HL, et al. Allotransplantation for patients age >/=40 years with non-Hodgkin lymphoma: encouraging progression-free survival. Biol. Blood Marrow Transplant. 2014;20:960–968. doi: 10.1016/j.bbmt.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigacci L, Puccini B, Dodero A, et al. Allogeneic hematopoietic stem cell transplantation in patients with diffuse large B cell lymphoma relapsed after autologous stem cell transplantation: a GITMO study. Ann. Hematol. 2012;91:931–939. doi: 10.1007/s00277-011-1395-9. [DOI] [PubMed] [Google Scholar]
- 21.van Kampen RJ, Canals C, Schouten HC, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin's lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J. Clin. Oncol. 2011;29:1342–1348. doi: 10.1200/JCO.2010.30.2596. [DOI] [PubMed] [Google Scholar]
- 22.Kim JW, Kim SW, Tada K, et al. Allogeneic stem cell transplantation in patients with de novo diffuse large B-cell lymphoma who experienced relapse or progression after autologous stem cell transplantation: a Korea-Japan collaborative study. Ann. Hematol. 2014;93:1345–1351. doi: 10.1007/s00277-014-2045-9. [DOI] [PubMed] [Google Scholar]
- 23.Fenske TS, Ahn KW, Graff TM, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br. J. Haematol. 2016 doi: 10.1111/bjh.14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vose JM, Bierman PJ, Anderson JR, et al. Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: clinical course and patient follow-up. Blood. 1992;80:2142–2148. [PubMed] [Google Scholar]
- 25.Nagle SJ, Woo K, Schuster SJ, et al. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am. J. Hematol. 2013;88:890–894. doi: 10.1002/ajh.23524. [DOI] [PubMed] [Google Scholar]
- 26.Costa LJ, Micallef IN, Inwards DJ, Johnston PB, Porrata LF, Ansell SM. Time of relapse after initial therapy significantly adds to the prognostic value of the IPI-R in patients with relapsed DLBCL undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2008;41:715–720. doi: 10.1038/sj.bmt.1705967. [DOI] [PubMed] [Google Scholar]
- 27.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 28.Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. J. Clin. Oncol. 1997;15:1110–1117. doi: 10.1200/JCO.1997.15.3.1110. [DOI] [PubMed] [Google Scholar]
- 29.Kahl BS, Hong F, Williams ME, et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: eastern cooperative oncology group protocol e4402. J. Clin. Oncol. 2014;32:3096–3102. doi: 10.1200/JCO.2014.56.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rummel M, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 31.Freedman A. Follicular lymphoma: 2014 update on diagnosis and management. Am. J. Hematol. 2014;89:429–436. doi: 10.1002/ajh.23674. [DOI] [PubMed] [Google Scholar]
- 32.Casulo C, Byrtek M, Dawson KL, et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J. Clin. Oncol. 2015;33:2516–2522. doi: 10.1200/JCO.2014.59.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenz G, Dreyling M, Schiegnitz E, et al. Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression-free survival in follicular lymphoma: results of a prospective, randomized trial of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:2667–2674. doi: 10.1182/blood-2004-03-0982. [DOI] [PubMed] [Google Scholar]
- 34.Deconinck E, Foussard C, Milpied N, et al. High-dose therapy followed by autologous purged stem-cell transplantation and doxorubicin-based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by GOELAMS. Blood. 2005;105:3817–3823. doi: 10.1182/blood-2004-10-3920. [DOI] [PubMed] [Google Scholar]
- 35.Sebban C, Mounier N, Brousse N, et al. Standard chemotherapy with interferon compared with CHOP followed by high-dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma: the GELF-94 randomized study from the Groupe d'Etude des Lymphomes de l'Adulte (GELA) Blood. 2006;108:2540–2544. doi: 10.1182/blood-2006-03-013193. [DOI] [PubMed] [Google Scholar]
- 36.Ladetto M, De Marco F, Benedetti F, et al. Prospective, multicenter randomized GITMO/IIL trial comparing intensive (R-HDS) versus conventional (CHOP-R) chemoimmunotherapy in high-risk follicular lymphoma at diagnosis: the superior disease control of R-HDS does not translate into an overall survival advantage. Blood. 2008;111:4004–4013. doi: 10.1182/blood-2007-10-116749. [DOI] [PubMed] [Google Scholar]
- 37.Schouten HC, Qian W, Kvaloy S, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J. Clin. Oncol. 2003;21:3918–3927. doi: 10.1200/JCO.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Sebban C, Brice P, Delarue R, et al. Impact of rituximab and/or high-dose therapy with autotransplant at time of relapse in patients with follicular lymphoma: a GELA study. J. Clin. Oncol. 2008;26:3614–3620. doi: 10.1200/JCO.2007.15.5358. [DOI] [PubMed] [Google Scholar]
- 39.Hamadani M. Reappraising the role of autologous transplantation for indolent B-cell lymphomas in the chemoimmunotherapy era: is it still relevant? Bone Marrow Transplant. 2013;48:1013–1021. doi: 10.1038/bmt.2012.182. [DOI] [PubMed] [Google Scholar]
- 40.Shea T, Johnson J, Westervelt P, et al. Reduced-intensity allogeneic transplantation provides high event-free and overall survival in patients with advanced indolent B cell malignancies: CALGB 109901. Biol. Blood Marrow Transplant. 2011;17:1395–1403. doi: 10.1016/j.bbmt.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinana JL, Martino R, Gayoso J, et al. Reduced intensity conditioning HLA identical sibling donor allogeneic stem cell transplantation for patients with follicular lymphoma: long-term follow-up from two prospective multicenter trials. Haematologica. 2010;95:1176–1182. doi: 10.3324/haematol.2009.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson KJ, Morris EC, Milligan D, et al. T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J. Clin. Oncol. 2010;28:3695–3700. doi: 10.1200/JCO.2009.26.9100. [DOI] [PubMed] [Google Scholar]
- 44.Rezvani AR, Storer B, Maris M, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin's lymphoma. J. Clin. Oncol. 2008;26:211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 45.Evens AM, Vanderplas A, LaCasce AS, et al. Stem cell transplantation for follicular lymphoma relapsed/refractory after prior rituximab: a comprehensive analysis from the NCCN lymphoma outcomes project. Cancer. 2013;119:3662–3671. doi: 10.1002/cncr.28243. [DOI] [PubMed] [Google Scholar]
- 46.Klyuchnikov E, Bacher U, Kroger NM, et al. Reduced-Intensity Allografting as First Transplantation Approach in Relapsed/Refractory Grades One and Two Follicular Lymphoma Provides Improved Outcomes in Long-Term Survivors. Biol. Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lunning MA, Migliacci JC, Hilden P, et al. The potential benefit of allogeneic over autologous transplantation in patients with very early relapsed and refractory follicular lymphoma with prior remission duration of </=12 months. Br. J. Haematol. 2016;173:260–264. doi: 10.1111/bjh.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klyuchnikov E, Bacher U, Woo Ahn K, et al. Long-term survival outcomes of reduced-intensity allogeneic or autologous transplantation in relapsed grade 3 follicular lymphoma. Bone Marrow Transplant. 2015 doi: 10.1038/bmt.2015.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vose JM. Mantle cell lymphoma: 2015 update on diagnosis, risk-stratification, and clinical management. Am. J. Hematol. 2015;90:739–745. doi: 10.1002/ajh.24094. [DOI] [PubMed] [Google Scholar]
- 50.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 51.Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT) Blood. 2010;115:1530–1533. doi: 10.1182/blood-2009-08-236570. [DOI] [PubMed] [Google Scholar]
- 52.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J. Clin. Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 53.Robak T, Huang H, Jin J, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N. Engl. J. Med. 2015;372:944–953. doi: 10.1056/NEJMoa1412096. [DOI] [PubMed] [Google Scholar]
- 54.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944–2952. doi: 10.1182/blood-2013-11-531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruan J, Martin P, Shah B, et al. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N. Engl. J. Med. 2015;373:1835–1844. doi: 10.1056/NEJMoa1505237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br. J. Haematol. 2010;150:200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 57.Merli F, Luminari S, Ilariucci F, et al. Rituximab plus HyperCVAD alternating with high dose cytarabine and methotrexate for the initial treatment of patients with mantle cell lymphoma, a multicentre trial from Gruppo Italiano Studio Linfomi. Br. J. Haematol. 2012;156:346–353. doi: 10.1111/j.1365-2141.2011.08958.x. [DOI] [PubMed] [Google Scholar]
- 58.Epner E, Unger J, Miller T, et al. A multi center trial of hyperCVAD+Rituxan in patients with newly diagnosed mantle cell lymphoma. Blood. 2007;110:387. [Google Scholar]
- 59.Kenkre VP, Long WL, Eickhoff JC, et al. Maintenance rituximab following induction chemo-immunotherapy for mantle cell lymphoma: long-term follow-up of a pilot study from the Wisconsin Oncology Network. Leuk. Lymphoma. 2011;52:1675–1680. doi: 10.3109/10428194.2011.580404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N. Engl. J. Med. 2012;367:520–531. doi: 10.1056/NEJMoa1200920. [DOI] [PubMed] [Google Scholar]
- 61.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 62.Geisler CH, Kolstad A, Laurell A, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br. J. Haematol. 2012;158:355–362. doi: 10.1111/j.1365-2141.2012.09174.x. [DOI] [PubMed] [Google Scholar]
- 63.Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J. Clin. Oncol. 2009;27:6101–6108. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tam CS, Bassett R, Ledesma C, et al. Mature results of the M.D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113:4144–4152. doi: 10.1182/blood-2008-10-184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d'Etude des Lymphomes de l'Adulte. Blood. 2013;121:48–53. doi: 10.1182/blood-2011-09-370320. [DOI] [PubMed] [Google Scholar]
- 66.Hermine O, Hoster E, Walewski J, et al. Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high dose ARA-C containing myeloablative regimen and autologous stem cell transplantation (ASCT) increases overall survival when compared to 6 courses of CHOP plus rituximab followed by myeloablative radiochemotherapy and ASCT in mantle cell lymphoma: final analysis of the MCL younger trial of the European Mantle Cell Lymphoma Network (MCL net) Blood. ASH Annual Meeting Abstracts. 2012;120:151. [Google Scholar]
- 67.Kolstad A, Laurell A, Jerkeman M, et al. Nordic MCL3 study: 90Y-ibritumomab-tiuxetan added to BEAM/C in non-CR patients before transplant in mantle cell lymphoma. Blood. 2014;123:2953–2959. doi: 10.1182/blood-2013-12-541953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fenske TS, Zhang MJ, Carreras J, et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: analysis of transplantation timing and modality. J. Clin. Oncol. 2014;32:273–281. doi: 10.1200/JCO.2013.49.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cowan AJ, Stevenson PA, Cassaday RD, et al. Pretransplantation Minimal Residual Disease Predicts Survival in Patients with Mantle Cell Lymphoma Undergoing Autologous Stem Cell Transplantation in Complete Remission. Biol. Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Budde LE, Guthrie KA, Till BG, et al. Mantle cell lymphoma international prognostic index but not pretransplantation induction regimen predicts survival for patients with mantle-cell lymphoma receiving high-dose therapy and autologous stem-cell transplantation. J. Clin. Oncol. 2011;29:3023–3029. doi: 10.1200/JCO.2010.33.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vandenberghe E, Ruiz de Elvira C, Loberiza FR, et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br. J. Haematol. 2003;120:793–800. doi: 10.1046/j.1365-2141.2003.04140.x. [DOI] [PubMed] [Google Scholar]
- 72.Cassaday RD, Guthrie KA, Budde EL, et al. Specific features identify patients with relapsed or refractory mantle cell lymphoma benefitting from autologous hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2013;19:1403–1406. doi: 10.1016/j.bbmt.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamadani M, Saber W, Ahn KW, et al. Allogeneic hematopoietic cell transplantation for chemotherapy-unresponsive mantle cell lymphoma: a cohort analysis from the center for international blood and marrow transplant research. Biol. Blood Marrow Transplant. 2013;19:625–631. doi: 10.1016/j.bbmt.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 75.Cook G, Smith GM, Kirkland K, et al. Outcome following Reduced-Intensity Allogeneic Stem Cell Transplantation (RIC AlloSCT) for relapsed and refractory mantle cell lymphoma (MCL): a study of the British Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2010;16:1419–1427. doi: 10.1016/j.bbmt.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Le Gouill S, Kroger N, Dhedin N, et al. Reduced-intensity conditioning allogeneic stem cell transplantation for relapsed/refractory mantle cell lymphoma: a multicenter experience. Ann. Oncol. 2012;23:2695–2703. doi: 10.1093/annonc/mds054. [DOI] [PubMed] [Google Scholar]
- 77.Kruger WH, Hirt C, Basara N, et al. Allogeneic stem cell transplantation for mantle cell lymphoma--final report from the prospective trials of the East German Study Group Haematology/Oncology (OSHO) Ann. Hematol. 2014;93:1587–1597. doi: 10.1007/s00277-014-2087-z. [DOI] [PubMed] [Google Scholar]
- 78.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched unrelated donors. Blood. 2015 doi: 10.1182/blood-2015-09-671834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bachanova V, Burns LJ, Wang T, et al. Alternative donors extend transplantation for patients with lymphoma who lack an HLA matched donor. Bone Marrow Transplant. 2015;50:197–203. doi: 10.1038/bmt.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kasamon YL, Bolanos-Meade J, Prince GT, et al. Outcomes of Nonmyeloablative HLA-Haploidentical Blood or Marrow Transplantation With High-Dose Post-Transplantation Cyclophosphamide in Older Adults. J. Clin. Oncol. 2015;33:3152–3161. doi: 10.1200/JCO.2014.60.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomblyn MR, Ewell M, Bredeson C, et al. Autologous versus reduced-intensity allogeneic hematopoietic cell transplantation for patients with chemosensitive follicular non-Hodgkin lymphoma beyond first complete response or first partial response. Biol. Blood Marrow Transplant. 2011;17:1051–1057. doi: 10.1016/j.bbmt.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sirvent A, Dhedin N, Michallet M, et al. Low nonrelapse mortality and prolonged long-term survival after reduced-intensity allogeneic stem cell transplantation for relapsed or refractory diffuse large B cell lymphoma: report of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biol. Blood Marrow Transplant. 2010;16:78–85. doi: 10.1016/j.bbmt.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 83.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol. Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khouri IF, Saliba RM, Erwin WD, et al. Nonmyeloablative allogeneic transplantation with or without 90yttrium ibritumomab tiuxetan is potentially curative for relapsed follicular lymphoma: 12-year results. Blood. 2012;119:6373–6378. doi: 10.1182/blood-2012-03-417808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robinson SP, Canals C, Luang JJ, et al. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: an analysis from the Lymphoma Working Party of the EBMT. Bone Marrow Transplant. 2013;48:1409–1414. doi: 10.1038/bmt.2013.83. [DOI] [PubMed] [Google Scholar]
- 86.Yano S, Mori T, Kanda Y, et al. Favorable survival after allogeneic stem cell transplantation with reduced-intensity conditioning regimens for relapsed/refractory follicular lymphoma. Bone Marrow Transplant. 2015;50:1299–1305. doi: 10.1038/bmt.2015.158. [DOI] [PubMed] [Google Scholar]