Abstract
Recent reports suggest abnormalities of neurotransmitter receptor trafficking, targeting, dendritic localization, recycling, and degradation in the brain in schizophrenia. We hypothesized that a potential explanation for these findings may be abnormal posttranslational modifications that influence intracellular targeting and trafficking of proteins between subcellular compartments. Dysregulation of protein palmitoylation is a strong candidate for such a process. S-palmitoylation is a reversible thioesterification of palmitoyl-groups to cysteine residues that can regulate trafficking and targeting of intracellular proteins. Using a biotin switch assay to study S-palmitoylation of proteins in human postmortem brain, we identified a pattern of palmitoylated proteins that cluster into 17 bands of discrete molecular masses, including numerous proteins associated with receptor signal transduction. Using mass spectrometry, we identified 219 palmitoylated proteins in human frontal cortex, and individually validated palmitoylation status of a subset of these proteins. Next, we assayed protein palmitoylation in dorsolateral prefrontal cortex from 16 schizophrenia patients and paired comparison subjects. S-palmitoylation was significantly reduced for proteins in most of the 17 schizophrenia bands. In rats chronically treated with haloperidol, the same pattern of palmitoylation was observed but the extent of palmitoylation was unchanged, suggesting that the diminution in protein palmitoylation in schizophrenia is not due to chronic antipsychotic treatment. These results indicate there are changes in the extent of S-palmitoylation of many proteins in the frontal cortex in schizophrenia. Given the roles of this posttranslational modification, these data suggest a potential mechanism reconciling previous observations of abnormal intracellular targeting and trafficking of neurotransmitter receptors in this illness.
1. Introduction
Multiple hypotheses have implicated myriad neurotransmitter systems in the pathophysiology of schizophrenia. Recent findings suggest abnormal receptor localization may play a role in abnormalities of both excitatory and inhibitory neurotransmitter pathways in this illness. For example, alterations have been found in transcripts associated with AMPA receptor localization and regulation (Beneyto and Meador-Woodruff, 2006; Drummond et al., 2013), and expression of some glutamate receptor subunits within specific subcellular compartments have been shown to be abnormal in this illness (Hammond et al., 2010; Kristiansen et al., 2010). Additionally, extent of N-linked glycosylation is associated with subcellular localization; abnormal glycan expression associated with N-glycosylation of glutamatergic receptor subunits (Tucholski et al., 2013a; Tucholski et al., 2013b), excitatory amino acid transporters (Bauer et al., 2010), and GABAA receptor subunits (Mueller et al., 2014), suggests that abnormal subcellular distribution of such proteins may contribute to disturbances of neurotransmission in schizophrenia. Given that these abnormalities in subcellular localization are found in multiple neurotransmitter systems, we hypothesized that posttranslational protein modifications (PTM) affecting targeting and localization of proteins may be altered in schizophrenia.
The intracellular distribution of neurotransmitter receptors and transporters, as well as other proteins, can be regulated by the uniquely reversible PTM, S-palmitoylation (El-Husseini and Bredt, 2002; Fukata and Fukata, 2010). This is in large measure due to the membrane stability facilitated by the hydrophobic 16-carbon palmitic fatty acid that can be covalently bound to cysteine residues. Palmitoylation status is tightly orchestrated by a large family of enzymes responsible for the addition or removal of palmitic acid, resulting in a cycle of palmitoylation/depalmitoylation that provides a mechanism for multiple cellular processes including subcellular targeting of proteins (Aicart-Ramos et al., 2011). The palmitoyl moiety is initially bound by a thioester linkage to cysteine residues by members of a family of palmitoyl acyl transferases (PATs) (Mitchell et al., 2006). Palmitoyl groups are dynamically removed by depalmitoylating enzymes including acyl-protein thioesterases (APTs) and palmitoyl protein thioesterases (PPTs) (Conibear and Davis, 2010). This reversibility of palmitoylation provides cyclical membrane association functionally required for a diverse number of proteins.
Many proteins associated with neurotransmission are known to be palmitoylated, including some glutamatergic receptor subunits (Hayashi et al., 2005; Hayashi et al., 2009), GABA receptor subunits (Fang et al., 2006; Keller et al., 2004; Rathenberg et al., 2004), proteins involved in release and recycling of neurotransmitters (El-Husseini and Bredt, 2002), and proteins associated with regulating trafficking to and stability of receptors at the synaptic membrane (Aicart-Ramos et al., 2011; Dejanovic et al., 2014; El-Husseini et al., 2000a; Huang and El-Husseini, 2005; Linder and Deschenes, 2007; Smotrys and Linder, 2004).
Interestingly, DiGeorge syndrome, associated with genetic deletion at 22q11.2, is associated with a 20–30 fold increase in risk of developing schizophrenia (Drew et al., 2011; Mukai et al., 2004). A mouse model for human 22q11 syndrome (Mukai et al., 2008; Mukai et al., 2015) has been shown to exhibit palmitoylation abnormalities. One of the deleted genes at 22q11.2 is a key palmitoyltransferase, ZDHHC8, which regulates proteins involved with axonal growth and arborization. ZDHHC8-deficient mice have impaired palmitoylation and axonal branching, which can be rescued by the reintroduction of active ZDHHC8 (Mukai et al., 2015). Palmitoylation has also been implicated in several neurodegenerative disorders (Antinone et al., 2013; Butland et al., 2014; Mitchell et al., 2014), but has yet to be well-characterized in human brain or studied in schizophrenia.
Given the role of palmitoylation in subcellular localization, and growing evidence for abnormalities in trafficking and targeting of proteins that regulate neurotransmission in schizophrenia, we hypothesized that abnormal protein palmitoylation could be an underlying mechanism contributing to the pathophysiology of schizophrenia. To address this, we first developed an assay to characterize protein palmitoylation in postmortem human brain, based on a previously described acyl-biotinyl exchange (ABE) technique (Drisdel and Green, 2004; Kang et al., 2008; Wan et al., 2007). Next, we studied extent of total protein palmitoylation in dorsolateral prefrontal cortex (DLPFC) in subjects with schizophrenia and paired comparison subjects. Given the integral roles palmitoylation plays in membrane targeting and interaction of proteins, we suggest that compromised protein palmitoylation may be a novel mechanism contributing to the abnormal subcellular targeting of proteins seen across multiple neurotransmitter pathways in schizophrenia brain.
2. Materials and methods
2.1 Human subjects
Samples of human brain used in this study were obtained from the Mount Sinai Medical Center brain collection (Table 1) as previously described (Davidson and Keefe, 1995; Funk et al., 2009; Harvey et al., 1992; Powchik et al., 1998). Schizophrenia patients had psychotic symptoms before the age of 40 and were hospitalized for at least 10 years. Patients were recruited prospectively and underwent extensive antemortem evaluation. Consent was obtained from the next of kin for each subject. Sixteen pairs of schizophrenia and comparison subjects were matched for sex, age, pH, and PMI (Table 1). All subjects were without a history of alcoholism, substance abuse, death by suicide, coma for more than 6 h before death, or any neuropathological evidence of any neurodegenerative disorders (Funk et al., 2009; Purohit et al., 1993). Comparison subjects were selected using a formal blinded chart review instrument. Assessment of subjects included the CERAD battery, the Clinical Dementia Rating Scale, and the Positive and Negative Symptom Scale (Powchik et al., 1998).
Table 1.
Paired subject demographics.
| Pair | Subject | Sex | Age | pH | PMI | Rx | Cause of death |
|---|---|---|---|---|---|---|---|
| 1 | Comparison | F | 73 | 6.3 | 3.4 | Ac. Myocardial Infarction | |
| Schizophrenia | F | 74 | 6.3 | 7 | On | Cardiopulmonary Arrest | |
| 2 | Comparison | F | 74 | 6.0 | 3 | Cardio respiratory failure | |
| Schizophrenia | F | 76 | 6.1 | 8.5 | On | Cardiopulmonary arrest, cancer of breast | |
| 3 | Comparison | F | 83 | 6.8 | 6.2 | Cardiopulmonary Arrest | |
| Schizophrenia | F | 79 | 6.8 | 9.9 | Off | Cardiac Arrest | |
| 4 | Comparison | F | 92 | 6.2 | 3.5 | ||
| Schizophrenia | F | 89 | 6.2 | 9.6 | On | Cardiopulmonary Arrest | |
| 5 | Comparison | F | 82 | 6.1 | 5.7 | Cardiopulmonary Arrest | |
| Schizophrenia | F | 81 | 5.9 | 12.5 | Off | ||
| 6 | Comparison | F | 74 | 6.3 | 4.8 | Cardiopulmonary arrest | |
| Schizophrenia | F | 76 | 6.1 | 21.2 | On | Cardiogenic shock | |
| 7 | Comparison | F | 75 | 6.0 | 6.5 | ||
| Schizophrenia | F | 76 | 6.0 | 9.7 | On | Cardiopulmonary Arrest | |
| 8 | Comparison | M | 58 | 6.7 | 12.3 | Arteriosclerotic heart disease | |
| Schizophrenia | M | 58 | 6.9 | 13.3 | On | Cardiopulmonary failure | |
| 9 | Comparison | M | 59 | 6.7 | 20.4 | Cardiopulmonary arrest | |
| Schizophrenia | M | 56 | 6.5 | 13.5 | Off | ||
| 10 | Comparison | M | 65 | 6.8 | 3.8 | Renal Failure | |
| Schizophrenia | M | 68 | 6.8 | 5.6 | On | Cardio respiratory failure | |
| 11 | Comparison | M | 64 | 6.3 | 4.2 | ||
| Schizophrenia | M | 63 | 6.3 | 6.2 | On | Cardiopulmonary arrest | |
| 12 | Comparison | M | 75 | 6.3 | 16 | ||
| Schizophrenia | M | 73 | 6.5 | 7.9 | On | Cardio respiratory failure | |
| 13 | Comparison | M | 92 | 6.4 | 20 | ||
| Schizophrenia | M | 93 | 6.6 | 17.7 | Off | Cardiopulmonary Arrest | |
| 14 | Comparison | M | 60 | 6.6 | 28.7 | ||
| Schizophrenia | M | 57 | 6.4 | 20.7 | On | Acute myocardial infarction, ASHD | |
| 15 | Comparison | M | 76 | 6.3 | 2.9 | Cardiopulmonary Arrest | |
| Schizophrenia | M | 77 | 6.4 | 24 | Off | ||
| 16 | Comparison | M | 73 | 6.2 | 14.9 | Cardio respiratory failure | |
| Schizophrenia | M | 73 | 6.2 | 8.8 | On | Cardiopulmonary Arrest | |
| Comparison | Mean: | 73 ± 10.5 | 6.4 ± 0.3 | 9.8 ± 8.0 | |||
| Schizophrenia | 73 ± 10.6 | 6.4 ± 0.3 | 12.3 ± 5.7 |
Abbreviations: Female (F); Male (M); Postmortem Interval (PMI, hours); Mean (group mean with SD);
Brains were collected and cut coronally in 10 mm slabs. The DLPFC (Brodmann areas 9/46) was dissected from the coronal slabs, snap frozen, pulverized into powder, and stored at −80°C until used. Samples were reconstituted and homogenized in ice-cold buffer (5 mM Tris-HCl (pH 7.4), 0.32 M sucrose, with a protease inhibitor tablet (Complete Mini, Roche Diagnostics, Mannheim, Germany) using a Power Gen 125 homogenizer (Thermo Fisher Scientific, Waltham, MA). Protein concentration was determined using a bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific).
2.2 Antipsychotic treated rats
Fourteen male Sprague-Dawley rats (250 g) were housed in pairs during a 9 month course of treatment; seven were used for each treatment condition. Treatment was either with sesame oil (vehicle) or haloperidol decanoate (28.5 mg/kg) administered via intramuscular injection every 3 weeks, for a total of 12 injections. This dose and duration of treatment has been previously described (Harte et al., 2005; Kashihara et al., 1986). The animals were sacrificed by decapitation, and brains were immediately harvested; the right frontal cortex was dissected on wet ice, snap frozen in liquid nitrogen and stored at −80°C.
2.3 Effect of postmortem interval in mice
To determine the effects of PMI on palmitoylation, we modeled in mice conditions typically seen at the time of death in humans. Fifteen female C57BL/6 mice were used for this study. The animals were sacrificed by decapitation, and brains were immediately harvested. Hour 0 samples (N=3) were immediately frozen in dry ice, and then stored at −80°C. Remaining brains were held at 4°C for progressively longer times (1 hour, 4 hours, 10 hours, N=4 at each time point), and then transferred to −80°C for storage. The right frontal cortex was dissected from each brain on wet ice and used for ABE assays. Rat and mice studies and procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
2.4 Acyl-Biotinyl-Exchange methodology
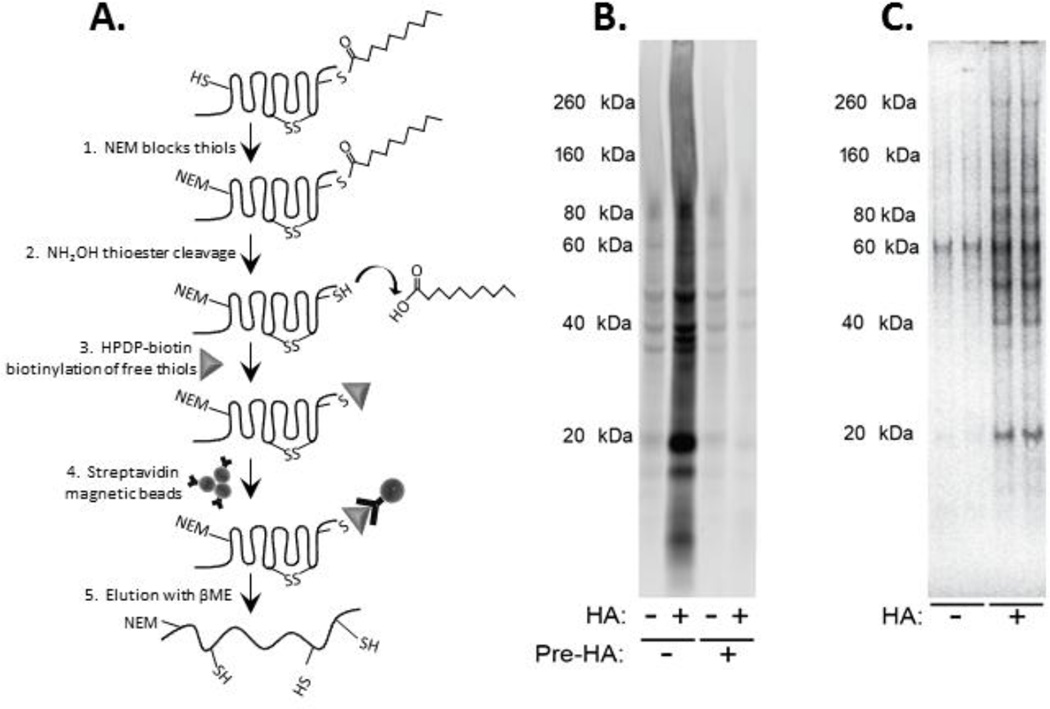
We modified a previously published ABE technique (Drisdel and Green, 2004; Kang et al., 2008; Wan et al., 2007) for efficient biotinylation and resulting purification of proteins from small (50–200 µg) quantities of human postmortem brain (Figure 1). The ABE methodology employs a hydroxylamine (HA)-dependent biotin-switch assay which allows for the purification and identification of S-palmitoylated proteins from brain tissue. Free thiols are first blocked with N-ethyl maleimide (NEM), then S-palmitoyl thioester bonds are cleaved with HA, allowing the newly-exposed cysteinyl thiols to be labeled with HPDP-biotin and purified by binding to streptavidin conjugated magnetic beads. Negative controls were treated the same, substituting additional reaction buffer for HA. As an additional negative control, to confirm HPDP-biotin specificity for intact palmitoyl groups, we pre-incubated samples with HA; this cleaved all palmitoyl groups prior to total thiol blockage with NEM, preventing biotinylation of any endogenously palmitoylated thiols.
Figure 1. Purification of palmitoylated proteins from human brain samples.
A) The acyl-biotinyl exchange (ABE) approach uses substitution of biotin for palmitoyl modifications through a sequence of three chemical steps: (1) unmodified cysteine thiols are blocked with N-ethyl maleimide (NEM), (2) palmitoyl thioester linkages are cleaved with hydroxylamine (HA), and (3) newly-exposed cysteinyl thiols are marked with a thiol-specific biotinylation reagent (Biotin-HPDP). (4) Biotinylated proteins are then affinity-purified on magnetic beads conjugated to streptavidin (5) and eluted with β-mercaptoethanol (β-ME). B) A representative streptavidin-blot to visualize biotinylated proteins after biotinylation in the presence (+HA) or absence (−HA) of hydroxylamine. Pre-incubation of the samples with HA (Pre-HA) resulted in disappearance of biotinylation of proteins in the +HA samples. C) A representative SDS-PA gel stained with SimplyBlue™ SafeStain (Invitrogen) to visualize palmitoylated proteins purified using the ABE method.
Homogenized brain samples (100 µg) were aliquoted on ice, and proteins were precipitated at room temperature (RT) using chloroform-methanol precipitation (Wan et al., 2007). When precipitating, a brief (~10 s) sonication pulse was used to facilitate washing of precipitated proteins with methanol. Precipitated proteins were then resuspended in HEN/SDS buffer (250 mM HEPES, pH 7.7, 1 mM EDTA, 2% SDS, 10 mM NEM, 1XPI) and incubated at 40°C for 60 min with occasional mixing. After blocking free thiols with NEM, any remaining NEM was removed by chloroform-methanol precipitation. Proteins were resuspended in −HA/SDS buffer (50 mM HEPES, pH 7.7, 1 mM EDTA, 0.2% SDS, 1XPI) and each sample was divided into two aliquots treated with or without HA. The +HA sample contained HA prepared in water (pH 7.7) to a final concentration of 0.7 M, while the −HA sample was treated with an equivalent volume of buffer. HPDP-biotin was added to both samples to a final concentration of 1 mM. Samples were incubated for 60 min RT, and then HA and unincorporated HPDP-biotin were removed by chloroform-methanol precipitation of proteins. These protein precipitates were resuspended in −HA buffer. To purify biotinylated proteins, samples from the previous step were diluted five times with −HA/Triton buffer (50 mM HEPES, pH 7.7, 1 mM EDTA, 0.2% Triton-X, 1XPI) and incubated with rotation for 30 min at RT with Streptavidin Dynabeads M-280 (Invitrogen, Carlsbad, CA). The samples were incubated in 6× protein sample buffer (170 mM Tris pH 6.8, 36% glycerol, 4.5% SDS, 0.018% bromophenol blue, 2% β-mercaptoethanol (βME) at 70°C for 10 min to elute bound proteins from the magnetic beads.
2.5 Western Blotting
After protein samples were denatured at 70°C for 10 min under reducing conditions, proteins were resolved with 4–12% gradient 1D-SDS-PAGE (Invitrogen, Carlsbad, CA), and transferred to 0.45 µm nitrocellulose (or PVDF) membranes (Bio-Rad, Hercules, CA) using a Bio-Rad semi-dry transblotter. The membranes were blocked in LiCor blocking buffer (LiCor, Lincoln, NE) and then incubated overnight at 4°C in LiCor blocking buffer with 0.1% Tween 20 and the primary antibodies listed in Table 2. All antibodies were individually optimized to determine ideal conditions within the linear range of detection for each assay, and that the primary antibody was present in excess. After antibody incubations, the membranes were rinsed in phosphate-buffered saline with 0.1% Tween (PBST) and probed with IR-dye labeled secondary antibodies (Li-Cor; 1:10,000) for 1 h at RT. The membranes were rinsed again with PBST and then with deionized water. Immunoblots were scanned using a LiCor Odyssey near-infrared scanner using the Odyssey V3.0.16 software package.
Table 2.
Antibodies used for Western blot analysis
| Antibody | Species | Catalog # | Company |
|---|---|---|---|
| PSD-95 | Rabbit | 3450 | Cell Signaling (Danvers, MA) |
| Rap1A/Rap1B | Rabbit | 2399 | Cell Signaling (Danvers, MA) |
| Ras | Rabbit | 3965 | Cell Signaling (Danvers, MA) |
| VGLUT1 | Rabbit | 135302 | Synaptic Systems (Goettingen Germany) |
| EAAT1 | Rabbit | ab416 | Abcam (Cambridge, MA) |
| GABARγ2 | Goat | sc-131935 | Santa Cruz (Dallas, TX) |
| ZDHHC8 | Goat | NBP100-57849 | Novus Biologicals (Littleton, CO) |
| Calnexin | Rabbit | 208880 | EMD Millipore (Billerica, MA) |
| MBP | Mouse | SMI-99P | Covance (Princeton, NJ) |
2.6 Proteomic analysis
2.6.1 In-gel digestion
Samples from −HA and +HA eluates were resolved on 4–12% gradient 1D-SDS-PAGE gels (Invitrogen, Carlsbad, CA) and proteins visualized with SimplyBlue™ SafeStain (Invitrogen). Gel lanes for each sample were sequentially cut into a series of small sections to facilitate sample processing. Excess stain was removed from the gels sections with an overnight wash in 100 mM ammonium bicarbonate/acetonitrile (50:50). After destaining, disulfide bonds were reduced with 25 mM dithiothreitol at 50°C for 30 min; subsequently, free thiols group were alkylated with 55 mM iodoacetamide for 30 min in the dark. Excess alkylating agent was removed and the gel pieces were washed twice in 100 mM ammonium bicarbonate for 30 min. The gel pieces were evaporated to dryness in a Savant SpeedVac (ThermoScientific, Waltham, MA) before the addition of 12.5 ng/µl Trypsin Gold Mass Spectrometry Grade (Promega, Madison, WI) to each gel sample and incubated overnight at 37°C. Proteins were extracted from the gel pieces with 5% formic acid/ 50% aqueous acetonitrile (50:50) twice for 15 min each. Extracts were pooled and evaporated to dryness. The samples were then resuspended in 20 µl of 0.1% formic acid prior to mass spectrometry analysis.
2.6.2 Nano liquid chromatography-tandem mass spectrometry
An aliquot (4 µl) of each digest from the processed excised gel samples was loaded onto a Nano cHiPLC 200 µm × 0.5 mm ChromXP C18-CL 3 µm 120 Å reverse-phase trap cartridge (Eksigent, Dublin, CA, USA) at 2 ml/min using an Eksigent autosampler. After washing the cartridge for 4 min with 0.1% formic acid in ddH2O, the bound peptides were flushed onto a Nano cHiPLC column 75 µm × 15 cm ChromXP C18-CL 3 µm 120 Å (Eksigent, Dublin, CA, USA) with a 15 min linear (5–35%) acetonitrile gradient in 0.1% formic acid at 300 nl/min using an Eksigent Nano1D + LC (Eksigent, Dublin, CA, USA). The column was washed with 90% acetonitrile-0.1% formic acid for 5 min and then re-equilibrated with 5% acetonitrile—0.1% formic acid for 10 min. The Applied Biosystems 5600 Triple-Tof mass spectrometer (AB-Sciex, Toronto, Canada) was used to analyze the protein digest. The IonSpray voltage was 2300 V and the declustering potential was 60 V. Ionspray and curtain gases were set at 10 psi and 20 psi, respectively. The interface heater temperature was 120°C. Eluted peptides were subjected to a time-of-flight survey scan from 400–1250 m/z to determine the top twenty most intense ions for MS/MS analysis. Product ion time-of-flight scans at 50 msec were carried out to obtain the tandem mass spectra of the selected parent ions over the range from m/z 400–2000. Spectra were centroided and de-isotoped by Analyst software, version TF (Applied Biosystems). A β-galactosidase trypsin digest was used to establish and confirm the mass accuracy of the mass spectrometer. In-house MASCOT database searches were carried out against the human genome on the UniProt database. The mass tolerances for precursor scans and MS/MS scans were set at 0.05 Daltons. One missed cleavage for trypsin was allowed. A fixed modification of carbamidomethylation was set for cysteine residues, and a variable mediation of oxidation was allowed for methionine residues.
2.7 Quantification of protein palmitoylation
2.7.1 Global protein palmitoylation
Humans and rat brain samples were assayed using the ABE methodology, where each of the biotinylated +HA and −HA samples were resuspended in −HA buffer, mixed with equal volumes of 6× protein sample buffer without β-mercaptoethanol, heated at 70°C for 10 min, and sample eluates were resolved on a gradient 1D-SDS-PAGE gel (Invitrogen, Carlsbad, CA). Proteins were then transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA); membranes were blocked in LiCor blocking buffer (LiCor, Lincoln, NE) and then incubated with IR-dye labeled streptavidin (Li-Cor; 1:5,000) for 1h at RT. The membranes were rinsed three times for 15 min with PBST, briefly with deionized water, and then scanned. Blots were then reprobed with an anti-PSD-95 monoclonal antibody overnight at 4°C.
Palmitoylation of proteins was quantified by measuring intensity of biotinylated proteins within 17 rectangles associated with bands of discrete molecular weights for each human subject, and within 16 discrete bands for each rat. Biotinylation intensity values within each rectangle used to determine palmitoylation status were normalized to the protein expression of total PSD-95 (input PSD-95) within each lane. These samples were not purified with streptavidin beads, therefore contained proteins that were both palmitoylated and un12 palmitoylated. The input PSD-95 values were also used to normalize palmitoylated PSD-95 on a separate set of blots; palmitoylated PSD-95 was purified with streptavidin beads along with other biotinylated/palmitoylated proteins. The normalized −HA and +HA data were used to calculate the fold increase (calculated as the ratio of intensity of bands in the +HA/−HA lanes) of protein biotinylation in schizophrenia and comparison subjects, as well as in haloperidol vs. vehicle treated rats.
2.7.2 Statistical analysis
Statistical analyses were performed using the Statistica software package (Statsoft, Tulsa, OK). All dependent measures passed the D'Agostino-Pearson normality test. No correlations were found between age, PMI and tissue pH and any dependent measures. Data were analyzed with two-tailed Student’s paired t-tests for human data, and unpaired t-tests for rat data. For all statistical tests, α= 0.05.
3. Results
3.1 Palmitoyl-proteins in human frontal cortex
To characterize and validate the palmitoylation assay, we first identified the population of candidate proteins that are palmitoylated in human frontal cortex using mass spectrometry to explore this modification in an unbiased fashion. We purified proteins that are selectively biotinylated in homogenates of human prefrontal cortex after ABE reactions with or without (+/−) HA on streptavidin-conjugated magnetic beads. Following elution from the beads and sample processing, proteins were identified using nanoscale liquid chromatography-tandem mass spectrometry (nano LC-MS/MS). All candidate palmitoyl-proteins were identified through analysis of the combined data sets of proteins identified in negative control (−HA) samples and in biotinylated samples (+HA). Proteins by MS in the −HA lanes were subtracted from the +HA lane, resulting in a total working list of 380 palmitoylated proteins in human DLFPC. Based on previously suggested criteria for the mouse brain palmitoproteome (Kang et al., 2008), we removed from the list likely false-positives. The sequence of each remaining palmitoyl-target protein was verified for presence of palmitoylatable cysteine residues with CSS-Palm 3.0 prediction program (http://csspalm.biocuckoo.org)(Ren et al., 2008); candidates not containing any Cys residues are predicted to not be palmitoylated and were removed from the list as additional false positives, resulting in a final working list of 219 candidate palmitoyl-proteins. Consistent with previous findings in rodent brain (Kang et al., 2008), these palmitoylated proteins cluster into 7 groups: 1) receptors, channels, and transporters; 2) neuronal proteins; 3) small GTPases; 4) membrane associated proteins; 5) cell adhesion and cytoskeletal proteins; 6) myelin associated proteins; and 7) metabolic proteins and enzymes. A complete list of these 219 proteins can be found in the supplemental table.
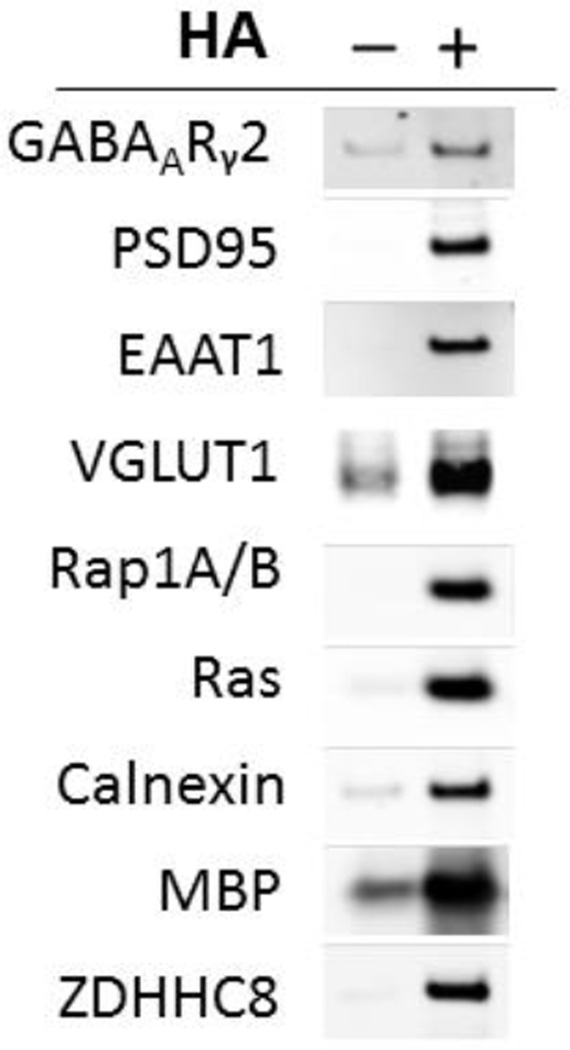
As a next step to characterize the ABE assay, we determined if we could detect palmitoylated forms of proteins that have been previously reported as substrates for this posttranslational modification. Proteins from −HA and +HA treated samples following affinity-purification of biotinylated proteins on streptavidin-magnetic beads were separated on SDS-PAGE and probed with antisera specific to GABAARγ2, PSD-95, EAAT1, Rap1A/B, Ras, calnexin, myelin basic protein (MBP), and ZDHHC8 in +HA samples, consistent with each being endogenously palmitoylated in human brain as has been previously reported (Choy et al., 1999; Fukata and Fukata, 2010; Kang et al., 2008; Keller et al., 2004; Rathenberg et al., 2004; Yang et al., 2010) (Figure 2). We also found that VGLUT1 is palmitoylated (Figure 2).
Figure 2. Confirmation of palmitoylation of selected candidate palmitoyl-proteins in human brain.
Western blotting was performed after ABE assay of palmitoylation status in human brain for selected proteins previously reported to be palmitoylated, including GABAARγ2, PSD-95, EAAT1, VGLUT1, Rap1A/B, Ras, Calnexin, myelin basic protein (MBP), and ZDHHC8. As can be seen by substantial enrichment in the +HA lanes, all of these proteins are palmitoylated.
3.2 Decreased protein S-palmitoylation in schizophrenia
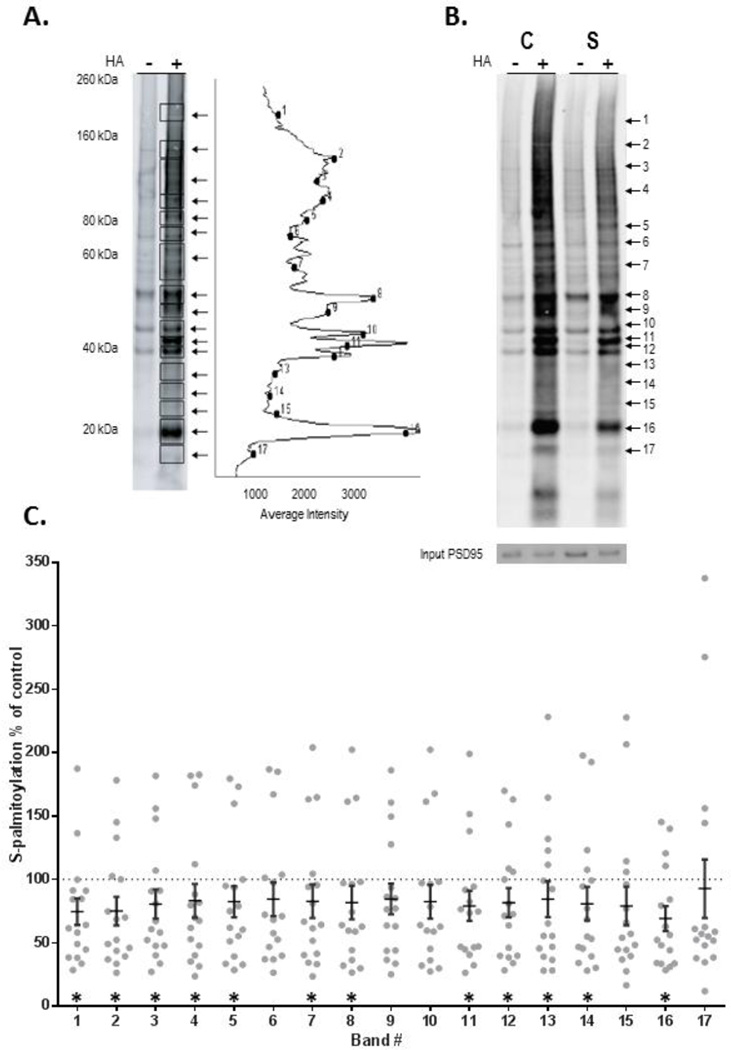
We compared S-palmitoylation of proteins in patients with schizophrenia and pair-matched comparison subjects. We measured intensity of biotinylation of proteins within seventeen rectangles associated with palmitoylation of proteins of discrete molecular masses from the −HA and +HA treated samples for each of the schizophrenia and paired comparison subjects, following the ABE assay and Western blotting with IR-dye labeled streptavidin (Figure 3). Secondary unpaired analyses resulted in similar results, with palmitoylation in all but one molecular mass band (15) significantly decreased. Biotinylation levels of proteins within these rectangles were normalized to expression of PSD-95 detected by Western blotting in each sample. Neither the expression of input PSD-95 (total) (t=1.3, p=n.s.), or of palmitoylated PSD-95 (t=1.0, p=n.s.) were changed between groups. We found an approximately 20% decrease of biotinylation of proteins of most molecular mass clusters in schizophrenia (Figure 3).
Figure 3. Decreased protein palmitoylation in schizophrenia DLPFC.
ABE assay and western blotting were used to quantify protein palmitoylation in DLPFC in schizophrenia. A) Palmitoylation of proteins was analyzed by measuring intensity of biotinylation of proteins within seventeen bands representing palmitoylated-proteins of different molecular weights.
B) Representative western blots from a paired schizophrenia (S) and comparison subject (C) probed with streptavidin and PSD-95.
C) Biotinylation levels of proteins within each of the rectangles were normalized to expression of input PSD-95 present in each sample, detected by subsequent western blotting. Normalized −HA and +HA data were used to calculate fold increase (+HA/−HA ratios) in protein biotinylation within the seventeen +HA samples compared to the corresponding −HA samples for each subject pair (N=16 pairs). Data are presented as the ratio of palmitoylation in each schizophrenia case divided by the matched comparison subject for each molecular weight band for each subject pair, with means ± SEM overlayed. *p<0.05
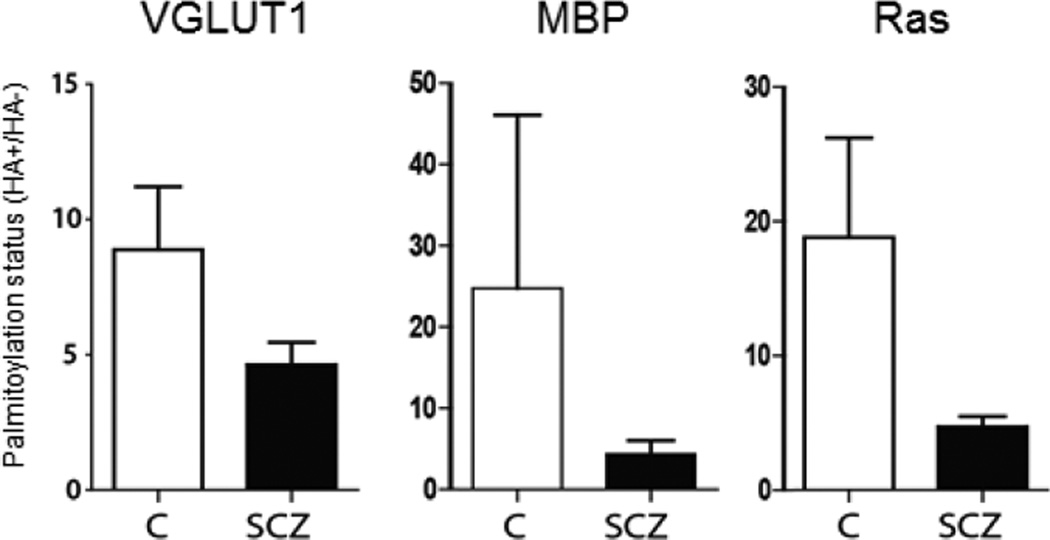
We next sought to determine if S-palmitoylation was changed in several key candidate proteins known to be palmitoylated (Figure 4). Decreased palmitoylation of VGLUT1, Ras and MBP were found, consistent with the global deficit of protein palmitoylation we observed in schizophrenia.
Figure 4. Protein palmitoylation of candidate proteins in schizophrenia.
ABE assay and western blotting were used in schizophrenia (SCZ) and comparison (C) subjects, to quantify protein palmitoylation of key proteins known to be palmitoylated, VGLUT1, MBP and Ras. Palmitoylation of each protein was decreased, consistent with the pattern of global decrease of protein palmitoylation in schizophrenia.
3.3 Lack of effect of antipsychotic treatment on palmitoylation
To determine if this change in protein palmitoylation is associated with chronic antipsychotic exposure, we conducted parallel assays in cortical samples from rats following long-term treatment with haloperidol. Protein palmitoylation in haloperidol-treated rats was unchanged from control animals (Figure 5). As in the human experiments, PSD-95 was used for normalization: antipsychotic treatment did not affect either input PSD-95 (t=0.04, p=n.s.) or palmitoylation of PSD-95 (t=0.54, p=n.s.) in these rats. We also performed a secondary analysis of medication status for the schizophrenia (receiving antipsychotic medications at the time of death vs. drug-free for six weeks or more at the time of death). We did not find any effect of antipsychotic treatment on palmitoylation of proteins in schizophrenia in this post-hoc analysis.
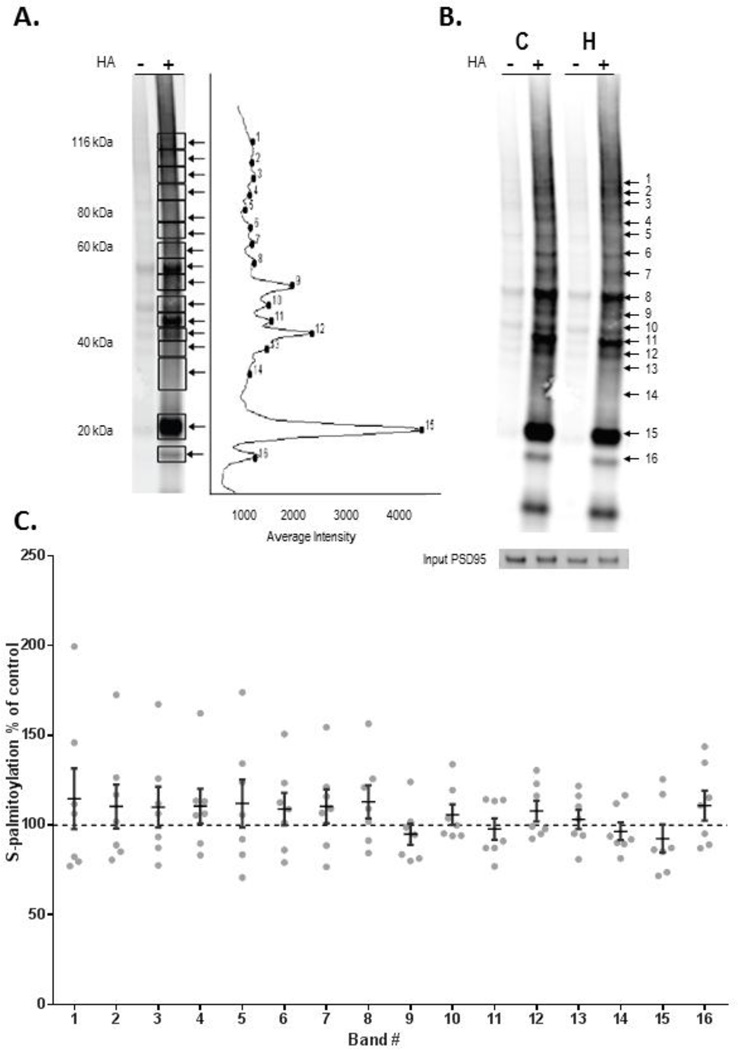
Figure 5. Protein palmitoylation is not affected by chronic antipsychotic treatment.
Rats treated for 9 months with haloperidol decanoate did not exhibit changes in palmitoylation, suggesting that decreased palmitoylation in schizophrenia is not likely due to antipsychotic treatment. ABE assay and western blotting were used to quantify protein palmitoylation in rat frontal cortex. A) Palmitoylation of proteins was analyzed by measuring intensity of biotinylation of proteins within sixteen bands representing palmitoylated-proteins of different molecular weights. B) Representative western blots probed with streptavidin and PSD-95 from haloperidol (H) and vehicle (C) treated rats.
C) Biotinylation levels of proteins within each of the rectangles were normalized to expression of PSD-95 present in each sample. Data are presented as the ratio of palmitoylation in frontal cortex from rats treated with haloperidol decanoate divided by values from control rats for each molecular weight band with means ± SEM overlayed. For no bands were there significant differences in palmitoylation between vehicle and haloperidol treated animals.
3.4 No effect of postmortem interval on palmitoylation
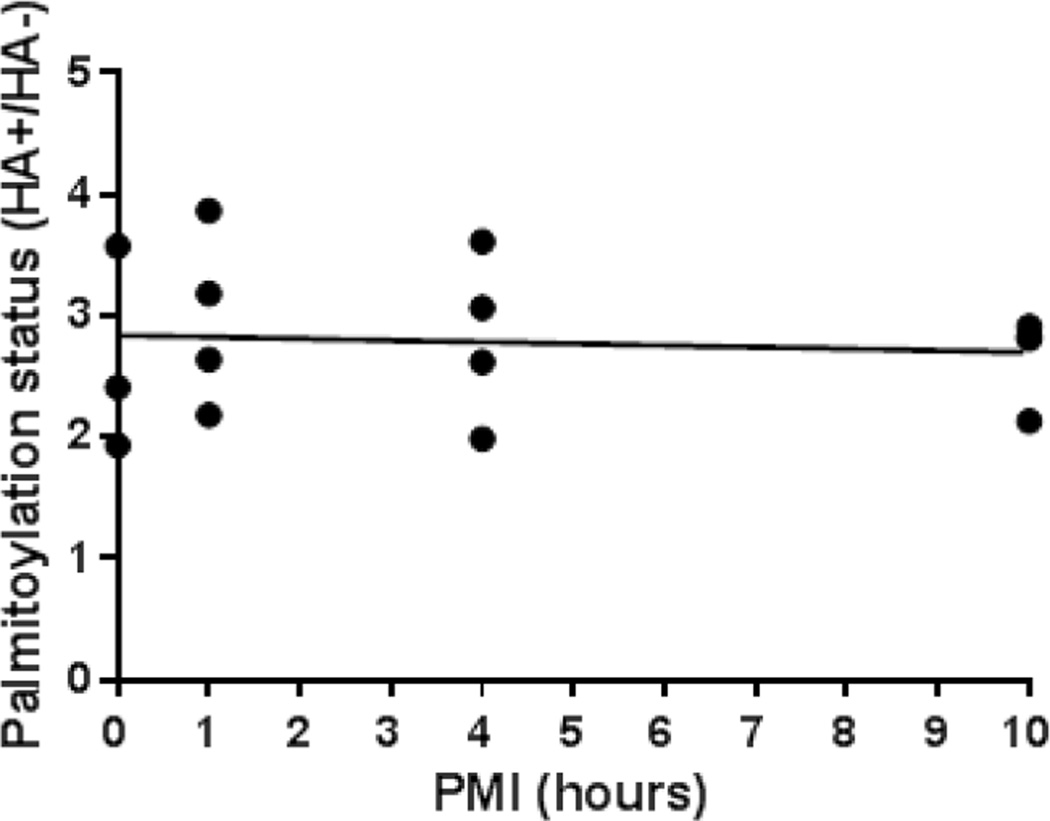
To determine if PMI had an effect on palmitoylation, we modelled delays from time of death to freezer storage time typical of human autopsy. We used the ABE assay to measure protein palmitoylation in frontal cortex from mice at PMIs at 0, 1, 4 and 10 hours. PMI did not affect palmitoylation status (Figure 6), suggesting that the decreased palmitoylation observed in schizophrenia is not substantially affected by PMI.
Figure 6. Protein palmitoylation is not affected by postmortem interval.
Frontal cortex from brains of mice held at 4°C for 0, 1, 4, and 10 hours postmortem did not affect protein palmitoylation status, suggesting that palmitoylation changes in schizophrenia are not affected by PMI. Palmitoylation status was determined from duplicate samples from each animal of total lane biotinylation intensity values normalized to an intralane loading control (PSD-95). Each dot represents the mean of duplicate samples from each animal. Spearman ρ=0.20, p=n.s.
4. Discussion
Palmitoylation is a posttranslational modification (PTM) that plays a key role in subcellular protein localization by increasing protein hydrophobicity and thereby targeting proteins to membranes; its unique reversibility regulates intracellular membrane interactions, as well as stabilizing proteins at the synapse or other subcellular compartments (Fukata and Fukata, 2010). In this study, we modified an Acyl-Biotinyl-Exchange (ABE) technique (Drisdel and Green, 2004; Kang et al., 2008; Wan et al., 2007) to assay S-palmitoylation of proteins in human postmortem brain (Figure 1). Using mass spectrometry, we identified 219 new and known palmitoylated candidate proteins. We then assayed protein S-palmitoylation in DLPFC in paired schizophrenia and comparison subjects, and found in schizophrenia a 20% decrease in palmitoylation across proteins of most molecular masses measured. Parallel studies in rodents treated chronically with haloperidol decanoate revealed that this drug did not change protein palmitoylation, suggesting that our findings in schizophrenia are not a medication effect. Thus, this widespread decrease in protein palmitoylation may be associated with the alterations of trafficking or stability of membrane proteins involved in neurotransmission or cell signaling events that have been hypothesized to be aspects of the pathophysiology of schizophrenia (Lewis and Gonzalez-Burgos, 2008). Abnormal palmitoylation can affect neurotransmission or cell signaling via altered protein localization. For example, PSD-95 palmitoylation stabilizes AMPA receptors at the synapse, while glutamate receptor activity triggers internalization of AMPA receptors by depalmitoylation of PSD-95 (El-Husseini et al., 2002). Since S-palmitoylation of AMPA and NMDA receptor subunits can regulate their trafficking, recycling and function (Yang et al., 2009), altered palmitoylation status is consistent with abnormalities of early endosomal expression of GluA1 (Hammond et al., 2010), as well as abnormal subcellular location of NR2B (Kristiansen et al., 2010) that we have previously reported in schizophrenia. Alterations in receptor localization in the cell may contribute to dysregulated excitatory synaptic transmission in schizophrenia.
A number of the palmitoyl-proteins identified by mass spectrometry in this study can be broadly classified as integral or peripheral membrane proteins. Integral membrane proteins such as G protein-coupled receptors (GPCR) are affected by palmitoylation in a receptor-dependent manner; constitutive palmitoylation is involved with dimerization and targeting of GPCRs to their terminal locations, where palmitoylation also can influence GPCR mediated signaling (Bulenger et al., 2005; Goddard and Watts, 2012; Qanbar and Bouvier, 2003). Considerable evidence has implicated abnormal GPCR expression and regulation in schizophrenia; including dysregulation of dopaminergic, serotonergic, glutamatergic, and adrenergic receptors (Catapano and Manji, 2007). Given the roles palmitoylation plays in GPCR function (Chini and Parenti, 2009), multiple neurotransmitter pathways could be implicated by decreased cellular levels of protein palmitoylation.
Mass spectrometry also identified palmitoylated peripheral membrane proteins associated with transient protein-protein interactions. Examples of these proteins include the α-subunits of heterotrimeric G-proteins (Gα) (Linder et al., 1993) and GTPases such as cell division cycle 42 (CDC42) (Kang et al., 2008), both of which have been reported to be abnormally expressed in schizophrenia (Gilks et al., 2012; Ide and Lewis, 2010). Increased expression of Gα and increased phosphoinositide signaling have been found in schizophrenia frontal cortex (Jope et al., 1998). Gα subunits require lipid modification by N-myristoylation and/or palmitoylation for membrane targeting and protein interactions (Chen and Manning, 2001), and altered palmitoylation may be associated with these findings of Gα abnormalities.
The mechanisms driving this significant change in palmitoylation in schizophrenia are not known. Lipid modifications by N-terminal myristoylation and/or C-terminal prenylation frequently precede and may even be required for S-palmitoylation to occur (Aicart-Ramos et al., 2011). The pathways involved with these lipid modifications may be abnormal and underlie the palmitoylation findings we now report in schizophrenia. We previously reported that protein expressions of the key enzymes responsible for N-myristoylation are not changed in schizophrenia (Pinner et al., 2014). We hypothesize that prenylation abnormalities may contribute to palmitoylation deficits in schizophrenia. The palmitoylation/depalmitoylation cycle is mediated not only by its constitutive enzymes and receptor activation, but can also be influenced by other cell signaling events through S-nitrosylation. Evidence for a competitive relationship between S-palmitoylation and S-nitrosylation was first suggested when the presence of NO donors decreased palmitoylation of the neuronal growth cone proteins GAP- 43 and SNAP-25, resulting in growth cone collapse. A similar collapse was seen after use of an inhibitor of palmitoylation (Hess et al., 1993). It is likely that other posttranslational modifications are dysregulated in schizophrenia, and defining these and elucidating associated mechanisms is a current area of active study.
A reduction in number of dendritic spines on pyramidal cells has been reported in schizophrenia brain (Glausier and Lewis, 2013). Interestingly, spine density and number of excitatory synaptic networks are decreased by reduced palmitoyltransferase activity of ZDHHC8 in a mouse model of the human 22q11 syndrome (Mukai et al., 2015), particularly relevant given that 22q11 increases the risk of schizophrenia 20 to 30-fold (Drew et al., 2011). Alterations of spine morphology found in ZDHHC8-deficient neurons are thought to be mediated by one of its substrates, CDC42 (Mukai et al., 2015). CDC42 is a Rho GTPase associated with dendritic morphogenesis and implicated in the dendritic spine pathology found in schizophrenia (Glausier and Lewis, 2013). Expression of CDC42 in the dendritic spine is associated with its palmitoylation status and exogenous glutamate levels; in cortical neurons, CDC42 is rapidly depalmitoylated and redistributes from dendritic spines in response to high levels of exogenous glutamate, resulting in spine collapse (Kang et al., 2008). Less palmitoylation of proteins involved in the formation of neuronal processes and spines may not only contribute to loss of pyramidal neuron dendritic spines, but also alter the maturation of excitatory and inhibitory synapses (El-Husseini et al., 2000b; Schweizer et al., 2003), which in turn could be linked to cognitive deficits in schizophrenia (Glausier and Lewis, 2013). Palmitoylation has also been found to be critical for sorting to the myelin membrane in oligodendrocyte cultures (Schneider et al., 2005). Accordingly, abnormal palmitoylation could be associated with the downregulation of myelin basic protein previously observed in schizophrenia (Martins-de-Souza et al., 2009) and contribute to oligodendroglial abnormalities implicated in schizophrenia (Davis et al., 2003; McCullumsmith et al., 2007).
Our characterization of the human palmitoyl-proteome resulted in a list of palmitoylated proteins that overlap with a previous study of protein palmitoylation in rat (Kang et al., 2008). While there are differences in proteins noted in our study and this earlier work, the most likely explanation for these differences is one of sample composition. Our study was conducted in homogenized human frontal cortex, which includes all cell types: glia, neurons, and extracellular proteins. Data in the earlier rat characterization were generated specifically from cultured rat embryonic cortical neurons and synaptosomal membrane fractions extracted from rat brain (Kang et al., 2008). Despite these substantial differences in starting material, there is significant overlap in the list of proteins identified in these studies.
Postmortem brain studies in schizophrenia have common limitations. Our subjects were aged and these results may not generalize to younger subjects. This study was performed in DLPFC and it is not yet known if these changes are region-specific. The effects of chronic antipsychotic treatment are a confound in these types of studies. Given that rats chronically treated with haloperidol did not exhibit changes in S-palmitoylation, the finding of decreased S-palmitoylation in schizophrenia is likely not due to antipsychotic treatment.
These data reflect changes in the extent of S-palmitoylation of many proteins in the frontal cortex in schizophrenia. Given the roles of functional effects for this lipid modification, these data suggest a potential mechanism underlying previous observations of abnormal intracellular targeting and subcellular location of cell surface expressed proteins in this illness.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr. Rosalinda Roberts and the Alabama Brain Bank for providing materials used to optimize conditions used in this study. The authors would also like to thank generosity and time of Dr. Michelle Olsen, Leanne Holt, and Madeline Scott for the mice used in the PMI study. Last, but not least, the authors thank Stefani Yates for her excellent technical expertise.
ROLE OF THE FUNDING
This work was funded by the National Institutes of Health Grants MH53327 (JHMW), MH064673 and MH066392 (VH), and MH094445 (REM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest.
CONTRIBUTORS
JT and JHMW designed the study. REM provided oversight of the animal experiments, and JHMW provided oversight of the human studies. JT and ALP performed the experiments, statistical analyses, and wrote initial drafts of the manuscript. VH provided the human tissue. All authors contributed to and have approved the final manuscript.
References
- Aicart-Ramos C, Valero RA, Rodriguez-Crespo I. Protein palmitoylation and subcellular trafficking. Biochimica et biophysica acta. 2011;1808(12):2981–2994. doi: 10.1016/j.bbamem.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Antinone SE, Ghadge GD, Lam TT, Wang L, Roos RP, Green WN. Palmitoylation of superoxide dismutase 1 (SOD1) is increased for familial amyotrophic lateral sclerosis-linked SOD1 mutants. The Journal of biological chemistry. 2013;288(30):21606–21617. doi: 10.1074/jbc.M113.487231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophrenia research. 2010;117(1):92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse. 2006;60(8):585–598. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends in pharmacological sciences. 2005;26(3):131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Butland SL, Sanders SS, Schmidt ME, Riechers SP, Lin DT, Martin DD, Vaid K, Graham RK, Singaraja RR, Wanker EE, Conibear E, Hayden MR. The palmitoyl acyltransferase HIP14 shares a high proportion of interactors with huntingtin: implications for a role in the pathogenesis of Huntington's disease. Human molecular genetics. 2014;23(15):4142–4160. doi: 10.1093/hmg/ddu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catapano LA, Manji HK. G protein-coupled receptors in major psychiatric disorders. Biochimica et biophysica acta. 2007;1768(4):976–993. doi: 10.1016/j.bbamem.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene. 2001;20(13):1643–1652. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- Chini B, Parenti M. G-protein-coupled receptors, cholesterol and palmitoylation: facts about fats. Journal of molecular endocrinology. 2009;42(5):371–379. doi: 10.1677/JME-08-0114. [DOI] [PubMed] [Google Scholar]
- Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, Philips MR. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98(1):69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- Conibear E, Davis NG. Palmitoylation and depalmitoylation dynamics at a glance. Journal of cell science. 2010;123(Pt 23):4007–4010. doi: 10.1242/jcs.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M, Keefe RS. Cognitive impairment as a target for pharmacological treatment in schizophrenia. Schizophrenia research. 1995;17(1):123–129. doi: 10.1016/0920-9964(95)00037-m. [DOI] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Archives of general psychiatry. 2003;60(5):443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- Dejanovic B, Semtner M, Ebert S, Lamkemeyer T, Neuser F, Luscher B, Meier JC, Schwarz G. Palmitoylation of gephyrin controls receptor clustering and plasticity of GABAergic synapses. PLoS biology. 2014;12(7):e1001908. doi: 10.1371/journal.pbio.1001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B, Mukai J, Fenelon K, Hsu PK, Gogos JA, Karayiorgou M. The 22q11.2 microdeletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2011;29(3):259–281. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisdel RC, Green WN. Labeling and quantifying sites of protein palmitoylation. BioTechniques. 2004;36(2):276–285. doi: 10.2144/04362RR02. [DOI] [PubMed] [Google Scholar]
- Drummond JB, Tucholski J, Haroutunian V, Meador-Woodruff JH. Transmembrane AMPA receptor regulatory protein (TARP) dysregulation in anterior cingulate cortex in schizophrenia. Schizophrenia research. 2013;147(1):32–38. doi: 10.1016/j.schres.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Craven SE, Chetkovich DM, Firestein BL, Schnell E, Aoki C, Bredt DS. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. The Journal of cell biology. 2000a;148(1):159–172. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000b;290(5495):1364–1368. [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108(6):849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- El-Husseini AED, Bredt DS. Protein palmitoylation: A regulator of neuronal development and function. Nature Reviews Neuroscience. 2002;3(10):791–802. doi: 10.1038/nrn940. [DOI] [PubMed] [Google Scholar]
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(49):12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nature reviews. Neuroscience. 2010;11(3):161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- Funk AJ, Rumbaugh G, Harotunian V, McCullumsmith RE, Meador-Woodruff JH. Decreased expression of NMDA receptor-associated proteins in frontal cortex of elderly patients with schizophrenia. Neuroreport. 2009;20(11):1019–1022. doi: 10.1097/WNR.0b013e32832d30d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks WP, Hill M, Gill M, Donohoe G, Corvin AP, Morris DW. Functional investigation of a schizophrenia GWAS signal at the CDC42 gene. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2012;13(7):550–554. doi: 10.3109/15622975.2012.666359. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard AD, Watts A. Regulation of G protein-coupled receptors by palmitoylation and cholesterol. BMC biology. 2012;10:27. doi: 10.1186/1741-7007-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Evidence for abnormal forward trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(10):2110–2119. doi: 10.1038/npp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte MK, Bachus SB, Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophrenia research. 2005;75(2–3):303–308. doi: 10.1016/j.schres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Davidson M, Powchik P, Parrella M, White L, Mohs RC. Assessment of dementia in elderly schizophrenics with structured rating scales. Schizophrenia research. 1992;7(1):85–90. doi: 10.1016/0920-9964(92)90078-j. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47(5):709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009;64(2):213–226. doi: 10.1016/j.neuron.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DT, Patterson SI, Smith DS, Skene JH. Neuronal growth cone collapse and inhibition of protein fatty acylation by nitric oxide. Nature. 1993;366(6455):562–565. doi: 10.1038/366562a0. [DOI] [PubMed] [Google Scholar]
- Huang K, El-Husseini A. Modulation of neuronal protein trafficking and function by palmitoylation. Current opinion in neurobiology. 2005;15(5):527–535. doi: 10.1016/j.conb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Ide M, Lewis DA. Altered cortical CDC42 signaling pathways in schizophrenia: implications for dendritic spine deficits. Biological psychiatry. 2010;68(1):25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, Song L, Grimes CA, Pacheco MA, Dilley GE, Li X, Meltzer HY, Overholser JC, Stockmeier CA. Selective increases in phosphoinositide signaling activity and G protein levels in postmortem brain from subjects with schizophrenia or alcohol dependence. Journal of neurochemistry. 1998;70(2):763–771. doi: 10.1046/j.1471-4159.1998.70020763.x. [DOI] [PubMed] [Google Scholar]
- Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO, Thompson JX, Roth AF, Drisdel RC, Mastro R, Green WN, Yates JR, 3rd, Davis NG, El-Husseini A. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 2008;456(7224):904–909. doi: 10.1038/nature07605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashihara K, Sato M, Fujiwara Y, Harada T, Ogawa T, Otsuki S. Effects of intermittent and continuous haloperidol administration on the dopaminergic system in the rat brain. Biological psychiatry. 1986;21(7):650–656. doi: 10.1016/0006-3223(86)90126-5. [DOI] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(26):5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Patel SA, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. 2010;64(7):495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(1):141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nature reviews. Molecular cell biology. 2007;8(1):74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- Linder ME, Middleton P, Hepler JR, Taussig R, Gilman AG, Mumby SM. Lipid modifications of G proteins: alpha subunits are palmitoylated. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-de-Souza D, Gattaz WF, Schmitt A, Maccarrone G, Hunyadi-Gulyas E, Eberlin MN, Souza GH, Marangoni S, Novello JC, Turck CW, Dias-Neto E. Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia. Journal of psychiatric research. 2009;43(11):978–986. doi: 10.1016/j.jpsychires.2008.11.006. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Gupta D, Beneyto M, Kreger E, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophrenia research. 2007;90(1–3):15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Hamel LD, Reddy KD, Farh L, Rettew LM, Sanchez PR, Deschenes RJ. Mutations in the X-linked intellectual disability gene, zDHHC9, alter autopalmitoylation activity by distinct mechanisms. The Journal of biological chemistry. 2014;289(26):18582–18592. doi: 10.1074/jbc.M114.567420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ. Protein palmitoylation by a family of DHHC protein S-acyltransferases. Journal of lipid research. 2006;47(6):1118–1127. doi: 10.1194/jlr.R600007-JLR200. [DOI] [PubMed] [Google Scholar]
- Mueller TM, Haroutunian V, Meador-Woodruff JH. N-Glycosylation of GABAA receptor subunits is altered in Schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(3):528–537. doi: 10.1038/npp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, Karayiorgou M, Gogos JA. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nature neuroscience. 2008;11(11):1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai J, Liu H, Burt RA, Swor DE, Lai WS, Karayiorgou M, Gogos JA. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nature genetics. 2004;36(7):725–731. doi: 10.1038/ng1375. [DOI] [PubMed] [Google Scholar]
- Mukai J, Tamura M, Fenelon K, Rosen AM, Spellman TJ, Kang R, MacDermott AB, Karayiorgou M, Gordon JA, Gogos JA. Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia. Neuron. 2015;86(3):680–695. doi: 10.1016/j.neuron.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinner AL, Haroutunian V, Meador-Woodruff JH. Alterations of the myristoylated, alanine-rich C kinase substrate (MARCKS) in prefrontal cortex in schizophrenia. Schizophrenia research. 2014;154(1–3):36–41. doi: 10.1016/j.schres.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP, Harvey PD, Davis KL. Postmortem studies in schizophrenia. Schizophrenia bulletin. 1998;24(3):325–341. doi: 10.1093/oxfordjournals.schbul.a033330. [DOI] [PubMed] [Google Scholar]
- Purohit DP, Davidson M, Perl DP, Powchik P, Haroutunian VH, Bierer LM, McCrystal J, Losonczy M, Davis KL. Severe cognitive impairment in elderly schizophrenic patients: a clinicopathological study. Biological psychiatry. 1993;33(4):255–260. doi: 10.1016/0006-3223(93)90291-k. [DOI] [PubMed] [Google Scholar]
- Qanbar R, Bouvier M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacology & therapeutics. 2003;97(1):1–33. doi: 10.1016/s0163-7258(02)00300-5. [DOI] [PubMed] [Google Scholar]
- Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Molecular and cellular neurosciences. 2004;26(2):251–257. doi: 10.1016/j.mcn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Ren J, Wen LP, Gao XJ, Jin CJ, Xue Y, Yao XB. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein Eng Des Sel. 2008;21(11):639–644. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Lander H, Schulz G, Wolburg H, Nave KA, Schulz JB, Simons M. Palmitoylation is a sorting determinant for transport to the myelin membrane. Journal of cell science. 2005;118(Pt 11):2415–2423. doi: 10.1242/jcs.02365. [DOI] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Luscher B. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Molecular and cellular neurosciences. 2003;24(2):442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Smotrys JE, Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annual review of biochemistry. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophrenia research. 2013a;146(1–3):177–183. doi: 10.1016/j.schres.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucholski J, Simmons MS, Pinner AL, McMillan LD, Haroutunian V, Meador-Woodruff JH. N-linked glycosylation of cortical N-methyl-D-aspartate and kainate receptor subunits in schizophrenia. Neuroreport. 2013b;24(12):688–691. doi: 10.1097/WNR.0b013e328363bd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: purification and identification. Nature protocols. 2007;2(7):1573–1584. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- Yang G, Xiong W, Kojic L, Cynader MS. Subunit-selective palmitoylation regulates the intracellular trafficking of AMPA receptor. The European journal of neuroscience. 2009;30(1):35–46. doi: 10.1111/j.1460-9568.2009.06788.x. [DOI] [PubMed] [Google Scholar]
- Yang W, Di Vizio D, Kirchner M, Steen H, Freeman MR. Proteome scale characterization of human S-acylated proteins in lipid raft-enriched and non-raft membranes. Molecular & cellular proteomics : MCP. 2010;9(1):54–70. doi: 10.1074/mcp.M800448-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.