Summary
Microbial infections are a major cause of infant mortality as a result of limitations in immune defences. Interleukin‐27 (IL‐27) is a heterodimeric cytokine produced primarily by leucocytes and is immunosuppressive toward lymphocytes and leucocytes. Our laboratory demonstrated that human neonatal macrophages express IL‐27 more abundantly than adult macrophages. Similarly in mice, IL‐27 expression is elevated early in life and maintained through infancy. To determine IL‐27‐regulated mechanisms that may limit immunity, we evaluated the expression of a number of genes in response to this cytokine in primary human neonatal macrophages. Indoleamine 2,3‐dioxygenase (IDO) gene expression was increased dose‐responsively by IL‐27. We have previously demonstrated inhibition of T‐cell proliferation and cytokine production by neonatal macrophage‐generated IL‐27, and IDO is often implicated in this negative regulation. An increase in IDO protein was demonstrated by immunofluorescence microscopy and was consistent with increased enzyme activity following treatment with IL‐27. Inclusion of a soluble receptor to neutralize endogenous IL‐27, decreased IDO expression and activity compared with untreated macrophages. In response to IL‐27, neonatal macrophages phosphorylate signal transdcuer and activator of transcription 1 (STAT‐1) and STAT‐3. Both transcription factors are recruited to the IDO regulatory region. STAT‐3 dominates during steady‐state regulation by lower levels of endogenous IL‐27 production. A shift to enhanced STAT‐1 recruitment occurs during increased levels of exogenously supplied IL‐27. These data suggest an interesting interplay of STAT‐1 and STAT‐3 to regulate IDO activity and immunosuppression in response to different levels of IL‐27 in the microenvironment of the immune response that may further our understanding of this interesting cytokine.
Keywords: cytokines, gene regulation, human, signal transduction, transcription factors
Introduction
Young infant mortality remains a major concern. Nearly 5 million deaths occurred within the first year of life worldwide in 2015.1 Approximately 26% of neonatal mortality is the result of infection.2, 3 This susceptibility in early life is generally considered to be reflective of differences in innate and adaptive immune function compared with adults. We previously reported differences in expression of the immune suppressive cytokine interleukin‐27 (IL‐27) between neonatal and adult macrophages.4 However, our understanding of how heightened production of IL‐27 may influence the neonatal macrophages that produce it, as well as other immune cells, has remained incomplete.
Interleukin‐27 is a heterodimeric cytokine composed of the subunits p28 and Epstein–Barr virus‐induced gene 3 (EBI3).5 EBI3 is expressed from a single gene.6 Interleukin‐27 is a pleiotropic cytokine that has both inflammatory and immune suppressive activity. The original description of IL‐27 identified a role for this cytokine in differentiation of naive T cells to a T helper type 1 (Th1) phenotype.7 This was accompanied by the observation that IL‐27 augmented IL‐12‐induced interferon‐γ (IFN‐γ) production from these lymphocytes.5 Interleukin‐27 has also been shown to activate expression of signal transducer and activator of transcription 1 (STAT‐1) ‐dependent inflammatory gene targets in human monocytes.8 Additional inflammatory activity includes antiviral properties in CD4+ T cells, macrophages and dendritic cells.9, 10, 11, 12 Since its early identification, IL‐27 has also been shown to oppose inflammation in animal models of infectious and chronic disease.13, 14, 15, 16, 17, 18, 19 This includes immune suppressive activity toward innate immune cells and lymphocyte subsets.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Our laboratory and others have demonstrated suppressive activity toward human macrophages that limits inflammatory cytokine production, inflammatory cytokine receptor expression and signalling, and lysosomal acidification with the consequence of compromising control of Mycobacterium tuberculosis, Staphylococcus aureus and Pseudomonas aeruginosa.4, 21, 22, 23, 24, 25, 26
The IL‐27 genes were recently shown to be expressed by neonatal macrophages at an elevated level compared with adult counterparts.4 Both IL‐27 gene and protein expression were further shown to remain elevated through infancy in mice with a reduction in levels as they progressed into adulthood.4 Neutralization of IL‐27 increased IFN‐γ production by neonatal T cells in mixed leucocyte reactions.4 Collectively, these data suggest an important role for IL‐27 in the regulation of immunity during early life. In the work presented here, we further probed the impact of IL‐27 on neonatal macrophage immunobiology and found that this cytokine specifically regulated expression of indoleamine 2,3‐dioxygenase (IDO). The activity of IDO is responsible for the rate‐limiting step in tryptophan catabolism and has been shown to promote immunological tolerance through the suppression of T‐cell responses (reviewed in ref. 27). Indeed, IDO has been associated with immunosuppressive activity of human macrophages in decidual tissue and at immune‐privileged sites.28, 29, 30, 31 Interleukin‐27‐induced IDO may contribute to the regulation of impaired T‐cell responses in early life, and measures to block IL‐27‐induced signalling may offer immunotherapeutic potential.
Experimental procedures
Cell culture
Human umbilical cord blood was obtained under Institutional Review Board approval from the Department of Obstetrics and Gynecology at the University of South Carolina and Palmetto Health Richland Hospital or the Cleveland Cord Blood Center. All donations were from healthy infants of gestational age > 37 weeks. All blood donors were anonymous and de‐identified. The cord blood was centrifuged for 15 min at 1500 g to isolate the buffy coat. Adult human buffy coats were purchased from the New York Blood Center (New York, NY). Eligible donors were 16 years of age or older, at least 110 lb (50 kg), and in good physical health. All adult donors were also anonymous and de‐identified. Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats as described previously.32 Briefly, the buffy coat was subjected to Ficoll (GE Healthcare, Chalfont St Giles, UK) density‐gradient centrifugation to isolate PBMCs. Monocytes were isolated from PBMCs by subsequent OptiPrep gradient centrifugation or CD14 immunomagnetic selection using reagents purchased from Miltenyi Biotec (Bergisch Gladbach, Germany.32 Monocytes were incubated for 1 hr in serum‐free Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mm glutamine and 25 mm HEPES to adhere to plastic culture dishes. Adherent cells were washed with PBS and then cultured for 5–8 days in DMEM supplemented with 2 mm glutamine, 25 mm HEPES, 20% fetal calf serum and 10% human serum at 37° with 5% CO2. Mature cord blood‐derived macrophages, referred to herein as neonatal macrophages, were cultured in DMEM that contained 2 mm glutamine, 25 mm HEPES and 1% human serum for all subsequent experiments. Murine macrophages were derived from bone marrow precursors of 6‐week‐old adult C57BL/6 mice. Bone marrow cells were cultured in DMEM that contained 2 mm glutamine, 25 mm HEPES, 10% fetal calf serum and 10% L‐cell‐conditioned medium for 7 days at 37° with 5% CO2. CD11b‐positive cells were isolated by immunomagnetic selection using reagents purchased from Miltenyi Bitoec.
Quantitative real‐time PCR
Macrophages (2 × 105/well) cultivated in 24‐well dishes were treated with IL‐27 for the time period indicated. In some experiments, samples received a soluble receptor to neutralize IL‐27 (sIL‐27R, 10 μg/ml) or fludarabine (250 μm) or niclosamide (2 μm; ID50 = 0·25 μm) to inhibit STAT‐1 and STAT‐3, respectively. The sIL27R was purchased from R&D Systems (Minneapolis, MN) and contains amino acids 34–516 of the IL‐27Ra (WSX‐1) chain fused through a linker domain with amino acids 100–330 of human IgG1 (50% Neutralization Dose 1–4 μg/ml). Medium was removed from cultures, the cells were lysed with PureZol™ (Bio‐Rad, Hercules, CA), and RNA was isolated according to commercial product protocol. First‐strand cDNA synthesis was performed using iScript™ cDNA synthesis reagents (Bio‐Rad) according to protocol. For human MRC1, FIZZ1, NOS2 and murine IDO1 and β‐actin gene expression analysis, real‐time cycling of reactions that included cDNA diluted 20‐fold from above, gene‐specific primer probe sets (Applied Biosystems, Foster City, CA), iQ™ Supermix (Bio‐Rad) was performed in triplicate using a CFX Connect™ (Bio‐Rad) or Step One Plus (Applied Biosystems) real‐time detection system. All other gene expression assays were performed with unlabelled forward and reverse primers described previously in a reaction similar to above with SsoFast™ Evagreen® Supermix (Bio‐Rad). Unlabelled human GAPDH and CD18 primer sequences were described previously.33 All remaining primer sequences were as follows: CD80, 5′‐tgacatcagcagagaact‐3′ (forward); CD80, 5′‐gaattgtggaaggcagtt‐3′ (reverse); CD86, 5′‐ctcaagataatgtcacagaact‐3′ (forward); CD86, 5′‐agatggtcatattgctcgta‐3′ (reverse); HLA‐DR, 5′‐agcagtcatcttcagcat‐3′ (forward); HLA‐DR, 5′‐atgttagagtacggagcaat‐3′ (reverse); human IDO1, 5′‐agaggagcagactacaag‐3′ (forward); human IDO1, 5′‐agcccacttcttcatcaa‐3′ (reverse); ARG1, 5′‐gaacagtgaacacagcag‐3′ (forward); ARG1, 5′‐ttacttaggtgggttaaggt‐3′ (reverse). Gene‐specific amplification was normalized to GAPDH as an internal reference gene for human data and β‐actin for murine data.
Immunoblot analysis
Human macrophages (2 × 105 cells/well) cultivated in 24‐well plates were left untreated or treated with varied concentrations of IL‐27 (1–100 ng/ml) for 6 or 8 hr at 37° with 5% CO2. Whole‐cell lysates were prepared by scraping cells in 40 μl of PBS supplemented with 1% Tx‐100. They were subsequently sonicated and stored at −80°. Equal amounts of cell lysates were separated on SDS–PAGE gels and transferred to nitrocellulose by standard techniques. Primary antibodies used in this study were rabbit polyclonal STAT1 (product 9172; Cell Signaling Technology, Danvers, MA), rabbit monoclonal STAT1 (clone D487, Cell Signaling Technology), mouse monoclonal STAT3 antibody (clone 124H6, Cell Signaling Technology), rabbit monoclonal phospho‐STAT3 Tyr705 (clone D3A7, Cell Signaling Technology) and rabbit polyclonal anti‐actin (product A2103, Sigma, St Louis, MO) antibodies. Primary antibodies against STAT proteins are cross‐reactive with both murine and human proteins. Primary antibodies were revealed with horseradish peroxidase‐conjugated anti‐mouse or anti‐rabbit secondary antibodies. ECL substrate (Amersham Biosciences, Little Chalfont, UK) was applied to visualize proteins.
Laser scanning confocal microscope
Human or murine macrophages were treated with IL‐27 (30 ng/ml) or sIL‐27R (10 μg/ml) for 72 hr. Macrophages were fixed with 4% paraformaldehyde and permeabilized with 0·05% saponin. The macrophages were immunostained using mouse anti‐IDO (clone 10.1; Millipore, Billerica, MA) that reacts with both human and mouse protein and visualized by anti‐mouse‐Alexa 488‐conjugated secondary antibodies (Life Technologies, Carlsbad, CA). The slides were examined using a Zeiss Meta 510 laser confocal microscope with a plan‐Apochromat 63× objective lens. A total of 10 fields containing 5–10 macrophages per field were examined in each experiment. The mean fluorescent intensity for each macrophage was calculated using image j software (U. S. National Institutes of Health, Bethesda, MD). Each cell from the image was selected and histogram analysis was performed.
IDO activity assay
Human macrophages were treated with increasing concentrations of IL‐27 (10–100 ng/ml) and incubated for 24–72 hr. Murine macrophages were treated with IL‐27 (30 ng/ml), IFN‐γ (100 ng/ml), lipopolysaccharide (LPS; 1 μg/ml), or a combination of IFN and LPS for 48 hr. At each time‐point, supernatants were replaced with RPMI (without phenol red) supplemented with 800 μg/ml of l‐tryptophan, 1% human serum, 2 mm glutamine, and 25 mm HEPES and incubated for an additional 48 hr. Supernatants were collected and analysed for kynurenine concentration indicative of IDO activity. Kynurenine standards were generated at final concentrations ranging from 1 to 5000 μm. Test samples or standards (100 μl) were mixed with 50 μl of 30% trichloroacetic acid and incubated for 30 min at 50°. Both samples and standards were centrifuged at 3716 g for 10 min to remove precipitated cellular proteins. The remaining aqueous layers (~100 μl) were mixed with an equal volume of freshly prepared Ehrlich reagent (2% p‐dimethylaminobenzaldehyde in glacial acetic acid) and incubated for 30 min at room temperature. Absorbance at 492 nm was measured with a Synergy HT Multi‐Mode microplate reader (Biotek, Winooski, VT).
ELISA for IL‐27 production
Supernatants were collected from adult or neonatal macrophage cultures. Interleukin‐27 was measured using the human IL‐27 ELISA kit (eBioscience, San Diego, CA) according to recommended protocol. These reagents measure the IL‐27 heterodimer without detection of free p28 or EBI3.
Chromatin isolation and immunoprecipitation
Neonatal macrophages were cultured in 24‐well plates and treated for 24 hr with medium alone, IL‐27 (30 ng/ml), or sIL‐27R (10 μg/ml). Chromatin was isolated by modification of a standard protocol.34 Formaldehyde was added directly to cell culture media at a final concentration of 2% for 2 min. Cross‐linking was stopped by addition of glycine to a final concentration of 0·1 m. Cells were washed with PBS and collected by scraping on ice. Cells were lysed by freezing on dry ice and rapid thawing with 100 μl Buffer C (20 mm HEPES pH 7·9, 25% glycerol, 0·42 m NaCl, 1·5 mm MgCl2, 0·2 mm EDTA). Nuclei were pelleted by centrifugation at 10 000 g for 10 min at 4° and suspended in 25 μl Breaking buffer (50 mm Tris–HCl pH 8·0, 1 mm EDTA, 0·15 m NaCl, 1% SDS, 2% Triton X‐100). Samples were sonicated for 50 seconds in a water bath to an average length of 400–700 bp and nuclear debris was cleared by centrifugation at 10 000 g for 10 min at 4°. Chromatin immunoprecipitation with antibodies specific for STAT‐1 and STAT‐3 (0·4 μg) or non‐immune serum was performed using the One Step CHIP kit (AbCam, Cambridge, UK) according to manufacturer instructions. Eluted DNA (30–40 ng) was subsequently used in quantitative PCR analysis with primers that amplify a 189‐bp sequence of the IDO regulatory region that includes three γ‐activation sequences. The IDOpromCHIP2 primer sequences were as follows: 5′‐tccttgaactgattcccaaagt‐3′ (forward); 5′‐gctctcgcgctattctctact‐3′ (reverse). Cycle threshold values were expressed relative to those returned using samples immunoprecipitated with non‐immune serum and then normalized to data obtained with samples that included STAT‐1‐associated chromatin from untreated macrophages using the formula 2−ΔΔCt.
Age‐dependent mouse experiment
Breeding colonies of C57BL/6 mice were maintained at the University of South Carolina Center for Colon Cancer Research Mouse Experimentation Core Facility. Male and female mice were used for experiments. Mice in this study were defined as neonates through 8 days of life, infants through 2 weeks, and adults at 6 weeks as described previously.4 Animals (n = 6) were identified at the appropriate age and killed by humane procedures to harvest the spleen. All procedures were approved by the USC Institutional Animal Care and Use Committee. The spleen was placed in RNA stabilization solution (RNAlater™, Qiagen, Hilden, Germany) and stored at −80°. Mouse spleens were homogenized in PureZOL™ and subsequent steps to isolate RNA were performed as described above.
Results
Neonatal macrophages secrete elevated levels of IL‐27 and respond through phosphorylation of STAT proteins
We have previously demonstrated that IL‐27 opposes inflammatory responses and control of bacterial pathogens.4, 21, 24, 25, 26 Furthermore, we showed that IL‐27 gene expression is elevated in neonatal macrophages relative to adult macrophages.4 This is consistent with elevated levels of secreted cytokine found in culture supernatants from neonatal macrophages (Fig. 1a). As IL‐27 has potent anti‐inflammatory activity, the elevated expression and secretion of IL‐27 may predispose neonatal macrophages to suboptimal responses. Signalling through the IL‐27 receptor leads to phosphorylation of STAT‐1 and STAT‐3.7, 16 However, neonatal cells have been reported to be deficient in Janus kinase–STAT signalling.35 Therefore, we evaluated the ability of cord blood macrophages to respond to IL‐27. Constitutive phosphorylation of STAT‐1 and STAT‐3 was observed in the absence of IL‐27 (Fig. 1b,c). This is consistent with steady‐state production of IL‐27 (Fig. 1a). Treatment with increasing concentrations of IL‐27 (10–100 ng/ml) for 8 hr resulted in a dose‐dependent increase in phosphorylation of STAT‐1 and STAT‐3 (Fig. 1b,c). This further demonstrates that neonatal macrophage activity may be influenced by IL‐27 in autocrine or paracrine fashion and STAT proteins may independently or cooperatively influence gene regulation.
Figure 1.
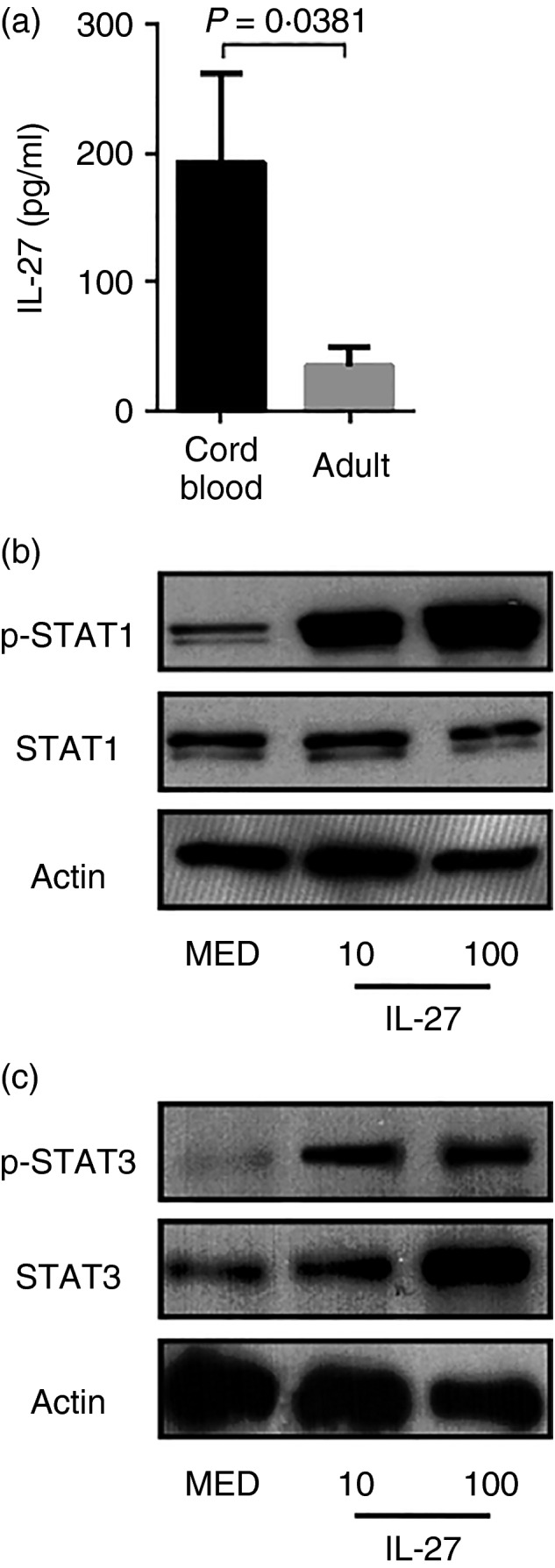
Elevated interleukin‐27 (IL‐27) production by neonatal macrophages. (a) Supernatants from neonatal (n = 11) and adult (n = 8) macrophage cultures were collected and IL‐27 concentration was determined by ELISA. Data are presented as the means of duplicate samples ± SEM. Statistical significance was determined by the non‐parametric Mann–Whitney U‐test. (b, c) Neonatal macrophages were treated with IL‐27 as indicated or left untreated for 8 hr. Cells were lysed to collect whole‐cell lysates and then subjected to immunoblot analysis for the indicated protein. Images representative of those from at least three separate donors are shown.
IL‐27 regulates IDO expression
We have hypothesized that IL‐27 may contribute to some of the deficiencies in immunological responses described in neonatal innate immune cells. To explore how IL‐27 may influence the neonatal immune response, we evaluated the expression of genes in a range of functions relevant to macrophage biology and immune function following treatment with IL‐27 (100 ng/ml) for 24 hr. These included categories such as activation state, antigen uptake, antigen presentation and cellular interactions (Table 1). Two genes exhibited statistically significant changes in expression levels in response to IL‐27 (Table 1). HLA‐DR expression was moderately increased. However, in experiments that evaluated the level of HLA‐DR cell surface protein levels, meaningful increases were not consistently detected (not shown). The most impressive finding was the dose‐responsive increase in IDO gene expression (Table 1, Fig. 2a). The levels were expressed relative to untreated cells, but transcripts were also detected in this condition. IDO has been associated with immunosuppressive activity of human macrophages in decidual tissue and at immune privileged sites.28, 29, 30, 31 Time–course experiments demonstrated that IDO expression was maximal by 24 hr in response to IL‐27 (30 ng/ml) and maintained at a high level through 72 hr (Fig. 2b). To further evaluate the specific role of IL‐27 in contributing to IDO gene expression at steady‐state levels, neonatal macrophages were treated with a soluble receptor (sIL‐27R, 10 μg/ml). Exogenous IL‐27 (30 ng/ml) served as a positive control. Expression of the IDO gene declined 30% over 24 hr when steady‐state levels of IL‐27 were neutralized (Fig. 2c). The pattern of IDO gene expression in response to exogenous IL‐27 over time is similar in adult macrophages (see Supplementary material, Fig. S1). However, in contrast to neonatal cells neutralizing IL‐27 did not reduce transcript levels relative to the untreated control (see Supplementary material, Fig. S1). Collectively, these data demonstrate that IL‐27 positively regulates IDO expression and further suggest regulation by endogenous levels of IL‐27 in neonatal macrophages.
Table 1.
Gene expression in response to interleukin‐27
| Gene | Interleukin‐27a | P valueb |
|---|---|---|
| HLA‐DR | 2·18 ± 1·31 | 0·002 |
| CD80 | 1·79 ± 1·57 | > 0·05 |
| CD86 | 1·35 ± 1·26 | > 0·05 |
| CD18 | 1·58 ± 1·56 | > 0·05 |
| IDO | 8·37 ± 2·07 | 0·01 |
| ARG1 | 4·12 ± 3·13 | > 0·05 |
| MRC1 | 0·76 ± 1·47 | > 0·05 |
| FIZZ1 | 0·36 ± 1·57 | > 0·05 |
| NOS2 | −1·63 ± 2·06 | > 0·05 |
Human neonatal macrophages were treated with interleukin‐27 (IL‐27) (100 ng/ml) for 24 hr. Gene expression is reported as log2 change in expression relative to untreated macrophages.
Statistical assessment was made using the Friedman test of repeated measures.
Figure 2.

Interleukin‐27 (IL‐27) induces indoleamine 2,3‐dioxygenase (IDO) expression. (a) Neonatal macrophages were treated with IL‐27 as indicated for 24 hr. RNA was harvested and quantitative PCR performed for IDO gene expression. Values were normalized to the mean expression of GAPDH within a duplicate sample group. Combined data from six separate donors is presented as the mean log2 change in gene expression relative to medium alone ± standard error. Significant changes in gene expression were determined using an analysis of variance and Tukey's multiple comparison's test; an asterisk indicates significance. (b) Macrophages were treated with IL‐27 (30 ng/ml) for 24, 48, and 72 hr. IDO gene expression was evaluated as above; combined data from three separate donors are shown. (c) Macrophages were treated with either soluble IL‐27 receptor (sIL‐27R) (10 μg/ml) or IL‐27 (30 ng/ml) for 24 hr. RNA collected at each time‐point was used for quantitative analysis of IDO gene expression expressed relative to untreated samples as described above. Combined data from three separate donors is shown. Statistical significance was determined using a paired samples t‐test. Each sample is statistically significant from medium alone (asterisks) and one another (different number of asterisks). (d) Macrophages were treated with IL‐27 (30 ng/ml), sIL‐27R (10 μg/ml), or interferon‐γ (IFN‐γ) (100 ng/ml) for 48 hr. Samples were subsequently fixed with 4% paraformaldehyde, permeabilized with 0·05% saponin, and immunostained for IDO. Primary antibodies for IDO were visualized with anti‐mouse IgG conjugated with Alexa fluor 468 (green). DAPI stain was used to visualize the nucleus (blue). Representative images from three experiments are shown. (e) The mean fluorescent intensity (MFI) was calculated as described in the Materials and methods section. The MFI obtained from the samples treated with IFN‐γ was set as 100% IDO expression. All other samples were expressed relative to this condition.
To confirm changes in gene expression, we evaluated IDO protein levels in response to IL‐27 by immunofluorescence. Consistent with the transcriptional analysis, there was constitutive expression of IDO protein in neonatal macrophages that increased significantly following 48 hr of treatment with IL‐27 (Fig. 2d). The amount of signal detected was comparable to that in response to a higher concentration of IFN‐γ, a known inducer of IDO, used as a positive control (Fig. 2d,e).36 Neutralization of endogenous IL‐27 levels for 48 hr significantly decreased protein expression below that of untreated neonatal macrophages (Fig. 2d,e). These results confirm the specific regulation of IDO by IL‐27 in neonatal macrophages and further demonstrate that steady‐state levels are controlling IDO expression.
IL‐27 regulates IDO activity
IDO performs the rate‐limiting step in catabolism of tryptophan to kynurenine through an N‐formyl kynurenine intermediate.37 To address the influences of increased gene and protein expression in response to IL‐27 on IDO enzyme activity, neonatal macrophages were treated with increasing concentrations of IL‐27 (10–100 ng/ml) for 24–72 hr. At that time, supernatants were replaced with media that contained 800 μg/ml of l‐tryptophan and incubated for an additional 48 hr. Supernatants were then analysed for the accumulation of kynurenine. Activity of IDO increased dose‐responsively with IL‐27 concentration (Fig. 3a). Time–course experiments paralleled transcriptional studies and demonstrated IL‐27‐induced IDO activity that was high at 24 hr and maintained through 72 hr (Fig. 3b). To demonstrate increased responsiveness at earlier time‐points, a higher concentration of IL‐27 (100 ng/ml) was required (Fig. 3c). Furthermore, neutralization of endogenous IL‐27 levels significantly decreased IDO activity (Fig. 3d). Induction of IDO activity in response to exogenous IL‐27 in adult macrophages over time follows a similar pattern as neonatal macrophages (see Supplementary material, Fig. S2). However, upon comparison to neonatal cells there are two additional observations to note. The steady‐state level of IDO activity in adult macrophages is more frequently lower than in neonatal cells and neutralizing endogenous IL‐27 did not reduce the level of kynurenine relative to the untreated control (see Supplementary material, Fig. S2). This suggests lower baseline levels of IL‐27. These data further demonstrate that IL‐27‐mediated changes in IDO expression correlate with enzymatic activity levels.
Figure 3.

Interleukin‐27 (IL‐27) increases indoleamine 2,3‐dioxygenase (IDO) activity in neonatal macrophages. (a) Macrophages were treated with IL‐27 or left untreated (MED) as indicated for 72 hr. (b) Macrophages were treated with IL‐27 (50 ng/ml) for 24, 48 or 72 hr. (c) Macrophages were treated with IL‐27 (100 ng/ml) for 2, 8 or 24 hr. (d) Macrophages were treated with soluble IL‐27 receptor (sIL‐27R) (10 μg/ml) or left untreated for 72 hr. Following the indicated treatment periods, the supernatants were replaced with RPMI containing tryptophan (800 μm) and incubated for a further 48 hr. The supernatants were collected and the accumulated kynurenine was measured as described in the Materials and methods section. Kynurenine concentrations × standard error from an individual experiment representative of at least three done with separate donors (a, b) or combined data from two separate donors (c, d) is shown in each panel. (d) A paired samples t‐test was used to determine statistical significance in the 95% confidence interval.
STAT‐1 and STAT‐3 are recruited to the IDO regulatory region with a shift in abundance
Phosphorylation of STAT‐1 and STAT‐3 was increased in neonatal macrophages treated with IL‐27 (Fig. 1b). The regulatory region upstream of the IDO transcriptional start site has multiple γ‐activation sequences; these elements can bind STAT‐1 or STAT‐3 homodimers.38 To address the importance of each transcription factor in IL‐27‐mediated regulation of gene expression, we isolated chromatin from neonatal macrophages that were left untreated (MED) or treated with IL‐27 or sIL‐27R for 24 hr. DNA bound to immunoprecipitated STAT‐1 and STAT‐3 was analysed by quantitative PCR in which a 189‐bp sequence within the IDO regulatory region that contains three γ‐activation sequences elements was amplified. Two of these γ‐activation sequence elements were previously characterized as active and important in conferring responsiveness to interferon‐γ.39 This analysis revealed a greater abundance of STAT‐3 relative to STAT‐1 at γ‐activation sequence sites within the IDO promoter during steady‐state (Fig. 4a). Upon treatment with exogenous IL‐27, a shift occurs in favour of STAT‐1 enrichment (Fig. 4a). Neutralization of steady‐state IL‐27 led to a decrease in both STAT‐1 and STAT‐3 relative to their respective levels under resting conditions, and an overall greater presence of STAT‐3 remained consistent (Fig. 4a). We do acknowledge that the relative antibody affinities could influence comparison of STAT‐1 and STAT‐3 levels; however, these data suggest an interesting mode of IL‐27 regulation in which both STAT‐1 and STAT‐3 are involved but with different degrees of importance dictated by the level of IL‐27 present in the microenvironment.
Figure 4.

Signal transducer and activator of transcription 1 (STAT‐1) and STAT‐3 are both recruited to the indoleamine 2,3‐dioxygenase (IDO) regulatory region. (a) Neonatal macrophages were treated with interleukin‐27 (IL‐27) (30 ng/ml), soluble IL‐27 receptor (sIL‐27R) (10 μg/ml), or left untreated (MED) for 24 hr. Chromatin was isolated as described in the Materials and methods and sheared by sonication. Nucleic acid associated with STAT‐1 and STAT‐3 was immunoprecipitated with specific antibodies. Quantitative amplification of a 189 bp segment that contains three γ‐activated sequences elements for STAT protein binding is reported as recruitment normalized to a non‐immune serum negative control and expressed relative to untreated samples immunoprecipitated for STAT‐1. Unpaired samples t‐tests were used to determine statistical significance in the 95% confidence interval. Samples with the same number of asterisks are considered statistically similar. (b, c) Neonatal macrophages were treated with IL‐27 (30 ng/ml) in the presence or absence of niclosamide (2 μm) or fludarabine (250 μm). Cells were lysed at 6 hr to collect whole‐cell lysates and then subjected to immunoblot analysis for the indicated protein. (d) RNA was collected at 48 hr and quantitative analysis of IDO transcripts is presented as the mean log2 change in gene expression of duplicate samples ± standard error. Values were normalized to the mean expression of GAPDH within a duplicate sample group and are expressed relative to untreated samples. Combined data from five experiments done with separate blood donors is shown. A two‐way analysis of variance was used to determine statistical significance in the 95% confidence interval.
To further address the molecular signals that contribute to IDO regulation by IL‐27, and in particular the switch to STAT‐1 involvement when IL‐27 levels increase, we used pharmacological inhibitors of STAT‐1 and STAT‐3. Neonatal macrophages were treated with IL‐27 for 48 hr in the presence or absence of fludarabine (250 μm) or niclosamide (2 μm). Fludarabine inhibits STAT‐1 activation 40 (Fig. 4b). Niclosamide inhibits STAT‐3 activation but is also cross‐reactive toward STAT‐1 41 (Fig. 4b,c). In the absence of IL‐27, treatment with niclosamide decreased IDO gene expression indicating a constitutive level of STAT‐1 and STAT‐3 signalling that was driving gene expression (Fig. 4d). However, treatment with fludarabine did not decrease steady‐state levels of gene expression and instead there was a slight increase (Fig. 4d). This is consistent with an enrichment of STAT‐3 under this condition. It could also suggest greater transactivation potential for STAT‐3. Treatment with niclosamide in the presence of supplied IL‐27, significantly decreased gene expression (Fig. 4d). This further supports the importance of STAT‐1 and STAT‐3 in driving IDO gene expression in response to IL‐27. Interestingly, upon treatment with greater levels of IL‐27, IDO gene expression was not significantly decreased by treatment with fludarabine, an inhibitor of STAT‐1 (Fig. 4d). STAT‐1 is enriched at the IDO regulatory region in response to higher levels of IL‐27 (Fig. 4a). Hence, these data suggest that STAT‐3 can compensate for STAT‐1 in the transactivation of IDO in response to high levels of IL‐27.
IL‐27‐mediated induction of IDO is specific to human macrophages
Using an age‐dependent mouse model, we previously reported that EBI3 gene expression in spleens from mice at 8 and 21 days of life was significantly higher than that of 56‐day‐old adult mice. This was further confirmed at the protein expression level.4 To determine if IDO expression also correlated with changes in IL‐27 levels in mice, RNA was isolated from the spleens of mice at 8, 21 or 56 days of age. The mean IDO gene expression from mice at different age groups was highest at day 56. However, there was no statistically significant difference in the amount of IDO gene expression between any age group (Fig. 5a). This suggested that IDO expression was not responsive to IL‐27 in mice. To further confirm this, macrophages were generated from adult murine bone marrow progenitors and treated with IL‐27 (50 ng/ml), IFN‐γ (100 ng/ml) as a positive control, or a combination of IFN‐γ and LPS (1 μg/ml) for 48 hr. Consistent with the analysis from mouse spleens, IL‐27 did not increase IDO gene expression or activity in macrophages relative to the untreated control (Fig. 5b,c). In contrast, IFN‐γ‐induced IDO gene expression and activity were further increased by the addition of LPS (Figs 5b and 6c). To further confirm these results with tissue macrophages, CD11b+ cells were isolated from mouse spleens and treated as above (Fig. 5d,e). Similar to bone marrow‐derived macrophages, IL‐27 did not induce detectable IDO transcripts or activity above baseline (Fig. 5d,e). This is in contrast to IDO transcripts detected in response to IFN‐γ (Fig. 5d). The addition of LPS, however, was required for detection of more pronounced levels of IDO activity (Fig. 5e). To confirm that murine macrophages were responding to IL‐27, we performed an immunoblot analysis of STAT‐1 and STAT‐3. Bone marrow‐derived macrophages phosphorylated STAT‐1 and STAT‐3 in response to increasing concentrations of IL‐27 (Fig. 5f). Similar results were obtained with CD11b+ macrophages (not shown). These data strongly suggest that the regulation of IDO by IL‐27 in human macrophages is not replicated in the murine system.
Figure 5.
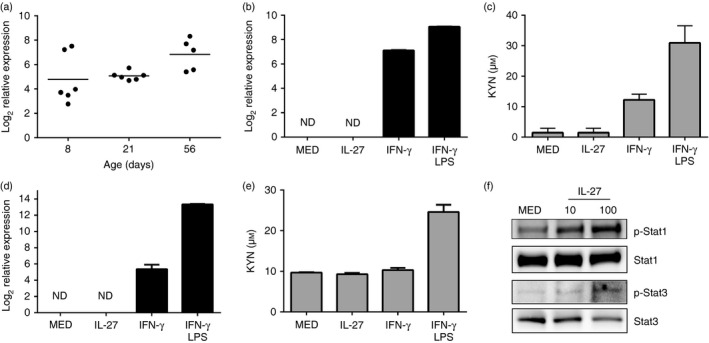
Interleukin‐27 (IL‐27) does not induce indoleamine, 2,3‐dioxygenase (IDO) activity in murine macrophages. (a) C57BL/6 mice (n = 5 or n = 6) were killed at the indicated time in days following birth. The spleens were removed and RNA was isolated as described in the Materials and methods. Quantitative analysis of IDO and β‐actin transcripts is presented as the log2 change in gene expression. Expression levels within an age group were normalized to β‐actin and expressed relative to that at 4 days. The data for each mouse and the group mean are shown at each age. A one‐way analysis of variance and Tukey's post‐hoc test for multiple comparisons were used to evaluate statistical significance. (b–e) Bone marrow‐derived macrophages (b, c) or CD11b+ macrophages (d, e) were treated with IL‐27 (30 ng/ml), interferon‐γ (IFN‐γ) (100 ng/ml), the combination of IFN‐γ with lipopolysaccharide (LPS) (1 μg/ml), or left untreated for 48 hr. (b, d) Quantitative analysis of IDO transcripts is presented as the mean log2 change in gene expression of duplicate samples ± standard error. Values were normalized to the mean expression of actin within a duplicate sample group and are expressed relative to untreated samples. The data shown are a representative of two independent experiments. (c, e) Following the indicated treatments, supernatants were replaced with RPMI containing tryptophan (800 μm) and incubated for a further 48 hr. The supernatants were collected and the accumulated kynurenine was measured as described in the Materials and methods section. Kynurenine concentrations ± standard error from an individual experiment representative of two is shown. (f) Bone marrow‐derived macrophages were treated with IL‐27 as indicated or left untreated for 6 hr. Cells were lysed to collect whole‐cell lysates and then subjected to immunoblot analysis for the indicated protein.
Figure 6.

Hypothetical model. In response to low or endogenous levels of interleukin‐27 (IL‐27), more signal transducer and activator of transcription 3 (STAT‐3) is present at the indoleamine 2,3‐dioxygenase (IDO) regulatory region. Upon addition of greater levels of IL‐27 the balance is shifted toward STAT‐1 without a significant change in the level of STAT‐3. The maintenance of STAT‐3 levels may be critical for enhanced transcriptional activity.
Discussion
Interleukin‐27 is a paradoxical cytokine that has both inflammatory and suppressive immune functions. Although its influences may be stimulus and cell‐type specific, a wide breadth of evidence has accumulated to demonstrate the importance of IL‐27 as an agent that opposes inflammatory immune responses.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 A number of limitations have been described in the neonatal immune response relative to adults. This includes limited production of Th1 cytokines, high production of anti‐inflammatory cytokines, and Th2‐polarizing activity.2, 42, 43 Although we have some understanding of the limitations in neonatal immunity that contribute to increased susceptibility to infection, a greater knowledge of mechanisms that account for these deficiencies is needed.
Recent evidence that demonstrates elevated IL‐27 expression in neonatal macrophages compared with adult counterparts suggests that IL‐27 may serve a unique function in regulating immunity early in life. In this report, we sought to uncover how IL‐27 may influence neonatal macrophages that are not only a considerable source of the cytokine but also equipped to respond in autocrine or paracrine fashion. We report the novel finding that IL‐27 specifically regulates expression of IDO in human neonatal macrophages and draw several additional conclusions from the results of this study. First, constitutive IDO protein and activity is observed in human neonatal macrophages. This is consistent with steady‐state production of IL‐27 and a corresponding constitutive phosphorylation of STAT‐1 and STAT‐3. Second, the induction of IDO by IL‐27 requires STAT‐3 and STAT‐1, although a shift in abundance of each transcription factor occurs depending on the level of IL‐27 present. Third, in a comparison of murine and human macrophages, the regulation of IDO expression by IL‐27 is specific to human cells.
Human neonatal macrophages express elevated levels of IL‐27 genes at steady‐state relative to adults.4 This was accompanied by increased levels of secreted cytokine in culture supernatants. Constitutive phosphorylation of STAT‐1 and STAT‐3 is further consistent with response to steady‐state levels of IL‐27 present in the culture. Neonatal macrophages have been reported to be impaired in Janus kinase–STAT signalling;35 however, we demonstrated increased STAT‐1 and STAT‐3 phosphorylation in response to added levels of cytokine. To address how IL‐27 may regulate neonatal macrophages, we examined the expression of select genes in different functional categories and found IDO to be consistently expressed at a significantly higher level in response to IL‐27. The regulation of IDO gene expression is dose‐dependent, maximal by 24 hr following stimulation, and sustained through 72 hr. Neutralization of steady‐state levels of IL‐27 led to a decrease in gene expression, further demonstrating the role of constitutive IL‐27 production in sustaining IDO expression. The enzymatic activity of IDO converts tryptophan to kynurenine through an N‐formylkynurenine intermediate.37 We demonstrated that changes in IDO protein paralleled the IL‐27‐specific control of gene expression. This was also evident for neutralization of endogenous IL‐27 that decreased protein levels relative to untreated macrophages. Interleukin‐27 dose‐dependently increased accumulation of kynurenine in culture supernatants. This activity was specific to IDO and could be blocked by a known inhibitor (data not shown). Interleukin‐27 also induces IDO gene expression and enzymatic activity in adult macrophages. However, since endogenous levels of IL‐27 are lower, steady‐state IDO activity is also lower. This was evident from the level of kynurenine associated with untreated adult macrophages. It was routinely lower than in neonatal cells consistent with less IL‐27 production.
Previously, IDO was shown to be induced by IL‐27 in an STAT‐1‐dependent manner in colorectal cancer epithelial cells.44 Additionally, a more recent report described the induction of IDO by IL‐27 in human epithelial ovarian cancer (EOC) cells.45 In this report, cultured EOC cells did not express constitutive levels of IDO and STAT‐1 and STAT‐3 were not constitutively phosphorylated. However, these activities could be induced in cultured cells by treatment with IL‐27, and IDO expression was dependent on STAT‐1.45 We demonstrate here an interesting interplay in regulation in human neonatal macrophages by both STAT‐1 and STAT‐3 that is influenced by the levels of IL‐27 present. At lower endogenous levels of IL‐27, STAT‐3 is more abundant at the IDO regulatory region. Our study is the first to demonstrate STAT‐3 association with the IDO regulatory region, which suggests transcriptional activation of IDO. This is further supported by experiments in which STAT‐1 inhibition by a chemical inhibitor had no effect on IDO gene expression. Because we were unable to identify a chemical inhibitor of STAT‐3 that did not have a cross‐reactive effect on STAT‐1 without compromising the viability of the macrophages, we were not able to perform the reciprocal experiment of specifically inhibiting STAT‐3. When the concentration of IL‐27 is elevated, STAT‐1 is recruited to the IDO regulatory region without an increase in STAT‐3 levels. However, when STAT‐1 is specifically inhibited under the same conditions there is no pronounced decrease in IDO gene expression. We acknowledge that the concentrations of fludarabine used do not completely inhibit STAT‐1 activation, so some involvement of STAT‐1 may remain; however, a significant decrease in STAT‐1 inhibition is demonstrated. These results suggest that in response to low levels of IL‐27, STAT‐3 drives a constitutive level of gene expression that further manifests in enzymatic activity. However, in the presence of high levels of IL‐27, STAT‐1 is facilitating enhanced promoter activity, that can mostly be compensated for by STAT‐3. A model for the role of STAT proteins is presented in Fig. 6. Our results are mostly consistent with the above mentioned reports in cancer cells.44, 45 In response to supplied IL‐27, STAT‐1 is predominantly driving gene expression. However, our results define a role for STAT‐3 in constitutive IDO expression driven by low levels of IL‐27. In contrast to the cultured EOS cells, Carbotti et al.45 demonstrated constitutive IDO expression that was consistent with phosphorylation of STAT‐1 and STAT‐3 in cells from EOC ascites. The authors did not explore STAT‐dependence in this context. It is fair to assume that abrogation of specific STAT proteins in that context is much more challenging. However, we highlight this result because the constitutive IDO expression in cells from EOC ascites may require a greater role for STAT‐3 consistent with our results.
In human adult macrophages we recently demonstrated negative regulation of lysosomal acidification by IL‐27‐induced STAT‐3 with consequences for the control of mycobacterial growth.25, 26 The data presented here highlight an additional role of IL‐27‐induced STAT‐3 on human neonatal macrophages, and also provides functional consequences of STAT‐1 phosphorylation in response to IL‐27. IDO is also regulated by type‐I IFN in human macrophages46, 47, 48 and IL‐27 has been shown to induce type I IFN.11 This would raise the possibility that IL‐27 may also at least partially work through type I IFN to activate STAT‐1 and regulate IDO, particularly in the context of high levels of IL‐27. Interleukin‐27 is an interesting cytokine with ascribed inflammatory and immune suppressive activity. The results here may contribute to our understanding of how a single cytokine may influence immune responses on both ends of the inflammatory spectrum. The concentration of cytokine in the local environment may be a critical factor in the immunological outcome and a predominance of STAT‐3 at lower concentrations and STAT‐1 at higher concentration may be a component of the molecular mechanism.
Elevated expression of IL‐27 genes in neonates was previously shown to be consistent between human and murine macrophages.4 It was further established in mice that IL‐27 levels remained elevated throughout infancy. This was important not only to establish the potential significance of the IL‐27 observations, but also suggested that the mouse would be a good model to further evaluate the impact in neonatal and infant immune responses. Although this is still likely to be the case, the regulation of IDO by IL‐27 in macrophages is specific to the human system and not maintained in mice. Hence, the immunological consequences of IL‐27‐regulated IDO will have to be pursued using human cells. This is in contrast to the induction of IDO by IL‐27 in murine intestinal epithelial cells.44 Furthermore, with murine macrophages, the level of IDO activity induced by IFN‐γ and LPS serving as a positive control, was still well below that observed in response to IL‐27 in neonatal human macrophages (Fig. 6d). These results were not the consequence of an inability of the macrophages to respond to IL‐27 as they phosphorylated both STAT‐1 and STAT‐3 in response to IL‐27. This further highlights differences in IDO regulation between murine and human macrophages.
Indoleamine dioxygenase has established roles in infection and immunological responses. Through depletion of pools of available tryptophan, IDO activity has been shown to limit the replication of susceptible intracellular pathogens in vitro, including Chlamydia spp. and Toxoplasma gondii.49, 50, 51, 52, 53 Although we should not exclude the possibility that IL‐27‐mediated induction of IDO may offer some protection for neonates in the control of intracellular growth of some bacteria and parasites, the immunomodulatory activity is likely to make the most significant impact. IDO has been associated with immunosuppressive activity of human macrophages in decidual tissue and at immune privileged sites.28, 29, 30, 31 Activity of IDO has been extensively linked to suppression of T cells.27, 54, 55, 56 The immunosuppressive effects of kynurenine and other downstream catabolites have also been shown to be selective for Th1 through induction of apoptosis and restriction of cell proliferation.57, 58 Functionally active IDO is constitutively expressed in thymic eosinophils during early life.59 These cells naturally home to the thymus in neonates.60 Increased numbers of IDO‐expressing eosinophils that steadily declined with increasing age were found in thymic tissue of newborns < 6 months of age.59 This was further supported by an increased concentration of kynurenine in thymic tissue homogenates and further correlated with Th2 but not Th1 markers.59 In tumour‐draining lymph nodes, IDO is critical for the maintenance of regulatory T cell populations; inhibition of IDO promotes a switch from Foxp3+ regulatory T cells to Th17 cells.61 Interleukin‐27‐induced IDO may contribute to immunological immaturity and Th2 dominance in early life. Indeed, the disparity in Th1 responses early in life cannot be attributed solely to intrinsic deficiencies in neonatal lymphocytes because adult antigen‐presenting cells are able to promote Th1 responses from cord blood lymphocytes.62 The nature of the antigen‐presenting cells is likely to contribute and IL‐27 and IDO may be involved. Future studies will determine if IL‐27 drives IDO expression in other human cell types and if circulating levels of IDO‐positive cells correlate with Th2 profiles and susceptibility to infection, particularly infectious scenarios such as sepsis that are a significant threat to neonates.
Author contributions
JYJ, JDK, LL, BK, MGP and JR performed experiments. CD and MMOP provided mice and technical expertise critical to experiments. JYJ and CMR conceived the ideas, designed experiments, analysed data and prepared the manuscript.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1. Interleukin‐27 induces indoleamine 2,3 dioxygenase (IDO) gene expression in adult macrophages.
Figure S2. Interleukin‐27 increases indoleamine 2,3‐dioxygenase (IDO) activity in adult macrophages.
Acknowledgements
We would like to extend special thanks to the Department of Obstetrics and Gynecology at the University of South Carolina School of Medicine and Palmetto Health Richland Hospital for their assistance with the collection and provision of umbilical cord blood.
This work was supported by institutional funds supplied by the University of South Carolina School of Medicine, the West Virginia School of Osteopathic Medicine, NIH/NIAID grant AI116423 to CMR, and an Institutional Development Award (IDeA) from the NIH/NIGMS under grant number P20GM12345.
We would also like to acknowledge grant support for the University of South Carolina Center for Colon Cancer Research Mouse Experimentation Core Facility from the NIH/NIGMS COBRE grant P30GM103336‐01A1 and NIH/NCI R01CA154731‐01A1 to MMOP.
References
- 1. World Health Organization . Infant mortality. URL http://www.who.int/gho/child_health/mortality/neonatal_infant_text/en/index.html [accessed on January 5, 2016]
- 2. Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? where? why? Lancet 2005; 365:891–900. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . The Global Burden of Disease: 2004 Update. Geneva: World Health Organization, 2008. [Google Scholar]
- 4. Kraft JD, Horzempa J, Davis C, Jung JY, Peña MMO, Robinson CM. Neonatal macrophages express elevated levels of interleukin‐27 that oppose immune responses. Immunology 2013; 139:484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al IL‐27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naïve CD4+ T cells. Immunity 2002; 16:779–90. [DOI] [PubMed] [Google Scholar]
- 6. Devergne O, Hummel M, Koeppen H, LeBeau MM, Nathanson EC, Kieff E, et al A novel interleukin‐12 p40‐related protein induced by latent Epstein–Barr virus infection in B lymphocytes. J Virol 1996; 70:1143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, et al Development of Th1‐type immune responses requires the type I cytokine receptor TCCR. Nature 2000; 407:916–20. [DOI] [PubMed] [Google Scholar]
- 8. Kalliolias GD, Ivashkiv LB. IL‐27 activates human monocytes via STAT1 and suppresses IL‐10 production but the inflammatory functions of IL‐27 are abrogated by TLRs and p38. J Immunol 2008; 180:6325–33. [DOI] [PubMed] [Google Scholar]
- 9. Fakruddin JM, Lempicki RA, Gorelick RJ, Yang J, Adelsberger JW, Garcia‐Pineres AJ, et al Noninfectious papilloma virus‐like particles inhibit HIV‐1 replication: implications for immune control of HIV‐1 infection by IL‐27. Blood 2007; 109:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imamichi T, Yang J, Huang DW, Brann TW, Fullmer BA, Adelsberger JW, et al IL‐27, a novel anti‐HIV cytokine, activates multiple interferon‐inducible genes in macrophages. AIDS 2008; 22:39–45. [DOI] [PubMed] [Google Scholar]
- 11. Greenwell‐Wild T, Vázquez N, Jin W, Rangel Z, Munson PJ, Wahl SM. Interleukin‐27 inhibition of HIV‐1 involves an intermediate induction of type I interferon. Blood 2009; 114:1864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Q, Swaminathan S, Yang D, Dai L, Sui H, Yang J, et al Interleukin‐27 is a potent inhibitor of cis HIV‐1 replication in monocyte‐derived dendritic cells via a type I interferon‐independent pathway. PLoS One 2013; 8:e59194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, et al The IL‐27R (WSX‐1) is required to suppress T cell hyperactivity during infection. Immunity 2003; 19:645–50. [DOI] [PubMed] [Google Scholar]
- 14. Pearl J, Khader SA, Solache A, Gilmartin L, Ghilardi N, DeSauvage F, et al IL‐27 signaling compromises control of bacterial growth in Mycobacteria‐infected mice. J Immunol 2004; 173:7490–6. [DOI] [PubMed] [Google Scholar]
- 15. Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, et al The IL‐27 receptor is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol 2004; 173:5626–34. [DOI] [PubMed] [Google Scholar]
- 16. Hölscher C, Hölscher A, Rückerl D, Yoshimoto T, Yoshida H, Mak T, et al The IL‐27 receptor chain WSX‐1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol 2005; 174:3524–44. [DOI] [PubMed] [Google Scholar]
- 17. Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, et al Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med 2006; 203:1875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al Interleukin 27 negatively regulates the development of interleukin 17‐producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 2006; 7:937–45. [DOI] [PubMed] [Google Scholar]
- 19. Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17‐producing T cells. Nat Immunol 2006; 7:929–36. [DOI] [PubMed] [Google Scholar]
- 20. Awasthi A, Carrier Y, Peron JPS, Bettelli E, Kamanaka M, Flavell RA, et al A dominant function for interleukin 27 in generating interleukin 10‐producing anti‐inflammatory cells. Nat Immunol 2007; 8:1380–9. [DOI] [PubMed] [Google Scholar]
- 21. Robinson CM, Nau GJ. Interleukin‐12 and Interleukin‐27 regulate macrophage control of Mycobacterium tuberculosis . J Infect Dis 2008; 198:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinson CM, Nau GJ. Cytokines involved in interferon‐γ production by human macrophages. J Innate Immun 2010; 2:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalliolias GD, Gordon RA, Ivashkiv LB. Suppression of TNF‐α and IL‐1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL‐27. J Immunol 2010; 185:7047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robinson CM, Jung JY, Nau GJ. Interferon‐γ, tumor necrosis factor, and interleukin‐18 cooperate to control growth of Mycobacterium tuberculosis in human macrophages. Cytokine 2012; 60:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jung JY, Robinson CM. Interleukin‐27 inhibits phagosomal acidification by blocking vacuolar ATPases. Cytokine 2013; 62:202–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jung JY, Robinson CM. IL‐12 and IL‐27 regulate the phagolysosomal pathway in mycobacteria‐infected human macrophages. Cell Commun Signal 2014; 12:16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3‐dioxygenase inhibit T cell responses. J Immunol 2002; 168:3771–6. [DOI] [PubMed] [Google Scholar]
- 28. Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281:1191–3. [DOI] [PubMed] [Google Scholar]
- 29. Heikkinen J, Möttönen M, Komi J, Alanen A, Lassila O. Phenotypic characterization of human decidual macrophages. Clin Exp Immunol 2003; 131:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gustafsson C, Mjösberg J, Matussek A, Geffers R, Matthiesen L, Berg G, et al Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One 2008; 3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang L, Baban B, Johnson BA III, Mellor AD. Dendritic cells, indoleamine 2,3‐dioxygenase and acquired immune privilege. Int Rev Immunol 2010; 29:133–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carlson PE Jr, Carroll JA, O'Dee DM, Nau GJ. Modulation of virulence factors in Francisella tularensis determines human macrophage responses. Microb Pathog 2007; 42:204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roberts LL, Robinson CM. Mycobacterium tuberculosis infection of human dendritic cells decreases integrin expression, adhesion, and migration to chemokines. Immunology 2014; 141:39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boyd KE, Wells J, Gutman J, Bartley SM, Farnham PJ. c‐Myc target gene specificity is determined by a post‐DNA binding mechanism. Proc Natl Acad Sci USA 1998; 95:13887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maródi L, Goda K, Palicz A, Szabó G. Cytokine receptor signaling in neonatal macrophages: defective STAT‐1 phosphorylation in response to stimulation with IFN‐γ. Clin Exp Immunol 2001; 126:456–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thomas SM, Garrity LF, Brandt CR, Schobert CS, Feng GS, Taylor MW, et al IFN‐γ‐mediated antimicrobial response. Indoleamine 2,3‐dioxygenase‐deficient mutant host cells no longer inhibit intracellular Chlamydia spp. or Toxoplasma growth. J Immunol 1993; 150:5529–34. [PubMed] [Google Scholar]
- 37. Shimuzu T, Nomiyama S, Hirata F, Hayashi O. Indoleamine 2,3‐dioxygenase: purification and some properties. J Biol Chem 1978; 253:4700–6. [PubMed] [Google Scholar]
- 38. Wen Z, Zhong Z, Darnell JE Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 1995; 82:241–50. [DOI] [PubMed] [Google Scholar]
- 39. Robinson CM, Shirey KA, Carlin JM. Synergistic transcriptional activation of indoleamine dioxygenase by IFN‐γ and tumor necrosis factor‐α . J Interferon Cytokine Res 2003; 23:413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frank DA, Mahaian S, Ritz J. Fludarabine‐induced immunosuppression is associated with inhibition of STAT‐1 signaling. Nat Med 1999; 5:444–7. [DOI] [PubMed] [Google Scholar]
- 41. Li R, You S, Hu Z, Chen ZG, Sica GL, Khuri FR, et al Inhibition of STAT3 by niclosamide synergizes with erlotinib against head and neck cancer. PLoS One 2013; 8:e74670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kollmann TR, Crabtree J, Rein‐Weston A, Blimkie D, Thommai F, Wang XY, et al Neonatal innate TLR‐mediated responses are distinct from those of adults. J Immunol 2009; 183:7150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maródi L. Down‐regulation of Th1 responses in human neonates. Clin Exp Immunol 2002; 128:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Diegelmann J, Olszak T, Göke B, Blumberg RS, Brand S. A novel role for interleukin‐27 (IL‐27) as mediator of intestinal epithelial barrier protection mediated via differential signal transducer and activator of transcription (STAT) protein signaling and induction of antibacterial and anti‐inflammatory proteins. J Biol Chem 2012; 287:286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carbotti G, Barisione G, Airoldi I, Mezzanzanica D, Bagnoli M, Ferrero S, et al IL‐27 induces the expression of IDO and PD‐L1 in human cancer cells. Oncotarget 2015; 6:43267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carlin JM, Borden EC, Sondel PM, Byrne GI. Biologic response modifier‐induced indoleamine 2,3‐dioxygenase activity in human peripheral blood mononuclear cell cultures. J Immunol 1987; 139:2414–8. [PubMed] [Google Scholar]
- 47. Carlin JM, Borden EC, Sondel PM, Byrne GI. Interferon‐induced indoleamine 2,3‐dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol 1988; 45:29–34. [DOI] [PubMed] [Google Scholar]
- 48. Carlin JM, Borden EC, Byrne GI. Interferon‐induced indoleamine 2,3‐dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J Interferon Res 1989; 9:329–37. [DOI] [PubMed] [Google Scholar]
- 49. Byrne GI, Lehmann LK, Kirschbaum JG, Borden EC, Lee CM, Brown RR. Induction of tryptophan catabolism is the mechanism for γ‐interferon‐mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. J Interferon Res 1986; 6:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pfefferkorn ER. Interferon γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA 1984; 81: 908–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmitz JL, Carlin JM, Borden EC, Byrne GI. β interferon inhibits Toxoplasma gondii growth in human monocyte‐derived macrophages. Infect Immun 1989; 57:3254–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shemer Y, Sarov I. Inhibition of growth of Chlamydia trachomatis by human γ interferon. Infect Immun 1985; 48:592–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Summersgill JT, Sahney NN, Gaydos CA, Quinn TC, Ramirez JA. Inhibition of Chlamydia pneumoniae growth in HEp‐2 cells pretreated with γ interferon and tumor necrosis factor α . Infect Immun 1995; 63:2801–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan‐derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3‐dioxygenase. J Exp Med 2002; 196:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al Inhibition of allogeneic T cell proliferation by indoleamine 2,3‐ dioxygenase‐expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med 2002; 196:447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bauer TM, Jiga LP, Chuang JJ, Randazzo M, Opelz G, Terness P. Studying the immunosuppressive role of indoleamine 2,3‐dioxygenase: tryptophan catabolites suppress rat allogeneic T‐cell responses in vitro and in vivo . Transpl Int 2005; 18:95–100. [DOI] [PubMed] [Google Scholar]
- 57. Fallarino F, Grohmann U, Vacca C, Orabona C, Spreca A, Fioretti MC, et al T cell apoptosis by kynurenines. Adv Exp Med Biol 2003; 527:183–90. [DOI] [PubMed] [Google Scholar]
- 58. Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double‐edged sword for TH1/TH2 regulation. Immunol Lett 2008; 121:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tulic MK, Sly PD, Andrews D, Crook M, Davoine F, Odemuyiwa SO, et al Thymic indoleamine 2,3‐dioxygenase‐positive eosinophils in young children: potential role in maturation of the naive immune system. Am J Pathol 2009; 175:2043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee I, Yu E, Good RA, Ikehara S. Presence of eosinophilic precursors in the human thymus: evidence for intra‐thymic differentiation of cells in eosinophilic lineage. Pathol Int 1995; 45:655–62. [DOI] [PubMed] [Google Scholar]
- 61. Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, et al Indoleamine 2,3‐dioxygenase controls conversion of Foxp3+ Tregs to TH17‐like cells in tumor‐draining lymph nodes. Blood 2009; 113:6102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Taylor S, Bryson YJ. Impaired production of γ‐interferon by newborn cells in vitro is due to a functionally immature macrophage. J Immunol 1985; 134:1493–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Interleukin‐27 induces indoleamine 2,3 dioxygenase (IDO) gene expression in adult macrophages.
Figure S2. Interleukin‐27 increases indoleamine 2,3‐dioxygenase (IDO) activity in adult macrophages.


