SUMMARY
During wakefulness, extracellular levels of metabolites in the brain increase. These include amyloid beta (Aβ), which contributes to the pathogenesis of Alzheimer’s disease (AD). Counterbalancing their accumulation in the brain, sleep facilitates the removal of these metabolites from the extracellular space by convective flow of the interstitial fluid from the para-arterial to the para-venous space. However, when the sleep-wake cycle is disrupted (characterized by increased brain levels of the wake-promoting neuropeptide orexin and increased neural activity), the central nervous system (CNS) clearance of extracellular metabolites is diminished. Disruptions to the sleep-wake cycle have furthermore been linked to increased neuronal oxidative stress and impaired blood–brain barrier function – conditions that have also been proposed to play a role in the development and progression of AD. Notably, recent human and transgenic animal studies have demonstrated that AD-related pathophysiological processes that occur long before the clinical onset of AD, such as Aβ deposition in the brain, disrupt sleep and circadian rhythms. Collectively, as proposed in this review, these findings suggest the existence of a mechanistic interplay between AD pathogenesis and disrupted sleep-wake cycles, which is able to accelerate the development and progression of this disease.
Keywords: Aging, Amyloid beta, Blood brain barrier, Circadian misalignment, Neurodegeneration, Orexin, Oxidative stress, Sleep disruption, Slow-wave sleep, Tau
Regulation of amyloid and tau levels in the brain across the sleep-wake cycle
The aggregation of amyloid beta (Aβ) peptides (predominantly Aβ peptides 1–40 and 1–42; Aβ40 and Aβ42, respectively) into plaques in the brain is a marker of Alzheimer’s disease (AD) and a key component of the ‘Amyloid cascade hypothesis’ [1]. In recent years, increasing evidence has accumulated to support the hypothesis that the production of Aβ peptides in the brain is closely connected to the 24-hr sleep-wake cycle, with high extracellular levels during wakefulness and low extracellular levels during sleep [2–4] (Fig. 1). A major driver for the production of Aβ appears to be neuronal activity, which is higher during wakefulness as compared with sleep. This hypothesis is supported by the observation that unilateral vibrissal stimulation increases, while unilateral vibrissal removal decreases, interstitial fluid (ISF) levels of Aβ in the contralateral barrel cortex of transgenic mice (Tg2576) [5]. In humans, ISF Aβ concentrations have been shown to increase in patients with acute brain damage as neurological status improves, and conversely to fall when neurological status declines [6].
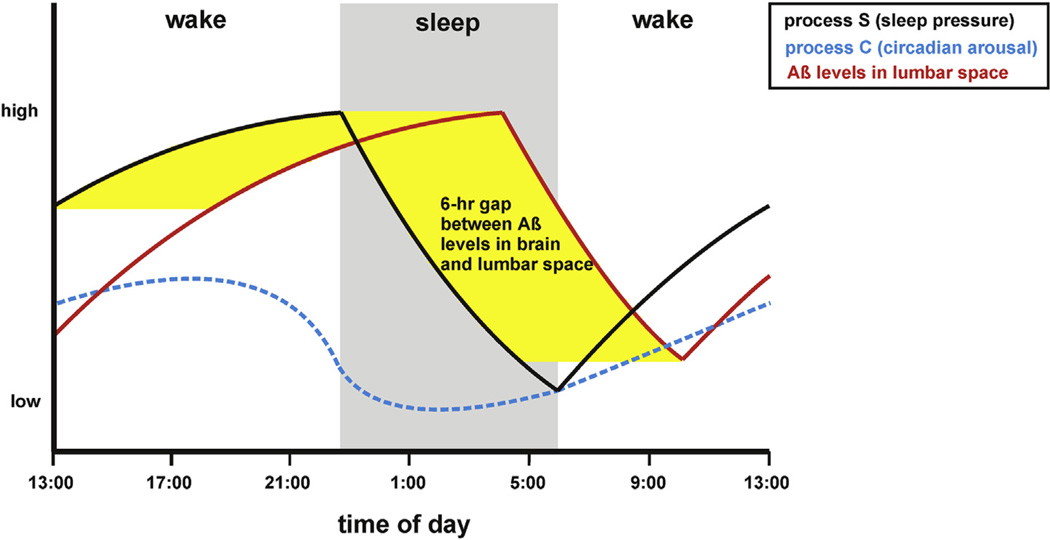
Fig. 1.
Temporal association between homeostatic sleep pressure and CSF concentrations of amyloid beta. The propensity to sleep is considered to be regulated by two interacting mechanisms: a circadian process (C) and a homeostatic process (S) [110]. Process C drives arousal and helps time the onset of normal sleep (driven by e.g., environmental light changes and meal patterns), whereas Process S drives sleep pressure and increases as wakefulness continues and decreases during slow-wave sleep (SWS), a sleep stage that predominates during the first 1/3 of the night. In humans, in a study where samples were collected via an indwelling lumbar catheter, both CSF Aβ40 and Aβ42 fluctuated by 25% with a diurnal pattern (labeled as Aβ in the figure) (higher during wakefulness and lower during sleep), with the lowest Aβ42 levels at around 10:00 h [2]. This corresponds to approximately 04:00 h in sleep time as there is a 6-h lag for brain Aβ to reach the lumbar space [12,57]. Abbreviations: Aβ, amyloid beta; Aβ40, amyloid beta peptide 1–40; Aβ42, amyloid beta peptide 1–42 CSF: cerebrospinal fluid.
During sleep, the brain remains metabolically and electrically active with preservation of cortico-cortical connectivity during light sleep, i.e., non-rapid eye movement (NREM) sleep stage 1 (N1) and NREM sleep stage 2 (N2) [7–9]. However, a reduction occurs in fronto-parietal functional connectivity with increasing depth of NREM sleep to the point of being significantly reduced in deep sleep [7–11], also called NREM sleep stage 3 (N3) or slow-wave sleep (SWS). Therefore, Aβ production could be postulated to decrease during SWS by virtue of the decreased neuronal activity in this sleep stage. Supporting this hypothesis, cerebrospinal fluid (CSF) Aβ42 levels have been shown to be lowest in humans at around 10:00 h (around 25% lower than peak values), corresponding to a nadir in ISF levels at 04:00 h (as there is a 6-h lag for brain soluble Aβ to reach the lumbar space [12,13]). This represents a time point after which most SWS has typically occurred and after which sleep is predominated by sleep stages N1–2 and rapid eye movement (REM) sleep.
Neuropeptides involved in the regulation of the sleep-wake cycle may additionally contribute to the characteristic 24-hr pattern of Aβ peptides in the brain. One such candidate is the hypothalamic neuropeptide orexin-A (hypocretin 1), the level of which increases during wakefulness [14]. A study in transgenic APPswe (Tg2576) mice – a mouse model of AD pathology, which carries the Swedish mutation (K595N/M596L) of the amyloid precursor protein (APP) resulting in higher Aβ peptide levels, and which does not develop behavioral signs of AD – showed that intracerebroventricular administration of orexin at the beginning of the light (i.e., inactive) period could acutely increase both wakefulness and Aβ levels in ISF. Conversely, intracerebroventricular treatment over 24 h with a dual orexin receptor antagonist (almorexant) decreased Aβ ISF levels [2]. Further supporting the role of orexin for Aβ accumulation, daily treatment with almorexant for 8 w reduced the formation of Aβ plaques in several brain regions in APPswe/PS1dE9 mice [2]. In a recent study performed in amyloid transgenic mice in which the orexin gene was knocked out(APP/PS1dE9/OR−/−) [15], lossoforexin resulted in decreased wakefulness and a subsequent reduction in amyloid pathology. In contrast to findings of animal studies, evidence from human studies about the role of orexin in the regulation of Aβ production in the brain is less consistent [16–23]. For instance, a recent study involving patients with the sleep disorder narcolepsy – a disease hallmarked by a progressive loss of brain orexin function [24] – revealed that CSF concentration of Aβ was significantly higher in the patient group with normal CSF orexin-A concentration than in those with low orexin-A concentrations [16]. Moreover, in a separate study, CSF levels of Aβ42 were found to be lower in narcoleptic patients compared with healthy controls [25]. Finally, in a case of narcolepsy-cataplexy that occurred post H1N1 vaccination, a strong decrease in CSF beta-amyloid was observed (152 mg/l, normal >500 mg/l [26]). In contrast to these studies involving narcoleptic patients, CSF concentrations of orexin-A and Aβ42 have been found to show no relationship in both AD patients and healthy controls [20].
Another key component of AD pathogenesis is the accumulation of intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau (P-tau) protein. Importantly, to date it is not known if the parenchymal levels of tau in the CNS exhibit the same 24-hr rhythmicity as Aβ. Notwithstanding, neuronal activity rapidly increases extracellular tau in mice [27], suggesting that mammalian ISF levels of tau may exhibit a similar neuronal activity-driven 24-hr sleep-wake pattern as Aβ.
Disruptions to sleep and circadian rhythms and the risk of AD
Given the evidence above, an obvious question is: do chronic disruptions to the sleep-wake cycle increase the risk of AD in humans? An increasing number of studies support such a notion, as insomnia [28], self-reported sleep disturbances [29], a decline in sleep duration [30], impaired sleep consolidation [31], delayed or decreased circadian rhythms [32], and sleep disordered breathing (SDB) [33], all have been shown to increase the risk of AD (for a detailed review, see [34]). While AD itself may cause sleep disruptions, the fact that sleep disruptions increase AD risk in non-demented older humans, supports the hypothesis that a chronically disrupted sleep-wake cycle can drive AD pathogenesis. Present evidence from human and animal experiments lends further support to this hypothesis. Experimentally induced sleep disruptions, to date almost exclusively carried out in rodent models, lead to an accumulation of AD-promoting metabolites Aβ and tau in the brain [2,3,35–37], increase central nervous system (CNS) oxidative stress [38–40], and reduce the structural and functional integrity of the blood–brain barrier (BBB) [41,42], all of which have been hypothesized to promote the development and progression of AD [1,43,44]. Finally, disrupted circadian rhythms, which have been found to even occur prior to the clinical onset of AD [45], have also been linked to neurodegeneration in rodent models [46,47].
Recent results from human and transgenic animal studies also support the idea that AD pathology itself can lead to sleep and circadian disruptions. It has for instance been demonstrated that pathophysiological processes associated with AD, such as Aβ deposition in the brain, alter sleep, as well as disrupt circadian rhythms [3,48,49]. Since Aβ deposition in the brain and cognitive dysfunctions are detectable years prior to the clinical onset of AD [50,51] this suggests that disruptions to the sleep-wake cycle may be a consequence rather than cause of AD pathogenesis. Alternatively, existing evidence leads us to propose that there exists a mechanistic interplay between AD pathogenesis and disruptions to sleep and interrelated circadian rhythms (as illustrated in Fig. 2).
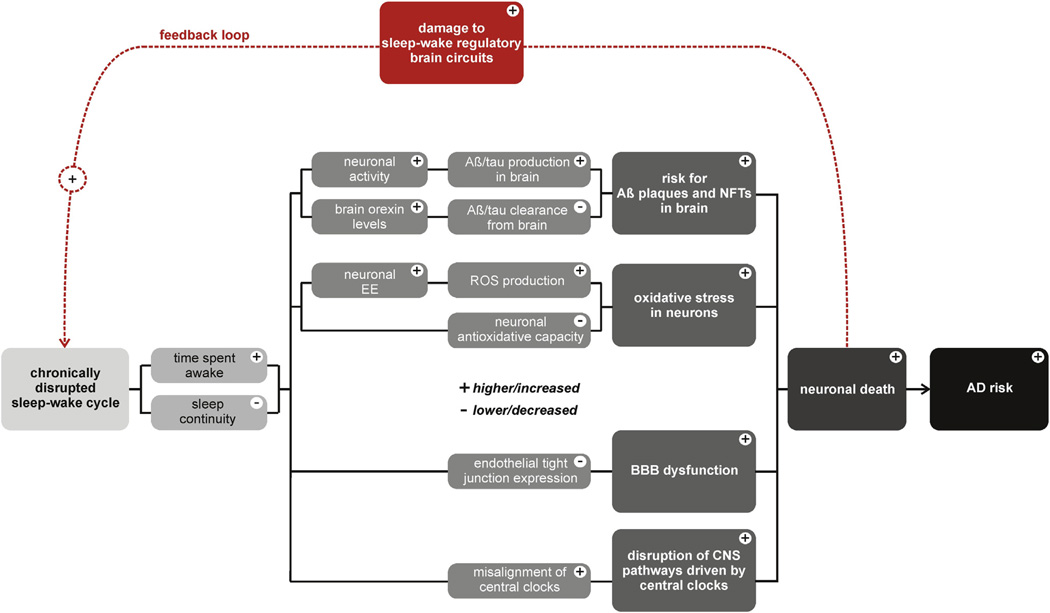
Fig. 2.
Overview of proposed mechanisms through which disruptions to the sleep-wake cycle form a positive feedback loop with AD pathogenesis in humans. Abbreviations: Aβ, amyloid beta; AD, Alzheimer disease; CNS, central nervous system; BBB, blood–brain barrier; EE, energy expenditure; NFTs, neurofibrillary tangles.
With this in mind, the objective of our review is to systematically frame recent experimental findings from human and animal experiments into a comprehensive overview on candidate mechanisms through which chronically disrupted sleep-wake cycles (e.g., fragmented sleep and circadian disruption) drive AD pathogenesis, and vice versa.
While there are several recent reviews on sleep-wake cycle disruptions and CNS deposition of Aβ peptides [52–56], to our best knowledge no one has yet comprehensively reviewed the recent literature including the role of tau and detailing the wider range of candidate mechanisms that may underlie the harmful association between sleep-wake disruptions and risk of AD.
Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease
Clearance of AD-promoting metabolites from the brain
Soluble Aβ levels are higher in the brain during wakefulness and lower during sleep, indicating that sleep maycurb processes leading to Aβ production, concomitantly promoting processes involved in Aβ clearance [2–4]. In contrast, under conditions of acute sleep deprivation, brain Aβ concentrations further increase during the night/inactive period, both in mice and in humans [2,35,57]. In one study, mice were subjected both to acute and chronic sleep deprivation. Acute sleep deprivation during 6h of day time (7:00–13:00h) resulted in around 17% higher ISF Aβ levels in Tg2576 mice. Chronic sleep deprivation of APPswe/PS1dE9 mice, which instead lasted for 21 d, more than doubled Aβ levels throughout the brain compared with control animals [2]. Whereas neither Aβ nor tau levels have been assessed in CSF in more long-term sleep deprivation experiments in humans, several studies have confirmed that sleep in humans is associated with lower Aβ levels in CSF. In a study of 26 healthy men (age 40–60y), the 13 individuals who were allowed to sleep for one night showed a 6% decrease in CSF Aβ42 levels across the night; an additional correlation analysis showed that total sleep duration correlated with decreased Aβ42 in CSF – neither of these effects were seen for Aβ40, P-tau or total tau (T-tau) [35]. Meanwhile, the 13 subjects who instead underwent sleep deprivation for one night had no overnight decrease in Aβ42 levels in CSF [35]. With these findings in mind, the question is what mechanism – decreased production or increased clearance – mainly accounts for the drop in brain Aβ levels during sleep, and which of the involved mechanisms is impaired to the greatest extent during sleep loss?
A recent study suggests that increased clearance during sleep may be a major driver for the overnight decrease in Aβ levels in the brain. The clearance of Aβ is driven by local degradation by a wide range of proteases [58], phagocytosis by glial cells [59], egress across the BBB, and Aβ reabsorption through the CSF [60]. Recently, the existence of a CNS paravascular circulation [61] was confirmed and extended by the use of in vivo two-photon imaging in mice [62]. It was determined that the CSF acts in the brain parenchyma like lymph, by flushing out interstitial substances in a process facilitated by glial cells. It was therefore named the glymphatic (a glia-dependent lymphatic) system [62]. Subsequently, another study found that this CSF-ISF exchange increased during sleep in wild-type mice [37], thereby enabling removal of metabolites that typically accumulate during wakefulness, encompassing AD-promoting soluble Aβ peptides. During wakefulness, however, the removal of such metabolites or inert tracers from the brain was not as efficient [37]. The study demonstrated that there was a 60% increase in the interstitial space of brain parenchyma in mice during sleep, as compared with the space found during wakefulness, and sleep was found to increase the convective flow of ISF from the para-arterial to the para-venous space [37] (see Fig. 3). The removal of substances was also greatly increased during sleep, possibly as a result of the concomitant expansion of the interstitial space, evidenced by a doubling in the rate of Aβ removal from brain parenchyma seen during wakefulness [37]. These effects were furthermore mimicked by infusion of noradrenergic receptor antagonists, suggesting that low adrenergic input is required for this convective clearance to occur. In contrast, awakening of sleeping mice sharply reduced the para-arterial and parenchymal influx of ISF (reduction of ~95%) [37]. This could suggest that sleep disruptions hallmarked by recurrent awakenings, increased time awake after sleep onset, or sleep fragmentation due to SDB, may diminish the ability of the glymphatic system to remove Aβ from the brain. Importantly, a caveat that must be considered is that the study by Xie et al. utilized exogenously administered Aβ to ascertain the clearance function of the glymphatic system during sleep [37]. Thus, it has not yet been shown to which extent the function of the glymphatic system during sleep is of relevance for clearance of endogenously produced Aβ. This also applies to a recent human study, which found MRI-based evidence for modulation of the extracellular space by sleep and wakefulness. Following 24 h of wakefulness combined with cognitively demanding task, increased subcortical but not global gray and white matter volumes were observed in the healthy participants, as well as decreased volume of the brain ventricles, compared with the volumes observed after normal sleep. These changes reverted after recovery sleep. However, any relationship to a possible glymphatic system or potential increased clearance of metabolites during human sleep, as compared with wakefulness, was not examined [63].
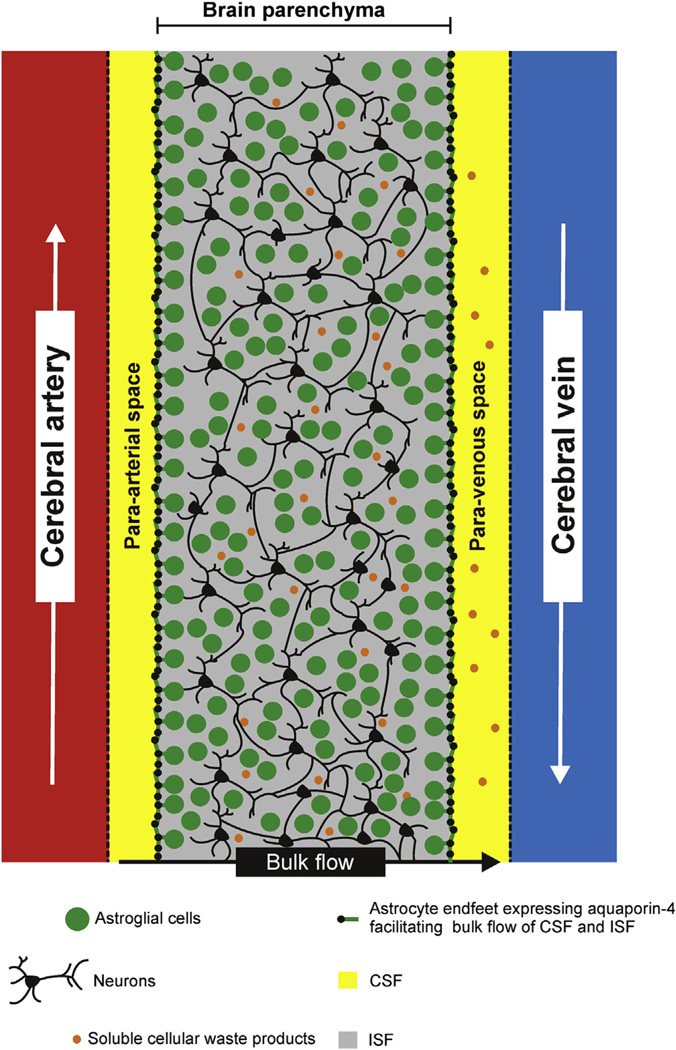
Fig. 3.
Scheme illustrating the glymphatic system (primarily based on [37]). Akin to other cells in the body, brain cells are surrounded by interstitial fluid (ISF), which contains nutrients, proteins and other solutes essential for brain cell survival, but also includes extracellular waste molecules that may be neurotoxic if not cleared properly (e.g., amyloid beta, Aβ). By utilizing real-time assessments of tetramethylammonium diffusion and two-photon imaging in mice, a brain-specific system with a similar function as lymph vessels, for removing ISF from the brain, was discovered in 2012 [62], and termed the “glymphatic” system as it depends on glial cell functioning. This system promotes clearanceof soluble metabolites from the brain [37,62,68,111]. Following entry of CSF through the para-arterial space that surrounds penetrating arteries in the brain, CSF exchanges with parenchymal ISF, moving across the parenchyma. ISF and interstitial solutes are then cleared via exit into the para-venous space surrounding large-caliber cerebral veins [62]. This system was found to depend on the function of astroglial cells that express the protein aquaporin-4 (AQP4; a water channel), in a highly polarized manner along the cells’ perivascular endfeet, thus ensheathing the cerebral vasculature. When AQP4 was deleted, CSF influx decreased, coupled with a 70% reduction in ISF solute clearance. This suggests that the system is involved in clearance of substances such as Aβ, the clearance of which was also markedly reduced following deletion of AQP4 [62]. Abbreviations: Aβ, amyloid beta; AD, Alzheimer disease; AQP4, aquaporin-4; CSF, cerebrospinal fluid; ISF, interstitial fluid.
While aggregation of P-tau into NFTs represents the other main pathologic feature of AD [64], clearance of tau has not been well characterized. The protein exists in six isoforms, ranging from 352 to 441 amino acids (ten times the size of Aβ peptides), without any known active transport mechanisms to blood like Aβ. CSF bulk flow and in situ degradation are the most likely mechanisms for clearance of tau released into the interstitial space, but a recent study in mice showed that extracellular tau could also be cleared from the brain via the glymphatic pathway under anesthesia [65]. To the best of our knowledge, no study has directly investigated the role of sleep in the degradation or removal of tau protein from the ISF, and whether its removal can be impaired by sleep loss. One clinical study has shown that CSF P-tau or T-tau levels are not affected by one night of sleep loss in healthy individuals [35], although as noted by the authors, the long turnover of tau (11 d; [27]) would likely have required a considerably longer sleep loss paradigm to study how these dynamics are affected by disrupted sleep.
Because sleep clears cellular waste from the extracellular space of the brain parenchyma [37], it is nonetheless likely that sleep promotes tau protein removal from the brain ISF and reduces its aggregation into NFTs. Suggestive of this, studies using mice that develop AD pathology (3xTg; produce amyloid plaques and NFTs) suggest that altered sleep-wake patterns can increase tau levels in the brain [36,66]. In one of these studies, six weeks of sleep restriction for 6 h/day increased cortical Aβ and P-tau to about 2-fold of control animals, although the changes were not significant [66]. In another study, two months of sleep disruption by prolonged daily light exposure (20/4-h light–dark cycle) led to a greater than 50% significant increase of the insoluble fraction of tau in the brain, as compared with control mice that were maintained on a 12/12-h light–dark cycle [36].
As reviewed in this section, the recent finding that sleep promotes the function of a glymphatic system, resulting in enhanced removal of AD-promoting metabolites, is intriguing, but still requires additional research. For instance, it is still unclear whether sleep-driven clearance of metabolites is also present in humans and whether it can increase the clearance rate of neurotoxic substances to the same extent as in mice. Moreover, further research is needed to disentangle the contribution of normal and disrupted sleep stages and circadian mechanisms to this function, for example to address whether interventions that enhance specific sleep stages can improve the clearance of metabolites from the brain in subjects genetically prone to develop AD. Given that activity profiles of neurotransmitter and neuropeptide systems vary across sleep stages (e.g., high cholinergic activity during REM sleep vs. low cholinergic activity during SWS [67]) further studies are needed to ascertain how sleep stage-specific neurotransmitter and neuro-peptide patterns contribute to the clearance of AD-promoting metabolites from the ISF. A recent study demonstrated that when old (18-month-old) and young (2- to 3-month-old) mice were compared, the older mice exhibited a marked reduction in the CSF-ISF exchange, with a 27% reduction in the pulsatility in arterioles in the brain, and a loss of perivascular aquaporin-4 (AQP4) polarization [68]. Furthermore, the older mice exhibited a 40% reduction of their ability to clear Aβ that had been injected intraparenchymally [68]. However, no age-associated decline was seen in the ability of sleep to increase the interstitial space. This suggests that deterioration of this part of the glymphatic system does not contribute to the age-associated increase in the risk of AD, to which disrupted sleep may contribute. However, the influence of sleep deprivation on the glymphatic system in young compared with old mice was not investigated and as such warrants further investigation.
CNS oxidative stress
In addition to the accumulation and deposition of Aβ and NFTs in the brain, CNS oxidative stress resulting from increases in reactive oxygen species (ROS) and reactive nitrogen species (RNS) has also been proposed to promote the development and progression of AD [43].In APPs we mice, oxidative stress precedes deposition of Aβ [69], and evidence of oxidative damage has been observed in human AD subjects studied postmortem, with the greatest pathology found early in the disease [70]. Defective mitochondria, which are a major source of ROS, are seen in AD as well as in what is the greatest risk factor for AD, i.e., aging, and may contribute to AD pathology by altering how APP and tau proteins are processed [71].Conversely, APP and Aβ have been shown to interfere with mitochondrial functions and enzymes [71],in what may develop into a positive feedback loop.
ROS and RNS play essential roles under physiological conditions (e.g., induction of host defense). However, excessive production of reactive species can induce cellular stress through lipid peroxidation and protein oxidation. Once initiated, such oxidative processes can lead to damage to vital cellular components such as proteins, lipids, and nucleic acids, which can finally result in cellular death [43].
Several animal studies have found loss of sleep to increase oxidative stress in the brain [38,39,72,73], suggesting it might be a candidate mechanism underlying the association between sleep loss and AD. For instance, in one such study in mice [72], three nights of extended wakefulness (via environmental enrichment during the rest cycle) increased signs of oxidative stress in locus coeruleus (LC) neurons, as reflected by increased production of superoxide. Another study found that sleep loss in mice (for 72 h using a multiple platform method) increased oxidative stress in the hippocampus and was linked to learning deficits, as both the oxidative stress and the learning deficits could be prevented if anti-oxidative agents (N-tertbutyl-alpha-phenylnitrone, vitamin E or melatonin) were administered prior to the sleep deprivation [73], supporting a detrimental role for oxidative stress following even short term sleep loss. It must however be noted that other studies in mice have not found signs of increased oxidative stress in the cortex using total sleep deprivation paradigms [74] or following REM sleep deprivation after which whole-brain extracts have been analyzed [75]. One explanation for these discrepant results could be that oxidative stress is primarily apparent in brain regions that fire at increased rates across sustained wakefulness, comprising the LC [72]. Supporting this notion, such brain regions appear to be more sensitive to cellular damage that can lead to cellular death following sleep disruptions in humans with and without AD [76,77].
An important question that remains unanswered is how can sleep loss cause oxidative stress in the brain? One explanation could be that cellular scavenger mechanisms against reactive species are compromised in function. Supporting this notion, three nights of extended wakefulness were shown to reduce the activity of sirtuin type 3 (SirT3) in the mouse brain [72]. SirT3 is a nicotinamide adenine dinucleotide-dependent enzyme that is localized to the mitochondrial membrane and which upregulates many antioxidant defenses. Anti-oxidative mechanisms may also decrease in certain brain regions following sleep loss, especially in older animals [78], which also display lower CNS levels of defensive mechanisms such as lower levels of SirT3 [79].
Blood-brain barrier (BBB) integrity
Neurons demand a nearly continuous supply of energy metabolites, as they have only limited energy reserves. This requires a continuous metabolite exchange between circulating plasma and the brain. This high rate of molecular exchange, however, also implies that neurons are exposed to many potentially harmful factors derived from the periphery. To prevent the brain from uncontrolled entry of blood factors and toxins as well as an unfavorable efflux of CNS metabolites into the periphery, the BBB consists of endothelial cells lining brain capillaries that tightly regulate the flow of nutrients, ions, and fluids between both compartments. As reviewed in Ref. [44], BBB dysfunctions (e.g., endothelial loss and loss of tight junction proteins) have been proposed to contribute to the development and progression of AD, as they impair Aβ clearance from the brain, lead to increased influx of circulating Aβ into the brain, and elevate expression and processing of the Aβ precursor protein.
Importantly, emerging evidence suggests that sleep disruption may impair the function of the BBB [41,42]. For instance, six days of sleep restriction in mice, resulting in a mild 13% increase in total wake time, led to a reduced expression of tight junction proteins by BBB endothelial cells [42]. This reduction was paralleled by increasing paracellular permeability of the BBB to small substances, which under physiological conditions mainly reach the brain via a saturable transport system located at the BBB [42]. The expression of glucose transporter 1 (GLUT1), a protein that mediates glucose uptake through cerebral BBB micro-vessels, was also reduced following the sleep restriction paradigm. Importantly, functional and structural alterations to the BBB have also been found in AD patients, including reduced expression of GLUT1 [80–82]. Further highlighting the importance of glucose transport, a recent study found that GLUT1-deficienct (Slc2a1+/−) mice with APPs we over-expression had increased BBB permeability, reduced dendritic spines and cognitive deficits [83].
As the increase in paracellular permeability following six days of sleep restriction in mice returned to baseline after 24 h of recovery sleep [42], this however suggests that impaired BBB integrity following short periods of sleep restriction is a reversible process. It is currently not known whether sleep restriction-induced BBB disruptions may accelerate neurodegenerative processes involved in AD, such as Aβ plaque formation. This appears especially relevant given that a recent study found that preclinical AD mouse models (e.g., PS2-APP and hTauP301S) do not per se display disrupted BBB function [84], as assessed by passive antibody uptake into the brain. This could suggest that the role of the BBB may be dissociated from the pathogenic burden posed by Aβ accumulation on e.g., circadian rhythms and sleep patterns, whereby BBB disruption (due to e.g., sleep restriction) may increase AD pathology, but not vice versa.
Circadian disruption
Accumulating evidence connects disruptions of circadian rhythms, as frequently found in AD patients [45], but also in shift workers who are also more likely to be short sleepers [85], to neurodegeneration and cognitive aging [86,87]. For instance, one study found that shift workers without the ability to recover for longer time periods between demanding periods of shift work (less than five versus over 14 d of recovery time) had increased temporal lobe atrophy observed on magnetic resonance imaging [86]. In addition, a 5-year prospective study of 1282 women found that delayed peak of activity rhythms, and decreased amplitude and robustness thereof, conferred an increased risk of mild cognitive impairment (MCI) or dementia [32].
Recent studies have begun delineating how disrupted circadian rhythms may contribute to neurodegeneration (summarized in [46] and [47]). Mice with ablated Clock or Bmal1 – genes regulating central and peripheral molecular clocks – exhibit impaired sleep parameters. Homozygous Clock mutants sleep approximately 2 h less than wild-type mice [88], and they exhibit increased signs of oxidative stress in the brain [89,90]. Neuronal- and glial-specific deletion of the master clock gene Bmal1 in mice also increased neurodegeneration, as evidenced by degeneration of synaptic terminals and impaired cortical functional connectivity, as well as neuronal oxidative damage and impaired induction of several redox defense genes, such as Aldh2 and Nqo1 [90]. This was observed even though such genetically targeted ablation (using a Nestin-Cre driver) does not fully abolish expression of these clock genes in the suprachiasmatic nucleus (SCN) – the pacemaker clock that entrains other circadian oscillators – and accordingly activity and sleep rhythms remained largely unaffected [90]. Similarly, disrupting the genetic clock machinery via knockout of Bmal1 or Per2 has been shown to impair murine hippocampal neurogenesis by perturbing the conversion of quiescent neural progenitor cells into newborn neurons in the mouse hippocampus [91].
Although these studies establish a connection between circadian disruption and neurodegeneration, which may be linked to AD, they leave several gaps in our knowledge warranting further investigation. For instance: does glial-specific deletion of master clock genes (e.g., Bmal1) alter the ability of the glympathic system to remove AD-promoting metabolites from the brain parenchyma, and as an extension, to what extent does the glymphatic system depend on properly aligned circadian rhythms? Circadian rhythm disturbances are frequently seen even in patients with preclinical AD [45]. The possible contribution of circadian disruption to AD disease progression has been showcased by a study in which bright- vs. low-light exposure in combination with melatonin vs. placebo was used to study the effect of synchronizing circadian rhythms in over 189 patients, 63% of whom had probable AD, over an average follow-up period of 15 months. Both bright light and melatonin had positive effects: the light therapy was for example able to slow cognitive decline (as measured by the mini-mental state examination) [92]. Thus, it may also be worth investigating if therapeutic synchronization of the circadian timing system (e.g., through bright light therapy) can help re-establish circadian Aβ dynamics in ISF and CSF in humans suffering from circadian rhythm disorders or at increased risk of AD (e.g., ApoE4 carriers), and if such therapies can curb the development and progression of this disease.
Notably, functional weakening of the circadian system, characterized by phase advance, increased fragmentation and reduced amplitudes of circadian rhythms, is a well-documented consequence of aging [46]. Given that synchronized activity of multiple circadian clocks in the brain has been suggested to be critically important for a number of CNS processes [46], it could be speculated that loss of phase coherence between these CNS clocks due to normal aging, promotes neurodegenerative processes associated with AD pathology. Supporting this view, it has been shown that, when comparing young and elderly adults who were either positron emission tomography (PET) amyloid positive or negative, 24-h fluctuations in CSF Aβ levels decreased with age and even more in those who were ‘amyloid positive’ [2,57]. The loss of the dynamic pattern was more pronounced for Aβ42 than for Aβ40, most likely due to its greater propensity to aggregate in amyloid plaques [93].
Disruptions to the sleep-wake cycle as a consequence of AD-related processes
Multiple lines of evidence suggest that neurodegenerative processes associated with AD can cause sleep and circadian disruptions in humans. This is particularly relevant to the interpretation of findings from epidemiological studies investigating the association between sleep disruption and AD features, as they do not typically account for the occurrence of preclinical AD in a substantial proportion of cognitively healthy older individuals who are included in such studies [50,51]. For instance, a recent study involving 45 older adults (12 with AD) demonstrated that individuals with AD had fewer intermediate nucleus neurons than controls at the time of death [76]. The intermediate nucleus is considered as the human homolog of the rodent ventrolateral preoptic nucleus, a brain region that promotes sleep by inhibiting wake-promoting brain regions, which include the lateral hypothalamic area, raphe nucleus, tuberomammillary nucleus and the LC (for more details concerning the neurobiology of sleep regulation, please see [94]). Additional support for the existence of a bidirectional rather than unidirectional link between sleep disruption and AD has been provided by a recent study involving 26 cognitively healthy (i.e., non-demented) elders. In this study, it was revealed that those with high Aβ burden in the medial prefrontal cortex (a typical feature of AD) had lower slow-wave activity (SWA) during NREM sleep. Furthermore, prefrontal Aβ burden was found to be associated with impaired sleep-dependent memory consolidation, most likely mediated through its effects on NREM SWA [48]. Adding further support to the hypothesis that amyloid deposition may alter sleep characteristics in the preclinical stage of AD, three additional studies performed in younger-old and middle-old cognitively healthy individuals have reported shorter sleep duration and/or lower sleep quality to be associated with greater CNS amyloid burden [94–97]. Finally, several studies utilizing post-mortem brain samples of AD patients have found a substantial decrease of neurons in the central pacemaker SCN compared with control subjects[98–100].One recent study did not find a decrease in two of the major SCN cell neuron populations in AD vs controls subjects [101], but still found an age-dependent decrease in SCN neuron numbers in both AD and control subjects. Notably, there was still a3-hdelay incircadianrhythms in AD compared with control subjects, suggesting damage to input or downstream output brain regions of the SCN in AD patients. Indeed, tau pathology is observed in its earliest stages in the sleep-wake regulating LC [102,103]. Together, these findings may explain why AD patients, in addition to their sleep problems, frequently have difficulty maintaining normal circadian rhythms [45].
In line with these clinical findings, many animal studies also demonstrate that AD-related features can affect sleep parameters. For instance, APPswe/PS1de9 mice did not show disrupted diurnal Aβ rhythms in ISF or disrupted sleep-wake before deposition of Aβ (at 3 mo of age) compared with wild-type mice. However, following the onset of Aβ plaque formation in the brain, the sleep-wake cycle of these animals markedly deteriorated, evidenced by reduced NREM and REM sleep, and increased wake time [3]. This indicates that molecular features of preclinical AD, such as Aβ accumulation in the brain, can lead to sleep disruptions. Evidence also suggests that tau, as the other main molecular driver of AD, can disrupt sleep in animals. For instance, mice that mimic human tauopathy (rTg4510) have slower membrane oscillations during SWS and under anesthesia, indicating that pathological tau accumulation disrupts such CNS states of highly synchronized synaptic activity [49]. Finally, reduced NREM sleep and increased sleep fragmentation have been observed in a phenotypic analysis of a mouse model that mimics the pathophysiology reminiscent of early, prodromal AD (PLB1Triple mice) [104].
Transgenic animal studies have further enriched our knowledge on how AD-related molecular features may contribute to circadian disruption. For instance, in a study examining Tg2576 mice, the period of wheel running was increased in constant darkness [105] – indicating a prolonged circadian period. Furthermore, NREM sleep contained higher electroencephalogram (EEG) frequencies in the transgenic mice compared to controls, with transgenic mice failing to demonstrate the increased delta (1–4 Hz) power that wild-type mice exhibited in response to sleep loss. Complementing these findings, a separate study investigating the role of Aβ in the regulation of clock molecules and circadian rhythm found that a mouse model of AD pathology (5XFAD, which harbors human APP and PS1 mutations) exhibited altered circadian rhythms in both young (2 mo old) but especially in older mice (8 mo), as assessed by both body temperature and home cage activity [106]. The mice also exhibited altered SCN expression patterns of the circadian clock genes, e.g., Bmal1, which through in vitro tests was corroborated to result from Aβ-induced degradation [106]. Similar findings have also been observed following overexpression of orthologs of APP cleavage enzymes in drosophila flies, in which the resulting increase in Aβ production resulted in disrupted circadian rhythms [107].
Collectively, evidence from both human and animal studies provide a strong rationale for hypothesizing that poor sleep and disrupted circadian rhythms may be a potential early marker of neuropathology during the long preclinical phase of AD.
Conclusions
Observational studies have found that patients with insomnia or sleep disruptions in mid-life to old age have an increased risk of pathological changes that precede AD (e.g., CNS increased amyloid burden, neurofibrillary tangles), as well as an increased risk of dementia and AD. In line with these findings, experimental studies have demonstrated that sleep disruptions result in higher levels of markers that are associated with AD, increase CNS oxidative stress, can damage the blood–brain barrier in mice, and disrupt clearance of AD-promoting Aβ peptides. Importantly, patients with impaired cognition or increased Aβ burden also show signs of impaired sleep, and animal models have shown that Aβ deposition can directly drive impaired sleep. Collectively, current evidence points toward the existence of a mechanistic loop between AD pathogenesis and disrupted sleep and circadian rhythms.
Given the alarming increase in the number of people who are afflicted by chronic sleep problems [108] and AD pathology [109], studies with long follow-up periods and repeated observations initiated prior to the clinical onset of AD are needed to further disentangle the contribution of sleep and AD pathology in their intertwined relationship. The ideal longitudinal studies will assess sleep and circadian rhythms both subjectively and objectively (e.g., by actigraphy and EEG-based sleep monitoring),simultaneously withAD biomarkers (CSF biomarker levels, imaging using PET and, potentially, of glymphatic flow) and AD- and sleep-modulating risk factors, such as genetics, co-morbidities, exercise and exposure to environmental light and stress. The extent to which general improvements in sleep, or e.g., targeted sleep-stage enhancement, can help in lowering the risk or reversing signs of accelerated cognitive aging, MCI/AD, or other neurodegenerative diseases represent an important parallel path of research in this emerging interdisciplinary field.
Practice points.
Disrupted sleep has been found to be associated with an increased risk of Alzheimer’s disease (AD) through several mechanisms:
Observational studies have found that patients with e.g., insomnia or sleep disruptions in mid-life to old age have an increased risk of pathological changes that precede AD (e.g., CNS increased amyloid burden, neurofibrillary tangles), as well as an increased risk of dementia and AD.
Sleep restriction results in higher levels of markers that are associated with AD, increases CNS oxidative stress and can damage the blood–brain barrier in mice.
Clearance of the AD-promoting Aβ peptides is greatly enhanced during sleep in mice and awakening the mice disrupts this process.
Importantly, patients with impaired cognition or increased Aβ burden also show signs of impaired sleep, and animal models have shown that Aβ deposition can directly drive impaired sleep, in what can turn into a positive feedback loop.
Research agenda.
Future research should address the following questions with regards to sleep and AD pathogenesis:
The contribution of sleep disruptions to AD pathogen-esis, and of AD pathogenesis to sleep disruption in humans, by conducting long follow-up studies that are initiated prior to the presence of significant amyloid and tau burden in the brain that may otherwise conceal the contribution of each factor.
How clearance of metabolites in the CNS is regulated in humans and what sleep stage(s), circadian and underlying neurobiological mechanisms are the greatest contributors to this function.
The extent to which general improvements in sleep, or e.g., targeted sleep-stage enhancement, can help in lowering the risk or reversing signs of accelerated cognitive aging, MCI/AD, or other neurodegenerative diseases.
The impact of genetic variants in influencing the extent by which sleep disruption can confer an increased risk of AD.
Acknowledgments
Work by the authors is supported by the Swedish Brain Foundation (J.C., H.S., C.B.), the Fredrik and lngrid Thuring Foundation (J.C.), the Novo Nordisk Foundation (Denmark, C.B.), AFA Insurance (Sweden, C.B.), the Swedish Society for Medical Research (J.C.), the Swedish Research Council (J.C., H.S., C.B.) and the National Institute of Health (NIH) R01HL118624, R21AG049348 (R.O.). A.V. received research support from the American Sleep Medicine Foundation and the Leon Levy Foundation. We would like to thank Professor Lennart Wetterberg, at the Department of Clinical Neuroscience, and Nils Landegren at the Department of Medicine, both at the Karolinska Institute, for their critical review of our manuscript. We would also like to thank Dr. Maria Cuartero-Toledo, Tyler Gumb and Clifton Lewis for their help with the preparation of the manuscript. We would also like to thank the anonymous academic peer reviewers who provided helpful and detailed comments on earlier drafts of this review manuscript. We apologize to the many researchers who have contributed to the field and who because of space constraints have not been cited herein.
Abbreviations
- Aβ
amyloid beta
- Aβ40
amyloid beta peptide 1–40
- Aβ42
amyloid beta peptide 1–42
- AD
Alzheimer’s disease
- ApoE
apolipoprotein E
- APP
amyloid precursor protein
- AQP4
aquaporin-4
- BBB
blood–brain barrier
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EEG
electroencephalogram
- GLUT1
glucose transporter 1
- ISF
interstitial fluid
- LC
locus coeruleus
- MCI
mild cognitive impairment
- NFT
neurofibrillary tangle
- NREM
non-rapid eye movement
- N1
NREM sleep stage 1
- N2
NREM sleep stage 2
- N3
NREM sleep stage 3
- PET
positron emission tomography
- P-tau
phosphorylated tau
- REM
rapid eye movement
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SDB
sleep-disordered breathing
- SirT3
sirtuin type 3
- SCN
suprachiasmatic nucleus
- SWA
slow-wave activity
- SWS
slow-wave sleep
- T-tau
Total tau
Footnotes
Conflict of interest
The authors do not have any conflicts of interest to disclose.
References
- 1.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 2.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004291. 150ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007;68:666–669. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- 5.Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, et al. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, et al. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci U S A. 2009;106:11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larson-Prior LJ, Power JD, Vincent JL, Nolan TS, Coalson RS, Zempel J, et al. Modulation of the brain’s functional network architecture in the transition from wake to sleep. Prog Brain Res. 2011;193:277–294. doi: 10.1016/B978-0-444-53839-0.00018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spoormaker VI, Schroter MS, Gleiser PM, Andrade KC, Dresler M, Wehrle R, et al. Development of a large-scale functional brain network during human non-rapid eye movement sleep. J Neurosci Off J Soc Neurosci. 2010;30:11379–11387. doi: 10.1523/JNEUROSCI.2015-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samann PG, Wehrle R, Hoehn D, Spoormaker VI, Peters H, Tully C, et al. Development of the brain’s default mode network from wakefulness to slow wave sleep. Cereb Cortex. 2011;21:2082–2093. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- 11.Wu CW, Liu PY, Tsai PJ, Wu YC, Hung CS, Tsai YC, et al. Variations in connectivity in the sensorimotor and default-mode networks during the first nocturnal sleep cycle. Brain Connect. 2012;2:177–190. doi: 10.1089/brain.2012.0075. [DOI] [PubMed] [Google Scholar]
- 12.Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Potter R, Sigurdson W, Kasten T, Connors R, Morris JC, et al. beta-amyloid dynamics in human plasma. Arch Neurol. 2012;69:1591–1597. doi: 10.1001/archneurol.2012.18107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci Off J Soc Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roh JH, Finn MB, Stewart FR, Mahan TE, Cirrito JR, Heda A, et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J Exp Med. 2014;211:2487–2496. doi: 10.1084/jem.20141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heier MS, Skinningsrud A, Paus E, Gautvik KM. Increased cerebrospinal fluid levels of nerve cell biomarkers in narcolepsy with cataplexy. Sleep Med. 2014;15:614–618. doi: 10.1016/j.sleep.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71:1498–1505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- 18.Slats D, Claassen JA, Lammers GJ, Melis RJ, Verbeek MM, Overeem S. Association between hypocretin-1 and amyloid-beta42 cerebrospinal fluid levels in Alzheimer’s disease and healthy controls. Curr Alzheimer Res. 2012;9:1119–1125. doi: 10.2174/156720512804142840. [DOI] [PubMed] [Google Scholar]
- 19.Portelius E, Soininen H, Andreasson U, Zetterberg H, Persson R, Karlsson G, et al. Exploring Alzheimer molecular pathology in Down’s syndrome ce-rebrospinal fluid. Neurodegener Dis. 2014;14:98–106. doi: 10.1159/000358800. [DOI] [PubMed] [Google Scholar]
- 20.Wennstrom M, Londos E, Minthon L, Nielsen HM. Altered CSF orexin and alpha-synuclein levels in dementia patients. J Alzheimers Dis. 2012;29:125–132. doi: 10.3233/JAD-2012-111655. [DOI] [PubMed] [Google Scholar]
- 21.Dauvilliers YA, Lehmann S, Jaussent I, Gabelle A. Hypocretin and brain beta-amyloid peptide interactions in cognitive disorders and narcolepsy. Front Aging Neurosci. 2014;6:119. doi: 10.3389/fnagi.2014.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt FM, Kratzsch J, Gertz HJ, Tittmann M, Jahn I, Pietsch UC, et al. Cerebrospinal fluid melanin-concentrating hormone (MCH) and hypocretin-1 (HCRT-1, orexin-A) in Alzheimer’s disease. PloS One. 2013;8:e63136. doi: 10.1371/journal.pone.0063136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fronczek R, van Geest S, Frolich M, Overeem S, Roelandse FW, Lammers GJ, et al. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol Aging. 2012;33:1642–1650. doi: 10.1016/j.neurobiolaging.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Scammell TE. Narcolepsy. N. Engl J Med. 2015;373:2654–2662. doi: 10.1056/NEJMra1500587. [DOI] [PubMed] [Google Scholar]
- 25.Liguori C, Placidi F, Albanese M, Nuccetelli M, Izzi F, Marciani MG, et al. CSF beta-amyloid levels are altered in narcolepsy: a link with the inflammatory hypothesis? J Sleep Res. 2014;23:420–424. doi: 10.1111/jsr.12130. [DOI] [PubMed] [Google Scholar]
- 26.Kallweit U, Hidalgo H, Engel A, Baumann CR, Bassetti CL, Dahmen N. Post H1N1 vaccination narcolepsy-cataplexy with decreased CSF beta-amyloid. Sleep Med. 2012;13:323. doi: 10.1016/j.sleep.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osorio RS, Pirraglia E, Aguera-Ortiz LF, During EH, Sacks H, Ayappa I, et al. Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59:559–562. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benedict C, Byberg L, Cedernaes J, Hogenkamp PS, Giedratis V, Kilander L, et al. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimer’s Dement J Alzheimer’s Assoc. 2015;11:1090–1097. doi: 10.1016/j.jalz.2014.08.104. [DOI] [PubMed] [Google Scholar]
- 30.Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2014;22:1262–1271. doi: 10.1016/j.jagp.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E epsilon4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013;70:1544–1551. doi: 10.1001/jamaneurol.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13:1017–1028. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 35.Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71:971–977. doi: 10.1001/jamaneurol.2014.1173. [DOI] [PubMed] [Google Scholar]
- 36.Di Meco A, Joshi YB, Pratico D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging. 2014;35:1813–1820. doi: 10.1016/j.neurobiolaging.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Almeida V, Lobo LL, Hipolide DC, de Oliveira AC, Nobrega JN, Tufik S. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport. 1998;9:2853–2856. doi: 10.1097/00001756-199808240-00031. [DOI] [PubMed] [Google Scholar]
- 39.Mathangi DCP, Shyamala R, Subhashini AS. Effect of REM sleep deprivation on the antioxidant status in the brain of Wistar rats. Ann Neurosci. 2012;19:161–164. doi: 10.5214/ans.0972.7531.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramanathan L, Gulyani S, Nienhuis R, Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002;13:1387–1390. doi: 10.1097/00001756-200208070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez-Gonzalez B, Hurtado-Alvarado G, Esqueda-Leon E, Santana-Miranda R, Rojas-Zamorano JA, Velazquez-Moctezuma J. REM sleep loss and recovery regulates blood-brain barrier function. Curr Neurovasc Res. 2013;10:197–207. doi: 10.2174/15672026113109990002. [DOI] [PubMed] [Google Scholar]
- 42.He J, Hsuchou H, He Y, Kastin AJ, Wang Y, Pan W. Sleep restriction impairs blood-brain barrier function. J Neurosci Off J Soc Neurosci. 2014;34:14697–14706. doi: 10.1523/JNEUROSCI.2111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis. 2010;19:341–353. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 44.Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol. 2014;24:371–386. doi: 10.1111/bpa.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med. 2007;8:623–636. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13:325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 48.Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, et al. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18:1051–1057. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menkes-Caspi N, Yamin HG, Kellner V, Spires-Jones TL, Cohen D, Stern EA. Pathological tau disrupts ongoing network activity. Neuron. 2015;85:959–966. doi: 10.1016/j.neuron.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 50.Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N. Engl J Med. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan W, Kastin AJ. Can sleep apnea cause Alzheimer’s disease? Neurosci Biobehav Rev. 2014;47:656–669. doi: 10.1016/j.neubiorev.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Lim MM, Gerstner JR, Holtzman DM. The sleep-wake cycle and Alzheimer’s disease: what do we know? Neurodegener Dis Manag. 2014;4:351–362. doi: 10.2217/nmt.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging. 2014;35(Suppl. 2):S29–S34. doi: 10.1016/j.neurobiolaging.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 55.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38. doi: 10.1016/j.smrv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju YE, et al. Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch Neurol. 2012;69:51–58. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saido T, Leissring MA. Proteolytic degradation of amyloid beta-protein. Cold Spring Harb Perspect Med. 2012;2:a006379. doi: 10.1101/cshperspect.a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeWitt DA, Perry G, Cohen M, Doller C, Silver J. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer’s disease. Exp Neurol. 1998;149:329–340. doi: 10.1006/exnr.1997.6738. [DOI] [PubMed] [Google Scholar]
- 60.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 62.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003748. 147ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernardi G, Cecchetti L, Siclari F, Buchmann A, Yu X, Handjaras G, et al. Sleep reverts changes in human gray and white matter caused by wake-dependent training. Neuroimage. 2016;129:367–377. doi: 10.1016/j.neuroimage.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 65.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci Off J Soc Neurosci. 2014;34:16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer’s disease. Brain Res. 2013;1529:200–208. doi: 10.1016/j.brainres.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 68.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid per-oxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci Off J Soc Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 71.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J, Zhu Y, Zhan G, Fenik P, Panossian L, Wang MM, et al. Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons. J Neurosci Off J Soc Neurosci. 2014;34:4418–4431. doi: 10.1523/JNEUROSCI.5025-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silva RH, Abilio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46:895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 74.Gopalakrishnan A, Ji LL, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep. 2004;27:27–35. doi: 10.1093/sleep/27.1.27. [DOI] [PubMed] [Google Scholar]
- 75.D’Almeida V, Hipolide DC, Azzalis LA, Lobo LL, Junqueira VB, Tufik S. Absence of oxidative stress following paradoxical sleep deprivation in rats. Neurosci Lett. 1997;235:25–28. doi: 10.1016/s0304-3940(97)00706-4. [DOI] [PubMed] [Google Scholar]
- 76.Lim AS, Ellison BA, Wang JL, Yu L, Schneider JA, Buchman AS, et al. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain J Neurol. 2014;137:2847–2861. doi: 10.1093/brain/awu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gelber RP, Redline S, Ross GW, Petrovitch H, Sonnen JA, Zarow C, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology. 2015;84:296–303. doi: 10.1212/WNL.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh R, Kiloung J, Singh S, Sharma D. Effect of paradoxical sleep deprivation on oxidative stress parameters in brain regions of adult and old rats. Biogerontology. 2008;9:153–162. doi: 10.1007/s10522-008-9124-z. [DOI] [PubMed] [Google Scholar]
- 79.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 81.Kalaria RN, Harik SI. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J Neurochem. 1989;53:1083–1088. doi: 10.1111/j.1471-4159.1989.tb07399.x. [DOI] [PubMed] [Google Scholar]
- 82.Horwood N, Davies DC. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in Alzheimer’s disease. Virchows Arch. 1994;425:69–72. doi: 10.1007/BF00193951. [DOI] [PubMed] [Google Scholar]
- 83.Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bien-Ly N, Boswell CA, Jeet S, Beach TG, Hoyte K, Luk W, et al. Lack of widespread BBB disruption in Alzheimer’s disease models: focus on therapeutic antibodies. Neuron. 2015;88:289–297. doi: 10.1016/j.neuron.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 85.Ohayon MM, Lemoine P, Arnaud-Briant V, Dreyfus M. Prevalence and consequences of sleep disorders in a shift worker population. J Psychosom Res. 2002;53:577–583. doi: 10.1016/s0022-3999(02)00438-5. [DOI] [PubMed] [Google Scholar]
- 86.Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 87.Marquie JC, Tucker P, Folkard S, Gentil C, Ansiau D. Chronic effects of shift work on cognition: findings from the VISAT longitudinal study. Occup Environ Med. 2015;72:258–264. doi: 10.1136/oemed-2013-101993. [DOI] [PubMed] [Google Scholar]
- 88.Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, et al. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci Off J Soc Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kondratova AA, Dubrovsky YV, Antoch MP, Kondratov RV. Circadian clock proteins control adaptation to novel environment and memory formation. Aging. 2010;2:285–297. doi: 10.18632/aging.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bouchard-Cannon P, Mendoza-Viveros L, Yuen A, Kaern M, Cheng HY. The circadian molecular clock regulates adult hippocampal neurogenesis by controlling the timing of cell-cycle entry and exit. Cell Rep. 2013;5:961–973. doi: 10.1016/j.celrep.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 92.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 93.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 94.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 95.Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36:2568–2576. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 99.Stopa EG, Volicer L, Kuo-Leblanc V, Harper D, Lathi D, Tate B, et al. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J Neuropathol Exp Neurol. 1999;58:29–39. doi: 10.1097/00005072-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 100.Baloyannis SJ, Mavroudis I, Mitilineos D, Baloyannis IS, Costa VG. The hypothalamus in Alzheimer’s disease: a Golgi and electron microscope study. Am J Alzheimers Dis Other Demen. 2015;30:478–487. doi: 10.1177/1533317514556876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang JL, Lim AS, Chiang WY, Hsieh WH, Lo MT, Schneider JA, et al. Su-prachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol. 2015;78:317–322. doi: 10.1002/ana.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 103.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 104.Platt B, Drever B, Koss D, Stoppelkamp S, Jyoti A, Plano A, et al. Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PloS One. 2011;6:e27068. doi: 10.1371/journal.pone.0027068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wisor JP, Edgar DM, Yesavage J, Ryan HS, McCormick CM, Lapustea N, et al. Sleep and circadian abnormalities in a transgenic mouse model of Alzheimer’s disease: a role for cholinergic transmission. Neuroscience. 2005;131:375–385. doi: 10.1016/j.neuroscience.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 106.Song H, Moon M, Choe HK, Han DH, Jang C, Kim A, et al. Abeta-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer’s disease. Mol Neurodegener. 2015;10:13. doi: 10.1186/s13024-015-0007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blake MR, Holbrook SD, Kotwica-Rolinska J, Chow ES, Kretzschmar D, Giebultowicz JM. Manipulations of amyloid precursor protein cleavage disrupt the circadian clock in aging Drosophila. Neurobiol Dis. 2015;77:117–126. doi: 10.1016/j.nbd.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ford ES, Cunningham TJ, Croft JB. Trends in self-reported sleep duration among us adults from 1985 to 2012. Sleep. 2015;38:829–832. doi: 10.5665/sleep.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alzheimer’s A. 2015 Alzheimer’s disease facts and figures. Alzheimer’s Dement J Alzheimer’s Assoc. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 110.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 111.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]