Summary
Lipid rafts are dynamic assemblies of proteins and lipids that harbour many receptors and regulatory molecules and so act as a platform for signal transduction. They float freely within the liquid‐disordered bilayer of cellular membranes and can cluster to form larger ordered domains. Alterations in lipid rafts are commonly found to be associated with the pathogenesis of several human diseases and recent reports have shown that the raft domains can also be perturbed by targeting raft proteins through microRNAs. Over the last few years, the importance of lipid rafts in modulating both innate and acquired immune responses has been elucidated. Various receptors present on immune cells like B cells, T cells, basophils and mast cells associate with lipid rafts on ligand binding and initiate signalling cascades leading to inflammation. Furthermore, disrupting lipid raft integrity alters lipopolysaccharide‐induced cytokine secretion, IgE signalling, and B‐cell and T‐cell activation. The objective of this review is to summarize the recent progress in understanding the role of lipid rafts in the modulation of immune signalling and its related therapeutic potential for autoimmune diseases and inflammatory disorders.
Keywords: autoimmune disease, B/T‐cell activation, cytokine signalling, IgE, lipid rafts, microRNA, Toll‐like receptor
Abbreviations
- BCR
B‐cell receptor
- DRMs
detergent‐resistant membrane fractions
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal‐regulated kinase
- IFN
interferon
- IL‐8
interleukin‐8
- ITAM motif
immunoreceptor tyrosine‐based activation
- LAT
linker of activation of T cells
- LBP
LPS‐binding protein
- Lck
lymphocyte‐specific protein tyrosine kinase
- LIF
leukaemia inhbitory factor
- LIFR
leukaemia inhbitory factor receptor
- LPS
lipopolysaccharide
- MAP
mitogen‐activated protein
- MBCD
methyl‐β cyclodextrin
- NF‐κB
nuclear factor‐κB
- SLE
systemic lupus erythematosus
- TCR
T‐cell receptor
- TGF
transforming growth factor
- TLR
Toll‐like receptor
- TIR domain
TLR/interleukin‐1 receptor homology domain
- TNF
tumour necrosis factor
- TRAF6
TNF receptor‐associated factor 6
Introduction
The plasma membrane is an important component of the cell and a large number of studies have been conducted into understanding the membrane structure and function. In 1972, Singer and Nicolson proposed the ‘Fluid mosaic model’ of biological membranes, which describes it as ‘protein icebergs floating in the sea of lipid’.1 In the early 1980s, a series of experimental findings revealed that the lipids are not uniformly distributed in the cell membrane, and the concept of membrane microdomain ‘lipid rafts’ was postulated.2, 3 Figure 1 depicts the timeline of pioneer studies in the lipid raft field.
Figure 1.
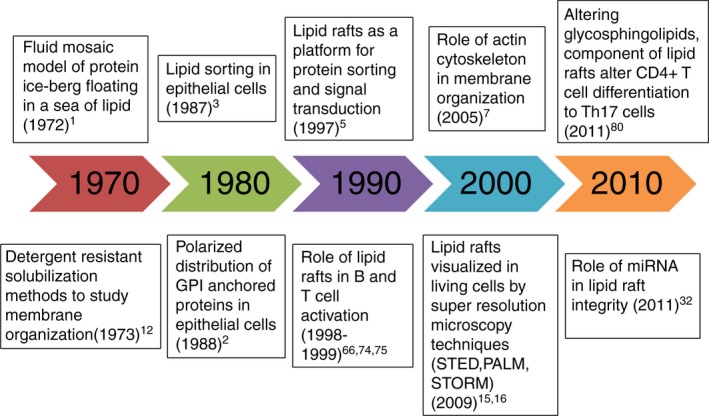
Timeline of pioneer discoveries in the field of lipid rafts and immune signalling.
Lipid rafts are defined as small (10–200 nm) heterogeneous, highly dynamic, sterol (cholesterol), sphingolipid‐ and protein‐enriched domains that compartmentalize the cellular processes.4 They incorporate several distinct classes of proteins – true resident proteins (glycosylphosphatidylinositol‐linked proteins, caveolin, flotillin), signalling proteins (doubly acylated proteins like Src family kinases), G‐protein‐coupled receptor (GPCR) proteins, cholesterol‐linked and palmitoylated proteins such as hedgehog and myristoylated proteins.5 Although the existence of lipid rafts remains controversial, there are several theories that explain its formation in biological membranes. According to one theory, self‐associative properties of sphingolipid and cholesterol facilitate selective lateral segregation in the membrane plane. Another hypothesis suggests that rafts are constructed of lipid shells, which are 1–10 nm thermodynamically stable mobile entities of proteins preferentially associated with certain types of lipids (protein–lipid shell). Lipid shells target the proteins they encase to pre‐existing raft/caveolar domains and they could be the quantal unit of rafts.6 Further, Kusumi et al.7 proposed the ‘picket–fence model’, which explains that the anchoring of transmembrane proteins to the underlying actin cytoskeleton restricts lateral diffusion of proteins and is therefore involved in compartmentalization of the membrane. The evidence suggests that lipid raft formation is driven and stabilized by lipid–lipid, lipid–protein and protein–protein interactions.
There are generally two types of lipid rafts: planar lipid rafts (also known as non‐caveolar) and caveolae. Planar rafts are continuous non‐invaginated membrane domains that lack distinguishing morphological features whereas caveolae are flask‐shaped invaginated membrane structures formed by polymerization of caveolin proteins. Caveolins are transmembrane palmitoylated proteins having a hairpin‐like structure that binds tightly to cholesterol.8 These are present in three isoforms (caveolin‐1, ‐2 and ‐3) and are transcribed from different genes. Out of the three caveolins, caveolin‐1 is known to modulate inflammatory responses. Medina et al.9 found that knockdown of the caveolin‐1 protein leads to defects in innate immunity and mice become more susceptible to infection. Caveolin‐2 is involved in lung functions while caveolin‐3 is a muscle‐specific isoform and its knockdown in mice results in muscular dystrophy symptoms.10
Two widely used methods to study lipid rafts are the isolation of detergent‐resistant membrane fractions (DRMs) and cholesterol depletion.11, 12 DRM analysis is not considered reliable because DRM fractions vary in lipid and protein composition depending upon the type of detergent, its concentration, temperature conditions and different cell types.13 Cholesterol depletion by methyl‐β cyclodextrin (MBCD) suffers from serious adverse effects; in addition to raft disruption, it also perturbs many other cellular functions.14 Due to the lack of proper methodologies for studying rafts, several questions about its existence have been raised. Later in 2000, advanced microscopy techniques such as fluorescence resonance energy transfer, fluorescence polarization anisotropy and single‐particle tracking, fluorescence correlation spectroscopy, supported the existence of dynamic cholesterol‐dependent nano‐clusters in the cell membrane. Furthermore, super‐resolution microscopy techniques including stimulated emission depletion, photoactivated localization microscopy and stochastic optical reconstruction microscopy that provide resolution substantially below the diffraction limit, demonstrated the presence of raft domains in the cell membrane.15, 16
MicroRNAs as modulators of lipid rafts in human diseases
Lipid rafts play a crucial role in protein sorting and receptor‐mediated signal transduction, providing a distinct environment for the functioning of receptors and intracellular molecules. These nanodomains have been known to be altered in various diseases such as neurological disorders, cancer, cardiovascular diseases, insulin resistance, microbial infections and inflammatory diseases. As shown in Fig. 2,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 various molecules involved in disease progression have also been found to be associated with lipid rafts and caveolae.
Figure 2.
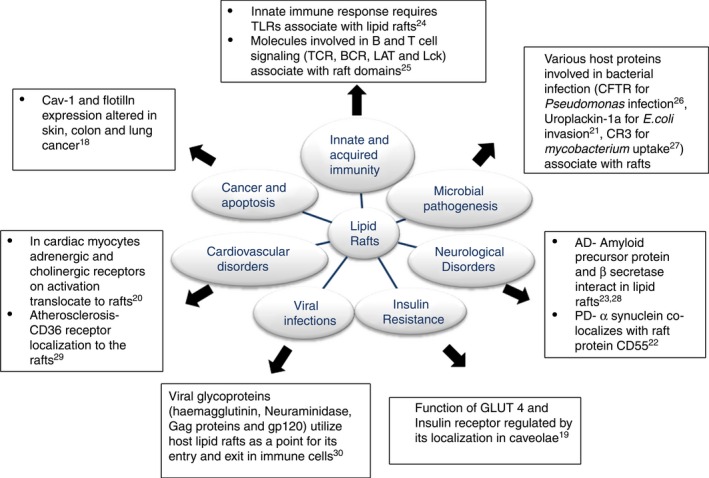
The role of lipid rafts in health and disease – Interaction of various molecules known to be involved in the pathogenesis of various diseases with lipid rafts. Abbreviations: AD, Alzheimer disease; PD, Parkinson's disease; CFTR, cystic fibrosis transmembrane conductance regulator; CR3, complement receptor 3; GLUT4, glucose transporter.
Recently, several reports have suggested that microRNAs act as critical regulators of lipid raft structure by targeting raft‐associated proteins (caveolin and flotillin). MicroRNAs are single‐stranded non‐coding RNAs (~ 22 nucleotides in length) that are ubiquitously present in plants and animals and act in a sequence‐specific manner to regulate gene expression at the post‐transcriptional level by cleavage or translational repression of their target mRNAs.30 Findings over the past few years have strongly supported the role of microRNAs in the regulation of crucial cellular processes such as cell proliferation, apoptosis, adipocyte and myoblast differentiation, fat metabolism, neuronal paternity, developmental regulation and cell differentiation in mammals.31 The first report on the role of microRNA in regulating lipid raft components was published in 2011 when Nohata et al.32 showed that miR‐133a attenuates metastasis in head and neck squamous cell carcinoma cells by targeting caveolin‐1. Other studies from our laboratory and other groups showed that microRNAs (miR‐195, miR‐203, miR‐199a‐3p and miR‐218) significantly inhibit migration and invasion in cancer cells by targeting caveolins.33, 34, 35, 36, 37 In an independent study, Li et al.38 found the anti‐metastatic activity of miR‐124 against breast cancer cells to be flotillin‐dependent. Later, Gong et al.39 found that miR‐138 negatively regulates nuclear factor‐κB (NF‐κB) activation in oesophageal cancer cells by perturbing assimilation of NF‐κB signalling intermediates [TNF receptor‐associated factor 2 (TRAF2), receptor‐interacting protein kinase‐1 (RIP1)] within the lipid raft domain by targeting caveolin‐1, flotillin‐1 and flotillin‐2. The microRNA miR‐124 was also found to regulate caveolae‐mediated endocytosis of pathogens as well as acrosome biogenesis during spermatogenesis by specifically targeting flotillin‐2.40, 41
Apart from cancer pathogenesis, the involvement of miRNAs in mediating regulation of lipid raft components has also been implicated in the pathogenesis of several other diseases (Table 1). Trajkovski et al.42 in their study found that miR‐103/107 regulates glucose homeostasis and insulin sensitivity in obese mice by modulating the levels of caveolin‐1. Furthermore, loss of function by antagonizing miR‐103/107 levels leads to the up‐regulation of caveolin‐1, which in turn stabilizes the insulin receptor and enhanced insulin signalling. In an independent study, Hoeke et al.43 observed that miR‐29a regulates cellular uptake of pathogens during intestinal salmonella infection by way of caveolin‐2‐mediated targeting of focal adhesion and the actin cytoskeleton pathway. Similar to these, Chen et al.44 and Lino Cardenas et al.45 showed that miR‐22 and miR‐199‐5p possess caveolin‐mediated regulatory and protective effects during cardiac and lung injury, respectively. Recently, Sang et al.46 reported the inhibitory action of miR‐150 against inflammatory cytokine production. miR‐150 induces immune tolerance not only by decreasing the levels of ARRB2/AKT/PDE4 (arrestin beta 2/ protein kinase B/ phosphodiesterase 4) but also by preventing their recruitment inside the lipid raft domain. As lipid rafts have been known to be crucial players in innate and acquired immunity,24 we herein review the involvement of these membrane domains in various immune signalling pathways with their mechanisms in detail. We also describe how alterations in lipid rafts can be used as therapeutics for immunological disorders.
Table 1.
List of microRNAs altering disease physiology by targeting raft proteins (caveolin and flotillin)
| miRNA | Target | Activity | References |
|---|---|---|---|
| miR‐133a | Caveolin‐1 | Regulates migration and invasion in head and neck squamous cell carcinoma | 32 |
| miR‐124 | Caveolin‐1 | Reduces caveolar density in porcine kidney cells and regulates caveolae‐mediated endocytosis of pathogens | 41 |
| Flotillin‐1 | Regulates migration and invasion in breast cancer cells | 38 | |
| Flotillin‐2 | Regulates mouse acrosome biogenesis during spermatogenesis | 40 | |
| miR‐203 | Caveolin‐1 | Regulates migration and invasion in pancreatic cancer cells | 33 |
| Regulates longevity and aging in breast tissue during caloric restriction | 37 | ||
| miR‐138 | Flotillin‐1, ‐2, Caveolin‐1 | Regulates nuclear factor‐κB signalling pathway involved in aggressiveness of oesophageal squamous cell carcinoma | 39 |
| miR‐103/107 | Caveolin‐1 | Regulates insulin sensitivity in obese mice | 42 |
| miR‐199‐5p | Caveolin‐1 | Regulator of tissue fibrosis during lung fibrogenesis | 45 |
| miR‐199a‐3p | Caveolin‐2 | Regulates proliferation and survival of endothelial and breast cancer cells | 34 |
| miR‐218 | Caveolin‐2 | Regulates migration and invasion in renal cell carcinoma | 36 |
| miR‐29a | Caveolin‐2 | Inhibits endocytosis of pathogens during salmonella infection | 43 |
| miR‐22 | Caveolin‐3 | Regulates endothelial nitric oxide synthase activity during cardiac injury | 44 |
| miR‐150 | – | Inhibits nuclear factor‐κB signalling by perturbing the recruitment of AKT/ARRB2/PDE4 into the lipid raft | 46 |
Lipid rafts involved in pathogen recognition via Toll‐like receptor signalling
Toll‐like receptors (TLRs) are part of the innate immune system that act as the primary sensors of pathogens, and the literature reveals that the integrity of lipid rafts is crucial for normal TLR signalling. There are 10 members of the TLR family, some of which are present on the cell surface (TLR1, TLR2, TLR4, TLR5 and TLR6) whereas others are present intracellularly (TLR3, TLR7, TLR8 and TLR9). Diverse TLRs recognize diverse pathogen‐associated molecular patterns and trigger secretion of pro‐inflammatory cytokines. Binding of the ligand recruits adaptor toll‐interleukin 1 receptor domain containing adaptor protein (TIRAP) through the TIR domain (TLR/interleukin‐1 receptor homology domain) to initiate downstream signalling. TIRAP recruits MyD88, interleukin‐1 receptor‐associated kinase and TRAF6 to the receptor complex and activates transforming growth factor‐β‐activated kinase, which then further activates transcriptional factors like NF‐κB and interferon regulatory factors resulting in the production of the cytokines tumour necrosis factor‐α (TNF‐α), interleukin 8 (IL‐8), IL‐6 etc.
The innate immune response towards bacterial lipopolysaccharide (LPS) is facilitated by its binding with LPS‐binding protein (LBP). The resulting LPS–LBP complex then binds to CD14, a lipid raft‐associated glycosylphosphatidylinositol‐anchored protein, which then transduces signals by associating with TLR4 and recruiting downstream signalling molecules as shown in Fig. 3(a).47 Microscopy studies (fluorescence resonance energy transfer and fluorescence recovery after photobleaching) have shown that LPS induces the clustering of TLR4 in lipid rafts. In addition to CD14, several other raft proteins such as CD36, CD44, heat‐shock protein 90 are also known to be involved in recognition of LPS and non‐microbial products that participate in TLR4 signalling.48, 49 The intracellular juxtamembrane domains of several Toll‐like receptors are known to possess Cholesterol Recognition Amino‐Acid Consensus sequences, which suggests a direct role of cholesterol in the activation process.50 Polyunsaturated fatty acids are known to inhibit TLR signalling by inhibiting receptor dimerization and TLR recruitment to the lipid rafts.51 Furthermore, it has been observed that increasing cellular cholesterol increases the number of lipid rafts, which in turn affects TLR signalling. Cholesterol‐loading by the cyclodextrin–cholesterol complex in the plasma membrane activates TLR4 signalling whereas in the endosomal membrane, the complex activates TLR3 signalling in macrophages. Zhu et al.52 in their recent study found that cholesterol transporter ATP‐binding cassette transporter A1 knockout macrophages showed MyD88‐dependent enhanced NF‐κB activation and enhanced secretion of pro‐inflammatory cytokines in response to TLR2, TLR4, TLR7, TLR9 agonists compared with wild‐type macrophages. This hyper‐responsiveness is due to increased lipid raft‐free cholesterol and increased TLR localization to lipid rafts.
Figure 3.
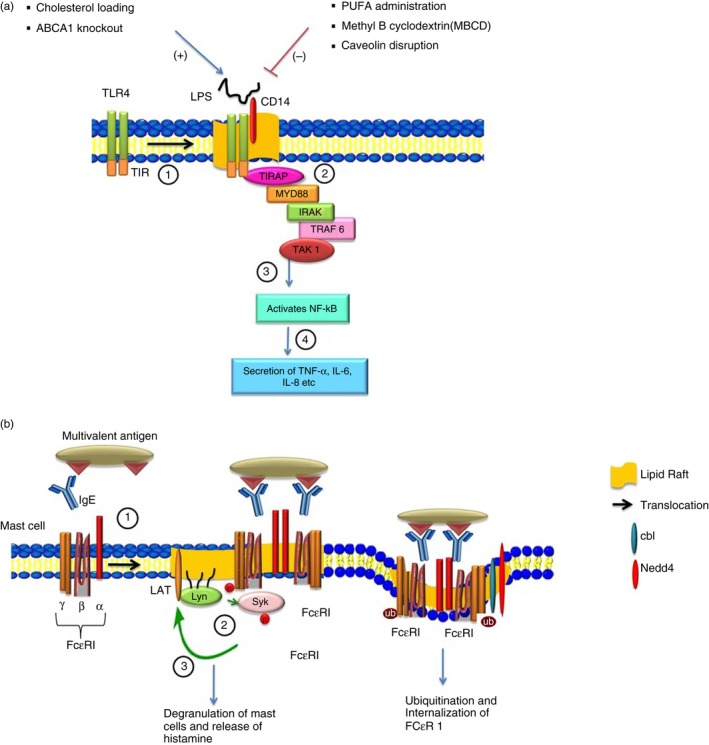
(a) The role of lipid rafts in Toll‐like receptor (TLR) signaling. (1) Binding of ligand such as lipopolysaccharide (LPS) with TLR4 results in its translocation to lipid rafts where it interacts with CD14. (2) In lipid rafts, the interaction of toll‐interleukin 1 receptor domain containing adaptor protein (TIRAP) with TLR4–CD14 complex through the TLR/interleukin‐1 receptor (TIR) domain recruits several adaptor molecules such as MYD88, interleukin‐1R‐associated kinase (IRAK) and tumour necrosis factor receptor‐associated factor 6 (TRAF6) and activates transforming growth factor‐β‐activated kinase 1 (TAK1). (3) TAK1 further activates nuclear factor‐κB (NF‐κB) and leads to the secretion of various cytokines like interleukin‐6 (IL‐6), IL‐8 etc. (4) Disruption of lipid rafts by polyunsaturated fatty acid (PUFA) administration and methyl‐β cyclodextrin (MBCD) or by caveolin disruption inhibits TLR signalling (−), while increasing the cellular cholesterol and ATP‐binding cassette transporter A1 (ABCA1) knockout enhances TLR signalling (+). (b) The role of lipid rafts in IgE signalling. (1) In mast cells, cross‐linking of IgE‐bound antigen with Fcε RI leads to its translocation to lipid rafts. (2) Within the raft domain, a doubly acylated Src‐like tyrosine kinase protein Lyn phosphorylates the immunoreceptor tyrosine‐based activation ( ITAM) domain and results in recruitment and phosphorylation of Syk kinase. (3) Syk further activates linker of activation of T cells (LAT) and recruits several other adaptor molecules in the lipid rafts resulting in the release of chemical mediators such as histamine. (4) Co‐localization of ubiquitin ligase Cbl and Nedd4 with Fcε RI inside the lipid raft domain results in ubiquitination and internalization of the receptor.
Raft protein caveolin‐1 is known to regulate TLR signalling by modulating endothelial nitric oxide synthase (eNOS) activity. The eNOS‐derived nitric oxide (NO) blocks platelet and neutrophil activation and inhibits mast cell‐induced inflammation. Stable binding of eNOS with caveolin‐1 protein suppresses its activity but in the presence of agonist, eNOS becomes dissociated from caveolin‐1 and synthesizes NO. Mirza et al.53 reported that the lungs of caveolin‐1 knockout mice show reduced TLR4 signalling through sustained eNOS activation and decreased NF‐κB activation in response to LPS compared with the lungs of wild‐type mice. Pulmonary hypertension, cardiomyopathy and resistance to LPS‐induced acute lung injury was observed in cav–/– mice, which suggests an inflammatory effect of caveolae.54 Similarly, Tsai et al.55 broadly showed that caveolin‐1 not only regulates TLR4 signalling but also regulates CD14, CD36 and MyD88 protein expression in macrophages and their response towards bacterial infection. All these studies suggest that recruitment of a TLR complex into lipid rafts and the expression of cav‐1 are crucial for modulating the innate immune response.
Lipid rafts in IgE signalling
IgE signalling was the first form of signalling that highlighted the involvement of lipid rafts during allergic and parasitic immune responses. This signalling is initiated when IgE binds to the Fc region of its specific receptor (FcεRI) which is constitutively expressed on mast cells and basophils. FcεRI is a tetrameric molecule that includes one α, one β and two γ chains, the α‐subunit binds to the Fc region of IgE and the β and γ subunits have immunoreceptor tyrosine‐based activation (ITAM) motifs in their cytoplasmic domain. Cross‐linking of these receptors with IgE‐bound antigen increases their association with the lipid rafts where Lyn, a doubly acylated Src‐like tyrosine kinase protein phosphorylates the ITAM domain of FcεRI and creates a novel binding site recognized by cytoplasmic Syk/Zap70 family tyrosine kinases through its tandem Src homology‐2 (SH2) domain.56 Lyn further phosphorylates Syk and activates linker of activation of T cells (LAT), a palmitoylated raft‐associated adaptor molecule that further recruits various molecules in the raft and leads to the degranulation of mast cells (Fig. 3b).57
Lipid raft‐associated adaptor proteins LAT and non‐T cell activation linker (NTAL) act as the positive and negative regulators of FcεRI signalling. In the absence of LAT alone, mast cells showed a slight decrease in secretion of cytokines whereas knockdown of NTAL alone enhances the activity of LAT. Knockdown of both LAT and NTAL completely blocks FcεRI signalling.58 SHP1/SHP2 (Src homology 2 domain tyrosine phosphatase) phosphatase and protein tyrosine phosphatase ε are two phosphatases that negatively regulate LAT/NTAL and are excluded from lipid rafts during antigen‐activated IgE signalling. Exclusion of phosphatases from raft domains provides an environment for the efficient activity of kinases. Young et al.59 found that co‐expression of phosphatases in lipid rafts inhibits Lyn kinase activity along with inhibition of FcεRI phosphorylation. Similarly, Sheets et al.60 observed that depletion of cholesterol by MBCD leads to loss of cross‐linking between FcεRI and Lyn kinase in mast cells, which subsequently attenuates IgE signalling. Another key constituent of lipid raft, flotillin‐1, was identified as an important component of FcεRI‐mediated mast cell activation.61 Ubiquitination of FcεRI regulates the strength of IgE signalling. Mono‐ubiquitination of the receptor functions as a signal for endocytosis inside the lipid rafts whereas polyubiquitination leads to proteasomal degradation.62 Phosphorylation‐dependent ubiquitination of FcεRI occurs in lipid rafts, which in turn leads to endocytosis of the cross‐linked FcεRI receptor. It regulates the half‐life of a receptor on the cell membrane and is therefore involved in regulating the duration of IgE signalling in lipid rafts. Lafont and Simons63 demonstrated co‐localization of ubiquitin ligase Cbl and Nedd4 with FcεRI in lipid rafts where Cbl was shown to mediate FcεRI ubiquitination and Nedd4 to ubiquitinate membrane proteins. Using cyclodextrin (for cholesterol depletion), Molfetta et al.64 showed that lipid raft integrity is essential for receptor ubiquitination and endocytosis, which later participates in downstream signalling.
Role of lipid rafts in lymphocyte activation
Immune cell activation is required for defence against microbes and several reports suggest a crucial role of lipid rafts in their activation. The B‐cell receptor (BCR) present on the surface of B cells comprises a ligand‐binding moiety (membrane‐bound immunoglobulin molecule which binds to antigen) and a signal transduction moiety (two disulphide‐linked heterodimer polypeptides Igα/Igβ each of which contains an ITAM). In the resting state, the BCR has little affinity for rafts and so are present in the non‐raft domain of the plasma membrane. Binding of antigen to BCR brings conformational change in the receptor and results in the translocation of BCR to rafts.65 In rafts, the Src family kinase Lyn phosphorylates the Igα/Igβ heterodimer and recruits the cytoplasmic protein kinase Syk, which further initiates the signalling cascade.66 In immature and anergic B cells, binding of antigen to BCR fails to instigate its association with rafts and further internalization, which in turn triggers apoptosis of the cell. These observations suggest that the association of BCR with rafts is crucial for B‐cell activation.67
As shown in Fig. 4(a), the B‐cell response is augmented or attenuated by the co‐receptors present on its surface. Complement receptor‐2 (CR2, also known as CD21) is a positive co‐receptor in conjunction with two other transmembrane proteins CD19 and CD81 and augments B‐cell activation. Similar to BCR, these co‐receptors reside in non‐lipid raft domains in the resting state and translocate to lipid rafts during B‐cell activation. These co‐receptors decrease the internalization of BCR and lead to prolonged persistence of BCR in lipid rafts and sustained signalling as detected by co‐localization of BCR with the DiIC16 raft marker.68 On the other hand, the low‐affinity co‐receptor FcγRIIB inhibits B‐cell activation when cross‐linked with BCR in rafts.69 During BCR cross‐linking, the immunoreceptor tyrosine‐based inhibitory motif in the cytoplasmic domain of FcγRIIB becomes phosphorylated and recruits SH2 domain‐containing inositol phosphatase, which further dephosphorylates the ITAM domain and so inhibits B‐cell activation.70
Figure 4.
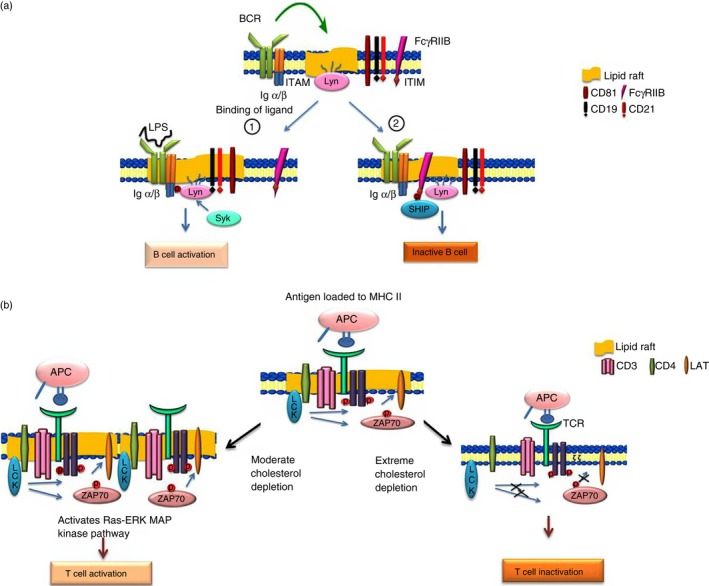
(a) The role of lipid rafts in B‐cell activation. Ligand binding localizes the B‐cell receptor (BCR) to lipid rafts where Lyn phosphorylates the immunoreceptor tyrosine‐based activation (ITAM) domain of BCR and recruits Syk and other molecules which lead to B‐cell activation. (1) In the presence of ligand (lipopolysaccharide; LPS), various co‐receptors such as CD19, CD21 and CD81 associate with the raft domain and positively regulate B‐cell signalling. (2) Interaction of the BCR with Fcγ RIIB co‐receptor leads to phosphorylation of the immunoreceptor tyrosine‐based inhibitory motif (ITIM) domain, which recruits SH2 domain containing inositol 5‐phosphatase (SHIP) phosphatase in the raft domain and negatively regulates B‐cell activation. (b) The role of lipid rafts in T‐cell activation. Cross‐linking of antigen loaded onto the MHC II molecule on antigen‐presenting cells (APC) with the T‐cell receptor (TCR) leads to TCR–CD3 translocation to lipid rafts where lymphocyte‐specific protein tyrosine kinase (Lck) not only phosphorylates the ITAM domain present on ζ chain of TCR but also phosphorylates ZAP70, both of which bind to the phosphorylated domain and activate LAT, resulting in T‐cell activation. Moderate cholesterol depletion leads to raft‐clustering and activation of the Ras–extracellular signal‐regulated kinase (ERK) mitogen‐activated protein (MAP) kinase resulting in T‐cell signalling while extreme cholesterol depletion alters T‐cell activation by affecting the localization and phosphorylation of Lck, LAT and ZAP 70.
Biochemical and microscopy studies have revealed the localization of the T‐cell receptor (TCR) outside the raft domain in a resting state and its translocation in the detergent‐resistant membrane fraction during T‐cell activation.71 In contrast, Dinic et al.72 found TCR in the raft domains of resting T cells and the aggregation of these domains upon TCR engagement. The disparate results concerning the association of TCR with lipid rafts is probably due to the differing methodologies used for the study. T‐cell signalling involves the molecules Lck (lymphocyte‐specific protein tyrosine kinase), dually acylated Src‐family kinase and LAT, which are constitutively present in the rafts. The TCR comprises an α/β heterodimer that associates with CD3 and the ζ homodimer. The α/β chain comprises the ligand‐binding site while CD3 and the ζ chain have a cytoplasmic ITAM domain that becomes phosphorylated by Lck. Upon T‐cell stimulation, the tyrosine kinase ZAP70 binds to the phosphorylated ITAM in rafts and activates LAT and recruits other signalling scaffolds for T‐cell activation. Moreover, the regulators of Lck – protein tyrosine kinase Csk (C‐terminal Src kinase) and the protein tyrosine phosphatase CD45 – are also compartmentalized in T‐cell signalling. Csk interacts with Cbp (Csk‐binding protein) a raft protein for regulating Lck activity whereas CD45 is excluded from the lipid rafts during T‐cell activation.73
There are several pieces of evidence showing that lipid rafts are not only required but are also important for T‐cell activation. A mutant form of Lck that is excluded from the raft domain is unable to activate TCR signalling.74 Lipid raft disruption by methyl‐β‐cyclodextrin modulates T‐cell activation by affecting the phosphorylation and localization of Lck, ZAP‐70 and LAT as shown in Fig. 4(b).75 On the other hand, a few studies have reported that moderate cholesterol depletion activates T‐cell signalling by lipid raft aggregation and activation of the Ras–extracellular signal‐regulated kinase (ERK) mitogen‐activated protein (MAP) kinase pathway.76, 77 This discrepancy could be due to the variation in the time duration of MBCD treatment and the extent of cholesterol depletion, which affects cell viability.
The role of lipid rafts in segregation and integration of key signalling molecules (cytoplasmic CD45, phospholipase Cγ1, SHP1) in T‐cell activation have been proven by artificially targeting them individually.78 Another raft protein, raftlin was also found to play an important role in B‐cell as well as T‐cell signalling.79 Moreover, lipid rafts were found to be crucial for T‐cell polarization as reduced polarization to T helper type 17 cells was observed while differentiation to the other T‐cell subtypes T helper type 1, T helper type 2 and regulatory T cells was not affected when the level of lipid raft glycosphingolipid was decreased in CD4+ T cells by using specific inhibitors of glucosylceramide synthase.80
B‐cell–T‐cell interactions lead to polarization and clustering of TCR and signalling molecules along with the lipid rafts at the immunological synapse, which is required for enhanced and sustained downstream signalling in both B and T cells. CD4 partitions into lipid rafts because of its lipid modification and this induces raft aggregation and formation of molecular clusters at the site of the immunological synapse whereas CD8+ T cells did not show this type of lipid raft polarization upon stimulation.81 Co‐receptors are known to enhance or impede the signalling by altering TCR association with raft domains. The CD28 co‐receptor positively regulates T‐cell activation by increasing cell surface lipid raft concentration as well as enhancing its localization to the immunological synapse, whereas CTLA4 (a negative regulator) inhibits intracellular transport to the T‐cell surface.82 Other co‐stimulatory molecules CD2, CD5, CD9, CD26 and CD44 have also been found to enhance TCR association with lipid rafts.83
Lipid rafts alter cytokine signalling
Cytokines play a wide variety of roles from proliferation, differentiation, migration and apoptosis of immune and non‐immune cells to self‐renewal of embryonic stem cells. Cytokine signalling is regulated by compartmentalization of multi‐subunit chains of cytokine receptors in the raft domains, which determines the specificity of the signalling and provides an efficient platform for concentrating kinases and adaptor molecules. Disruption of lipid rafts on cells alters cytokine signalling and so attenuates the cytokine response.
The main receptor that transduces TNF‐α signals inside the cell is TNF receptor 1 (TNFR1) and there are contradicting reports on its localization in the lipid raft domain. In the absence of stimulation, a small fraction of endogenous TNFR1 is localized to lipid rafts while the majority of the fraction is present in the non‐raft domain. Binding of a ligand recruited more fractions of TNFR1 to lipid rafts in the HT1080 fibrosarcoma cell line and HeLa cells.84 However, TNFR1 was not detected in lipid rafts of resting fibroblasts whereas in U937 and NIH3T3 cells TNFR1 localized exclusively to lipid rafts even in unstimulated conditions.85 These anomalies could be due to the differences in lipid raft isolation methods. Upon ligand binding, the TNF receptor‐associated death domain adaptor binds to the intracellular domain of the receptor and recruits TRAF2 and receptor‐interacting protein kinase to the raft domain, which further leads to downstream activation of NF‐κB and to cell survival.
Treatment of HT1080 cells with MBCD inhibited TNF‐α‐induced NF‐κB activation and rendered these cells susceptible to apoptosis. Doan et al.86 reported that TNFR1 is capable of initiating signalling in both raft and non‐raft membrane fractions. Raft TNFR1 initiates p42mapk/ERK2 signalling while non‐raft TNFR1 initiates NF‐κB signalling. Hence, TNF‐α‐induced p42mapk/ERK2 activation was dependent on lipid raft integrity while the activation of NF‐κB occurs independently of cholesterol and lipid raft integrity. Moreover, TNFR1 contains a specific amino acid sequence in the death domain which is required and sufficient for their translocation to lipid rafts. Deletion of these sequences leads to loss of raft localization and uniform distribution of the receptor in the plasma membrane.87
Fas, a member of the TNFR superfamily was found to be constitutively present in lipid rafts of thymocytes and the B‐cell line SKW6.4 whereas other studies reported that FasL‐induced apoptosis was independent of lipid rafts in Jurkat and HT1080 cells.88 Another member of the TNFR superfamily, CD40, is involved in mediating immune and inflammatory responses like the development of memory B cells and antibody class switching. CD40 interacts with TRAF2 and TRAF3 in rafts and activates NF‐κB. Hostager89 observed that cholesterol depletion inhibits translocation of TRAF molecules to rafts and so alters CD40 signalling.
Interferons are key players in innate and acquired immune responses against viral infections and have anti‐proliferative ability. Decreased interferon‐γ (IFN‐γ) signalling was observed in Leishmania donovani‐infected macrophages of Kala‐azar patients. Sen et al.90 showed that L. donovani increases membrane fluidity and perturbs IFNGR1 and IFNGR2 subunit assembly of the receptor, which occurs in lipid rafts in normal macrophages. Restoration of macrophage membrane cholesterol by exogenous liposomal delivery restores IFN‐γ signalling. Binding of IFN‐γ to the receptor activates receptor‐associated Janus kinase, which in turn phosphorylates Stat (signal transducer and activator of transcription) in the caveolar membrane.91
Leukaemia inhibitory factor (LIF) and ciliary neurotrophic factor (CNTF) are IL‐6 family member cytokines known to have neuroprotective functions. The literature reveals that CNTFR is present in the detergent‐resistant membrane fractions as it is attached by a glycosylphosphatidylinositol anchor to the extracellular membrane while LIFR and gp130 are transmembrane proteins not residing in raft domain.92 LIF interacts with the low‐affinity receptor (LIFR) and gp130 to transduce their signals while CNTF requires CNTFR along with LIFR and gp130 for signal transduction. Stimulation of IMR‐32 neuronal cells with CNTF resulted in translocation of LIFR and gp130 to lipid rafts and cholesterol depletion inhibited this translocation.93 Lee et al.94 observed that depletion of cholesterol by MBCD in embryonic stem cells resulted in decreased expression of LIF‐induced pluripotency genes Oct4, Sox2, Rex1 and FoxD3 and so showed the involvement of lipid rafts in LIF‐induced embryonic stem (ES) cell renewal.
Transforming growth factor (TGF‐β) regulates proliferation and differentiation of the cell. Receptor complex for TGF‐β signalling (TβR1) and (TβR2) have both been reported to be present in lipid rafts in epithelial cells.95 On ligand binding, TβR2 phosphorylates TβR1, which in turn activates Smad proteins, MAP kinase and phosphoinositide 3 kinase molecules in rafts and leads to epithelial to mesenchymal transition. Zuo et al.96 found that nystatin, a cholesterol‐sequestering agent, inhibits TGF‐β‐induced epithelial to mesenchymal transition in epithelial cells by inhibiting the localization of TGF‐β receptors in lipid rafts and activation of the MAP kinase pathway.
Role of lipid rafts in autoimmune disorders
Systemic lupus erythematosus (SLE) and rheumatoid arthritis are autoimmune disorders characterized by impaired humoral and cellular immune responses to self‐antigens. The underlying molecular mechanism involves impaired TCR signalling in SLE patients. Peripheral blood T cells isolated from SLE patients were found to have more cholesterol and GM1 content in their plasma membrane compared with healthy individuals. This suggests an activated state of T cells in SLE, as T cells activated in vitro synthesize more GM1. Reports also suggest that aggregated lipid rafts present on the T‐cell surface contain TCR as well as other co‐stimulatory molecules. Differential expression and localization of CD45 tyrosine phosphatase, CD3 and Lck in lipid rafts were increased in T cells from SLE patients.97 Hence, lipid rafts could be considered as a therapeutic target in SLE treatment. Deng et al.98 reported that disruption of lipid rafts by cyclodextrin delayed disease progression whereas aggregation of rafts using cholera toxin accelerates disease progression in mice.
Rheumatoid arthritis is a chronic, systemic inflammatory disease that mainly affects synovial joints and leads to a disabling and painful condition. The synovium of the inflamed joints harbours many responsive T cells along with activated neutrophils, macrophages, B lymphocytes and dendritic cells. Grinnell et al.99 observed that T cells isolated from synovial fluid of inflamed joints of rheumatoid arthritis patients had an activated phenotype but were hyporesponsive to stimuli compared with T cells from healthy individuals. This hyporesponsiveness of T cells is due to dysfunctional TCR signalling initiated by an increase in oxidative stress in inflamed joints due to the generation of free radicals and depletion of antioxidant glutathione. Under oxidative stress, LAT becomes dissociated from the raft domain, which ultimately leads to abrogation of TCR signalling.100 This signifies the importance of localization of T‐cell signalling components in lipid rafts for efficient T‐cell activation.
Conclusion and future perspective
The integrity of rafts and spatial segregation of receptors in rafts regulates a variety of signal transduction pathways. Specific targeting of lipid rafts could be used as a potential therapeutic target in inflammatory and autoimmune diseases. This review summarizes the existing evidence on the involvement of lipid rafts in immune signalling.
Rational targeting of membrane rafts or caveolae and perturbing their interactions with signalling molecules may soon become a promising therapy for treatment and cure of diseases. To our knowledge, no previous report has addressed the possible association of microRNA with lipid rafts in human diseases. Furthermore, it may be important to develop techniques suitable for studies of membrane microdomains in other physiological functions. Further studies are required to understand the role of lipid rafts in the pathogenesis of other immune disorders.
Disclosures
The authors have no financial or commercial conflicts of interest.
Acknowledgements
This work was supported by grant BSC0302 (TOUCH) from the Council of Scientific and Industrial Research (CSIR). P.V. was supported by a Senior Research Fellowship (SRF) and V.Y. was supported by a Research Associate (RA) fellowship from CSIR.
References
- 1. Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science 1972; 175:720–31. [DOI] [PubMed] [Google Scholar]
- 2. Lisanti MP, Sargiacomo M, Graeve L, Saltiel AR, Rodriguez‐Boulan E. Polarized apical distribution of glycosyl‐phosphatidylinositol‐anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci USA 1988; 85:9557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van MG, Stelzer EH, Wijnaendts‐van‐Resandt RW, Simons K. Sorting of sphingolipids in epithelial (Madin–Darby canine kidney) cells. J Cell Biol 1987; 105:1623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pike LJ. Rafts defined: a report on the keystone symposium on lipid rafts and cell function. J Lipid Res 2006; 47:1597–8. [DOI] [PubMed] [Google Scholar]
- 5. Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000; 1:31–9. [DOI] [PubMed] [Google Scholar]
- 6. Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 2002; 296:1821–5. [DOI] [PubMed] [Google Scholar]
- 7. Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H et al Paradigm shift of the plasma membrane concept from the two‐dimensional continuum fluid to the partitioned fluid: high‐speed single‐molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct 2005; 34:351–78. [DOI] [PubMed] [Google Scholar]
- 8. Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 2007; 8:185–94. [DOI] [PubMed] [Google Scholar]
- 9. Medina FA, de Almeida CJ, Dew E, Li J, Bonuccelli G, Williams TM et al Caveolin‐1‐deficient mice show defects in innate immunity and inflammatory immune response during Salmonella enterica serovar Typhimurium infection. Infect Immun 2006; 74:6665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galbiati F, Razani B, Lisanti MP. Caveolae and caveolin‐3 in muscular dystrophy. Trends Mol Med 2001; 7:435–41. [DOI] [PubMed] [Google Scholar]
- 11. London E, Brown DA. Insolubility of lipids in triton X‐100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim Biophys Acta 2000; 1508:182–95. [DOI] [PubMed] [Google Scholar]
- 12. Steck TL, Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct 1973; 1:220–32. [DOI] [PubMed] [Google Scholar]
- 13. Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA 2003; 100:5795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta 2007; 1768:1311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S et al Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 2009; 457:1159–62. [DOI] [PubMed] [Google Scholar]
- 16. Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 2010; 11:688–99. [DOI] [PubMed] [Google Scholar]
- 17. Arkhipova KA, Sheyderman AN, Laktionov KK, Mochalnikova VV, Zborovskaya IB. Simultaneous expression of flotillin‐1, flotillin‐2, stomatin and caveolin‐1 in non‐small cell lung cancer and soft tissue sarcomas. BMC Cancer 2014; 14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capozza F, Combs TP, Cohen AW, Cho YR, Park SY, Schubert W et al Caveolin‐3 knockout mice show increased adiposity and whole body insulin resistance, with ligand‐induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol 2005; 288:C1317–31. [DOI] [PubMed] [Google Scholar]
- 19. Das M, Das DK. Lipid raft in cardiac health and disease. Curr Cardiol Rev 2009; 5:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duncan MJ, Li G, Shin JS, Carson JL, Abraham SN. Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem 2004; 279:18944–51. [DOI] [PubMed] [Google Scholar]
- 21. Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of α‐synuclein. J Neurosci 2004; 24:6715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hooper NM. Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein. Biochem Soc Trans 2005; 33:335–8. [DOI] [PubMed] [Google Scholar]
- 23. Hueber AO. Role of membrane microdomain rafts in TNFR‐mediated signal transduction. Cell Death Differ 2003; 10:7–9. [DOI] [PubMed] [Google Scholar]
- 24. Katagiri YU, Kiyokawa N, Fujimoto J. A role for lipid rafts in immune cell signaling. Microbiol Immunol 2001; 45:1–8. [DOI] [PubMed] [Google Scholar]
- 25. Kowalski MP, Pier GB. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa‐induced cellular activation. J Immunol 2004; 172:418–25. [DOI] [PubMed] [Google Scholar]
- 26. Peyron P, Bordier C, N'Diaye EN, Maridonneau‐Parini I. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol‐anchored proteins. J Immunol 2000; 165:5186–91. [DOI] [PubMed] [Google Scholar]
- 27. Riddell DR, Christie G, Hussain I, Dingwall C. Compartmentalization of β‐secretase (Asp2) into low‐buoyant density, noncaveolar lipid rafts. Curr Biol 2001; 11:1288–93. [DOI] [PubMed] [Google Scholar]
- 28. Zeng Y, Tao N, Chung KN, Heuser JE, Lublin DM. Endocytosis of oxidized low density lipoprotein through scavenger receptor CD36 utilizes a lipid raft pathway that does not require caveolin‐1. J Biol Chem 2003; 278:45931–6. [DOI] [PubMed] [Google Scholar]
- 29. Zhang J, Pekosz A, Lamb RA. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J Virol 2000; 74:4634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281–97. [DOI] [PubMed] [Google Scholar]
- 31. Chhabra R, Dubey R, Saini N. Cooperative and individualistic functions of the microRNAs in the miR‐23a~27a~24‐2 cluster and its implication in human diseases. Mol Cancer 2010; 9:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nohata N, Hanazawa T, Kikkawa N, Sakurai D, Sasaki K, Chiyomaru T et al Identification of novel molecular targets regulated by tumor suppressive miR‐1/miR‐133a in maxillary sinus squamous cell carcinoma. Int J Oncol 2011; 39:1099–107. [DOI] [PubMed] [Google Scholar]
- 33. Miao L, Xiong X, Lin Y, Cheng Y, Lu J, Zhang J et al miR‐203 inhibits tumor cell migration and invasion via caveolin‐1 in pancreatic cancer cells. Oncol Lett 2014; 7:658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shatseva T, Lee DY, Deng Z, Yang BB. MicroRNA miR‐199a‐3p regulates cell proliferation and survival by targeting caveolin‐2. J Cell Sci 2011; 124:2826–36. [DOI] [PubMed] [Google Scholar]
- 35. Singh R, Yadav V, Kumar S, Saini N. MicroRNA‐195 inhibits proliferation, invasion and metastasis in breast cancer cells by targeting FASN, HMGCR, ACACA and CYP27B1. Sci Rep 2015; 5:17454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamasaki T, Seki N, Yoshino H, Itesako T, Hidaka H, Yamada Y et al MicroRNA‐218 inhibits cell migration and invasion in renal cell carcinoma through targeting caveolin‐2 involved in focal adhesion pathway. J Urol 2013; 190:1059–68. [DOI] [PubMed] [Google Scholar]
- 37. Orom UA, Lim MK, Savage JE, Jin L, Saleh AD, Lisanti MP et al MicroRNA‐203 regulates caveolin‐1 in breast tissue during caloric restriction. Cell Cycle 2012; 11:1291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li L, Luo J, Wang B, Wang D, Xie X, Yuan L et al Microrna‐124 targets flotillin‐1 to regulate proliferation and migration in breast cancer. Mol Cancer 2013; 12:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gong H, Song L, Lin C, Liu A, Lin X, Wu J et al Downregulation of miR‐138 sustains NF‐κB activation and promotes lipid raft formation in esophageal squamous cell carcinoma. Clin Cancer Res 2013; 19:1083–93. [DOI] [PubMed] [Google Scholar]
- 40. Wu Y, Zhong A, Zheng H, Jiang M, Xia Z, Yu J et al Expression of flotilin‐2 and acrosome biogenesis are regulated by MiR‐124 during spermatogenesis. PLoS ONE 2015; 10:e0136671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang S, Liu X, Li X, Sun S, Sun F, Fan B et al MicroRNA‐124 reduces caveolar density by targetingcaveolin‐1 in porcine kidney epithelial PK15 cells. Mol Cell Biochem 2013; 384:213–9. [DOI] [PubMed] [Google Scholar]
- 42. Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M et al MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011; 474:649–53. [DOI] [PubMed] [Google Scholar]
- 43. Hoeke L, Sharbati J, Pawar K, Keller A, Einspanier R, Sharbati S et al Intestinal Salmonella typhimurium infection leads to miR‐29a induced caveolin 2 regulation. PLoS ONE 2013; 8:e67300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Z, Qi Y, Gao C. Cardiac myocyte‐protective effect of microRNA‐22 during ischemia and reperfusion through disrupting the caveolin‐3/eNOS signaling. Int J Clin Exp Pathol 2015; 8:4614–26. [PMC free article] [PubMed] [Google Scholar]
- 45. Lino Cardenas CL, Henaoui IS, Courcot E, Roderburg C, Cauffiez C, Aubert S et al miR‐199a‐5p Is upregulated during fibrogenic response to tissue injury and mediates TGFβ‐induced lung fibroblast activation by targeting caveolin‐1. PLoS Genet 2013; 9:e1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sang W, Wang Y, Zhang C, Zhang D, Sun C, Niu M et al MiR‐150 impairs inflammatory cytokine production by targeting ARRB‐2 after blocking CD28/B7 costimulatory pathway. Immunol Lett 2015; 172:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990; 249:1431–3. [DOI] [PubMed] [Google Scholar]
- 48. Plociennikowska A, Hromada‐Judycka A, Borzecka K, Kwiatkowska K. Co‐operation of TLR4 and raft proteins in LPS‐induced pro‐inflammatory signaling. Cell Mol Life Sci 2015; 72:557–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Triantafilou M, Lepper PM, Olden R, Dias IS, Triantafilou K. Location, location, location: is membrane partitioning everything when it comes to innate immune activation? Mediators Inflamm 2011; 2011:186093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruysschaert JM, Lonez C. Role of lipid microdomains in TLR‐mediated signalling. Biochim Biophys Acta 2015; 1848:1860–7. [DOI] [PubMed] [Google Scholar]
- 51. Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH Fatty acids modulate Toll‐like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species‐dependent manner. J Biol Chem 2009; 284:27384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ et al Macrophage ABCA1 reduces MyD88‐dependent Toll‐like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res 2010; 51:3196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB et al Caveolin‐1 deficiency dampens Toll‐like receptor 4 signaling through eNOS activation. Am J Pathol 2010; 176:2344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lei MG, Morrison DC. Differential expression of caveolin‐1 in lipopolysaccharide‐activated murine macrophages. Infect Immun 2000; 68:5084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsai TH, Chen SF, Huang TY, Tzeng CF, Chiang AS, Kou YR et al Impaired Cd14 and Cd36 expression, bacterial clearance, and Toll‐like receptor 4‐Myd88 signaling in caveolin‐1‐deleted macrophages and mice. Shock 2011; 35:92–9. [DOI] [PubMed] [Google Scholar]
- 56. Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest 2002; 110:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rivera J, Arudchandran R, Gonzalez‐Espinosa C, Manetz TS, Xirasagar S. A perspective: regulation of IgE receptor‐mediated mast cell responses by a LAT‐organized plasma membrane‐localized signaling complex. Int Arch Allergy Immunol 2001; 124:137–41. [DOI] [PubMed] [Google Scholar]
- 58. Draber P, Halova I, Levi‐Schaffer F, Draberova L. Transmembrane adaptor proteins in the high‐affinity IgE receptor signaling. Front Immunol 2011; 2:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Young RM, Zheng X, Holowka D, Baird B. Reconstitution of regulated phosphorylation of FcεRI by a lipid raft‐excluded protein‐tyrosine phosphatase. J Biol Chem 2005; 280:1230–5. [DOI] [PubMed] [Google Scholar]
- 60. Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn‐mediated tyrosine phosphorylation of FcεRI and their association with detergent‐resistant membranes. J Cell Biol 1999; 145:877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Silveira E, Souza AM, Mazucato VM, Jamur MC, Oliver C. Lipid rafts in mast cell biology. J Lipids 2011; 2011:752906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shih SC, Sloper‐Mould KE, Hicke L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J 2000; 19:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lafont F, Simons K. Raft‐partitioning of the ubiquitin ligases Cbl and Nedd4 upon IgE‐triggered cell signaling. Proc Natl Acad Sci USA 2001; 98:3180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Molfetta R, Gasparrini F, Peruzzi G, Vian L, Piccoli M, Frati L et al Lipid raft‐dependent FcεRI ubiquitination regulates receptor endocytosis through the action of ubiquitin binding adaptors. PLoS ONE 2009; 4:e5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gupta N, DeFranco AL. Visualizing lipid raft dynamics and early signaling events during antigen receptor‐mediated B‐lymphocyte activation. Mol Biol Cell 2003; 14:432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cheng PC, Dykstra ML, Mitchell RN, Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Med 1999; 190:1549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sproul TW, Malapati S, Kim J, Pierce SK. Cutting edge: B cell antigen receptor signaling occurs outside lipid rafts in immature B cells. J Immunol 2000; 165:6020–3. [DOI] [PubMed] [Google Scholar]
- 68. Cherukuri A, Shoham T, Sohn HW, Levy S, Brooks S, Carter R et al The tetraspanin CD81 is necessary for partitioning of coligated CD19/CD21‐B cell antigen receptor complexes into signaling‐active lipid rafts. J Immunol 2004; 172:370–80. [DOI] [PubMed] [Google Scholar]
- 69. Aman MJ, Tosello‐Trampont AC, Ravichandran K. Fcγ RIIB1/SHIP‐mediated inhibitory signaling in B cells involves lipid rafts. J Biol Chem 2001; 276:46371–8. [DOI] [PubMed] [Google Scholar]
- 70. Drevot P, Langlet C, Guo XJ, Bernard AM, Colard O, Chauvin JP et al TCR signal initiation machinery is pre‐assembled and activated in a subset of membrane rafts. EMBO J 2002; 21:1899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Beck‐Garcia K, Beck‐Garcia E, Bohler S, Zorzin C, Sezgin E, Levental I et al Nanoclusters of the resting T cell antigen receptor (TCR) localize to non‐raft domains. Biochim Biophys Acta 2015; 1853:802–9. [DOI] [PubMed] [Google Scholar]
- 72. Dinic J, Riehl A, Adler J, Parmryd I. The T cell receptor resides in ordered plasma membrane nanodomains that aggregate upon patching of the receptor. Sci Rep 2015; 5:10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol 2003; 21:107–37. [DOI] [PubMed] [Google Scholar]
- 74. Kabouridis PS, Magee AI, Ley SC. S‐acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J 1997; 16:4983–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 1998; 9:239–46. [DOI] [PubMed] [Google Scholar]
- 76. Kabouridis PS, Janzen J, Magee AL, Ley SC. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur J Immunol 2000; 30:954–63. [DOI] [PubMed] [Google Scholar]
- 77. Mahammad S, Dinic J, Adler J, Parmryd I. Limited cholesterol depletion causes aggregation of plasma membrane lipid rafts inducing T cell activation. Biochim Biophys Acta 2010; 1801:625–34. [DOI] [PubMed] [Google Scholar]
- 78. D'Oro U, Ashwell JD. Cutting edge: the CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J Immunol 1999; 162:1879–83. [PubMed] [Google Scholar]
- 79. Saeki K, Miura Y, Aki D, Kurosaki T, Yoshimura A. The B cell‐specific major raft protein, Raftlin, is necessary for the integrity of lipid raft and BCR signal transduction. EMBO J 2003; 22:3015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhu Y, Gumlaw N, Karman J, Zhao H, Zhang J, Jiang JL et al Lowering glycosphingolipid levels in CD4+ T cells attenuates T cell receptor signaling, cytokine production, and differentiation to the Th17 lineage. J Biol Chem 2011; 286:14787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Balamuth F, Brogdon JL, Bottomly K. CD4 raft association and signaling regulate molecular clustering at the immunological synapse site. J Immunol 2004; 172:5887–92. [DOI] [PubMed] [Google Scholar]
- 82. Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J Exp Med 2001; 194:1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yashiro‐Ohtani Y, Zhou XY, Toyo‐Oka K, Tai XG, Park CS, Hamaoka T et al Non‐CD28 costimulatory molecules present in T cell rafts induce T cell costimulation by enhancing the association of TCR with rafts. J Immunol 2000; 164:1251–9. [DOI] [PubMed] [Google Scholar]
- 84. Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFα‐mediated NF‐κB activation. Immunity 2003; 18:655–64. [DOI] [PubMed] [Google Scholar]
- 85. Ko YG, Lee JS, Kang YS, Ahn JH, Seo JS. TNF‐α‐mediated apoptosis is initiated in caveolae‐like domains. J Immunol 1999; 162:7217–23. [PubMed] [Google Scholar]
- 86. Doan JE, Windmiller DA, Riches DW. Differential regulation of TNF‐R1 signaling: lipid raft dependency of p42mapk/erk2 activation, but not NF‐κB activation. J Immunol 2004; 172:7654–60. [DOI] [PubMed] [Google Scholar]
- 87. Cottin V, Doan JE, Riches DW. Restricted localization of the TNF receptor CD120a to lipid rafts: a novel role for the death domain. J Immunol 2002; 168:4095–102. [DOI] [PubMed] [Google Scholar]
- 88. Hueber AO, Bernard AM, Herincs Z, Couzinet A, He HT. An essential role for membrane rafts in the initiation of Fas/CD95‐triggered cell death in mouse thymocytes. EMBO Rep 2002; 3:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hostager BS. Roles of TRAF6 in CD40 signaling. Immunol Res 2007; 39:105–14. [DOI] [PubMed] [Google Scholar]
- 90. Sen S, Roy K, Mukherjee S, Mukhopadhyay R, Roy S. Restoration of IFNgammaR subunit assembly, IFN‐γ signaling and parasite clearance in Leishmania donovani infected macrophages: role of membrane cholesterol. PLoS Pathog 2011; 7:e1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Takaoka A, Mitani Y, Suemori H, Sato M, Yokochi T, Noguchi S et al Cross talk between interferon‐γ and ‐α/β signaling components in caveolar membrane domains. Science 2000; 288:2357–60. [DOI] [PubMed] [Google Scholar]
- 92. Buk DM, Waibel M, Braig C, Martens AS, Heinrich PC, Graeve L et al Polarity and lipid raft association of the components of the ciliary neurotrophic factor receptor complex in Madin–Darby canine kidney cells. J Cell Sci 2004; 117:2063–75. [DOI] [PubMed] [Google Scholar]
- 93. Port MD, Gibson RM, Nathanson NM. Differential stimulation‐induced receptor localization in lipid rafts for interleukin‐6 family cytokines signaling through the gp130/leukemia inhibitory factor receptor complex. J Neurochem 2007; 101:782–93. [DOI] [PubMed] [Google Scholar]
- 94. Lee MY, Ryu JM, Lee SH, Park JH, Han HJ. Lipid rafts play an important role for maintenance of embryonic stem cell self‐renewal. J Lipid Res 2010; 51:2082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Di Guglielmo GM, Le RC, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF‐β receptor signalling and turnover. Nat Cell Biol 2003; 5:410–21. [DOI] [PubMed] [Google Scholar]
- 96. Zuo W, Chen YG. Specific activation of mitogen‐activated protein kinase by transforming growth factor‐beta receptors in lipid rafts is required for epithelial cell plasticity. Mol Biol Cell 2009; 20:1020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jury EC, Kabouridis PS. T‐lymphocyte signalling in systemic lupus erythematosus: a lipid raft perspective. Lupus 2004; 13:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Deng GM, Tsokos GC. Cholera toxin B accelerates disease progression in lupus‐prone mice by promoting lipid raft aggregation. J Immunol 2008; 181:4019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Grinnell S, Yoshida K, Jasin HE. Responses of lymphocytes of patients with rheumatoid arthritis to IgG modified by oxygen radicals or peroxynitrite. Arthritis Rheum 2005; 52:80–3. [DOI] [PubMed] [Google Scholar]
- 100. Gringhuis SI, Papendrecht‐van der Voort EA, Leow A, Nivine Levarht EW, Breedveld FC, Verweij CL et al Effect of redox balance alterations on cellular localization of LAT and downstream T‐cell receptor signaling pathways. Mol Cell Biol 2002; 22:400–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


