Summary
Schistosoma japonicum infection can induce granulomatous inflammation and cause tissue damage in the mouse liver. The cytokine secretion profile of T helper (Th) cells depends on both the nature of the activating stimulus and the local microenvironment (e.g. cytokines and other soluble factors). In the present study, we found an accumulation of large numbers of IFN‐γ + IL‐4+ CD4+ T cells in mouse livers. This IFN‐γ + IL‐4+ cell population increased from 0·68 ± 0·57% in uninfected mice to 7·05 ± 3·0% by week 4 following infection and to 9·6 ± 5·28% by week 6, before decreasing to 6·3 ± 5·9% by week 8 in CD4 T cells. Moreover, IFN‐γ + IL‐4+ Th cells were also found in mouse spleen and mesenteric lymph nodes 6 weeks after infection. The majority of the IFN‐γ + IL‐4+ Th cells were thought to be related to a state of immune activation, and some were memory T cells. Moreover, we found that these S. japonicum infection‐induced IFN‐γ + IL‐4+ cells could express interleukin‐2 (IL‐2), IL‐9, IL‐17 and high IL‐10 levels at 6 weeks after S. japonicum infection. Taken together, our data suggest the existence of a population of IFN‐γ + IL‐4+ plasticity effector/memory Th cells following S. japonicum infection in C57BL/6 mice.
Keywords: CD4 T cells, cytokines, interferon‐γ, interleukin‐4, liver, Schistosoma japonicum
Abbreviations
- APC
allophycocyanin
- BFA
Brefeldin A
- BSA
Bovine Serum Albumin
- DAPI
4′,6‐diamidino‐2‐phenylindole
- DN
Double Negative
- ELISA
Enzyme Linked Immunosorbent Assay
- FACS
Fluorescence Activating Cell Sorter
- FITC
Fluorescein Isothiocyanae
- HBSS
Hank's balanced salt solution
- IFN
interferon
- IL
interleukin
- mAb
monoclonal antibody
- MLN
Mesenteric lymph node
- PE
phycoerythrin
- PerCP
Peridinin‐Chlorophyll‐Protein Complex
- PMA
Phorbol‐12‐myristate‐13‐acetate
- RT‐PCR
Reverse Transcription‐Polymerase Chain Reaction
- S. japonicum
Schistosoma japonicum
- Th
T helper
Introduction
Murine and human studies have demonstrated that the normal liver contains significant numbers of resident lymphocytes.1 They are known as intrahepatic immune cells, and they are phenotypically and functionally distinct from those in the peripheral blood and may mature locally.2 For example, natural killer (NK) cells, NKT cells, γδT cells and memory T cells are highly enriched in the liver, whereas the proportions of naive T and B lymphocytes have been reported to be relatively small.3, 4 Intrahepatic immune cells are known to perform diverse immunological functions, generating immunological tolerance to a large number of dietary antigens that are received directly from the gastrointestinal tract and, at the same time, respond to blood‐ and food‐borne pathogens with effective immunological defences.5, 6
Schistosome infection is initiated by cercariae, which burrow into the skin, transform into schistosomula, and then enter the vasculature and migrate to the portal system, where they mature into adult worms.7 Eggs are released by female parasites, and they begin to lodge in the interlobular portal venules 4–6 weeks after infection. During the infection, the liver is the principal site that is affected because many of the eggs are carried by the blood flow into this organ, and the sinusoids of the liver are too small for the eggs to traverse. Moreover, these eggs secrete antigens that can induce granulomatous inflammation, which can sequester the pathogenic egg antigens but also leads to host tissue damage,8 leading to severe circulatory impairments such as portal hypertension.9
The process of egg granuloma formation is dependent upon sensitized CD4+ T helper (Th) cells, which are the central effectors of the immune response.10 After encountering antigen, these Th cells differentiate into functionally distinct cell subsets that are distinguished by specific cytokine production profiles.11 For example, Th1 cells produce high levels of interferon‐γ (IFN‐γ); Th2 cells produce interleukin‐4 (IL‐4), IL‐5 and IL‐13; Th9 cells produce IL‐9 and IL‐10,12 and Th17 cells produce high levels of IL‐17.13
However, recent studies challenging the single‐fate model have highlighted a significant degree of flexibility and plasticity between T‐cell destinies in vitro and to a lesser extent in vivo.14 Many types of plasticity cells were reported, such as Th17/Th1 conversion cells, Th1/Th2 conversion cells, and so on.14 For example, IFN‐γ + IL‐4+ cells could be readily observed in vivo in mice.15 Lymphocytic choriomeningitis virus‐specific Th1 or Th2 cells could give rise to comparable frequencies of IFN‐γ‐producing cells following lymphocytic choriomeningitis virus infection. The Th2‐polarized cells could give rise to a substantial population of IFN‐γ and IL‐4 co‐expressing cells.16 Plasticity is regarded as a differentiated state of CD4 T cells.17
The aim of this study was to investigate some characteristics of IFN‐γ and IL‐4 co‐expressing CD4 T cells in the C57BL/6 mouse liver during Schistosoma japonicum infection.
Materials and methods
Ethics statement
Female C57BL/6 mice, 6–8 weeks old, were purchased from Zhongshan University Animal Centre (Guangzhou, China) and maintained in an animal care facility under pathogen‐free conditions. All protocols for animal use were approved as appropriate and humane by the Guangzhou Medical University institutional animal care and use committee (2011‐44). Every effort was made to minimize suffering.
Parasite infection
Schistosoma japonicum cercariae were shed from naturally infected Oncomelania hupensis snails, which were purchased from Jiangsu Institute of Parasitic Disease (Wuxi, China). Thirty mice were infected percutaneously with 40 ± 5 cercariae. The infected mice were killed at 4, 6 and 8 weeks after infection. Ten pathogen‐free mice constituted the control group.
Antibodies
The following monoclonal antibodies (all from BD/Pharmingen, San Diego, CA) were used for cell phenotype determinations: allophycocyanin (APC)‐Cy7‐conjugated anti‐mouse CD3 (145‐2C11), Peridinin chlorophyll protein‐conjugated anti‐mouse CD4 (RM4‐5), phycoerythrin (PE) ‐conjugated anti‐mouse CD25 (3C7), FITC‐conjugated anti‐mouse CD45RB (16A), FITC‐conjugated anti‐mouse CD62L (MEL‐14), APC‐conjugated anti‐mouse CD69 (H1.2F3), PE‐conjugated anti‐mouse CD127 (SB/199), APC‐conjugated anti‐mouse IL‐2 (JES6‐5H4), PE‐conjugated anti‐mouse IL‐4 (11B11), APC‐conjugated anti‐mouse IL‐9 (D9302C12), APC‐conjugated anti‐mouse IL‐10 (JES5‐16E3), PE‐conjugated anti‐mouse IL‐17A (TC11‐18H10), APC‐conjugated anti‐mouse IFN‐γ (XMG1.2), FITC‐conjugated anti‐mouse IFN‐γ (XMG1.2), APC‐conjugated anti‐mouse IL‐10 (JES5‐16E3) and an isotype‐matched rat IgG2a monoclonal antibody (clone RTK2758).
Lymphocyte isolation
Mice were killed at 4, 6 or 8 weeks after infection. The precava was cut, and sterile normal saline was injected to remove blood from the liver through the ventriculus sinister. The liver was removed, pressed through 200‐gauge stainless‐steel mesh, and suspended in Hanks' balanced salt solution (HBSS). Hepatic mononuclear cells were isolated with Ficoll–Hypaque (Dakewe, Shenzhen, China) density‐gradient centrifugation for 20 min at 800 g. The lung was excised and cut into small pieces and incubated in 5 ml of digestion buffer (collagenase IV/DNase I mix, Invitrogen, CA, USA) for 30 min at 37°. The digested lung tissue was pressed through 200‐gauge stainless‐steel mesh and was then suspended in HBSS. Lymphocytes were isolated with Ficoll–Hypaque density‐gradient centrifugation. The mesenteric lymph nodes (MLN) were harvested. Single cell suspensions were prepared by passing through 200‐gauge stainless‐steel mesh and were suspended in HBSS. The isolated cells were washed twice in HBSS and re‐suspended at 2 × 106 cells/ml in complete RPMI‐1640 medium supplemented with 10% heat‐inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mm glutamine, and 50 μm 2‐mercaptoethanol.
ELISA for cytokines
Single‐cell suspensions were prepared and plated in a 96‐well plate at 4 × 105 cells/200 μl per well. Anti‐CD3 (1 μg/ml) and anti‐CD28 (1 μg/ml) were added to each well, and the plate was incubated at 37°. Cell culture supernatants were collected 72 hr later. The culture supernatant cytokines were analysed using cytokine assay kits for IFN‐γ (BD Pharmingen, San Diego, CA, USA) and IL‐4 (BD Pharmingen) detection. ELISAs were performed in accordance with the manufacturer's instructions. Samples were read at 450 nm with a micro‐plate reader (Model ELX‐800, BioTek, Winooski, VT, USA).
RNA preparation for RT‐PCR
Total RNA was isolated from the liver cells of infected and normal mice using Trizol Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) following the manufacturer's instructions. The cDNA was synthesized, and mRNA expression was determined with a PrimeScript® RT‐PCR Kit (Takara, Tokyo, Japan) according to the manufacturer's instructions. The primers were synthesized from Invitrogen (Shanghai, China) as follows: for IFN‐γ, 5'‐AGG CCA TCA GCA ACA ACA TAA G‐3' (forward) and 5'‐TCA GCA GCG ACT CCT TTT CCG‐3' (reverse); for IL‐4, 5'‐GGT CTC AAC CCC CAG CTA GT‐3' (forward) and 5'‐TGA TGC TCT TTA GGC TTT CCA‐3' (reverse); for T‐bet, 5'‐CAA GTG GGT GCA GTG TGG AAA G‐3' (forward) and 5'‐TGG AGA GAC TGC AGG ACG ATC‐3' (reverse); for GATA‐3', 5'‐GGA GGA CTT CCC CAA GAG CA‐3' (forward) and 5'‐CAT GCT GGA AGG GTG GTG A‐3' (reverse). Amplification was performed using the S1000 Thermal Cycler (Bio‐Rad, Hercules, CA) under the following conditions: 45 seconds at 94°, 45 seconds at 60°, and 45 seconds at 72°, with 32 cycles for GATA‐3; PCR cycles were 180 seconds at 94°, 45 seconds at 51·6°, and 60 seconds at 72°, with 30 cycles for IFN‐γ, IL‐4 and T‐bet. The RT‐PCR products were analysed on a 1·0% multiwelled agarose gel. Electrophoresis was carried out in 1 × TAE buffer at 100 V for 30 min. The gel was visualized in a ChemiDoc XRS Universal Hood II (Bio‐Rad).
Histology studies
Livers were removed from the mice, perfused three times with 0·01 m PBS (pH 7·4), fixed in 10% formalin, embedded in paraffin, and sectioned. The sections were then examined by light microscopy under 100 × and 400 × magnification after standard haematoxylin & eosin staining for visualization of cellular changes.
The fixed livers were embedded in OCT compound (Sakura Finetec Japan, Tokyo, Japan), frozen in liquid nitrogen and stored at −70°. Frozen tissues were cut (7 μm), mounted on slides, fixed in ice‐cold acetone for 20 min, and permeabilized with 0·1% Triton/PBS. Slides were then treated with the blocking buffer (10% fetal calf serum in PBS) for 1 hr. Slides were incubated with FITC‐conjugated anti‐mouse IFN‐γ and PE‐conjugated anti‐mouse IL‐4 in a 1 : 20 dilution overnight at 4°. Nucleic acid staining was carried out by labelling with DAPI for 10 min. Following three washes with PBS, coverslips were mounted in gel‐mount. Fluorescent staining patterns were detected and acquired by serial imaging on a CARL ZEISS Axio Imager confocal microscope.
Cell surface marker and intracellular cytokine expression detection
The isolated mononuclear cells from the control and S. japonicum‐infected mice were stimulated with 20 ng/ml PMA plus 1 μg/ml ionomycin for 5 hr at 37° under a 5% CO2 atmosphere. Brefeldin A (10 μg/ml; Sigma, St Louis, MO, USA) was added during the last 4 hr of incubation. The cells were washed twice in PBS and then stained (30 min at 4° in the dark) with conjugated antibodies specific for the cell surface antigens CD3, CD4, CD8, CD25, CD42L, CD45RB, CD62L, CD69 and CD127. The stained cells were washed twice in PBS, fixed with 4% paraformaldehyde, and permeabilized overnight at 4° in PBS buffer containing 0·1% saponin (Sigma), 0·1% BSA and 0·05% NaN3. These treated cells were then stained (30 min at 4° in the dark) with conjugated antibodies that were specific for the following intracellular cytokines: IFN‐γ, IL‐2, IL‐4, IL‐9, IL‐10 and IL‐17. The expression phenotypes of these antibody‐labelled lymphocytes were analysed by flow cytometry (BD Calibur or FACS Aria ll cell sorter). The results were analysed with the cellquest program (BD). Isotype‐matched controls for the cytokines were included in each staining protocol.
Statistical analyses
The differences between the means were evaluated with unpaired, two‐tailed t‐tests. A P < 0·05 was considered significant.
Results
IFN‐γ and IL‐4 were induced in schistosome‐infected liver lymphocytes
To explore the IFN‐γ and IL‐4 production that was induced by schistosome infection, single mononuclear liver cell suspensions of normal and schistosome‐infected mice (4–6 weeks after infection) were prepared and cultured in the presence of anti‐CD3 plus anti‐CD28. Seventy‐two hours later, the culture supernatants were collected, and the IFN‐γ and IL‐4 levels were detected with ELISAs. The results (Fig. 1a) indicated that the IFN‐γ and IL‐4 concentrations in the anti‐CD3/anti‐CD28‐stimulated liver supernatants from infected mice were 14·1 ± 2·8 ng/ml and 967 ± 561 pg/ml, respectively, which were obviously higher than those from normal mice and unstimulated controls (P < 0·05). Moreover, after a 5‐hr incubation, the cells from each group were collected. The total RNA from the cultured cells was purified, cDNA was synthesized, and IFN‐γ, IL‐4 and β‐actin gene expression levels were detected by RT‐PCR. As shown in Fig. 1(b), the IFN‐γ, T‐bet, IL‐4 and GATA‐3 gene stripes of the stimulated infected cells were brighter than the others. To detect and locate IFN‐γ + IL‐4+ cells elicited by S. japonicum infection in mice liver, immunofluorescence histological analysis was performed (Fig. 2).The result showed that some IFN‐γ + IL‐4+ cells resided around hepatic granulomatous inflammation and schistosome eggs. This meant that schistosome infection could induce mouse liver T lymphocytes to produce a large amount of IFN‐γ and IL‐4.
Figure 1.
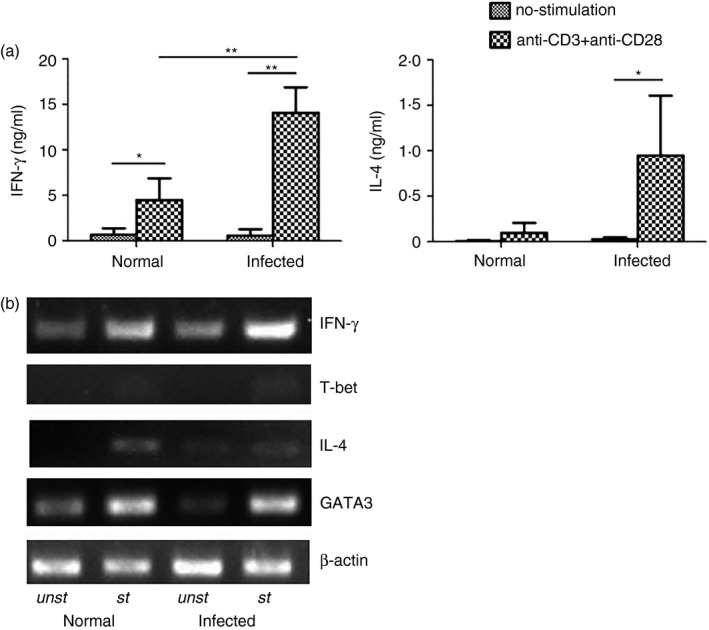
Interferon‐γ (IFN‐γ) and interleukin‐4 (IL‐4) are induced in schistosome‐infected liver lymphocytes. Thirty female C57BL/6 mice were infected with 40 ± 5 Schistosoma japonicum per cercariae, and then the mice were killed 6 weeks after infection. Mononuclear cells were isolated from the livers of uninfected mice and from schistosome‐infected C57BL/6 mice 6 weeks after S. japonicum infection. The cells were cultured in the presence of anti‐CD3 plus anti‐CD28, and the culture supernatants were collected 72 hr after incubation for IFN‐γ and IL‐4 detection by ELISA (a) The cells were harvested 5 hr after stimulation with anti‐CD3 plus anti‐CD28 (st) or without stimulation (unst). Total RNA from the cultured cells was collected and purified, cDNA was synthesized, and the IFN‐γ, IL‐4, T‐bet, GATA‐3, and β‐actin expression levels were detected with RT‐PCR (b). The data are representative of three experiments (*P < 0·05; **P < 0·01).
Figure 2.
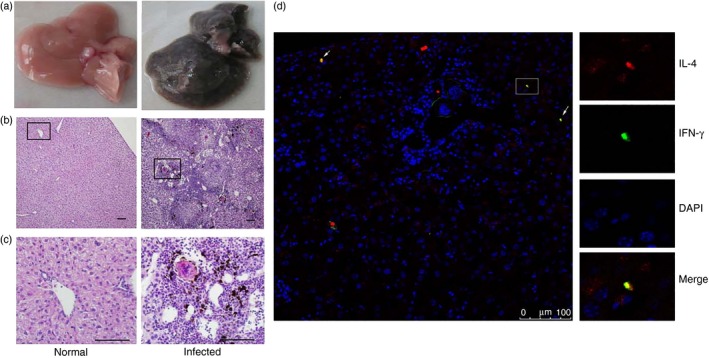
Detection of IFN‐γ + IL‐4+ cells in livers of C57BL/6 mice by immunofluorescence. Female C57BL/6 mice were infected with 40 ± 5 Schistosoma japonicum per cercariae per mouse. Six weeks after the infection, the mice were killed. (a) Livers were washed, and removed from the different groups of mice. (b,c) Tissue sections were fixed in 10% formaldehyde solution, embedded in paraffin, cut into 5‐μm slices, and stained with haematoxylin & eosin (b, 100×, c, 400×). (d) Interferon‐γ (IFN‐γ) was identified by green fluorescence (FITC) and interleukin‐4 (IL‐4) was identified by red fluorescence (phycoerythrin; PE), whereas nuclei are visualized using DAPI staining (blue). Merged images of IFN‐γ and IL‐4 staining are shown around granulomatous (white arrows).
Dynamic changes in IFN‐γ and IL‐4 co‐expressing Th cells
To measure the changes in CD4+ T cells co‐expressing IFN‐γ and IL‐4, lymphocytes were isolated from normal and schistosome‐infected C57BL/6 mouse livers at 4, 6 and 8 weeks after infection. These cells were then stimulated with PMA and ionomycin and detected with FACS as described in the Materials and methods. The CD3+ CD4+ Th cells were gated first, and the proportion of co‐expressing IFN‐γ and IL‐4 cells was detected, as shown in Fig. 3(a). The proportion of IFN‐γ and IL‐4 co‐expressing Th cells increased from 0·68 ± 0·57% in the uninfected mice to 7·05 ± 3·0% by week 4 following infection and to 9·6 ± 5·28% by week 6, before decreasing to 6·3 ± 5·9% by week 8. Over this same period, the fraction of IFN‐γ + IL‐4− CD4+ T cells increased from 4·22 ± 1·58% in the uninfected mice to 8·2 ±2·3% in week 4 following infection and to 10·18 ±1·49% in week 6, before decreasing to 5·92 ± 3·52% by week 8. The proportion of IFN‐γ − IL‐4+ CD4+ T cells had a distinct temporal profile, which increased only slightly from 2·97 ± 1·81% in the uninfected mice to 3·97 ± 2·12% by week 4 and then rose dramatically to 14·69 ± 5·62% by week 6 and 19·21 ± 14% by week 8 (Fig. 3b).
Figure 3.
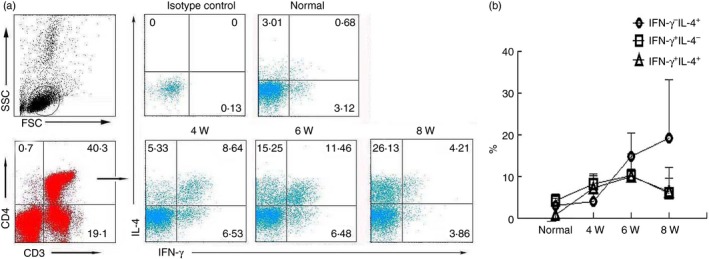
Dynamic changes in CD4+ T cells expressing interferon‐γ (IFN‐γ) and interleukin‐4 (IL‐4) alone or in combination. In three independent experiments, 20 female C57BL/6 mice were infected with 40 ± 5 Schistosoma japonicum cercariae per mouse. At 0 (before infection), 4, 6 and 8 weeks post‐infection, four mice at each time‐point were randomly chosen and killed. Mononuclear cells were isolated from the liver, and stimulated with PMA and ionomycin for 5 hr at 37° under a 5% CO 2 atmosphere. Then, cells were stained for 30 min at 4° in the dark with anti‐CD3‐allophycocyanin‐Cy7, CD4‐Peridinin chlorophyll protein and then intracellularly stained with allophycocyanin‐IFN‐γ, phycoerythrin‐IL‐4 and isotype IgG2a control antibodies. The relative proportion of each phenotype was measured with intracellular cytokine staining and FACS. The numbers in the histogram quadrants are the relative proportions of each expression phenotype. (a) One representative experiment is shown. (b) The proportion of CD4+ cells expressing IFN‐γ alone, IL‐4 alone, or co‐expressing both is plotted. The error bars indicate the standard errors of the means. Similar results were obtained in two additional experiments.
IFN‐γ + IL‐4+ Th cell distribution
Lymphocytes were isolated from blood‐free C57BL/6 mouse livers, lungs, spleens and MLNs 6 weeks after schistosome infection and then stimulated with PMA and ionomycin, as described in the Materials and methods. The cells were stained with intracellular cytokine staining (ICS) and detected by FACS. The CD3 and CD4 co‐expressing Th cells were gated first. Then, the IFN‐γ and IL‐4 expression levels were examined. As shown in Fig. 4, the proportion of the IFN‐γ + IL‐4+ cells in the CD4 cell populations of the infected mouse livers, lungs and spleens was 7·09 ± 3·38%, 4·94 ± 1·67% and 3·39 ± 0·54%, respectively, which was significantly higher than that in the normal mouse tissues (P < 0·05). Though the percentage of IFN‐γ + IL‐4+ cells was higher in the liver CD4+ T‐cell population than that in the lungs and spleens, the difference was not significant (P > 0·05). However, the percentage of IFN‐γ + IL‐4+ CD4+ cells was lowest in the MLN with no significant difference between the MLN‐derived cells from the infected and normal mice (P > 0·05).
Figure 4.
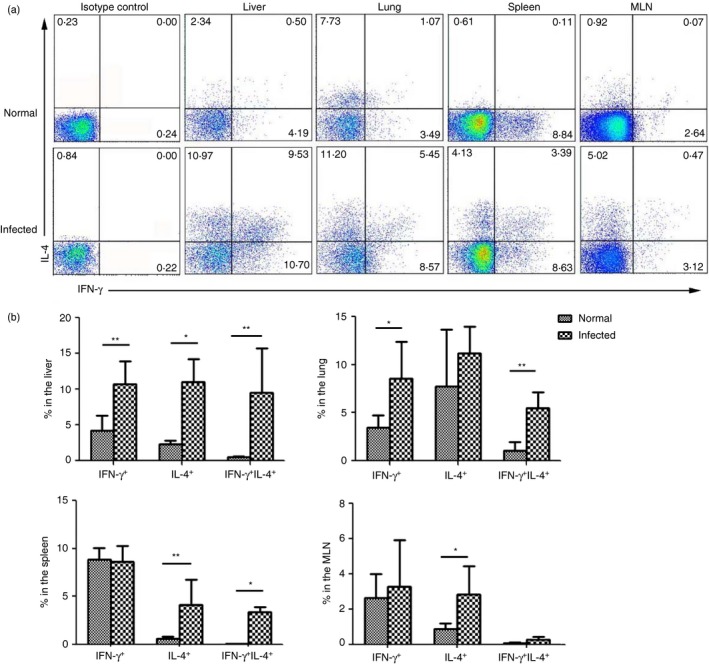
IFN‐γ + IL‐4+ T helper (Th) cell proportions in different tissues. Mononuclear cells were isolated from the liver, lung, spleen and mesenteric lymph nodes of schistosome‐infected C57BL/6 mice (10 mice per group) at 6 weeks after infection. The cells were stimulated with PMA and ionomycin for 5 hr. The cells were stained with anti‐CD3‐allophycocyanin‐Cy7, anti‐CD4‐Peridinin chlorophyll protein and then intracellularly stained with anti‐interferon‐γ (IFN‐γ)‐allophycocyanin, anti‐interleukin‐4 (IL‐4)‐phycoerythrin and isotype IgG2a control antibodies for FACS analysis. The numbers in the histogram quadrants are the relative proportions of each expression phenotype. (a) One representative result is shown. (b) The proportion of CD4+ cells expressing IFN‐γ alone, IL‐4 alone, or co‐expressing both is plotted. The error bars indicate the standard errors of the means. Similar results were obtained from four or five independent experiments. *P < 0·05 compared with livers and lungs.
The state of the IFN‐γ + IL‐4+ Th cells
The states of the infected hepatic IFN‐γ − IL‐4−, IFN‐γ + IL‐4+, IFN‐γ + IL‐4− and IFN‐γ − IL‐4+ cells were determined by measuring the CD25, CD45RB, CD62L, CD69 and CD127 molecule expression by cell surface staining, as described in the Materials and Methods, which therefore characterized the differentiation state (i.e. naive versus effector or memory) of the different cell subsets. As shown in Fig. 5(a), we found that the IFN‐γ + IL‐4+ cells mostly expressed effector cell markers (CD45RB−: 95·02 ± 0·71%; CD62L−: 94·35 ± 0·07%; CD127−: 94·8 ± 4·10%). The CD25 and CD69 expression levels on the IFN‐γ + IL‐4+ Th cells after infection were 38·0 ± 5·2% and 59·0 ± 7·9%, respectively (Fig. 5).
Figure 5.
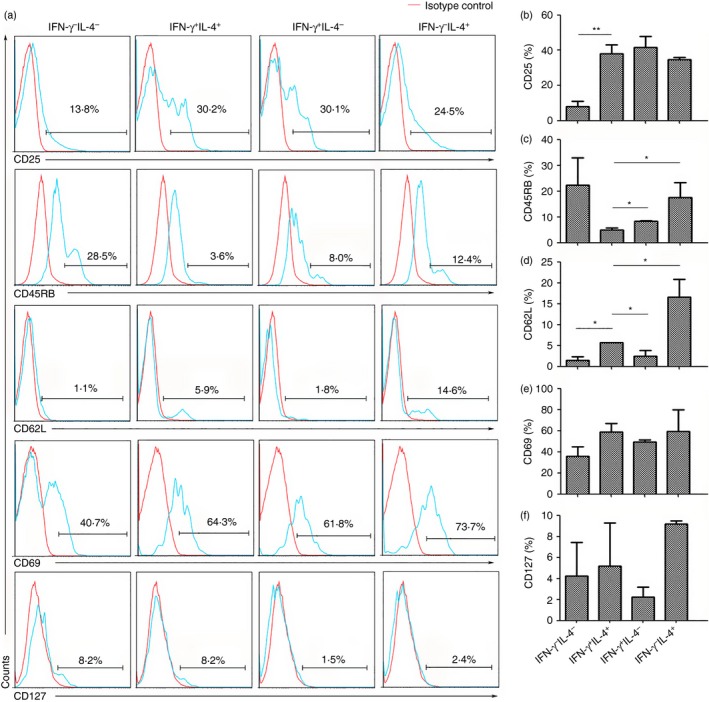
The CD25, CD45RB, CD62L, CD69 and CD127 expression levels on the IFN‐γ + IL‐4+ T helper (Th) cell population. Six weeks after Schistosoma japonicum infection, mice (six per group) were killed. The cells were stimulated with PMA and ionomycin. The cells were stained with anti‐CD3‐allophycocyanin‐Cy7 and CD4‐Peridinin chlorophyll protein antibodies and then intracellularly stained with anti‐interferon‐γ (IFN‐γ)‐FITC and anti‐IL‐4‐phycoerythrin (PE) antibodies. The conjugated antibodies, including the anti‐CD25‐PE, anti‐CD45RB‐FITC, anti‐CD62L‐FITC, anti‐CD69‐allophycocyanin, anti‐CD127‐PE and isotype IgG2a control antibodies were measured in five separate staining panels by intracellular cytokine staining and FACS. The cells were gated on the IFN‐γ − IL‐4− double‐negative cell, IFN‐γ + IL‐4+ CD4 T cell, IFN‐γ + IL‐4− Th1 cell and IFN‐γ − IL‐4+ Th2 cell populations for analysis of different molecules in the infected livers with a flow cytometer. (a) One representative result is shown. (b–f) The percentages of the different markers on the IFN‐γ − IL‐4− cells, IFN‐γ + IL‐4+ cells, IFN‐γ + IL‐4− cells and IFN‐γ − IL‐4+ cells in the hepatic mononuclear cells were calculated (*P < 0·05; **P < 0·01).
IFN‐γ + IL‐4+ Th cell cytokine expression profiles
To explore the cytokine expression profiles in the IFN‐γ + IL‐4+ Th cell population, lymphocytes were isolated from blood‐free C57BL/6 mouse livers 6 weeks after schistosome infection and then stimulated with PMA and ionomycin as described. The cells were stained and detected by intracellular cytokine staining and FACS. The CD3 and CD4 co‐expressing Th cells were gated first. Then, the IFN‐γ and IL‐4 expression levels were examined. Finally, the IFN‐γ − IL‐4− (double‐negative), IFN‐γ + IL‐4+ (double‐positive), IFN‐γ + IL‐4− (Th1) and IFN‐γ − IL‐4+ (Th2) populations were examined for IL‐2, IL‐9, IL‐10 and IL‐17 expression. As shown in Fig. 6, in the IFN‐γ + IL‐4+ cell population, 25·6 ± 6·46% of the cells expressed IL‐2, 1·4 ± 0·18% expressed IL‐9, 29·27 ±11·74% expressed IL‐10, and 1·3 ± 0·5% expressed IL‐17. The proportion of IL‐10‐expressing IFN‐γ + IL‐4+ cells was significantly higher than that of the other cytokines (P < 0·05). Consistent with the IFN‐γ + IL‐4+ cells, the Th1 cells produced more IL‐10 than the other cytokines. In contrast to the aforementioned cells, however, the difference in the IL‐2 expression among cells was not obvious. Moreover, the double‐negative cells after S. japonicum infection could hardly secrete any cytokines compared with the other cells.
Figure 6.
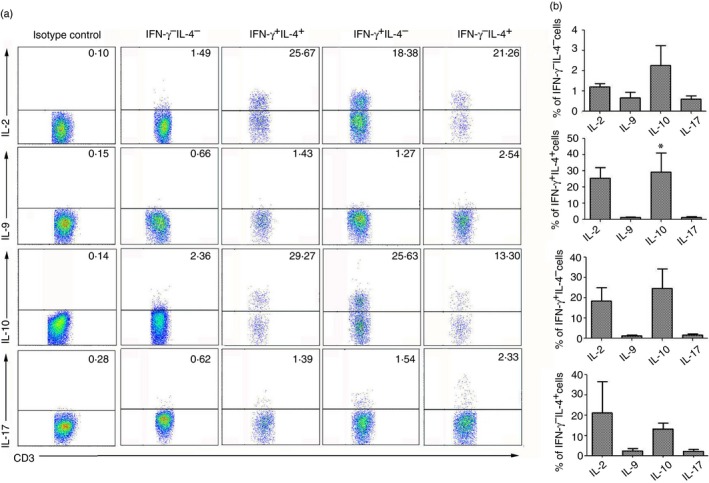
The different cytokine proportions that were expressed by the IFN‐γ + IL‐4+ T helper (Th) cell population. Female C57BL/6 mice were infected with 40 ± 5 Schistosoma japonicum cercariae per mouse and killed 6 weeks after infection. The mononuclear cell population was isolated from schistosome‐infected C57BL/6 mouse livers. The cells were stimulated with PMA and ionomycin and stained with anti‐CD3‐allophycocyanin‐Cy7 and CD4‐Peridinin chlorophyll protein antibodies and then intracellularly stained with anti‐interferon‐γ (IFN‐γ) ‐FITC and phycoerythrin (PE‐) interleukin‐4 (IL‐4) antibodies. The anti‐IL‐2‐PE, anti‐IL‐9‐PE, anti‐IL‐10‐PE, anti‐IL‐17‐PE and isotype IgG2a control antibody expression levels were measured in separate staining panels by intracellular cytokine staining and FACS using specific fluorophore‐conjugated antibodies. The numbers in quadrants are percentages of each expression phenotype. (a) One representative experiment is shown. (b) An average of three independent experiments was conducted. The error bars indicate the standard errors of the means (*P < 0·05).
Discussion
Schistosome infection can induce granulomatous inflammation in mouse livers.8 It has been reported that S. japonicum infection can induce Th1‐, Th2‐ and Th17‐mediated cell responses in infected animals.18, 19 Schistosoma mansoni eggs rapidly induce a CD4+ Th2 response, by way of Th2 cell maturation through an early transient Th0‐like stage.20 Interferon‐γ and IL‐4 are the dominant Th1 and Th2 cytokines, respectively, and play crucial roles in schistosome egg granuloma formation.21, 22 Both ELISA (Fig. 1a) and RT‐PCR (Fig. 1b) results indicated that S. japonicum infection could induce IFN‐γ and IL‐4 production. These results were consistent with a report by Wen et al.23 Moreover, immunofluorescent histological result showed that there are recruited IFN‐γ + IL‐4+ cells around granulomas in the liver (Fig. 2d).
Interestingly, when isolated liver lymphocytes were stimulated with PMA and ionomycin, a large group of CD4+ T cells that co‐expressed IFN‐γ and IL‐4 were detected with FACS. As shown in Fig. 3, the percentages of IFN‐γ and IL‐4 co‐expressing Th cells were increased at week 4, peaked at week 6, and decreased at week 8 after infection. This may correlate with the intensity of the pathogenic immune response that is induced by S. japonicum eggs.18 Inflammatory cells infiltrated into the liver lobules at week 4 following infection, and a robust immune response was observed at week 6. Additionally, it is interesting that there was no difference in the relative proportion of the IFN‐γ + IL‐4+, IFN‐γ + IL‐4−, and IFN‐γ − IL‐4+ cells at 6 weeks after infection. This suggested that nearly half of the IFN‐γ + or IL‐4+ Th cells that were isolated from the liver and re‐stimulated with PMA plus ionomycin might be IFN‐γ + IL‐4+ co‐expressing cells, not Th1 or Th2 cells. Similar results were observed by Deaton et al.24 in mice infected with the S. mansoni.
Except for the liver, most schistosome eggs are deposited into the intestinal mucosa, and some eggs can migrate into the lung, leading to immune response induction at these sites.22 The MLN is the main draining lymph node in the mouse enterocoelia, and it contains many immune cell types.25 It has been reported that an S. japonicum infection can induce significant CD4+ Th cell responses in B6 mouse MLNs.26, 27 In this study, an increased IFN‐γ + IL‐4+ CD4+ T‐cell percentage was also detected in isolated lymphocytes from C57BL/6 mouse lungs, spleens and MLNs 6 weeks after S. japonicum infection. As shown in Fig. 4, as in the liver, very few IFN‐γ + IL‐4+ Th cells were detected in normal mouse lungs, spleens and MLNs. However, after 6 weeks of S. japonicum infection, IFN‐γ + IL‐4+ Th cells were prominent in the mouse lungs and spleens. This demonstrated the existence of S. japonicum infection‐induced IFN‐γ + IL‐4+ Th cells in C57BL/6 mouse livers at early time‐points following infection. Moreover, nearly half of the IL‐4+ Th cells in the lung and one‐third of the IL‐4+ Th cells in the spleen were IFN‐γ + IL‐4+ cells, which indicated the importance of IFN‐γ + IL‐4+ Th cells in this process. Although an obviously high percentage of IL‐4+ cells was induced in the mouse MLNs, very few IFN‐γ and IL‐4 co‐expressing Th cells were induced. This may be related to the fact that the responsibility of T cells in different tissues and organs was different.28
In the past, IFN‐γ + IL‐4+ Th cells were named ‘Th0 cells’, which is a special cell population that is in the process of cell differentiation.29 When cell differentiation is active, IFN‐γ + IL‐4+ Th cells might be active. Recently, the concept of ‘cell plasticity’ was accepted.15 Interferon‐γ and IL‐4 co‐expressing CD4 T cells are thought to be a type of plastic cell that exists in many models. Long‐lived virus‐specific Th2 memory populations can produce IFN‐γ if reactivated in vivo in the context of an innate response that favours Th1 cell development.16
Early studies showed that the CD45RB expression level, which is an isoform of CD45, on mouse CD4 T cells is believed to distinguish naive (CD45RBhigh) from activated/memory (CD45RBlow) cells.30 l‐Selectin (CD62L), a vascular adhesion molecule, has been associated with differentiating naive from activated/memory T cells.31 Interleukin‐7 receptor (CD127) is down‐regulated on all human T cells after activation.32 In the past, IFN‐γ and IL‐4 co‐expressing Th cells were named the ‘Th0 cell’. Th0 cells appear like a naive T‐cell type before differentiation. Our results (Fig. 5) showed that the majority of IFN‐γ + IL‐4+ Th cells did not express CD45RB and CD62L. This finding indicated that IFN‐γ and IL‐4 co‐expressing CD4 T cells were not naive T cells. Moreover, the CD25 and CD69 expression levels on the IFN‐γ and IL‐4 cells reflected that this cell population was in an activated state, as reported by Miyamoto et al.33 More interesting is that the IFN‐γ‐ and IL‐4‐expressing cells expressed similar levels of these cell surface markers as Th1 and Th2 cells, though some differences existed between these cell populations as shown in Fig. 5. This means that most of the IFN‐γ + IL‐4+ cells, like the Th1 and Th2 cells, should be a type of activated effector T cell, not a naive T cell.
It was reported that plastic T cells possess the ability to secrete many types of cytokines, including IFN‐γ, IL‐4, IL‐9, IL‐17 and transforming growth factor‐β.14 Some typical cytokines that are secreted by Th cells were detected in the IFN‐γ + IL‐4+ cells. The results (Fig. 6) showed that the level of IL‐2 was higher in the double‐positive and Th1 cells compared with the Th2 cells. This indicated that IFN‐γ + IL‐4+ Th cells possess the ability to grow and differentiate.34 It was reported that IL‐10 is primarily a pro‐Th2 factor rather than an immune suppressor in filariasis.35 Several studies demonstrated that IL‐10 also regulates the growth and/or differentiation of T helper cells, B cells, NK cells, cytotoxic T cells and mast cells.36 The higher level of IL‐10 that is secreted by IFN‐γ + IL‐4+ cells might imply that this population of IFN‐γ + IL‐4+ cells could continue to differentiate into Th2 cells. Additionally, IL‐9 is a cytokine with pleiotropic activities that modulates fibrosis development.37 It is now known that under specific conditions, the regulatory T, Th1, Th17 and the Th9 subsets of T cells also produce IL‐9.38 Consistent with these reports, Th1, Th2 and even IFN‐γ + IL‐4+ cells produced IL‐9 in our results. Additionally, the observed IFN‐γ + IL‐4+ cells also produced IL‐17.
In conclusion, the results presented in this paper demonstrated that S. japonicum infection could induce a large number of IFN‐γ + IL‐4+ CD4 T cells in the C57BL/6 mouse liver. This IFN‐γ + IL‐4+ cell population is a type of activated multifunction effector/memory Th cell that possesses the ability to secrete multiple types of cytokines.
Disclosures
The authors have no financial conflict of interest.
Acknowledgements
This work was supported by a grant from the College Scientific Research Project in Guangzhou City (2012C117).
References
- 1. Curry MP, Norris S, Golden‐Mason L, Doherty DG, Deignan T, Collins C et al Isolation of lymphocytes from normal adult human liver suitable for phenotypic and functional characterization. J Immunol Methods 2000; 242:21–31. [DOI] [PubMed] [Google Scholar]
- 2. Blom KG, Qazi MR, Matos JB, Nelson BD, DePierre JW, Abedi‐Valugerdi M. Isolation of murine intrahepatic immune cells employing a modified procedure for mechanical disruption and functional characterization of the B, T and natural killer T cells obtained. Clin Exp Immunol 2009; 155:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006; 43:S54–62. [DOI] [PubMed] [Google Scholar]
- 4. Klugewitz K, Adams DH, Emoto M, Eulenburg K, Hamann A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol 2004; 25:590–4. [DOI] [PubMed] [Google Scholar]
- 5. Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev 2000; 174:21–34. [DOI] [PubMed] [Google Scholar]
- 6. Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol 2003; 3:51–62. [DOI] [PubMed] [Google Scholar]
- 7. Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol 2002; 2:499–511. [DOI] [PubMed] [Google Scholar]
- 8. Lenzi HL, Romanha WS, Santos RM, Rosas A, Mota EM, Manso PP et al Four whole‐istic aspects of schistosome granuloma biology: fractal arrangement, internal regulation, autopoietic component and closure. Mem Inst Oswaldo Cruz 2006; 101(Suppl. 1):219–31. [DOI] [PubMed] [Google Scholar]
- 9. Wilson MS, Mentink‐Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol 2007; 85:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jankovic D, Cheever AW, Kullberg MC, Wynn TA, Yap G, Caspar P et al CD4+ T cell‐mediated granulomatous pathology in schistosomiasis is downregulated by a B cell‐dependent mechanism requiring Fc receptor signaling. J Exp Med 1998; 187:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wan YY. Multi‐tasking of helper T cells. Immunology 2010; 130:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA et al IL‐4 inhibits TGF‐β‐induced Foxp3+ T cells and together with TGF‐β, generates IL‐9+ IL‐10+ Foxp3− effector T cells. Nat Immunol 2008; 9:1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL‐17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 2007; 25:821–52. [DOI] [PubMed] [Google Scholar]
- 14. Coomes SM, Pelly VS, Wilson MS. Plasticity within the αβ + CD4+ T‐cell lineage: when, how and what for? Open Biol 2013; 3:120157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krawczyk CM, Shen H, Pearce EJ. Functional plasticity in memory T helper cell responses. J Immunol 2007; 178:4080–8. [DOI] [PubMed] [Google Scholar]
- 16. Lohning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Hofer T et al Long‐lived virus‐reactive memory T cells generated from purified cytokine‐secreting T helper type 1 and type 2 effectors. J Exp Med 2008; 205:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baxter AG, Jordan MA. Plasticity is the differentiated state of CD4 T cells. Cell Mol Immunol 2013; 10:375–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie H, Chen D, Luo X, Gao Z, Fang H, Huang J. Some characteristics of IL‐5‐producing T cells in mouse liver induced by Schistosoma japonicum infection. Parasitol Res 2013; 112:1945–51. [DOI] [PubMed] [Google Scholar]
- 19. Chen D, Luo X, Xie H, Gao Z, Fang H, Huang J. Characteristics of IL‐17 induction by Schistosoma japonicum infection in C57BL/6 mouse liver. Immunology 2013; 139:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vella AT, Pearce EJ. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0‐like stage. J Immunol 1992; 148:2283–90. [PubMed] [Google Scholar]
- 21. Lohning M, Grogan JL, Coyle AJ, Yazdanbakhsh M, Meisel C, Gutierrez‐Ramos JC et al T1/ST2 expression is enhanced on CD4+ T cells from schistosome egg‐induced granulomas: analysis of Th cell cytokine coexpression ex vivo . J Immunol 1999; 162:3882–9. [PubMed] [Google Scholar]
- 22. Chen D, Xie H, Luo X, Yu X, Fu X, Gu H et al Roles of Th17 cells in pulmonary granulomas induced by Schistosoma japonicum in C57BL/6 mice. Cell Immunol 2013; 285:149–57. [DOI] [PubMed] [Google Scholar]
- 23. Wen X, He L, Chi Y et al Dynamics of Th17 cells and their role in Schistosoma japonicum infection in C57BL/6 mice. PLoS Negl Trop Dis 2011; 5:e1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deaton AM, Cook PC, De Sousa D, Phythian‐Adams AT, Bird A, MacDonald AS. A unique DNA methylation signature defines a population of IFN‐γ/IL‐4 double‐positive T cells during helminth infection. Eur J Immunol 2014; 44:1835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chung Y, Chang WS, Kim S, Kang CY. NKT cell ligand α‐galactosylceramide blocks the induction of oral tolerance by triggering dendritic cell maturation. Eur J Immunol 2004; 34:2471–9. [DOI] [PubMed] [Google Scholar]
- 26. Turner JD, Jenkins GR, Hogg KG, Aynsley SA, Paveley RA, Cook PC et al CD4+ CD25+ regulatory cells contribute to the regulation of colonic Th2 granulomatous pathology caused by schistosome infection. PLoS Negl Trop Dis 2011; 5:e1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luo XP, Chen DH, Xie HY, Gao ZY, Fang HL, Huang J. Immune response of Th17 cells in mesenteric lymph node of mice infected by Schistosoma japonicum . Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2012; 30:258–61, 267. [PubMed] [Google Scholar]
- 28. Huang J, Luo X, Chen D, Fang H, Xie H. Proportion and characteristics of γδT cells in different tissues and organs of C57BL/6 mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2013; 29:449–52, 457. [PubMed] [Google Scholar]
- 29. Miner KT, Croft M. Generation, persistence, and modulation of Th0 effector cells: role of autocrine IL‐4 and IFN‐γ . J Immunol 1998; 160:5280–7. [PubMed] [Google Scholar]
- 30. Ten HT, The OF, Berkhout M, Bruggeman JP, Vyth‐Dreese FA, Slors JF et al Expression of CD45RB functionally distinguishes intestinal T lymphocytes in inflammatory bowel disease. J Leukoc Biol 2004; 75:1010–5. [DOI] [PubMed] [Google Scholar]
- 31. Choudhary D, Hegde P, Voznesensky O, Choudhary S, Kopsiaftis S, Claffey KP et al Increased expression of l‐selectin (CD62L) in high‐grade urothelial carcinoma: a potential marker for metastatic disease. Urol Oncol 2015; 33:387. e17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu W, Putnam AL, Xu‐Yu Z, Szot GL, Lee MR, Zhu S et al CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203:1701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miyamoto C, Mattos NR, Cesare SD, Belfort JR, Burnier MJ. Use of CD25 as an immunohistochemical marker for acquired ocular toxoplasmosis. Arq Bras Oftalmol 2010; 73:443–6. [DOI] [PubMed] [Google Scholar]
- 34. Liao W, Lin JX, Leonard WJ. IL‐2 family cytokines: new insights into the complex roles of IL‐2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol 2011; 23:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitre E, Chien D, Nutman TB. CD4+ (and not CD25+) T cells are the predominant interleukin‐10‐producing cells in the circulation of filaria‐infected patients. J Infect Dis 2008; 197:94–101. [DOI] [PubMed] [Google Scholar]
- 36. Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS et al Interleukin‐10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti‐inflammatory macrophage function. Immunity 2014; 40:706–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoyle GW, Brody AR. IL‐9 and lung fibrosis: a Th2 good guy? Am J Respir Cell Mol Biol 2001; 24:365–7. [DOI] [PubMed] [Google Scholar]
- 38. Nowak EC, Weaver CT, Turner H, Begum‐Haque S, Becher B, Schreiner B et al IL‐9 as a mediator of Th17‐driven inflammatory disease. J Exp Med 2009; 206:1653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


