Abstract
We present a race-specific model of propofol-induced loss of consciousness that is based on pharmacodynamic data collected and adapted from the peer-reviewed literature. In the proposed race-specific model that includes EC05 and EC95 concentrations, the median (EC50) (and where available 95% CI) propofol concentrations at the effect site compartment for propofol-induced loss of consciousness for Caucasians, Chinese, Blacks, and Indians are 2.8 (2.7–2.9), 2.2 (2.2–2.3), 2.0, and 1.9 µg/mL, respectively.
Keywords: propofol, loss of consciousness, race-specific model, effect site compartment, EC50
Propofol is on the World Health Organization’s Model List of Essential Medicines, which is “a list of minimum medicine needs for a basic health-care system, listing the most efficacious, safe and cost-effective medicines for priority conditions.”1 Approved for use in more than 50 countries, propofol is routinely used worldwide and thus on patients of different races. During propofol sedation, an ever-present concern is that because of variability in patient pharmacodynamics and sensitivity to propofol, sedation can inadvertently slide into general anesthesia where a patient loses self-protective reflexes such as spontaneous breathing. This situation can become life threatening if the provider is unaware that it has occurred or is not skilled in restoring ventilation and oxygenation. The tragic consequences of loss of self-protective reflexes during sedation for routine procedures were brought to the attention of the lay public by a spate of presumably preventable propofol-related deaths during cosmetic surgery in Florida2 and, more recently, with the death of comedienne Joan Rivers.
Inter-racial variability to propofol anesthesia is well described.3–9 In an international study with patients of different races undergoing total intravenous anesthesia (TIVA) with propofol and fentanyl, Ortolani et al. evaluated propofol consumption and recovery time.4 Bispectral Index (BIS) monitoring, derived from electroencephalography, was used to maintain the same anesthesia depth in all patients. The BIS value, the response to verbal stimuli, and eye-opening time were the pharmacodynamic parameters used to assess recovery. After propofol administration was stopped, the mean time for the BIS values to return to baseline was 11 minutes for Caucasians from Italy, 12.5 minutes for Chinese from Malaysia, 15.9 minutes for Malays, and 22.1 minutes for Indians from Malaysia. Mean time to eye-opening was 11.63 minutes in Caucasians, 13.23 minutes in Chinese, 16.97 minutes in Malays, and 22.3 minutes in Indians. The amount of propofol consumed to maintain the same anesthesia depth was significantly lower in Indians compared to the other three groups.4
EC05, EC50, and EC95 (also expressed as C05, C50, and C95) represent the effective concentration for 5%, 50%, and 95% of a population. CP50 (Cp50, CP50), etc. are also used but the “P” or “p” refers to the plasma compartment and this nomenclature should be used only when referring to the plasma (central) compartment. EC50 is considered the TIVA equivalent of the inhalational anesthesia concept of Minimum Alveolar Concentration (MAC, the alveolar concentration of a volatile anesthetic or anesthetic gas at which 50% of patients will not move in response to incision).8,9,13 For inhalational anesthesia, a clinical rule of thumb is that 1.3 MAC (1.3 times the median value) is effective for preventing movement upon incision in 95% of a population. EC50 is the effective concentration of an intravenous anesthetic agent at which 50% of patients will not move in response to skin incision. EC50 is therefore the median, not the mean, of a population response. We describe in detail how we developed and validated a race-specific model of propofol-induced LOC based on data from peer-reviewed publications.
In this article, we focus on inter-racial variability, which is the difference between races in the median dose of propofol required for loss of consciousness (LOC) within a race. Our motivation in developing a race-specific model of propofol-induced LOC is to provide a tool for simulation-based training to make clinicians aware of inter-racial differences in propofol sensitivity. The ultimate goal is to make it less likely that patients from races known to be sensitive (some by a factor of 1.5) to propofol suffer inadvertent overdosing.
Methods
This section consists of two main headings: 1) Modeling of propofol pharmacokinetics/pharmacodynamics (PK/PD) and 2) Modeling of inter-racial variability in propofol LOC. First, we developed and validated an implementation of the Marsh model in Director (Adobe, Inc., San Jose, CA). Subsequent to validation, we developed a race-specific PD model of propofol EC05, EC50 and EC95 concentrations at the effect site compartment (ESC) at LOC for Indian (South Asian), Black, Chinese, and Caucasian patients.
Modeling of Propofol Pharmacokinetics/Pharmacodynamics
We selected the Marsh propofol model10 because (a) it has been used to drive a target-controlled infusion (TCI) pump (Diprifusor™, Zeneca, Grangemouth, UK) in studies of patients from three different races (Caucasians,9 Chinese,8 and Indians7) and (b) Barakat et al.11 found good correlations between its predicted propofol effect site concentration with the Observer Assessment of Alertness/Sedation (OAAS) (r = 0.87) in 20 patients and bispectral index BIS (r = 0.97). The OAAS is a well-accepted sedation scoring system12. An OAAS score of 2 or less is considered unconsciousness7,13 and corresponds to lack of response to verbal commands (also called loss of response to verbal contact). The Marsh propofol model uses three compartments: central (plasma/blood), fast equilibrating (highly perfused), and slow equilibrating (poorly perfused) in addition to an effect site “compartment.” Because the receptors (effect site) for propofol are in the brain, there is a short time delay between propofol blood (plasma/central compartment) concentration and the resulting clinical pharmacodynamics. An effect site compartment (ESC) is used to implement this time delay in the Marsh model.
In our propofol model, we used a time step of 1 second and parameters identical to the Marsh model. Our propofol PK parameters are therefore as follows: V = 15.9 L, K10 = 0.119/min, K12 = 0.112/min, K21 = 0.055/min, K13 = 0.042/min, K31 = 0.0033/min, and Keo = 0.26/min.
Description of Target Controlled Infusion (TCI)
During Target Controlled Infusion (TCI), open loop control of an infusion pump is performed by a computer. The control is open loop because the parameter being controlled (i.e., blood propofol concentration) is not actually measured. Because there is no feedback regarding actual blood propofol concentration, the error between the desired and actual values cannot be calculated and applied, as would be the case with a closed loop control system, to minimize error. After users enter the patient’s weight, age, and target (desired) blood propofol concentration, the computer uses a predetermined compartmental PK model (such as the Marsh10 or the Schnider19 model) to calculate and infuse the propofol administration profile that will theoretically achieve the desired target concentration in a given compartment. The predicted (not actual) concentrations of propofol in different compartments (blood, ESC) of the model are calculated and can be displayed by the TCI system. An example of a commercial TCI pump is the Diprifusor™, which incorporates the Marsh propofol model and uses the plasma compartment as the target site. Some TCI pumps target the effect site compartment instead. TCI pumps are used outside the United States. They are not approved by the Food and Drug Administration and, therefore, are not used clinically in the U.S. The Marsh model is based on data collected from 20 children [aged 1–12 years; American Society of Anesthesiologists (ASA) Physical Status Classification I (normal, healthy) or II (mild systemic disease)] of the Caucasian race (patient race was missing in the Marsh paper but was confirmed as Caucasian in a December 2015 email communication from Professor Gavin N.C. Kenny, MB ChB, MD).
Validation of the Propofol Model
We used Tivatrainer© (version 8, build 5)14 as a validation tool. It is a commercial pharmacokinetic (PK) simulation program created to teach total intravenous anesthesia (TIVA) as its name implies and to explain PK concepts. Users can edit PK parameters, chart the resulting time profiles of drug concentrations in different compartments, and export data to a spreadsheet. Tivatrainer© provides PK models for different drugs such as remifentanil and propofol, including the manual, non-TCI Marsh and Fresenius models as well as the propofol Diprifusor™ TCI that uses the Marsh model. We selected a time step of 1 second when using Tivatrainer© as a validation tool to match the time step implemented in our simulation. Tivatrainer, when running the Diprifusor model, reports propofol concentrations at time intervals of 8 seconds.
To validate our implementation of the Marsh model, we compared its time profiles for blood/plasma (central) compartment and effect site compartment propofol concentrations under identical conditions to those generated by the Tivatrainer© Marsh model in manual (not TCI) mode. The time profiles were almost identical, accounting for round-off error in the numerical computation. The difference between model outputs was 1% to 2% with a bolus and 0.2% with an infusion.
Modeling of Inter-Racial Variability in Propofol Pharmacodynamics (LOC)
Assumptions
We assumed that propofol pharmacokinetics are identical in different races and only altered the pharmacodynamics to simulate the LOC response of each race to propofol. British Blacks and Whites, Chinese from Beijing and Indians from Chandigarh are representative of all Blacks, Caucasians, Chinese and Indians.
We changed only the pharmacodynamics in modeling inter-racial variability in propofol response. For all races, on the assumption that the liver is a near perfect propofol clearance filter in all races, we left the pharmacokinetic parameters unchanged from the values in the Marsh model. Therefore, the time profile of propofol ESC concentration is identical for all races for the same propofol dosing profile. The assumption of similar PK across races is supported by the findings of Li et al.,15 who measured actual propofol blood levels in Chinese patients and compared them to those predicted by the Marsh Diprifusor™ model; they found the relationship acceptable for clinical use. This suggests that the propofol pharmacokinetics of Caucasians and Chinese are similar and therefore not the source of the inter-racial variability in propofol response observed by Irwin,8 Xu,16 and Dahaba17 in independent studies.
In adapting data from multiple studies6–9, 16 to model inter-racial variability in propofol LOC for the purposes of simulation-based training, we used median (EC50; not mean) concentration at the effect site compartment to represent a “typical” patient from that race with respect to propofol-induced LOC. We used EC50 instead of EC05 or EC95 to model LOC because we wanted to represent a median patient response at the 50th percentile within a race rather than one at the 5th or 95th percentile. Where data were reported for EC05 and EC95 or can be graphically extracted from population distribution plots, they are included in Table 1 as well as the 95% confidence interval (where available) for the EC values.
Table 1.
A Race-Specific Model of Effect Site Compartment EC05, EC50, EC95 Propofol Concentrations Predicted by the Marsh Diprifusor Model Corresponding to Loss of Consciousness.
| Race Location Sample size |
ESC
EC05 (95%CI) (µg/mL) |
ESC
EC50 (95%CI) (µg/mL) |
ESC
EC95 (95%CI) (µg/mL) |
ESC EC50 X/Cauc. Cauc./X |
Pla.
EC05 (95%CI) (µg/mL) |
Pla.
EC50 (95%CI) (µg/mL) |
Pla.
EC95 (95%CI) (µg/mL) |
Source Year of Publication |
|---|---|---|---|---|---|---|---|---|
| Indians Chandigarh n=18 |
1.38 (-) |
1.88 (1.87–1.91) |
2.37 (2.37–2.38) |
0.67 1.5 |
1.88 from Puri Fig. 1 | 2.31 (2.16–2.45) |
2.8 (2.61–3.19) |
Puri et al. [7] 2011 (adapted) |
| Blacks UK n=50 |
1.08 (-) |
2.02 (-) |
2.95 (-) |
0.72 1.4 |
- | - | - | Natarajan et al. [6] 2011 (adapted) |
| Chinese Beijing n=405 | 1.3 (1.2–1.4) |
2.2 (2.2–2.3) |
3.2 (3.1–3.3) |
0.8 1.3 |
2.9 (2.7–3.0) |
3.8 (3.8–3.9) |
4.8 (4.7–4.9) |
Xu et al. [16] 2009 |
| Caucasians Glasgow n=40 | 1.5 (1.3–1.7) |
2.8 (2.7–2.9) |
4.1 (3.9–4.3) |
1.0 1.0 |
3.1 (2.6–3.5) |
5.2 (5.0–5.4) |
7.3 (7.0–7.8) |
Milne et al. [9] 2003 |
| Chinese Hong Kong n=60 |
1.89 (-) |
2.66 (2.59–2.72) |
3.82 (3.68–4.00) |
- |
2.98 (-) |
3.92 (3.8–4.04) |
5.38 (5.16–5.66) |
Irwin et al. [8] 2002 |
| Chinese Zhejiang n=30 | - | 1.6 ±
0.4 (mean±sd) Not EC50 |
- | - | - | - | - | Dahaba et al. [17] 2011 |
| Caucasians Austria n=30 | - | 2.4 ±
1.5 (mean±sd) Not EC50 |
- | - | - | - | - | Dahaba et al. [17] 2011 |
ESC, effect site compartment; Pla., plasma (central) compartment; Cauc., Caucasian.
All studies used the Marsh Diprifusor TCI software v 2.0 except Puri (Marsh TCI Diprifusor of unspecified version) and Natarajan (non-TCI pump). EC values in italics mean they are adapted from the literature. The bottom 3 rows present results from studies that were not used to create the proposed model.
The peer-reviewed publications we employed to develop the model used different propofol administration techniques (TCI and non-TCI), different time intervals between step increases in TCI target concentration (30 seconds to 6 minutes), different compartments (ESC, plasma), and different statistical measures {EC05, EC50, and EC95 [95% confidence interval (CI)]; mean ± SD} for reporting propofol concentrations at LOC. To develop a race-specific model of propofol LOC, we converted available data, where needed, to a consistent, common format: EC05, EC50, EC95 propofol concentration at the effect site compartment at LOC abbreviated as ECXX ESC @ LOC. Below, we detail our approach and rationale for adapting the existing peer-reviewed data.
Propofol ESC Concentration at LOC for Caucasians
Milne et al.9 anesthetized 40 (19 males, 21 females) unpremedicated ASA status I and II Caucasian patients. Intravenous propofol was delivered by Diprifusor™ TCI (software version 2) incorporating the Marsh10 model that predicted and displayed blood and ESC propofol concentrations. Propofol infusion was initiated to provide an initial blood concentration of 1.5 µg/mL that was subsequently increased (in a stair-step fashion) by 0.5 µg/mL every 30 seconds until patients lost their eyelash reflex and no longer responded to a verbal command. This point was defined as LOC. The EC05, EC50, and EC95 (95%CI) ESC concentrations at which LOC occurred for Caucasians were 1.5 (1.3–1.7), 2.8 (2.7–2.9), and 4.1 (3.9–4.3) µg/mL, respectively. The EC05, EC50, and EC95 (95%CI) plasma concentrations at which LOC occurred were 3.1 (2.6–3.5), 5.2 (5.0–5.4), and 7.3 (7.0–7.8) µg/mL, respectively.
Dahaba et al. found, in a study of 30 Caucasian patients in Austria (mean ± SD), a mean ESC concentration @ LOC of 2.4 ± 1.5 µg/mL.17 The data from Dahaba et al. are not used in our model because they are reported as mean ± SD instead of median (EC50). More details of Dahaba et al.’s study are found below.
Propofol ESC Concentration at LOC for Blacks (adapted)
Most of the studies used to develop the race-specific model used the Diprifusor™ TCI pump running the Marsh model to administer propofol to patients of different races. Natarajan et al.6 did not use a Diprifusor™ TCI pump in their study comparing propofol response between 50 British white and 50 British black patients (ASA status I and II, aged 18–65 years). An Asena Mk III (Alaris Medical Systems, San Diego, CA) non-TCI pump was used to administer a constant (Propofol-Lipuro 1%) propofol infusion of 40 mg/kg/h. The investigators found that the mean (SD) dose of propofol required for a loss of verbal response is lower (P < 0.001) in black patients at 1.17 (0.25) mg/kg compared to white patients at 1.41 (0.4) mg/kg.6
To obtain the equivalent mean ESC concentration at LOC for blacks from the Natarajan et al. data, we simulated a 40 mg/kg/h constant infusion, as used by Nataranjan et al., on our implementation of the Marsh model and also on the Marsh model running in manual (non-TCI) mode in Tivatrainer.14 The propofol effect site concentrations when the administered propofol dose was 1.17 and 1.41 mg/kg on our implementation of the Marsh model were considered the mean ESC propofol concentrations for a loss of verbal response in blacks (0.85 µg/mL) and whites (1.18 µg/mL), respectively. We obtained similar mean ESC concentrations when repeating Natarajan et al.’s protocol on Tivatrainer (0.87 and 1.21 µg/mL), an additional validation of our implementation of the Marsh model.
The mean ESC propofol concentration at LOC in whites derived from Natarajan et al. (1.18 µg/mL) is much smaller than Milne et al.’s EC50 ESC @ LOC (2.8 µg/mL) and may be explained by the different administration techniques (non-TCI vs TCI) and protocols (continuous vs. stair step). Because the data for the other three races are derived from TCI pumps, we opted to keep the Milne et al. TCI data for Caucasians instead of using the mean ESC concentration derived from the Natarajan et al. non-TCI data for whites. To derive an EC ESC @ LOC for blacks, we used the ratio of black-to-white mean ESC @ LOC from Natarajan et al. to arrive at a scaling factor: 0.85/1.18 = 0.72. We then multiplied the ESC EC05, EC50, and EC95 for Caucasians from Milne et al. (1.5, 2.8, and 4.1) by that scaling factor (0.72) to obtain derived EC05, EC50, EC95 ESC @ LOC for blacks of 1.08, 2.02, 2.95 µg/mL, respectively. We do not provide 95%CI for the derived EC values for Blacks because they are not available or derivable from the results presented by Natarajan et al.
Propofol ESC Concentration at LOC for Chinese
Irwin et al. studied 60 (30 males, 30 females) unpremedicated ASA I and II, normal body mass index, adult Hong Kong Chinese patients.8 Induction of anesthesia with intravenous propofol was delivered by Diprifusor™ TCI (software version 2) incorporating the Marsh model (Graseby 3500 pump, Graseby Medical Limited, Hertfordshire, UK). In the Irwin et al. study, an initial propofol blood (target) concentration of 1.5 µg/mL was chosen based on a previous study that found that a median target concentration of 0.9 µg/mL was required to produce adequate sedation using a similar TCI system. The target blood concentration was then increased by 0.5 µg/mL every 2 minutes until LOC occurred. LOC was determined by the confluence of three end points: no response to verbal command, loss of eyelash reflex, and the inability to hold a 20-mL syringe. The propofol concentrations at the target (blood) and effect site compartments were then recorded. Irwin et al.’s Table 3 confusingly denoted the ESC concentrations @ LOC as CP50 and CP95, respectively, instead of the correct nomenclature: EC50 and EC95. In addition, the legend for Irwin’s Table 3 incorrectly states that the data is presented as “mean (95%CI)” when it is actually EC (95%CI), as can be confirmed by a quick comparison of the left plot in Irwin’s Figure 4 to the ESC data in Table 3. The EC50 (95%CI) blood compartment and ESC concentrations at LOC for Chinese were 3.92 µg/mL (3.8–4.04 µg/mL) and 2.66 µg/mL (2.59–2.72 µg/mL), respectively. The blood and ESC EC95 (95%CI) concentrations in micrograms per milliliter were 5.38 (5.16 – 5.66) and 3.82 (3.68–4.00), respectively. Irwin did not explicitly report EC05 values, but they can be obtained graphically from the distribution plots in Irwin’s Figures 3 and 4. The blood and ESC EC05 concentrations in micrograms per milliliter were 2.98 and 1.89, respectively; no 95%CI are included because the EC05 values were graphically extracted.
Xu et al. studied 405 (97 males, 308 females) unpremedicated healthy (ASA I and II) Chinese patients at five Beijing hospitals.16 Propofol (1% Diprivan™) was administered via TCI (Diprifusor™ with Marsh model, software version 2; Graseby 3500 pump) to an initial predicted blood concentration of 1.2 µg/mL that thereafter was increased by 0.3 µg/mL every 30 seconds until the OAAS score was 1. Xu et al. explained that they chose an interval of 30 seconds (instead of 2 minutes) between step increments in target concentration for two reasons: 1) to adopt the same time interval used by Milne et al. (30 seconds) to facilitate direct comparison of data from Chinese patients with the Milne et al. data from Caucasian patients and 2) because, at 2 minutes, the time interval between step increases in target concentration used by Irwin et al. was four times longer.16 Data from earlier studies (Struys et al.,18 Schnider et al.,19 and Wakeling et al.20) indicate that longer time intervals tend to produce higher EC50s. The ESC C05, C50 (EC50), and C95 at LOC for Chinese patients obtained by Xu et al. were (brackets indicate 95%CI): 1.3 µg/mL (1.2–1.4 µg/mL), 2.2 µg/mL (2.2–2.3 µg/mL), and 3.2 µg/mL (3.1–3.3 µg/mL), respectively. The EC05, EC50, and EC95 (95%CI) plasma concentrations in micrograms per milliliter at which LOC occurred for Chinese were 2.9 (2.7–3.0), 3.8 (3.8–3.9), 4.8 (4.7–4.9), respectively. The EC50 ESC concentration obtained by Xu et al. (2.2 µg/mL) was lower than the 2.66 µg/mL found by Irwin et al., consistent with the concern that longer time intervals (in this case, 4×) produce higher EC50s.
Dahaba et al. conducted a multinational prospective study directly comparing 30 (15 male/15 female) Caucasian patients in Austria and 30 (15 male, 15 female) Chinese patients in China using propofol from the same production batch.17 In both groups, identical exclusion criteria were used. The demographics of both groups were also very similar, including ASA status (I and II), age range (30–50) and body mass index. Using Diprifusor™ TCI (Marsh model, software version 2), the estimated plasma (blood) concentration (Cp) of propofol for both groups was set at 1 µg/mL and increased by 1 µg/mL every minute to gradually reach 5 µg/mL after 5 minutes. To reduce potential variations in the observers’ subjective use of the OAAS to determine LOC, a consistent set of identical verbal commands in the respective languages was used. Patients were tapped gently on the shoulder every 10 seconds and asked whether they were still awake until an OAAS score of 1 (no eyelash reflex and no response to verbal commands; considered as LOC) was reached. Dahaba et al. reported their findings in terms of mean ± SD instead of the median EC50. There were significant differences (all at P = 0.0001) between Austrians versus Chinese (mean ± SD) for time to LOC (3.9 ± 0.5 minutes vs. 2.8 ± 0.6 minutes), plasma concentration @ LOC (4.6 ± 2.8 µg/mL vs. 3.3 ± 0.8 µg/mL), and ESC concentration @ LOC (2.4 ± 1.5 µg/mL vs. 1.6 ± 0.4 µg/mL).
There are discrepancies for ESC EC50 at LOC for Chinese patients between the 2002 Irwin et al.8 (2.66 µg/mL), 2009 Xu et al.16 (2.2 µg/mL), and 2011 Dahaba et al.17 (1.6 µg/mL) data. We confirmed with Tivatrainer the concern stated by Xu et al.: longer time intervals between step increases in TCI target concentration do increase the apparent ESC concentration at which LOC appears to occur when targeting the plasma concentration during TCI. We include the Irwin et al., Xu et al., and Dahaba et al. data in Table 1 so that readers can make their own choice. However, we recommend using the data from Xu et al. for Chinese patients for the following reasons: a larger sample size of 405 patients from a multi-center study (five different medical centers in the Beijing area) versus 60 for the Irwin et al. and 30 for the Dahaba et al. studies. The 2009 Xu et al. study specifically addressed a potential limitation (4-fold longer time interval) of the earlier Irwin et al. study and was designed for direct comparison with the Milne et al.9 data for Caucasians. Importantly, Dahaba et al. reported the LOC data in terms of mean ± SD instead of EC50. Additionally, the mean ESC concentration of 1.6 µg/mL at LOC in Chinese (lower than the 1.88 µg/mL derived for Indians below) is inconsistent with the order of increasing sensitivity to propofol found by Ortolani: Caucasians, Chinese, Malays, and Indians.4
We therefore adopt for the model for simulation-based training the EC50 ESC propofol concentration at LOC for Chinese found by Xu et al.: 2.2 µg/mL.
Propofol ESC Concentration at LOC for Indians (South Asians)
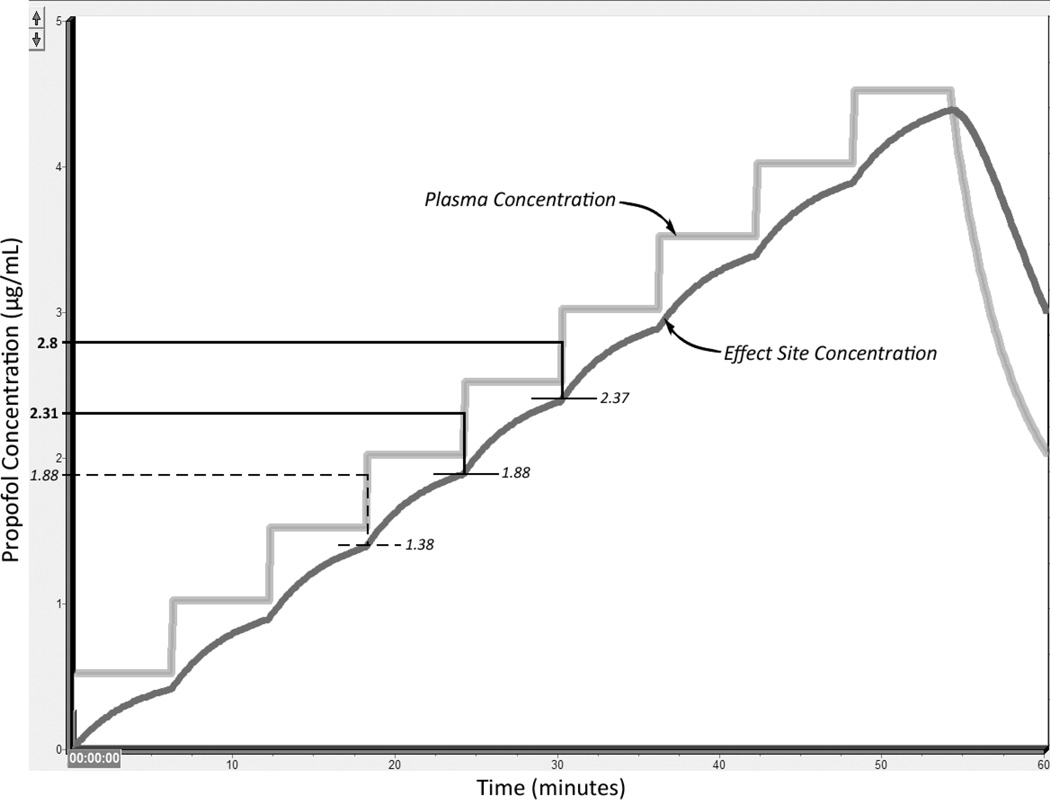
Puri et al.7 used a TCI pump (Master TCI, Fresenius Kabi Group, Bad Homburg, Germany) that used the Marsh model and displayed blood (plasma) concentration instead of effect site concentration to administer propofol to 18 ASA I and II Indian patients. Thus, the EC data reported by Puri et al. for Indians at LOC is for blood, not ESC, concentration. We needed an ESC EC50 at LOC for Indians to normalize the Puri et al. data to those of Xu et al. and Milne et al., who reported EC50 effect site concentrations. Puri et al. attempted to compensate for the fact that their pump did not display ESC concentration by using a time interval of 6 minutes between step increases in target concentration. The rationale was that “maintaining the target plasma concentration for 6 min before stepping up to the higher concentration ensured achieving a similar target concentration at the effect-site also.” We repeated with Tivatrainer’s Marsh Diprifusor™ TCI model the Puri et al. protocol (initial target blood concentration of 0.5 µg/mL followed by increases in target blood concentration by steps of 0.5 µg/mL and allowing 6 minutes of equilibration after each new target concentration is set). We found when conducting a Tivatrainer simulation of the Puri et al. protocol that the 6-minute interval did not ensure achieving similar target concentrations at the blood and ESC compartments. For example, Tivatrainer predicted these sets of blood/ESC concentrations at the end of each successive 6-minute time interval: 0.5/0.4, 1.0/0.88, 1.5/1.37, 2.0/1.87, and 2.5/2.37 µg/mL, indicating that similar blood and ESC concentrations were not achieved by the end of the 6-minute interval (Figure 1). Puri et al. found the predicted EC50 (95%CI) and EC95 (95%CI) plasma concentrations at LOC to be 2.31 µg/mL (2.16–2.45 µg/mL) and 2.8 µg/mL (2.61–3.19 µg/mL), respectively. When the blood (plasma) concentration was 2.31 and 2.8 µg/mL, the ESC concentration was 1.88 and 2.37 µg/mL, the values we consider as the adapted EC50 and EC95 ESC concentration at LOC for Indians. Puri did not explicitly report an EC05 value, but it can be manually derived from the loss of verbal contact (LVC) distribution on the probability to target concentration plot in Puri’s Figure 1 as 1.88 µg/mL at the plasma compartment, which translates through Tivatrainer as 1.38 µg/mL at the ESC. We do not provide a 95%CI for Indian EC05 ESC @ LOC in Table 1 because the value of 1.88 µg/mL was obtained graphically from Puri’s Figure 1.
Figure 1.
This plot is based on a screen capture of the output of the Marsh model on Tivatrainer14 when running in TCI (Diprifusor), non-manual mode to replicate the Puri et al.7 protocol. The times at which the plasma concentration matches the results obtained by Puri et al. (EC05, EC50, and EC95 of 1.88, 2.31, and 2.8 µg/mL, respectively) are used to determine the corresponding effect site compartment EC05, EC50, and EC95 values. The temporal resolution of the concentration outputs when running the Marsh Diprifusor TCI model in Tivatrainer is coarse at 8 seconds, making the changes in plasma concentration appear almost vertical, i.e., “instantaneous.” The plasma EC05 value (1.88 µg/mL on the y axis) is shown as a dashed, instead of a solid, line because this value was not explicitly reported by Puri et al. but was instead graphically derived from Figure 1 in Puri’s paper. Numbers in italic font indicate they are derived. Numbers in regular (non-italic) font indicate they are copied verbatim from the studies used to create the race-specific model.
Results
The race-specific propofol EC05, EC50, and EC95 plasma and ESC concentrations at LOC for four different races (Caucasian, Chinese, Blacks, and Indians) based on the Marsh Diprifusor model and adapted from the prior studies are tabulated in Table 1. The 95%CI for the EC values are included if they were reported in the papers or were able to be derived. There is no overlap of the 95%CI for EC50 ESC @ LOC between Caucasians (2.7–2.9 µg/mL), Chinese (2.2–2.3 µg/mL), or Indians (1.87–1.91 µg/mL), or for EC95 ESC @ LOC between Caucasians (3.9–4.3 µg/mL), Chinese (3.1–3.3 µg/mL), or Indians (2.37–2.38 µg/mL).
Discussion
The race-specific model of propofol-induced LOC detailed in this article was designed for simulation-based education and training. However, it lends itself to exploration for reduced clinical dosing of propofol for non-Caucasians, especially for propofol-induced procedural sedation, given that the proposed model is based on multiple independent clinical studies that are consistent in their findings that other races studied so far are more sensitive to propofol than Caucasians. The lack of overlap and indeed the clear demarcation in the 95%CI for the EC50 and EC95 ESC concentrations at LOC for the Caucasian, Chinese, and Indian study populations (Table 1) indicate that the differences presented in the model are meaningful.
A typical current clinical practice in the United States is a bolus of 0.5 to 1.0 mg/kg of propofol for initiating conscious sedation followed by a titrated infusion (personal communication from co-author NG, an anesthesiologist who regularly administers propofol in his clinical practice). We simulated the high end of the range (1 mg/kg) on the Marsh and Fresenius non-TCI models in Tivatrainer. The Fresenius models clinical observations more realistically by having the peak propofol effect in response to a bolus sooner (via a faster Keo equilibrium constant of 1.2/min between the central and effect site compartment) compared to 0.267/min for the Marsh model.
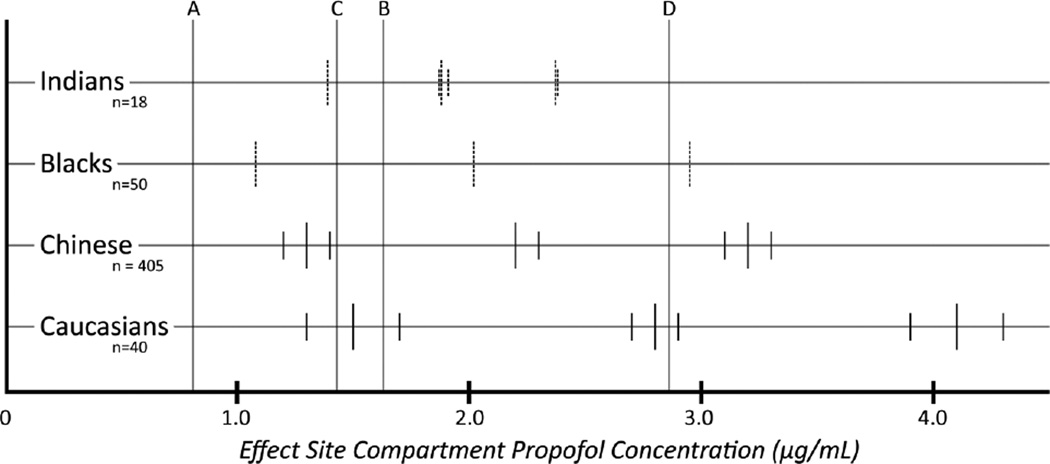
The peak ESC concentration was 1.63 µg/mL (Marsh) and 2.86 µg/mL (Fresenius) in response to a bolus of 1 mg/kg and 0.81 µg/mL (Marsh) and 1.43 µg/mL (Fresenius) in response to a bolus of 0.5 mg/kg for conscious sedation. In Figure 2, we compare these peak propofol ESC concentrations to the EC values in Table 1. With the Marsh model ESC peak concentration (1.63 µg/mL) for a 1.0 mg/kg bolus and race-blind dosing based on studies in Caucasian populations for conscious sedation, about 5% of Caucasians will momentarily lose consciousness; more than 5%, but less than 50% of Chinese, Black, and Indian patients will momentarily lose consciousness. With the Fresenius model, ESC peak concentration (2.86 µg/mL) for a 1.0 mg/kg bolus, about 50% of Caucasians, more than 50%, but less than 95% of Chinese; almost 95% of blacks; and more than 95% of Indians will momentarily lose consciousness.
Figure 2.
The effect site compartment EC05, EC50, and EC95 (left to right along each horizontal line corresponding to a race) propofol concentrations at loss of consciousness for four different races. Lines A and B represent the peak ESC concentration (0.81 and 1.63 µg/mL) predicted by the Marsh model (manual mode, non-TCI) for a propofol bolus of 0.5 mg/kg (low end of dosing range for sedation) and 1.0 mg/kg (high end of dosing range for sedation), respectively. Lines C and D represent the peak ESC concentration (1.43 and 2.86 µg/mL) predicted by the Fresenius (modified Marsh model with faster equilibration between the plasma and effect site compartments) model (manual mode, non-TCI) for a propofol bolus of 0.5 mg/kg and 1.0 mg/kg, respectively. Where available or derivable, the 95%CI is also displayed. The short line in the middle of each set is the EC value with the two shorter lines on each side representing the 95%CI. The short vertical lines are shown as dashed lines where the values that they represent are derived instead of being copied verbatim from the prior studies used to create the model.
The model suggests that when a TCI Diprifusor pump is used for propofol-induced sedation, the initial plasma (not ESC) target concentrations should take race into account by pragmatically setting the plasma target concentration at the EC50 value for a given patient’s race listed in Table 1. Subsequently, the target plasma concentration can be dialed up or down according to how the individual patient responds. Alternatively, based on situational awareness and risk assessment, the initial plasma concentration TCI target could be set at EC05 in high-risk situations (if no one is readily available to help re-establish ventilation and oxygenation) and at EC95 where airway equipment and skilled personnel are immediately at hand. Given that Table 1 was generated using the Marsh Diprifusor TCI model that targets the plasma compartment, it is probably not advisable to use the ESC EC50 values in Table 1 as targets when using a TCI pump that targets the effect site compartment instead of the plasma compartment.
Providing specific race-dependent clinical dosing guidelines when not using TCI at all (i.e., in manual mode) is more involved and challenging as there is no computer model to perform the computation for users. For non-TCI dosing, the general recommendation is to be at least cognizant of the increased sensitivity of Indians > Blacks > Chinese > Caucasians to propofol and to reduce at least the initial loading dose of propofol, especially for procedural propofol-induced conscious sedation. The reduction of doses could be according to the ESC EC50 ratios that have been calculated and are displayed in Table 1.
A limitation of the study is that the model uses data predicted or derived from the Marsh Diprifusor™ TCI model to infer, not measure, plasma and effect site compartment propofol concentrations. Actual measurements of propofol concentrations in the blood in different races are needed and will help elucidate if the inter-racial differences in EC05, EC50, and EC95 propofol concentration @ LOC are due to PK, PD or both. However, from purely clinical and patient safety perspectives, it can be argued that it is immaterial whether the differences are pharmacokinetically or pharmacodynamically mediated. The model provides actionable data that is inherently safe for application to clinical settings because it suggests smaller doses for non-Caucasians (rather than the higher dosing guidelines based on studies in Caucasian populations) to prevent accidental overdose, unintended general anesthesia, and the accompanying complications and risks.
African Americans represent a significant segment of the U.S. population. Our inability to find race-specific propofol LOC data derived from TCI models for African Americans points to a significant gap in the peer-reviewed literature. The need to urgently address this gap is hampered by the fact that TCI pumps are not Food and Drug Administration approved in the United States. On the assumption (that we readily acknowledge may be flawed) that a similar race on different continents has similar propofol PK/PD, obtaining race-specific propofol data for African Americans may also benefit much of the African population.
The Hispanic population represented 17.4% of the U.S. population in 2014. A literature search for propofol PK/PD parameters produced no results for the Hispanic population, given that it is an ethnic group, not a racial group. An ethnic group shares a common language and culture and can encompass multiple races, as is the case with the Hispanic population.
India will soon have the world’s largest population; paradoxically, Puri’s study of Indians has the smallest patient sample size (n = 18). Significantly, it would appear that definitive data on Indian propofol PK/PD is most needed because Indians thus far appear to be the race most sensitive to propofol.4 Furthermore, the dosing guidelines in package inserts for propofol manufactured in India seem to acknowledge the higher sensitivity of Indians. The Diprivan (AstraZeneca Pharmaceuticals LP; document number 30193-00 Rev 08/05 235003) package insert for 1% propofol sold in North America recommends that for induction of general anesthesia in ASA I/II adult patients under 55 years, propofol should be “titrated at approximately 40 mg every 10 seconds against the response of the patient.” The Critifol (Bharat Serums and Vaccines, Ltd; document number IN90110D0) package insert for 1% propofol manufactured in India recommends that for induction of general anesthesia, propofol should be “titrated approximately 2 ml (20 mg) every 10 seconds in an average healthy adult against the response of the patient,” effectively recommending titration at half the rate recommended in the North American package insert. The Diprivan package insert for North America clearly states: “Fifty-five of these trials, 20 for anesthesia induction and 35 for induction and maintenance of anesthesia or MAC sedation, were carried out in the US or Canada and provided the basis for dosage recommendations and the adverse event profile during anesthesia or MAC sedation.”
In our era of globalization, are package inserts with specific regional or national dosing knowledge vestiges of a more insular past and less connected world? Should package inserts be universal and have race-specific dosing guidelines, where appropriate, in acknowledgment of the new world we live in? The far-flung Indian and Chinese diasporas come to mind, especially for those members who live in countries with predominantly Caucasian populations. In addition to the small Indian patient sample size, ESC concentrations had to be deduced by the current authors. The small span between the EC05 and EC95 values for Indians in Figure 2 stand out as being narrow compared to the distribution for Chinese and Caucasians. All these factors speak to the urgency of obtaining definitive propofol data, for not only Indians, but also all other races, preferably from study protocols that actually measure propofol concentration from assays of blood samples.
Conclusion
We detailed how we used and adapted propofol LOC data for different races in the peer-reviewed literature to create a race-specific model of propofol-induced LOC based on the Marsh Diprifusor model. We anticipate that our model will be updated as new data become available. Therefore, we described how we generated the model in sufficient detail that it can be readily updated as new data become available such as EC values @ LOC for races missing from the model.
A result of globalization is that patients encountered by any given clinician may not be racially homogeneous, especially in large cosmopolitan cities. Other relevant developments are the availability of generic propofol, the widening popularity of day (outpatient) surgery with the increased use of propofol for procedural sedation, and the administration of propofol by or under the supervision of non-anesthesiologists who may not be adequately skilled in restoring ventilation. Our model suggests reduced propofol doses (compared to dosing guidelines based on studies conducted on populations of primarily Caucasian patients) for Indian, Black, and Chinese patients. Because it is a reduced, rather than an increased, dosing recommendation, the model would not promote unintended loss of consciousness or even general anesthesia during propofol sedation, but instead tend to mitigate this adverse outcome.
Acknowledgments
We thank Corinna Yu, MD, who conducted an extensive literature search about racial differences during anesthesia that she generously shared with us, Prof. Kenny for confirming the Caucasian race of the patients in the Marsh, Morton, Kenny 1991 paper describing the “Marsh” model, and Corey Astrom for editorial help.
Research reported in this publication was supported by the National Library of Medicine Division of the National Institutes of Health (NIH/NLM 1R21LM010829-01A1) and the National Center for Advancing Translational Sciences of the National Institutes of Health under Award numbers UL1 TR000064 and UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The authors report no conflicts of interests or disclosures.
Author Contributions
S.L. created the race-specific model and wrote the paper. D.L. wrote the code for replicating the Marsh model and ran simulations to create the race-specific model. H.D. and N.G. analyzed the data and edited the article. B.L reviewed the article. J.Q. contributed to the study and edited the article. S.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.World Health Organization. 19th WHO Model List of Essential Medicines. [Accessed December 2015]; http://www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf.
- 2.Ayer DW. The Who’s Next Club: A Cosmetic Surgery Disaster. Strategic Book Publishing; 2011. [Google Scholar]
- 3.Ortolani O, Conti A, Sall-Ka B, et al. The recovery of Senegalese African blacks from intravenous anesthesia with propofol and remifentanil is slower than that of Caucasians. Anesth Analg. 2001;93:1222–1226. doi: 10.1097/00000539-200111000-00036. [DOI] [PubMed] [Google Scholar]
- 4.Ortolani O, Conti A, Chan YK, Sie MY, Ong GS. Comparison of propofol consumption and recovery time in Caucasians from Italy, with Chinese, Malays and Indians from Malaysia. Anaesth Intensive Care. 2004;32:250–255. doi: 10.1177/0310057X0403200215. [DOI] [PubMed] [Google Scholar]
- 5.Ortolani O, Conti A, Ngumi ZW, et al. Ethnic differences in propofol and fentanyl response: a comparison among Caucasians, Kenyan Africans and Brazilians. Eur J Anaesthesiol. 2004;21:314–319. doi: 10.1017/s0265021504004119. [DOI] [PubMed] [Google Scholar]
- 6.Natarajan A, Strandvik GF, Pattanayak R, et al. Effect of ethnicity on the hypnotic and cardiovascular characteristics of propofol induction. Anaesthesia. 2011;66:15–19. doi: 10.1111/j.1365-2044.2010.06568.x. [DOI] [PubMed] [Google Scholar]
- 7.Puri GD, Mathew PJ, Sethu Madhavan J, Hegde HV, Fiehn A. Bi-spectral index, entropy and predicted plasma propofol concentrations with target controlled infusions in Indian patients. J Clin Monit Comput. 2011;25:309–314. doi: 10.1007/s10877-011-9309-x. [DOI] [PubMed] [Google Scholar]
- 8.Irwin MG, Hui TW, Milne SE, Kenny GN. Propofol effective concentration 50 and its relationship to bispectral index. Anaesthesia. 2002;57:242–248. doi: 10.1046/j.0003-2409.2001.02446.x. [DOI] [PubMed] [Google Scholar]
- 9.Milne SE, Troy A, Irwin MG, Kenny GN. Relationship between bispectral index, auditory evoked potential index and effect-site EC50 for propofol at two clinical end-points. Br J Anaesth. 2003;90:127–131. doi: 10.1093/bja/aeg035. [DOI] [PubMed] [Google Scholar]
- 10.Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991;67:41–48. doi: 10.1093/bja/67.1.41. [DOI] [PubMed] [Google Scholar]
- 11.Barakat AR, Sutcliffe N, Schwab M. Effect site concentration during propofol TCI sedation: a comparison of sedation score with two pharmacokinetic models. Anaesthesia. 2007;62:661–666. doi: 10.1111/j.1365-2044.2007.05059.x. [DOI] [PubMed] [Google Scholar]
- 12.Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251. [PubMed] [Google Scholar]
- 13.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–847. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Engbers F, Sutcliffe N, Kenny G. TIVA Trainer. 8 (build 5) Leiden: European Society for Intravenous Anaesthesia; 2008. [Google Scholar]
- 15.Li YH, Yang J, Tian J, Xu J. Evaluation of predictive performance of Diprifusor target-controlled infusion system for propofol in Chinese patients. Chinese J Anesthesiol. 2004;24:889–892. [Google Scholar]
- 16.Xu Z, Liu F, Yue Y, et al. C50 for propofol-remifentanil target-controlled infusion and bispectral index at loss of consciousness and response to painful stimulus in Chinese patients: a multicenter clinical trial. Anesth Analg. 2009;108:478–483. doi: 10.1213/ane.0b013e31818f8a30. [DOI] [PubMed] [Google Scholar]
- 17.Dahaba AA, Zhong T, Lu HS, et al. Geographic differences in the target-controlled infusion estimated concentration of propofol: bispectral index response curves. Can J Anesth. 2011;58:364–370. doi: 10.1007/s12630-011-9453-2. [DOI] [PubMed] [Google Scholar]
- 18.Struys MMRF, De Smet T, Depoorter B, et al. Comparison of plasma compartment versus two methods for effect compartment-controlled target-controlled infusion for propofol. Anesthesiology. 2000;92(2):399–406. doi: 10.1097/00000542-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Schnider TW, Minto CF, Shafer SL, et al. The influence of age on propofol pharmacodynamics. Anesthesiology. 1999;90:1502–1516. doi: 10.1097/00000542-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Wakeling HG, Zimmerman JB, Howell S, Glass PS. Targeting effect compartment or central compartment concentration of propofol: what predicts loss of consciousness? Anesthesiology. 1999;90:92–97. doi: 10.1097/00000542-199901000-00014. [DOI] [PubMed] [Google Scholar]