Abstract
Cognitive flexibility, a core aspect of executive functioning, is required for the speeded shifting between different tasks and sets. Using an interindividual differences approach, we examined whether cognitive flexibility, as assessed by the Delis–Kaplan card-sorting test, is associated with gray matter volume (GMV) and functional connectivity (FC) of regions of a core network of multiple cognitive demands as well as with different facets of trait impulsivity. The core multiple-demand network was derived from three large-scale neuroimaging meta-analyses and only included regions that showed consistent associations with sustained attention, working memory as well as inhibitory control. We tested to what extent self-reported impulsivity as well as GMV and resting-state FC in this core network predicted cognitive flexibility independently and incrementally. Our analyses revealed that card-sorting performance correlated positively with GMV of the right anterior insula, FC between bilateral anterior insula and midcingulate cortex/supplementary motor area as well as the impulsivity dimension “Premeditation.” Importantly, GMV, FC and impulsivity together accounted for more variance of card-sorting performance than every parameter alone. Our results therefore indicate that various factors contribute individually to cognitive flexibility, underlining the need to search across multiple modalities when aiming to unveil the mechanisms behind executive functioning.
Keywords: Cognitive flexibility, Anterior insula, Salience network, Functional connectivity, Gray matter, Impulsivity
Introduction
Executive functioning is a construct that is widely used across many fields of psychology. Despite its significance, however, it lacks a clear formal definition. One important contribution to this observation is the fact that there is not one type of “executive functioning” but rather different aspects of mental functioning are all consolidated under this term. That is, the construct of executive functioning refers to different functions and aspects, important for planned, controlled and goal-directed behavior. One of the core aspects of executive functions is cognitive flexibility, which can be regarded as the ability to consider multiple concepts successively or simultaneously and thus to switch between tasks and sets in a quick and flexible manner (Diamond 2013). Cognitive flexibility is thus a prerequisite for various other mental functions and an important factor which is not only associated to aspects of intelligence, but, e.g., also to creativity (Diamond 2013).
One of the main reasons why “executive processes” such as cognitive flexibility have remained rather elusive concepts is that most tasks used to probe them require the interaction of several mental processes that can hardly be dissociated from each other. Put differently, executive functioning involves several different cognitive components while conversely different lower-level processes contribute to (higher-order) executive functioning. Thus, the construct has been operationalized by different subcomponents interacting with each other dependent on task demands. Miyake et al. (2000) for example postulate that three separable functions (updating, shifting and inhibition) provide the main contribution to higher-order executive functioning. Similarly, Alvarez and Emory (2006) also specify three components in their review, namely working memory, attention and inhibition, underlying executive functioning. Executive functioning may thus be regarded as an interplay of processes mediating attentional, inhibitory as well as working memory-related capacities. While the contribution of these may vary depending on the particular task, all executive functions, however, should depend on resources from attention, inhibition and working memory.
There are a lot of studies investigating the neural correlates of executive functioning, and the involvement of individual regions varies substantially across the different executive tasks employed in these. However, there is a lot of evidence that the prefrontal cortex is one of the key regions in executive functioning (Buchsbaum et al. 2005; for review see Alvarez and Emory 2006; Nyhus and Barcelo 2009). Human lesion studies, for example, demonstrate that disruptions of prefrontal cortex go along with deficits in goal-directed planning and behavior (Tsuchida and Fellows 2013; for review see Alvarez and Emory 2006). In addition, functional magnetic resonance imaging (fMRI) studies report mainly but by far not exclusively frontal activation during tasks requiring executive control (Buchsbaum et al. 2005; for review see Alvarez and Emory 2006; Nyhus and Barcelo 2009), indicating a distributed network centered on the frontal cortex subserving executive functions. The contribution of individual regions to the different tasks and processes related to executive functioning, however, is still a matter of conjecture. Based on the above-mentioned concept of lower-level sub-processes contributing to higher-level executive functioning, we would argue that a core network of executive functions can be defined as encompassing those regions which are convergently involved in attention, inhibition as well as working memory. That is, regions, which play a key role in those processes, contribute to executive control. In line with this view, a multiple-demand system (Duncan 2010) has been suggested, consisting of frontal but also parietal brain regions, which show conjoint activation across multiple cognitive tasks. However, how this network is related to performance in specific tests of executive functioning (like a test measuring cognitive flexibility) remains to be investigated.
Most studies investigating the neural correlates of cognitive flexibility have primarily focused on brain regions, which are more activated during cognitively demanding conditions, as compared to less demanding ones. However, it can be assumed that task performance also depends on structural factors like the amount of gray and white matter in a given region as well as the structural and functional coupling between task-relevant regions. In other words, anatomical and functional brain organization, which is measured in the absence of an external task, might have an influence on executive performance. For example, it has been shown that gray matter volume (GMV; Fine et al. 2009; for review see Kanai and Rees 2011), structural (Bonnelle et al. 2012; for review see Kanai and Rees 2011) but also functional connectivity (FC; Hampson et al. 2006; Seeley et al. 2007) are related to cognitive performance.
In addition to GMV and FC, there are other task-unspecific factors, which might affect executive functioning, for example individual personality characteristics. Indeed, correlations have been found between different personality dimensions (Flehmig et al. 2010; Aiken-Morgan et al. 2012; Soubelet and Salthouse 2011; Sutin et al. 2011) and neuropsychological measures of executive functioning. Previous personality research, however, has strongly focused on the Big Five, while other personality factors have been rather neglected. In particular, impulsivity, however, long treated as an aspect of Extraversion (Eysenck 1967), has recently received more and more attention. In fact, it has been argued that most of the variance of the relationship found between Extraversion and cognitive performance can be explained by impulsivity (Dickman 1993). In line with this view, virtually all disorders associated with high impulsivity go along with executive deficits (Gonzalez-Gadea et al. 2013; Haaland and Landro 2009; Verdejo-Garcia and Perez-Garcia 2007; Albein-Urios et al. 2012; van der Plas et al. 2009; Cunha et al. 2010). Finally, according to Dickman (1993), high- and low-impulsive individuals differ in their ability of shifting attention. Thus, cognitive flexibility as a core aspect of executive functioning might especially be influenced by impulsivity.
The above-described relationships now raise the question whether different, task-unspecific measures of brain architecture as well as interindividual differences in impulsivity represent converging or complementary contributions to executive functioning. Thus, the conjoint investigation of all these aspects should shed further light on how interindividual differences in executive functioning can be explained by different neural and personality factors.
The aim of the current study is therefore to investigate how regions of the multiple-demand system (regions involved in attention, inhibition and working memory) relate to the specific performance in a particular test (i.e., a test measuring cognitive flexibility). In particular, it will be assessed whether GMV and FC within this core network as well as impulsivity are related to cognitive flexibility. Furthermore, the study aims to clarify whether personality traits and neural parameters together predict behavioral performance better than each parameter/score alone.
Methods
Participants
Data were obtained from the Nathan Kline Institute ‘Rockland’ sample, which is available online as part of the International Neuroimaging Datasharing Initiative (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html). Reanalysis of this data was approved by the Ethics Committee of the Heinrich Heine University Düsseldorf. The ‘Rockland’ sample is a cross-sectional sample, consisting of neuroimaging and neuropsychological test data from healthy individuals between 4 and 85 years. For the current project, only data from participants aged between 18 and 85 years without any previous neurologic or psychiatric disorder and without any clinically relevant test characteristics [for example, a score in the beck depression inventory (BDI) higher than 18] were included. Furthermore, three subjects were excluded because of incomplete data. In total, the sample consisted of 128 healthy subjects between 18 and 85 years (mean age 42.35, SD ±18.23 years; 52 female).
Definition of the multiple-demand network
To determine the multiple-demand network, which is involved in tasks requiring working memory, attention and inhibition, we performed a conjunction across the results of three large-scale neuroimaging meta-analyses related to vigilant attention (Langner and Eickhoff 2013), working memory (Rottschy et al. 2012) and inhibitory control (Cieslik et al. 2013), respectively. That is, by using the conservative minimum statistics (Nichols et al. 2005), the intersection of the thresholded maps derived from the three meta-analyses was computed and thresholded by a minimum cluster size of 50 voxels, yielding a core network defined by regions significantly involved in all three processes.
Measures
MRI: gray matter volume
Structural T1-weighted magnetic resonance images were acquired on a Siemens 3-T (TimTrio) scanner [repetition time (TR) = 2,500 ms, echo time (TE) = 3.5 ms, flip angle = 8°, in-plane resolution = 1.0 × 1.0 mm]. Images were analyzed and processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/download/) using standard settings. Within a unified segmentation model (Ashburner and Friston 2005), images were corrected for bias-field inhomogenities; brain tissue classified into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF); adjusted for partial volume effects and spatially normalized to the Montreal Neurological Institute (MNI) template. Furthermore, the segmented images were non-linearly modulated in order to adjust them to the amount of expansion and contraction, which was applied during normalization. Voxel-wise gray matter volume (GMV) was then calculated and extracted for each participant and every cluster of the core multiple-demand network. This was done by integrating the modulated voxel-wise gray matter probabilities under each cluster. Importantly, as we did not multiply the segmented images by linear components but rather modulated the images by the nonlinear components only, the calculated GMV represents the amount of gray matter corrected for individual brain size.
Functional MRI: resting state
Resting-state fMRI images were acquired on a Siemens 3-T (TrioTim) scanner using blood-oxygen-level-dependent (BOLD) contrast [gradient-echo Echo-planar Images (EPI) pulse sequence, TR = 2,500 ms, TE = 30 ms, flip angle = 80°, in-plane resolution = 3.0 × 3.0 mm, 38 axial slices (3.0 mm thickness), covering the entire brain] and likewise processed using SPM8: The first four dummy scans were discarded to allow for magnetic field saturation. EPI images were corrected for head movement by affine registration using a two-pass procedure in which images were first aligned to the initial volumes and subsequently to the mean of all images. Next, for each participant, the mean EPI image was spatially normalized into MNI space by using the “unified segmentation” approach (Ashburner and Friston 2005). The ensuing deformation was then applied to the individual EPI volumes and images smoothed by a 5-mm full-width at half-maximum Gaussian kernel to improve the signal-to-noise ratio and to compensate for residual anatomical variation. The activity time series of each voxel was processed as follows (Müller et al. 2013): Spurious correlations were reduced by excluding variance that could be explained by the following nuisance variables: (1) the six motion parameters derived from image realignment; (2) their first derivatives; (3) mean GM, WM and CBF intensities (i.e., each tissue-class-related signal separately). All nuisance variables entered the model as first- and second-order terms (cf. Satterthwaite et al. 2013 for an evaluation of this framework). Finally, the data were band-pass filtered with cutoff frequencies of 0.01 and 0.08 Hz. The time course for each of the regions of the multiple-demand network (see Fig. 1) was then extracted for each participant as the first eigenvariate of all GM voxels within the volume of interest. For each participant, linear Pearson's correlation coefficients between all extracted time series were computed, and these voxel-wise correlation coefficients were transformed into Fisher's Z-scores. These scores represent the functional connectivity (FC) for each connection within the core multiple-demand network in the individual participant.
Fig. 1.
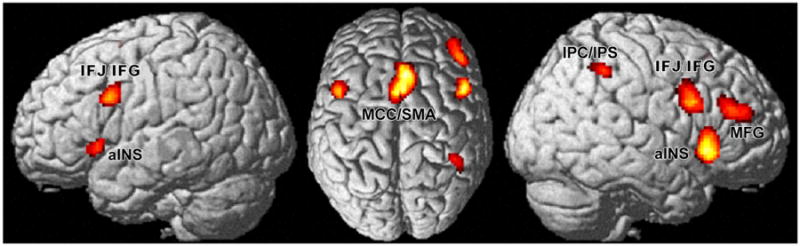
The core multiple-demand network, consisting of regions that showed significant convergence across studies in three different meta-analyses, including midcingulate cortex extending into supplementary motor area (MCC/SMA), left and right anterior insula (aINS), left and right inferior frontal junction/gyrus (IFJ/IFG), right middle frontal gyrus (MFG) as well as right inferior parietal cortex extending into intraparietal sulcus (IPC/IPS)
As this resulted in 21 functional connectivity scores within the network, we first performed a principal component analysis (PCA) in order to determine sub-networks. This data reduction is not only highly warranted to reduce the need for multiple comparisons but at the same time also addressed another important issue in the analysis of resting-state connectivity, namely the fact that FC values for different connections are highly intercorrelated. The applied PCA thus allowed us to extract and test the relevant patterns of FC within the assessed network rather than assessing multiple highly related individual connections. Based on the PCA decomposition, we then calculated mean functional connectivity scores for each resulting component. In particular, based on the factor loadings, we first assigned each individual connection to one of the components. Then, for each component, a mean functional connectivity score across the Fisher's Z-scores of all assigned connections (weighted with its factor loading) was calculated.
Measure of impulsivity
The revised version of the UPPS Impulsive Behavior scale (Whiteside and Lynam 2001) was used as a measure of the individual amount of impulsivity. The UPPS consists of 59 items, which assess five different facets of impulsivity: Premeditation, Perseverance, negative Urgency, positive Urgency and Sensation Seeking. While Premeditation reflects the tendency of an individual to carefully think and plan actions, Perseverance assesses the ability of an individual to finish tasks and to avoid boredom. Importantly, higher scores on those two scales reflect higher impulsivity and can therefore be regarded as lack of Premeditation or Perseverance, respectively. Negative Urgency measures a person's tendency to engage in rash actions as a result of negative affect, whereas positive Urgency reflects the tendency of rash actions in response to positive affect. Finally, Sensation Seeking measures an individual tendency and openness for excitement and adventure (Whiteside and Lynam 2001).
Delis–Kaplan executive function system: card-sorting test
As card-sorting procedures are the most frequently used tests for assessing executive functioning (Alvarez and Emory 2006), we chose the sorting test of the Delis–Kaplan executive function system (Delis et al. 2001) out of the possible behavioral data available for the NKI sample. The sorting test measures different components of problem-solving abilities but is thought to reflect in particular cognitive flexibility (Delis et al. 1992). The test consists of two different conditions, a spontaneous and a structured condition. In the spontaneous sorting condition, participants are asked to sort a set of cards into two piles and describe the strategy they are using for the sort. This should be repeated several times but by using a different strategy in every new sort. Subjects are instructed to work quickly (maximum of 4 min) and to generate as many different sorts as possible (but are only allowed to attempt 10 sorts). Importantly, no feedback is given, and the task is just to sort the card sets using as many different sorting strategies as possible. That is, participants have to switch to a new sorting strategy on each trial in order to achieve good scores. In contrast, in the structured sorting condition, the examiner sorts the card set according to different strategies, and the participant has to identify the employed strategy. Here, we used the three primary measures of the sorting test for our analyses: (1) the freesorting description score (“freesorting score”) and (2) the number of confirmed sorts score from the spontaneous sorting condition and the (3) sort-recognition score from the structured condition. While the freesorting score reflects the abstraction level of the correctly described strategies, the number of confirmed sorts score describes the total number of different strategies a subject has used in the spontaneous condition. The sort-recognition score, on the other hand, reflects the number of sorts correctly identified in the structured condition.
Statistical analyses
Statistical analyses were conducted using SPSS 15.0 (SPSS Inc., Chicaco, IL, USA). We used a two-step approach in order to analyze the data. In a first step, we aimed to explore whether and how gray matter volume of all network nodes, functional connectivity of the FC components as well as all subscales of the UPPS are related to performance in the card-sorting test. For this first analysis, partial correlations (controlling for age and sex) were calculated between each of the three scores of the card-sorting test (i.e., number of confirmed sorts, freesorting score, sort-recognition) and (1) GMV of each region of the core multiple-demand network, (2) FC scores of the sub-networks revealed by PCA and (3) the five impulsivity scores of the UPPS. The results of each analysis were Bonferroni-corrected for the number of tests performed, seperately for each card-sorting score. To explore how the different parameters are intercorrelated, we additionally correlated all measures with each other.
With these first explorative analyses, we determined the variables that we used in the second analysis step to test our specific hypothesis. In this second step, we tested whether GMV, functional connectivity and personality together explained more variance of card-sorting performance than each parameter alone.
This was done by predicting card-sorting performance with a hierarchical linear regression analysis. Only those card-sorting measures, featuring correlations with impulsivity, FC and GMV were further assessed. In a first step, age and sex were included in the hierarchical regression, while subsequently all variables showing a significant (corrected for multiple comparisons) association with sorting performance were included in the analysis. The order of inclusion followed the strengths of the correlations. A hierarchical linear regression including only those variables that feature a significant correlation with sorting performance was chosen given the strong intercorrelations (multicollinearity) between the different variables of GMV, FC and impulsivity. To rule out suppressor effects, a stepwise linear regression analyses with simultaneous consideration of all variables was additionally performed.
Results
The multiple-demand network
In order to determine a network of regions consistently involved in three key sub-components of executive functioning, a conjunction across the three networks derived from meta-analyses investigating attention, working memory and inhibitory control was calculated (extent threshold of 50 voxel). This yielded seven clusters, mainly located in the frontal lobe (Fig. 1): midcingulate cortex extending into pre- and supplementary motor area (MCC/SMA), left and right anterior insula (aINS), left and right inferior frontal junction/gyrus (overlapping area 44; Amunts et al. 1999; Clos et al. 2013), right middle frontal gyrus as well as right intraparietal sulcus (overlapping PFm and hlP2; Caspers et al. 2006; Choi et al. 2006).
Correlations between card-sorting performance and gray matter volume
Correlations of the three card-sorting measures with GMV of the above-defined regions of the multiple-demand network revealed positive correlations between GMV of the right aINS and number of confirmed sorts (r = .22, p < .05; Fig. 2c) as well as freesorting score (r = .20, p < .05). However, both correlations did not survive correction for multiple comparisons.
Fig. 2.
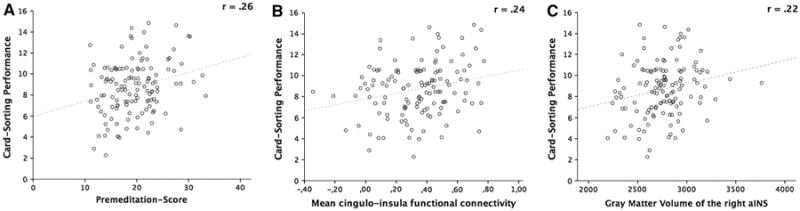
Significant positive correlations of card-sorting performance (number of confirmed sorts) with: a Premeditation; b mean cingulo-insula functional connectivity and c gray matter volume of the right anterior insula (aINS). All measures are corrected for effects of age and sex. Note that c does not survive correction for multiple comparisons
Correlations between card-sorting performance and functional connectivity
The principal component analysis revealed seven components, which explained 75 % of the original 21 functional connectivity scores. Details about the composition of the different components (sub-networks) are shown in supplement table S1.
Correlations of card-sorting performance and FC of those seven sub-networks revealed significant positive correlations of component six (cingulo-insula functional connectivity) with number of confirmed sorts (r = .24, p < .05, Bonferroni-corrected; Fig. 2b) as well as the freesorting score (r = .24, p < .05, Bonferroni-corrected). None of the other FC components showed a significant association of functional connectivity with card-sorting performance.
Correlations between card-sorting performance and impulsivity scores
Correlations of card-sorting performance and impulsivity scores revealed significant positive correlations between Premeditation and both the number of confirmed sorts (r = .26, p < .05, Bonferroni-corrected; Fig. 2a) and freesorting score (r = .24, p < .05, Bonferroni-corrected). Further correlations of number of confirmed sorts with Perseverance (r = .20, p < .05) and Sensation Seeking (r = .18, p < .05) as well as of freesorting score with Perseverance (r = .18, p < .05) did not remain significant after correcting for multiple comparisons.
Intercorrelations of gray matter volume, functional connectivity and impulsivity
While correlations between GMV and FC, GMV and impulsivity, as well as FC and impulsivity were rather low and only partly significant at an uncorrected level (see Supplementary Tables S2 and S3), intercorrelations were high and significant between GMV of the different regions, between the strengths of the different functional connectivity components as well as between the impulsivity subscales (see Supplementary Tables S4–S6).
Hierarchical regression predicting card-sorting performance
We then examined whether FC and self-reported impulsivity together predicted card-sorting performance better than each parameter alone. That is, we investigated whether Premeditation explained unique variance in card-sorting performance beyond that explained by cingulo-insula FC.
At last, although GMV of the right aINS did not survive correction for multiple comparisons, in a final step, we also included that parameter to assess if it explained any unique variance of card-sorting performance beyond that explained by Premeditation and cingulo-insula FC. The order of predictor inclusion in the regression model followed the strengths of the correlations with the criterion. We calculated one regression predicting the number of confirmed sorts, which will be presented in the following section. The analysis with the freesorting score as criterion led to similar results and will be presented in the supplement table S7.
The hierarchical linear regression was calculated using SPSS (Table 1). In the first step, age, sex and Premeditation scores were entered into the model, which accounted for 16.3 % of the interindividual variance in card-sorting performance (F3,124 = 8.07, p < .05). In the next step, cingulo-insula FC was additionally entered, leading to an increase of 4.7 % in explained variance (F4,123 = 8.20, p <.05). In the third step, GMV of right aINS was entered, which additionally accounted for 4.6 % of the variance (F5,122 = 8.40, p < .05). In sum, 25.6 % of the interindividual variance in card-sorting performance could be explained by age, sex, Premeditation, cingulo-insula FC and GMV of the right aINS (Table 1).
Table 1. Hierarchical linear regression analysis predicting card-sorting performance (measured by number of confirmed sorts in the Delis–Kaplan card-sorting test).
| Predictor | β | R2 | ΔR2 |
|---|---|---|---|
| Step 1 | |||
| Age | −.259* | .163 | .163 |
| Sex | −.150 | ||
| Premeditation | .254* | ||
| Step 2 | |||
| Age | −.163 | .210 | .047 |
| Sex | −.165* | ||
| Premeditation | .244* | ||
| Cingulo-insula functional connectivity | .239* | ||
| Step 3 | |||
| Age | .047 | .256 | .046 |
| Sex | −.080 | ||
| Premeditation | .259* | ||
| Cingulo-insula functional connectivity | .224* | ||
| Gray matter right anterior insula | .307* | ||
Significant at p < .05
Stepwise linear regression with simultaneous consideration of all variables resulted in the inclusion of the three variables in the hierarchical regression (GMV of the right aINS, cingulo-insula FC, Premeditation) with no further improvement of the model by including any other variable (R2 = 0.25, p < .05).
At last, two further hierarchical regression analyses were calculated with one additionally entering squared effects of the three predictors and the other including interaction terms of age and the three predictors. This was done to rule out potential nonlinear and interaction effects with age. These alternative analyses did not reveal any further improvements and only non-significant effects of the steps where squared or interaction terms were entered (see Supplementary Tables S8 and S9).
Discussion
In this study, we used a two-step approach to (1) explore whether and how structural (regional GMV) and functional (interregional FC) brain characteristics as well as personality factors are associated to performance in a test measuring cognitive flexibility and (2) test the hypothesis that GMV, FC and personality characteristics together explain more variance in cognitive flexibility than each parameter alone. In contrast to previous studies, GMV and FC were investigated within an a priori-defined network of brain regions associated with executive functioning. This network was formed by regions that were consistently found across three large-scale neuroimaging meta-analyses. In this way, we restricted our analysis to regions that were robustly associated with executive functioning across a range of paradigms requiring cognitive control (i.e., vigilant attention, working memory and inhibitory control). Our analyses revealed that card-sorting performance as a measure of cognitive flexibility was positively correlated with cingulo-insula FC as well as the impulsivity dimension Premeditation. GMV of the right aINS was also positively correlated with card-sorting performance, but did not survive the stringent significant level corrected for multiple comparisons. However, results revealed that GMV, FC and Premeditation together accounted for more variance of card-sorting performance than each parameter alone.
The multiple-demand network derived from three different meta-analyses
There has been a long-standing controversy about the exact definition of executive functioning. As a result, the associated neural correlates likewise vary depending on the different tasks used to measure executive functioning. However, Duncan (2010) noticed that a similar network, consisting of frontal and parietal regions, is consistently been recruited during tasks requiring different cognitive demands. The current study therefore aimed to identify such core multiple-demand network by using a novel meta-analytic approach. According to Alvarez and Emory (2006), working memory, attention and inhibition are the key sub-components of executive functioning, with the specific contribution of each of these components to the overall performance depending on the particular task. We therefore defined the multiple-demand system by including only those regions that were robustly associated with all three sub-components across many neuroimaging studies. To this end, we performed a conjunction across three meta-analyses dealing with working memory (Rottschy et al. 2012), attention (Langner and Eickhoff 2013) and inhibitory control (Cieslik et al. 2013). This approach yielded a set of seven regions consistently implicated across all three meta-analyses. This network greatly resembles the multiple-demand network suggested by Duncan (2010), encompassing MCC/SMA, bilateral inferior frontal junction/gyrus, right middle frontal gyrus but also bilateral aINS and right inferior parietal cortex and intraparietal sulcus (IPC/IPS). Thus, by using the results of different meta-analyses (each integrating several dozens of neuroimaging findings), we were able to robustly identify the core multiple-demand network and therefore the regions of interest for the current study. In a next step, we then assessed how task-unspecific parameters of this network like gray matter volume and functional connectivity relates to performance in a specific task measuring one of the core aspects of executive functioning, namely cognitive flexibility.
Functional connectivity and cognitive flexibility
When correlating the seven FC components within the meta-analytically defined multiple-demand network, a significant relationship was found between card-sorting performance and the component including FC between bilateral aINS and the midcingulate cortex/supplementary motor area (MCC/SMA). Thus, our results indicate that FC between the aINS with MCC/SMA has a crucial influence on individual executive functioning, with stronger interaction between these regions going along with better card-sorting performance. Importantly, with regard to the MCC/SMA, it has to be noted that previous studies often refer to this region as the dorsal anterior cingulate cortex (ACC), although the location of their discussed cingulate region corresponds well to the midcingulate cortex. This discrepancy is most likely attributable to now obsolete two-region classification of Brodmann that is still used at times, while the current study employs the more recent four-region division of Vogt et al. (2004). Thus, for the sake of avoiding confusion in the further discussion, “MCC” instead of “dorsal ACC” is used for labeling this region.
A high probability for co-activation of the bilateral aINS with the MCC/SMA is a well replicated finding (for example Lie et al. 2006; Hoffstaedter et al. 2013; Chechko et al. 2012; Veldhuizen et al. 2011; Jackson et al. 2005; Langner et al. 2012). Thus, bilateral aINS and the MCC/SMA are thought to form a core “salience network,” relevant for entering and maintaining a cognitive set (Dosenbach et al. 2006) and initiating cognitive control signals (Menon and Uddin 2010) in order to regulate activity in other systems/networks (Sridharan et al. 2008). Even though these interpretations differ in some aspects from each other, they all have in common that they grant the aINS and the MCC/SMA a key role in guiding goal-directed planning and behavior. Interestingly, when looking at FC between all regions within our multiple-demand network, we find precisely the interaction within the salience network, i.e., between the aINS and the MCC/SMA, to be significantly associated with card-sorting performance.
Similarly, but by investigating FC during fluid reasoning, Ebisch et al. (2013) reported a stronger interaction between right aINS and MCC/SMA being associated with fewer errors during fluid reasoning. Thus, connectivity between right aINS and MCC/SMA seems to be important for exerting cognitive control to avoid errors. In contrast, Ham et al. (2013) demonstrated that effective connectivity from MCC/SMA to left aINS is modulated by the occurrence of errors. That is, the connectivity strength between those two regions changed after participants made mistakes, indicating adaptation processes and stronger need for executive control. This assumption was supported by significant correlations between connectivity strength and the extent of change in behavior after an error occurred (Ham et al. 2013). This indicates that the MCC/SMA may be crucial for executive functioning as it projects and signals to left aINS when an adjustment of behavior is required. In context of the card-sorting test, adaptation and adjustments are in particular relevant, as the individual has to take previous sorting strategies and behavior into account.
That is, one has to keep in mind which sorting strategies have already been adopted and adjust behavior according to it.
Moreover, regions of the salience network, that is aINS and MCC/SMA, have recently received more and more attention in clinical studies. Specifically, in schizophrenia, it has been shown that this network plays a major role in the disorder, indicated by reports of aberrant connectivity within the salience network (Pu et al. 2012; Manoliu et al. 2013), but also by altered connectivity between salience and other resting-state networks, for example the central executive network (Manoliu et al. 2013). Thus, with regard to the results of the current study, it might be speculated that these connectivity deficits in schizophrenia might be associated to poorer executive functioning in this patient group (Orellana and Slachevsky 2013).
In addition, Bonnelle et al. (2012) showed in patients with traumatic brain injury that white matter integrity between aINS and MCC/SMA is not only related to (de)activation abnormalities in the default mode network but also to behavioral deficits. In particular, fractional anisotropy between right aINS and MCC/SMA correlated with performance of tasks requiring executive control, i.e., a stop signal and a stroop task.
In summary, our results point especially to the salience network as having an important influence on cognitive flexibility. These results thus fit well with the common view that the salience network is crucial in situations where goal-directed behavior is required but also with clinical studies demonstrating alterations within this network in disorders going along with executive deficits.
Gray matter volume and cognitive flexibility
In addition to cingulo-insula FC, correlation of card-sorting performance with GMV of the regions of the multiple-demand network revealed a positive correlation of cognitive flexibility with GMV of the right aINS. However, it should be taken into account that this correlation could only be found on the uncorrected level and this relationship should therefore be interpreted with caution. Nevertheless, hierarchical regression analysis shows that in addition to FC and impulsivity, GMV of the right aINS explains another 5 % of variance in card-sorting performance, which can not be explained by the other two predictors. Given that the amount of uniquely explained variance is virtually identical to that explained by the cingulo-insular FC (which was significant when correcting for multiple comparisons), we would argue that GMV of the right aINS should also be considered as a relevant influence on interindividual differences in cognitive flexibility.
Several studies have demonstrated that the (inferior) frontal cortex plays a major role in executive processes (Nyhus and Barcelo 2009; for review see Alvarez and Emory 2006). In contrast, the adjacent aINS has rather been neglected in this context. However, given its close proximity to the inferior frontal cortex, the aINS as identified by our meta-analytic approach is possibly often part of some of those reported inferior frontal clusters and therefore not specifically mentioned. Alternatively and especially in the context of cognitive control, the aINS might sometimes be mislabeled as inferior frontal cortex (Swick et al. 2011). In addition, even though there are studies reporting the insula in executive functioning tasks (Lie et al. 2006; Specht et al. 2009), its specific role has in this context hardly been discussed. Nevertheless, there is growing evidence that the aINS plays a major role in cognitive and attentional control (Menon and Uddin 2010; Dosenbach et al. 2006; Kurth et al. 2010). In particular, according to Dosenbach et al. (2006), the aINS is part of a system managing central and core executive functions by signaling the beginning of a task, sustaining activity during the task and receiving error signals in order to optimize performance. Importantly, this system is domain-unspecific and can be found across different types of tasks (Dosenbach et al. 2006; Kurth et al. 2010). Additionally, it has been suggested that the aINS is part of the salience network (Seeley et al. 2007) and plays a crucial role in the initiation of cognitive control signals to modulate activity in other networks (Sridharan et al. 2008). That is, when detecting something salient, the aINS, in particular on the right side, activates task-relevant networks while concurrently deactivating regions that are irrelevant for the particular task, for example, the default mode network (Sridharan et al. 2008). The current finding of a positive correlation between right aINS GMV and card-sorting performance might therefore indicate that subjects with greater GMV of the aINS are better at detecting salient and/or task-relevant signals, are therefore more efficient in activating executive and deactivating default mode regions and, as a result, perform better on executive tasks. Conversely, a decrease in GMV of the right aINS might go along with less efficiency to switch between task-irrelevant and task-relevant networks and, therefore, worse executive functions. This idea is in line with Yuan et al. (2012), who also argue for a key role of the fronto-insula cortex in the development of reasoning abilities based on a significant correlation between insula volume and fluid intelligence (Yuan et al. 2012). As measures of fluid intelligence and measures of executive functioning are highly intercorrelated (Diamond 2013), our results thus further support the involvement of the salience network, especially the right aINS, in executive functioning.
This view is also in line with clinical studies reporting structural differences in the aINS between patients and controls. In particular, decreased aINS GMV was found in patients with addiction (Moreno-Lopez et al. 2012), schizophrenia (Ellison-Wright et al. 2008; Bora et al. 2011; Radua et al. 2012; Pu et al. 2012; Shepherd et al. 2012), ADHD (Lopez-Larson et al. 2012) as well as borderline personality disorder (Soloff et al. 2012). Our current results suggest that this decrease in aINS volume might be related to executive functioning deficits. In line with this assumption, poorer executive functions have been reported in all these patient groups (Albein-Urios et al. 2012; Cunha et al. 2010; van der Plas et al. 2009; Haaland and Landro 2009; Willcutt et al. 2005; Orellana and Slachevsky 2013; Behrwind et al. 2011). Thus, the observed decrease in anterior insular GMV may be regarded as one neural substrate of executive deficits in these patient groups.
In summary, our results demonstrate that GMV of the right aINS influences how an individual performs on a test of cognitive flexibility. In particular, greater GMV of the right aINS possibly leads to better detection of salient events and more efficiency in up-regulating task-relevant executive and down-regulating task-irrelevant networks, which then as a result leads to better cognitive flexibility.
The role of the frontal cortex
It is noteworthy that we did not find any correlations of card-sorting performance with FC or GMV of the inferior frontal junction/gyrus and middle frontal gyrus. This finding, at the first glance, seems to contradict previous lesion and fMRI studies, implicating a key role for the frontal cortex in executive functioning (Buchsbaum et al. 2005; for review see Alvarez and Emory 2006; Nyhus and Barcelo 2009). However, such interpretation may be a misunderstanding of the current study. In particular, it should be noted that these regions were revealed as key neural correlates of tasks probing executive functioning by the conjunction across the three meta-analyses. Thus, our results do support the crucial role of frontal cortices in diverse tasks of executive functioning. What we did not find, however, is an association between the interindividual differences in volume or FC of these regions on the one hand and interindividual differences in performance in a prototypical executive functioning task on the other. That is, while these regions are implicated in cognitive functioning, the current parameters extracted from them do not relate to individual performance levels. In this context, we note that a previous study investigating the relationship of brain structure and executive functioning by Fine et al. (2009) reported a significant prediction of card-sorting performance by the (entire) left frontal lobe volume in a group of patients and healthy subjects. When comparing this study with the current one, we, in contrast to Fine et al.'s study, functionally defined a specific core network for which we assessed GMV, while Fine et al. rather unspecifically investigated full lobe volumes. Furthermore, that study did not investigate the volume of the aINS, or, more precisely, it is not clear whether or not the insular cortex was not analyzed or included as part of the temporal or frontal lobe. Thus, the current study now indicates that when specifically investigating GMV of regions associated with executive functioning, only GMV of the right aINS significantly correlates with card-sorting performance.
However, given that the frontal cortex has been discussed as playing a main role in executive processes, the absence of significant associations with frontal regions is still somewhat surprising. We would argue that frontal regions might be more domain-specific and play a minor role in the (rather global) executive capacities required for card-sorting performance. In line with this view, Dosenbach et al. (2006) demonstrated that the anterior prefrontal cortex, in contrast to aINS and MCC/SMA, did not show activity across different types of tasks but rather only in certain conditions. Thus, our results do not rule out potential relationships of performance in other executive tasks with structure and function of frontal regions of the multiple-demand network. Rather, they underline the overarching role of the cingulo-insular network in executive functioning previously highlighted, e.g., by Dosenbach et al. (2006).
Alternatively, the inclusion of age (and gender) as a control variable could provide another possible explanation for this apparent discrepancy. It has been demonstrated that substantial age-related volume changes can be found in the (pre)frontal cortex (Raz and Rodrigue 2006; Drag and Bieliauskas 2010), which is possibly associated with the age-related decline in executive functioning. Thus, in the current study, GMV and FC of the frontal regions did not explain any specific variance in individual cognitive flexibility on top of what is already explained by age. Thus, the frontal regions of the multiple-demand network seem to show no (significant) relation to interindividual differences in card-sorting performance after accounting for general age effects.
At last, it has to be noted that we focused on the relationship between cognitive flexibility and parameters of brain structure and function measured in the absence of a task. Thus, based on our results, we can not draw any conclusions with regard to how brain activity measured during actually performing the card-sorting test relates to performance.
In summary, on the one hand, our results of the conjunction across the results of three different meta-analyses, showing a network which encompasses mainly frontal regions, strongly supports that activity in those regions is crucial for executive functioning. However, on the other hand, when specifically focusing on task-unspecific brain parameters and their relationship to performance in cognitive flexibility, it is more the structure and functional connectivity of the cingulo-insular “salience network” that relates to interindividual differences.
Impulsivity and cognitive flexibility
At last, a positive correlation was found between the impulsivity dimension Premeditation and card-sorting performance. It has to be noted that higher scores on this impulsivity scale reflect less premeditation and therefore greater impulsivity. Thus, the positive correlation between Premeditation and card-sorting performance indicates that participants who described themselves as often acting spontaneously without considering possible consequences perform better than participants reporting higher levels of thoughtfulness and deliberation. At first glance, this result might seem rather surprising, given that many studies reported poorer executive functioning and cognitive performance in disorders related to increased impulsivity such as ADHD, addiction or Borderline Personality Disorder (Gonzalez-Gadea et al. 2013; Haaland and Landro 2009; Verdejo-Garcia and Perez-Garcia 2007; Albein-Urios et al. 2012; Cunha et al. 2010; van der Plas et al. 2009). However, these patients only represent the extreme end of the continuum of impulsivity, and results are possibly confounded by factors other than the personality trait, i.e., other aspects of the disorder that may influence cognitive performance. We here analyzed a sample of healthy subjects, which vary in the amount of impulsivity but without any extreme scores. It may therefore be speculated that performance in a cognitive shift task improves with higher impulsivity only until a certain (non-pathological) level of impulsivity and goes back down beyond that (i.e., with extremely high impulsivity, as in certain disorders). Because of the lack of extreme scores in the current sample, we might not have captured such an inverted U-shaped relationship. Thus, it seems that in the healthy population, a certain degree of impulsivity has a positive effect on cognitive performance.
However, the relationship between impulsivity and cognitive performance might vary depending on the task to be performed. According to the “attentional fixity” theory (Dickman 1993), high-impulsive individuals perform poorer on tasks where attention has to be fixed, whereas their performance is better in tasks where attentional and cognitive shifts are required. As the card-sorting test, as a measure of cognitive flexibility, requires frequent and speeded shifts of cognitive sets (Swanson 2005), the observed positive correlation agrees well with this theory. That is, low-impulsive individuals, who tend to focus attention, need more time in order to obtain a new strategy and therefore achieve less different sorts, whereas high impulsivity goes along with better attention switching and less time needed to change strategy. As a result, individuals scoring high on impulsivity are able to apply more different sorting rules than low-impulsive ones and, as our results show, therefore perform better on tests of cognitive flexibility.
In summary, our results thus indicate that within the non-pathological range, higher amounts of impulsivity go along with more flexible thinking. Importantly, this effect might be highly dependent on the performed task and is thus probably only true in situations where attention has to be shifted.
Different neural parameters and impulsivity account for the same but also different variance
Finally, we tested whether GMV of the right aINS, cingulo-insula FC as well as Premeditation account for equal or specific variance of card-sorting performance. That is, are the contributions converging or complementary? Our results point to the latter, as we could demonstrate that all three parameters together explain more variance than every variable alone, indicating that they explain different aspects of variance. This highlights that the basis of cognitive performance is highly complex and a synergy of several different factors has to be taken into account. That is, in order to understand cognitive performance, one should not only take into account different brain regions but also different modalities, i.e., structure, function or other individual characteristics. In light of the findings, it thus appears that dysexecutive symptoms found in various psychiatric and neurological diseases cannot be explained in a simple way. Rather, there are several modalities, which individually have an influence, and in addition to “dysconnectivity,” also GMV and personality traits contribute to overall executive performance. That is, impaired executive capacities in these patients are most probably associated with multiple different factors with each factor possibly being differently affected across patients. Our results therefore indicate that various factors contribute individually to cognitive flexibility, underlining the need to search across multiple modalities when aiming to unveil the mechanisms behind dysexecutive symptoms found in several psychiatric and neurological diseases.
We side with the idea that the right aINS detects salient events and is especially responsible for entering the required cognitive state. This is mainly achieved by activation of the rest of task-relevant executive and deactivation of the task-irrelevant default mode network. The higher the GMV of the right aINS, the more effectively these processes may be implemented, resulting in fewer errors and better performance. In contrast, FC between aINS and MCC/SMA might modulate adaptation processes that are relevant for the specific task, for example, adjusting behavior to previous events such as preceding behaviors or the occurrence of an error. The stronger the interaction between MCC/SMA and aINS, the better are the adjustments and, therefore, the better is the cognitive outcome.
Impulsivity, on the other hand, most likely influences performance indirectly. For example, it might have an impact on default mode function, given the relevance of socio-affective processes within this network (Schilbach et al. 2012). Thus, high-impulsive individuals might have a lesser tendency to remain in the default mode state than low impulsive. That is, high impulsivity might go along with more flexible switching between networks and with less stable representations of default and task states, which in turn facilitates the aINS in switching between networks. As efficient regulation of the default mode network is crucial for cognitive performance (Kelly et al. 2008; Bonnelle et al. 2012), this then in turn influences how efficiently an executive task is conducted. However, how precisely impulsivity relates to the default mode network remains to be investigated.
In summary, the current findings indicate that cognitive flexibility can be predicted by GMV and FC of specific brain regions as well as by subjective impulsivity. Impairments of the aINS and the MCC/SMA, not only on the structural level but also on their functional interconnectivity, may lead to inefficiency in adequately up-regulating regions of the multiple-demand network and down-regulating the default mode network, which appears to be additionally affected by the degree of self-reported impulsivity. Together, this might then lead to poorer cognitive flexibility. Our results therefore highlight that multiple modalities as well as factors and parameters which are not necessarily task-specific must be taken into account in order to be able to understand the neural basis of executive functioning.
Supplementary Material
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, EI 816/4-1; S.B.E. and LA 3071/3-1; R.L. and S.B.E.), the National Institute of Mental Health (R01-MH074457; S.B.E.) and the Helmholtz Initiative on systems biology (The Human Brain Model; S.B.E.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-014-0797-6) contains supplementary material, which is available to authorized users.
Conflict of interest All authors declare that they have no conflicts of interest.
Contributor Information
Veronika I. Müller, Email: v.mueller@fz-juelich.de, Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Düsseldorf, Germany; Institute of Neuroscience and Medicine, INM-1, Research Centre Jülich, Wilhelm-Johnen-Straße, Jülich, Germany.
Robert Langner, Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Düsseldorf, Germany; Institute of Neuroscience and Medicine, INM-1, Research Centre Jülich, Wilhelm-Johnen-Straße, Jülich, Germany.
Edna C. Cieslik, Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Düsseldorf, Germany; Institute of Neuroscience and Medicine, INM-1, Research Centre Jülich, Wilhelm-Johnen-Straβe, Jülich, Germany
Claudia Rottschy, Institute of Neuroscience and Medicine, INM-1, Research Centre Jülich, Wilhelm-Johnen-Straβe, Jülich, Germany; Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Aachen, Germany.
Simon B. Eickhoff, Institute of Clinical Neuroscience and Medical Psychology, Heinrich Heine University, Düsseldorf, Germany; Institute of Neuroscience and Medicine, INM-1, Research Centre Jülich, Wilhelm-Johnen-Straβe, Jülich, Germany
References
- Aiken-Morgan AT, Bichsel J, Allaire JC, Savla J, Edwards CL, Whitfield KE. Personality as a source of individual differences in cognition among older African Americans. J Res Personal. 2012;46(5):465–471. doi: 10.1016/j.jrp.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albein-Urios N, Martinez-Gonzalez JM, Lozano O, Clark L, Verdejo-Garcia A. Comparison of impulsivity and working memory in cocaine addiction and pathological gambling: implications for cocaine-induced neurotoxicity. Drug Alcohol Depend. 2012;126(1–2):1–6. doi: 10.1016/j.drugalcdep.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16(1):17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and inter-subject variability. J Comp Neurol. 1999;412(2):319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Behrwind SD, Dafotakis M, Halfter S, Hobusch K, Berthold-Losleben M, Cieslik EC, Eickhoff SB. Executive control in chronic schizophrenia: a perspective from manual stimulus-response compatibility task performance. Behav Brain Res. 2011;223(1):24–29. doi: 10.1016/j.bbr.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. P Natl Acad Sci USA. 2012;109(12):4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yucel M, Velakoulis D, Pantelis C. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127(1–3):46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25(1):35–45. doi: 10.1002/Hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33(2):430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Chechko N, Kellermann T, Zvyagintsev M, Augustin M, Schneider F, Habel U. Brain circuitries involved in semantic interference by demands of emotional and non-emotional distractors. Plos One. 2012;7(5) doi: 10.1371/journal.pone.0038155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol. 2006;495(1):53–69. doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Bamberger K, Rottschy C, Eickhoff SB. Unterschiedliche neuronale Netzwerkes für die Verarbeitung kognitiver Interferenz—eine ALE-Meta-Analyse. Klinische Neurophysiologie. 2013;44(1):72. [Google Scholar]
- Clos M, Amunts K, Laird AR, Fox PT, Eickhoff SB. Tackling the multifunctional nature of Broca's region meta-analytically: co-activation-based parcellation of area 44. Neuroimage. 2013;83C:174–188. doi: 10.1016/j.neuroimage.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha PJ, Nicastri S, de Andrade AG, Bolla KI. The frontal assessment battery (FAB) reveals neurocognitive dysfunction in substance-dependent individuals in distinct executive domains: abstract reasoning, motor programming, and cognitive flexibility. Addict Behav. 2010;35(10):875–881. doi: 10.1016/j.addbeh.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Delis DC, Squire LR, Bihrle A, Massman P. Componential analysis of problem-solving ability—performance of patients with frontal-lobe damage and amnesic patients on a new sorting test. Neuropsychologia. 1992;30(8):683–697. doi: 10.1016/0028-3932(92)90039-O. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer J. Delis Kaplan executive function system. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman SJ. Impulsivity and information processing. In: McCown WG, Johnson JL, Shure MB, editors. The impulsive client: theory, research, and treatment. American Psychological Association; Washington DC: 1993. pp. 151–184. [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HSC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. J Geriatr Psychiatr Neur. 2010;23(2):75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Ebisch SJH, Mantini D, Romanelli R, Tommasi M, Perrucci MG, Romani GL, Colom R, Saggino A. Long-range functional interactions of anterior insula and medial frontal cortex are differently modulated by visuospatial and inductive reasoning tasks. Neuroimage. 2013;78:426–438. doi: 10.1016/j.neuroimage.2013.04.058. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiat. 2008;165(8):1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ. The biological basis of personality. Thomas, Springfield; 1967. [Google Scholar]
- Fine EM, Delis DC, Dean D, Beckman V, Miller BL, Rosen HJ, Kramer JH. Left frontal lobe contributions to concept formation: a quantitative MRI study of performance on the Delis–Kaplan executive function system sorting test. J Clin Exp Neuropsychol. 2009;31(5):624–631. doi: 10.1080/13803390802419017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flehmig HC, Steinborn MB, Westhoff K, Langner R. Neuroticism and speed-accuracy tradeoff in self-paced speeded mental addition and comparison. J Individ Differ. 2010;31(3):130–137. doi: 10.1027/1614-0001/A000021. [DOI] [Google Scholar]
- Gonzalez-Gadea ML, Baez S, Torralva T, Castellanos FX, Rattazzi A, Bein V, Rogg K, Manes F, Ibanez A. Cognitive variability in adults with ADHD and AS: disentangling the roles of executive functions and social cognition. Res Dev Disabil. 2013;34(2):817–830. doi: 10.1016/j.ridd.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Haaland VO, Landro NI. Pathological dissociation and neuropsychological functioning in borderline personality disorder. Acta Psychiatr Scand. 2009;119(5):383–392. doi: 10.1111/j.1600-0447.2008.01323.x. [DOI] [PubMed] [Google Scholar]
- Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ. Cognitive control and the salience network: an investigation of error processing and effective connectivity. J Neurosci. 2013;33(16):7091–7098. doi: 10.1523/Jneurosci.4692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26(51):13338–13343. doi: 10.1523/Jneurosci.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB. The “What” and “When” of self-initiated movements. Cereb Cortex. 2013;23(3):520–530. doi: 10.1093/cercor/bhr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24(3):771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci. 2011;12(4):231–242. doi: 10.1038/Nrn3000. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5–6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull. 2013;139(4):870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Kellermann T, Eickhoff SB, Boers F, Chatterjee A, Willmes K, Sturm W. Staying responsive to the world: modality-specific and -nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Hum Brain Mapp. 2012;33(2):398–418. doi: 10.1002/Hbm.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage. 2006;30(3):1038–1049. doi: 10.1016/j.neuroimage.200510031. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, King JB, Terry J, McGlade EC, Yurgelun-Todd D. Reduced insular volume in attention deficit hyperactivity disorder. Psychiat Res-Neuroim. 2012;204(1):32–39. doi: 10.1016/j.pscychresns.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Doll A, Bauml JG, Muhlau M, Schwerthoffer D, Scherr M, Zimmer C, Forstl H, Bauml J, Wohlschlager AM, Koch K, Sorg C. Insular dysfunction reflects altered between-network connectivity and severity of negative symptoms in schizophrenia during psychotic remission. Front Hum Neurosci. 2013;7:216. doi: 10.3389/fnhum.2013.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez L, Catena A, Fernandez-Serrano MJ, Delgado-Rico E, Stamatakis EA, Perez-Garcia M, Verdejo-Garcia A. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012;125(3):208–214. doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Eickhoff SB. Dysregulated left inferior parietal activity in schizophrenia and depression: functional connectivity and characterization. Front Hum Neurosci. 2013;7:268. doi: 10.3389/fnhum.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nyhus E, Barcelo F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. 2009;71(3):437–451. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Orellana G, Slachevsky A. Executive functioning in schizophrenia. Front Psychiatry. 2013;4:35. doi: 10.3389/fpsyt.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu WD, Li L, Zhang HR, Ouyang X, Liu HH, Zhao JP, Li LJ, Xue ZM, Xu K, Tang HB, Shan BC, Liu ZN, Wang F. Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophr Res. 2012;141(1):15–21. doi: 10.1016/j.schres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire PK, Fusar-Poli P. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav R. 2012;36(10):2325–2333. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav R. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60(1):830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS ONE. 2012;7(2):e30920. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/Jneurosci.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AM, Matheson SL, Laurens KR, Carr VJ, Green MJ. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72(9):775–784. doi: 10.1016/j.biopsych.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res. 2012;46(4):516–525. doi: 10.1016/j.jpsychires.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubelet A, Salthouse TA. Personality-cognition relations across adulthood. Dev Psychol. 2011;47(2):303–310. doi: 10.1037/A0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht K, Lie CH, Shah NJ, Fink GR. Disentangling the prefrontal network for rule selection by means of a non-verbal variant of the Wisconsin Card Sorting Test. Hum Brain Mapp. 2009;30(5):1734–1743. doi: 10.1002/Hbm.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. P Natl Acad Sci USA. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Terracciano A, Kitner-Triolo MH, Uda M, Schlessinger D, Zonderman AB. Personality traits prospectively predict verbal fluency in a lifespan sample. Psychol Aging. 2011;26(4):994–999. doi: 10.1037/A0024276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. The Delis–Kaplan executive function system. Can J School Psychol. 2005;20(1/2):117–128. [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56(3):1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Are core component processes of executive function dissociable within the frontal lobes? Evidence from humans with focal prefrontal damage. Cortex. 2013;49(7):1790–1800. doi: 10.1016/j.cortex.2012.10.014. [DOI] [PubMed] [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31(6):706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Douglas D, Aschenbrenner K, Gitelman DR, Small DM. The anterior insular cortex represents breaches of taste identity expectation. J Neurosci. 2011;31(41):14735–14744. doi: 10.1523/Jneurosci.1502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology. 2007;190(4):517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Vogt LJ. Cingulate gyrus. In: Paxinos G, Mai JK, editors. The human nervous system. Elsevier; Amsterdam: 2004. pp. 915–949. [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Indiv Differ. 2001;30(4):669–689. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiat. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Yuan ZN, Qin W, Wang DW, Jiang TZ, Zhang YT, Yu CS. The salience network contributes to an individual's fluid reasoning capacity. Behav Brain Res. 2012;229(2):384–390. doi: 10.1016/j.bbr.2012.01.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


