SUMMARY
Ferrets are widely employed to study the pathogenicity, transmissibility, and tropism of influenza viruses. However, inherent variations in inoculation methods, sampling schemes, and experimental designs are often overlooked when contextualizing or aggregating data between laboratories, leading to potential confusion or misinterpretation of results. Here, we provide a comprehensive overview of parameters to consider when planning an experiment using ferrets, collecting data from the experiment, and placing results in context with previously performed studies. This review offers information that is of particular importance for researchers in the field who rely on ferret data but do not perform the experiments themselves. Furthermore, this review highlights the breadth of experimental designs and techniques currently available to study influenza viruses in this model, underscoring the wide heterogeneity of protocols currently used for ferret studies while demonstrating the wealth of information which can benefit risk assessments of emerging influenza viruses.
INTRODUCTION
The use of small mammalian models permits the study of complex pathogen-host interactions and multifactorial traits that may not be possible outside a living host. Well-established mammalian models further provide a framework for the evaluation of interventions and therapeutics to mitigate disease. For influenza virus, a principal respiratory pathogen capable of causing a spectrum of human illness from mild to life-threatening, these models have contributed invaluable information toward the prevention and control of human infection. Evaluating the pathogenesis, transmission, and tropism of emerging influenza viruses represents a key component of public health risk assessment. However, it is critical that experiments performed with mammalian models be designed precisely, conducted fastidiously, and interpreted responsibly, with any limitations affecting the conclusions of the study disclosed. Only then can the findings contribute to the understanding of human health and disease.
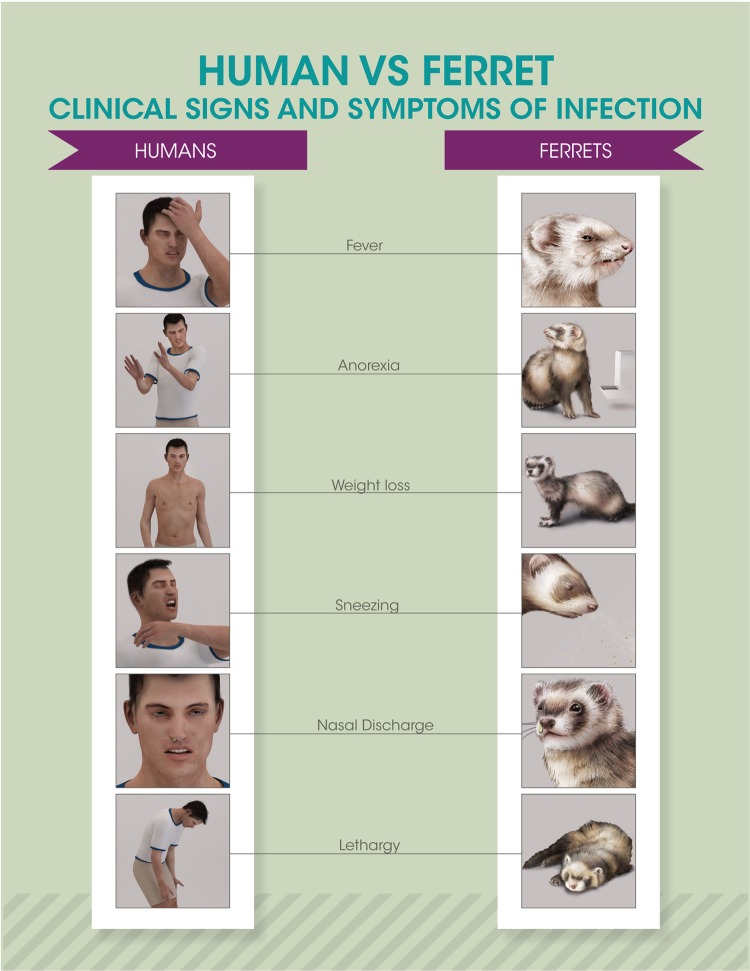
Several small mammalian models are routinely employed for the study of influenza viruses, each with its own advantages and limitations depending on the research question addressed. Among these species, which include the mouse, ferret, guinea pig, cotton rat, and swine, the ferret is considered best suited for the coincident study of mammalian pathogenesis and transmission (1). Similar to the case for humans, inoculation of ferrets with influenza virus leads to predominant infection in the upper respiratory tract, with numerous clinical signs and symptoms shared between the two species, as shown in Fig. 1 (2). The utility of ferrets to model human infection with influenza virus is further evidenced by commonalities between the species with regard to comparable shedding of virus-containing respirable aerosols following infection, induction of innate and adaptive immune responses, and the capacity for highly virulent viruses to cause severe lower respiratory tract disease and spread to extrapulmonary tissues, among other considerations (3–5). Close physiologic links between ferret and human respiratory tissues and comparable binding patterns of human and avian influenza viruses to sialic acid receptors distributed throughout the respiratory tract further strengthen the applicability of ferrets to study human disease (6, 7). However, the paucity of commercially available ferret-specific reagents, especially those which characterize the host response, has limited the ability of researchers to study in detail innate and adaptive responses to influenza virus infection and vaccination in this species. Sequencing of the ferret genome represents a critical addition to the utility of the ferret model for respiratory disease study (8); advances in this area of research in the ferret will continue to broaden the applicability of this species in influenza virus research.
FIG 1.
Clinical signs and symptoms of influenza virus infection. Selected commonalities in influenza virus infection between humans and ferrets are depicted.
When evaluating research performed in the ferret, there are a myriad of factors and decisions which can ultimately affect the experimental results presented. As described in more detail throughout this review, results can be influenced by numerous parameters, including, but not limited to, stock generation and passage history, the age, gender, and immunological background of the ferrets used, the housing and environmental conditions under which the experiment is performed, the route and dose of virus inoculation, the timing and choice of sample collection, and the design of the transmission setup. As there is an ongoing need to improve the basis for assessment of the risks posed by influenza viruses (9), understanding the role that these choices in experimental design can play in research studies and how these mammalian models can be further improved for use in risk assessment is critical.
STANDARDIZATION OF MATERIALS
A great deal of heterogeneity is introduced into the experimental design of mammalian laboratory experiments before the first animal is inoculated. With no universal standard established for ferret studies evaluating the relative risk of influenza viruses, virus-specific, host-specific, and equipment-specific variation is inherent in published studies. This represents a double-edged sword: while these laboratory-specific conditions limit the ease of directly comparing results obtained by different research groups, the body of work performed by multiple laboratories contributing to initial assessments of pathogenesis and transmission of influenza viruses permits a robust collection of data which surpasses potential biases of experimental conditions and strain-specific influences. For example, none of the six published reports in 2013 characterizing the airborne transmissibility of novel H7N9 viruses isolated from China possessed identical experimental designs, with differences in ferret ages, genders, inoculation doses and volumes, total number of viruses tested or ferrets used to conduct each experiment, or criteria to determine transmissibility present between studies (Table 1). It is, therefore, not surprising that relative rates of virus transmission ranged from 33 to 75% in individual studies. However, these data collectively illustrate the limited transmissibility of this virus by respiratory droplets and provide a more comprehensive consensus of this property than can be provided by any one isolated study.
TABLE 1.
Respiratory droplet transmission studies with H7N9 influenza viruses, 2013
| Study authors | Ferret gender | Ferret age | Inoculum vol (μl)a | Inoculum doseb | Total no. of: |
% overall RD transmissione | Reference | |
|---|---|---|---|---|---|---|---|---|
| Virusesc | Ferretsd | |||||||
| Zhu et al. | Male | 1.5 yr | 500 | 106 TCID50 | 1 | 3 | 66 | 88 |
| Zhang et al. | Female | 4 mo | 500 | 106 EID50 | 3 | 12 | 66 | 75 |
| Belser et al. | Male | 7–8 mo | 1,000 | 106 PFU | 2 | 9 | 33 | 89 |
| Watanabe et al. | Female | 5–8 mo | 500 | 106 PFU | 1 | 3 | 33 | 90 |
| Richard et al. | Female | 1–2 yr | 500 | 106 TCID50 | 1 | 4 | 75 | 91 |
| Xu et al. | Male | 6–12 mo | NR | 106 TCID50 | 1 | 3 | 33 | 92 |
Volume of virus delivered intranasally to anesthetized ferrets. NR, not reported.
Dose of virus used to inoculate ferrets. TCID50, 50% tissue culture infectious dose; EID50, 50% egg infectious dose.
Total number of unique wild-type H7N9 viruses isolated from human cases included in the study.
Total number of pairs of inoculated and contact ferrets used in the study to determine virus transmissibility.
Percentage of transmission events detected among all pairs of inoculated and contact ferrets tested. All transmission events were defined as virus isolation from contact ferrets, with the exception of one ferret in the study by Zhu et al. (88) that seroconverted in the absence of virus detection. RD, respiratory droplet.
Beyond the parameters compared in Table 1, which are discussed in more detail below, there are numerous additional properties that can vary between studies reporting on a given virus strain. Influenza viruses are propagated, titrated, and standardized to equivalent inoculation doses using either eggs (typically reported as a 50% egg infectious dose [EID50]) or cells (typically reported as PFU or 50% tissue culture infectious dose [TCID50]). Variation in the age, source, inoculation dose, and incubation time of eggs used to grow viruses can alter the resulting infectivity of the propagated stock. Similarly, the cell type, quantity and type of exogenous trypsin added to culture, inoculation dose, and incubation time can influence the infectivity of cell-grown stocks. Furthermore, the quality of the resulting virus stock can be altered by the temperature maintained during stock preparation and storage, the number of freeze-thaws that may have taken place since initial stock generation, the presence of quasispecies in the stock, and the passage history of the virus prior to inoculation (10). While the importance of these parameters is evident, they can nonetheless be overlooked when efforts to directly compare in vivo results from one laboratory to the next are made.
Characterization of influenza viruses in the ferret typically occurs in the same subspecies (Mustela putorius furo), but as is illustrated in Table 1, the age and gender of ferrets differ between studies. While young adult ferrets (generally 4 to 12 months old) are most frequently used, the age of animals employed can vary depending on the questions addressed in the study, ranging from ferrets that are newly weaned (≤8 weeks old) to aged ferrets (>4 years of age) (11, 12). The choice of anesthetic used for inoculation and sampling postinoculation (typically ketamine cocktails of various compositions and dosages administered intramuscularly, inhaled isoflurane, or both) can vary between laboratories, leading to differences in the depth of sedation and the respiration rates of sedated animals (13–16).
The repertoire of human antibodies to influenza virus is influenced by numerous factors, including prior viral infection, vaccination, and individual host factors, complicating the study of primary immune responses elicited following exposure to a given strain (17). However, due to the high susceptibility of ferrets to influenza virus infection, difficulties in controlling for immunological backgrounds of seropositive animals, and need to study primary virus infection, ferrets in mammalian pathotyping and transmission studies should be prescreened to ensure seronegativity to circulating influenza A and B viruses (following either direct exposure or passively acquisition of maternal antibody) (18); the increased commercial availability of seronegative ferrets in recent years has made this a nearly ubiquitous requirement for influenza virus research in this species. Thus, it is important to pay close attention to the age, gender, vendor, and serostatus of ferrets when comparing results between laboratories. Given the seasonality of many influenza viruses, laboratories must additionally monitor and regulate ambient temperature and relative humidity levels year-round to ensure experimental reproducibility during all seasons regardless of external environmental conditions.
INOCULATION ROUTE
As influenza virus is a respiratory pathogen, inhalation of virus-containing aerosols is considered a predominant route of virus spread among people. However, it represents just one of several possible modes of influenza virus transmission, which encompass direct contact, indirect contact with virus-containing surfaces, droplet transmission (droplets of ≥5 μm in size, which typically travel less than 1 m from the infected host), and airborne transmission (droplets and droplet nuclei of <5 μm in diameter, which can remain suspended in the air for an extended period of time) (19). Thus, beyond virus inhalation, potential means of human exposure to influenza virus include, but are not limited to, contact with fomites, the splashing of contaminated fluid onto the face, and the lack of wearing appropriate respiratory and/or eye protection in a potentially contaminated environment, all of which represent past documented routes of avian-to-human transmission of virus (20). Ingestion of virus-containing matter represents an additional potential means of exposure (21, 22).
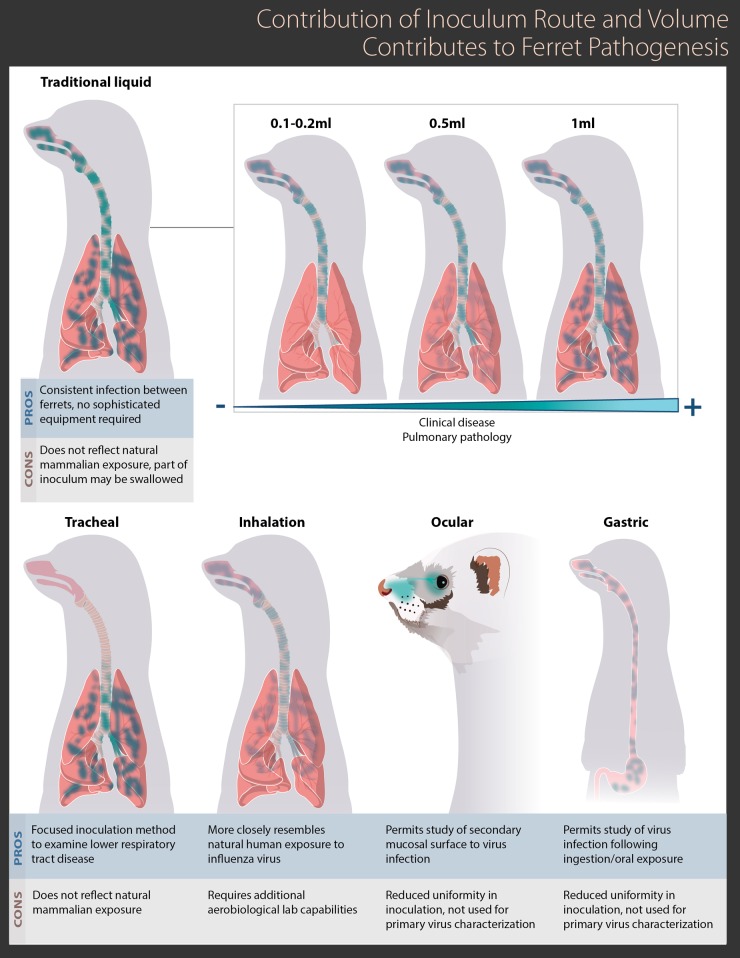
It is understandable that the way a person is exposed to influenza virus could influence the resulting disease that occurs once infection is initiated. For example, recent studies describing inoculation of ferrets by the aerosol exposure route (either whole-body or nose-only exposure) have been driven by efforts to more closely mimic a typical human airborne exposure to virus in this species (23, 24), and studies examining the potential of virus infection of ferrets following consumption of infected meat have met a public health need (25, 26). Modulation of the inoculation route can affect where the virus is deposited following exposure (24, 27), where initial virus replication takes place (22, 26), and the timing and extent of virus dissemination to other tissues (26, 28, 29). As shown in Fig. 2, there are a wide array of established inoculation methods in the ferret, which include (but are not limited to) the intranasal, tracheal, ocular, and gastric routes, all of which can modulate where virus is deposited, replicates, and spreads throughout the course of infection. Further variability may be present within each of these routes; for example, ocular inoculation of ferrets may be conducted with either a liquid suspension or via aerosol exposure, and gastric inoculation may be conducted via the use of an endoscope or by ingestion (26–28). Thus, care should be taken to choose the inoculation route which best emulates and reflects the parameter examined in the study.
FIG 2.
Contribution of inoculum route and volume to influenza virus virulence in the ferret. Examples of variance in location and viral load between multiple different inoculum routes employed in the ferret model are shown. Green shading indicates locations with high viral loads. Selected advantages and disadvantages for each inoculum route are highlighted.
Despite this body of knowledge regarding the important role that the inoculation route can play in the resulting infection, the established, traditional means of inoculating ferrets for the study of influenza virus pathogenesis remains the intranasal route, that is, the instillation of diluted virus in a liquid matrix into the nares of the ferret. The ubiquity of the use of this route stems from its ease and reliability in establishing a uniform, reproducible infection in the animal. However, inoculation of ferrets by the intranasal route can be performed with a range of volumes, typically between 500 μl and 1 ml, though much lower volumes have been reported as well (27, 30, 31). The inoculum volume can greatly affect the coverage in the mammalian respiratory tract initially exposed during the inoculation, and the tendency of ferrets to swallow during inoculation can affect the ultimate dose that stays within the respiratory tract (Fig. 2). Tracheal inoculation, where the upper respiratory tract is bypassed via the use of an otoscope or other method, can also greatly affect the location and coverage of respiratory tract tissues initially exposed to the virus inoculum (29).
While modulating the inoculation route can provide a wealth of information about the behavior of influenza viruses in a mammalian host, there are clear conceptual differences between directly inoculating a ferret with a fixed quantity of virus during a defined period of time and the “natural” contraction of disease which occurs in humans. Underscoring this, recent studies have shown that at low doses, aerosol inoculation can more closely emulate a naturally acquired airborne infection than inoculation using a liquid suspension (24). A ferret model of household contact was developed by using a directly inoculated ferret to transmit virus to susceptible contacts and then using these contact ferrets to test the efficacy of prophylactic regimens (32); while this approach yields ferrets that are more “naturally” infected, potentially augmenting the applicability of the results to human situations, it is difficult to control precisely the timing and viral input leading to infection in these contacts, increasing variability in the experimental protocol. As discussed in more detail below, ferrets infected “naturally” can still possess differences in virus diversity based on the transmission mode employed (33).
INOCULATION DOSE
Standard inoculation of ferrets by the intranasal route typically employs a dose of 105 to 107 PFU, EID50, or TCID50. This input ensures the greatest likelihood of virus infectivity and replication in the ferret, leading to a robust infection that reveals the maximum capacity of the virus to cause severe disease; doses above 107 may result in less severe disease due to the presence of putative defective interfering particles in the inoculum. However, this dose is unrealistically high compared with typical infectious doses in humans, which have been shown to be as low as 3 TCID50 following exposure to small-particle influenza virus aerosols (34, 35). Similarly, studies quantitating the 50% infectious dose of influenza viruses in the ferret (FID50) have revealed that both avian and human influenza viruses are capable of high infectivity in this species, with doses under 10 PFU leading to productive ferret infection following inhalation of virus-containing aerosols (24, 36). Low-dose infection has also been documented following inoculation of ferrets by the ocular route (28).
Beyond affecting pathogenicity in the ferret, the inoculation dose also appears to be a contributing factor in determining the transmission efficiency of a virus in this species. Transmission of human seasonal influenza viruses between cohoused ferrets has been documented following inoculation of donor animals at doses of ≤10 PFU (24, 37). However, while only limited studies have been conducted on this topic to date, it appears that virus transmissibility in ferrets is dependent on both the level and duration of peak virus shedding in the upper respiratory tracts of inoculated animals, thresholds which may not be achieved at very low inoculation doses in this model (24). In support of this, placement of ferrets in direct contact with animals inoculated with a high (106 EID50) but not a low (103 EID50) dose of an H5N1 virus resulted in seroconversion of the contact ferrets, possibly due to reduced viral shedding by ferrets inoculated with the lower dose (38).
Inoculation of ferrets for standard pathotyping and/or transmission studies is performed with one virus strain. However, coinfection studies to assess viral fitness and generation of natural reassortants during infection have been performed, typically to study the risk of concurrent seasonal and zoonotic influenza virus infection (39, 40) or the comparative fitness of wild-type versus mutation-bearing viruses (41). These studies can be performed by inoculating ferrets with either an equal or variable ratio of each virus in the inoculum, depending on the questions addressed. Furthermore, while the use of healthy young adult ferrets is standard in the field, manipulation of the health or immune status of ferrets prior to and/or during infection (including inoculation of pregnant ferrets, coinfection with Streptococcus pneumoniae, or administration of chemotherapy or other immunosuppressive agents) can provide useful information about the course of disease or efficacy of vaccination or antiviral administration in comparable human populations (42–46), which may concurrently require modulation of typical inoculation parameters.
ASSESSMENT OF DISEASE SEVERITY
As highlighted in Fig. 1, the presentation of multiple clinical signs and symptoms of infection is shared between ferrets and humans. Typical assessments of ferret health postinoculation generally include measures of weight loss, anorexia (aversion to food), fever, activity level, sneezing, dyspnea, nasal or ocular discharge, diarrhea, and neurological complications. However, the detection, measurement, and analysis of these signs and symptoms can vary greatly between laboratories, and the quantitative assessment of these parameters can be difficult due to potential subjectivity in assessments, a paucity of sustained periods of observation, and the influence of confounders (such as scheduling of anesthesia administration). Fever (generally defined as a rise over baseline temperature) typically peaks by day 2 postinoculation when ferrets are inoculated by traditional means with a high dose of virus, but this can vary based on inoculation conditions and the virus used to infect (47). Body temperature is typically monitored by use of a rectal thermometer or the use of subcutaneous implants (with observations collected 1 or 2 times a day). Alternatively, telemetry systems permitting automated collection of continuous core temperature data or collection at preset time points are also available; depending on the implant, additional parameters (e.g., activity or respiration) may be measured simultaneously. Typically, surgical implantation of the transponder device is required (48, 49). The incidence of sneezing in ferrets is generally noted during routine periods of observation. Consistent documentation of this can be challenging if animals are cohoused or if they are housed inside containment devices that limit a direct line of sight.
Measuring ferret activity levels during the acute phase of infection can provide a valuable piece of information when assessing disease severity. Daily scoring of activity level is a traditional method to evaluate this property (50); computation of a relative inactivity index from these observations provides a quantitative way to compare activity levels between viruses of high or low virulence in this model (51). These types of scoring systems can be expanded beyond activity level to encompass several clinical signs and symptoms of infection (52). The effect of infection on endurance has also been assessed by training ferrets to use a modified treadmill, so that ferrets experiencing more severe disease exhibit reduced activity on the treadmill compared with that of ferrets experiencing a milder illness (48). Video tracking analyses to quantify changes in activity following infection have also been recently described (53).
As is standard with all laboratory animal manipulations, several endpoint criteria are employed when conducting studies with influenza viruses in the ferret. Weight loss endpoints are typically enforced when a ferret falls below 20 to 30% of its preinfection weight. The development of neurological symptoms (typically observed as hind limb weakness/paralysis, torticollis, or behavioral shifts) is considered a humane endpoint, as is the onset of pronounced lethargy. If an endpoint is reached during the acute phase of infection, a postmortem necropsy may be performed to examine the possible spread of virus to extrapulmonary tissues, including the brain, as discussed in more detail below.
SAMPLE COLLECTION AND TIMING
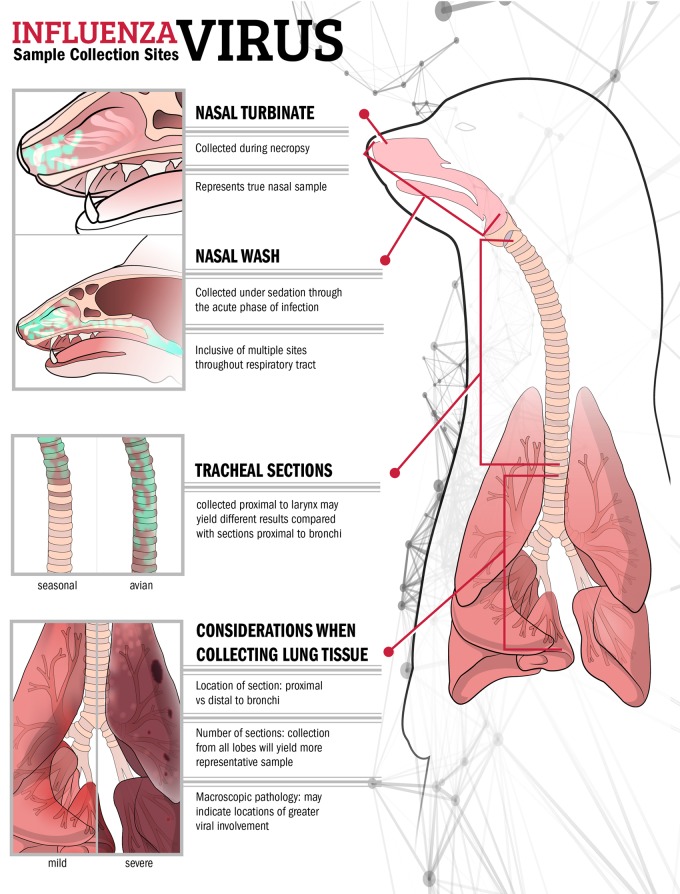
There are several samples which can be collected throughout the acute phase of infection in ferrets (Fig. 3). Nasal wash, where sterile liquid is introduced into the nasal passages of the ferret (which may or may not induce sneezing, depending on the technique employed) and aspirate is collected for subsequent titration, is a frequently collected sample (49, 51). High viral titers in nasal wash are indicative of efficient replication in the respiratory tract, but due to the expulsion of the liquid matrix, especially if sneezing is induced, this sample is representative of multiple anatomical sites in the respiratory tract beyond the nasal passages. Swabbing of the nose and/or throat represents an alternative to nasal washing, permitting repeated sampling under anesthesia of these more discrete locations (54). High viral loads in bronchoalveolar lavage fluid have been reported in ferrets following influenza virus infection, but this sample is infrequently collected during routine pathotyping experiments (16). Rectal swabs may be collected to assess the presence of virus in the gastrointestinal tract (55). Conjunctival swabs and washes may also be collected to identify the presence of virus in the ocular milieu (27). These samples are typically titrated for the presence of infectious virus, but molecular analyses, such as sequencing, may also be performed. The presence of immune mediators may also be detected by reverse transcription-PCR (RT-PCR) or enzyme-linked immunosorbent assay (ELISA) (4). Peripheral blood and serum collected in small volumes may be sampled to observe viremia or perform other lymphohematopoietic assessments, including determination of lymphopenia, leukopenia, and/or serum abnormalities (56). Collection of these samples is generally noninvasive to the ferret, and the samples are typically collected on alternate days postinoculation until virus has cleared the animal. Daily sampling is possible but generally is not conducted due to additional concerns associated with the need for frequent anesthesia of animals.
FIG 3.
Frequently collected samples from the ferret respiratory tract during the acute phase of infection. Examples of variance in location precision and viral load between different sites and types of samples collected during influenza virus infection in the ferret, and different viral loads and pathology present between mild/seasonal and virulent/avian influenza virus infection, are shown. Green shading indicates locations of high viral loads in nasal and tracheal samples. Sites sampled during nasal wash collection may extend beyond the upper respiratory tract, as shown in the inset.
Scheduled necropsy of ferrets provides an opportunity to better identify the scope of virus dissemination throughout the animal. Collection of samples from discrete sites throughout the respiratory tract (including the nasal turbinates, soft palate, trachea, and lung) provides more precision in identifying sites of virus replication in the ferret than, for example, collection of a nasal wash sample, which is likely inclusive of multiple sites in the respiratory tract (Fig. 3) (57). Unlike the case for smaller models, such as the mouse, where collection of whole tissues is possible, the larger size of ferrets necessitates sampling of most tissues. As such, uniformity in collection of these samples between ferrets is critical, as is detail in describing the methodology for multiple sampled sites. For example, viral loads may differ in tracheal samples collected from the upper or lower region, distal regions of the lung may possess reduced viral loads compared with those in regions excised proximal to the bronchi, and viral loads likely are not uniform between the five lobes of the ferret lung (Fig. 3).
The collection of extrapulmonary tissues may provide evidence of the capacity for systemic spread of virus, an important property to monitor given the detection of disseminated infection in selected cases of highly pathogenic avian influenza (HPAI) in humans (58). Necropsies are typically scheduled during peak times of viral replication (i.e., day 3 postinoculation), though earlier and later times are also employed, depending on the questions addressed in the study (47). Necropsy of ferrets which succumb to infection or must be euthanized due to reaching humane endpoints midexperiment may provide additional information regarding the severity of disease (56). While viral titers in the anterior or posterior brain are generally observed only with highly virulent viruses, detectable virus in the olfactory bulbs is not uncommon with viruses that have reduced virulence in ferrets, including seasonal influenza viruses, given the proximity of this tissue to the nasal cavity (51).
TRANSMISSION MODELS
In addition to bearing a hemagglutinin to which there is little or no preexisting immunity and causing disease in humans following infection, the capacity for sustained human-to-human transmission is a necessary feature for the generation of a pandemic virus and as such is included as a parameter in the influenza risk assessment tool (IRAT) (59, 60). Many viruses considered to possess pandemic potential meet the first two criteria, illustrating the importance of surveillance and monitoring efforts to detect whether novel viruses associated with disease in humans or to which humans may be exposed have acquired a transmissible phenotype. Unlike studies which examine molecular determinants of virulence, for which many in vitro assays are available, transmissibility represents a polygenic trait which must be studied in vivo. A quantitative link between estimates of transmission efficiency generated using the ferret model and secondary attack rates in humans has further highlighted the role these analyses can serve in assessing the relative risk of influenza viruses with pandemic potential (61), though the ferret model is not 100% predictive of this property and there remains a need for the concurrent use of other laboratory and genotypic assays to study virus transmissibility. However, describing existing transmission models to understand the precise mode of transmission being modeled in a given study, understanding the limitations of these models, and appreciating the wide heterogeneity of experimental setups currently employed in laboratories worldwide are necessary before extrapolating experimentally generated data to humans or placing results in context with the published literature.
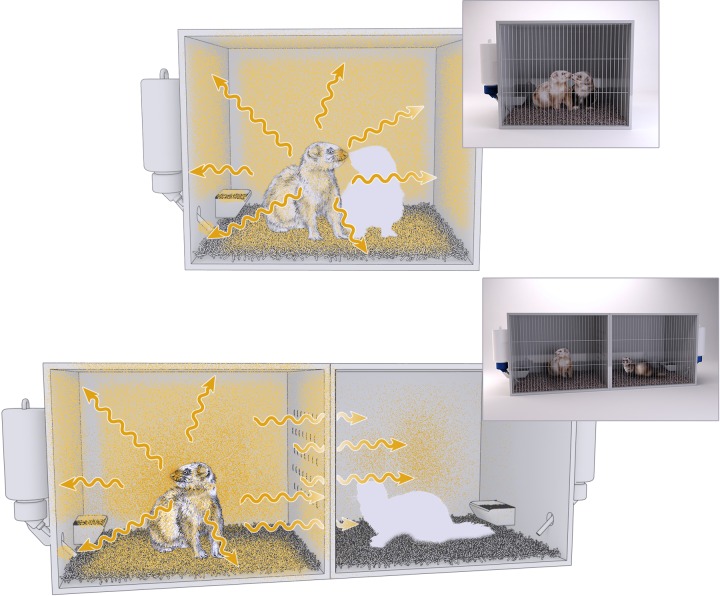
There are currently two established, widely employed models for examining virus transmissibility between ferrets. The first is a model where an inoculated and naive ferret are cohoused in the same shared space to evaluate transmission which occurs in the presence of direct contact (DC model) (38, 55, 62) (Fig. 4). In this model, the inoculated and contact ferrets are in direct contact with each other, share common food, water, and bedding, and breathe the same air. Therefore, if the naive ferret does become infected, any part of this three-dimensional space could potentially represent the causative source of the transmission. This model represents the most permissive transmission model employed in the field, as it encapsulates multiple modes of transmission, but it cannot identify any dominant mode of transmission responsible.
FIG 4.
Differences in exposure to influenza virus between established ferret transmission models. Naive ferrets (white silhouette) are cohoused with inoculated ferrets (top, direct contact model) or placed adjacent to inoculated ferrets (bottom, respiratory droplet model). Areas of potential exposure to influenza virus are depicted in yellow. Arrows indicate dispersion of respiratory droplets expelled from the inoculated ferret.
The second well-characterized ferret transmission model separates inoculated and contact ferrets by a partition which permits airflow exchange between ferrets but eliminates all other sources of direct or indirect contact (38, 63) (Fig. 4). In other words, the naive ferret shares no common housing spaces with the inoculated ferret, ensuring that if the contact ferret becomes infected, the source must have been respiratory droplets originating from the inoculated ferret (RD model). This model represents a far more stringent evaluation of virus transmissibility, as many viruses that demonstrate the capacity for transmission when ferrets are placed in direct contact are not transmitted by the respiratory droplet or aerosol route (64, 65), potentially due to differences in bottleneck stringency between different routes of transmission (33).
For both models, transmission is typically assessed by the detection of both infectious virus in contact ferrets during the experimental period and evidence of seroconversion to homologous virus during the convalescent phase of infection (38). Seroconversion in the absence of virus detection is not unusual, and many laboratories document this as a transmission event (38, 66–68). Conversely, low-level detection of virus in contact ferrets in the absence of seroconversion can often be attributed to direct contact with virus-containing fomites that did not lead to productive infection in the animal and is not generally considered a true transmission event (64). Transmission studies utilizing bioluminescent influenza viruses represent an additional variable, as a transmission event can be identified by the presence of virus by in vivo imaging in the absence of infectious virus detection in nasal wash samples (69). Statistical modeling has shown that the predictive power of ferret transmission experiments can change based on the criteria used to assess transmission events, underscoring the need to be precise when describing this property (61).
TRANSMISSION EXPERIMENTAL DESIGNS
In both models described here, there is typically a 24-hour interval between inoculating the donor ferret and establishing contact with the naive ferret, primarily to eliminate inadvertent transmission of inoculum material to the contact. Furthermore, once contact is established, the animals typically remain in continuous exposure for the duration of the experiment. However, several studies have modulated this parameter, creating contact pairs for short periods of time during the acute phase of infection to better understand the temporal dynamics of virus transmission in the ferret model (49, 70) or establishing contact at different times throughout the acute phase of infection to determine links between virus shedding in inoculated ferrets and virus transmissibility (71). Donor chains, where virus is serially transmitted or passaged from ferret to ferret, have also been employed to model the selective pressure that may occur by sustained transmission of viruses through a population (72–74).
There is a great deal of variability in the cage engineering utilized for studies that examine virus transmissibility by respiratory droplets. Cages can have solid walls on all sides except the shared plane between inoculated and contact ferrets (38) or can be made of wire mesh on some or all sides (73, 75, 76), potentially permitting virus-containing aerosols originating from inoculated ferrets to reach contact ferrets by multiple dimensions. Unsurprisingly, the perforation size between these cages can vary between laboratories (most often it is 0.05 to 0.5 cm2 [38, 73, 77], though larger distances have been reported [76]). The distance between these paired cages is typically proximal (separated by only 3 mm to 10 cm) (38, 77), depending on the construction of the cages and the experimental design, though transmission from inoculated to susceptible ferrets has been reported at distances up to 5 feet (63). Furthermore, airflow in transmission caging can vary between laboratories, with examples of directional flow from the inoculated ferret to the contact ferret (70, 75, 77) or top-to-bottom (31, 38), bottom-to-top (73), front-to-back (76), or unspecified (14) directionality. Modulation of temperature and relative humidity, the study of which was previously limited to the guinea pig transmission model (78, 79), has also recently been shown to affect virus transmissibility in ferrets (80). However, ambient temperature and humidity readings are not always described or specified in reports of studies utilizing the RD model, further complicating direct comparison of results.
Transmission schemes can also be altered to study the ability of countermeasures to reduce virus transmission. The capacity of antiviral prophylaxis to block or mitigate virus transmissibility has been studied by treating inoculated (68) or contact (32) ferrets with oseltamivir (Tamiflu). Vaccination or prior infection of donor ferrets can be useful when determining the efficacy of virus transmission under immune pressure (studied with both homologous and heterologous challenge viruses) (81–85) or when determining the efficacy of novel vaccine or antiviral candidates to limit transmission (68, 86). These and other modifications to transmission schemes illustrate the high plasticity of this model and utility of these data for numerous applications beyond initial virus characterization but, given the wide range of alterations possible, similarly underscore the need for precision in how these data are presented and contextualized.
CONCLUSIONS
Ferrets have become a ubiquitous, essential tool for evaluating the pathogenicity, transmissibility, and tropism of influenza viruses. However, with this ubiquity comes pronounced heterogeneity in experimental methods utilized in the field to assess these parameters. As highlighted in Table 1, this heterogeneity can serve as a benefit, as multiple laboratories contributing to initial assessments of pathogenesis and transmission can eliminate biases inherent in experimental conditions and strain-specific influences. The use of additional techniques and methods in the field not covered in this review further emphasizes this point. As influenza viruses from avian, swine, and other hosts continue to cause human infection, the continued assessment in animal models of selected viruses believed to pose a pandemic risk is essential. Increased use of nontraditional inoculation routes and doses should be embraced when possible to better emulate the range of real-world exposures likely to result in human infection. Greater precision in descriptions of experimental methods when publishing and sharing research will improve the ability to conduct larger-scale analyses of trends present among particular virus subtypes or other features with statistical rigor not generally possible in individual studies (61, 87).
ACKNOWLEDGMENT
The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Belser JA, Katz JM, Tumpey TM. 2011. The ferret as a model organism to study influenza A virus infection. Dis Model Mech 4:575–579. doi: 10.1242/dmm.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith H, Sweet C. 1988. Lessons for human influenza from pathogenicity studies with ferrets. Rev Infect Dis 10:56–75. doi: 10.1093/clinids/10.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Gustin KM, Katz JM, Tumpey TM, Maines TR. 2013. Comparison of the levels of infectious virus in respirable aerosols exhaled by ferrets infected with influenza viruses exhibiting diverse transmissibility phenotypes. J Virol 87:7864–7873. doi: 10.1128/JVI.00719-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maines TR, Szretter KJ, Perrone L, Belser JA, Bright RA, Zeng H, Tumpey TM, Katz JM. 2008. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol Rev 225:68–84. doi: 10.1111/j.1600-065X.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 5.Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HH, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol 79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maher JA, DeStefano J. 2004. The ferret: an animal model to study influenza virus. Lab Anim (NY) 33:50–53. doi: 10.1038/laban1004-50. [DOI] [PubMed] [Google Scholar]
- 7.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. 2007. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol 171:1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng X, Alfoldi J, Gori K, Eisfeld AJ, Tyler SR, Tisoncik-Go J, Brawand D, Law GL, Skunca N, Hatta M, Gasper DJ, Kelly SM, Chang J, Thomas MJ, Johnson J, Berlin AM, Lara M, Russell P, Swofford R, Turner-Maier J, Young S, Hourlier T, Aken B, Searle S, Sun X, Yi Y, Suresh M, Tumpey TM, Siepel A, Wisely SM, Dessimoz C, Kawaoka Y, Birren BW, Lindblad-Toh K, Di Palma F, Engelhardt JF, Palermo RE, Katze MG. 2014. The draft genome sequence of the ferret (Mustela putorius furo) facilitates study of human respiratory disease. Nat Biotechnol 32:1250–1255. doi: 10.1038/nbt.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell CA, Kasson PM, Donis RO, Riley S, Dunbar J, Rambaut A, Asher J, Burke S, Davis CT, Garten RJ, Gnanakaran S, Hay SI, Herfst S, Lewis NS, Lloyd-Smith JO, Macken CA, Maurer-Stroh S, Neuhaus E, Parrish CR, Pepin KM, Shepard SS, Smith DL, Suarez DL, Trock SC, Widdowson MA, George DB, Lipsitch M, Bloom JD. 2014. Improving pandemic influenza risk assessment. eLife 3:e03883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McWhite C, Meyer A, Wilke CO. 2016. Serial passaging causes extensive positive selection in seasonal influenza A hemagglutinin. bioRxiv doi: 10.1101/038364. [DOI] [Google Scholar]
- 11.Huang SS, Banner D, Degousee N, Leon AJ, Xu L, Paquette SG, Kanagasabai T, Fang Y, Rubino S, Rubin B, Kelvin DJ, Kelvin AA. 2012. Differential pathological and immune responses in newly weaned ferrets are associated with a mild clinical outcome of pandemic 2009 H1N1 infection. J Virol 86:13187–13201. doi: 10.1128/JVI.01456-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paquette SG, Huang SS, Banner D, Xu L, Leomicronn A, Kelvin AA, Kelvin DJ. 2014. Impaired heterologous immunity in aged ferrets during sequential influenza A H1N1 infection. Virology 464-465:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govorkova EA, Rehg JE, Krauss S, Yen HL, Guan Y, Peiris M, Nguyen TD, Hanh TH, Puthavathana P, Long HT, Buranathai C, Lim W, Webster RG, Hoffmann E. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J Virol 79:2191–2198. doi: 10.1128/JVI.79.4.2191-2198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaraket H, Baranovich T, Kaplan BS, Carter R, Song MS, Paulson JC, Rehg JE, Bahl J, Crumpton JC, Seiler J, Edmonson M, Wu G, Karlsson E, Fabrizio T, Zhu H, Guan Y, Husain M, Schultz-Cherry S, Krauss S, McBride R, Webster RG, Govorkova EA, Zhang J, Russell CJ, Webby RJ. 2015. Mammalian adaptation of influenza A(H7N9) virus is limited by a narrow genetic bottleneck. Nat Commun 6:6553. doi: 10.1038/ncomms7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustin KM, Maines TR, Belser JA, van Hoeven N, Lu X, Dong L, Isakova-Sivak I, Chen LM, Voeten JT, Heldens JG, van den Bosch H, Cox NJ, Tumpey TM, Klimov AI, Rudenko L, Donis RO, Katz JM. 2011. Comparative immunogenicity and cross-clade protective efficacy of mammalian cell-grown inactivated and live attenuated H5N1 reassortant vaccines in ferrets. J Infect Dis 204:1491–1499. doi: 10.1093/infdis/jir596. [DOI] [PubMed] [Google Scholar]
- 16.Lee DH, Kim JI, Lee JW, Chung WH, Park JK, Lee YN, Han JS, Kim HY, Lee SW, Song CS. 2014. Quantitative measurement of influenza virus replication using consecutive bronchoalveolar lavage in the lower respiratory tract of a ferret model. J Vet Sci 15:439–442. doi: 10.4142/jvs.2014.15.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews SF, Kaur K, Pauli NT, Huang M, Huang Y, Wilson PC. 2015. High preexisting serological antibody levels correlate with diversification of the influenza vaccine response. J Virol 89:3308–3317. doi: 10.1128/JVI.02871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suguitan AL Jr, Zengel JR, Jacobson S, Gee S, Cetz J, Cha P, Chen Z, Broome R, Jin H. 2014. Influenza H1N1pdm-specific maternal antibodies offer limited protection against wild-type virus replication and influence influenza vaccination in ferrets. Influenza Other Respir Viruses 8:169–176. doi: 10.1111/irv.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. 2007. Transmission of influenza A in human beings. Lancet Infect Dis 7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 20.Belser JA, Bridges CB, Katz JM, Tumpey TM. 2009. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg Infect Dis 15:859–865. doi: 10.3201/eid1506.090072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu X, Cho D, Hall H, Rowe T, Sung H, Kim W, Kang C, Mo I, Cox N, Klimov A, Katz J. 2003. Pathogenicity and antigenicity of a new influenza A (H5N1) virus isolated from duck meat. J Med Virol 69:553–559. doi: 10.1002/jmv.10344. [DOI] [PubMed] [Google Scholar]
- 22.Paquette SG, Banner D, Huang SS, Almansa R, Leon A, Xu L, Bartoszko J, Kelvin DJ, Kelvin AA. 2015. Influenza transmission in the mother-infant dyad leads to severe disease, mammary gland infection, and pathogenesis by regulating host responses. PLoS Pathog 11:e1005173. doi: 10.1371/journal.ppat.1005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lednicky JA, Hamilton SB, Tuttle RS, Sosna WA, Daniels DE, Swayne DE. 2010. Ferrets develop fatal influenza after inhaling small particle aerosols of highly pathogenic avian influenza virus A/Vietnam/1203/2004 (H5N1). Virol J 7:231. doi: 10.1186/1743-422X-7-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustin KM, Belser JA, Wadford DA, Pearce MB, Katz JM, Tumpey TM, Maines TR. 2011. Influenza virus aerosol exposure and analytical system for ferrets. Proc Natl Acad Sci U S A 108:8432–8437. doi: 10.1073/pnas.1100768108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertran K, Swayne DE. 2014. High doses of highly pathogenic avian influenza virus in chicken meat are required to infect ferrets. Vet Res 45:60. doi: 10.1186/1297-9716-45-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipatov AS, Kwon YK, Pantin-Jackwood MJ, Swayne DE. 2009. Pathogenesis of H5N1 influenza virus infections in mice and ferret models differs according to respiratory tract or digestive system exposure. J Infect Dis 199:717–725. doi: 10.1086/596740. [DOI] [PubMed] [Google Scholar]
- 27.Belser JA, Gustin KM, Maines TR, Pantin-Jackwood MJ, Katz JM, Tumpey TM. 2012. Influenza virus respiratory infection and transmission following ocular inoculation in ferrets. PLoS Pathog 8:e1002569. doi: 10.1371/journal.ppat.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belser JA, Gustin KM, Katz JM, Maines TR, Tumpey TM. 2014. Influenza virus infectivity and virulence following ocular-only aerosol inoculation of ferrets. J Virol 88:9647–9654. doi: 10.1128/JVI.01067-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bodewes R, Kreijtz JH, van Amerongen G, Fouchier RA, Osterhaus AD, Rimmelzwaan GF, Kuiken T. 2011. Pathogenesis of influenza A/H5N1 virus infection in ferrets differs between intranasal and intratracheal routes of inoculation. Am J Pathol 179:30–36. doi: 10.1016/j.ajpath.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore IN, Lamirande EW, Paskel M, Donahue D, Qin J, Subbarao K. 2014. Severity of clinical disease and pathology in ferrets experimentally infected with influenza viruses is influenced by inoculum volume. J Virol 88:13879–13891. doi: 10.1128/JVI.02341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamelin ME, Baz M, Bouhy X, Beaulieu E, Dube K, Mallett C, Boivin G. 2011. Reduced airborne transmission of oseltamivir-resistant pandemic A/H1N1 virus in ferrets. Antivir Ther 16:775–779. doi: 10.3851/IMP1794. [DOI] [PubMed] [Google Scholar]
- 32.Oh DY, Lowther S, McCaw JM, Sullivan SG, Leang SK, Haining J, Arkinstall R, Kelso A, McVernon J, Barr IG, Middleton D, Hurt AC. 2014. Evaluation of oseltamivir prophylaxis regimens for reducing influenza virus infection, transmission and disease severity in a ferret model of household contact. J Antimicrob Chemother 69:2458–2469. doi: 10.1093/jac/dku146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varble A, Albrecht RA, Backes S, Crumiller M, Bouvier NM, Sachs D, Garcia-Sastre A, tenOever BR. 2014. Influenza A virus transmission bottlenecks are defined by infection route and recipient host. Cell Host Microbe 16:691–700. doi: 10.1016/j.chom.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tellier R. 2007. Transmission of influenza A in human beings. Lancet Infect Dis 7:759–760. (Author reply, 7:761–753.) doi: 10.1016/S1473-3099(07)70271-2. [DOI] [PubMed] [Google Scholar]
- 35.Alford RH, Kasel JA, Gerone PJ, Knight V. 1966. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med 122:800–804. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 36.Marriott AC, Dove BK, Whittaker CJ, Bruce C, Ryan KA, Bean TJ, Rayner E, Pearson G, Taylor I, Dowall S, Plank J, Newman E, Barclay WS, Dimmock NJ, Easton AJ, Hallis B, Silman NJ, Carroll MW. 2014. Low dose influenza virus challenge in the ferret leads to increased virus shedding and greater sensitivity to oseltamivir. PLoS One 9:e94090. doi: 10.1371/journal.pone.0094090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts KL, Shelton H, Scull M, Pickles R, Barclay WS. 2011. Lack of transmission of a human influenza virus with avian receptor specificity between ferrets is not due to decreased virus shedding but rather a lower infectivity in vivo. J Gen Virol 92:1822–1831. doi: 10.1099/vir.0.031203-0. [DOI] [PubMed] [Google Scholar]
- 38.Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, Falcon A, Nguyen TH, Mai le Q, Sedyaningsih ER, Harun S, Tumpey TM, Donis RO, Cox NJ, Subbarao K, Katz JM. 2006. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci U S A 103:12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson S, Van Hoeven N, Chen LM, Maines TR, Cox NJ, Katz JM, Donis RO. 2009. Reassortment between avian H5N1 and human H3N2 influenza viruses in ferrets: a public health risk assessment. J Virol 83:8131–8140. doi: 10.1128/JVI.00534-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song MS, Baek YH, Pascua PN, Kwon HI, Kim EH, Park SJ, Kim SM, Kim YI, Choi WS, Kim EG, Kim CJ, Choi YK. 2016. Growth and pathogenic potential of naturally selected reassortants after coinfection with pandemic H1N1 and highly pathogenic avian influenza H5N1 viruses. J Virol 90:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurt AC, Nor'e SS, McCaw JM, Fryer HR, Mosse J, McLean AR, Barr IG. 2010. Assessing the viral fitness of oseltamivir-resistant influenza viruses in ferrets, using a competitive-mixtures model. J Virol 84:9427–9438. doi: 10.1128/JVI.00373-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huber VC, McCullers JA. 2006. Live attenuated influenza vaccine is safe and immunogenic in immunocompromised ferrets. J Infect Dis 193:677–684. doi: 10.1086/500247. [DOI] [PubMed] [Google Scholar]
- 43.van der Vries E, Stittelaar KJ, van Amerongen G, Veldhuis Kroeze EJ, de Waal L, Fraaij PL, Meesters RJ, Luider TM, van der Nagel B, Koch B, Vulto AG, Schutten M, Osterhaus AD. 2013. Prolonged influenza virus shedding and emergence of antiviral resistance in immunocompromised patients and ferrets. PLoS Pathog 9:e1003343. doi: 10.1371/journal.ppat.1003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweet C, Toms GL, Smith H. 1977. The pregnant ferret as a model for studying the congenital effects of influenza virus infection in utero: infection of foetal tissues in organ culture and in vivo. Br J Exp Pathol 58:113–123. [PMC free article] [PubMed] [Google Scholar]
- 45.Peltola VT, Boyd KL, McAuley JL, Rehg JE, McCullers JA. 2006. Bacterial sinusitis and otitis media following influenza virus infection in ferrets. Infect Immun 74:2562–2567. doi: 10.1128/IAI.74.5.2562-2567.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh DY, Hurt AC. 2016. Using the ferret as an animal model for investigating influenza antiviral effectiveness. Front Microbiol 7:80. doi: 10.3389/fmicb.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maines TR, Belser JA, Gustin KM, van Hoeven N, Zeng H, Svitek N, von Messling V, Katz JM, Tumpey TM. 2012. Local innate immune responses and influenza virus transmission and virulence in ferrets. J Infect Dis 205:474–485. doi: 10.1093/infdis/jir768. [DOI] [PubMed] [Google Scholar]
- 48.Kugel D, Kochs G, Obojes K, Roth J, Kobinger GP, Kobasa D, Haller O, Staeheli P, von Messling V. 2009. Intranasal administration of alpha interferon reduces seasonal influenza A virus morbidity in ferrets. J Virol 83:3843–3851. doi: 10.1128/JVI.02453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts KL, Shelton H, Stilwell P, Barclay WS. 2012. Transmission of a 2009 H1N1 pandemic influenza virus occurs before fever is detected, in the ferret model. PLoS One 7:e43303. doi: 10.1371/journal.pone.0043303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reuman PD, Keely S, Schiff GM. 1989. Assessment of signs of influenza illness in the ferret model. J Virol Methods 24:27–34. doi: 10.1016/0166-0934(89)90004-9. [DOI] [PubMed] [Google Scholar]
- 51.Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol 76:4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meunier I, Embury-Hyatt C, Stebner S, Gray M, Bastien N, Li Y, Plummer F, Kobinger GP, von Messling V. 2012. Virulence differences of closely related pandemic 2009 H1N1 isolates correlate with increased inflammatory responses in ferrets. Virology 422:125–131. doi: 10.1016/j.virol.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 53.Oh DY, Barr IG, Hurt AC. 2015. A novel video tracking method to evaluate the effect of influenza infection and antiviral treatment on ferret activity. PLoS One 10:e0118780. doi: 10.1371/journal.pone.0118780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richard M, Herfst S, van den Brand JM, Lexmond P, Bestebroer TM, Rimmelzwaan GF, Koopmans M, Kuiken T, Fouchier RA. 2015. Low virulence and lack of airborne transmission of the Dutch highly pathogenic avian influenza virus H5N8 in ferrets. PLoS One 10:e0129827. doi: 10.1371/journal.pone.0129827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yen HL, Lipatov AS, Ilyushina NA, Govorkova EA, Franks J, Yilmaz N, Douglas A, Hay A, Krauss S, Rehg JE, Hoffmann E, Webster RG. 2007. Inefficient transmission of H5N1 influenza viruses in a ferret contact model. J Virol 81:6890–6898. doi: 10.1128/JVI.00170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belser JA, Gustin KM, Maines TR, Blau DM, Zaki SR, Katz JM, Tumpey TM. 2011. Pathogenesis and transmission of triple-reassortant swine H1N1 influenza viruses isolated before the 2009 H1N1 pandemic. J Virol 85:1563–1572. doi: 10.1128/JVI.02231-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lakdawala SS, Jayaraman A, Halpin RA, Lamirande EW, Shih AR, Stockwell TB, Lin X, Simenauer A, Hanson CT, Vogel L, Paskel M, Minai M, Moore I, Orandle M, Das SR, Wentworth DE, Sasisekharan R, Subbarao K. 2015. The soft palate is an important site of adaptation for transmissible influenza viruses. Nature 526:122–125. doi: 10.1038/nature15379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, Naghdaliyev A, Peiris JS, Shindo N, Soeroso S, Uyeki TM, Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus. 2008. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 358:261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 59.Belser JA, Maines TR, Tumpey TM, Katz JM. 2010. Influenza A virus transmission: contributing factors and clinical implications. Expert Rev Mol Med 12:e39. doi: 10.1017/S1462399410001705. [DOI] [PubMed] [Google Scholar]
- 60.Trock SC, Burke SA, Cox NJ. 2015. Development of framework for assessing influenza virus pandemic risk. Emerg Infect Dis 21:1372–1378. doi: 10.3201/eid2108.141086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buhnerkempe MG, Gostic K, Park M, Ahsan P, Belser JA, Lloyd-Smith JO. 2015. Mapping influenza transmission in the ferret model to transmission in humans. eLife 4:e07969. doi: 10.7554/eLife.07969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herlocher ML, Elias S, Truscon R, Harrison S, Mindell D, Simon C, Monto AS. 2001. Ferrets as a transmission model for influenza: sequence changes in HA1 of type A (H3N2) virus. J Infect Dis 184:542–546. doi: 10.1086/322801. [DOI] [PubMed] [Google Scholar]
- 63.Andrewes CH, Glover RE. 1941. Spread of infection from the respiratory tract of the ferret. I. Transmission of influenza A virus. Br J Exp Pathol 22:91–97. [Google Scholar]
- 64.Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, Donis R, Busch J, McBride R, Paulson JC, Katz JM, Tumpey TM. 2008. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc Natl Acad Sci U S A 105:7558–7563. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Hoeven N, Pappas C, Belser JA, Maines TR, Zeng H, Garcia-Sastre A, Sasisekharan R, Katz JM, Tumpey TM. 2009. Human HA and polymerase subunit PB2 proteins confer transmission of an avian influenza virus through the air. Proc Natl Acad Sci U S A 106:3366–3371. doi: 10.1073/pnas.0813172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linster M, van Boheemen S, de Graaf M, Schrauwen EJ, Lexmond P, Manz B, Bestebroer TM, Baumann J, van Riel D, Rimmelzwaan GF, Osterhaus AD, Matrosovich M, Fouchier RA, Herfst S. 2014. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 157:329–339. doi: 10.1016/j.cell.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pizzorno A, Abed Y, Bouhy X, Beaulieu E, Mallett C, Russell R, Boivin G. 2012. Impact of mutations at residue I223 of the neuraminidase protein on the resistance profile, replication level, and virulence of the 2009 pandemic influenza virus. Antimicrob Agents Chemother 56:1208–1214. doi: 10.1128/AAC.05994-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oxford JS, Lambkin R, Guralnik M, Rosenbloom RA, Petteruti MP, Digian K, LeFante C. 2007. In vivo prophylactic activity of QR-435 against H3N2 influenza virus infection. Am J Ther 14:462–468. doi: 10.1097/MJT.0b013e3180a7206e. [DOI] [PubMed] [Google Scholar]
- 69.Karlsson EA, Meliopoulos VA, Savage C, Livingston B, Mehle A, Schultz-Cherry S. 2015. Visualizing real-time influenza virus infection, transmission and protection in ferrets. Nat Commun 6:6378. doi: 10.1038/ncomms7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koster F, Gouveia K, Zhou Y, Lowery K, Russell R, MacInnes H, Pollock Z, Layton RC, Cromwell J, Toleno D, Pyle J, Zubelewicz M, Harrod K, Sampath R, Hofstadler S, Gao P, Liu Y, Cheng YS. 2012. Exhaled aerosol transmission of pandemic and seasonal H1N1 influenza viruses in the ferret. PLoS One 7:e33118. doi: 10.1371/journal.pone.0033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inagaki K, Song MS, Crumpton JC, DeBeauchamp J, Jeevan T, Tuomanen EI, Webby RJ, Hakim H. 2016. Correlation between the interval of influenza virus infectivity and results of diagnostic assays in a ferret model. J Infect Dis 213:407–410. doi: 10.1093/infdis/jiv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutton TC, Finch C, Shao H, Angel M, Chen H, Capua I, Cattoli G, Monne I, Perez DR. 2014. Airborne transmission of highly pathogenic H7N1 influenza virus in ferrets. J Virol 88:6623–6635. doi: 10.1128/JVI.02765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sorrell EM, Wan H, Araya Y, Song H, Perez DR. 2009. Minimal molecular constraints for respiratory droplet transmission of an avian-human H9N2 influenza A virus. Proc Natl Acad Sci U S A 106:7565–7570. doi: 10.1073/pnas.0900877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336:1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, Kong H, Gu C, Li X, Liu J, Wang G, Chen Y, Liu L, Liang L, Li Y, Fan J, Wang J, Li W, Guan L, Li Q, Yang H, Chen P, Jiang L, Guan Y, Xin X, Jiang Y, Tian G, Wang X, Qiao C, Li C, Bu Z, Chen H. 2013. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 341:410–414. doi: 10.1126/science.1240532. [DOI] [PubMed] [Google Scholar]
- 76.Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, van de Vijver D, Boucher CA, Koopmans M, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA. 2009. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325:481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lowen AC, Mubareka S, Steel J, Palese P. 2007. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog 3:1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steel J, Palese P, Lowen AC. 2011. Transmission of a 2009 pandemic influenza virus shows a sensitivity to temperature and humidity similar to that of an H3N2 seasonal strain. J Virol 85:1400–1402. doi: 10.1128/JVI.02186-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gustin KM, Belser JA, Veguilla V, Zeng H, Katz JM, Tumpey TM, Maines TR. 2015. Environmental conditions affect exhalation of H3N2 seasonal and variant influenza viruses and respiratory droplet transmission in ferrets. PLoS One 10:e0125874. doi: 10.1371/journal.pone.0125874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Houser KV, Pearce MB, Katz JM, Tumpey TM. 2013. Impact of prior seasonal H3N2 influenza vaccination or infection on protection and transmission of emerging variants of influenza A(H3N2)v virus in ferrets. J Virol 87:13480–13489. doi: 10.1128/JVI.02434-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guarnaccia T, Carolan LA, Maurer-Stroh S, Lee RT, Job E, Reading PC, Petrie S, McCaw JM, McVernon J, Hurt AC, Kelso A, Mosse J, Barr IG, Laurie KL. 2013. Antigenic drift of the pandemic 2009 A(H1N1) influenza virus in A ferret model. PLoS Pathog 9:e1003354. doi: 10.1371/journal.ppat.1003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carter DM, Bloom CE, Nascimento EJ, Marques ET, Craigo JK, Cherry JL, Lipman DJ, Ross TM. 2013. Sequential seasonal H1N1 influenza virus infections protect ferrets against novel 2009 H1N1 influenza virus. J Virol 87:1400–1410. doi: 10.1128/JVI.02257-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ellebedy AH, Ducatez MF, Duan S, Stigger-Rosser E, Rubrum AM, Govorkova EA, Webster RG, Webby RJ. 2011. Impact of prior seasonal influenza vaccination and infection on pandemic A (H1N1) influenza virus replication in ferrets. Vaccine 29:3335–3339. doi: 10.1016/j.vaccine.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pascua PN, Song MS, Lee JH, Park KJ, Kwon HI, Baek YH, Hong SP, Rho JB, Kim CJ, Poo H, Ryoo TS, Sung MH, Choi YK. 2009. Evaluation of the efficacy and cross-protectivity of recent human and swine vaccines against the pandemic (H1N1) 2009 virus infection. PLoS One 4:e8431. doi: 10.1371/journal.pone.0008431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baker SF, Guo H, Albrecht RA, Garcia-Sastre A, Topham DJ, Martinez-Sobrido L. 2013. Protection against lethal influenza with a viral mimic. J Virol 87:8591–8605. doi: 10.1128/JVI.01081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stark GV, Long JP, Ortiz DI, Gainey M, Carper BA, Feng J, Miller SM, Bigger JE, Vela EM. 2013. Clinical profiles associated with influenza disease in the ferret model. PLoS One 8:e58337. doi: 10.1371/journal.pone.0058337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu H, Wang D, Kelvin DJ, Li L, Zheng Z, Yoon SW, Wong SS, Farooqui A, Wang J, Banner D, Chen R, Zheng R, Zhou J, Zhang Y, Hong W, Dong W, Cai Q, Roehrl MH, Huang SS, Kelvin AA, Yao T, Zhou B, Chen X, Leung GM, Poon LL, Webster RG, Webby RJ, Peiris JS, Guan Y, Shu Y. 2013. Infectivity, transmission, and pathology of human-isolated H7N9 influenza virus in ferrets and pigs. Science 341:183–186. doi: 10.1126/science.1239844. [DOI] [PubMed] [Google Scholar]
- 89.Belser JA, Gustin KM, Pearce MB, Maines TR, Zeng H, Pappas C, Sun X, Carney PJ, Villanueva JM, Stevens J, Katz JM, Tumpey TM. 2013. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature 501:556–559. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S, Murakami S, Yamayoshi S, Iwatsuki-Horimoto K, Sakoda Y, Takashita E, McBride R, Noda T, Hatta M, Imai H, Zhao D, Kishida N, Shirakura M, de Vries RP, Shichinohe S, Okamatsu M, Tamura T, Tomita Y, Fujimoto N, Goto K, Katsura H, Kawakami E, Ishikawa I, Watanabe S, Ito M, Sakai-Tagawa Y, Sugita Y, Uraki R, Yamaji R, Eisfeld AJ, Zhong G, Fan S, Ping J, Maher EA, Hanson A, Uchida Y, Saito T, Ozawa M, Neumann G, Kida H, Odagiri T, Paulson JC, Hasegawa H, Tashiro M, Kawaoka Y. 2013. Characterization of H7N9 influenza A viruses isolated from humans. Nature 501:551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Richard M, Schrauwen EJ, de Graaf M, Bestebroer TM, Spronken MI, van Boheemen S, de Meulder D, Lexmond P, Linster M, Herfst S, Smith DJ, van den Brand JM, Burke DF, Kuiken T, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2013. Limited airborne transmission of H7N9 influenza A virus between ferrets. Nature 501:560–563. doi: 10.1038/nature12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu L, Bao L, Deng W, Dong L, Zhu H, Chen T, Lv Q, Li F, Yuan J, Xiang Z, Gao K, Xu Y, Huang L, Li Y, Liu J, Yao Y, Yu P, Li X, Huang W, Zhao X, Lan Y, Guo J, Yong W, Wei Q, Chen H, Zhang L, Qin C. 2014. Novel avian-origin human influenza A(H7N9) can be transmitted between ferrets via respiratory droplets. J Infect Dis 209:551–556. doi: 10.1093/infdis/jit474. [DOI] [PubMed] [Google Scholar]