ABSTRACT
Spiroplasma bacteria are highly motile bacteria with no cell wall and a helical morphology. This clade includes many vertically transmitted insect endosymbionts, including Spiroplasma poulsonii, a natural endosymbiont of Drosophila melanogaster. S. poulsonii bacteria are mainly found in the hemolymph of infected female flies and exhibit efficient vertical transmission from mother to offspring. As is the case for many facultative endosymbionts, S. poulsonii can manipulate the reproduction of its host; in particular, S. poulsonii induces male killing in Drosophila melanogaster. Here, we analyze the morphology of S. poulsonii obtained from the hemolymph of infected Drosophila. This endosymbiont was not only found as long helical filaments, as previously described, but was also found in a Y-shaped form. The use of electron microscopy, immunogold staining of the FtsZ protein, and antibiotic treatment unambiguously linked the Y shape of S. poulsonii to cell division. Observation of the Y shape in another Spiroplasma, S. citri, and anecdotic observations from the literature suggest that cell division by longitudinal scission might be prevalent in the Spiroplasma clade. Our study is the first to report the Y-shape mode of cell division in an endosymbiotic bacterium and adds Spiroplasma to the so far limited group of bacteria known to utilize this cell division mode.
IMPORTANCE
Most bacteria rely on binary fission, which involves elongation of the bacteria and DNA replication, followed by splitting into two parts. Examples of bacteria with a Y-shape longitudinal scission remain scarce. Here, we report that Spiroplasma poulsonii, an endosymbiotic bacterium living inside the fruit fly Drosophila melanogaster, divide with the longitudinal mode of cell division. Observations of the Y shape in another Spiroplasma, S. citri, suggest that this mode of scission might be prevalent in the Spiroplasma clade. Spiroplasma bacteria are wall-less bacteria with a distinctive helical shape, and these bacteria are always associated with arthropods, notably insects. Our study raises the hypothesis that this mode of cell division by longitudinal scission could be linked to the symbiotic mode of life of these bacteria.
Observation
Spiroplasma bacteria are members of the Mollicutes class, a wall-less eubacterial group related to Gram-positive bacteria (1). These bacteria exhibit a distinctive helical shape and high motility. Spiroplasma bacteria are widely associated with arthropods and found in 5 to 10% of all insect species. While some species are horizontally transmitted insect pathogens or commensals in the gut, other lineages exhibit transovarial vertical transmission from mother to offspring. Spiroplasma poulsonii is one of the species exhibiting vertical transmission, and together with Wolbachia, are two natural endosymbionts known from Drosophila melanogaster.
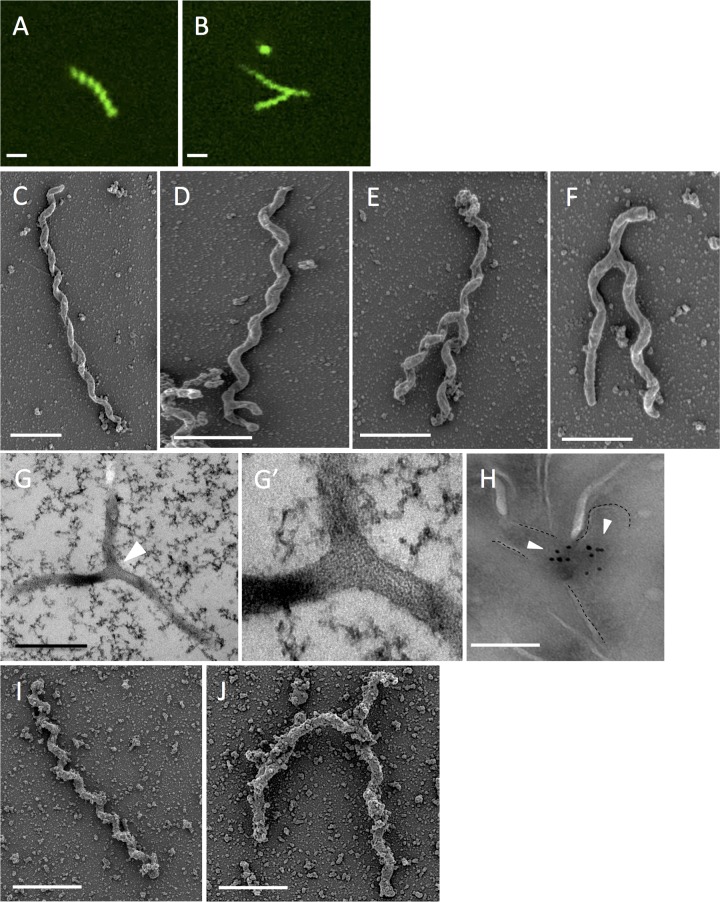
S. poulsonii bacteria are primarily found in the hemolymph of adult flies, where they are neither detected nor affected by the Drosophila immune system. The proliferation of Spiroplasma is constrained by the availability of hemolymph lipids (2, 3). S. poulsonii uses the yolk uptake machinery to colonize the germ line during oogenesis to ensure vertical transmission (4). Similar to other facultative endosymbionts, S. poulsonii can affect the sex ratio of its host by inducing male killing (causing death of male embryos at early time points) (5). It has been proposed that reproductive manipulation and the ability of Spiroplasma to protect Drosophila against certain parasites are among the main driving forces that maintain this facultative endosymbiont in fly populations (6). There have been a number of studies recently investigating how Spiroplasma impacts its insect host; however, little is known about the biology of S. poulsonii itself. This led us to investigate the morphology of S. poulsonii (strain MSRO; for experimental methods, see Text S1 in the supplemental material) derived from fly hemolymph extracts. We chose 1- to 2-week-old females, since this time period corresponds to the exponential growth phase of this endosymbiont (2). Use of the nucleic acid stain SYTO9 reveals the presence of Spiroplasma with a classical helical and linear morphology (Fig. 1A). Surprisingly, up to 30% of bacteria show a Y-shape morphology, which has not been previously characterized (Fig. 1B). Scanning electron microscopy (SEM) of hemolymph samples from Spiroplasma-infected D. melanogaster females also confirms the presence of Y-shaped Spiroplasma. Typical linear bacteria have 5 to 15 helices, with the length of the bacteria ranging from 0.8 µm to 5 µm and the width ranging from 50 to 100 nm (Fig. 1C). SEM confirmed the presence of Y-shaped bacteria with one main branch of various sizes, splitting into two thinner arms of identical length (Fig. 1D, E, and F). To exclude the possibility that the Y-shaped form results from the twisting of two bacteria, we performed high-pressure freezing followed by transmission electron microscopy (TEM) on hemolymph samples. Our results confirm that the Y shapes correspond to one bacterium with cytoplasmic continuity between the two arms and the main trunk (Fig. 1G, white arrowhead; high-magnification view shown in Fig. 1G′).
FIG 1 .
Presence of Y-shaped S. poulsonii in Drosophila hemolymph samples. (A and B) Fluorescence microscopy images showing SYTO9-stained S. poulsonii from freshly extracted hemolymph from 1-week-old Drosophila melanogaster flies. Bars, 1 µm. (C to F) SEM of S. poulsonii extracted from 1-week-old Spiroplasma-infected female flies. S. poulsonii can be found as one elongated body (C) or with a Y-shape conformation (D to F) with variation in the length of the arms. Bars, 1 µm. (G and zoom in G′) TEM of S. poulsonii from freshly extracted Drosophila hemolymph. The white arrowhead shows the branching. Bar, 1 µm. (H) Immunogold labeling pattern of the FtsZ protein with an anti-FtsZ antibody. Y-shaped bacteria have two FtsZ protein aggregates. Bar, 200 nm. (I and J) SEM of in vitro-cultured S. citri. S. citri can be found as one elongated body (I) or with a Y-shaped conformation (J). Bars, 1 µm. The presence of aggregates might be due to protein enrichment in the medium used to cultivate S. citri.
Cytokinesis is the process by which the cytoplasm of one cell is divided in two cells. In bacteria, it involves the guanosine triphosphatase (GTPase) FtsZ (filamenting temperature-sensitive Z), which assembles at the inner face of the bacterial membrane to form a Z-ring, which subsequently recruits molecules to provide the contractile force for membrane invagination (7, 8). One gene encoding a FtsZ protein is present in the S. poulsonii genome (9). To assess whether the S. poulsonii Y shape is indeed linked to cell division, we performed immunogold labeling against FtsZ protein using a commercially available antibody. Figure 1H shows accumulation of signals corresponding to FtsZ in the branching area of Y-shape bacteria, with gold particles forming two small aggregates at the beginning of the two arms (white arrowheads). No gold particle aggregate was observed in linear bacteria.
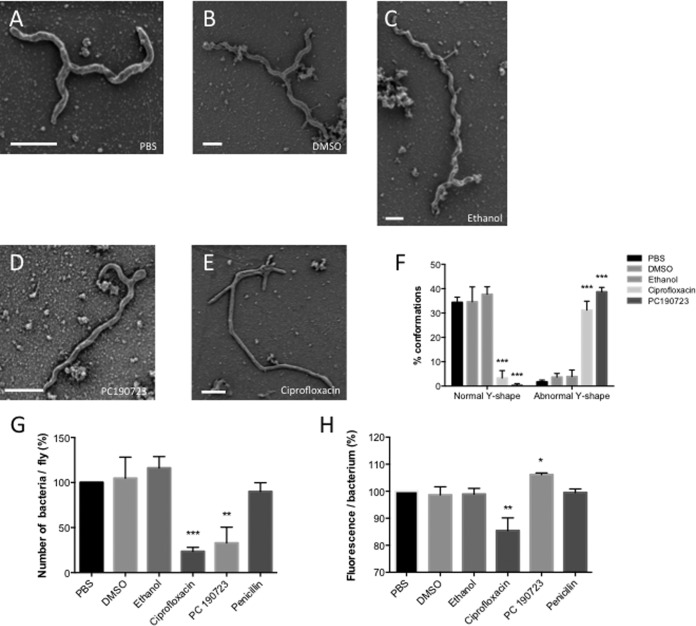
To confirm our conclusion, we analyzed the impacts of antibiotics that affect cell division on S. poulsonii morphology. Antibiotics were directly injected into the body cavities of Spiroplasma-infected flies, and hemolymph samples were collected 4 days later for observations and 1 week later for bacterial quantification in flies. Injection of PC190723 (dissolved in dimethyl sulfoxide [DMSO]), a cell division inhibitor known to block the GTPase activity of FtsZ leads to aberrant Y-shape morphology with an aborted arm situated at one extremity of the main trunk (Fig. 2D; quantified in Fig. 2F). Injection of ciprofloxacin (dissolved in ethanol), a fluoroquinolone antibiotic which inhibits DNA gyrase activity, and therefore DNA replication, results in abnormal shaped Spiroplasma with no spiral and multibranching with 3 to 5 arms of various sizes linked to the main trunk (Fig. 2E; quantified in Fig. 2F). We speculate that this morphology results from multiple scission departures that aborted precociously. This also suggests that DNA replication is not mandatory for the initiation of branching. Injection of DMSO or ethanol, the two antibiotic solvents, or penicillin, an antibiotic that targets peptidoglycan, a cell wall component absent in Spiroplasma, did not affect S. poulsonii morphology, indicating that the effects are caused by the two molecules (Fig. 2A to C). Figure 2G shows that PC190723 and ciprofloxacin antibiotic that affect Spiroplasma morphology also reduce bacterial growth as measured by quantitative PCR (qPCR).
FIG 2 .
Impacts of various antibiotics on the Y-shaped form of Spiroplasma. (A to E) SEM of S. poulsonii extracted from Spiroplasma-infected female flies injected with phosphate-buffered saline (PBS) (A), DMSO (B), ethanol (C), PC190723 (D), or ciprofloxacin (E). One-week-old females were injected with molecules, and hemolymph was extracted after 4 days. Bars, 500 nm. (F) Quantification of normal and abnormal Y-shape S. poulsonii by SEM in hemolymph samples collected from treated flies. ***, P < 0.005. Values are means plus standard deviations (SD) (error bars) of data pooled from three independent experiments with 100 bacteria for each count. Twenty flies were used to extract fresh hemolymph for each experiment. (G) Evaluation of bacterial count per fly following each treatment. Spiroplasma bacteria were counted 1 week after injection. **, P < 0.01; ***, P < 0.005. Values are means plus SD of data pooled from three independent experiments with 20 flies for each count. (H) Quantification of the amount of fluorescence per bacteria. DNA fluorescence per Spiroplasma was measured 4 days after injection. *, P < 0.05; **, P < 0.01. Values are means plus SD of data pooled from three independent experiments with 20 flies tested for each count.
SYTO9 DNA staining demonstrates the presence of DNA all along the arms of both linear and Y-shaped Spiroplasma (Fig. 1A and B). To investigate the link between DNA synthesis and Spiroplasma morphology, we analyze by qPCR the presence of DNA in bacteria treated with PC190723 and ciprofloxacin. Remarkably, PC190723-treated bacteria presented more fluorescence, which is consistent with the notion that FtsZ target drug inhibits cell division, but not DNA replication. In contrast, a lower DNA signal was observed upon treatment with the DNA synthesis inhibitor ciprofloxacin. Taken together, our data suggest that the production of a new arm occurs before replication of DNA but that the final scission of the two cells required DNA synthesis completion. Unfortunately, due to the high motility of S. poulsonii and their quick adherence (within minutes) to the substratum, we were unable to perform live imaging of S. poulsonii division.
Most bacteria rely on binary fission, which involved elongation of the bacteria and DNA replication before splitting into two parts. Examples of bacteria with a Y-shape division remain scarce. Bacteria with Y-shape division include Actinomyces (10) and Bifidobacterium (11). It is interesting to connect our observation to a recent study revealing the presence of the Y shape in another symbiotic bacterium. Leisch et al. have shown that the gammaproteobacterium ectosymbiont, which attaches to the surface of the marine nematode Laxus oneistus, grows laterally and divides longitudinally (13). This mode of cell division is thought to be important for maintaining the attachment of both daughter cells to the nematode surface (12). In contrast to Spiroplasma, longitudinal scission takes place simultaneously on both extremities of the ectosymbiont. Y-shaped bacteria have also been observed in another symbiotic context that involves nitrogen-fixing Rhizobium and leguminous plants. Y-shaped Rhizobium bacteria are abundant in the nitrogen fixation zone of nodules, suggesting that this morphology could be induced by the plant host (14).
Our report adds S. poulsonii to the short list of bacteria with a longitudinal mode of cell division and is the first to show this feature in an insect endosymbiont. Screening of the literature reveals previous observations of branched morphologies in tick-associated Spiroplasma ixodetis (15), plant-associated Spiroplasma citri (16), and an unidentified Spiroplasma subspecies found in the Chinese mitten crab Eriocheir sinensis (17). However, Garnier et al. proposed that Spiroplasma citri divides by elongating before constricting to give two daughter cells (18). Careful analysis of S. citri cultures reveals the presence of the Y shape (Fig. 1I and J), suggesting that this mode of division could be rather prevalent in the Spiroplasma clade. An open question is to determine the nature of the selective pressure that leads to this mode of cell division. The prevalence of the Y shape in bacteria establishing symbiotic interactions with eukaryotes may allow the coupling of longitudinal fission with signals from the host, as proposed for Rhizobium or the nematode ectosymbionts. Alternatively, this mode of septation might be related to the motility of Spiroplasma, which utilizes long contractile fibers that run from one end of the cell to the other (19). Indeed, we can speculate that division begins at one extremity of the bacteria, following by longitudinal opening (“zip-progression”) of the cell, which could explain the presence of a main trunk and two arms of the same length, before the separation of the two daughter cells on the other extremity. A longitudinal scission would allow the preservation of the fiber network such that fibers are not cut in half. Future studies should search for the presence of other Y-shaped bacteria in other microbial symbioses and characterize their functional relevance.
SUPPLEMENTAL MATERIAL
Experimental methods. Download
ACKNOWLEDGMENTS
We thank Laure Béven (INRA Bordeaux, France), Haig Eskandarian, Melanie Blokesch, and David Adams (EPFL, Switzerland) for helpful discussions. We thank Jeremy Herren (ICIPE, Kenya) for comments on the manuscript and helpful suggestions, and Wen Bin Chng (EPFL, Switzerland) for careful reading.
Footnotes
Citation Ramond E, Maclachlan C, Clerc-Rosset S, Knott GW, Lemaitre B. 2016. Cell division by longitudinal scission in the insect endosymbiont Spiroplasma poulsonii. mBio 7(4):e00881-16. doi:10.1128/mBio.00881-16.
REFERENCES
- 1.Whitcomb RF. 1980. The genus Spiroplasma. Annu Rev Microbiol 34:677–709. doi: 10.1146/annurev.mi.34.100180.003333. [DOI] [PubMed] [Google Scholar]
- 2.Herren JK, Paredes JC, Schüpfer F, Arafah K, Bulet P, Lemaitre B. 2014. Insect endosymbiont proliferation is limited by lipid availability. Elife 3:e02964. doi: 10.7554/eLife.02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herren JK, Lemaitre B. 2011. Spiroplasma and host immunity: activation of humoral immune responses increases endosymbiont load and susceptibility to certain Gram-negative bacterial pathogens in Drosophila melanogaster. Cell Microbiol 13:1385–1396. doi: 10.1111/j.1462-5822.2011.01627. [DOI] [PubMed] [Google Scholar]
- 4.Herren JK, Paredes JC, Schüpfer F, Lemaitre B. 2013. Vertical transmission of a Drosophila endosymbiont via cooption of the yolk transport and internalization machinery. mBio 4:e00532-12. doi: 10.1128/mBio.00532-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson DL, Sakaguchi B, Hackett KJ, Whitcomb RF, Tully JG, Carle P, Bové JM, Adams JR, Konai M, Henegar RB. 1999. Spiroplasma poulsonii sp. nov., a new species associated with male-lethality in Drosophila willistoni, a neotropical species of fruit fly. Int J Syst Bacteriol 49:611–618. doi: 10.1099/00207713-49-2-611. [DOI] [PubMed] [Google Scholar]
- 6.Xie J, Tiner B, Vilchez I, Mateos M. 2011. Effect of the Drosophila endosymbiont Spiroplasma on parasitoid wasp development and on the reproductive fitness of wasp-attacked fly survivors. Evol Ecol 53:1065–1079. doi: 10.1007/s10682-010-9453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams DW, Errington J. 2009. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol 7:642–653. doi: 10.1038/nrmicro2198. [DOI] [PubMed] [Google Scholar]
- 8.Lutkenhaus J, Pichoff S, Du S. 2012. Bacterial cytokinesis: from Z ring to divisome. Cytoskeleton 69:778–790. doi: 10.1002/cm.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paredes JC, Herren JK, Schüpfer F, Marin R, Claverol S, Kuo C-H, Lemaitre B, Béven L. 2015. Genome sequence of the Drosophila melanogaster male-killing Spiroplasma strain MSRO endosymbiont. mBio 6:e02437-14. doi: 10.1128/mBio.02437-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden GHW. 1996. Actinomyces, Propionibacterium propionicus, and Streptomyces. In Baron S (ed), Medical microbiology, 4th ed. University of Texas Medical Branch, Galveston, TX. [PubMed] [Google Scholar]
- 11.Husain I, Poupard JA, Norris RF. 1972. Influence of nutrition on the morphology of a strain of Bifidobacterium bifidum. J Bacteriol 111:841–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polz MF, Felbeck H, Novak R, Nebelsick M, Ott JA. 1992. Chemoautotrophic, sulfur-oxidizing symbiotic bacteria on marine nematodes: morphological and biochemical characterization. Microb Ecol 24:313–329. doi: 10.1007/BF00167789. [DOI] [PubMed] [Google Scholar]
- 13.Leisch N, Verheul J, Heindl NR, Gruber-Vodicka HR, Pende N, den Blaauwen T, Bulgheresi S. 2012. Growth in width and FtsZ ring longitudinal positioning in a gammaproteobacterial symbiont. Curr Biol 22:R831–R832. doi: 10.1016/j.cub.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Kondorosi E, Mergaert P, Kereszt A. 2013. A paradigm for endosymbiotic life: cell differentiation of Rhizobium bacteria provoked by host plant factors. Annu Rev Microbiol 67:611–628. doi: 10.1146/annurev-micro-092412-155630. [DOI] [PubMed] [Google Scholar]
- 15.Tully JG, Rose DL, Yunker CE, Carle P, Bové JM, Williamson DL, Whitcomb RF. 1995. Spiroplasma ixodetis sp. nov., a new species from Ixodes pacificus ticks collected in Oregon. Int J Syst Bacteriol 45:23–28. doi: 10.1099/00207713-45-1-23. [DOI] [PubMed] [Google Scholar]
- 16.Fudl-Allah AE-SA, Calavan EC. 1974. Cellular morphology and reproduction of the mycoplasmalike organism associated with citrus stubborn disease. Phytopathology 64:1309–1313. [Google Scholar]
- 17.Wang W, Chen J, Du K, Xu Z. 2004. Morphology of Spiroplasmas in the Chinese mitten crab Eriocheir sinensis associated with tremor disease. Res Microbiol 155:630–635. doi: 10.1016/j.resmic.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Garnier M, Clerc M, Bové JM. 1984. Growth and division of Spiroplasma citri: elongation of elementary helices. J Bacteriol 158:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaevitz JW, Lee JY, Fletcher DA. 2005. Spiroplasma swim by a processive change in body helicity. Cell 122:941–945. doi: 10.1016/j.cell.2005.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental methods. Download