Abstract
Background
Intensive bimanual therapy can improve hand function in children with unilateral spastic cerebral palsy (USCP). We compared the effects of structured bimanual skill training vs. unstructured bimanual practice on motor outcomes and motor map plasticity in children with USCP.
Objective
We hypothesized that structured skill training would produce greater motor map plasticity than unstructured practice.
Methods
Twenty children with USCP (average age 9,5; 12 males) received therapy in a day-camp-setting, 6 h/day, 5 days/week, for 3 weeks. In structured skill training (n=10), children performed progressively more difficult movements and practiced functional goals. In unstructured practice (n=10), children engaged in bimanual activities but did not practice skillful movements or functional goals. We used the Assisting Hand Assessment (AHA), Jebsen-Taylor test of Hand Function (JTTHF) and Canadian Occupational Performance Measure (COPM) to measure hand function. We used single-pulse transcranial magnetic stimulation (TMS) to map the representation of first dorsal interosseous (FDI) and flexor carpi radialis (FCR) muscles bilaterally.
Results
Both groups showed significant improvements in bimanual hand use (AHA; p<0.05) and hand dexterity (JTTHF; p<0.001). However, only the structured skill group showed increases in the size of the affected hand motor map and amplitudes of motor evoked potentials (p<0.01). Most children who showed the most functional improvements (COPM) had the largest changes in map size.
Conclusions
These findings uncover a dichotomy of plasticity: the unstructured practice group improved hand function but did not show changes in motor maps. Skill training is important for driving motor cortex plasticity in children with USCP.
Keywords: Rehabilitation, Pediatric, Transcranial Magnetic Stimulation, Neuroplasticity, Hemiplegia
Introduction
Unilateral spastic cerebral palsy (USCP) is caused by damage to the developing brain within the first two years of life. USCP results in weakness and motor deficits on one side of the body. Improving hand function is a main goal for most children with USCP1. Intensive bimanual therapy improves hand function in children with USCP2-6. In our intensive bimanual training model (Hand-Arm Bimanual Intensive Therapy: HABIT), children spend three weeks using both upper extremities in play-based activities, six hours/day4. Task difficulty systematically increases as motor proficiency progresses, further enhancing improvement7. We recently showed that HABIT improves unimanual skill, bimanual hand use, and functional hand use8. Given the functional impact of training, it is important to determine how training affects the brain.
Skill training drives plasticity of the motor system9-12. Skill training involves progressively greater task difficulty, whereas unskilled use of the hand involves repeating a movement that can be done without learning and does not increase in difficulty7. Animal models of USCP2 and stroke13-15 have demonstrated the importance of skill training in rehabilitation. Skill training improves motor outcomes and expands the motor map of the affected extremity, while repetitive unskilled use of the affected extremity does not produce map changes or motor recovery11,16. The brain circuits that underlie motor control in USCP have been well-characterized in an animal model17. Specifically, work in the USCP model demonstrated that skill training, but not unskilled motor use, drives plasticity of cortical motor maps and corticospinal connections16. This study leverages our understanding of critical ingredients in USCP rehabilitation in the animal model to test whether skill training changes motor maps and hand function in children with USCP. Several studies have shown that constraint-induced movement therapy produces changes in motor cortex physiology in children with CP18-20 and stroke patients21-23. Although several studies have examined the effects of constraint and bimanual therapies in children with USCP6,24-27, to our knowledge, this study is the first to specifically examine the critical ingredients that drive motor cortex plasticity associated with bimanual training in children with USCP.
We compared the effects of bimanual therapy that incorporates structured skill training versus unstructured play-like hand use on manual skill in children with USCP. Each group received 90 hours of therapy, six hours/day, three weeks. During training, children actively used both hands in play-based activities. Structured skill training incorporated three key components: 1) progression of task difficulty; 2) repeated practice of isolated movements that are components of a more complex task; and 3) repetition of functional goals, such as tying shoes. Children in the unstructured practice group performed bimanual movements during play, but did not progress skill of activities, nor practice isolated movements or functional goals. In the structured skill training group, task difficulty was increased by imposing greater spatial or temporal constraints, or by providing tasks that required increased skilled use and problem solving7,8. An example is placing a game board farther away from the child, to encourage a farther reach as a child’s arm extension increased. In the unstructured practice group, no constraints were placed on how a child completed an activity (e.g., game board kept in close position).
We used single-pulse transcranial magnetic stimulation (TMS) to assess topography and excitability of the motor cortex map of the affected hand before, immediately after, and six months after training. We hypothesized that structured skill training, but not unstructured practice, would drive changes in size and excitability of the motor map.
Methods
Participants
We recruited participants from regional clinics, our website (http://www.tc.edu/centers/cit/), ClinicalTrials.gov (NCT00305006), and online communities. Inclusion criteria: 1) congenital USCP, 2) ability to lift arms 15cm above table surface and grasp light objects, and 3) cognition similar to their age-specific peers in school. Exclusion criteria: 1) health problems that would interfere with participation, 2) history of seizures after 2 years of age with active use of anti-seizure medications, 3) uncorrected visual problems, 4) severe spasticity (Ashworth ≥3), 5) surgery on more-affected hand within one year, 6) botulinum toxin in upper extremity within six months, 7) non-removable metallic objects in body. Informed assent was obtained from participants and consent from caregivers. Procedures were approved by the Institutional Review Boards of Teachers College, Columbia University, where hand training was performed, and the New York State Psychiatric Institute, where brain imaging and motor mapping were acquired.
Interventions
Day camps were conducted at Teachers College, Columbia University during summers 2010 to 2012. Eighteen children were randomized to either structured HABIT (n=8, structured skill group) or unstructured bimanual play (n=10, unstructured practice group), performed in separate rooms. Motor outcomes from these children have been published in a larger randomized clinical trial that included twenty-two children8. Eighteen of the children in the RCT also participated in this TMS mapping study. Two teenagers (ages 15, 17) were assigned to the structured skill group because they were self-motivated to maximize skill demand of activities. This balanced group size at 10 each. Clinical characteristics did not differ between groups (Table 1, Chi-square p>0.05). Details of materials, methods, randomization, location, and adherence are presented in Supplementary Materials.
Table 1.
Baseline Participant Characteristics
| Characteristics | Structured (n=10) | Unstructured (n=10) |
|---|---|---|
| Mean age in years, months (SD) | 9,11 (3,5) | 8,11 (2,5) |
| Gender | ||
| Male | 6 (60%) | 6 (60%) |
| Female | 4 (40%) | 4 (40%) |
| Paretic Upper Extremity | ||
| Right | 6 (60%) | 6 (60%) |
| Left | 4 (40%) | 4 (40%) |
| Lesion Location | ||
| Right | 4 | 4 |
| Left | 6 | 6 |
| Laterality of Motor Map by TMS | ||
| Contralateral | 1 (10%) | 3 (30%) |
| Bilateral | 5 (50%) | 1 (10%) |
| Ipsilateral | 4 (40%) | 6 (60%) |
| Race | ||
| Asian | 0 | 1 (10%) |
| Caucasian | 9 (90%) | 7 (70%) |
| Hispanic | 1 (10%) | 1 (10%) |
| Mixed | 0 | 1 (10%) |
| Manual Ability Classification System | ||
| I | 2 (20%) | 2 (20%) |
| II | 5 (50%) | 6 (60%) |
| III | 3 (30%) | 2 (20%) |
| Baseline AHA, mean (SD), logits | 58.5 (8.3) | 63.3 (11.5) |
| Baseline JTTHF, mean (SD), sec | 286.7 (239) | 225.2 (166) |
| Baseline COPM Performance, mean (SD) | 3.6 (1.8) | 3.1 (0.9) |
| Baseline COPM Satisfaction, mean (SD) | 3.2 (2.2) | 3.6 (1.5) |
Abbreviations: SD, standard deviation; AHA, Assisting Hand Assessment; JTTHF, Jebsen-Taylor Test of Hand Function; COPM, Canadian Occupational Performance Measure
Intervention description
Procedures
All interventionists, parents, children, and motor skill assessors were blinded to therapy group and study hypotheses. Children were randomized to receive structured or unstructured HABIT. Participants engaged in age-appropriate bimanual training 6 hours/day for 15 days (90 hours). In both groups, activities were chosen that required use of both hands. If a child stopped using one hand during therapy, interventionists immediately reminded the child to use both hands.
Structured HABIT
(n=10) Structured HABIT4,28,29 differed from unstructured bimanual training in three ways (Table 2): 1) progression of task difficulty; 2) repeated practice of isolated movements; and 3) practice of functional goals. Progression of task difficulty: Task difficulty was graded by either increasing the temporal and spatial complexity of the movements or by increasing the complexity of how the affected hand was used. Repeated practice of isolated movements: Part-task practice (shaping) emphasized practice of a single movement component. Task performance, time on task, and number of repetitions were logged. Practice of functional goals: Play goals, such as dribbling a basketball, and activity of daily living goals, such as tying shoes, were determined by the participant and their family before training. Goals were practiced during training.
Table 2.
Similarities and Differences Between Treatment Groups
| Treatment Characteristic | Structured | Unstructured |
|---|---|---|
| Treatment Duration | 90 hrs | 90 hrs |
| Ratio interventionist to child | At least 1:1 | At least 1:1 |
| Homework (1 hr/day) during training and for 6m after training | Yes | Yes |
| Focus on bimanual practice | Yes | Yes |
| Skill progression during therapy | Yes | No |
| Practiced shaping of movements | Yes | No |
| Practiced functional goals | Yes | No |
Unstructured Bimanual Training
Children (n=10) were engaged in intensive use of both hands, without focus on skill. Bimanual activities were selected according to the child’s interest. Interventionists were trained only to provide activities that required use of both hands in a playful context. They were told that the emphasis was on having fun rather than rehabilitation. The supervisor ensured interventionists provided no increase in task complexity, no guidance on how to use the affected hand, and no gradation of task demands. The participants did not practice functional goals or part-task practice of movement components.
Behavioral Measures
Participants were evaluated prior to treatment, within two days after treatment, and six months later by a physical therapist blinded to group allocation. Three outcome measures were used to quantify unimanual capacity, bimanual performance, and functional goals, based on the International Classification of Functioning, Disability, and Health.
We quantified unilateral dexterity using the Jebsen-Taylor Test of Hand Function (JTTHF)30. Participants use one hand to perform functional movements, including flipping cards, manipulating/placing small objects, simulated eating, checker stacking, and manipulating cans. The outcome is the time (seconds) to perform movements.
To quantify how the hands function together, we employed the Assisting Hand Assessment (AHA)31,32. The AHA quantifies the effectiveness of assisting hand use in performing bimanual activities in children with unilateral upper limb disabilities. The AHA has excellent validity, reliability (0.97-0.99) and responsiveness to change8,31. The test was videotaped and scored off-site by an experienced blinded evaluator. Scores were computed as transformed logits (AHA units).
To measure performance and satisfaction levels in functional goals, we employed the Canadian Occupational Performance Measure (COPM)33. The COPM is a standardized measure that identifies goals and detects changes in self-care, productivity and leisure performance areas. Through interview, the caregiver identified the child’s functional goals and ranked their importance. They rated satisfaction and performance of each goal (maximum 5). Mean performance and satisfaction scores were analyzed. Both groups of children set goals, but only the structured group practiced goals during the intervention.
TMS Motor Mapping
We used single pulse TMS to evoke movements of selected digit and wrist muscles of the affected hand to address whether training changed the motor map. We measured motor responses with surface electromyography (EMG) of the first dorsal interosseous (FDI) and flexor carpi radialis (FCR) muscles bilaterally. TMS details are presented in Supplementary Materials.
Each child’s TMS map was colocalized to their structural MRI, to allow motor mapping that was consistent between each time point. Details are presented in Supplementary Materials.
TMS-evoked motor responses were recorded with surface EMG electrodes. Electrodes were connected to a Brainvision ExG amplifier (NeuroConn, Germany). TMS pulses were first delivered to the affected hemisphere to search for an EMG response (motor evoked potential; MEP) of the affected FDI. If an MEP of the affected FDI could not be elicited in the affected hemisphere, the uninjured hemisphere was probed for MEPs of the affected FDI. In all cases, if an MEP of the affected FDI or FCR was not found in the injured hemisphere, it was found in the uninjured hemisphere.
We determined the threshold for provoking an MEP. The TMS coil was held at the location that provoked the largest FDI response (“hotspot”). MEPs were evoked beginning at a suprathreshold stimulus intensity. Stimulator output was lowered at 2% increments. The lowest stimulator output at which MEP responses of the affected FDI could be elicited from five of ten pulses was defined as the motor threshold (MT). An MEP was categorized as a response if the latency between the TMS pulse and MEP onset was less than 40ms, and if the amplitude of the MEP was at least 50μV.
After the MT was determined, a circular grid was superimposed onto the child’s MRI using Brainsight. The grid was centered at the affected FDI hotspot. Grid spacing was 1 cm. The grid consisted of five concentric rings, resulting in a grid with a 5 cm radius (81 grid points). Single TMS pulses (3-6 per site when an MEP was found, 1-2 per site when no MEP was found) were delivered to each grid point, starting at the hotspot and moving concentrically along the grid, ending at the outermost ring. Stimuli were delivered at an intensity of 110% affected FDI MT, frequency <0.1Hz. This intensity has been used previously to map stroke patients after constraint therapy34. Average MEPs per site were calculated using peak-to-peak amplitude.
TMS maps were done before training, within two days of the end of training, and six months after training. For each child, the same intensity of stimulation (110% pre-training resting MT) was used in all TMS sessions. TMS data analyses methods are presented in Supplementary Materials.
Statistics
Statistics were performed using SPSS (IBM, V.21). Intention-to-treat analyses were conducted. Missing data (one six-month follow-up, unstructured group) were interpolated based on the group average for the six-month time point. A 2 (group) × 3 (time) ANOVA with repeated measures was performed on all measures. We performed post-hoc analyses when a main ANOVA effect was found, correcting for multiple comparisons (Bonferroni). The group × test session interaction effect tested if improvements along test sessions differed between groups. Linear regression examined associations between behavior and TMS measures.
Results
Changes in Hand Function after Bimanual Training
Hand function data from the 18 children randomized to group are published as part of a randomized clinical trial8. Changes in bimanual hand use were measured with the AHA (Fig. 1A). AHA improved after training (F(2,17)=4.03, p=0.037, 1.9-unit improvement pre-post training, retained at six months), though not a clinically meaningful difference. There was no interaction between training type and AHA improvement (F(2,17)=0.57, p=0.57). Both groups improved equally well in bimanual use of the affected hand.
Figure 1.
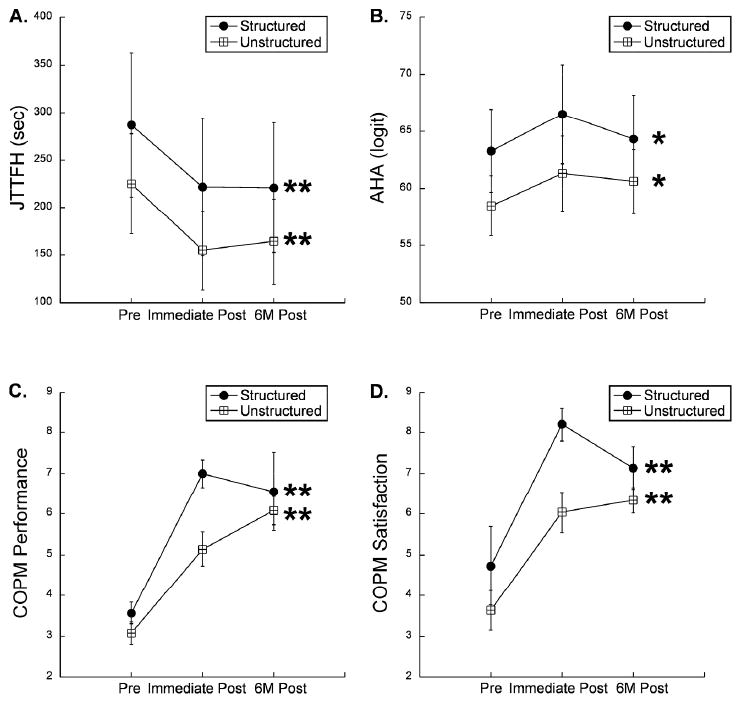
Changes in skill of the affected hand after intensive bimanual training. Structured and unstructured groups improved equally well in bimanual use of the affected hand (A), movement speed of the affected hand (B), performance of functional goals (C), and satisfaction of performance of functional goals (D). Asterisks indicate within-group differences from baseline to post-intervention time points. * p<0.05, ** p<0.01.
Changes in unimanual dexterity were assessed with the JTTHF. There was an overall improvement in the JTTHF in the affected hand after training across all subjects a clinically meaningful amount (Fig. 1B, F(2,17)=8.74, p<0.001, 17% improvement pre-post training, retained at six months). There was no interaction between training type and JTTHF improvement (F(2,17)=0.08, p=0.92). Both groups improved equally well in unimanual performance with the affected hand.
Improvements on functional goals were measured with the COPM. There was an overall improvement in COPM-Performance (Fig. 1C, F(2,17)=42.19, p<0.001). There was a trend toward a significant interaction between COPM-Performance and training type (F(2,17)=3.2, p=0.068). The structured skill group trended toward greater improvement than the unstructured group in functional use of the affected hand. There was also an overall improvement in COPM-Satisfaction (Fig. 1D, F(2,17)=15.77, p<0.001) but no interaction between COPM-Satisfaction and training type (F(2,17)=1.76, p=0.20). Both groups improved a clinically meaningful amount in COPM-Performance and Satisfaction.
Improvements in all measures were maintained six months after therapy, as there were no statistically significant changes between the immediate post-training and six months post-training measures for either group.
Laterality of Motor Map Controlling the Impaired Hand
We determined the location of the motor map of the affected hand (Table 1, Figure 2). Children were categorized as contralateral if 100% of TMS responses in the affected hand resided in the same hemisphere as the lesion. Children were categorized as ipsilateral if 100% of TMS responses in the affected hand resided in the opposite hemisphere as the lesion. There was no difference in the distribution of hand map laterality (contralateral or ipsilateral to affected hand) between the two groups (Table 1, Fisher’s Exact, p=0.85). Latency of MEP response was not different for contralateral or ipsilateral responses across all subjects (p>0.3).
Figure 2.
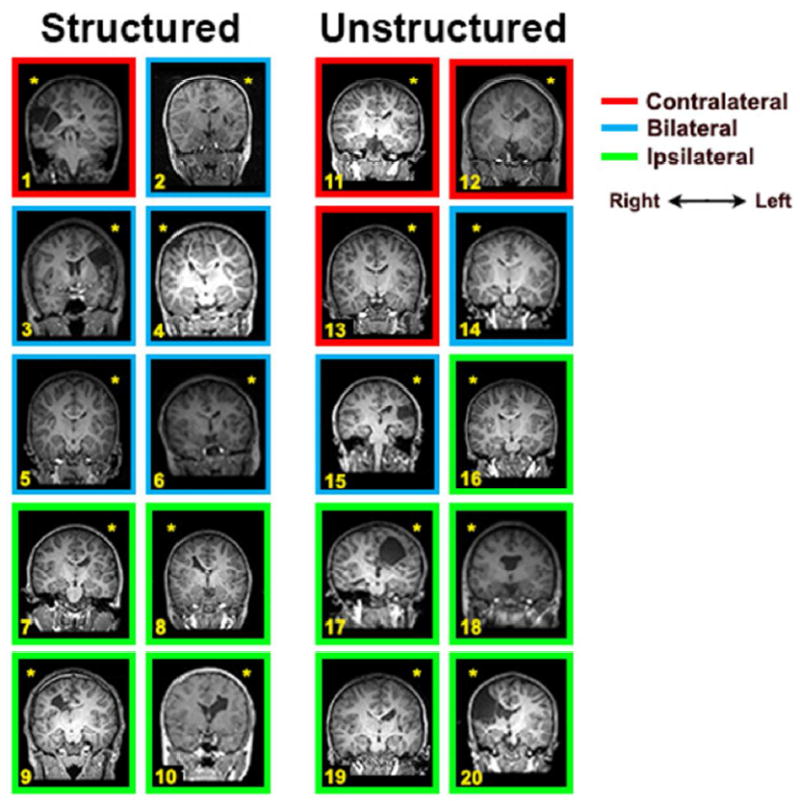
Coronal MRI slices showing a cross-section of each child’s lesion. The color of the frame surrounding the MRI indicates the CST laterality of the child (red = contralateral, blue = bilateral, yellow = ipsilateral). The yellow asterisk indicates the side of injury.
Six children (5 structured, one unstructured) had motor maps of the affected hand in both hemispheres – that is, bilateral motor maps. There was a strong asymmetry in map size of the two sides. In five of the six cases (4 structured, 1 unstructured), the map in the injured hemisphere was approximately double the size of the map in the other hemisphere (ratio, injured:uninjured hemispheres=1.95, SD=0.11). For these cases, we used the map in the injured hemisphere for analysis. In the remaining child with bilateral maps (structured group), the map of the affected FDI and FCR was 4.75x larger in the uninjured hemisphere than the injured hemisphere, and the map in the uninjured hemisphere was used for analyses.
Changes in Motor Maps after Bimanual Training
We examined changes in motor maps after structured skill training or unstructured practice. Fig. 3 shows representative motor maps from one child per group. Map size increased in the structured (1A-1C), but not the unstructured group (2A-C).
Figure 3.
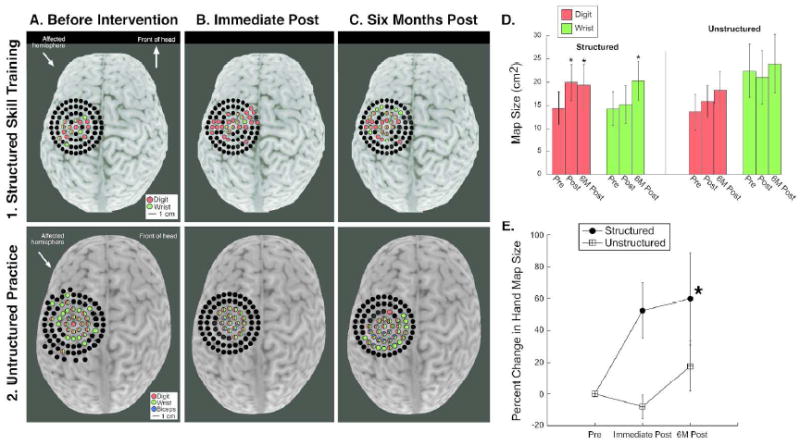
Changes in motor maps after training. Colored dots represent movement evoked by TMS at that site.A1-C1. Structured skill training, representative maps of the affected hand from one child. A2-C2. Unstructured practice, representative maps of the affected hand from one child. D. Quantification of map changes. The size of the motor map of the affected hand increased significantly only in the structured training group (* p<0.05).
After training, there was a significant interaction between map size (FDI+FCR) of the affected hand and training type (F(2,17)=4.7, p=0.036). There was a significant increase in map size of the affected hand in the structured skill group (pre-post-training p=0.009, 23.3% increase, pre-training to six months post-training p=0.047, 34.9% increase) but not in the unstructured practice group (p>0.3; <10% increase) (Fig. 3D-E). Map size of the affected FDI increased significantly in the structured skill group (pre-post-training p=0.046, 27.4% increase, pre-training to six months post-training p=0.05, 34.2% increase), but not in the unstructured group (p>0.2). The map size for the affected FCR did not change significantly in the structured skill group immediately after training (p=0.85, 6.1% increase) but was significantly greater than baseline six months after training (p=0.049, 42.2% increase). In the unstructured group, FCR map size did not change after training (p>0.3).
Amplitude of motor responses to TMS increased significantly (Fig. 4) after structured skill training but not unstructured practice (Fig. 4D-E). There was a significant interaction between average MEP of the affected FDI and treatment group (F(2,17)=4.21, p=0.033). The structured skill group showed a significant increase in FDI MEP size from pre-training to six months post-training (p<0.0001, 19% increase), though not from pre-training to immediately following or immediately following to six months later. There was no significant change in FDI MEP size in the unstructured practice group (p>0.8). There was a significant interaction between average MEP of the affected FCR and group (F(2,17)=4.96, p=0.019). The structured skill group showed a significant increase in FCR MEP size from pre-training to six months post-training (p<0.0001, 78.1% increase) but not pre-training to immediate post-training. There was no significant change in FCR MEP size in the unstructured practice group (p>0.9).
Figure 4.
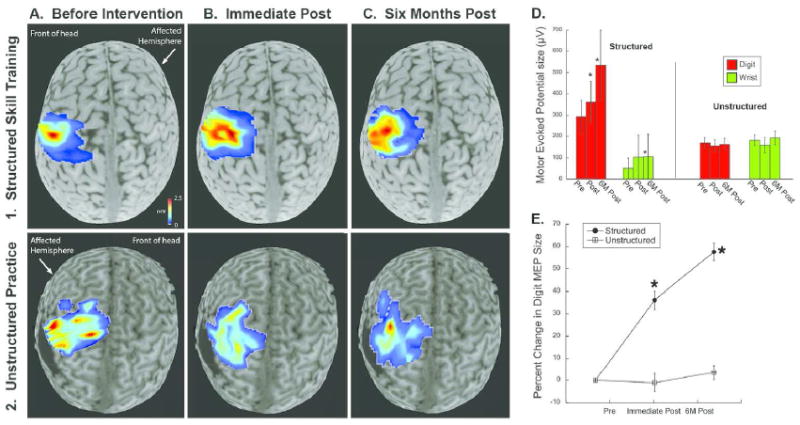
Changes in magnitude of motor evoked potentials in TMS maps after structured skill training. A1-C1. Maps of the affected hand located contralateral to the affected hemisphere in a representative case. Red color indicates stronger MEP response. A2-C2. Representative maps of the affected hand in a representative case from the unstructured practice group. D-E. MEP amplitude of the representation of the affected FDI increased significantly after structured but not unstructured training (* p<0.05).
Plasticity of Ipsilateral versus Contralateral Maps
We determined the location of the motor map of the affected hand (Table 1). There was no difference in the distribution of hand map laterality (contralateral or ipsilateral to affected hand) between groups (Fisher’s, p=0.85). Representative maps are shown in Fig. 2 (contralateral) and Fig. 3 (ipsilateral). Both ipsilateral and contralateral maps expanded after structured skill HABIT (F(2,8)=4.6, p=0.048). The magnitude of map changes were not different in children with an ipsilateral versus contralateral CST (F(2,7)=0.32, p=0.74).
Associations Between Map Changes and Hand Function Changes
Children in the structured skill group who showed the most improvement in COPM-Performance also had the largest changes in hand map size (Supplemental Fig. 1, F(1,8)=7.5, p=0.013, r=0.54, r2=0.30). Children with larger improvements in JTTHF showed larger expansions of the hand motor map (F(1,8)=5.6, p=0.045, r=0.64, r2=0.41). There was not a significant association between hand map change and improvement in the AHA (p=0.95, r=0.02). In the unstructured group, there were no significant associations between map changes and JTTHF, AHA, or COPM changes (p>0.3, r<0.35).
Importantly, baseline hand function and amount of recovery was not related to whether the child’s map of the impaired hand was located in the injured or uninjured hemisphere (JTTHF F(1,18)=4.82, p=0.10; AHA, F(1,18)=1.4, p=0.24; COPM performance F(1,18)=0.42, p=0.48, no interactions).
Stability of Motor Maps in the Absence of Intervention
We show the stability of motor maps in the absence of intervention (Supplementary Materials).
TMS Safety Outcomes
Of the 70 TMS sessions conducted in this study, two participants reported mild headache after four (5.7%) of the sessions, three participants reported discomfort in the headband used for neuronavigation after five (7.1%) of the sessions, and two participants reported discomfort with sitting for an extended period of time after three (4.3%) sessions. All side effects resolved without treatment within one hour after TMS. No serious adverse events occurred.
Discussion
This is the first study, to our knowledge, to examine the critical ingredients of bimanual training that drive changes in motor cortex physiology in children with USCP. We compared bimanual structured skill training and unstructured practice. The study uncovered three main findings: 1) Skill training increased the size and strength of the motor map of the affected hand; 2) Unimanual skill of the affected hand and performance of functional goals were correlated with increased map size; 3) Motor maps were plastic whether they resided in the hemisphere contralateral or ipsilateral to the affected hand.
Our findings are consistent with studies showing that repetitive practice of a specific motor skill drives map plasticity2,11,12,35-37. In animal models of stroke and USCP, and in human stroke patients, constraint of the unaffected forelimb, plus skill training of the affected forelimb, can improve motor skill and expand the motor map of the affected limb22,23,38-42. In human stroke patients, the magnitude of map expansion was correlated with improvements in skill43. Training has also been shown to increase fMRI activations in secondary motor and cerebellar regions44. Importantly, constraint alone, without skill training, does not change motor maps11,16.
Though further work is needed to uncover the mechanisms of cortical plasticity associated with skill-based neurorehabilitation, work in animal models has determined that skill training, but not unskilled motor activity, increases densities of synapses, dendritic arbors, and spines in the motor networks45. These changes are associated with increases in neurotrophin expression46, and increased density of spinal interneurons16.
We found that structured and unstructured therapy both resulted in equal improvements in unimanual movement speed and bimanual use. It is possible that the two groups had differences in movement kinematics or other more detailed measures of movement quality, since the clinical outcome measures we used do not quantify the quality of movements. Further studies should examine kinematics of motor recovery in children with USCP.
We found that functional gains and changes in cortical plasticity were independent of hemisphere of control, i.e. ipsilateral or contralateral to the affected hand. CIMT studies in children with USCP suggested that in children with ipsilateral control of the affected hand, improvements in movement speed47 and changes in M1 excitability18 were less robust than for children with a contralateral CST. However, we found that ipsilateral control of the affected limb showed similar amounts of plasticity as contralateral control. Ipsilateral pathways have the capacity to be adaptive, functional, and plastic48-50, though a larger study is needed to better understand differences in plasticity of different hemispheres of control.
This study uncovers a dichotomy of neuroplasticity. The unstructured practice group, which improved hand function, did not show motor cortex plasticity in TMS maps. The high dose (90 hours) may have washed out group differences. Lower dosages could result in differences between groups. While we did find a positive association between functional gains and cortical plasticity, it is possible that with lower doses, associations between motor outcomes and plasticity would be more apparent in the structured skill group than the unstructured group.
Changes in motor function can occur without causing changes in motor maps2,9. Some improvements in motor skill can be driven by existing motor networks, while more robust changes in motor skill are associated with rewiring of motor pathways51. It is also likely that plasticity occurred in brain regions other than M1, such as secondary motor areas, sensory networks52-54, subcortical brain structures55, and spinal interneuronal systems2,37,56. It is likely that plasticity in these systems underlies the motor improvements seen in the unstructured practice group. Further studies, specifically those that can measure plasticity in different brain regions, are needed to examine plasticity in other systems during rehabilitation.
The relative timing of motor map changes and improvements in motor skill has been studied in animal models of stroke and USCP. Behavioral improvements after infarct plateaued before changes in motor maps could be detected57,58. In contrast, in USCP, map changes were found after pharmacotherapy59 or CIMT2 that preceded motor recovery. It is possible that intensity of the structured skill group was sufficient to drive motor map plasticity, but that a longer duration of unstructured practice may have been needed to change motor maps.
The current findings show that skill training is a critical ingredient for driving motor cortical plasticity in children with USCP. While both groups improved in clinical outcomes, the structured skill training group showed the most improvement in functional gains. This work and other evidence60 indicate that skill training is an important ingredient in neurorehabilitation strategies.
Limitations
This study has several limitations. The relatively low number of participants limits generalizability. While we did match groups on baseline JTTHF and age, there were differences between groups in the distribution of CST projection patterns. Second, this study only measured M1 plasticity. Methods that examine cortical plasticity throughout the brain, such as functional MRI and electroencephalography, can determine other locations of plasticity associated with hand rehabilitation. We discuss challenges of applying our methods to children with USCP in Supplemental Materials. Finally, clinical outcomes reported here measure indirect aspect of movement quality. Further study of movement quality is needed to explore the relationship between cortical plasticity and rehabilitation outcomes.
Conclusions
Structured and unstructured bimanual training improved hand function in children with USCP. Skill training produced stronger improvements in functional goals. There was a dichotomy between these improvements and cortical plasticity. Only skilled training induced motor map plasticity. Further work is needed to examine the interplay between cortical physiology and motor skill improvements.
Supplementary Material
Acknowledgments
Funded by NS062116 (KMF), Columbia Professional Schools Diversity Award (KMF), and NIH CTSA Award (KMF) (KL2 RR024157, UL1 RR024156, TL1 RR024158). Each of these sponsors provided funding based on the design of the study. Funds were used to pay for equipment and personnel needed for data collection, management, analysis, and interpretation. None of these sponsors were involved in the preparation of the manuscript or decision to submit for publication. We thank Ya-Ching Hng, Electra Petra, Ashley Chinnan, and the volunteer interventionists. We thank Stephen Dashnaw and Glenn Castillo for MRI acquisition. We thank Drs. Peter Bulow and Joshua Berman for performing screening exams. We thank the participants and their families.
References
- 1.Makki D, Duodu J, Nixon M. Prevalence and pattern of upper limb involvement in cerebral palsy. Journal of children’s orthopaedics. 2014 May;8(3):215–219. doi: 10.1007/s11832-014-0593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friel K, Chakrabarty S, Kuo HC, Martin J. Using motor behavior during an early critical period to restore skilled limb movement after damage to the corticospinal system during development. J Neurosci. 2012 Jul 4;32(27):9265–9276. doi: 10.1523/JNEUROSCI.1198-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon AM, Chinnan A, Gill S, Petra E, Hung YC, Charles J. Both constraint-induced movement therapy and bimanual training lead to improved performance of upper extremity function in children with hemiplegia. Developmental medicine and child neurology. 2008 Dec;50(12):957–958. doi: 10.1111/j.1469-8749.2008.03166.x. [DOI] [PubMed] [Google Scholar]
- 4.Gordon AM, Hung YC, Brandao M, et al. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: a randomized trial. Neurorehabilitation and neural repair. 2011 Oct;25(8):692–702. doi: 10.1177/1545968311402508. [DOI] [PubMed] [Google Scholar]
- 5.Gordon AM, Schneider JA, Chinnan A, Charles JR. Efficacy of a hand-arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: a randomized control trial. Developmental medicine and child neurology. 2007 Nov;49(11):830–838. doi: 10.1111/j.1469-8749.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 6.Sakzewski L. Bimanual therapy and constraint-induced movement therapy are equally effective in improving hand function in children with congenital hemiplegia. J Physiother. 2012;58(1):59. doi: 10.1016/S1836-9553(12)70075-9. [DOI] [PubMed] [Google Scholar]
- 7.Charles J, Gordon AM. Development of hand-arm bimanual intensive training (HABIT) for improving bimanual coordination in children with hemiplegic cerebral palsy. Developmental medicine and child neurology. 2006 Nov;48(11):931–936. doi: 10.1017/S0012162206002039. [DOI] [PubMed] [Google Scholar]
- 8.Brandao MB, Ferre C, Kuo HC, et al. Comparison of Structured Skill and Unstructured Practice During Intensive Bimanual Training in Children With Unilateral Spastic Cerebral Palsy. Neurorehabil Neural Repair. 2013 Dec 27;28(5):452–461. doi: 10.1177/1545968313516871. [DOI] [PubMed] [Google Scholar]
- 9.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000 Jul;74(1):27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 10.Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999 Nov 15;19(22):10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friel KM, Heddings AA, Nudo RJ. Effects of postlesion experience on behavioral recovery and neurophysiologic reorganization after cortical injury in primates. Neurorehabilitation and neural repair. 2000;14(3):187–198. doi: 10.1177/154596830001400304. [DOI] [PubMed] [Google Scholar]
- 12.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. Journal of neurophysiology. 1998;80(6):3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 13.Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14(4):2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochemical research. 2003 Nov;28(11):1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- 15.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996 Jun 21;272(5269):1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 16.Friel K, Chakrabarty S, Kuo HC, Martin J. Using motor behavior during an early critical period to restore skilled limb movement after damage to the corticospinal system during development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012 Jul 4;32(27):9265–9276. doi: 10.1523/JNEUROSCI.1198-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friel KM, Chakrabarty S, Martin JH. Pathophysiological mechanisms of impaired limb use and repair strategies for motor systems after unilateral injury of the developing brain. Developmental medicine and child neurology. 2013 Nov;55(Suppl 4):27–31. doi: 10.1111/dmcn.12303. [DOI] [PubMed] [Google Scholar]
- 18.Juenger H, Kuhnke N, Braun C, et al. Two types of exercise-induced neuroplasticity in congenital hemiparesis: a transcranial magnetic stimulation, functional MRI, and magnetoencephalography study. Developmental medicine and child neurology. 2013 Oct;55(10):941–951. doi: 10.1111/dmcn.12209. [DOI] [PubMed] [Google Scholar]
- 19.Cao J, Khan B, Hervey N, et al. Evaluation of cortical plasticity in children with cerebral palsy undergoing constraint-induced movement therapy based on functional near-infrared spectroscopy. Journal of biomedical optics. 2015 Apr;20(4):046009. doi: 10.1117/1.JBO.20.4.046009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inguaggiato E, Sgandurra G, Perazza S, Guzzetta A, Cioni G. Brain reorganization following intervention in children with congenital hemiplegia: a systematic review. Neural plasticity. 2013;2013:356275. doi: 10.1155/2013/356275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kononen M, Tarkka IM, Niskanen E, et al. Functional MRI and motor behavioral changes obtained with constraint-induced movement therapy in chronic stroke. Eur J Neurol. 2012 Apr;19(4):578–586. doi: 10.1111/j.1468-1331.2011.03572.x. [DOI] [PubMed] [Google Scholar]
- 22.Liepert J, Miltner WH, Bauder H, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998 Jun 26;250(1):5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- 23.Park SW, Butler AJ, Cavalheiro V, Alberts JL, Wolf SL. Changes in serial optical topography and TMS during task performance after constraint-induced movement therapy in stroke: a case study. Neurorehabilitation and neural repair. 2004 Jun;18(2):95–105. doi: 10.1177/0888439004265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoare BJ, Wasiak J, Imms C, Carey L. Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database Syst Rev. 2007;2:CD004149. doi: 10.1002/14651858.CD004149.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Boyd R, Sakzewski L, Ziviani J, et al. INCITE: A randomised trial comparing constraint induced movement therapy and bimanual training in children with congenital hemiplegia. BMC neurology. 2010;10:4. doi: 10.1186/1471-2377-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aarts PB, Jongerius PH, Geerdink YA, van Limbeek J, Geurts AC. Effectiveness of modified constraint-induced movement therapy in children with unilateral spastic cerebral palsy: a randomized controlled trial. Neurorehabilitation and neural repair. 2010 Jul-Aug;24(6):509–518. doi: 10.1177/1545968309359767. [DOI] [PubMed] [Google Scholar]
- 27.Facchin P, Rosa-Rizzotto M, Visona Dalla Pozza L, et al. Multisite trial comparing the efficacy of constraint-induced movement therapy with that of bimanual intensive training in children with hemiplegic cerebral palsy: postintervention results. Am J Phys Med Rehabil. 2011 Jul;90(7):539–553. doi: 10.1097/PHM.0b013e3182247076. [DOI] [PubMed] [Google Scholar]
- 28.Charles JR, Wolf SL, Schneider JA, Gordon AM. Efficacy of a child-friendly form of constraint-induced movement therapy in hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol. 2006 Aug;48(8):635–642. doi: 10.1017/S0012162206001356. [DOI] [PubMed] [Google Scholar]
- 29.Gordon AM, Charles J, Schneider JA, Chinnan A. Efficacy of Hand-Arm bimanual intensive therapy (HABIT) for Children with Hemiplegic Cerebral Palsy: A Randomized Control Trial. Developmental medicine and child neurology. 2007 doi: 10.1111/j.1469-8749.2007.00830.x. in press. [DOI] [PubMed] [Google Scholar]
- 30.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Archives of physical medicine and rehabilitation. 1969;50(6):311–319. [PubMed] [Google Scholar]
- 31.Holmefur M, Krumlinde-Sundholm L, Eliasson AC. Interrater and intrarater reliability of the Assisting Hand Assessment. Am J Occup Ther. 2007;61(1):79–84. doi: 10.5014/ajot.61.1.79. [DOI] [PubMed] [Google Scholar]
- 32.Krumlinde-Sundholm L, Holmefur M, Kottorp A, Eliasson AC. The Assisting Hand Assessment: current evidence of validity, reliability, and responsiveness to change. Dev Med Child Neurol. 2007 Apr;49(4):259–264. doi: 10.1111/j.1469-8749.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 33.Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990 Apr;57(2):82–87. doi: 10.1177/000841749005700207. [DOI] [PubMed] [Google Scholar]
- 34.Sawaki L, Butler AJ, Leng X, et al. Differential patterns of cortical reorganization following constraint-induced movement therapy during early and late period after stroke: A preliminary study. NeuroRehabilitation. 2014;35(3):415–426. doi: 10.3233/NRE-141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleim JA, Barbay S, Cooper NR, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002 Jan;77(1):63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 36.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16(2):785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakrabarty S, Shulman B, Martin JH. Activity-dependent codevelopment of the corticospinal system and target interneurons in the cervical spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009 Jul 8;29(27):8816–8827. doi: 10.1523/JNEUROSCI.0735-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf SL, Blanton S, Baer H, Breshears J, Butler AJ. Repetitive task practice: a critical review of constraint-induced movement therapy in stroke. Neurologist. 2002 Nov;8(6):325–338. doi: 10.1097/01.nrl.0000031014.85777.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liepert J, Graef S, Uhde I, Leidner O, Weiller C. Training-induced changes of motor cortex representations in stroke patients. Acta Neurol Scand. 2000 May;101(5):321–326. doi: 10.1034/j.1600-0404.2000.90337a.x. [DOI] [PubMed] [Google Scholar]
- 40.Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. Journal of neurophysiology. 1998 Feb;79(2):1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 41.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000 Jun;31(6):1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 42.Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cogn Behav Neurol. 2006 Mar;19(1):41–47. doi: 10.1097/00146965-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Sawaki L, Butler AJ, Leng X, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabilitation and neural repair. 2008 Sep-Oct;22(5):505–513. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 45.Warraich Z, Kleim JA. Neural plasticity: the biological substrate for neurorehabilitation. PM & R : the journal of injury, function, and rehabilitation. 2010 Dec;2(12 Suppl 2):S208–219. doi: 10.1016/j.pmrj.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Boyce VS, Mendell LM. Neurotrophins and spinal circuit function. Frontiers in neural circuits. 2014;8:59. doi: 10.3389/fncir.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Developmental medicine and child neurology. 2008 Dec;50(12):898–903. doi: 10.1111/j.1469-8749.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- 48.Mackey A, Stinear C, Stott S, Byblow WD. Upper limb function and cortical organization in youth with unilateral cerebral palsy. Frontiers in neurology. 2014;5:117. doi: 10.3389/fneur.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carmel JB, Kimura H, Martin JH. Electrical stimulation of motor cortex in the uninjured hemisphere after chronic unilateral injury promotes recovery of skilled locomotion through ipsilateral control. J Neurosci. 2014 Jan 8;34(2):462–466. doi: 10.1523/JNEUROSCI.3315-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reitmeir R, Kilic E, Kilic U, et al. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain. 2011 Jan;134(Pt 1):84–99. doi: 10.1093/brain/awq344. [DOI] [PubMed] [Google Scholar]
- 51.Marshall RS, Zarahn E, Alon L, Minzer B, Lazar RM, Krakauer JW. Early imaging correlates of subsequent motor recovery after stroke. Ann Neurol. 2009 May;65(5):596–602. doi: 10.1002/ana.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loubinoux I, Carel C, Alary F, et al. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test--retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2001 May;21(5):592–607. doi: 10.1097/00004647-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Loubinoux I, Carel C, Pariente J, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003 Dec;20(4):2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 54.Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, et al. Prognostic value of FMRI in recovery of hand function in subcortical stroke patients. Cereb Cortex. 2007 Dec;17(12):2980–2987. doi: 10.1093/cercor/bhm023. [DOI] [PubMed] [Google Scholar]
- 55.Carey LM, Abbott DF, Egan GF, et al. Evolution of brain activation with good and poor motor recovery after stroke. Neurorehabil Neural Repair. 2006 Mar;20(1):24–41. doi: 10.1177/1545968305283053. [DOI] [PubMed] [Google Scholar]
- 56.Chakrabarty S, Martin JH. Postnatal development of a segmental switch enables corticospinal tract transmission to spinal forelimb motor circuits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010 Feb 10;30(6):2277–2288. doi: 10.1523/JNEUROSCI.5286-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishibe M, Urban ET, Barbay S, Nudo RJ. Rehabilitative Training Promotes Rapid Motor Recovery but Delayed Motor Map Reorganization in a Rat Cortical Ischemic Infarct Model. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314543499. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eisner-Janowicz I, S B, Hoover E, et al. Early and late changes in the distal forelimb representation of the supplementary motor area after injury to frontal motor areas in the squirrel monkey. J Neurophysiol. 2008;100:1498–1512. doi: 10.1152/jn.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chakrabarty S, Friel KM, Martin JH. Activity-dependent plasticity improves M1 motor representation and corticospinal tract connectivity. Journal of neurophysiology. 2009 Mar;101(3):1283–1293. doi: 10.1152/jn.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitago T, Krakauer JW. Motor learning principles for neurorehabilitation. Handbook of clinical neurology. 2013;110:93–103. doi: 10.1016/B978-0-444-52901-5.00008-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


