SUMMARY
Since the discovery in 1973 of the first of the bacterial lipoproteins (Lpp) in Escherichia coli, Braun's lipoprotein, the ever-increasing number of publications indicates the importance of these proteins. Bacterial Lpp belong to the class of lipid-anchored proteins that in Gram-negative bacteria are anchored in both the cytoplasmic and outer membranes and in Gram-positive bacteria are anchored only in the cytoplasmic membrane. In contrast to the case for Gram-negative bacteria, in Gram-positive bacteria lipoprotein maturation and processing are not vital. Physiologically, Lpp play an important role in nutrient and ion acquisition, allowing particularly pathogenic species to better survive in the host. Bacterial Lpp are recognized by Toll-like receptor 2 (TLR2) of the innate immune system. The important role of Lpp in Gram-positive bacteria, particularly in the phylum Firmicutes, as key players in the immune response and pathogenicity has emerged only in recent years. In this review, we address the role of Lpp in signaling and modulating the immune response, in inflammation, and in pathogenicity. We also address the potential of Lpp as promising vaccine candidates.
INTRODUCTION
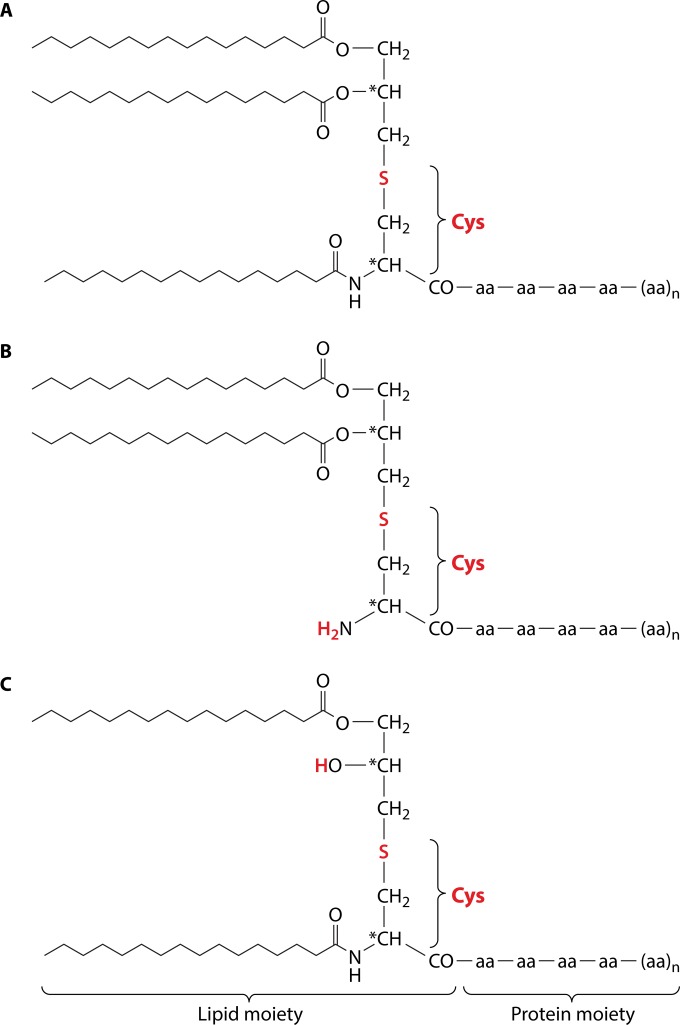
Bacterial lipoproteins (Lpp) represent a major class of surface proteins in Staphylococcus aureus. Recent proteome analysis of S. aureus COL revealed 14 cell wall-associated proteins, 19 sortase-anchored proteins that are covalently anchored to the murein, and 63 Lpp (1). The proteome-identified proteins reached almost the theoretical genome-based number. This means that 65% of the surfacome represents Lpp. These proteins are distinguished by a lipid moiety at the N terminus by which they are anchored either in the outer leaflet of the cytoplasmic membrane or, in Gram-negative bacteria, also in the inner leaflet of the outer membrane (2, 3). Lpp are synthesized as precursors and are processed into mature forms at the cytoplasmic membrane. In 1973, Hantke and Braun discovered that the Escherichia coli murein Lpp contain an unusual S-glyceryl-cysteine residue (N-acyl-S-diacylglyceryl-cysteine) modified with three fatty acids (FA) at its N terminus (4) (Fig. 1A). The precursor Lpp contain at the N terminus an 18- to 36-amino-acid-long signal peptide, which is distinguished from the normal signal peptides by its C-terminal lipobox, which comprises a conserved three-amino-acid sequence in front of the invariable cysteine [(LVI)(ASTG)(GA)↓C] (5). Normally, Lpp are translocated across or into the cytoplasmic membrane via the Sec machinery, leaving the lipidated N terminus anchored in the outer leaflet of the cytoplasmic membrane. There are, however, examples where they are translocated via the twin-arginine translocation (Tat) pathway (6). After insertion into the cytoplasmic membrane, Lpp are modified and processed by three enzymatic reactions. The first step is the transfer of the diacylglyceryl group from phosphatidylglycerol to the sulfhydryl group of the invariant cysteine residue in the lipobox. This reaction is catalyzed by the phosphatidylglycerol-pro-Lpp diacylglyceryl transferase, Lgt (7). When the Lpp are translocated through the cytoplasmic membrane, the specific signal peptidase II (Lsp) recognizes the diacylglyceryl-modified lipo-signal peptide and cleaves between the amino acid at position −1 and the lipid-modified cysteine residue at +1 (8). In most Gram-negative bacteria and in some high-GC Gram-positive bacteria, the N terminus of the diacylglyceryl-modified cysteine residue is fatty acylated by an N-acyltransferase (Lnt) to form N-acyl diacylglyceryl-cysteine (9). All three enzymes (Lgt, Lsp, and Lnt) involved in lipidation and Lpp processing are localized in the cytoplasmic membrane. The molecular mechanism of bacterial lipoprotein modification has been reviewed recently (10).
FIG 1.
Variable structures of the lipid moiety of lipoproteins (Lpp). In most Gram-negative bacteria Lpp are triacylated due to the thioether-linked diacyl glycerol residue and an acyl group at the N terminus of cysteine (A), in some low-GC Gram-positive bacteria the N-acyl group is missing (B), and in representatives of the lactic acid bacterial group an N-acyl-S-monoacylglyceryl-cysteine (named the lyso structure) has been identified (C). *, chiral center.
In Gram-positive bacteria lacking an outer membrane, Lpp are mainly anchored in the outer leaflet of the cytoplasmic membrane (Fig. 2A). On average, the number of Lpp in Staphylococcus aureus is between 55 and 70. For example, the genome of S. aureus N315 encodes about 55 putative Lpp (11, 12), while in S. aureus USA300 there are roughly 63 Lpp identified by the PRED-LIPO program. Approximately 50% of the Lpp were annotated as transporters for amino acids, peptides, iron, zinc, or molybdenum or as chaperones. Many of the proposed Lpp showed no similarity to known proteins, and their function awaits elucidation. The enzymes involved in modification and processing of the Lpp are essentially the same as described in Escherichia coli (10). However, a real Lnt homolog has been found only in high-GC Gram-positive bacteria, such as mycobacteria and streptomycetes, and not in the low-GC Gram-positive Firmicutes phylum. Therefore, it has been assumed that in these bacteria, Lpp were only diacylated (12). However, Kurokawa and colleagues showed that in S. aureus, the 33-kDa SitC, a component of the proposed iron ABC transporter SitABC, is triacylated and induces interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) release in human monocytes and NF-κB activation in Toll-like receptor 2 (TLR2)-transfected HEK293 cells (13). However, SitC not only induces a TLR2-dependent release of IL-6 in primary murine keratinocytes (MK), but it also colocalizes with TLR2, is internalized by the host cells, and triggers time- and concentration-dependent intracellular accumulation of TLR2 (14). Particularly the intracellular TLR2 accumulation is an interesting effect that deserves further analysis regarding localization and contribution to signaling of other microbe-associated molecular patterns (MAMPs).
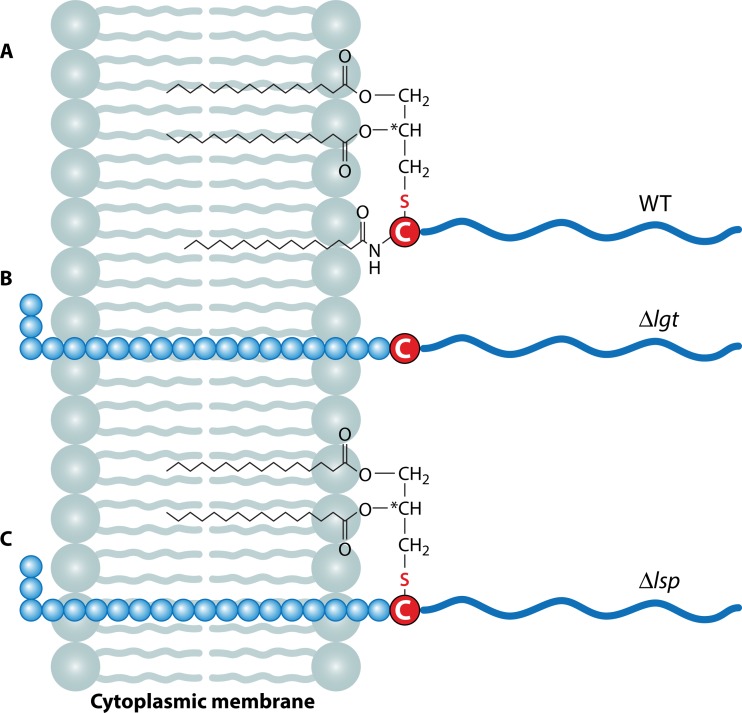
FIG 2.
Membrane incorporation of mature Lpp and unmodified/unprocessed pre-Lpp. (A) Matured and processed Lpp are localized with the triacyl or diacyl groups of the lipid moiety in the outer leaflet of the cytoplasmic membrane. (B) In the Δlgt mutant, the gene encoding the diacylglyceryl transferase enzyme is deleted; this mutant is unable to carry out the lipidation at the cysteine residue, and because of this lack of modification, the lipoprotein leader peptidase (Lsp) cannot process the signal peptide because this enzyme works only with modified pre-Lpp. (C) In the Δlsp mutant, the gene encoding the lipoprotein leader peptidase (Lsp) is deleted; in this mutant, lipidation at the cysteine residue can occur, but there is no processing of the signal peptide. Blue circles, amino acids of the lipo-signal peptide; blue wavy lines, protein part protruding into the cell wall; red-circled “C,” cysteine residues; zigzag lines, O- or N-acylated fatty acids.
The occurrence of triacylated SitC indicates that S. aureus has an Lnt-like enzyme, which adds a fatty acid to the amino group of the S-(diacyl-propyl)-cysteine residue. However, in S. aureus the degree of acylation of the lipid moiety is influenced by environmental conditions such as growth phase and pH; in stationary growth phase or at low pH (6.0), SitC was found almost exclusively in its diacyl structure lacking the alpha-aminoacylation (Fig. 1B) (15). An N-acyl-S-diacylglyceryl-cysteine was also found in five other Gram-positive bacteria, including Bacillus subtilis (16). Interestingly, in other low-GC Gram-positive bacteria, such as Enterococcus faecalis, Bacillus cereus, Streptococcus sanguinis, and Lactobacillus bulgaricus, an N-acyl-S-monoacylglyceryl-cysteine (named the lyso structure) has been identified (16) (Fig. 1C). How the lyso structure is formed is unknown.
MATURATION OF PRE-Lpp IS CRUCIAL FOR PATHOGENICITY AND INFLAMMATION
One of the first reports on the importance of Lpp maturation for immune signaling came from comparative studies of wild-type (WT) S. aureus and its Δlgt mutant (12). Although the Δlgt mutant lacks lipidation of pre-Lpp, they are still anchored in the cytoplasmic membrane via the unprocessed signal peptide (Fig. 2B). A similar situation has been observed with the lsp mutants, though the cysteine residue is lipidated, but the lipo-signal peptide is not processed (Fig. 2C). However, the anchoring in the membrane in these mutants is less strong and, particularly toward the stationary growth, increased release of unlipidated and unprocessed prelipoproteins was observed (12). Although the mutants grow well in complex medium, they show growth defects under nutrient-limited conditions due to impaired ion uptake. Another important phenotype of the lgt mutant is its markedly decreased induction of proinflammatory cytokines (IL-6, IL-8, and monocyte chemoattractant protein 1 [MCP-1]) in human monocytic (MonoMac6), epithelial (pulmonary A549), and endothelial (human umbilical vein endothelial) cells compared to that of the wild type (12). Furthermore, the Δlgt mutants of various S. aureus strains were severely affected in pathogenicity (17). In contrast to the wild-type strains, mutants were affected in induction of early and strong cytokines via the TLR2-MyD88 signaling pathway in murine peritoneal macrophages, and they showed decreased pathogenicity in a C57BL/6 mouse sepsis model and decreased IL-1β chemokine-mediated inflammation. The Δlgt mutants were also severely affected in iron acquisition under infectious conditions, and iron overload of the host restored the growth deficit of Δlgt mutants in MyD88−/− but not C57BL/6 mice (17, 18). This result contradicts earlier results showing that an lgt mutant of S. aureus was hypervirulent, escaped immune recognition, and caused lethal infections with disseminated abscess formation (19). In many other Gram-positive bacteria, inactivation of Lpp maturation by deletion of either the lgt or the lsp gene impaired growth and pathogenicity. In Mycobacterium tuberculosis, the lspA mutant was markedly attenuated in virulence, showing decreased intracellular multiplication in mouse macrophages and decreased growth in lungs and spleens of BALB/c mice (20). However, in many low-GC Gram-positive pathogens, mutations in either lgt or lsp also led to impaired pathogenicity. This was the case in various pathogenic streptococcal species such as Streptococcus pneumoniae (21, 22), Streptococcus agalactiae (23, 24), Streptococcus pyogenes, and Streptococcus equi (25, 26). In Listeria monocytogenes, the lgt mutant was impaired in invasion and intracellular survival, was markedly attenuated in a mouse infection model, was unable to induce TLR2-dependent activation of NF-κB, and exhibited increased susceptibility to cationic peptides (27, 28).
There are many other reports indicating that matured Lpp play a crucial role in pathogenicity. On the host side, TLR2-MyD88 signaling plays an important role in systemic infection with S. aureus, as TLR2−/− and MyD88−/− mice are much more susceptible to infection. TLR2−/− mice have an increased mortality compared to that of WT mice in systemic S. aureus infection, which was attributed to a higher bacterial burden and dysregulated inflammatory responses (29, 30). However, MyD88 is also an important signaling adaptor for IL-1 receptor (IL-1R) family members, and resident skin cells utilize IL-1R/MyD88 signaling to promote neutrophil recruitment upon S. aureus infection (31). Neutrophil recruitment is an essential innate immune response in the host defense against S. aureus infections and is required for bacterial clearance (32, 33). The IL-1R signaling by resident skin cells is therefore crucial for neutrophil recruitment to the site of infection. IL-1R is activated by IL-1β that is produced and activated by the inflammasome of bone marrow-derived cells (34).
On the bacterial side, it has been shown that both S. aureus and the synthetic Lpp Pam2Cys and Pam3Cys alone induced severe bone loss in the femurs of mice after intraperitoneal (i.p.) administration and in a calvarial bone implantation model. However, the Δlgt mutant did not show such effects, indicating that Lpp are responsible for bone destruction during bacterial infections through augmentation of osteoclast differentiation and activation (35). Lpp also induce the inflammatory mediator nitric oxide (NO) in host cells. S. aureus and its mutants lacking lipoteichoic acid (ΔltaS) or d-alanylation of teichoic acids (ΔdltA) stimulated NO production in a murine macrophage cell line; however, the Δlgt mutant failed to induce NO production in a dose-dependent manner (36). These results suggest that not lipoteichoic acid (LTA) but Lpp of S. aureus induce NO production in murine macrophages through activation of TLR2. It is to be expected that Lpp in other pathogenic Gram-positive bacteria, such as Streptococcus pyogenes or Listeria monocytogenes, exert similar effects. S. aureus and synthetic Lpp, but not the Δlgt mutant, LTA, or peptidoglycan (PGN), induced IL-8 expression in the human intestinal epithelial cell-line Caco-2 (37).
Like many other bacteria, S. pneumoniae Δlgt mutants hardly can grow in blood, bronchoalveolar lavage fluid, or cation-depleted medium, and virulence is attenuated in mouse models such as nasopharyngeal colonization, where sepsis or pneumonia was significantly decreased (38). Furthermore, in Δlgt mutants, TLR2 signaling is significantly decreased, and it appears that the leukocyte responses to Lpp are required for TLR2- and IL-1R-associated kinase-4-mediated inflammatory responses to S. pneumoniae (39). Like in many lactic acid bacteria, in S. pneumoniae Mn2+ and Zn2+ ions are also essential, playing a role as cofactors in many proteins and enzymes. PsaA, for example, mediates Mn2+ and Zn2+ transport (40); it is therefore not surprising that psaA mutants were severely affected in growth, virulence, adherence, and the oxidative stress response (41). Two other crucial Lpp are Etrx1 and Etrx2, which are involved in methionine sulfoxide reductase. Deletion of both genes drastically attenuated pneumococcal virulence in an acute mouse pneumonia model, and the mutants were more sensitive to H2O2 and free methionine sulfoxides (MetSO) (42). Streptococcus sanguinis, an important cause of infective endocarditis, contains 52 putative lipoprotein genes in strain SK36. Mutations in either lgt or lspA led to impaired growth and attenuation in virulence. Deletion of the ssaB gene, encoding an Lpp involved in metal transport, drastically reduced endocarditis virulence, while mutations in other lpp genes showed only a minor effect on virulence (43). In Enterococcus faecalis, an opportunistic pathogen responsible for nosocomial infections, Lpp constitute about 25% of the surface-associated proteins. An lgt mutant had significantly decreased virulence (44).
In the two main groups of Gram-positive endospore-forming bacteria, Lpp turned out to be crucial for spore germination and probably also for spore formation. For example, the lgt mutant of Bacillus anthracis not only showed a decreased innate immune stimulation but also was affected in spore germination both in vitro and in mouse skin, which was most likely the reason for the markedly decreased virulence; on the other hand, vegetative cells of the lgt mutant still produced anthrax toxin, rendering the cells dangerous (45). In the anaerobe Clostridium difficile, which causes severe gastrointestinal disease, the lipoproteome was determined by an elegant detection method using alkyne-tagged lipid analogs (46). With this method, most of the predicted Lpp could be detected. C. difficile is remarkable in that there are two active type II signal peptidases (LspA and LspA2) present. Like in B. anthracis, Lpp biogenesis here is also important for sporulation and therefore also for transmission of this pathogen.
The many phenotypic effects of mutations in either lgt or lsp are summarized in Table 1. In all cases but one, an S. aureus Newman transposon mutant of the Phoenix library, the mutations affecting Lpp maturation caused a decreased pathogenicity in the various bacterial species, indicating that maturation of pro-Lpp is important for virulence and pathogenicity.
TABLE 1.
Phenotypes of lgt and lsp mutants of Gram-positive bacteriaa
| Species (mutation[s]) | Phenotype | Reference(s) |
|---|---|---|
| Staphylococcus aureus (Δlgt) | Release of unmodified pre-Lpp is enhanced | 12 |
| No [14C]palmitic acid-labeled Lpp is observed | 12 | |
| Growth is affected in nutrient-poor medium | 12 | |
| Decreased induction of proinflammatory cytokines (IL-6, IL-8, and MCP-1) in human monocytic (MonoMac6), epithelial (pulmonary A549), and endothelial (human umbilical vein endothelial) cells | 12 | |
| No induction of early cytokines via the TLR2-MyD88 signaling pathway in murine peritoneal macrophages, decreased pathogenicity in a C57BL/6 mouse sepsis model, decreased IL-1β chemokine-mediated inflammation, impaired iron uptake under infectious conditions | 17 | |
| Attenuated in S. aureus-induced bone destruction, affected in osteoclast differentiation and bone resorption | 35 | |
| Affected to induce the inflammatory mediator nitric oxide (NO) in host cells | 36 | |
| No induction of IL-8 expression in the human intestinal epithelial cell line Caco-2 | 37 | |
| Staphylococcus aureus (Tnlgt) | Hypervirulent, escapes immune recognition, causes lethal infections with disseminated abscess formation | 19 |
| Streptococcus pneumoniae (lgt and lsp) | Affected in virulence in animal models | 21, 22 |
| Streptococcus agalactiae (lgt) | More sensitive to oxidative stress, reduced retention of group B carbohydrate and the polysaccharide capsule, decreased adherence to human endothelial cells of fetal origin | 23 |
| Streptococcus equi (lgt) | Attenuated virulence in a mouse model, significantly attenuated in a pony infection model | 25 |
| Streptococcus pyogenes (lsp) | Growth defect under zinc-limited conditions, significantly attenuated in virulence | 26 |
| Streptococcus sanguinis (lgt and lsp) | Impaired growth and attenuated virulence | 129 |
| Enterococcus faecalis (lgt) | Impaired growth and attenuated virulence | 44 |
| Listeria monocytogenes (lgt) | Impaired in invasion and intracellular survival, increased susceptibility to cationic peptides, markedly attenuated in mouse infection model | 27, 28 |
| Bacillus anthracis (lgt) | Impaired spore germination and attenuated virulence | 45 |
| Clostridium difficile (lgt) | Impaired sporulation | 46 |
Lgt, phosphatidylglycerol-pro-Lpp diacylglyceryl transferase; Lsp, type II signal peptidase.
ROLE OF TANDEM Lpp ISLANDS IN S. AUREUS
Most S. aureus genomes carry a νSaα island (nonphage and nonstaphylococcal cassette chromosome genomic island) that is inserted at specific loci in the chromosome (47). This island is not found in coagulase-negative species such as Staphylococcus carnosus (48). The genetic organization of νSaα is highly conserved and is composed of two gene clusters: one cluster carries a number of highly homologous exotoxin-encoding genes (set), and the other one encodes lipoproteins, referred to as lipoprotein-like (lpl), with a typical lipobox-containing signal sequence (49). When the entire lpl gene cluster was deleted in S. aureus USA300, the mutant showed a decreased TLR2-dependent stimulation of proinflammatory cytokines in human monocytes, macrophages, and keratinocytes (50). However, more important was the finding that the lpl cluster contributed to invasion of S. aureus into human keratinocytes and mouse skin. This was confirmed when the lpl gene cluster was transformed into S. carnosus, which became invasive in the presence of the lpl genes (50). In a murine kidney abscess model, the bacterial burden in the kidneys was decreased with the lpl deletion mutant. The increased invasion and pathogenicity suggest that the lpl gene cluster is an important virulence factor. As the number of lpl genes is particular high in epidemic S. aureus strains, it is assumed that the lpl gene cluster might contribute to increased dissemination and epidemic spreading, by shielding the pathogen from immune defense and antibiotic treatment (50).
Lpp IN TLR2-DEPENDENT IMMUNE ACTIVATION
While in Gram-negative bacteria lipopolysaccharides (LPS) are the major players in activating the innate immune system via TLR4 interaction, it appears that in Gram-positive bacteria this function is exerted by Lpp triggering the TLR2-MyD88 signaling pathway (51). TLR2 activation leads via a cascade of intermediary steps to NF-κB activation (52, 53). Depending on the degree of acylation, Lpp are recognized by different TLR2 heterodimers. Diacylated Lpp are recognized by TLR2 and TLR6 (54, 55), while triacylated Lpp are recognized by TLR2 and TLR1 heterodimers (56, 57). One of the first steps in TLR2 activation is the phosphorylation on tyrosine residues. Deficiencies in this phosphorylation are associated with defective dimerization and impaired recruitment of the TIR domain-containing adaptor MyD88 (58). The signal transduction from Lpp-bound TLR2 to the activation of the nuclear factor NF-κB involves a cascade of phosphorylation events (59). TIR domain-containing adaptors, such as MyD88 and TRIF, are essential for the induction of inflammatory cytokines triggered by all TLRs. TIRAP and MAL (MyD88-adaptor-like) are involved in the MyD88-dependent pathway via TLR2 signaling (60). Mal facilitates the direct recruitment of TRAF6 to the plasma membrane, which is necessary for TLR2- and TLR4-induced transactivation of NF-κB and regulation of the subsequent proinflammatory response (61). TIRAP recruits MyD88, which in turn recruits both kinases IRAK1 and IRAK4. IRAK4 then is phosphorylated and in turn phosphorylates IRAK1. MyD88 forms a complex with IRAK kinase family members, referred to as the Myddosome (62). Further steps involving IRAK1, TRAF6, TAK1, and IKK protein kinase complexes that lead to the phosphorylation of the NF-κB inhibitory protein IκBα, which undergoes proteasome degradation, thus allowing NF-κB to translocate into the nucleus to induce proinflammatory gene expression, have been reviewed by Kawasaki and Kawai (63). Once in the nucleus, transcription factors induce expression of proinflammatory cytokines, chemokines, and interferons.
There is no doubt that natural Lpp and/or lipopeptides are the main TLR2 agonists. However, it has been reported that TLR2 can also be activated by lipoteichoic acid (LTA) from S. aureus and Streptococcus agalactiae, peptidoglycan (PGN) of Gram-positive bacteria, lipoarabinomannan from mycobacteria, phospholipomannan from Candida albicans, porins from Neisseria, tGPI-mutin from Trypanosoma, or hemagglutinin protein from measles virus (for a review, see reference 64). That means that of all pattern recognition receptors (PRR) in innate immunity, TLR2 recognizes the structurally broadest range of different bacterial compounds known. These molecules are structurally so diverse that it seems unlikely that TLR2 has the capability to react with all agonists to the same degree, if at all. Most likely some of the compounds so far reported as TLR2 agonists were contaminated with highly active lipoproteins and/or lipopeptides.
For example, it has been reported that LTA from S. aureus triggers cytokine release by TLR2 activation (65–67). However, doubts that LTA is really inducing the TLR2 signaling pathway came from the finding that LTA isolated from an lgt mutant of S. aureus was 100-fold less potent than those of wild-type or complemented strains, although the LTA structure of the lgt mutant was the same as that of the wild type (68). These results indicate that the “highly” purified LTA fraction of S. aureus was still contaminated with Lpp and that Lpp-free LTA shows hardly any TLR2 activation. However, not only the staphylococcal LTA but also the LTAs of Enterococcus hirae and Streptococcus pyogenes show no cytokine-stimulating activity (69, 70). These results indicate that in these Gram-positive bacteria, Lpp represent the major TLR2 activating MAMPs (12, 68, 71). What can be learned from these studies is that even purified macromolecules such as LTA, wall teichoic acid (WTA), or polymeric peptidoglycan (PGNpol) always bear the risk of being contaminated with Lpp. As Lpp might be sticky like LPS, and as they are such potent TLR2 agonists, it is difficult to completely avoid contamination. One way to avoid Lpp contamination is to isolate the corresponding macromolecules from an lgt mutant.
Showing no signaling activity does not mean that LTA does not bind to TLR2. Recently, the ligand binding properties of the recombinant human TLR2 ectodomain (hTLR2ED) were investigated (72). It turned out that the ectodomain binds synthetic bacterial and mycoplasmal lipopeptides, S. aureus lipoteichoic acid (obtained from Invivogen, San Diego, CA), and synthetic lipoarabinomannan precursors from Mycobacterium in the absence of its coreceptors TLR1 and TLR6. However, the problem with commercial LTA is that it is not free from contaminating Lpp and other MAMPs; therefore, the results must be considered with some caution. It had earlier already been demonstrated that TLR1 and TLR6 are not necessary for TLR2 activation by distinct lipopeptides (73). All the data obtained in recent years suggest that Lpp and lipopeptides are the real TLR2 agonists and that they are sensed at picomolar levels (74).
TLRs are crucial in recognizing invading microorganisms and triggering their clearance. Therefore, successful skin-pathogenic bacteria, such as S. aureus, have an interest to avoid as much as possible the activation of the innate immune system. Indeed, it has been found that S. aureus secretes a potent TLR2 antagonist, staphylococcal superantigen-like protein 3 (SSL3), which prevents lipopeptide binding to TLR2 (75). SSL3 forms a complex with TLR2 by hydrophobic interaction in such a way that the entrance to the Lpp binding pocket in TLR2 is blocked. Another example of how S. aureus subverts the host innate immune detection by masking their MAMPs is the modification of peptidoglycan by O-acetylation. This modification protects S. aureus murein from lysozyme degradation (76); the corresponding enzyme, O-acetyltransferase (OatA), occurs mainly in pathogenic species (77). Because of the lysozyme-degraded peptidoglycan, a Δoat mutant drastically activates NLRP3 inflammasomes and IL-1β secretion in phagocytes, and mice developed sizeable abscess lesions (78). The induction of the inflammasomes is, however, beneficial for the host, as the Δoat mutant was cleared much better than the wild-type cells. How microbial pathogens employ their own strategies in order to evade, inhibit, or otherwise manipulate the innate immune response has recently been reviewed by Reddick and Alto (79).
IMPACT OF THE STRUCTURE OF THE LIPID MOIETY ON IMMUNE TOLERANCE
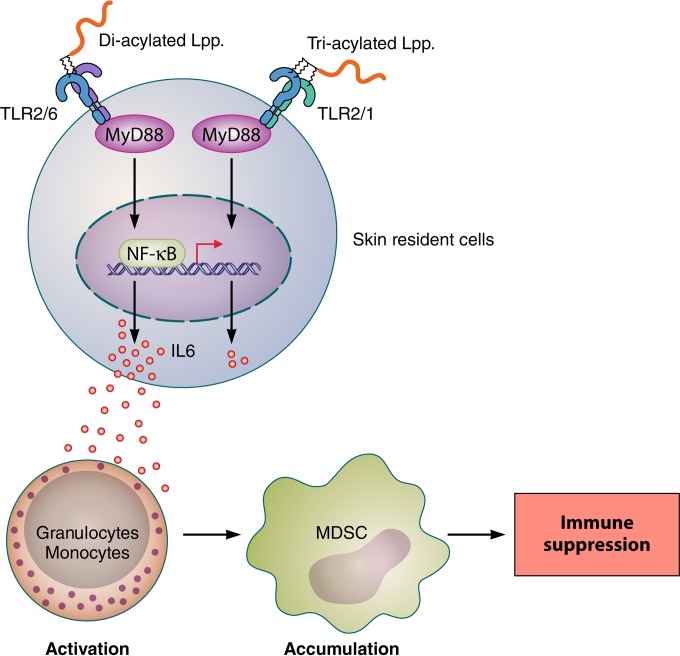
Another example of the different outcomes in immune stimulation is represented by di- and triacylated Lpp. Skin is constantly exposed to resident, mostly harmless, bacteria and their released MAMPs. One would therefore expect a permanent immune activation and accompanied inflammation, which is not the case. Therefore, the immune stimulation of the skin microbiota, living in a sort of symbiosis with the skin, must be perfectly fine-tuned. But how does the immune system distinguish between the skin microbiota and intruding pathogenic microorganisms? A small step forward in answering the question is the recent finding that whether the skin is exposed to di- or triacylated Lpp makes a profound difference in immune response (80). Only diacylated Lpp potently suppressed immune responses through induction of IL-6, which induces granulocytic and monocytic myeloid-derived suppressor cells (MDSCs). The immune suppression was dependent on lipidated Lpp, as the S. aureus Δlgt mutant failed to induce immune suppression (Fig. 3). This study shows that cutaneous bacteria can dampen the immune response by inducing MDSCs via activation of TLR2 to -6.
FIG 3.
Skin immune tolerance is caused by di- but not triacylated Lpp. Diacylated Lpp are sensed by the TLR2/TLR6 heterodimer, while triacylated Lpp are sensed by the TLR2/TLR1 heterodimer. Originally it was thought that the degree of acylation does not make much difference in signaling. However, diacylated Lpp such as Pam2Cys caused a multifold-higher induction of IL-6 than that caused by Pam3Cys in skin resident cells. IL-6 expands into mouse sera, causing induction of suppressive MDSCs derived from normal human peripheral blood mononuclear and granulocytic cells. Finally, the accumulation of MDSCs induces immune suppression. Lpp, lipoproteins/lipopeptides; MyD88, myeloid differentiation primary response protein 88; MDSCs, myeloid-derived suppressor cells; NF-κB, nuclear factor kappa-light-chain enhancer; NOD, nucleotide binding oligomerization domain-containing protein; RIP2, receptor-interacting serine/threonine protein kinase 2.
Finally, it has been demonstrated that cooperation of plasmacytoid dendritic cells (pDCs) and B cells enhances B cell-derived IL-10 production (81). IL-10 is a cytokine associated with immunosuppression and induction of IgG4, an isotype frequently dominating the IgG response to S. aureus. As IL-10 release is partially dependent on TLR2-active lipoproteins, they contribute directly or indirectly to B cell-mediated immune tolerance (82).
INTERFERENCE OF TLR2 LIGANDS WITH OTHER MAMPs AND CORRESPONDING PATHWAYS
An interesting topic is whether Lpp in combination with other MAMPs can exert an additive or even synergistic effect in immune stimulation. Indeed, Lpp have a costimulating effect with peptidoglycan (PGN). Bacterial PGN, another important MAMP, is sensed by NOD1 and NOD2 (83). As staphylococcal PGN contains l-lysine and not meso-diaminopimelic acid (mDAP) in its peptide subunit, it is recognized mainly by NOD2 (84). In contrast to TLR2, NOD proteins lack transmembrane domains and are localized in the cytoplasm. Because of this localization, it is thought that NODs are stimulated mainly by PGN released by phagocytized or invading bacteria, while TLR2 is stimulated mainly by external Lpp.
The role of externally applied PGN in innate immune stimulation is still puzzling. Presumably, some of the immune-stimulating results with PGN were due to contamination with Lpp. Therefore, PGNs were isolated from an lgt mutant (85). Polymeric PGN from the S. aureus Δlgt mutant was internalized in mouse keratinocytes (MK) in an endocytosis-like process and induced intracellular accumulation of NOD2 and TLR2 (85). However, monomeric PGN (PGNmonoΔlgt) completely lacked NF-κB activation and failed to functionally activate murine DCs (86), and it also failed to activate the immune response in bone marrow-derived dendritic cells (BMDCs), J774 cells (derived from mouse BALB/c monocytes/macrophages), and MM6 cells (a human monocytic cell line) (87). Polymeric PGN may be more effective than monomeric PGN because of a more efficient endocytosis (phagocytosis) of the polymers, which may be exported to the cytosol by solute carrier family proteins (SLC15A4) (88).
Peptidoglycan (NOD2 Ligand) Acts Synergistically with TLR2 Ligands
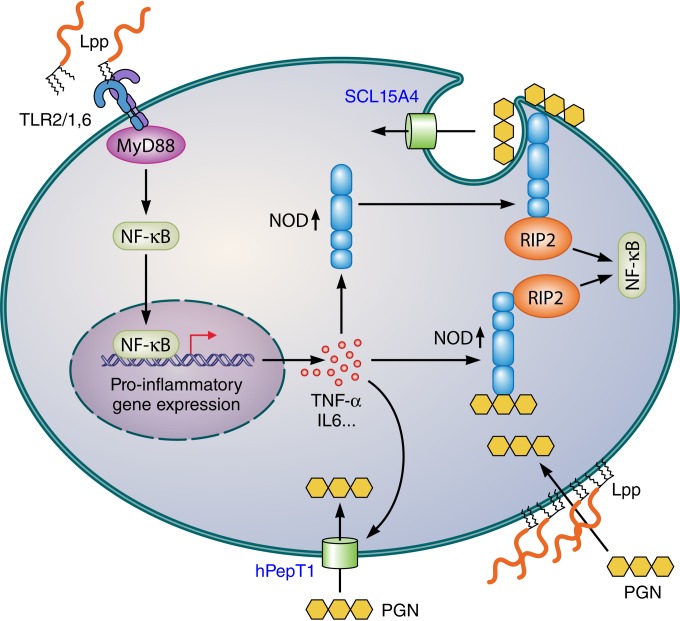
The most interesting observation was an apparent synergistic effect of TLR2 and NOD2 ligands. When PGNpolΔlgt was applied together with Pam3Cys, the immune activation was 3 to 4 times higher than with Pam3Cys alone (87). The question is how a TLR2 ligand can boost the activity of a NOD2 ligand or vice versa. We assume that activation of the TLR2-MyD88 signaling pathways by Lpp is the first reaction because the TLR2 ectodomain is immediately available for the Lpp ligand. Activation of the TLR2-MyD88-dependent signaling pathway might trigger a number of activities that may lead to enhanced NOD activation, as follows. (i) Activation of TLR2 in epithelial or endothelial cells leads to NF-κB activation, which upregulates TNF-α production; both activities in turn trigger the upregulation of NOD2 and increase muramyl dipeptide (MDP) responsiveness (MDP is a synthetic immunoreactive peptide consisting of N-acetylmuramic acid attached to a short amino acid chain of l-Ala-d-isoGln [89, 90]). (ii) NOD1 and NOD2 can be recruited to the plasma membrane, where they detect bacterial invasion or autophagosome-like vesicles (91) at the point of entry (92); it is likely that endocytosed PGN fragments are recognized by NODs recruited to the membrane. Whether the recruitment of NODs to the plasma membrane is induced by TLR2 activation will be shown in the future. (iii) There are various membrane transporters described in epithelial cells, e.g., hPepT1 and SCL15A4; the latter is thought to be an endosomal oligopeptide transporter that transports bacterial peptides such as N-formylmethionylleucylphenylalanine (fMLP) as well as the NOD agonists MDP and l-Ala-d-Glu-meso-diaminopimelic acid (tri-DAP) (93, 94). As hPepT1 expression was upregulated during inflammation triggered by proinflammatory mediators (such as IL-1β, IL-2, IL-6, IL-8, IL-15, gamma interferon [IFN-γ], TNF-α, and many more) one should expect that activation of the TLR2-MyD88 pathway should also lead to hPepT1 upregulation and consequently to an increased uptake of NOD agonists. (iv) NF-κB activation by NOD1 and NOD2 relies on a common downstream adaptor molecule, RIP2, a serine/threonine kinase (95); therefore, the RIP2 adaptor molecule would not compete with the MyD88 adaptor molecule of TLR2 activation, which also could explain the synergistic effect of Lpp and PGN activation. (v) One also should consider that Lpp not only induce the TLR2-MyD88 pathway but also at low doses (<1 μM) trigger membrane scrambling in erythrocyte cells, as indicated by increased cytosolic Ca2+ levels and ceramide formation (96). The membrane scrambling activity is independent of TLR2, as erythrocytes do not express TLR2. Surprisingly, this side effect of Lpp has so far not been connected with immune stimulation. A model for the mechanisms underlying the synergistic effect of TLR2 and NOD agonists is shown in Fig. 4.
FIG 4.
Possible mechanisms for the synergistic immune stimulation of TLR2 and NOD2 ligands. Stimulation with di- and triacylated Lpp triggers the TLR2-MyD88-dependent signaling pathway, resulting in NF-κB activation and induction of proinflammatory cytokines. The latter, particularly TNF-α, upregulate NOD expression as well as oligopeptide transporters, e.g., hPepT1 and SCL15A4, that also transport NOD agonists. PGN is also taken up in an endocytosis-like process, from where it can be translocated into cytoplasm by the endosomal transporter SCL15A4. How NODs are recruited to the plasma membrane is unknown. Lpp not only act as TLR2 agonists but are also able to cause membrane scrambling; whether this effect contributes to signaling or to facilitating PGN uptake is unknown. Both TLR2 and NOD activation lead finally to NF-κB activation, but the downstream adaptor molecules, RIP2 and MyD88, are different, thus excluding competition for a common adaptor protein and allowing synergistic NF-κB activation. PGN, peptidoglycan; Lpp, lipoproteins/lipopeptides; MyD88, myeloid differentiation primary response protein 88; NF-κB, nuclear factor kappa-light-chain enhancer; NOD, nucleotide binding oligomerization domain-containing protein; RIP2, receptor-interacting serine/threonine protein kinase 2.
RNA (TLR7, -8, and -9 Ligand) Acts Antagonistically with TLR2 Ligands
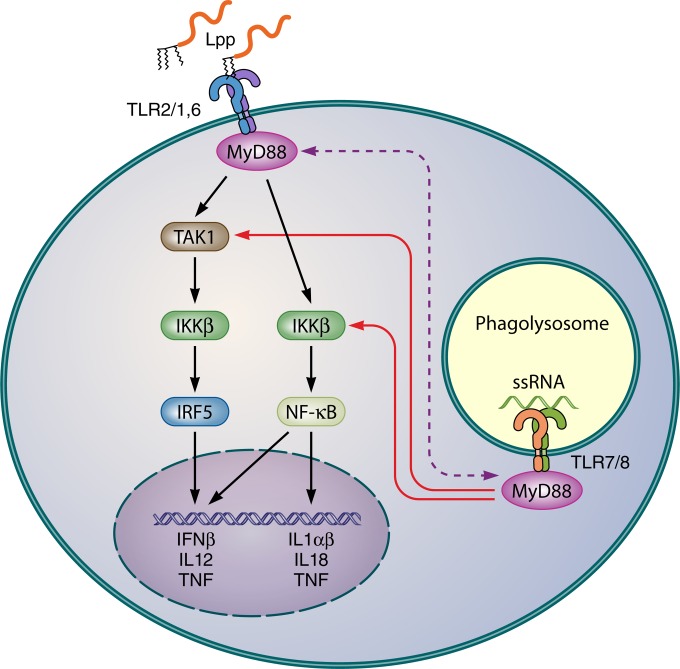
The various forms of bacterial RNA can be recognized by TLR7, TLR8, and TLR9 in concert with the cytosolic adaptor protein MyD88. Double-stranded RNAs of certain viruses are recognized by TLR3 in concert with TRIF as an adaptor protein which triggers IFN-β production via NF-κB activation (97, 98). It has been observed that in whole-blood samples the culture supernatant of the S. aureus lgt mutant induced TNF-α even more strongly than the wild-type (12). This suggests that in whole blood other MAMPs in addition to Lpp play a role. Indeed, one such player is RNA. S. aureus RNA induces the production of IFN-β by TLR8 sensing and triggers IRF5 nuclear accumulation in human primary monocytes and macrophages (99). The induction of IFN-β production by whole bacteria in human primary monocytes and monocyte-derived macrophages (MDMs) occurs via a TAK1-IKKβ-IRF5 signaling pathway. Surprisingly, TLR2 activation suppressed the S. aureus-induced production of IFN-β. How TLR2 activation antagonizes IFN-β production is unclear. One explanation could be that the TLR2 stimulus competes with TLR8 for the use of MyD88. Another explanation refers to the different localizations of TLR8 and TLR2 within the cell: TLR2 is localized mainly in the cell membrane, while TLR8 is localized mainly in the endosome (100, 101). This means that free Lpp can immediately activate TLR2 at the host cell surface, while TLR8 activation usually requires bacterial uptake and release of RNA in the host cell. Figure 5 illustrates the potential interaction of TLR2 and TLR7/8 ligands. Monocytes and phagocytes are ideal host cells to study the effects of simultaneously applied Lpp and RNA, as they have high mRNA levels of TLR2, TLR4, and TLR8 and low levels of TLR3, TLR7, and TLR9 (102). While in Gram-negative bacteria IFN-β is induced by LPS-mediated activation of endosomal TLR4 signaling (103, 104), in Gram-positive bacteria RNA and DNA are crucial for IFN-β production (105–107).
FIG 5.
Possible mechanism for the antagonistic effect of Lpp and RNA in innate immune stimulation in monocytes and macrophages. The induction of IFN-β production by whole bacteria in human primary monocytes and monocyte-derived macrophages (MDMs) is triggered by S. aureus RNA sensed by TLR7 and TLR8, which activates the TAK1-IKKβ-IRF5 signaling pathway. TLR2 activation by Lpp suppresses the RNA-induced production of IFN-β. As both TLR2 and TLR8 use the same adaptor molecule MyD88 in TAK1-dependent and TAK1-independent pathways, a depletion of MyD88 is the consequence (99). Another explanation for TLR2's overruling the TLR8 signaling is their different locations; Lpp can immediately activate TLR2 at the host cell surface, while TLR8 activation usually affords phagocytosis and phagolysosomally mediated release of RNA, which is unfavorable in the competition for MyD88. IKKβ, a serine kinase which plays a key role in the NF-κB signaling pathway by phosphorylating inhibitors in the inhibitor/NF-κB complex; IRF5, interferon regulatory factor 5; Lpp, lipoproteins/lipopeptides; MyD88, myeloid differentiation primary response protein 88; NF-κB, nuclear factor kappa-light-chain enhancer; ssRNA, single-strand RNA; TAK1, ubiquitin-dependent kinase of MKK and IKK. TLR7 and TLR8 are activated by ribonucleoside analogs.
SKIN UNSATURATED FATTY ACIDS BOOST THE IMMUNE RESPONSE
S. aureus is one of the most potent skin pathogens; it colonizes approximately 30 to 50% of healthy adults intermittently and 10 to 20% persistently (108). Sebaceous glands and nasal sebum, where S. aureus frequently has first contact, produce both saturated (particularly C14:0, C16:0, and C18:0) and unsaturated (particularly C16:1, C18:1, and C18:2) fatty acids (FA) in concentrations from 10 to 46 μM (109–111). This concentration is not enough to inhibit S. aureus growth (112, 113). S. aureus can synthesize only saturated FA with a chain length between C10:0 and C22:0 (114) but can incorporate exogenous unsaturated FA into phospholipids by use of the fatty acid kinase complex FakAB1B2 (115). The question is whether the unsaturated FA were also incorporated into the lipid moiety of Lpp and whether such Lpp effect immune stimulation. Indeed, unsaturated FA were incorporated into Lpp, particularly linoleic acid, and caused an increase of TLR2-dependent immune stimulation (116). These results show that not only the number but also the structure of the FA incorporated in the lipid moiety of Lpp is crucial for immune stimulation. Therefore, a more profound structural analysis of the lipid moiety of commensal and pathogenic bacteria might give clues for about the immune tolerance of commensals.
Lpp AS VACCINE CANDIDATES
Clinical trials in the United States showed that Lyme disease could be prevented by vaccination with OspA, a major surface Lpp encoded by all Borrelia burgdorferi species (117, 118). The development of a protective vaccine against S. aureus turned out to be very difficult, and many vaccine trials failed at the end. The reasons are that there is no correlate of protection known yet, and S. aureus pathogenic mechanisms are very complex, with a plethora of toxins and immune evasion factors expressed by the pathogen (119). Nevertheless, there were many potential vaccine candidates identified, particularly toxins. In more recent years Lpp also were considered as vaccine candidates. It has been already shown that S. aureus mutants defective in lipidation of pro-Lpp (Δlgt) are severely affected in virulence, particularly by their impaired uptake of iron (17). By surface proteome analysis it has been shown that during murine host infection and in convalescent human serum, the majority of the in vivo-expressed surface-associated proteins are Lpp involved in nutrient uptake and metal ion acquisition. Among the 7 highly abundant Lpp, only MntC (SitC), which is the manganese binding protein of the MntABC system, was essential for virulence of methicillin-resistant S. aureus (MRSA) during murine systemic infection (120); MntC was previously referred as SitC, as it was proposed that it was involved in iron transport (12–14, 121). MntC (SitC) is highly conserved in the genus Staphylococcus, and expression studies revealed that it is expressed early during the infectious cycle. Therefore, it is a promising vaccine candidates. Indeed, it has been shown that active immunization with MntC reduced the bacterial load of S. aureus and S. epidermidis infection in an acute murine bacteremia model, and anti-MntC monoclonal antibodies were protective in a rat passive immunization model and induced neutrophil respiratory burst activity (122). Moreover, MntC from S. aureus and SitA from S. pseudintermedius are orthologous, show high sequence identity, and have been well characterized biochemically and structurally, suggesting that they may be suitable for industrial-scale production as staphylococcal vaccine antigens (1, 123).
Another potential vaccine candidate is the Lpp FhuD2, which is involved in ferric-hydroxamate uptake. FhuD2 binds ferrichrome with nanomolar affinity, and the structure of FhuD2-ferrichrome has been determined (124, 125). Immunization with FhuD2 alone or together with hydroxamate siderophores was protective in a murine staphylococcal infection model (126). However, a breakthrough was reported only recently with a combination of five antigens formulated with a novel adjuvant containing a TLR7-dependent agonist adsorbed to alum. This vaccine provided close to 100% protection against four different staphylococcal strains. The new formulation induced not only high antibody titers but also a Th1-skewed immune response and IL-17-secreting T cells (127). Among the five antigens were two Lpp, FhuD2 and conserved staphylococcal antigen 1A (Csa1A). Csa1A belongs to a family of 10 to 20 conserved staphylococcal antigens (Csa) classified as DUF576 and taxonomically restricted to staphylococci. The structures of the Lpp Csa1A and Csa1B were determined, and it has been shown that they conferred protective immunity against S. aureus in animal models (128).
CONCLUSION
Lpp in Gram-positive bacteria fulfill a similar important function in immune modulation as the LPS in Gram-negative bacteria. However, unlike for LPS, we are only now beginning to better understand the multiple effects of Lpp in Gram-positive bacteria. We have learned that they play a crucial role in pathogenicity and that when the maturation of pre-Lpp is affected, then pathogenicity is also affected. It emerged that the degree of Lpp acylation has an enormous influence on the immune response. Exposure of the skin to diacylated Lpp induces immune suppression, while exposure to triacylated Lpp does not. Also, the fatty acid structure of the lipid moiety influences the proinflammatory response. However, we know very little about the structural diversity of the lipid moiety of Lpp in commensal and pathogenic Gram-positive bacteria. This review should sharpen our awareness that the bacterial growth phase and environmental conditions have an impact on expression and Lpp structure and thus on the immune response. We also have learned that bulky bacterial PRR ligands, although highly purified, bear the risk of being still contaminated with small amounts of Lpp or RNAs that may be finally responsible for the experimentally observed immune reaction; particularly, commercial bacterial polymeric PGN and LTA are not pure enough to yield reliable results. Only using defined mutants affected in synthesis of certain TLR ligands could help to solve the problem of contamination, as exemplified by the lgt mutants, where at least Lpp stimulation is excluded. A real challenge in the future will be to find out how the harmless commensals are tolerated by the immune system and how they differ from intruding pathogenic bacteria. Much work lies before us to better understand the structural diversity of the lipid moiety of Lpp and their impact on the immune response, inflammation, and pathogenicity.
ACKNOWLEDGMENTS
Our special thanks go to Fabio Bagnoli and to Jos van Strijp for their critical and helpful suggestions.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG; GO 371/9-1, SFB766, and TRR34).
Biographies

Minh Thu Nguyen is a postdoctoral fellow at the Department of Microbial Genetics at the University of Tübingen. She obtained her B.Sc. in biology at the Vietnam National University (Hanoi) College of Natural Sciences and her M.Sc. in chemical engineering at Sungkyunkwan University, South Korea. Since 2010 she has been at the University of Tübingen, supported by a scholarship from the Vietnamese government and subsequently by the German Research Foundation (DFG). She has received several awards and scholarships and has seven peer-reviewed publications, three times as first author. During her Ph.D. work, she found out that Staphylococcus aureus tandem lipoproteins increased virulence and invasiveness into host cells, and she unraveled a new mechanism of how skin controls bacterial colonization and infection. Currently, she is focusing on the structural modifications of the lipid moiety of lipoproteins and their impact on the innate and adaptive immune responses.

Friedrich Götz is Professor and since 1987 head of the Department of Microbial Genetics at the University of Tübingen, Germany. He studied biology and chemistry at the University of Munich, Germany, and received his Ph.D. in microbiology in 1978. From 1979 to 1981 he was a postdoctoral fellow (EMBO long-term fellowship) at the University of Uppsala Biomedical Center, Sweden. He received several awards, was president of the Association of General and Applied Microbiology (VAAM), and is a member of the European Academy of Microbiology (EAM). He has a broad interest in Staphylococcus physiology and the interaction of the bacterium with the host. Currently, he focuses on immune modulation and the various mechanisms of adaptation to the host and the environment.
REFERENCES
- 1.Abate F, Malito E, Cozzi R, Lo Surdo P, Maione D, Bottomley MJ. 2014. Apo, Zn2+-bound and Mn2+-bound structures reveal ligand-binding properties of SitA from the pathogen Staphylococcus pseudintermedius. Biosci Rep 34:e00154. doi: 10.1042/BSR20140088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun V, Rehn K. 1969. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem 10:426–438. [DOI] [PubMed] [Google Scholar]
- 3.Braun V, Wolff H. 1970. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem 14:387–391. doi: 10.1111/j.1432-1033.1970.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 4.Hantke K, Braun V. 1973. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem 34:284–296. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi S, Wu HC. 1990. Lipoproteins in bacteria. J Bioenerg Biomembr 22:451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 6.Lee PA, Tullman-Ercek D, Georgiou G. 2006. The bacterial twin-arginine translocation pathway. Annu Rev Microbiol 60:373–395. doi: 10.1146/annurev.micro.60.080805.142212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tokunaga M, Tokunaga H, Wu HC. 1982. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc Natl Acad Sci U S A 79:2255–2259. doi: 10.1073/pnas.79.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain M, Ichihara S, Mizushima S. 1982. Mechanism of signal peptide cleavage in the biosynthesis of the major lipoprotein of the Escherichia coli outer membrane. J Biol Chem 257:5177–5182. [PubMed] [Google Scholar]
- 9.Sankaran K, Gupta SD, Wu HC. 1995. Modification of bacterial lipoproteins. Methods Enzymol 250:683–697. doi: 10.1016/0076-6879(95)50105-3. [DOI] [PubMed] [Google Scholar]
- 10.Buddelmeijer N. 2015. The molecular mechanism of bacterial lipoprotein modification—how, when and why? FEMS Microbiol Rev 39:246–261. doi: 10.1093/femsre/fuu006. [DOI] [PubMed] [Google Scholar]
- 11.Schmollinger M, Fischer I, Nerz C, Pinkenburg S, Götz F, Kaufmann M, Lange KJ, Reuter R, Rosenstiel W, Zell A. 2004. ParSeq: searching motifs with structural and biochemical properties. Bioinformatics 20:1459–1461. doi: 10.1093/bioinformatics/bth083. [DOI] [PubMed] [Google Scholar]
- 12.Stoll H, Dengjel J, Nerz C, Götz F. 2005. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect Immun 73:2411–2423. doi: 10.1128/IAI.73.4.2411-2423.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurokawa K, Lee H, Roh KB, Asanuma M, Kim YS, Nakyama H, Shiratsuchi A, Choi Y, Takeuchi O, Kang HJ, Dohmae N, Nakanishi Y, Akira S, Sekimizu K, Lee BL. 2009. The triacylated ATP binding cluster transporter substrate-binding lipoprotein of Staphylococcus aureus functions as a native ligand for the Toll-like receptor 2. J Biol Chem 284:8406–8411. doi: 10.1074/jbc.M809618200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller P, Müller-Anstett M, Wagener J, Gao Q, Kaesler S, Schaller M, Biedermann T, Götz F. 2010. The Staphylococcus aureus lipoprotein SitC colocalizes with Toll-like receptor 2 (TLR2) in murine keratinocytes and elicits intracellular TLR2 accumulation. Infect Immun 78:4243–4250. doi: 10.1128/IAI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurokawa K, Kim MS, Ichikawa R, Ryu KH, Dohmae N, Nakayama H, Lee BL. 2012. Environment-mediated accumulation of diacyl lipoproteins over their triacyl counterparts in Staphylococcus aureus. J Bacteriol 194:3299–3306. doi: 10.1128/JB.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurokawa K, Ryu KH, Ichikawa R, Masuda A, Kim MS, Lee H, Chae JH, Shimizu T, Saitoh T, Kuwano K, Akira S, Dohmae N, Nakayama H, Lee BL. 2012. Novel bacterial lipoprotein structures conserved in low-GC content gram-positive bacteria are recognized by Toll-like receptor 2. J Biol Chem 287:13170–13181. doi: 10.1074/jbc.M111.292235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmaler M, Jann NJ, Ferracin F, Landolt LZ, Biswas L, Götz F, Landmann R. 2009. Lipoproteins in Staphylococcus aureus mediate inflammation by TLR2 and iron-dependent growth in vivo. J Immunol 182:7110–7118. doi: 10.4049/jimmunol.0804292. [DOI] [PubMed] [Google Scholar]
- 18.Schmaler M, Jann NJ, Götz F, Landmann R. 2010. Staphylococcal lipoproteins and their role in bacterial survival in mice. Int J Med Microbiol 300:155–160. doi: 10.1016/j.ijmm.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Bubeck Wardenburg J, Williams WA, Missiakas D. 2006. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc Natl Acad Sci U S A 103:13831–13836. doi: 10.1073/pnas.0603072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sander P, Rezwan M, Walker B, Rampini SK, Kroppenstedt RM, Ehlers S, Keller C, Keeble JR, Hagemeier M, Colston MJ, Springer B, Böttger EC. 2004. Lipoprotein processing is required for virulence of Mycobacterium tuberculosis. Mol Microbiol 52:1543–1552. doi: 10.1111/j.1365-2958.2004.04041.x. [DOI] [PubMed] [Google Scholar]
- 21.Khandavilli S, Homer KA, Yuste J, Basavanna S, Mitchell T, Brown JS. 2008. Maturation of Streptococcus pneumoniae lipoproteins by a type II signal peptidase is required for ABC transporter function and full virulence. Mol Microbiol 67:541–557. doi: 10.1111/j.1365-2958.2007.06065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petit CM, Brown JR, Ingraham K, Bryant AP, Holmes DJ. 2001. Lipid modification of prelipoproteins is dispensable for growth in vitro but essential for virulence in Streptococcus pneumoniae. FEMS Microbiol Lett 200:229–233. doi: 10.1111/j.1574-6968.2001.tb10720.x. [DOI] [PubMed] [Google Scholar]
- 23.Bray BA, Sutcliffe IC, Harrington DJ. 2009. Impact of lgt mutation on lipoprotein biosynthesis and in vitro phenotypes of Streptococcus agalactiae. Microbiology 155:1451–1458. doi: 10.1099/mic.0.025213-0. [DOI] [PubMed] [Google Scholar]
- 24.Henneke P, Dramsi S, Mancuso G, Chraibi K, Pellegrini E, Theilacker C, Hubner J, Santos-Sierra S, Teti G, Golenbock DT, Poyart C, Trieu-Cuot P. 2008. Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J Immunol 180:6149–6158. doi: 10.4049/jimmunol.180.9.6149. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton A, Robinson C, Sutcliffe IC, Slater J, Maskell DJ, Davis-Poynter N, Smith K, Waller A, Harrington DJ. 2006. Mutation of the maturase lipoprotein attenuates the virulence of Streptococcus equi to a greater extent than does loss of general lipoprotein lipidation. Infect Immun 74:6907–6919. doi: 10.1128/IAI.01116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weston BF, Brenot A, Caparon MG. 2009. The metal homeostasis protein, Lsp, of Streptococcus pyogenes is necessary for acquisition of zinc and virulence. Infect Immun 77:2840–2848. doi: 10.1128/IAI.01299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgartner M, Karst U, Gerstel B, Loessner M, Wehland J, Jansch L. 2007. Inactivation of Lgt allows systematic characterization of lipoproteins from Listeria monocytogenes. J Bacteriol 189:313–324. doi: 10.1128/JB.00976-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machata S, Tchatalbachev S, Mohamed W, Jansch L, Hain T, Chakraborty T. 2008. Lipoproteins of Listeria monocytogenes are critical for virulence and TLR2-mediated immune activation. J Immunol 181:2028–2035. doi: 10.4049/jimmunol.181.3.2028. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi O, Hoshino K, Akira S. 2000. TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 30.Yimin Kohanawa M, Zhao S, Ozaki M, Haga S, Nan G, Kuge Y, Tamaki N. 2013. Contribution of Toll-like receptor 2 to the innate response against Staphylococcus aureus infection in mice. PLoS One 8:e74287. doi: 10.1371/journal.pone.0074287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. 2006. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Molne L, Verdrengh M, Tarkowski A. 2000. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun 68:6162–6167. doi: 10.1128/IAI.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdrengh M, Tarkowski A. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun 65:2517–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O'Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. 2007. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol 179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Yang J, Park OJ, Kang SS, Kim WS, Kurokawa K, Yun CH, Kim HH, Lee BL, Han SH. 2013. Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation. J Bone Miner Res 28:2381–2391. doi: 10.1002/jbmr.1973. [DOI] [PubMed] [Google Scholar]
- 36.Kim NJ, Ahn KB, Jeon JH, Yun CH, Finlay BB, Han SH. 2015. Lipoprotein in the cell wall of Staphylococcus aureus is a major inducer of nitric oxide production in murine macrophages. Mol Immunol 65:17–24. doi: 10.1016/j.molimm.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Kang SS, Noh SY, Park OJ, Yun CH, Han SH. 2015. Staphylococcus aureus induces IL-8 expression through its lipoproteins in the human intestinal epithelial cell, Caco-2. Cytokine 75:174–180. doi: 10.1016/j.cyto.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 38.Chimalapati S, Cohen JM, Camberlein E, MacDonald N, Durmort C, Vernet T, Hermans PW, Mitchell T, Brown JS. 2012. Effects of deletion of the Streptococcus pneumoniae lipoprotein diacylglyceryl transferase gene lgt on ABC transporter function and on growth in vivo. PLoS One 7:e41393. doi: 10.1371/journal.pone.0041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomlinson G, Chimalapati S, Pollard T, Lapp T, Cohen J, Camberlein E, Stafford S, Periselneris J, Aldridge C, Vollmer W, Picard C, Casanova JL, Noursadeghi M, Brown J. 2014. TLR-mediated inflammatory responses to Streptococcus pneumoniae are highly dependent on surface expression of bacterial lipoproteins. J Immunol 193:3736–3745. doi: 10.4049/jimmunol.1401413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N, Yang XY, Guo Z, Zhang J, Cao K, Han J, Zhang G, Liu L, Sun X, He QY. 2014. Varied metal-binding properties of lipoprotein PsaA in Streptococcus pneumoniae. J Biol Inorg Chem 19:829–838. doi: 10.1007/s00775-014-1114-9. [DOI] [PubMed] [Google Scholar]
- 41.Johnston JW, Myers LE, Ochs MM, Benjamin WH Jr, Briles DE, Hollingshead SK. 2004. Lipoprotein PsaA in virulence of Streptococcus pneumoniae: surface accessibility and role in protection from superoxide. Infect Immun 72:5858–5867. doi: 10.1128/IAI.72.10.5858-5867.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saleh M, Bartual SG, Abdullah MR, Jensch I, Asmat TM, Petruschka L, Pribyl T, Gellert M, Lillig CH, Antelmann H, Hermoso JA, Hammerschmidt S. 2013. Molecular architecture of Streptococcus pneumoniae surface thioredoxin-fold lipoproteins crucial for extracellular oxidative stress resistance and maintenance of virulence. EMBO Mol Med 5:1852–1870. doi: 10.1002/emmm.201202435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biswas L, Biswas R, Nerz C, Ohlsen K, Schlag M, Schafer T, Lamkemeyer T, Ziebandt AK, Hantke K, Rosenstein R, Gotz F. 2009. Role of the twin-arginine translocation pathway in Staphylococcus. J Bacteriol 191:5921–5929. doi: 10.1128/JB.00642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reffuveille F, Serror P, Chevalier S, Budin-Verneuil A, Ladjouzi R, Bernay B, Auffray Y, Rince A. 2012. The prolipoprotein diacylglyceryl transferase (Lgt) of Enterococcus faecalis contributes to virulence. Microbiology 158:816–825. doi: 10.1099/mic.0.055319-0. [DOI] [PubMed] [Google Scholar]
- 45.Okugawa S, Moayeri M, Pomerantsev AP, Sastalla I, Crown D, Gupta PK, Leppla SH. 2012. Lipoprotein biosynthesis by prolipoprotein diacylglyceryl transferase is required for efficient spore germination and full virulence of Bacillus anthracis. Mol Microbiol 83:96–109. doi: 10.1111/j.1365-2958.2011.07915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charlton TM, Kovacs-Simon A, Michell SL, Fairweather NF, Tate EW. 2015. Quantitative lipoproteomics in Clostridium difficile reveals a role for lipoproteins in sporulation. Chem Biol 22:1562–1573. doi: 10.1016/j.chembiol.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Tsuru T, Kobayashi I. 2008. Multiple genome comparison within a bacterial species reveals a unit of evolution spanning two adjacent genes in a tandem paralog cluster. Mol Biol Evol 25:2457–2473. doi: 10.1093/molbev/msn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenstein R, Nerz C, Biswas L, Resch A, Raddatz G, Schuster SC, Götz F. 2009. Genome analysis of the meat starter culture bacterium Staphylococcus carnosus TM300. Appl Environ Microbiol 75:811–822. doi: 10.1128/AEM.01982-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babu MM, Priya ML, Selvan AT, Madera M, Gough J, Aravind L, Sankaran K. 2006. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J Bacteriol 188:2761–2773. doi: 10.1128/JB.188.8.2761-2773.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen MT, Kraft B, Yu W, Demicrioglu DD, Hertlein T, Burian M, Schmaler M, Boller K, Bekeredjian-Ding I, Ohlsen K, Schittek B, Götz F. 2015. The νSaα specific lipoprotein like cluster (lpl) of S. aureus USA300 contributes to immune stimulation and invasion in human cells. PLoS Pathog 11:e1004984. doi: 10.1371/journal.ppat.1004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 52.Rawadi G, Garcia J, Lemercier B, Roman-Roman S. 1999. Signal transduction pathways involved in the activation of NF-kappa B, AP-1, and c-fos by Mycoplasma fermentans membrane lipoproteins in macrophages. J Immunol 162:2193–2203. [PubMed] [Google Scholar]
- 53.Norgard MV, Arndt LL, Akins DR, Curetty LL, Harrich DA, Radolf JD. 1996. Activation of human monocytic cells by Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NF-kappa B. Infect Immun 64:3845–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bulut Y, Faure E, Thomas L, Equils O, Arditi M. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol 167:987–994. doi: 10.4049/jimmunol.167.2.987. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 56.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. 2002. Role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 57.Takeda K, Takeuchi O, Akira S. 2002. Recognition of lipopeptides by Toll-like receptors. J Endotoxin Res 8:459–463. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Lee DS, Madrenas J. 2013. Evolving bacterial envelopes and plasticity of TLR2-dependent responses: basic research and translational opportunities. Front Immunol 4:347. doi: 10.3389/fimmu.2013.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takeda K, Akira S. 2004. TLR signaling pathways. Semin Immunol 16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Horng T, Barton GM, Flavell RA, Medzhitov R. 2002. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 61.Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A. 2009. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J Biol Chem 284:24192–24203. doi: 10.1074/jbc.M109.023044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin SC, Lo YC, Wu H. 2010. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawasaki T, Kawai T. 2014. Toll-like receptor signaling pathways. Front Immunol 5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 65.Morath S, Geyer A, Hartung T. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med 193:393–397. doi: 10.1084/jem.193.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morath S, Stadelmaier A, Geyer A, Schmidt RR, Hartung T. 2002. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. J Exp Med 195:1635–1640. doi: 10.1084/jem.20020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem 274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto M, Tawaratsumida K, Kariya H, Kiyohara A, Suda Y, Krikae F, Kirikae T, Götz F. 2006. Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J Immunol 177:3162–3169. doi: 10.4049/jimmunol.177.5.3162. [DOI] [PubMed] [Google Scholar]
- 69.Hashimoto M, Yasuoka J, Suda Y, Takada H, Yoshida T, Kotani S, Kusumoto S. 1997. Structural feature of the major but not cytokine-inducing molecular species of lipoteichoic acid. J Biochem 121:779–786. doi: 10.1093/oxfordjournals.jbchem.a021653. [DOI] [PubMed] [Google Scholar]
- 70.Takada H, Kawabata Y, Arakaki R, Kusumoto S, Fukase K, Suda Y, Yoshimura T, Kokeguchi S, Kato K, Komuro T, et al. . 1995. Molecular and structural requirements of a lipoteichoic acid from Enterococcus hirae ATCC 9790 for cytokine-inducing, antitumor, and antigenic activities. Infect Immun 63:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hashimoto M, Tawaratsumida K, Kariya H, Aoyama K, Tamura T, Suda Y. 2006. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int Immunol 18:355–362. [DOI] [PubMed] [Google Scholar]
- 72.Jimenez-Dalmaroni MJ, Radcliffe CM, Harvey DJ, Wormald MR, Verdino P, Ainge GD, Larsen DS, Painter GF, Ulevitch R, Beutler B, Rudd PM, Dwek RA, Wilson IA. 2015. Soluble human TLR2 ectodomain binds diacylglycerol from microbial lipopeptides and glycolipids. Innate Immun 21:175–193. doi: 10.1177/1753425914524077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. 2006. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem 281:9049–9057. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- 74.Zähringer U, Lindner B, Inamura S, Heine H, Alexander C. 2008. TLR2—promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213:205–224. doi: 10.1016/j.imbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Koymans KJ, Feitsma LJ, Brondijk TH, Aerts PC, Lukkien E, Lossl P, van Kessel KP, de Haas CJ, van Strijp JA, Huizinga EG. 2015. Structural basis for inhibition of TLR2 by staphylococcal superantigen-like protein 3 (SSL3). Proc Natl Acad Sci U S A 112:11018–11023. doi: 10.1073/pnas.1502026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bera A, Herbert S, Jakob A, Vollmer W, Götz F. 2005. Why are pathogenic staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol Microbiol 55:778–787. [DOI] [PubMed] [Google Scholar]
- 77.Bera A, Biswas R, Herbert S, Götz F. 2006. The presence of peptidoglycan O-acetyltransferase in various staphylococcal species correlates with lysozyme resistance and pathogenicity. Infect Immun 74:4598–4604. doi: 10.1128/IAI.00301-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, Reyes CN, Miao EA, Aderem A, Götz F, Liu GY, Underhill DM. 2010. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe 7:38–49. doi: 10.1016/j.chom.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reddick LE, Alto NM. 2014. Bacteria fighting back: how pathogens target and subvert the host innate immune system. Mol Cell 54:321–328. doi: 10.1016/j.molcel.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skabytska Y, Wolbing F, Gunther C, Koberle M, Kaesler S, Chen KM, Guenova E, Demircioglu D, Kempf WE, Volz T, Rammensee HG, Schaller M, Röcken M, Götz F, Biedermann T. 2014. Cutaneous innate immune sensing of Toll-like receptor 2-6 ligands suppresses T cell immunity by inducing myeloid-derived suppressor cells. Immunity 41:762–775. doi: 10.1016/j.immuni.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 81.Bekeredjian-Ding I, Inamura S, Giese T, Moll H, Endres S, Sing A, Zähringer U, Hartmann G. 2007. Staphylococcus aureus protein A triggers T cell-independent B cell proliferation by sensitizing B cells for TLR2 ligands. J Immunol 178:2803–2812. doi: 10.4049/jimmunol.178.5.2803. [DOI] [PubMed] [Google Scholar]
- 82.Parcina M, Miranda-Garcia MA, Durlanik S, Ziegler S, Over B, Georg P, Foermer S, Ammann S, Hilmi D, Weber KJ, Schiller M, Heeg K, Schneider-Brachert W, Götz F, Bekeredjian-Ding I. 2013. Pathogen-triggered activation of plasmacytoid dendritic cells induces IL-10-producing B cells in response to Staphylococcus aureus. J Immunol 190:1591–1602. doi: 10.4049/jimmunol.1201222. [DOI] [PubMed] [Google Scholar]
- 83.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. 2014. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol 14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 84.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 85.Müller-Anstett MA, Müller P, Albrecht T, Nega M, Wagener J, Gao Q, Kaesler S, Schaller M, Biedermann T, Götz F. 2010. Staphylococcal peptidoglycan co-localizes with Nod2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PLoS One 5:e13153. doi: 10.1371/journal.pone.0013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Volz T, Nega M, Buschmann J, Kaesler S, Guenova E, Peschel A, Röcken M, Götz F, Biedermann T. 2010. Natural Staphylococcus aureus-derived peptidoglycan fragments activate NOD2 and act as potent costimulators of the innate immune system exclusively in the presence of TLR signals. FASEB J doi: 10.1096/fj.09-151001. [DOI] [PubMed] [Google Scholar]
- 87.Schäffler H, Demircioglu DD, Kuhner D, Menz S, Bender A, Autenrieth IB, Bodammer P, Lamprecht G, Götz F, Frick JS. 2014. NOD2 stimulation by Staphylococcus aureus-derived peptidoglycan is boosted by Toll-like receptor 2 costimulation with lipoproteins in dendritic cells. Infect Immun 82:4681–4688. doi: 10.1128/IAI.02043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iyer JK, Coggeshall KM. 2011. Primary innate immune cells respond efficiently to polymeric peptidoglycan, but not to peptidoglycan monomers. J Immunol 186:3841–3845. doi: 10.4049/jimmunol.1004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gutierrez O, Pipaon C, Inohara N, Fontalba A, Ogura Y, Prosper F, Nunez G, Fernandez-Luna JL. 2002. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem 277:41701–41705. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 90.Rosenstiel P, Fantini M, Brautigam K, Kuhbacher T, Waetzig GH, Seegert D, Schreiber S. 2003. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology 124:1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 91.Mauthe M, Yu W, Krut O., Kronke M, Götz F, Robenek H, Proikas-Cezanne T. 2012. WIPI-1 positive autophagosome-like vesicles entrap pathogenic Staphylococcus aureus for lysosomal degradation. Int J Cell Biol 2012:179207. doi: 10.1155/2012/179207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Philpott DJ, Girardin SE. 2010. Nod-like receptors: sentinels at host membranes. Curr Opin Immunol 22:428–434. doi: 10.1016/j.coi.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 93.Dalmasso G, Nguyen HT, Charrier-Hisamuddin L, Yan Y, Laroui H, Demoulin B, Sitaraman SV, Merlin D. 2010. PepT1 mediates transport of the proinflammatory bacterial tripeptide l-Ala-γ-d-Glu-meso-DAP in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 299:G687-696. doi: 10.1152/ajpgi.00527.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chiu YC, Lin CY, Chen CP, Huang KC, Tong KM, Tzeng CY, Lee TS, Hsu HC, Tang CH. 2009. Peptidoglycan enhances IL-6 production in human synovial fibroblasts via TLR2 receptor, focal adhesion kinase, Akt, and AP-1-dependent pathway. J Immunol 183:2785–2792. doi: 10.4049/jimmunol.0802826. [DOI] [PubMed] [Google Scholar]
- 95.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 96.Wang K, Mahmud H, Foller M, Biswas R, Lang KS, Bohn E, Götz F, Lang F. 2008. Lipopeptides in the triggering of erythrocyte cell membrane scrambling. Cell Physiol Biochem 22:381–386. doi: 10.1159/000187116. [DOI] [PubMed] [Google Scholar]
- 97.Gurtler C, Bowie AG. 2013. Innate immune detection of microbial nucleic acids. Trends Microbiol 21:413–420. doi: 10.1016/j.tim.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawasaki T, Kawai T, Akira S. 2011. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol Rev 243:61–73. doi: 10.1111/j.1600-065X.2011.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bergstrom B, Aune MH, Awuh JA, Kojen JF, Blix KJ, Ryan L, Flo TH, Mollnes TE, Espevik T, Stenvik J. 2015. TLR8 senses Staphylococcus aureus RNA in human primary monocytes and macrophages and induces IFN-beta production via a TAK1-IKKbeta-IRF5 signaling pathway. J Immunol doi: 10.4049/jimmunol.1403176. [DOI] [PubMed] [Google Scholar]
- 100.Ishii N, Funami K, Tatematsu M, Seya T, Matsumoto M. 2014. Endosomal localization of TLR8 confers distinctive proteolytic processing on human myeloid cells. J Immunol 193:5118–5128. doi: 10.4049/jimmunol.1401375. [DOI] [PubMed] [Google Scholar]
- 101.Triantafilou M, Gamper FG, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. 2006. Membrane sorting of Toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J Biol Chem 281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 102.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 103.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. 2008. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Husebye H, Aune MH, Stenvik J, Samstad E, Skjeldal F, Halaas O, Nilsen NJ, Stenmark H, Latz E, Lien E, Mollnes TE, Bakke O, Espevik T. 2010. The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity 33:583–596. doi: 10.1016/j.immuni.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deshmukh SD, Kremer B, Freudenberg M, Bauer S, Golenbock DT, Henneke P. 2011. Macrophages recognize streptococci through bacterial single-stranded RNA. EMBO Rep 12:71–76. doi: 10.1038/embor.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, Beninati C. 2009. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol 10:587–594. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- 107.Gratz N, Hartweger H, Matt U, Kratochvill F, Janos M, Sigel S, Drobits B, Li XD, Knapp S, Kovarik P. 2011. Type I interferon production induced by Streptococcus pyogenes-derived nucleic acids is required for host protection. PLoS Pathog 7:e1001345. doi: 10.1371/journal.ppat.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 109.Do TQ, Moshkani S, Castillo P, Anunta S, Pogosyan A, Cheung A, Marbois B, Faull KF, Ernst W, Chiang SM, Fujii G, Clarke CF, Foster K, Porter E. 2008. Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. J Immunol 181:4177–4187. doi: 10.4049/jimmunol.181.6.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lampe MA, Burlingame AL, Whitney J, Williams ML, Brown BE, Roitman E, Elias PM. 1983. Human stratum corneum lipids: characterization and regional variations. J Lipid Res 24:120–130. [PubMed] [Google Scholar]
- 111.Wille JJ, Kydonieus A. 2003. Palmitoleic acid isomer (C16:1delta6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacol Appl Skin Physiol 16:176–187. doi: 10.1159/000069757. [DOI] [PubMed] [Google Scholar]
- 112.Arsic B, Zhu Y, Heinrichs DE, McGavin MJ. 2012. Induction of the staphylococcal proteolytic cascade by antimicrobial fatty acids in community acquired methicillin resistant Staphylococcus aureus. PLoS One 7:e45952. doi: 10.1371/journal.pone.0045952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kenny JG, Ward D, Josefsson E, Jonsson IM, Hinds J, Rees HH, Lindsay JA, Tarkowski A, Horsburgh MJ. 2009. The Staphylococcus aureus response to unsaturated long chain free fatty acids: survival mechanisms and virulence implications. PLoS One 4:e4344. doi: 10.1371/journal.pone.0004344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.White DC, Frerman FE. 1968. Fatty acid composition of the complex lipids of Staphylococcus aureus during the formation of the membrane-bound electron transport system. J Bacteriol 95:2198–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parsons JB, Broussard TC, Bose JL, Rosch JW, Jackson P, Subramanian C, Rock CO. 2014. Identification of a two-component fatty acid kinase responsible for host fatty acid incorporation by Staphylococcus aureus. Proc Natl Acad Sci U S A 111:10532–10537. doi: 10.1073/pnas.1408797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nguyen MT, Hanzelmann D, Hartner T, Peschel A, Götz F. 2016. Skin-specific unsaturated fatty acids boost the Staphylococcus aureus innate immune response. Infect Immun 84:205–215. doi: 10.1128/IAI.00822-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sigal LH, Zahradnik JM, Lavin P, Patella SJ, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Molloy PJ, Seidner AL, Sabetta JR, Simon HJ, Klempner MS, Mays J, Marks D, Malawista SE. 1998. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant Outer-Surface Protein A Lyme Disease Vaccine Study Consortium. N Engl J Med 339:216–222. [DOI] [PubMed] [Google Scholar]
- 118.Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, Nowakowski J, Schmid CH, Laukamp S, Buscarino C, Krause DS. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med 339:209–215. [DOI] [PubMed] [Google Scholar]
- 119.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kehl-Fie TE, Zhang Y, Moore JL, Farrand AJ, Hood MI, Rathi S, Chazin WJ, Caprioli RM, Skaar EP. 2013. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect Immun 81:3395–3405. doi: 10.1128/IAI.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cockayne A, Hill PJ, Powell NB, Bishop K, Sims C, Williams P. 1998. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect Immun 66:3767–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anderson AS, Scully IL, Timofeyeva Y, Murphy E, McNeil LK, Mininni T, Nunez L, Carriere M, Singer C, Dilts DA, Jansen KU. 2012. Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. J Infect Dis 205:1688–1696. doi: 10.1093/infdis/jis272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gribenko A, Mosyak L, Ghosh S, Parris K, Svenson K, Moran J, Chu L, Li S, Liu T, Woods VL Jr, Jansen KU, Green BA, Anderson AS, Matsuka YV. 2013. Three-dimensional structure and biophysical characterization of Staphylococcus aureus cell surface antigen-manganese transporter MntC. J Mol Biol 425:3429–3445. doi: 10.1016/j.jmb.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 124.Mariotti P, Malito E, Biancucci M, Lo Surdo P, Mishra RP, Nardi-Dei V, Savino S, Nissum M, Spraggon G, Grandi G, Bagnoli F, Bottomley MJ. 2013. Structural and functional characterization of the Staphylococcus aureus virulence factor and vaccine candidate FhuD2. Biochem J 449:683–693. doi: 10.1042/BJ20121426. [DOI] [PubMed] [Google Scholar]
- 125.Podkowa KJ, Briere LA, Heinrichs DE, Shilton BH. 2014. Crystal and solution structure analysis of FhuD2 from Staphylococcus aureus in multiple unliganded conformations and bound to ferrioxamine-B. Biochemistry 53:2017–2031. doi: 10.1021/bi401349d. [DOI] [PubMed] [Google Scholar]
- 126.Mishra RP, Mariotti P, Fiaschi L, Nosari S, Maccari S, Liberatori S, Fontana MR, Pezzicoli A, De Falco MG, Falugi F, Altindis E, Serruto D, Grandi G, Bagnoli F. 2012. Staphylococcus aureus FhuD2 is involved in the early phase of staphylococcal dissemination and generates protective immunity in mice. J Infect Dis 206:1041–1049. doi: 10.1093/infdis/jis463. [DOI] [PubMed] [Google Scholar]
- 127.Bagnoli F, Fontana MR, Soldaini E, Mishra RP, Fiaschi L, Cartocci E, Nardi-Dei V, Ruggiero P, Nosari S, De Falco MG, Lofano G, Marchi S, Galletti B, Mariotti P, Bacconi M, Torre A, Maccari S, Scarselli M, Rinaudo CD, Inoshima N, Savino S, Mori E, Rossi-Paccani S, Baudner B, Pallaoro M, Swennen E, Petracca R, Brettoni C, Liberatori S, Norais N, Monaci E, Bubeck Wardenburg J, Schneewind O, O'Hagan DT, Valiante NM, Bensi G, Bertholet S, De Gregorio E, Rappuoli R, Grandi G. 2015. Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci U S A 112:3680–3685. doi: 10.1073/pnas.1424924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schluepen C, Malito E, Marongiu A, Schirle M, McWhinnie E, Lo Surdo P, Biancucci M, Falugi F, Nardi-Dei V, Marchi S, Fontana MR, Lombardi B, De Falco MG, Rinaudo CD, Spraggon G, Nissum M, Bagnoli F, Grandi G, Bottomley MJ, Liberatori S. 2013. Mining the bacterial unknown proteome: identification and characterization of a novel family of highly conserved protective antigens in Staphylococcus aureus. Biochem J 455:273–284. doi: 10.1042/BJ20130540. [DOI] [PubMed] [Google Scholar]
- 129.Das S, Kanamoto T, Ge X, Xu P, Unoki T, Munro CL, Kitten T. 2009. Contribution of lipoproteins and lipoprotein processing to endocarditis virulence in Streptococcus sanguinis. J Bacteriol 191:4166–4179. doi: 10.1128/JB.01739-08. [DOI] [PMC free article] [PubMed] [Google Scholar]